Abstract
Objective
There is heterogeneity in the prognosis of gastric cancers staged according to the tumornodes- metastasis (TNM) system. This study evaluated the prognostic potential of an immune score system to supplement the TNM staging system.
Methods
An immunohistochemical analysis was conducted to assess the density of T cells, B cells, and myeloid-derived suppressor cells (MDSCs) in cancer tissues from 100 stage IIIA gastric cancer patients; the expression of the high-mobility group protein B1 (HMGB1) was also evaluated in cancer cells. The relationship between the overall survival (OS), disease-free survival (DFS), and immunological parameters was analyzed.
Results
An immune score system was compiled based on the prognostic role of the density of T cells, B cells, MDSCs, and the expression of HMGB1 in cancer tissues. The median 5-year survival of this group of patient was 32%. However, the 5-year survival rates of 80.0%, 51.7%, 0%, 5.8%, and 0% varied among the patients with an immune score of 4 to those with an immune score of 0 based on the immune score system, respectively. Similarly, differences in DFS rates were observed among the immune score subgroups.
Conclusions
An immune score system could effectively identify the prognostic heterogeneity within stage IIIA gastric cancer patients, implying that this immune score system may potentially supplement the TNM staging system, and help in identifying a more homogeneous group of patients who on the basis of prognosis can undergo adjuvant therapy.
Keywords: Immune score, gastric cancer, CD33, STAT1, T cell, B cell, high-mobility group protein B1 (HMGB1)
Introduction
The American Joint Committee on Cancer/Union for International Cancer Control (AJCC/UICC) tumor-nodes-metastasis (TNM) staging system provides the most reliable guidelines for the prognostication and treatment of carcinomas. In fact, clinical outcome can significantly vary among patients within the same TNM stage. Some patients with advanced-stage cancer may remain stable for years, and although rare, partial or full regression of metastatic tumors may occur spontaneously (1,2). In contrast, relapse, rapid tumor progression, and patient death occur in approximately 10-25% of patients with TNM I/II stage cancers, despite performing complete surgical resection and even though there is no evidence of residual tumor burden or distant metastasis (2,3).
The TNM system is based on the tumors' biological behaviors, without considering host responses. It is becoming increasingly evident that immune response against cancers is an important factor in deciding the clinical outcomes (4-7). Multiple inflammatory cells, especially lymphocytes and macrophages commonly infiltrate into solid tumor tissues. Rather than considering a change in the expression of a single immunological marker, the change in a combined index or the immune score (Im) would better represent the immune response. The Im system, which provides an Im ranging from 0 to 4 (Im0 to Im4), was initially suggested and developed for colorectal cancers (8,9), and is based on the enumeration of two lymphocyte populations (CD3/CD45RO, CD3/CD8), both in the core of the tumor and in the invasive margin of tumors. The Im appears to be the strongest prognostic factor for disease-free survival (DFS), disease specific survival, and overall survival (OS) in colorectal cancers (10-13).
In this study, we developed a new Im system considering that the immune response consists of multiple effector cells and immune cells infiltrating tumor tissue, each of which may act as promoters or inhibitors of tumor progression, depending on the tumor microenvironment (4,14,15). The Im system included T cells, B cells, and myeloid-derived suppressor cells (MDSCs), and high-mobility group protein B1 (HMGB1) expression profile, to assay the prognostic role of the immune response in gastric cancers.
Materials and Methods
Tissue specimens
Between 2003 and 2006, 100 samples of pathologically confirmed cancer tissue were obtained from patients with stage IIIA (2009 AJCC/UICC staging system) gastric cancer at the Sun Yat-Sen University Cancer Center. This study was conducted in accordance with the Helsinki Declaration, and all patients signed a consent form approved by the Research Ethics Committee of the Sun Yat-Sen University Cancer Center.
Immunohistochemistry and scoring systems
Paraffin-embedded tissues were sectioned continuously with a thickness of 4 μm and baked for 1 h at 65 ℃. Briefly, the sections were de-paraffinized using xylene and then rehydrated with graded alcohol to distilled water. The sections were immersed in EDTA antigen retrieval buffer (pH 8.0), placed under high pressure for 3 minutes for antigen retrieval, and then allowed to cool to room temperature. After blocking with sheep serum, the sections were incubated overnight at 4 ℃ with either a rabbit polyclonal antibody against human HMGB1 at a dilution of 1:1,000 (Abcam, Cambridge, MA, USA) or a mouse monoclonal antibody against human cluster of differentiation 8 (CD8) and CD20 (Zymed, San Diego, CA, USA), all of which were diluted 1:400. Following incubation with the secondary antibodies, the sections were developed using diaminobenzidine tetrahydrochloride and counterstained with hematoxylin. Co-expression of CD33 and phospho-signal transducers and activators of transcription (p-STAT1) were detected by sequential, double-immunohistochemical staining using the double-staining En VisonTM G/2 Doublestain System (DakoCytomation, Glostrup, Denmark), according to the manufacturer's instructions and our previous report (6). Endogenous peroxidases and alkaline phosphatase enzymes were blocked with the dual endogenous enzyme blocking reagent provided in the double-stain kit; the sections were treated with normal goat serum for 20 min to reduce nonspecific binding, and incubated overnight at 4 ℃ with rabbit polyclonal anti-CD33 antibody (1:100; Protein Tech Group, Chicago, USA) and rabbit monoclonal anti-p-STAT1 (1:400; Cell Signaling, Boston, USA). Staining was visualized with diaminobenzidine (brown) and permanent red (red). As a negative control, the antibodies were replaced by phosphate-buffered solution (16,17).
