Abstract
Objective
To investigate the characteristics of recurrences that occurred 5 or more years after curative resection for gastric cancer.
Methods
We analyzed recurrences among 1,299 patients with gastric cancer who underwent curative operations at the Department of Surgery, Inje University Seoul Paik Hospital between September 1998 and December 2002. Recurrences were classified as within 2 years (early), 2–5 years (intermediate), and more than 5 years (late) after gastrectomy. The clinicopathologic findings of the patients with late recurrence were compared with those of patients in the other two recurrence groups, with special reference to the patterns of recurrence. Both univariate and multivariate analyses were performed, incorporating factors such as operation type, T-stage, N-stage, stage, lymphatic invasion, neural invasion, histology, tumor size, and recurrence site.
Results
At the time of last follow-up, recurrence occurred in 266 (20.5%) patients. Recurrence times were classified as <2 years (182 patients), 2–5 years (61 patients), or >5 years (23 patients). The late recurrence rate was 8.6%. The occurrence of recurrence >5 years after gastrectomy was significantly correlated with age, operation type, T-stage, N-stage, stage, lymphatic invasion, neural invasion, histology, tumor size, location and recurrence site (P<0.05). The main recurrence patterns in the 23 patients with late recurrence were locoregional metastasis (10 patients, 43.5%), peritoneal seeding (8 patients, 34.8%), hematogenous metastasis (2 patients, 8.7%), and multiple metastasis (3 patients, 13.0%). A multivariate analysis showed that larger tumor size and younger age were independent prognostic factors for late recurrence. Additionally, locoregional and peritoneal recurrences were significantly more common than hematogenous recurrences.
Conclusions
Although late recurrence was uncommon, younger age and larger tumor size were associated with high risk. Follow-up surveillance is recommended for locoregional and peritoneal metastasis.
Keywords: Curative surgery, gastric cancer, gastrectomy, late recurrence, recurrence
Introduction
As the second common cancer and the third leading cause of cancer-related death in Korea, gastric cancer remains a major public health issue (1). Curative surgery including systemic lymph node dissection is the current treatment of choice for gastric cancer. Nonetheless, patients with curative gastrectomy continue to experience recurrence during follow-up. More recent studies have shown that the overall 5-year survival rate of gastric cancer has improved because of curative gastrectomy with chemotherapy (2,3). However, death from gastric cancer is almost entirely caused by recurrent diseases (4). Recurrence of gastric cancer is strongly dependent on the TNM stage and extent of surgical resection (5). The gastric cancer classification system of the Union for International Cancer Control (UICC) accurately predicts the overall survival, but is unable to provide information in terms of overall recurrence, time-specific recurrence, and site-specific first recurrence. These valuable data are the basis for the development of causative recurrence-oriented and site-specific target therapies.
The definitions of early and late recurrence have varied across studies. Some studies have defined early recurrence as recurrence within 2 years after surgery, while other studies have used intervals of 1 year or 3 years after surgery in their definitions of early recurrence (6). Late recurrence has been defined as recurrence 2 or more years after surgery (7). Most follow-up programs end 5 years after primary treatment.
Recently, we began to observe frequent recurrences at more than 5 years after curative resection and adjuvant treatment. The goal of this study was to review late recurrence (more than 5 years after the primary surgery) patterns and to understand the characteristics of late recurrences after complete resection of gastric adenocarcinoma at a single institution. Further, the characteristics of patients with late recurrence were compared with those of patients with recurrence less than 5 years postoperatively.
Materials and methods
Patients
A total of 1, 299 patients underwent curative gastrectomy for gastric adenocarcinoma in the Korea Gastric Cancer Center, Inje University Seoul Paik Hospital, between September 1998 and December 2002. The exclusion criteria included metachronous gastrointestinal cancer, a previous history of surgery for gastric cancer, and a palliative operation. Patients did not receive any prior systemic chemotherapy or radiotherapy before surgery.
