Abstract
Indigo blue is a natural dye used for thousands of years by civilizations to dye fabric blue and it is naturally obtained from Isatis tinctoria. I. tinctoria is not only used for extraction of indigo blue color but also used medicinally in Traditional Chinese Medicine because of its active compounds. Sodium dithionite (Na2S2O4) is used in dye bath for indigo blue extraction, but this reducing agent and its derivatives are major pollutants of textile industry and subsequently have hazardous influences on public health. Herein, the present study was designed to obtain the high yield of natural indigo dye but with low possible toxic effect. In this context, genotoxic effects of particular combinations of natural dye solutions obtained from Isatis tinctoria subsp. tomentolla with Na2S2O4 as reducing agent were investigated. Dye solutions were obtained using two different pH levels (pH 9 and 11) and three different concentrations of Na2S2O4 (2.5, 5 and 10 mg/ml). In addition to the dye solutions and reducing agent, aqueous extracts of I. tinctoria were assessed for their genotoxicity on human lymphocytes. For in vitro testing of genotoxicity, chromosomal aberrations (CAs), sister chromatid exchanges (SCEs) and mitotic indexes (MI) assays were used. Accordingly, Na2S2O4 caused significant increases in CA and SCE as well decrease in MI but the genotoxic effects of sodium dithionite were reduced with natural indigo dye. As a result, aqueous extracts of Isatis leaves removed the toxic effects of sodium dithionite and showed anti-genotoxic effect. For the optimal and desired quality but with less toxic effects of natural dye, 2.5 mg/ml (for wool yarn) and 5 mg/ml (for cotton yarn) of Na2S2O4 doses were found to be the best doses for reduction in the dye bath at Ph 9.
Keywords: Indigo, Isatis spp., Sodium dithionite, Genotoxic
Introduction
The blue dye indigo is one common natural dye stuff and it is a general name addressed to the different plant species including Isatis indigotica and Isatis tinctoria (Brassicaceae), Baphicacanthus cusia (Acanthaceae), Polygonum tinctorium (Polygonaceae), Indigofera suffrutticosa and Indigofera tinctoria (Fabaceae) but interestingly, indigo plants do not contain indigo blue but instead they accumulate the indigo precursors including indican and isatan and also some indole derivatives such as isatin, indirubin, hydroxyl indoles and their glucosides (Gilbert et al. 2004; Zou and Koh 2007). The indigo blue is one of the world’s most important industrial chemicals but it has been progressively replaced with synthetic ones. Natural and synthetic indigo dyes are called vat dyes which means that they require to be suitable the addition of reducing agents to their water soluble leuco-form before dyeing under alkaline conditions. For this process, sodium dithionite (Na2S2O4) has been considered as a best reducing agent in traditional and industrial dying but the reducing agent releases sodium sulphate, sulphite and thiosulphate ions into the media. Those ions not only cause environmental concerns as major pollutants of the modern textile industry waste waters but they present also no negligible risks of toxicity against living organisms (Vourema et al. 2008; Dogan et al. 2005).
Numerous studies concerning the genotoxic effects of synthetic indigo and its dye solutions have been performed (Dixit and Goyal 2013; Bhattacharjee 2014; Bazin et al. 2012; Sarıkaya et al. 2012) and mutagenic activity of synthetic indigo was revealed using Ames test (Rannug et al. 1992). Furthermore, the lethal doses of patent blue and indigocarmin was reported to be 50 mg/ml (Sarıkaya et al. 2010). Although indigo was also used as food dye, given the statistically significant occurrence of tumors, sythetic indigo (blue 2) is not recommended for human consumption (Kobylewski and Jacobson 2010).
Natural Isatis plants, some indigo precursors and indirubin were also tested for their genotoxicity (Candido-Bacani et al. 2011) and indirubin and its derivatives were found to possess low toxicity (Zhen et al. 2007). In natural Isatis plants, some indigo precursors and indirubin known as red isomer of indigo were also tested for their genotoxicity (Candido-Bacani et al. 2011) and indirubin and its derivatives were reported to possess low toxicity (Zhen et al. 2007). Various biological activities including antifebrile, anti-inflammatory, antiviral, antimicrobial, detoxifying purposes, restenosis and skin psoriasis (Hoessel et al. 1999; Chiang et al. 2013; Liau et al. 2007) and antileukemic activity against myelocytic leukemia in Traditional Chinese Medicine (Hoessel et al. 1999) have been reported for indirubin and its derivatives (Hoessel et al. 1999). Studies with respect to the monitoring the possible toxic effects of plant species are of great concern for public and environmental health for desired sustainable life comfort. According to previous reports, genotoxic effects of synthetic indigo and its dye solutions and natural indigo plants were examined (Candido-Bacani et al. 2011; Calvo et al. 2011) but to the best of our knowledge, no previous reports in relation to the genotoxic and cytotoxic effects of natural indigo dye solutions with sodium dithionite using human peripheral blood lymphocytes (PBLs) have been published.
Chromosome Aberration (CA) and Sister Cromatid Exchange (SCE) assay in PBLs are recognized as a useful indicator for cytogenetic and genotoxic tests respectively (Timocin et al. 2016; İstifli and Topaktaş 2013). High level of CA in PBLs is associated with an increased risk of cancer and also predicts genotoxic risk of potentially mutagenic and carcinogenic chemicals (Kocaman et al. 2014; Norppa et al. 2006). SCEs involve the exchange of DNA segments between two sister chromatids and increase of SCE frequency in PBLs indicates its genotoxicity (Kocaman et al. 2014; Albertini et al. 2000).