The density of immune cells within the tumor specimens and the expression of HMGB1 in cancer cells were scored according to our previous report (7). Two scoring systems were used for quantification. Rui-Qing Peng's method (7) was used to score the density of TILs (tumor-infiltrating lymphocytes) as follows: (I) immune cells were counted in at least ten different fields of each section, the areas of highest density were chosen before cell counting; (II) the cells were counted in the intratumoral compartment (within the tumor cell nests); (III) necrotic areas were excluded; (IV) two observers counted the cells at the same time, in the same field, using a multiple-lens microscope; (V) the results were expressed as the mean ± standard error of the mean. The expression of HMGB1 was interpreted via immunoreactivity using the 0-4 semi-quantitative scoring systems for both the intensity of staining and the percentage of positive cells (labeling frequency percentage) (7). The intensity of staining was grouped into the following four categories: no staining/background of negative controls (score =0), weak staining detectable above background (score =1), moderate staining (score =2), and intense staining (score =3). The labeling frequency was scored as follows: 0 (≤1%), 1 (1-24%), 2 (25-49%), 3 (50-74%), and 4 (≥75%). The sum index was obtained by totaling the intensity and percentage scores, as follows: (-), (+), (++), and (+++) indicated the sum-indices of 0-1, 2-3, 4-5, and 6-7, respectively; (-) and (+) were defined as no or modest expression, respectively, and (++) and (+++) were defined as strong expression. Two pathologists independently scored each section. If there was an inconsistency in the scoring, a third pathologist was consulted to achieve a consensus.
Statistical analysis
All statistical analysis was performed with the SPSS 16.0 and R statistical software packages. The median value was used as a cut off between the different groups of all immunohistochemical variables in our results. The Wilcoxon-Mann-Whitney test was used to identify markers with significantly different expression among patient groups. The chi-squared test was used to analyze the relationship between HMGB1 expression, CD33+p-STAT1+ expression, and clinicopathological characteristics. The OS was defined as death from any cause, and DFS was defined as the time prior to relapse of the primary tumor. Survival curves were calculated using the Kaplan-Meier method and analyzed by the log-rank test. The multivariate Cox proportional hazards regression model was applied to analyze hazard ratios; a two-sided P<0.05 was considered statistically significant.
Results
Patient characteristics