Study design
All patients underwent curative gastrectomy with more than D1 + lymph node dissection, as defined by the Japanese Gastric Cancer Association (8). The patients with stage II or higher disease received adjuvant immunochemotherapy. Adjuvant immunochemotherapy was administered as follows. At the fifth postoperative day, OK 432 Streptococcus pyogenes preparation) 1.0 Klinische Einheit (KE) was administered. At the eighth postoperative day, mitomycin C (MMC) 4 mg/50 kg and 5-flurouracil (5-FU) 800 mg/50 kg injection were administered twice per week for 2 consecutive weeks. After 6 consecutive weeks, MMC and 5-FU injection were administered once per week. After finishing the injection chemotherapy regimen, we switched to oral 5-FU 800 mg/50 kg per day for 2 years. The medical records and computerized records of the patients were collected, focusing on the clinicopathological data. Based on the pathologic examination, we analyzed the following information: clinical stage, T-stage, N-stage, tumor size, operation method, histologic type, vascular invasion, lymphatic invasion, neural invasion, Lauren’s classification, tumor location, and recurrence pattern.
Follow-up assessments were performed every 3 months for the first 2 years after surgery, and then yearly thereafter. The follow-up data included medical history, physical examination, routine blood test including tumor markers (carcinoembryonic antigen and carbohydrate antigen 19-9), upper endoscopy, chest radiograph, and other imaging studies (abdominal sonogram and computed tomography). Biopsy and radiologic imaging studies could confirm the recurrence. Recurrence of cancer was classified as locoregional, peritoneal seeding, hematogenous and multiple metastases.
Locoregional metastases included dominant masses in the gastric bed, upper abdominal retroperitoneal lymph nodes, or anastomotic recurrence. Peritoneal seeding was defined as cancer recurrence in the abdominal cavity. Hematogenous metastasis was defined as any metastatic lesion in the liver, lung, bone, ovary, spleen, testis, or other distant organs. Multiple metastases were further defined according to the specific organ involved.
The results of the postoperative follow-up study were evaluated based on demographic information, as available in December 2012. The follow-up period ranged from 1 to 171 months (median, 64.9 months). The study was reviewed and approved by the Seoul Paik Hospital Institutional Review Board.
Statistical analysis
The statistical analysis was performed using SPSS software version 12.0 (SPSS Inc., Chicago, IL, USA). Survival time was calculated from the day of surgery to the last day of follow-up or the date of tumor-related death. Categorical variables were analyzed by the chi-squared test and Fisher’s exact test. The Kruskal-Wallis test was used for nonparametric analysis of variance testing. In the multivariate analysis, a logistic regression analysis was applied to identify independent clinicopathological factors which were associated with recurrence. The results of the statistical tests were considered statistically significant for P<0.05.
Results
Time to recurrence and clinicopathological findings
Among the 1, 299 patients who had undergone curative gastrectomy, recurrence was observed in 266 (20.5%) patients. Recurrence times were classified as <2 years (182 patients), 2−5 years (61 patients), or >5 years (23 patients). The mean age was 56.7±11.6 years (range, 20−86 years), the mean tumor size was 5.1±3.4 cm (range, 0.15−23 cm), and the median follow-up period was 64.9 months.
The relationships between time to recurrence and clinicopathologic findings are shown in Table 1. There were statistically significant correlations between recurrence times and age (P=0.003), N-stage (P=0.001), stage (P=0.004), and vascular invasion (P<0.001).
1.
