Abstract
Objective
Combined small cell lung cancer (C-SCLC) is an uncommon subgroup of small cell lung cancer (SCLC) and few clinical data can be referred. Our study is to investigate the clinical features and prognostic factors of C-SCLC, as well as the role of multimodality treatment.
Methods
Between January 2004 and December 2012, patients with histologically diagnosed C-SCLC were retrospectively analyzed. The survivals were evaluated with the Kaplan-Meier method. Univariate and multivariate analyses were used to evaluate potential prognostic factors.
Results
One hundred and fourteen patients were enrolled, with a median age of 59 (range: 20−79) years old. The most common combined component was squamous cell carcinoma (52.6%). Among these patients, the disease was stage I, II, III and IV in 9.6%, 19.3%, 46.5% and 24.6% of the patients, respectively. Eighty patients (70.2%) received at least two of the three modalities containing chemotherapy, radiotherapy and surgery. The median follow-up was 32.5 months. The median time of overall survival (OS) was 26.2 months. On univariate analysis, smoking (P=0.029), Karnofsky performance score (KPS) <80 (P=0.000), advanced TNM stage (P=0.000), no surgery (P=0.010), positive resection margin (P=0.000), positive lymph nodes ≥4 (P=0.000), positive lymph node ratio >10% (P=0.000) and non-multimodality treatment (P=0.004) were associated with poor OS. Multivariate analysis confirmed that smoking, advanced TNM stage, positive resection margin and positive lymph nodes ratio >10% were poor prognostic features.
Conclusions
C-SCLC has a relatively early stage and good prognosis, which may due to the underestimated diagnosis in non-surgical patients. Multimodality therapy is recommended, especially for limited disease. Smoking, advanced TNM stage, positive resection margin and positive lymph nodes ratio >10% are poor prognostic factors.
Keywords: Combined small cell lung carcinoma, diagnosis, prognosis, multimodality therapy
Introduction
Combined small cell lung carcinoma (C-SCLC) is defined as small cell lung cancer (SCLC) combined with an additional component that consists of any of the histological types of non-small cell lung cancer (NSCLC), usually adenocarcinoma (AC), squamous cell carcinoma (SCC) or large cell carcinoma (LCC) (1-3). C-SCLC is comparatively uncommon and accounts for only 1%−3% of all SCLCs (2, 4, 5), but the incidence has increased in recent decades (6-9).
Due to few studies and reports on C-SCLC, the clinical characteristics, optimized treatment model and prognostic factors are not yet clear. With the increase of early diagnosis of lung cancer, more SCLC patients undergo surgery and have pathological examinations, which have led to more C-SCLC diagnoses recently (6-9). Therefore, it is particularly important to understand this disease further. Our study aimed to investigate the clinical features and prognostic factors of C-SCLC, as well as the role of multimodality treatment.
Materials and methods
Study population
From January 2004 to December 2012, patients with histologically diagnosed C-SCLC were enrolled. C-SCLC is defined as SCLC combined with an additional component that consists of any of the histological types of NSCLC. Pathological diagnosis was based on specimens from surgery, percutaneous transthoracic biopsy, transbronchial biopsy, lymph node biopsy, or metastatic tumor biopsy. Pre-treatment evaluations included physical and hematological examinations, chest computed tomography (CT) scans or positron emission tomography-computed tomography (PET-CT), bronchoscopy, ultrasound, brain magnetic resonance imaging (MRI) and bone scans. C-SCLC was staged according to the 7th edition of the American Joint Committee on Cancer (AJCC) tumor-node-metastasis (TNM) classification system and the Veteran’s Administration Lung Cancer Study Group (VALSG) classification system. The main treatments included surgery, chemotherapy and radiotherapy. The types of surgical resection included wedge resection, sleeve resection, lobectomy, and pneumonectomy. Chemotherapy regimens were selected according to the pathological type. Radiotherapy was delivered using the two-dimensional conventional radiotherapy (2D-CRT), three-dimensional conformal radiotherapy (3D-CRT) and intensity modulated radiotherapy (IMRT) techniques. The target volume contained the primary tumor or tumor bed plus regional lymph nodes.