Among 100 patients, there were 72 men and 28 women, and the median age was 59.5 years (range from 29 to 82 years). Based on the Borrmann classification, 55 patients (55%) had type II lesions, 38 had type III, and only7 had type I and IV lesions. All the patients presented with lymph node metastasis before treatment, 36 patients had N1 stage, and 34 and 30 patients had N2 and N3 stages, respectively. Based on the National Comprehensive Cancer Network guidelines, 5-fluorouracil-based adjuvant therapy was administered. Of the total number of patients, 54 had died and 44 presented with disease progression during the follow-up.
The density of CD8+, CD20+, CD33+/p-STAT1+ cells and the expression of HMGB1 in gastric cancer tissues
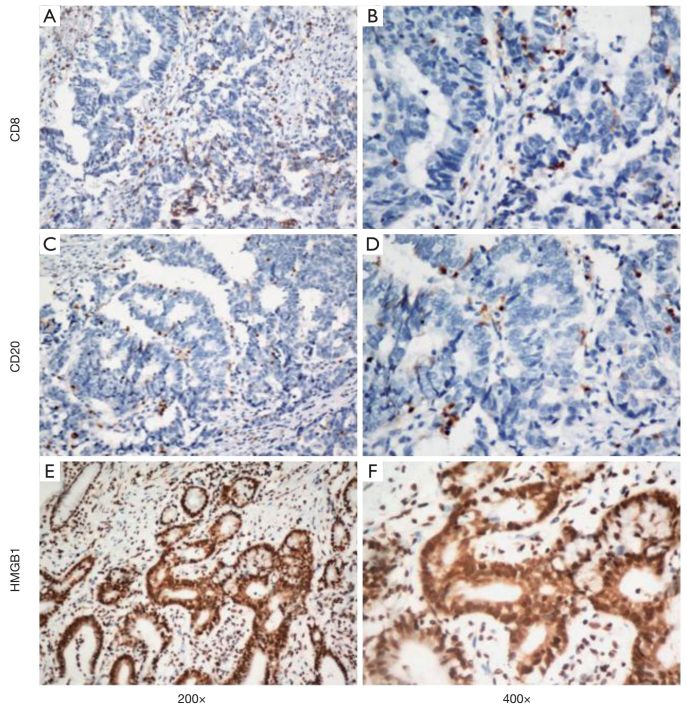
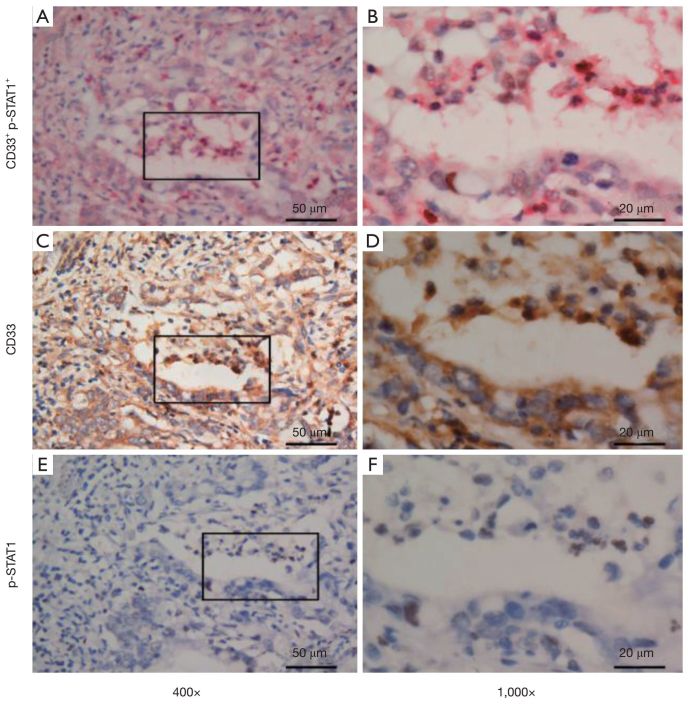
Strong membrane staining was observed for CD8+ and CD20+ lymphoid cells (Figure 1A-D). The infiltrating lymphoid cells in the intratumoral compartment were counted. Moreover, the HMGB1 showed both membrane and nuclear staining within the tumor cells (Figure 1E, F). To characterize MDSC infiltration in gastric cancer tissue, we defined MDSCs as CD33+/p-STAT1+ double-positive staining cells (6). These CD33+/p-STAT1+ double-positive cells have been found in most gastric cancer tissue but not in the non-tumorous stomach. Immunostaining revealed cytomembrane staining for CD33, and nuclear staining for p-STAT1 (Figure 2). CD33/p-STAT1 double-positive cells were observed in a subset of cells around the tumor nests.
1.
Single immunohistochemical staining for CD8, CD20, and HMGB1. (A, B) CD8+ T lymphocytes in the gastric cancer tissue (A, 200×; B, 400×); (C, D) CD20+ B lymphocytes in the gastric cancer tissue (C, 200×; D, 400×); (E, F) the expression of HMGB1 in the gastric cancer tissue (E, 200×; F, 400×). CD8, cluster of differentiation 8; HMGB1, high-mobility group protein B1.
2.
Double and single immunohistochemical staining for CD33 and p-STAT1. (A, B) Double-stained with CD33+ (red) and p-STAT1+ (brown) cells in gastric cancer tissue (A, 400×; B, 1,000×); (C, D) CD33 single-stained cells in parallel with CD33/p-STAT1 double-positive cells (C, 400×; D, 1,000×); (E, F) p-STAT1 single-stained cells in parallel with CD33/p-STAT1 double-positive cells (E, 400×; F, 1,000×). p-STAT1, phospho-signal transducers and activators of transcription.
Univariate analyses of the relationship between OS and DFS, and clinical and immunological parameters among stage IIIA gastric cancer patients
The median follow-up time for the 100 cases was 36.5 months, with a range from 2 to 88 months. At the completion of the study, 47 patients were alive, and 53 patients had died. Fifty-one deaths were cancer-related, and two deaths were due to causes unrelated to cancer. The estimated 5-year survival and 5-year recurrent free survival rates were 32% and 28%, respectively.
Univariate analysis showed that the age and immunological parameters were statistically significant prognostic factors for OS and DFS (Table 1). However, clinical prognosis was not associated with sex, tumor location, Borrmann classification, tumor size and the expression of estrogen receptor (ER), progesterone receptor (PR), p53 and carcinoembryonic antigen (CEA) in tumor tissue.
1.