The activity and quality of plants and their commercial or industrial products are based on their metabolite content. The possible anti-genotoxic potency of the Isatis leaves might be attributed to the metabolite profile and their quantity in addition to the optimized extraction conditions such as pH, concentration, temperature etc. Hereby, the current study was addressed to determine the possible alleviation roles of Isatis leaves on adverse effects of sodium dithionite used as a reducing agent in dye solutions. In this context, (1) the metabolite profile of Isatis leaves was monitored using high pressure liquid chromatography, (2) genotoxic effects of dye solutions and control groups were assessed, (3) cotton and wool yarns were dyed with indigo dye solutions, (4) and finally, best indigo dye solutions were classified based on the dye quality and genotoxic results.
Material and method
Plant material
Isatis tinctoria subsp. tomentella was used as a natural indigo source. The seeds of the plant were collected from Göksun-Kahramanmaraş (1200 m) and the plants were scientifically identified by Prof. Dr. Ahmet Ilçim (plant taxonomist) using Flora of Turkey and East Aegean Islands (Davis 1965). Collected seeds were grown at the Institute of Agriculturel Reasearch Station of the Eastern Mediterranean Crossing Region between 2010–2011 years. Rosette leaves of the plants were harvested in May, 2011 and then immediately transported to the laboratory and preserved until use.
Indigo precursors and indirubin analyses in I.tinctoria subsp. tomentolla leaves by High Performance Liquid Chromatography (HPLC).
The indigo precursors (indican and isatan-β) and its red isomer indirubin were extracted from fresh leaves of I. tinctoria according to the method proposed by Zou and Koh (2007). Since isatan-β is unstable and easily-affected by environmental conditions and there is no chemical standard for isatan-β available, the presence of isatan-β was revealed by our research group using LC–MS (Karaman et al. 2016).
To measure indican and indirubin yields, firstly, 4 g of fresh leaves were ground using a mortar and extracted with 40 ml THF by ultrasonication for 20 min in triplicate (40 × 3). The solvent was evaporated in a vacuum evaporator. Separation of the compounds in the extracts was performed by a liquid chromatography system (HPLC) Agilent 1100 series (Agilent Technologies, Santa Clara, CA, USA) equipped with an UV detector and C18 column at 280 nm. The elution solvent was water/acetonitrile (40:60), with a flow rate of 1 ml/min and analyzed for 37 min. The extract was resolved with 2 ml acetonitrile then the solution was filtered through a 0.45 -µl filter and 20 µl of the solutions were injected in to HPLC. The indican and indirubin peaks were identified using synthetic indican and indirubin references (purchased from Sigma-Aldrich) Indican was quantified by a calibration curve obtained from 5, 10, 50, 100, 250, 500 and 1000 ppm and indirubin was prepared 5, 10, 25, 50, 100 and 200 nM as a standard solutions. The reference chromogram peaks of isatan-β, indican and indirubin were observed at a retention time of 2.7, 2.4 and 4.96 min, respectively.
Preparation of plant extracts and dye solution
The rinsed Isatis leaves were cut into 1 cm wide-pieces and placed into the bottles. Distilled water was added to sealed bottles and then left to fermentation at room temperature for a day. At the end of the fermentation period, leaves was separated by filtration and the fluids were allocated to two equal volumes, one part of fluids were left for analysis as aqueous extract (AE) and the other fluids sample was splitted into two parts and then the pH of each fluids part was set to 9.0 and 11.0, respectively, using 2 N NaOH (Merck) solution (Stoker et al. 1998). After adjustment of pH, 100 mL solution were measured and then various (2.5,5 and 10 mg/ml) concentration of Na2S2O4 (Merck) were added for reduction of indigo at 60 °C for one hour (Karaman et al. 2013). The following methods were used for genotoxic analysis and dye process.
Dying procedures
The cotton and wool yarns were dyed at different pH (9 and 11) and Na2S2O4 (2.5, 5 and 10 mg/ml) concentrations. For this purpose 1 g of yarns were dipped into 100 ml dye solutions and then were incubated for 60 min at 50 °C in water bath. During the dyeing process, stirring and aeration were performed manually every 15 min for 1 min. Finally, dyed yarns were rinsed with tap water (approx. 500 mL) three times for the removal of excess unbound dyes and yarns were exposed to air (Balfour-Paul 1998). The colors detected from different dye solutions were evaluated by comparing each sample with standard blue scale. Also CIE-L*a*b* coordinates of dying yarns were measured with Spectra Flash Spectrophotometer in the Kipaş Textile Company laboratory (Kahramanmaraş, Turkey).
Lymphocyte cultures
Peripheral blood cells were obtained from six healthy non smoking (3 male and 3 female) donors (aged 23–24). The same subjects were used for all performed assays according to IPC guidelines (Albertini et al. 2000). Ethics committee of the Kahramanmaraş Sutcu Imam University was informed and approved the protocol for the study.