Follow-up
Patients were followed-up every 3 months for the first year and then every 3 to 6 months thereafter. Overall survival (OS) was defined as the time between initial treatment and death or the last follow-up. Locoregional recurrence-free survival (LRFS) was defined as the time between initial treatment and the recurrence of the primary tumor or regional lymph node, death or the last follow-up. Distant metastasis-free survival (DMFS) was measured from the date of initial treatment to the date of distant metastasis (DM), death or the last follow-up.
Statistical analysis
The Kaplan-Meier method was used to estimate OS, LRFS and DMFS. The difference in survival between patients with different clinical, pathological and treatment characteristics was compared using the log-rank test. Significant predictors for death of C-SCLC from univariate analysis, as well as age and gender were included in the multivariate Cox’s proportional hazards mode (backward) to estimate the association between the predictors and outcomes, using hazard ratio (HR) and its 95% confidence interval (95% CI) as the indicators. Statistical significance was set as P<0.05 (two-sided).
Ethics
This retrospective study was approved by the Institutional Ethics Review Board at the Institutional Review Board of the Cancer Hospital & Institute, Chinese Academy of Medical Sciences & Peking Union Medical College (CAMS & PUMC). Informed consent was exempted by the board due to the retrospective nature of this research. Patient records were anonymized and de-identified prior to analysis.
Results
Patient characteristics
A total of 114 patients from the Department of Radiation Oncology, Cancer Institute & Hospital, CAMS & PUMC were enrolled in this study. Patient characteristics and treatments are listed in Table 1. The median age was 59 (range: 20−79) years old, and the male to female ratio was 4.4:1. Most patients had a history of smoking (n=90, 78.9%). C-SCLC developed predominantly in central sites (n=92, 80.7%). Most patients only had one kind of component (n=104, 91.2%). The most common combined component was SCC (n=60, 52.6%), followed by AC (n=37, 32.5%) and LCC (n=13, 11.4%). The disease was primarily categorized as stage III (n=53, 46.5%) and limited disease (LD; n=83, 72.8%).
1.
Patients’characteristics and univariate analysis of C-SCLC (N=114)
| Characteristics | n (%) | MST (month) | OS (%) | χ2 | P | ||
| 1-year | 3-year | 5-year | |||||
| C-SCLC, combinedsmall cell lung carcinoma; MST, median survivaltime; OS, overallsurvival; KPS, Karnofskyperformance score; AJCC, the AmericanJoint Committee on Cancer; VALSG, the Veteran’sAdministration Lung Cancer Study Group; ED, extended disease; LD, limited disease; SCC, squamouscell carcinoma; *, of the 66 patients receiving surgery, 65 patientshad detailed de-scriptions of MLN and resection margin for the analysis of prognostic factors;MLN, metastatic lymph node. | |||||||
| Gender | 2.307 | 0.129 | |||||
| Male | 93 (81.6) | 22.7 | 75.3 | 37.2 | 32.9 | ||
| Female | 21 (18.4) | 82.0 | 81.0 | 65.4 | 54.5 | ||
| Age (year) | 2.384 | 0.123 | |||||
| ≤60 | 68 (59.6) | 34.3 | 81.2 | 47.3 | 41.1 | ||
| >60 | 46 (40.4) | 18.9 | 69.7 | 36.3 | 32.3 | ||
| Smoking history | 4.772 | 0.029 | |||||
| Absence | 24 (21.1) | 82.0 | 87.5 | 64.1 | 56.1 | ||
| Presence | 90 (78.9) | 21.5 | 73.4 | 36.1 | 31.5 | ||
| KPS 21.353 | 0.000 | ||||||
| ≥80 | 101 (88.6) | 34.3 | 82.9 | 46.7 | 42.7 | ||