Univariate analyses of OS and DFS among patients with UICC-TNM stage IIIA gastric cancer according to clinical and immune parameters
| Parameter | OS | DFS | |||||||||
| Predictive accuracy (%) | HR | 95% CI | P | Predictive accuracy (%) | HR | 95% CI | P | ||||
| C-index§ | CT* | C-index | CT | ||||||||
| §, C-index, Harrell’s concordance index; *, CT, time-dependent c-index. OS, overall survival; DFS, disease-free survival; UICC, Union for International Cancer Control; TNM, tumor-nodes-metastasis; ER, estrogen receptor; PR, progesterone receptor; CEA, carcinoembryonic antigen; CD8, cluster of differentiation 8; HMGB1, high-mobility group protein B1. | |||||||||||
| Clinical parameters | |||||||||||
| N stage | 53.6 | 54.3 | 0.828 | 0.586-1.169 | 0.283 | 53.6 | 54.3 | 0.835 | 0.594-1.175 | 0.301 | |
| Age | 57.7 | 57.3 | 1.903 | 1.094-3.311 | 0.023 | 58.0 | 57.7 | 1.964 | 1.118-3.390 | 0.019 | |
| Gender | 56.1 | 55.7 | 1.636 | 0.934-2.864 | 0.085 | 55.8 | 55.4 | 1.587 | 0.906-2.778 | 0.106 | |
| Tumor location | 53.6 | 53.3 | 0.914 | 0.738-1.134 | 0.414 | 54.0 | 53.7 | 0.909 | 0.733-1.128 | 0.386 | |
| Borrmann classification | 51.8 | 52.4 | 1.124 | 0.750-1.685 | 0.570 | 51.9 | 52.4 | 1.129 | 0.751-1.696 | 0.561 | |
| Tumor size | 52.7 | 53.2 | 1.255 | 0.733-2.148 | 0.408 | 53.0 | 53.6 | 1.278 | 0.746-2.187 | 0.372 | |
| ER | 51.5 | 51.8 | 0.580 | 0.141-2.387 | 0.450 | 51.7 | 52.0 | 0.563 | 0.137-2.317 | 0.426 | |
| PR | 52.3 | 52.6 | 0.658 | 0.262-1.652 | 0.373 | 52.7 | 53.0 | 0.634 | 0.253-1.592 | 0.332 | |
| P53 | 51.1 | 50.6 | 0.962 | 0.561-1.650 | 0.889 | 51.2 | 50.7 | 0.963 | 0.562-1.651 | 0.891 | |
| CEA | 56.8 | 56.8 | 0.589 | 0.334-1.037 | 0.067 | 56.7 | 56.7 | 0.606 | 0.344-1.068 | 0.083 | |
| Immune parameters | |||||||||||
| CD8 | 67.2 | 67.8 | 0.446 | 0.245-0.811 | 0.008 | 67.2 | 67.7 | 0.452 | 0.249-0.821 | 0.009 | |
| CD20 | 66.9 | 66.7 | 0.533 | 0.304-0.934 | 0.028 | 66.4 | 66.2 | 0.566 | 0.323-0.991 | 0.046 | |
| HMGB1 | 68.0 | 68.4 | 4.813 | 2.698-8.587 | < 0.001 | 68.1 | 68.5 | 4.759 | 2.668-8.496 | < 0.001 | |
| CD33+p-STAT1+ | 68.5 | 69.2 | 5.318 | 2.965-9.538 | < 0.001 | 68.7 | 69.2 | 5.330 | 2.971-9.561 | <0.001 | |
| Immune score | 77.2 | 80.1 | / | / | / | / | / | / | / | / | |
Compilation of the Im
To quantify the risk of relapse in individual patients, we calculated the "Im" based on the density of CD8, CD20, CD33+/p-STAT1+ and the expression of HMGB1. A Score1 was computed on the basis of lymphocyte infiltration (CD8+ T lymphocytes and CD20+ B lymphocytes) (18). Patients with two low (2-Lo) densities of CD8 and CD20 in the intratumoral compartment were classified as having a score of 0. Patients with one high (1-Hi) density for one marker were classified as having a score of 1. Patients with two high (2-Hi) densities for the markers were classified as having a score of 2. In contrast to the density of CD8+ T cells and CD20+ B lymphocytes, the density of CD33+/p-STAT1+ cells and the expression of HMGB1 in cancer cells were found to negatively influence the OS and DFS (Table 1). Therefore, a score 2 was inversely assigned based on the density of CD33+/p-STAT1+ cells and the expression of HMGB1. Patients with a 2-Hi expression of CD33+/p-STAT1+ and HMGB1 [(++) and (+++)] in the intratumoral compartment were classified as having a score of 0. Patients with a 1-Hi expression for one marker were classified as having a score of 1. Patients with a 2-Lo expression for the marker were classified as having a score of 0.
The Im, which ranged from Im0 to Im4, was calculated by combining score 1 with score 2 (Figure 3). A patient with 2-Hi densities of CD8 and CD20 and with 2-Lo expression of CD33+/p-STAT1+ and HMGB1 was assigned a score of Im4. In contrast, a patient with 2-Lo densities of CD8 and with 2-Hi expression of CD33+/p-STAT1+ and HMGB1 was assigned a score of Im0. According to these criteria, 21%, 17%, 13%, 29%, and 20% of patients were classified into the subgroups of Im0, Im1, Im2, Im3, and Im4, respectively.
3.
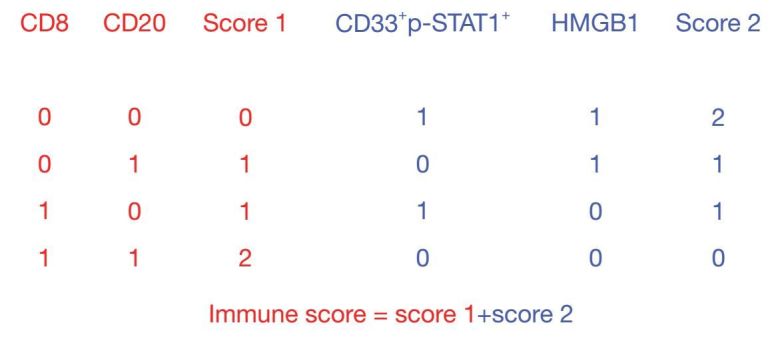
Compilation of the immune score.