Chromosomal aberrations (CA) and sister-chromatid exchange (SCE) assay
The tests were conducted according to method proposed by Çelik et al. (2010). The tests were conducted according to the method published by Çelik et al. (2010). Whole-blood sample (0.3 ml) was cultured in 4 ml Chromosome Medium B (containing fetal bovine serum, heparin, antibiotics, phytohaemagglutinin) (Biochrome, Berlin Germany) supplemented with 10 µg/mL-1 of 5-bromodeoxyuridine (CAS no: 14930-96-2) for SCE assays and incubated in the dark at 37 °C for 72 h. Two series of experiments were carried out. 80 μl test samples which were dye solutions prepared at two different pH values (9,11) and three different Na2S2O4 concentrations (2.5, 5 and 10 mg/ml) were added to tubes and cultered for 48 h (Çelik et al. 2010; Al-Zubairi et al. 2010; Anupama et al. 2008; López Nigro and Carballo 2008). A negative control (untreated blood cells) and a positive control mytomycin-c (MMC, CAS no: 9754) were used for testing. Furthermore, to reveal the genotoxic effect of Isatis plant, AE (aqueous extract), Na2S2O4+ AE and MMC (control mitomycin-c) + AE were also tested. In our preliminary study 10 mg/ml Na2S2O4 caused elevated CA and SCE so we decided to use 5 mg/ml Na2S2O4 dose for comparing as control group. To arrest the cell cycle at metaphase stage, colchicine (0.06 μg/ml) was added to each tube during the last 2 h. The cultured peripheral blood lymphocytes were harvested by treating with KCl (75 Mm), which spreads chromosomes and hemolyzes the red blood cells, and then fixed with freshly prepared methanol: acetic acid (3:1 v/v) for 45 min at +4 °C. At the end of this procedure, the following steps including I) washing twice with fixative, II) slide-staining with 5 % Giemsa (pH = 6.8) prepared in Sorensen buffer solution for 20–25 min, III) washing with distilled water, IV) drying at room temperature, V) mounting with depex were followed. Chromosomal abnormalities were counted from 100 well-spread metaphases per donor (totally 600 metaphases per concentration). The mean frequency of abnormal cells of structural and numerical CAs and the number of CAs per cell (CA/cell) were calculated. The mitotic index (MI: number of metaphase/total interphases and metaphases) was scored by recording the number of metaphases in 1000 cells for each donor. MI was calculated according to the OECD Guideline (1997). For the SCE study, the slides were stained using the Fluorescence Plus Giemsa method (FPG) technique according to the method described by Hazen et al. (1985). For this purpose, 1-day-old slides were covered with Sorensen Buffer (pH 6.8) and irradiated with UV light (254 nm) for 30 min. After irradiation slides were incubated in 1 × SSC (150 mM NaCl, 15 mM sodium citrate) at 58–60 °C for 60 min and stained with Giemsa (5 % in Sorensen Buffer) for 20 min (Kaya and Topaktaş 2007; Kocaman et al. 2014; Timuroğlu et al. 2014).
The SCEs were measured from 25 cells per donor. Replication Index (RI) was calculated according to the following formula RI = [(1 × M1) + (2 × M2) + (3 × M3)]/N, where M1, M2 and M3 represent the number of cells undergoing first, second and third mitosis, respectively, and N represents the total number of metaphases scored which were measured for 100 cells from each donor (Schneider et al. 2004; Timoroğlu et al. 2014). All chemicals used were of highest purity.
Statistical analysis
All variables were analyzed by SPSS software with z-test for the percentage of abnormal cell, CA/cell, RI and MI, and t-test was used for SCE. Results were expressed as the means ± standard error. The multiple comparison of mean data among negative control, positive control, Na2S2O4 and exposed groups was performed by one-way analysis of variance followed by Duncan test.
Result and discussion
Determination of indigo precursors and indirubin in I.tinctoria subsp. tomentolla leaves
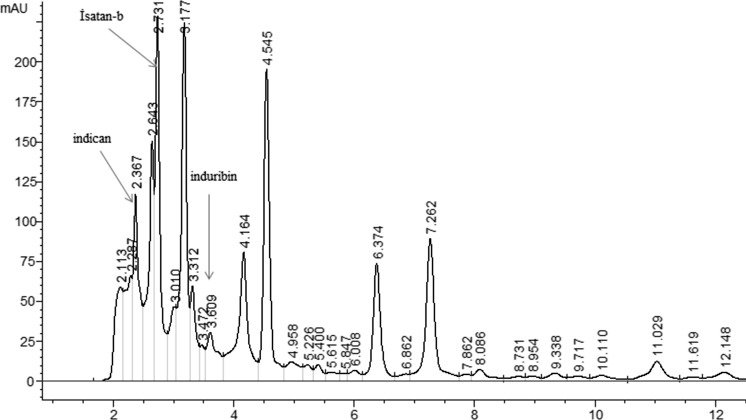
HPLC chromatogram of I. tinctoria is given in Fig. 1. Indican, indirubin and isatan-β compounds were identified and quantified according to HPLC results. Indican amount was found to be 4.26 mg/g and indirubin yield was determined to be 117.48 µg/g in the fresh leaves.
Fig. 1.
HPLC chromotogram of I. tinctoria subsp. tomentolla leaves
The indican results obtained herein were close to the previous studies (Kokubun et al. 1998; Gilbert et al. 2004; Zou and Koh 2007; Rocha et al. 2011; Campeol et al. 2006; Angelini et al. 2007). Indirubin results were similar or lower than those in the previous studies (Liau et al. 2007; Gilbert et al. 2004; Zou and Koh 2007; Chen et al. 2011). Indigo content (2.80 mg/g) was previously reported by our research group (Karaman et al. 2016). The results were higher than in the reports by Gilbert et al. (2004) and Campeol et al. (2006) but lower than in the study by Kokubun et al. (1998). The difference in literature concerned their content and quantification might be attributed to the plant species, extraction method, and harvesting time.