| <80 | 13 (11.4) | 8.0 | 30.8 | 15.4 | 0.0 | ||
| Tumor location | 0.401 | 0.527 | |||||
| Central type | 92 (80.7) | 26.2 | 76.7 | 41.2 | 34.8 | ||
| Peripheral type | 22 (19.3) | 26.2 | 76.4 | 50.0 | 50.0 | ||
| Tumor location | 3.092 | 0.079 | |||||
| Left lung | 52 (45.6) | 18.1 | 62.3 | 39.3 | 30.5 | ||
| Right lung | 62 (54.4) | 34.4 | 88.3 | 46.2 | 43.7 | ||
| Stage, AJCC 7th 18.010 | 0.000 | ||||||
| I | 11 (9.6) | − | 100 | 85.7 | 85.7 | ||
| II | 22 (19.3) | 62.2 | 85.9 | 65.6 | 57.4 | ||
| III | 53 (46.5) | 22.7 | 73.8 | 35.7 | 32.7 | ||
| IV | 28 (24.6) | 14.3 | 65.8 | 21.7 | 10.9 | ||
| Stage, VALSG | 10.661 | 0.001 | |||||
| ED | 31 (27.2) | 14.3 | 65.8 | 23.3 | 11.6 | ||
| LD | 83 (72.8) | 37.6 | 79.3 | 50.4 | 45.8 | ||
| Component | 1.656 | 0.198 | |||||
| SCC | 60 (52.6) | 37.6 | 82.1 | 51.3 | 43.7 | ||
| Non-SCC | 46 (40.4) | 21.5 | 67.8 | 35.0 | 31.1 | ||
| Chemotherapy | 0.364 | 0.547 | |||||
| Yes | 96 (84.2) | 26.5 | 79.1 | 42.4 | 37.7 | ||
| No | 18 (15.8) | 18.9 | 62.9 | 47.2 | 35.4 | ||
| Radiotherapy | 0.234 | 0.629 | |||||
| Yes | 48 (42.1) | 32.2 | 82.8 | 42.4 | 35.3 | ||
| No | 66 (57.9) | 22.7 | 71.7 | 42.4 | 37.6 | ||
Diagnosis
In this study, the diagnosis was made or confirmed after surgery in 66 patients (57.9%). The remaining 48 patients (42.1%) were diagnosed by biopsy. Of the 32 patients who had biopsy before surgery, only 4 (12.5%) were diagnosed as C-SCLC preoperatively. The other 28 patients were preoperatively diagnosed as NSCLC (15/32, 46.9%), pure SCLC (12/32, 37.5%), or non-cancer (1/32, 3.1%), respectively.
Treatments
Chemotherapy was performed in the majority of the patients (n=96, 84.2%), followed by surgery (n=66, 57.9%) and radiotherapy (n=48, 42.1%). Eighty patients (70.2%) received at least two of the three modalities. Chemotherapy was platinum-based combination regimen with a median of 5 (range: 1−27) cycles. Cisplatin/carboplatin combined with etoposide was the most common regimen (n=55, 57.3%), followed by platinum combined with paclitaxel (n=12, 12.5%), irinotecan (n=7, 7.3%), pemetrexed (n=5, 5.2%), and other regimens (n=17, 17.7%). Of 66 patients receiving surgery, there were 3 cases of wedge resection, 54 lobectomy, 7 pneumonectomy, and 2 sleeve resection. Four patient received neoadjuvant chemotherapy and 49 patients received adjuvant chemotherapy (74.2%). As for radiotherapy of the chest, the median dose was 56 Gy (range: 40−66 Gy). There was only 8 out of 83 LD patients (9.6%) received the prophylactic cranial irradiation (PCI).
Survival and failure pattern
The median follow-up period was 32.5 months (range: 1.3−110.4 months). The median time of OS, progression-free survival (PFS) and LRFS was 26.2, 13.1 and 26.5 months, respectively. The 1-, 3-and 5-year OS was 76.6%, 42.8% and 37.4%, respectively, with a corresponding PFS of 52.2%, 33.9%, and 29.0%, respectively, and LRFS of 68.5%, 43.0% and 37.3%, respectively. The median DMFS of patients with stage I-III disease was 22.7 months, with 1-, 3-and 5-year DMFS of 61.2%, 46.6% and 35.7%, respectively.
Tumor relapse occurred in 51 patients (44.7%) by the last follow-up. Locoregional recurrence (LRR) and DM were identified in 17 (14.9%) and 43 (37.7%) patients, respectively. Concurrent LRR and DM were identified in 9 patients (7.9%). The most common site of DM was the brain (12.3%), followed by the bone (10.5%), liver (9.6%) and non-regional lymph nodes (8.8%).