Relationship between time to recurrence and the clinicopathologic findings
| Parameter | Recurrence | P | ||
| <2 years (n=182) | 2–5 years (n=61) | >5 years (n=23) | ||
| Gender | 0.934 | |||
| Male | 120 | 41 | 16 | |
| Female | 62 | 20 | 7 | |
| Age (year) | 0.003 | |||
| ≤56 | 78 | 37 | 17 | |
| >56 | 104 | 24 | 6 | |
| Operation | 0.198 | |||
| Total | 84 | 21 | 12 | |
| Subtotal | 98 | 40 | 11 | |
| T-stage | 0.586 | |||
| T1 | 4 | 4 | 1 | |
| T2 | 39 | 17 | 5 | |
| T3 | 104 | 30 | 14 | |
| T4 | 35 | 10 | 3 | |
| N-stage | 0.001 | |||
| N0 | 7 | 5 | 1 | |
| N1 | 28 | 11 | 11 | |
| N2 | 48 | 22 | 7 | |
| N3 | 99 | 23 | 4 | |
| Stage | 0.004 | |||
| I | 5 | 3 | 2 | |
| II | 14 | 10 | 1 | |
| III | 52 | 21 | 14 | |
| IV | 111 | 27 | 6 | |
| Vascular invasion | <0.001 | |||
| Negative | 76 | 40 | 19 | |
| Positive | 106 | 21 | 4 | |
| Lymphatic invasion | 0.065 | |||
| Negative | 4 | 5 | 2 | |
| Positive | 178 | 56 | 21 | |
| Neural invasion | 0.607 | |||
| Negative | 17 | 7 | 1 | |
| Positive | 165 | 54 | 22 | |
| Histology | 0.066 | |||
| Differentiated | 47 | 13 | 1 | |
| Undifferentiated | 135 | 48 | 22 | |
| Lauren | 0.482 | |||
| Intestinal | 62 | 18 | 7 | |
| Diffuse | 93 | 38 | 14 | |
| Mixed | 27 | 5 | 2 | |
| Location | 0.103 | |||
| Lower | 85 | 37 | 10 | |
| Middle | 42 | 11 | 4 | |
| Upper | 23 | 10 | 4 | |
| Entire | 32 | 3 | 3 | |
| Recurrence site | 0.397 | |||
| Locoregional | 42 | 19 | 10 | |
| Hematogene | 32 | 7 | 2 | |
| Peritoneum | 74 | 25 | 8 | |
| Multiple | 34 | 10 | 3 | |
| Tumor size (cm) | 0.063 | |||
| <5 | 30 | 17 | 2 | |
| ≥ | 152 | 44 | 21 | |
In the comparison of recurrences <2 years and ≥2 years after curative gastrectomy, significant correlations were observed with age (P=0.001), N-stage (P=0.007), stage (P=0.010), vascular invasion (P<0.001), and lymphatic invasion (P=0.019). Further multivariate logistic regression analysis showed that age (P<0.001), N-stage (P=0.032) and vascular invasion (P=0.001) were independent predictors of recurrence within 2 years (Table 2).
2.
Comparison of recurrences <2 years and ≥2 years after curative gastrectomy
| Parameter | Recurrence | P | |||
| <2 years | ≥2 years | Univariate | Multivariate | ||
| Gender | 0.757 | – | |||
| Male | 120 | 57 | |||
| Female | 62 | 27 | |||
| Age (year) | 0.001 | <0.001 | |||
| ≤56 | 78 | 54 | |||
| >56 | 104 | 30 | |||
| Operation method | 0.294 | – | |||
| Total gastrectomy | 84 | 33 | |||
| Partial gastrectomy | 98 | 51 | |||
| T stage | 0.302 | – | |||
| T1 | 4 | 5 | |||
| T2 | 39 | 22 | |||
| T3 | 104 | 44 | |||
| T4 | 35 | 13 | |||
| N stage | 0.007 | 0.032 | |||
| N0 | 7 | 6 | |||
| N1 | 28 | 22 | |||
| N2 | 48 | 29 | |||
| N3 | 99 | 27 | |||
| Stage | 0.010 | – | |||
| I | 5 | 5 | |||
| II | 14 | 11 | |||
| III | 52 | 35 | |||
| IV | 111 | 33 | |||
| Vascular invasion | <0.001 | 0.001 | |||
| Negative | 76 | 59 | |||
| Positive | 106 | 25 | |||
| Lymphatic invasion | 0.019 | – | |||
| Negative | 4 | 7 | |||
| Positive | 178 | 77 | |||
| Neural invasion | 0.962 | – | |||
| Negative | 17 | 8 | |||
| Positive | 165 | 76 | |||
| Histology | 0.099 | – | |||
| Differentiated | 47 | 14 | |||
| Undifferentiated | 135 | 70 | |||
| Lauren classification | 0.178 | – | |||