Chromosome aberration (CA) test
Results with respect to the chromosome abberation test are collectively represented in Table 1. Accordingly, eight types of CA (chromatid and chromosome breaks, sister union, dicentric chromosome, chromatid exchanges, fragment, ring chromosome, dicentric chromosome and polyploidy) were obtained in the studied materials. The chromatid-type abberations were observed much more frequently than the chromosome-type abberations. Figure 2a shows the chromatid breaks in indigo dye solutions. The highest chromosomal aberrations were found by positive control MMC followed by Na2S2O4 (5 mg/ml) treatment (p < 0.001). The differences of frequency of abnormal cells and the number of CA per cell were not significant for the aqueous extracts of Isatis (AE) and the negative control. The lowest percentage of CA was obtained from negative control and AE group, respectively. Although Na2S2O4 application alone had significant toxic effects on abnormal cell and CA, AE + Na2S2O4 (5 mg/ml) treatment showed low CA and abnormal cells. Also this treatment was statistically insignificant when compared to the negative control (Table 1). Na2S2O4 application alone and increasing concentrations of dye (5 and 10 mg/ml) caused aberrations and high numbers of abnormal cells, and CA per cell at both pH (pH 9 and pH 11) levels when compared with negative and positive control. Generally, in 2.5 mg/ml Na2S2O4 treatments at the two different pH, there were no significant differences in the abnormal cell ratio and the number of CA as compared to negative control. It is obvious from Table 1 that water extract of Isatis leaves also decreased the toxic effect of MMC, resulting in the number of chromatid break, chromatid exchange, dicentric chromosome fragment and polyploidy cells in the AE +MMC reduced by half compared to MMC application alone. The current study showed that Na2S2O4 application significantly increased the frequency of CA and abnormal cell in human lymphocytes. High chromotid and chromosome breaks have been observed as main abberations at higher Na2S2O4 applications in dye solutions. Abnormal cell and CA/cell proportion were higher at increasing Na2S2O4 concentrations (5 and 10 mg/ml) in the study. Sodium dithionite is known mildy hazardous chemical and toxic effect of Na2S2O4 and its derivates for wastewater and ecology were reported by Božic and Kokol (2008). Gottlieb et al. (2003) studied toxicity of some textile dyes using sodium dithionite as a reducing agent. Güloglu et al. (2004) studied the effects of SO2 released through air because of the harmful mixture of a sodium hydrosulphite of a sodium hydrosulphite on textile workers and they reported that the effects of SO2 gases were depended on the intensity and the time of duration.
Table 1.
Total chromosomal aberrations in human lymphocytes treated with I.tinctoria subsp. tomentolla dye solution
| Test Substance | pH | Conc. | Aberrations | Abnormal cell ± SE (%) | CA/cell ± SE (%) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Na2S2O4 (mg/ml) | B′ | B″ | CE | DS | SU | F | R | P | ||||
| Negative Control | – | – | 7 | 2 | 1 | 2 | 3 | – | – | 2 | 2.83 ± 0.07 | 0.03 ± 0.01 |
| Positive Control (MMC) | – | 0.2 µg/m | 67 | 14 | 18 | 12 | 15 | 9 | – | 5 | 23.33 ± 1.71 | 0.24 ± 0.02 |
| Na2S2O4 | 5 | 16 | 7 | 7 | 5 | 5 | 2 | 2 | 4 | 8.00 ± 1.10 a1b1 | 0.08 ± 0.01a1b1 | |
| AE | – | – | 6 | 2 | 3 | 1 | 3 | 1 | 1 | 1 | 3.00 ± 0.65b1c1 | 0.03 ± 0.01b1c1 |
| AE +MMC | – | – | 25 | 11 | 9 | 4 | 11 | 4 | 1 | 2 | 11.16 ± 1.28a1b1c3 | 0.11 ± 0.01a1b1c1 |
| AE +Na2S2O4 | – | 5 | 9 | 3 | 4 | 4 | 5 | 3 | 1 | 3 | 4.95 ± 0.89b1c3 | 0.05 ± 0.01b1c3 |
| Indigo dye solution | 9 | 2.5 | 7 | 6 | 4 | 5 | 4 | 1 | 1 | – | 4.16 ± 0.8b1c2 | 0.04 ± 0.01b1c2 |
| Indigo dye solution | 9 | 5 | 10 | 6 | 10 | 4 | 7 | 1 | 1 | 2 | 6.8 ± 1.02a1b1 | 0.06 ± 0.01a2b1 |
| Indigo dye solution | 9 | 10 | 11 | 12 | 6 | 10 | 9 | 2 | – | 5 | 9.16 ± 1.60a1b1c1 | 0.09 ± 0.01a1b1 |
| Indigo dye solution | 11 | 2.5 | 7 | 4 | 5 | 6 | 4 | 1 | – | 2 | 4.97 ± 0.91b1c3 | 0.04 ± 0.01b1c2 |
| Indigo dye solution | 11 | 5 | 10 | 7 | 7 | 5 | 7 | 2 | 1 | 4 | 7.61 ± 1.04a1b1 | 0.07 ± 0.01a1b1 |
| Indigo dye solution | 11 | 10 | 18 | 10 | 7 | 9 | 7 | 5 | – | 4 | 10 ± 1.22a1b1 | 0.1 ± 0.01a1b1 |
B′ chromatid break, B″ chromosome break, CE chromatid exchange, DS dicentric chromosome, SU chromatid union, F fragment, R ring chromosome, P polyploidy, Na2S2O4 Sodium dithionite, MMC mitomycin-C, 600 metaphases were scored for six treatment. AE Aqueous extract of Isatis leaves, SE Standard Error
a Significantly different from negative control, b Significantly different from positive control (MMC) and c Significantly different from Na2S2O4
a1b1c1 p < 0.001, a2b2c2 p < 0.01, a3b3c3 p < 0.05
Fig. 2.
Photomicrograph showing human metaphase stage in indigo dye solutions with different Na2S2O4 concentrations (a 2.5 mg/ml at pH 11, b 10 mg/ml at pH 9, B′ chromatid break, SCE: Sister-chromatid exchanges)
Sister-chromatid exchange (SCE) and mitotic indices (MI) results
Table 2 presents the experimental results of SCE, RI (Replication index) and MI. Figure 2b presents a photomicrograph of SCE. As expected, MMC treated cultures exhibited a many fold induction of SCEs followed by AE + MMC and Na2S204 applications. Also high numbers of SCEs were observed by increasing the concentrations of Na2S2O4 in dye solutions (5 and 10 mg/ml) at two pH levels when compared with positive, negative controls and Na2S2O4. Aqueous extract of Isatis with MMC and Na2S2O4 treatments showed lower frequency rate of the SCE than MMC and Na2S2O4 treatments alone. Dye solutions with 2.5 mg/ml Na2S2O4 concentrations at two pH applications had no statistically significant effects on frequency rate of the SCE in comparison with the negative control but the results were significant when compared to both MMC and Na2S2O4 alone treatments (p < 0.001), suggesting that Isatis exracts might reduce the efficiency of MMC and Na2S2O4 applications on the human lymphocytes.