Prognosis
On univariate analysis, smoking (P=0.029), Karnofsky performance score (KPS) <80 (P=0.000), advanced TNM stage (P=0.000), extended disease (ED) (P=0.001), no surgery (P=0.010), positive margin (P=0.000), positive lymph nodes ≥4 (P=0.000), positive lymph node ratio >10% (P=0.000), and non-multimodality treatment (P=0.004) were significantly associated with poor OS (Table 1). The OS curves in different stages are shown in Figure 1. When analyzing the LD group and the ED group respectively, we found no factors associated with OS in the ED group. But in the LD group, KPS<80 (P=0.000), advanced TNM stage (P=0.013) and non-multimodality treatment (P=0.047) were significantly associated with poor OS. For the LD patients who received surgery, positive resection margin (P=0.000), positive lymph nodes ≥4 (P=0.001) and positive lymph node ratio >10% (P=0.000) were the predictive factors of poor prognosis. Multivariate analysis showed that smoking (HR, 2.215; 95% CI, 1.115−4.401; P=0.023) and advanced TNM stage (P=0.002) were the independent factors of poor prognosis for the whole patient group. Positive margin (HR, 2.927; 95% CI, 1.071−8.002; P=0.036) and positive lymph node ratio >10% (HR, 3.124; 95% CI, 1.321−7.390; P=0.009) were the independent factors of poor prognosis for the patients receiving surgery (Table 2, 3).
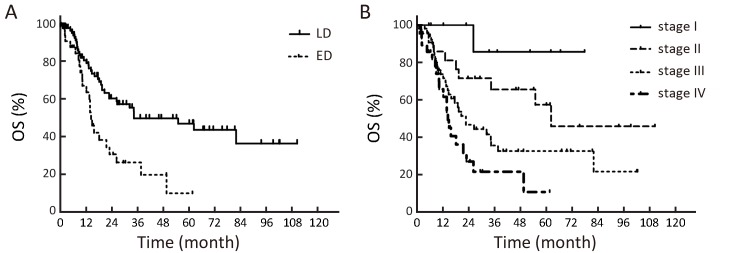
1.
Kaplan-Meier estimates of overall survival (OS) in different stages. (A) OS among patients with limited disease (LD) and extended disease (ED); (B) OS among patients with stage I, II, III and IV disease.
2.
Multivariate analysis of all patients (N=114)
| Characteristics | HR | 95% CI | P |
| HR, hazard ratio; 95% CI, 95% confidence interval; AJCC, the American Joint Committee on Cancer. | |||
| Gender | 0.355 | ||
| Male | Ref. | ||
| Female | 0.649 | 0.260−1.621 | |
| Age (year) | 0.203 | ||
| ≤60 | Ref. | ||
| >60 | 1.397 | 0.835−2.337 | |
| Smoking history | 0.023 | ||
| Absence | Ref. | ||
| Presence | 2.215 | 1.115−4.401 | |
| Stage, AJCC 7th | 0.002 | ||
| I | Ref. | ||
| II | 3.762 | 0.475−29.772 | |
| III | 8.520 | 1.161−62.522 | |
| IV | 14.440 | 1.925−108.326 | |
| Surgery | 0.139 | ||
| No | Ref. | ||
| Yes | 0.652 | 0.370−1.149 | |
3.