Relationship between the Im and patient survival and recurrence
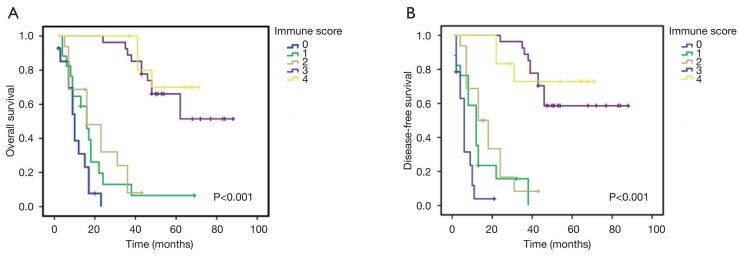
Next, we evaluated whether the Im was associated with patient prognosis. As shown in Figure 4A, the OS durations were significantly different among the Im groups (P<0.001). The cumulative 5-year survival rates were 70.0% and 51.7% among the patients with Im4 and Im3, respectively. Conversely, the survival rates were only 0%, 5.8%, and 0% among patients with Im0, Im1, and Im2, respectively.
4.
Kaplan-Meier analysis of the disease-free survival (A) and overall survival rates (B) corresponding to each immune score (Im) group. A higher Im (Im3 or Im4) was correlated with longer overall survival and disease-free survival than a lower Im (Im0, Im1, or Im2).
Similarly, there were significant differences in the DFS durations among the Im subgroups, as shown in Figure 4B; the median DFS time was 33 months in this group of patients, with a range of 1-88 months. The cumulative 5-year DFS was 75% and 44.8% among the patients with Im4 and Im3, respectively. Conversely, all the patients belonging to the Im0, Im1, and Im2 subgroups showed a 5-year DFS rate of 0%.
The Cox multivariate regression analysis was performed by entering the density of CD8+, CD20+, and CD33+/p-STAT1+ cells, the expression of HMGB1, and Im into a model. Only the Im remained significantly associated with OS and DFS (Table 2). In addition, we built a final model combining the Im with the N stage, age, sex, tumor location, Borrmann classification, tumor size, and the expression of the ER, PR, and p53, as well as immunological parameters. This model showed that only the Im remained significantly associated with OS and DFS (Table 2). In addition, the Im remained significantly correlated with OS and DFS upon multivariate analysis, whereas other clinical parameters were not significant.
2.
Multivariate analyses of OS and DFS among patients with UICC-TNM stage IIIa gastric cancer according to clinical and immune parameters
| Variable | OS | DFS | |||||
| HR | 95% CI | P | HR | 95% CI | P | ||
| OS, overall survival; DFS, disease-free survival; UICC, Union for International Cancer Control; TNM, tumor-nodes-metastasis; CD8, cluster of differentiation 8; HMGB1, high-mobility group protein B1; ER, estrogen receptor; PR, progesterone receptor; CEA, carcinoembryonic antigen. | |||||||
| Immune parameters | |||||||
| CD8 | 0.684 | 0.275-1.697 | 0.412 | 0.699 | 0.286-1.707 | 0.432 | |
| CD20 | 0.921 | 0.320-2.647 | 0.878 | 1.217 | 0.406-3.651 | 0.726 | |
| HMGB1 | 0.996 | 0.379-2.615 | 0.994 | 1.030 | 0.396-2.683 | 0.951 | |
| CD33+p-STAT1+ | 0.584 | 0.202-1.684 | 0.319 | 0.670 | 0.238-1.888 | 0.448 | |
| Immune score | 0.367 | 0.177-0.760 | 0.007 | 0.361 | 0.171-0.762 | 0.008 | |
| Final model | |||||||
| N stage | 0.641 | 0.417-0.985 | 0.043 | 0.665 | 0.436-1.013 | 0.057 | |
| Age | 0.964 | 0.503-1.848 | 0.912 | 1.083 | 0.568-2.065 | 0.809 | |
| Gender | 1.646 | 0.816-3.317 | 0.164 | 1.181 | 0.589-2.371 | 0.639 | |
| Tumor location | 0.896 | 0.708-1.134 | 0.360 | 0.896 | 0.706-1.138 | 0.368 | |
| Borrmann classification | 0.895 | 0.529-1.512 | 0.678 | 0.822 | 0.486-1.390 | 0.465 | |
| Tumor size | 1.292 | 0.650-2.567 | 0.464 | 1.569 | 0.779-3.162 | 0.208 | |
| ER | 2.539 | 0.434-14.855 | 0.301 | 2.104 | 0.366-12.101 | 0.405 | |
| PR | 0.641 | 0.204-2.011 | 0.446 | 0.608 | 0.193-1.913 | 0.395 | |
| P53 | 1.762 | 0.820-3.790 | 0.147 | 1.601 | 0.766-3.343 | 0.211 | |
| CEA | 0.548 | 0.292-1.030 | 0.062 | 0.579 | 0.314-1.069 | 0.081 | |
| CD8 | 0.670 | 0.261-1.723 | 0.406 | 0.551 | 0.216-1.405 | 0.212 | |
| CD20 | 1.414 | 0.341-3.818 | 0.830 | 1.203 | 0.364-3.970 | 0.762 | |
| HMGB1 | 0.741 | 0.261-2.105 | 0.573 | 0.874 | 0.311-2.453 | 0.797 | |
| CD33+p-STAT1+ | 0.570 | 0.181-1.792 | 0.336 | 0.679 | 0.222-2.080 | 0.498 | |
| Immune score | 0.292 | 0.127-0.675 | 0.004 | 0.324 | 0.142-0.743 | 0.008 | |
Discussion
In this study, we have proposed an Im system for gastric cancer. We observed that the OS and DFS of stage IIIA gastric cancer patients were significantly correlated with the Im. A lower Im was associated with poorer clinical outcomes, implicating that the Im system was a useful prognostic tool for gastric cancer patients, supplementing the TNM staging system.