Table 2.
Sister-chromatid exchanges (SCE) and mitotic indices (MI) in human lymphocytes treated with I. tinctoria subsp. tomentol la and its dye solution
| Test Substance | pH | Na2S2O4 (mg/ml) | Min- Max SCE | SCE/Cell ± SE | M1 | M2 | M3 | Rl ± E | MI ± SE |
|---|---|---|---|---|---|---|---|---|---|
| Negative control | – | – | 1–6 | 3,70 ± 0,09 | 114 | 146 | 340 | 2.37 ± 0.03 | 8.3 ± 0.35 |
| Positive control (MMC) | – | 0.2 µg/ml | 10–28 | 19.76 ± 0.25 | 310 | 218 | 72 | 1.60 ± 0.03 | 5 ± 0.28 |
| Na2S2O4 | 5 | 6–10 | 6.27 ± 0.11a1b1 | 376 | 144 | 80 | 1.66 ± 0.02 | 4.31 ± 0.25a1 | |
| AE | – | – | 1–7 | 3.983 ± 0.08b1c1 | 116 | 157 | 327 | 2.35 ± 0.03 | 7.78 ± 0.32b1c1 |
| AE +MMC | – | – | 8–22 | 16.01 ± 0.18a1b2c1 | 260 | 238 | 102 | 1.73 ± 0.03 | 6.65 ± 0.03a1b1c1 |
| AE +Na2S2O4 | – | 5 | 3–8 | 4.10 ± 0.09b1c1 | 200 | 122 | 178 | 1.67 ± 0.03 | 7.40 ± 0.34b1c1 |
| Indigo dye solution | 9 | 2.5 | 1–9 | 4.01 ± 0.07b1 c1 | 130 | 178 | 292 | 2.27 ± 0.03 | 8.20 ± 0.35b1c1 |
| Indigo dye solution | 9 | 5 | 2–11 | 4.20 ± 0.09a2b1c2 | 228 | 199 | 173 | 1.90 ± 0.03 | 7.9 ± 0.30b1c1 |
| Indigo dye solution | 9 | 10 | 2–13 | 5.42 ± 0.12a1b1c3 | 289 | 213 | 98 | 1.68 ± 0.02 | 5.85 ± 0.28a1c1 |
| Indigo dye solution | 11 | 2.5 | 1–8 | 4.08 ± 0.20b1 c1 | 137 | 176 | 287 | 2.25 ± 0.03 | 8.15 ± 0.35b1c1 |
| Indigo dye solution | 11 | 5 | 3–13 | 4.76 ± 0.09a1b1c3 | 213 | 238 | 149 | 1.89 ± 0.03 | 7.45 ± 0.32b1c1 |
| Indigo dye solution | 11 | 10 | 5–11 | 5.99 ± 0.10a1b1 | 306 | 218 | 76 | 1.65 ± 0.02 | 5.6 ± 0.28a1c2 |
A total 600 metaphases were scored for SCE test. 600 metaphases were scored for RI, and 6000 metaphases were scored for the MI; RI replication index, MI mitotic index, M 1, M 2 and M 3 metaphases 1, 2 and 3, respectively, Na2S2O4 Sodium dithionite, MMC mitomycin-C, AE Aqueous extract of Isatis leaves, SE Standard Error
aSignificantly different from negative control, b Significantly different from positive control (MMC) and c Significantly different from Na2S2O4
a1b1c1 p < 0.001, a2b2c2 p < 0.01, a3b3c3 p < 0.05
A reduction at higher Na2S2O4 applications (50 and 100 mg/ml) in dye solution at two pH levels was observed for Replication Index (RI) when compared with the negative control, the positive control and Na2S2O4 but the differences in the results were not found to be statistically significant for all control groups.
Aqueous extract of Isatis with MMC and Na2S2O4 increased the MI in human lymphocytes when compared to the chemicals alone (Table 2). Surprisingly the lowest MI value were observed for the Na2S2O4 application instead of the MMC treatment. Dye solutions including high concentration of Na2S2O4 (100 mg/ml) at both pH levels also showed low mitotic index (MI) relative to negative and positive control and Na2S2O4 alone (p < 0.001). Overall these results indicate that increasing concentration of Na2S2O4 in dye solutions significantly increased SCE frequencies and decreased the MI as compared with the control groups.
SCE and MI results of the experiment seem to be parallel with chromosomal abberations results with regard to MMC, Na2S2O4 and their combination with Isatis extract. According to the CA results, higher Na2S2O4 concentrations (5 and 10 mg/ml) in dye solutions gave high SCE results in comparison with the control groups. These results showed that natural indigo from I. tinctoria subsp. tomentolla reduced genotoxic effects of MMC and Na2S2O4. A possible explanation for this might be that the natural indigo dye contains some precursors of indigo besides indirubin. These chemicals are known as antigenotoxic and anti cancerogenic in Traditional Chinese Medicine. Hoessel et al. (1999) reported that indirubin exhibited no toxic effect on bone marrow even in long-term studies. Also synthetic indirubin was reported to be a good antitumour agent and had only minor toxicity in animal models (Hoessel et al. 1999). Mutagenic effect of the natural chemicals and some Isatis sp. were studied by some authors. Hesbert et al. (1984) compared the genotoxicity of natural indigo with synthetic indigo using Salmonella/microsome mutagenicity test and clastogenic potential was observed by micronucleus test in the bone marrow of male mice. The authors found that natural indigo exhibited no significant genotoxic effect. Dominici et al. (2010) studied the genotoxicity of water and DMSO solutions of indigo naturalis prepared from Indigofera tinctoria leaves using the cytokinesis-blocked micronucleus (CBMN) assay on human metabolically active HepG2 cell line. For both solutions, cytotoxicity was below 10 % and not higher than the control. The results of this study indicated that indigo naturalis exhibited neither cytotoxicity, nor genotoxicity for all tested concentrations, which may justify excluding Indigofera and its components from the list of carcinogenic agents.