Multivariate analysis of surgical patients with detailed postoperative pathological reports (N=65)
| Characteristics | HR | 95% CI | P |
| HR, hazard ratio; 95% CI, 95% confidence interval; MLN, metastatic lymph node. | |||
| Gender | 0.285 | ||
| Male | Ref. | ||
| Female | 0.451 | 0.105−1.939 | |
| Age (year) | 0.069 | ||
| ≤60 | Ref. | ||
| >60 | 2.004 | 0.948−4.238 | |
| Resection margin | 0.036 | ||
| Negative | Ref. | ||
| Positive | 2.927 | 1.071−8.002 | |
| MLN ratio | 0.009 | ||
| ≤10% | Ref. | ||
| >10% | 3.124 | 1.321−7.390 | |
Discussion
C-SCLC is an uncommon tumor with an incidence of approximately 1%−3% of all SCLC (1-3). In the Cancer Institute & Hospital, CAMS & PUMC, the incidence was 4.5%. But this figure may be also underestimated due to the diagnosis based on limited specimens. As Fraire et al. (2) suggested, the true frequency of the combined type might be influenced by small or crushed biopsy samples, the number of histological slides examined, and whether surgical (or necropsy) specimens were available. Fushimi et al. (10) also reported that the frequency of C-SCLC in the primary sites was statistically higher in autopsy specimens (14.3%) than in biopsy or cytology specimens (8.6%) (P<0.05). In previous published studies, a high proportion (12%−26%) of SCLC patients who underwent surgical resection showed a combination with non-SCLC component. Thus, pathological specimens retrieved after surgery may improve the diagnosis rate of C-SCLC. Recently, the diagnostic rate of C-SCLC has improved with the increase of early diagnosis of lung cancer, which leads to more pathological examinations after surgery. In 2013, Babakoohi et al. (6) reported that of 428 cases of SCLC, 22 were C-SCLC. The ratio was 5.1%, which was higher than the historically reported. Luo et al. (7, 9) reported that in their own hospital, the number of C-SCLC cases increased from 88 during 2002−2007 to 176 during 2006−2010. In a recent report from the Tianjin Medical University Cancer Institute and Hospital, 90 cases of C-SCLC were diagnosed between January 2006 and December 2008, and they occurred in 32.1% of all SCLCs (8). In our study, the majority of patients received a definite diagnosis after surgery (58%), but most of them (87.5%) were not diagnosed as C-SCLC before surgery. This finding not only indicates the importance of sufficient histopathological examinations in C-SCLC diagnosis, but also prompts the underestimation of the incidence of C-SCLC in non-surgery patients.
C-SCLC has similar clinical features as pure SCLC. According to the reports, more than 70% C-SCLC patients are current or past smokers. The patients were overwhelmingly male (68.2%−96.2%). However, the median age ranged from 58.0 to 66.4 years, which was younger than that of SCLC. More C-SCLC occurs in the central areas (59.1%−86.4%) (5, 7-9). Our data also showed that the main population was male with a smoking history. The median age was 59 years, and most patients had central disease (80.7%). Moreover, our study showed that the predominant combined components were SCC and AC, which was in accordance with other studies (2, 4, 5, 11). As to the stage, only 40% of SCLCs constituted LD at diagnosis due to the high frequency of DM. However, more C-SCLCs were in the limited stage. In our study, ED only represented 27% of all C-SCLCs. According to the TNM staging system, only 10% of SCLCs were stage I-II (12, 13), but this figure increased to 29% in C-SCLC in our study. This difference may be attributed to the fact that most of the cases in our study received a definite diagnosis after surgery. In general, the patients who were fit for surgery had comparatively early disease.
Conventionally, the treatment of C-SCLC refers to the guidelines for SCLC, and multimodality therapy is often recommended. However, the optimized treatment model is not yet clear because of the very small number of reports that have focused on it. Although the treatment patterns were not uniform in our study, most patients received multimodality therapy including surgery, radiotherapy and chemotherapy, and the univariate analysis showed that patients with multimodality therapy exhibited significantly better survival, especially for the LD. In the LD group, the 5-year OS of patients treated by surgery was 48.9%, which was significantly higher than that in non-surgery group (36.6%) and much better than that historically reported. Babakoohi et al. (6) also noted that patients with C-SCLC were more likely to undergo surgery. When they examined the difference in OS for those who underwent surgery, the risk of death in patients with SCLC was 2.5 times than that in C-SCLC patients. These data suggest that surgery is important not only in the diagnosis of C-SCLC, but also in the improvement of treatment results, especially for patients with LD. Though nearly 84% patients received chemotherapy in our study, DM was still the most common treatment failure. This finding indicated the relatively lower sensitivity of C-SCLC to chemotherapy, which might be ascribed to the component of both SCLC and NSCLC. More individualized chemotherapy regimens considering both SCLC and NSCLC components need to be further evaluated. For patients who are unfit for surgery or those with a positive margin after surgery, radiotherapy is usually suggested. However, unlike pure SCLC, the role of radiotherapy in improving the OS of C-SCLC has not yet been established.