T cells mediated adaptive immunity play a major role in antitumor immunity (19,20). Most study showed that high densities of cytotoxic T cells and memory T cells are associated with the favorable prognosis in gastric cancer (21,22). CD8+ T cell are the main effector cells, and the CD4+ T cell also can induce and activate CD8+ T cell in tumor microenvironment. Previous studies have focused on the role of CD8+ and CD45RO + T cells in an Im system. The rationale for compiling T cells, B cells, MDSCs, and HMGB1expression into this Im system was based on the observation that the immune response, which includes both cellular and humoral immunity, is essential in controlling cancer progression; thus, both the tumor-inhibitory and tumor-promotional factors were compiled into this Im system. In general, among the immune cell subtypes, CD8+ T cells, CD45RO + T cells, and Th1 cells show anti-cancerous potential. Conversely, the Th2, Th17, and Treg cells exert a more complicated influence, depending on the tumor types (4,10,15,23). Furthermore, in contrast to the B cells present in draining lymph nodes, the CD20+ TIL cells represent anti-cancer immunity for melanomas, ovarian, breast, and head and neck cancers (24-27). In this study, the densities of both CD8+ T cells and CD20+ B cells were associated with a better prognosis of gastric cancer, and were represented as positive Im scores.
Several other subtypes of immune cells, such as the MDSCs and tumor-associated macrophages (TAMs), are connected with the tumor progression. The MDSCs originate from myeloid cells, which are transformed into potent immunosuppressive cells upon being recruited to the tumor microenvironment. Circulating MDSCs have a negative prognostic role in multiple solid tumors (28-30). Although MDSCs subtypes are heterogeneous, we have previously identified the CD33 and p-STAT1 double-positive cells as a specific type of MDSCs; we observed that the density of CD33 and p-STAT1 double-positive cells was associated with a poor prognosis of gastric cancer (6). As TAMs are known to be associated with MDSCs, and MDSCs are responsible for amplifying the immunosuppressive activity of macrophages, MDSCs were compiled into this scoring system instead of TAMs as the representatives of tumor-promotional immunity (31,32).
With the exception of immune cells, damage-associated molecular patterns (DAMPs) are known to participate during the prime phase of the immune response. Some DAMPs are actively secreted by cells undergoing immunogenic cell death (e.g., calreticulin and adenosine triphosphate), whereas others are emitted passively (e.g., HMGB1). The same DAMPs may contribute to both, the inhibition or progression of cancer (33). As the immune staining for HMGB1 exhibited more reproducibility than the staining for calreticulin and adenosine triphosphate, HMGB1 expression was selected as a represent active immunological modulator in order to enhance the prognostic potential of immune cells including T cells, B cells, and MDSCs. The overexpression of HMGB1 was observed in tumor cells derived from colon, breast, lung, cervical, hepatocellular, and gastric cancers. Higher levels of HMGB1 have been associated with greater tumor angiogenesis, growth, invasion, metastasis, and immunosuppressive activity (34,35). In this study, the expression of HMGB1 was inversely associated with the prognosis, and was characterized by negative Im scores.
Using the TNM staging system, gastric cancer patients with IIIA stage have an overall 5-year survival rate of 19.8% (36). In this study, although the 5-year survival rate was 32%, the OS time varied significantly among the Im subgroups. According to the immune score system, the patients can be divided into two groups, the high and low immune score group. The high immune score group include Im3 and Im4, and low immune score group include Im0, Im1 and Im2. The OS and DFS time varied significantly between high group and low group, especially the Im0 and Im4. The cumulative 5-year survival rate was 80.0% and 51.7% among the patients with Im4 and Im3, respectively. Conversely, the 5-year survival rate was only 0%, 5.8%, and 0% among patients with Im0, Im1, and Im2, respectively. Similarly, the DFS rates were markedly among the Im subgroups. The cumulative 5-year DFS was 75%, and 44.8% among the patients with Im4 and Im3, respectively. However, patients belonging to the Im0, Im1, and Im2 subgroups showed a 5-year DFS rate of 0%. These data imply that the clinical outcome was heterogeneous, even within the same TNM stage. These big differences outcome may be the different immune responses to gastric cancer. This type of heterogeneity should have a considerable influence when the role of adjuvant therapy is assayed. Im systems have the potential to identify this diversity effectively. This Im system might be beneficial while selecting patients for adjuvant therapy, especially for adjuvant immunotherapy.
This study has the following limitations: first, it included only 100 patients with stage IIIA cancer; a larger number of patients are needed for validating these observations. Second, the selection of the hot spots of immune cell infiltration was arbitrary; therefore, a digital method might improve the reproducibility.
Conclusions
In conclusion, this study proved the existence of prognostic heterogeneity among stage IIIA gastric patients; an Im system was designed that might be helpful in identifying such types of heterogeneity.