Studies by Jongen and Alink (1982) support the present results, they examined the mutagenic potential of two natural and seven synthetic commercial indigo products and they reported that natural products showed no mutagenicity in Salmonella typhimurium strains TA98 and TA100 but all of the synthetic products were found mutagenic. Also our results coincided with previous studies by Candido-Bacani et al. (2011) which evaluated genotoxic and mutagenic effects of acute (24 h) and repeated (14 day) exposure to isatin as indigo precursor in vivo, and the results showed that the mutagenic and genotoxic effects of isatin depended on dose and—exposure period.
In contrast, a study by Calvo et al. (2011) reported the mutagenic activity of the methanolic extracts of Indigofera truxillensis and I. suffruticosa in the Salmonella/microsome assays using TA100, TA98, TA102 and TA97a strains. The methanolic extract of I. truxillensis showed mutagenic activity in the TA98 strain. The alkoloid fraction of both the species including indigo, indirubin and indigo were found mainly responsible for the mutagenic activity.
In our experiment, the significant increase in the CAs and SCEs were observed at high concentrations of Na2S2O4 in dye solutions. This study supports the clastogenic potential of this reducing agent. Our result can be confirmed by a report on the genotoxic effects of synthetic indigo dye that Rannug et al. (1992) tested for the mutagenic effect of pure cotton and jeans fabrics extracts and synthetic indigo derivates using the Salmonella typhimurium strains TA98 and TA100. The mutagenicity of the indigo dyed fabrics was reported to be dependent on type and treatment of the fabrics. Mutagenic effects on TA98 ± S9 and TA100 ± S9 have been observed on both bleached and non bleached jeans. The greatest effects were seen in the presence of S9. Normal washing of the fabrics after bleaching reduced mutagenicity. They reported that considering the amount of indigo in the extracts and its low mutagenicity, the toxic effect of jeans extracts must be caused by other unknown components. In another study by Karslı-Çeppioğlu and Yurdun (2012) exhibitited results similar to those published by Rannug et al. (1992) that reported indigo and indigoid dyes would be genotoxic at higher concentrations. It is probable that a genotoxic effect might occur among those individuals that have worked with these dyestuffs. Dixit and Goyal (2013) stated that the reproductive toxicity was caused by indigo carmine in Swiss albino mice. An other report by Bazin et al. (2012) on toxic effects of synthetic indigo dye, Bazin et al. (2012) indicated agonistic and antagonistic estrogen activity of several textile dyes and extracts of blue jeans textiles wastewater samples and that several textile dyes were potential endocrine disrupting agents.
Dying performance
The main colors obtained from cotton and wool yarns at different pH and Na2S2O4 levels are given in Fig. 3. Dark blue colour was observed in wool yarns at both pH values and all Na2S2O4 concentrations, whereas lighter blue color was obtained from cotton yarns at both pH levels and all Na2S2O4 concentrations. While light blue colour observed at 2.5 mg/ml Na2S2O4 at the cotton yarn, dark blue colours were obtained from 5 mg/ml Na2S2O4 concentration at both pH levels. Table 3 shows CIE Lab coordinates of the dying yarns. Because the lighter blue colour obtained from 2.5 mg/ml Na2S2O4, the colorimetric speration failed at this concentration. Therefore dyeing yarns with 5 mg/ml and 10 mg/ml Na2S2O4 treatments were analzed for colorimetric specification with CIELab system. The coordinates describe the shade of the dyeing: L∗ value describes lightness, a∗ value defines the red/green value (positive mark = red, negative mark = green) and b∗ marks the yellow/blue value (positive sign = yellow, negative sign = blue). Dyed cotton yarns showed darker color (L = 49,74) at pH 9 and 50 mM Na2S2O4 treatment. Wool yarn showed high L* values at 10 mg/ml Na2S2O4 treatment. Dyed cotton and wool yarns showed blue shade with negative values of b* and all yarns showed green shade with negative values of a* coordinate. Komboonchoo and Bechtold (2009) reported that optimum dye quality would require optimized dyeing baths of indigo which depends on pH, temperature, ratio of dyestuff and reducing agent, etc.
Fig. 3.
The wool and cotton yarn dyeing at different pH values and Na2S2O4 concentrations obtained from I. tinctoria subsp. tomentella
Table 3.
CIE L*a*b* cordinates of the dying yarns obtained from natural indigo dye
| Cottton yarn | Wool yarn | ||||||
|---|---|---|---|---|---|---|---|
| pH | Na2S2O4 (mg/ml) | L | a | b | L | a | b |
| 9 | 5 | 49.74 | −6.72 | −14.91 | 23.06 | −2.29 | −11.81 |
| 9 | 10 | 55.92 | −5.27 | −10.22 | 20.62 | −1.46 | −11.57 |
| 11 | 5 | 54.57 | −7.55 | −12.80 | 29.81 | −5.49 | −10.80 |
| 11 | 10 | 51.68 | −7.26 | −14.03 | 26.60 | −3.69 | −13.03 |
In the final part of the experiment, taken together dying yarns and the results of genotoxic analysis the better dye solution could be prepared with 2.5 mg/ml Na2S2O4 for wool yarn and 5 mg/ml Na2S2O4 for cotton yarn because of dye quality and efficiency. One of the more significant findings to emerge from this study is that antigenotoxic effects of Isatis leaves in water and dye solutions were revealed.