Despite the deficiency of large sample studies, there is no difference in prognosis between C-SCLC and pure SCLC in most of the published literature. Hage et al. (5) presented that the cumulative 5-year survival of patients with pure SCLC in pathological stage I was 39%, which was not significantly different from that of C-SCLC (P=0.629). The survival was also similar between C-SCLC and pure SCLC in stages II and III. Nicholson et al. (4) found that there was no difference in survival between the two groups either. However, recently, Babakoohi et al. (6) and Qin et al. (11) held different viewpoints from previous studies. They suggested that the OS of patients with C-SCLC was significantly better than that of patients with pure SCLC. Qin et al. (11) showed that the median OS of C-SCLC and pure SCLC was 31 months and 15 months, respectively. The prognosis of C-SCLC was significantly better than that of pure SCLC (P<0.001). Similarly, in the Cancer Institute & Hospital, CAMS & PUMC, the LD patients had a longer median OS than that of SCLC (34.4 months vs. 23.0 months), as well as the ED (14.3 months vs. 13.7 months). To some extent, this finding suggests a superior survival of C-SCLC. Conversely, most patients with C-SCLC received surgery and had comparatively early disease, which might have been correlated with better survival.
The prognostic factors for C-SCLC are primarily stage (2, 14, 15), the type of non-SCLC component (8, 16-18) and the tumor location (8). In our study, smoking, advanced TNM stage, positive margins and positive lymph node ratio >10% were poor prognostic factors. In univariate analysis, both the VALSG and the TNM classification system are associated with OS. As we all know, the VALSG classification system is commonly used in SCLC. However, in the 7th edition of AJCC, because of the excellent relationship between the TNM stage and prognosis of SCLC, the International Association for the Study of Lung Cancer recommended that the TNM classification system should be used for both NSCLC and SCLC (19). Our research also confirmed that the TNM classification system, but not the VALSG classification system, was closely related to the prognosis of C-SCLC on multivariate analysis. The resected margin status and the proportion of positive lymph nodes partly reflect the degree of tumor malignancy, treatment difficulty and tolerability. In this respect, positive margins and positive lymph node ratio >10% should predict a poor prognosis, which implies the importance of complete resection. Our study did not verify the prognostic significance of non-SCLC components or tumor location, which may due to the difference of patient populations between our study and other studies.
As a retrospective analysis, there were some limitations in our study. Influenced by specimen size, the number of histological slides and whether there were surgical specimens, the diagnosis of C-CSLC might be missed and underestimated. Additionally, the lack of uniform treatment, such as chemotherapy regimens and radiotherapy dose, was another deficiency of our study. Finally, because this study solely utilized single institutional data with limited cases, study bias might exist, and the results should be interpreted cautiously.
Conclusions
C-SCLC is a rare type of SCLC that has a relatively early stage and good prognosis which may due to the underestimated diagnosis in non-surgical patients. Smoking, advanced TNM staging, positive margins and positive lymph node ratio >10% are poor prognostic factors. Multimodality therapy is recommended, especially for LD. However, these observations should be improved by further large-scale studies.
Acknowledgments
This study was supported by the Capital Health Development Research Grant for Youth Scholars (2011-4002-05) and the Funding for Talents Training Project in Beijing (2012D009008000001).
Footnotes
The authors have no conflicts of interest to declare.
References
- 1.Travis WD, Colby TV, Corrin B, et al. World Health Organization International Histological Classification of Tumours. Histological typing of lung and pleural tumors. 3rd edition. Berlin: Springer, 1999.