Acknowledgments
Funding: This study received support from the National Nature Science Foundation of China (Grant No. 81272341, 81401156). Research Program of Guangzhou Municipal Health Bureau Foundation of China (Grant No. 20141A011085, 20141A011088). The PhD Start-up Fund Guangzhou Medical University (Grant No. 2013C49).
Footnotes
The authors have no conflicts of interest to declare.
References
- 1.Nagtegaal ID, Quirke P, Schmoll HJ. Has the new TNM classification for colorectal cancer improved care? Nat Rev Clin Oncol. 2011;9:119–23. doi: 10.1038/nrclinonc.2011.157. [DOI] [PubMed] [Google Scholar]
- 2.Mlecnik B, Bindea G, Pagès F, et al. Tumor immunosurveillance in human cancers. Cancer Metastasis Rev. 2011;30:5–12. doi: 10.1007/s10555-011-9270-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Quiet CA, Ferguson DJ, Weichselbaum RR, et al. Natural history of node-positive breast cancer: the curability of small cancers with a limited number of positive nodes. http://ascopubs.org/doi/abs/10.1200/jco.1996.14.12.3105. J Clin Oncol. 1996;14:3105–11. doi: 10.1200/JCO.1996.14.12.3105. [DOI] [PubMed] [Google Scholar]
- 4.Fridman WH, Pagès F, Sautès-Fridman C, et al. The immune contexture in human tumours: impact on clinical outcome. Nat Rev Cancer. 2012;12:298–306. doi: 10.1038/nrc3245. [DOI] [PubMed] [Google Scholar]
- 5.Fridman WH, Dieu-Nosjean MC, Pagès F, et al. The immune microenvironment of human tumors: general significance and clinical impact. Cancer Microenviron. 2013;6:117–22. doi: 10.1007/s12307-012-0124-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dong J, Li J, Liu SM, et al. CD33+/p-STAT1+ double-positive cell as a prognostic factor for stage IIIa gastric cancer. Med Oncol. 2013;30:442. doi: 10.1007/s12032-012-0442-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Peng RQ, Wu XJ, Ding Y, et al. Co-expression of nuclear and cytoplasmic HMGB1 is inversely associated with infiltration of CD45RO+ T cells and prognosis in patients with stage IIIB colon cancer. BMC Cancer. 2010;10:496. doi: 10.1186/1471-2407-10-496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Galon J, Pagès F, Marincola FM, et al. Cancer classification using the Immunoscore: a worldwide task force. J Transl Med. 2012;10:205. doi: 10.1186/1479-5876-10-205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Galon J, Mlecnik B, Bindea G, et al. Towards the introduction of the 'Immunoscore' in the classification of malignant tumours. J Pathol. 2014;232:199–209. doi: 10.1002/path.4287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Anitei MG, Zeitoun G, Mlecnik B, et al. Prognostic and predictive values of the immunoscore in patients with rectal cancer. Clin Cancer Res. 2014;20:1891–9. doi: 10.1158/1078-0432.CCR-13-2830. [DOI] [PubMed] [Google Scholar]
- 11.Galon J, Angell HK, Bedognetti D, et al. The continuum of cancer immunosurveillance: prognostic, predictive, and mechanistic signatures. Immunity. 2013;39:11–26. doi: 10.1016/j.immuni.2013.07.008. [DOI] [PubMed] [Google Scholar]
- 12.Angell H, Galon J. From the immune contexture to the Immunoscore: the role of prognostic and predictive immune markers in cancer. Curr Opin Immunol. 2013;25:261–7. doi: 10.1016/j.coi.2013.03.004. [DOI] [PubMed] [Google Scholar]
- 13.Kim ST, Jeong H, Woo OH, et al. Tumor-infiltrating lymphocytes, tumor characteristics, and recurrence in patients with early breast cancer. Am J Clin Oncol. 2013;36:224–31. doi: 10.1097/COC.0b013e3182467d90. [DOI] [PubMed] [Google Scholar]
- 14.Salama P, Phillips M, Grieu F, et al. Tumor-infiltrating FOXP3+ T regulatory cells show strong prognostic significance in colorectal cancer. J Clin Oncol. 2009;27:186–92. doi: 10.1200/JCO.2008.18.7229. [DOI] [PubMed] [Google Scholar]
- 15.Ladoire S, Martin F, Ghiringhelli F. Prognostic role of FOXP3+ regulatory T cells infiltrating human carcinomas: the paradox of colorectal cancer. Cancer Immunol Immunother. 2011;60:909–18. doi: 10.1007/s00262-011-1046-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hussein MR, Ismael HH. Alterations of p53, Bcl-2, and hMSH2 protein expression in the normal breast, benign proliferative breast disease, in situ and infiltrating ductal breast carcinomas in the upper Egypt. Cancer Biol Ther. 2004;3:983–8. doi: 10.4161/cbt.3.10.1136. [DOI] [PubMed] [Google Scholar]