Conclusion
In the present study we tested the genotoxic effect of natural indigo dye solutions to determine lower toxic dose of Na2S2O4. Sodium dithionite is most commonly used as a reducing agent in textile industries due to low cost and dying properties. Toxic sulfides produced by Na2S2O4 not only cause serious environmental problems but also posses potential risks for textile workers. Hence, determination the non-toxic concentration of reducing agent for workers health but yielding the desired dye quality are of great concerns. From the study, we concluded that 2.5 mg/ml (for wool yarn) or 5 mg/ml (for cotton yarn) Na2S2O4 could be used as reducing agent in indigo dye solution, lower doses exhibited no genotoxic effect on PBLs.
Antigenotoxic effects of Isatis leaves in dye solutions were revealed in this study. Indigo and indirubin in natural dye solution might reduce the toxic effects of Na2S2O4 but still further studies are required to determine and better understand the efficacy of natural indigo. The result obtained with respect to Na2S2O4 dose also could be usable for synthetic indigo bath dye for reducing toxic effects. Further studies should be focused on the determination of the different chemicals besides sodium dithionite. The study should be repeated using natural compounds or enzymes for reduction of indigo in dye solution. Also genotoxic and allergen effect of colored yarn and textile material can be investigated in future studies.
Acknowledgments
The authors wish to thank for financial support from The Scientific And Technological Research Council of Turkey (Project number 109O655). We are also grateful to Prof. Dr. Ahmet İLÇİM for his valuable assistance in taxonomic identification of the plant.
References
- Albertini RJ, Anderson D, Douglas GR, Hagmar L, Hemminki K, Merlo F, Waters MD. IPCS guidelines for the monitoring of genotoxic effects of carcinogens in humans. Mutat Res/Rev Mutat Res. 2000;463:111–172. doi: 10.1016/S1383-5742(00)00049-1. [DOI] [PubMed] [Google Scholar]
- Al-Zubairi AS, Abdul AB, Syam MM. Evaluation of the genotoxicity of zerumbone in cultured human peripheral blood lymphocytes. Toxicol In Vitro. 2010;24:707–712. doi: 10.1016/j.tiv.2010.01.011. [DOI] [PubMed] [Google Scholar]
- Angelini LG, Tozzi S, Di Nasso NN. Differences in leaf yield and indigo precursors production in woad (Isatis tinctoria L.) and Chinese woad (Isatis indigotica Fort.) genotypes. Field Crops Res. 2007;101:285–295. doi: 10.1016/j.fcr.2006.12.004. [DOI] [Google Scholar]
- Anupama M, Murgan SS, Balakrishna MP. Broccoli flower head extract reduces mitomycin-C induced sister chromatid exchange in cultured human lymphocytes. Food Chem Toxicol. 2008;46:3351–3353. doi: 10.1016/j.fct.2008.08.009. [DOI] [PubMed] [Google Scholar]
- Balfour-Paul J. Indigo. London: British Museum Press; 1998. pp. 115–145. [Google Scholar]
- Bazin I, Hassine AIH, Hamouda YH, Mnif W, Bartegi A, Lopez-Ferber M, Gonzalez C. Estrogenic and anti-estrogenic activity of 23 commercial textile dyes. Ecotoxicol Environ Saf. 2012;85:131–136. doi: 10.1016/j.ecoenv.2012.08.003. [DOI] [PubMed] [Google Scholar]
- Bhattacharjee M. Studies on mitodepressive effect of Indigocarmine. Int J Innov Sci Eng Technol. 2014;1:157–160. [Google Scholar]
- Božič M, Kokol V. Ecological alternatives to the reduction and oxidation processes in dyeing with vat and sulphur dyes. Dyes Pigment. 2008;76:299–309. doi: 10.1016/j.dyepig.2006.05.041. [DOI] [Google Scholar]
- Calvo TR, Cardosa CRP, Moura ACS, Santos LC, Colus IMS, Vilegas W, Varanda EA. Mutagenic activity of Indigofera truxiensis ana I.suffruticosa aerial parts. Evid-Based Complement Altern Med. 2011;2011:1–9. doi: 10.1093/ecam/nep123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campeol E, Angelini L, Tozzı S, Bertolaccı M. Seasonal variation of indigo precursors in Isatis tinctoria L. and Polygonum tinctorium Ait. as affected by water deficit. Environ Exp Bot. 2006;58:223–233. doi: 10.1016/j.envexpbot.2005.09.006. [DOI] [Google Scholar]
- Candido-Bacani PDM, DosReis MB, Serpeloni JM, Calvo TR, Vilegas W, Varanda EA, De SyllosColus IM. Mutagenicity and genotoxicity of isatin in mammalian cells in vivo. Mutat Res Genet Toxicol Environ Mutagen. 2011;719:47–51. doi: 10.1016/j.mrgentox.2010.11.006. [DOI] [PubMed] [Google Scholar]
- Çelik M, Aksoy H, Yılmaz S. Evaluation of beauvericin genotoxicity with the chromosomal aberrations, sister-chromatid exchanges and micronucleus assays. Ecotoxicol Environ Saf. 2010;73:1553–1557. doi: 10.1016/j.ecoenv.2010.07.036. [DOI] [PubMed] [Google Scholar]
- Chen H-L, Tsao H-H, Lo JG, Chiu KH, Jen JF. Supercritical fluid extraction coupled with solvent-less spray collection mode for rapid separation of indirubin and tryptanthrin from folium isatidis. Sep Sci Technol. 2011;46:972–977. doi: 10.1080/01496395.2010.537725. [DOI] [Google Scholar]