| Intestinal | 62 | 25 | |||
| Diffuse | 93 | 52 | |||
| Mixed | 27 | 7 | |||
| Location | 0.052 | - | |||
| Lower | 85 | 47 | |||
| Middle | 42 | 15 | |||
| Upper | 23 | 16 | |||
| Entire | 32 | 6 | |||
| Recurrence site | 0.176 | - | |||
| Locoregional | 42 | 29 | |||
| Hematogeneous | 32 | 9 | |||
| Peritoneal | 74 | 33 | |||
| Multiple | 34 | 13 | |||
| Tumor size (cm) | 0.230 | - | |||
| <5 | 30 | 19 | |||
| ≥5 | 152 | 65 | |||
Clinicopathological findings and recurrence pattern of patients with late recurrence (more than 5 years)
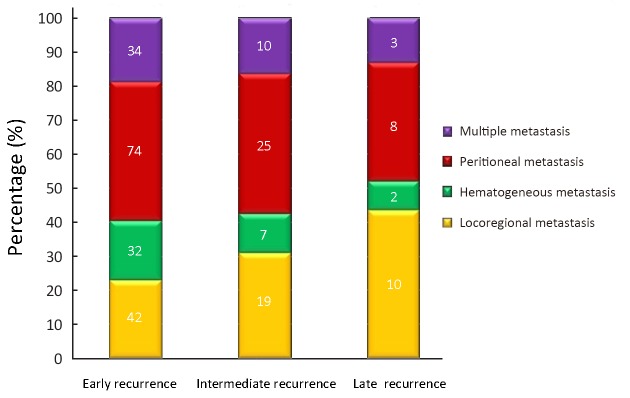
The rate of late recurrence was 8.6%; and late recurrence was experienced by 23 of the 1, 299 patients who had undergone curative gastric resection and adjuvant chemotherapy. These patients had not shown any signs of recurrence before 5 years postoperatively, and had participated in a regular follow-up program. Among these 23 patients, recurrences were noticed at 5 years postoperatively in 9 patients, at 6 years postoperatively in 5 patients, at 7 years postoperatively in 3 patients, at 8 years postoperatively in 2 patients, at 9 years postoperatively in 1 patient, and more than 10 years postoperatively in 3 patients. The recurrence patterns are shown according to the recurrence time in Figure 1. The main recurrence patterns in the 23 patients with late recurrence were locoregional metastasis (10 patients, 43.5%), peritoneal seeding (8 patents, 34.8%), hematogenous metastasis (2 patients, 8.7%), and multiple metastasis (3 patients, 13.0%). The main patterns in early and intermediate recurrence group were hematogenous and peritoneal metastasis; and locoregional and peritoneal metastasis were observed in the late recurrence group.
1.
Histogram of recurrence pattern according to recurrence time.
For patients who recurred after 5 years in the case of significant differences in the univariate analysis, the variables were age (P=0.002), operation type (P=0.007), T-stage (P<0.001), N-stage (P<0.001), stage (P<0.001), lymphatic invasion (P=0.002), neural invasion (P<0.001), histology (P=0.001), tumor size (P<0.001), recurrence site (P<0.001) and location (P=0.004). Further multivariate logistic regression analysis showed that larger size (P=0.028) and younger age (P=0.022) were significantly related risk factors (Table 3).
3.
Multivariate analysis of clinicopathologic variables for patients who recurred after 5 years
| Parameter | Hazard ratio | 95% CI | P |
| 95% CI, 95% confidence interval. | |||
| Tumor size | 5.887 | 1.215–28.511 | 0.028 |
| Age | 0.323 | 0.123–0.848 | 0.022 |
Discussion
The 5-year survival rate of gastric cancer has increased in recent years. This can be interpreted as a consequence of increasing rates of early gastric cancer, which have resulted from earlier diagnosis of gastric cancer (9). The current survival rate of advanced gastric cancer following curative surgery is better than past survival rates because of postsurgical chemotherapy and adjuvant immunotherapy. Gastric cancer recurs most frequently within 5 years after surgery, with 60%−70% of recurrence occurring within 2 years. Nevertheless, death is attributable to recurrence in 60%−70% of advanced gastric cancer cases (10).