- 2.Fraire AE, Johnson EH, Yesner R. Prognostic significance of histopathologic subtype and stage in small cell lung cancer. Hum Pathol. 1992;23:520–8. doi: 10.1016/0046-8177(92)90129-Q. [DOI] [PubMed] [Google Scholar]
- 3.Mangum MD, Greco FA, Hainsworth JD. Combined small-cell and non-small-cell lung cancer. http://ascopubs.org/doi/abs/10.1200/jco.1989.7.5.607. J Clin Oncol. 1989;7:607–12. doi: 10.1200/JCO.1989.7.5.607. [DOI] [PubMed] [Google Scholar]
- 4.Nicholson SA, Beasley MB, Brambilla E. Small cell lung carcinoma (SCLC): a clinicopathologic study of 100 cases with surgical specimens. Am J Surg Pathol. 2002;26:1184–97. doi: 10.1097/00000478-200209000-00009. [DOI] [PubMed] [Google Scholar]
- 5.Hage R, Elbers JR, Brutel de la Rivière A. Surgery for combined type small cell lung carcinoma. Thorax. 1998;53:450–3. doi: 10.1136/thx.53.6.450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Babakoohi S, Fu P, Yang M. Combined SCLC clinical and pathologic characteristics. Clin Lung Cancer. 2013;14:113–9. doi: 10.1016/j.cllc.2012.07.002. [DOI] [PubMed] [Google Scholar]
- 7.Luo J, Li A, Wu F. Navelbine-ifosfamide-cisplatin versus etoposide-cisplatin as the first-line treatment of advanced combined small-cell lung cance: retrospective analysis of 167 cases. Zhong Liu (in Chinese) 2012;32:194–8. [Google Scholar]
- 8.Lyu X, Sun L, Zhan Z. Clinicopathological characteristics and prognosis of combined small cell lung cancer. Zhongguo Zhong Liu Lin Chuang (in Chinese) 2011;38:769–72, 777. [Google Scholar]
- 9.Luo J, Ni J, Zheng H. Clinical analysis of 88 cases with combined small cell carcinoma. Zhong Liu (in Chinese) 2009;29:156–9. [Google Scholar]
- 10.Fushimi H, Kikui M, Morino H. Histologic changes in small cell lung carcinoma after treatment. Cancer. 1996;77:278–83. doi: 10.1002/(ISSN)1097-0142. [DOI] [PubMed] [Google Scholar]
- 11.Qin A, Qian Y, Cao W. Analysis and comparison of the prognosis of combined and pure small cell lung cancer. Zhongguo Zhong Liu Lin Chuang (in Chinese) 2014;41:720–3. [Google Scholar]
- 12.Brock MV, Hooker CM, Syphard JE. Surgical resection of limited disease small cell lung cancer in the new era of platinum chemotherapy: Its time has come. J Thorac Cardiovasc Surg. 2005;129:64–72. doi: 10.1016/j.jtcvs.2004.08.022. [DOI] [PubMed] [Google Scholar]
- 13.Davis S, Crino L, Tonato M. A prospective analysis of chemotherapy following surgical resection of clinical stage I-II small-cell lung cancer. Am J Clin Oncol. 1993;16:93–5. doi: 10.1097/00000421-199304000-00001. [DOI] [PubMed] [Google Scholar]
- 14.Zakowski MF. Pathology of small cell carcinoma of the lung. http://www.seminoncol.org/article/S0093-7754(03)70038-4/pdf. Semin Oncol. 2003;30:3–8. doi: 10.1053/sonc.2003.50015. [DOI] [PubMed] [Google Scholar]
- 15.Simon GR, Wagner H, American College of Chest Physicians. Small cell lung cancer. Chest. 2003;123:259S–71S. doi: 10.1378/chest.123.1_suppl.259S. [DOI] [PubMed] [Google Scholar]
- 16.Ruffini E, Rena O, Oliaro A. Lung tumors with mixed histologic pattern. Clinico-pathologic characteristics and prognostic significance. Eur J Cardiothorac Surg. 2002;22:701–7. doi: 10.1016/S1010-7940(02)00481-5. [DOI] [PubMed] [Google Scholar]
- 17.Nappi O, Glasner SD, Swanson PE. Biphasic and monophasic sarcomatoid carcinomas of the lung. A reappraisal of 'carcinosarcomas' and 'spindle-cell carcinomas'. Am J Clin Pathol. 1994;102:331–40. doi: 10.1093/ajcp/102.3.331. [DOI] [PubMed] [Google Scholar]
- 18.Fishback NF, Travis WD, Moran CA. Pleomorphic (spindle/giant cell) carcinoma of the lung. A clinicopathologic correlation of 78 cases. Cancer. 1994;73:2936–45. doi: 10.1002/(ISSN)1097-0142. [DOI] [PubMed] [Google Scholar]
- 19.Goldstraw P. IASLC staging handbook in thoracic oncology. Editorial Rx Press: Florida; 2009. [Google Scholar]