- 17.Hussein MR, Hassan HI. Analysis of the mononuclear inflammatory cell infiltrate in the normal breast, benign proliferative breast disease, in situ and infiltrating ductal breast carcinomas: preliminary observations. J Clin Pathol. 2006;59:972–7. doi: 10.1136/jcp.2005.031252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mlecnik B, Tosolini M, Kirilovsky A, et al. Histopathologic-based prognostic factors of colorectal cancers are associated with the state of the local immune reaction. J Clin Oncol. 2011;29:610–8. doi: 10.1200/JCO.2010.30.5425. [DOI] [PubMed] [Google Scholar]
- 19.Bergman MP, D'Elios MM. Cytotoxic T cells in H. pylori-related gastric autoimmunity and gastric lymphoma. J Biomed Biotechnol. 2010;2010:104918. doi: 10.1155/2010/104918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Amedei A, Della Bella C, Silvestri E, et al. T cells in gastric cancer: friends or foes. Clin Dev Immunol. 2012;2012:690571. doi: 10.1155/2012/690571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim YJ, Lim J, Kang JS, et al. Adoptive immunotherapy of human gastric cancer with ex vivo expanded T cells. Arch Pharm Res. 2010;33:1789–95. doi: 10.1007/s12272-010-1111-7. [DOI] [PubMed] [Google Scholar]
- 22.Lee HE, Chae SW, Lee YJ, et al. Prognostic implications of type and density of tumour-infiltrating lymphocytes in gastric cancer. Br J Cancer. 2008;99:1704–11. doi: 10.1038/sj.bjc.6604738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pagès F, Kirilovsky A, Mlecnik B, et al. In situ cytotoxic and memory T cells predict outcome in patients with early-stage colorectal cancer. J Clin Oncol. 2009;27:5944–51. doi: 10.1200/JCO.2008.19.6147. [DOI] [PubMed] [Google Scholar]
- 24.Nielsen JS, Sahota RA, Milne K, et al. CD20+ tumor-infiltrating lymphocytes have an atypical CD27- memory phenotype and together with CD8+ T cells promote favorable prognosis in ovarian cancer. Clin Cancer Res. 2012;18:3281–92. doi: 10.1158/1078-0432.CCR-12-0234. [DOI] [PubMed] [Google Scholar]
- 25.Mahmoud SM, Lee AH, Paish EC, et al. The prognostic significance of B lymphocytes in invasive carcinoma of the breast. Breast Cancer Res Treat. 2012;132:545–53. doi: 10.1007/s10549-011-1620-1. [DOI] [PubMed] [Google Scholar]
- 26.Pretscher D, Distel LV, Grabenbauer GG, et al. Distribution of immune cells in head and neck cancer: CD8+ T-cells and CD20+ B-cells in metastatic lymph nodes are associated with favourable outcome in patients with oro- and hypopharyngeal carcinoma. BMC Cancer. 2009;9:292. doi: 10.1186/1471-2407-9-292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ladányi A, Kiss J, Mohos A, et al. Prognostic impact of B-cell density in cutaneous melanoma. Cancer Immunol Immunother. 2011;60:1729–38. doi: 10.1007/s00262-011-1071-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Diaz-Montero CM, Finke J, Montero AJ, et al. Myeloid-derived suppressor cells in cancer: therapeutic, predictive, and prognostic implications. Semin Oncol. 2014;41:174–84. doi: 10.1053/j.seminoncol.2014.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lakshmi Narendra B, Eshvendar Reddy K, Shantikumar S, et al. Immune system: a double-edged sword in cancer. Inflamm Res. 2013;62:823–34. doi: 10.1007/s00011-013-0645-9. [DOI] [PubMed] [Google Scholar]
- 30.Khaled YS, Ammori BJ, Elkord E. Myeloid-derived suppressor cells in cancer: recent progress and prospects. Immunol Cell Biol. 2013;91:493–502. doi: 10.1038/icb.2013.29. [DOI] [PubMed] [Google Scholar]
- 31.Ostrand-Rosenberg S, Sinha P, Beury DW, et al. Cross-talk between myeloid-derived suppressor cells (MDSC), macrophages, and dendritic cells enhances tumor-induced immune suppression. Semin Cancer Biol. 2012;22:275–81. doi: 10.1016/j.semcancer.2012.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gao J, Wu Y, Su Z, et al. Infiltration of alternatively activated macrophages in cancer tissue is associated with MDSC and Th2 polarization in patients with esophageal cancer. PLoS One. 2014;9:e104453. doi: 10.1371/journal.pone.0104453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Krysko DV, Garg AD, Kaczmarek A, et al. mmunogenic cell death and DAMPs in cancer therapy. Nat Rev Cancer. 2012;12:860–75. doi: 10.1038/nrc3380. [DOI] [PubMed] [Google Scholar]
- 34.Kang R, Chen R, Zhang Q, et al. HMGB1 in health and disease. Mol Aspects Med. 2014;40:1–116. doi: 10.1016/j.mam.2014.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Parker KH, Sinha P, Horn LA, et al. HMGB1 enhances immune suppression by facilitating the differentiation and suppressive activity of myeloid-derived suppressor cells. Cancer Res. 2014;74:5723–33. doi: 10.1158/0008-5472.CAN-13-2347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Washington K. 7th edition of the AJCC cancer staging manual: stomach. Ann Surg Oncol. 2010;17:3077–9. doi: 10.1245/s10434-010-1362-z. [DOI] [PubMed] [Google Scholar]