The recurrence rate after 5 years appears to have declined. In a study by Wu et al. (11), cumulative recurrence rates were 53.5%, 80.0%, 89.0%, 94.7%, 96.3%, 98.0%, and 99.5% at 1, 2, 3, 4, 5, 6, and 7 years, respectively. Lee et al. (12) reported that 22.1% (348/1, 573 patients with recurrence) of their patients experienced recurrence at more than 5 years after surgery. In the present report, the clinicopathologic factors of the late recurrence cases were compared with those of the early and intermediate recurrence cases. Among 266 patients with recurrence, the rate of early recurrence was 68.4%, that of intermediate recurrence was 22.9%, and that of late recurrence was 8.6%. Although no clear definition has been established for late recurrence of gastric cancer, most reports have described cases recurring over 5 years after surgery. No clear consensus has been reached regarding the mechanisms underlying such late recurrence. However, Choiet al. (13) discussed several possible contributing factors, including: 1) a very minute amount of residual cancer tissue; 2) a site of residual cancer that is not conducive to progression; 3) slow growth of the tumor; and 4) high levels of host resistance.
Shiraishi et al. (7) have compared early and late recurrence after gastrectomy for gastric cancer patients, and have reported that tumor size, lymphatic invasion, level of lymph node metastasis, stage of disease, and extent of lymph node dissection are the factors that are most significantly associated with early recurrence (within 2 years after gastrectomy). In contrast, Moon et al. (14) have described the changing patterns of prognostic indicators during 15 years of follow-up of advanced gastric cancer after gastrectomy and adjuvant chemotherapy. They have suggested that tumor factors, including stage, were clinical prognostic indicators within 5 years post-gastrectomy, but observed no such indicators after 10 years. In the present study, when an analysis was performed to determine whether or not recurrence had appeared at 2 years, age, N-stage, and vascular invasion were related risk factors. When a comparison was performed to determine whether or not recurrence had appeared at 5 years, N-stage and vascular invasion were related risk factors. In patients with recurrence after 5 years, larger tumor size and younger age were significantly related risk factors.
In clinical practice, recurrence patterns are classified as locoregional, peritoneal, and hematogenous (15). Peritoneal recurrence is reported the most common recurrence pattern, accounting for 40% of cases and usually occurred within 2 years after surgery (16). In another recent study, hematogenous metastasis was the most common type in patients who experienced recurrence within 1 year, and locoregional and peritoneal metastasis were the most common type in patients who experienced recurrence more than 1 year after curative resection (17). In this present study, recurrence within 2 years mainly presented as hematogenous and peritoneal metastasis, but recurrence after 5 years mainly manifested as locoregional and peritoneal metastasis. On the other hand, Buzzoni et al. (18) reported that the long-term recurrence rate at 7 years was 15.8% for locoregional recurrence and 34.5% for distant recurrence. Although the results vary slightly, early detection of recurrence is very important.
Because gastric cancer has various patterns of recurrence, close follow-up is important for early detection of recurrence, and subsequent tailored treatment should be provided as soon as possible. Despite poor survival after recurrence of gastric cancer and unfavorable response to chemotherapy in some patients, other patients do demonstrate a partial or even complete response after chemotherapy and have long-term survival.
Conclusions
The results of this study show that recurrence after 5 years is not common. The characteristics of late recurrence of gastric cancer could be summarized as larger tumor size and younger age at surgery. Locoregional and peritoneal metastases were the common patterns of late recurrence, and locoregional metastasis was observed more frequently than peritoneal metastasis. Although there is no consensus about the appropriate surveillance program after 5 years for curative surgery, the possibility of recurrence should be considered. We recommend that a study for locoregional metastasis should be performed on gastric surgery patients more than 5 years after they undergo resection, especially those with larger tumor size and younger age at surgery.
Acknowledgements
None.
Footnotes
The authors have no conflicts of interest to declare.
References
- 1.Department of Population Tendency of Korea National Statistical Office. Mortality and the cause of death in 2013. Seoul: Korea National Statistical Office, 2013.
- 2.Lee JW, Ali B, Yoo HM. Conditional survival analysis in Korean patients with gastric cancer undergoing curative gastrectomy. BMC Cancer. 2015;15:1005. doi: 10.1186/s12885-015-2022-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Park JH, Ryu MH, Kim HJ. Risk factors for selection of patients at high risk of recurrence or death after complete surgical resection in stage I gastric cancer. Gastric Cancer. 2016;19:226–33. doi: 10.1007/s10120-015-0464-5. [DOI] [PubMed] [Google Scholar]
- 4.Nakagawa M, Kojima K, Inokuchi M. Patterns, timing and risk factors of recurrence of gastric cancer after laparoscopic gastrectomy: reliable results following long-term follow-up. Eur J Surg Oncol. 2014;40:1376–82. doi: 10.1016/j.ejso.2014.04.015. [DOI] [PubMed] [Google Scholar]
- 5.Roukos DH, Kappas AM. Perspectives in the treatment of gastric cancer. Nat Clin Pract Oncol. 2005;2:98–107. doi: 10.1038/ncponc0099. [DOI] [PubMed] [Google Scholar]
- 6.Li JH, Zhang SW, Liu J. Review of clinical investigation on recurrence of gastric cancer following curative resection. Chin Med J (Engl) 2012;125:1479–95. [PubMed] [Google Scholar]
- 7.Shiraishi N, Inomata M, Osawa N. Early and late recurrence after gastrectomy for gastric carcinoma. Univariate and multivariate analyses. Cancer. 2000;89:255–61. doi: 10.1002/(ISSN)1097-0142. [DOI] [PubMed] [Google Scholar]
- 8.Japanese Gastric Cancer Association Japanese gastric cancer treatment guidelines 2010 (ver. 3) Gastric Cancer. 2011;14:113–23. doi: 10.1007/s10120-011-0042-4. [DOI] [PubMed] [Google Scholar]
- 9.Choi JY, Ha TK, Kwon SJ. Clinicopathologic characteristics of gastric cancer patients according to the timing of recurrence after curative surgery. J Gastric Cancer. 2011;11:46–54. doi: 10.5230/jgc.2011.11.1.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kang WM, Meng QB, Yu JC. Factors associated with early recurrence after curative surgery for gastric cancer. World J Gastroenterol. 2015;21:5934–40. doi: 10.3748/wjg.v21.i21.6434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wu CW, Lo SS, Shen KH. Incidence and factors associated with recurrence patterns after intended curative surgery for gastric cancer. http://link.springer.com/article/10.1007/s00268-002-6279-7. World J Surg. 2003;27:153–8. doi: 10.1007/s00268-002-6279-7. [DOI] [PubMed] [Google Scholar]
- 12.Lee JH, Kim HI, Kim MG. Recurrence of gastric cancer in patients who are disease-free for more than 5 years after primary resection. Surgery. 2016;159:1090–8. doi: 10.1016/j.surg.2015.11.002. [DOI] [PubMed] [Google Scholar]
- 13.Choi JY, Ha TK, Kwon SJ. Clinicopathologic characteristics of gastric cancer patients according to the timing of the recurrence after curative surgery. J Gastric Cancer. 2011;11:46–54. doi: 10.5230/jgc.2011.11.1.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moon YW, Jeung HC, Rha SY. Changing patterns of prognosticators during 15-year follow-up of advanced gastric cancer after radical gastrectomy and adjuvant chemotherapy: a 15-year follow-up study at a single Korean institute. Ann Surg Oncol. 2007;14:2730–7. doi: 10.1245/s10434-007-9479-4. [DOI] [PubMed] [Google Scholar]
- 15.Bilici A, Selcukbiricik F. Prognostic significance of the recurrence pattern and risk factors for recurrence in patients with proximal gastric cancer who underwent curative gastrectomy. Tumour Biol. 2015;36:6191–9. doi: 10.1007/s13277-015-3304-7. [DOI] [PubMed] [Google Scholar]
- 16.Cho JM, Jang YJ, Kim JH. Pattern, timing and survival in patients with recurrent gastric cancer. Hepatogastroenterology. 2014;61:1148–53. [PubMed] [Google Scholar]
- 17.Eom BW, Yoon H, Ryu KW. Predictors of timing and patterns of recurrence after curative resection for gastric cancer. Dig Surg. 2010;27:481–6. doi: 10.1159/000320691. [DOI] [PubMed] [Google Scholar]
- 18.Buzzoni R, Bajetta E, Di Bartolomeo M. Pathological features as predictors of recurrence after radical resection of gastric cancer. Br J Surg. 2006;93:205–9. doi: 10.1002/(ISSN)1365-2168. [DOI] [PubMed] [Google Scholar]