- Chiang YR, Li A, Leu YL, Fang JY, Lin YK. An in vitro study of the antimicrobial effects of indigo naturalis prepared from Strobilanthes formosanus moore. Molecules. 2013;18:14381–14396. doi: 10.3390/molecules181114381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis PH (1965) Flora of Turkey and the East Aegean Islands, vol 1. Edinburgh University Press, Edinburgh, pp 287–366
- Dixit A, Goyal RP. Evaluation of reproductive toxicity caused by Indigo carmine on male swiss albino mice. Pharmacology. 2013;1:218–224. [Google Scholar]
- Doğan EE, Yesilada E, Ozata L, Yologlu S. Genotoxicity testing of four textile dyes in two crosses of drosophila using wing somatic mutation and recombination test. Drug Chem Toxicol. 2005;28:289–301. doi: 10.1081/DCT-200064473. [DOI] [PubMed] [Google Scholar]
- Dominici L, Cerbone B, Villarini M, Fatigoni C, Moretti M. In vitro testing for genotoxicity of indigo naturalis assessed by micronucleus test. Natural Prod Comm. 2010;5:1039–1042. [PubMed] [Google Scholar]
- Gilbert KG, Maule HG, Rudolph B, Lewis M, Vandenburg H, Sales E, Tozzi S, Cooke DT. Quantitative analysis of indigo and indigo precursors in leaves of Isatis spp. and Polygonum tinctorium. Biotechnol Prog. 2004;20:1289–1292. doi: 10.1021/bp0300624. [DOI] [PubMed] [Google Scholar]
- Gottlieb A, Shaw C, Smith A, Wheatley A, Forsythe S. The toxicity of textile reactive azo dyes after hydrolysis and decolourisation. J Biotechnol. 2003;101:49–56. doi: 10.1016/S0168-1656(02)00302-4. [DOI] [PubMed] [Google Scholar]
- Güloglu C, Kadiroglu AK, Kara İH. Evaluation of acute cases of so2 gas poisoning due to reaction of sodium hydrosulfite with water. Middle East J Family Med. 2004;5:1–6. [Google Scholar]
- Hazen MJ, Villanueva A, Juarranz A, Cañete M, Stockert JC (1985) Photosensitizing dyes and fluorochromes as substitutes for 33258 Hoechst in the fluorescence-plus-Giemsa (FPG) chromosome technique. Histochemistry 83:241–244 [DOI] [PubMed]
- Hesbert A, Bottin MC, De Ceaurriz J, Protois JC, Cavelier C. Testing natural indigo for genotoxicity. Toxicol Lett. 1984;21:119–125. doi: 10.1016/0378-4274(84)90232-7. [DOI] [PubMed] [Google Scholar]
- Hoessel R, Leclerc S, Endıcott JA, Lawrıe NM, Tunnah A, Leost M. Indirubin, the active constituent of Chinese antileukemia medicine, inhibits cylin-dependent kinase. Nat Cell Biol. 1999;1:60–67. doi: 10.1038/9035. [DOI] [PubMed] [Google Scholar]
- İstifli ES, Topaktaş M. Genotoxicity of pemetrexed in human peripheral blood lymphocytes. Cytotechnology. 2013;65:621–628. doi: 10.1007/s10616-012-9516-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jongen WMF, Alink GM. Enzyme-mediated mutagenicity in Salmonella typhimurium of contaminants of synthetic indigo products. Food Chem Toxicol. 1982;20:917–920. doi: 10.1016/S0015-6264(82)80228-9. [DOI] [PubMed] [Google Scholar]
- Karaman Ş, İlçim A, Çelik M, Çömlekçioğlu N, Dıraz E (2013) Cultivation trials, dying process and dyestuffs content of native Isatis species which are distrubuted in Kahramanmaras and near surrounding area. TUBİTAK Project Number 109O655, 80–90
- Karaman Ş, Dıraz E, Çömlekçioğlu N, İlçim A, Durdu H, Tansi S. High yielding indigo sources in native Isatis (Brassicaceae) taxa from Turkey. Genet Resour Crop Evol. 2016;63:531–543. doi: 10.1007/s10722-015-0269-8. [DOI] [Google Scholar]
- Karslı-Çeppioğlu S, Yurdun T. In vitro testing for genotoxicity of indigoid dyes by comet assay. J Marmara Univ Inst Health Sci. 2012;2:108–112. [Google Scholar]
- Kaya FF, Topaktaş M. Genotoxic effects of potassium bromate on human peripheral lymphocytes in vitro. Mutat Res. 2007;626:48–52. doi: 10.1016/j.mrgentox.2006.08.006. [DOI] [PubMed] [Google Scholar]
- Kobylewski S, Jacobson MF. Food dyes a rainbow of risks. Washington, DC: Center for Science in the Public Interest; 2010. [Google Scholar]
- Kocaman AY, Rencuzogulları E, Topaktas M. In vitro investigation of the genotoxic and cytotoxic effects of thiacloprid in cultured human peripheral blood lymphocytes. Environ Toxicol. 2014;29:631–641. doi: 10.1002/tox.21790. [DOI] [PubMed] [Google Scholar]
- Kokubun T, Edmonds J, John P. Indoxyl derivatives in woad in relation to medieval indigo production. Phytochemistry. 1998;49:79–87. doi: 10.1016/S0031-9422(97)01069-8. [DOI] [Google Scholar]
- Komboonchoo S, Bechtold T. Natural dyeing of wool and hair with indigo carmine (CI Natural Blue 2), a renewable resource based blue dye. J Clean Prod. 2009;17:1487–1493. doi: 10.1016/j.jclepro.2009.05.007. [DOI] [Google Scholar]
- Liau BC, Jong TT, Lee MR, Chen SS. LC-APCI-MS method for detection and analysis of tryptanthrin, indigo, and indirubin in Daqingye and Banlangen. J Pharm Biomed Anal. 2007;43:346–351. doi: 10.1016/j.jpba.2006.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- López Nigro MM, Carballo MA. Genotoxicity and cell death induced by tinidazole (TNZ) Toxicol Lett. 2008;180:46–52. doi: 10.1016/j.toxlet.2008.05.017. [DOI] [PubMed] [Google Scholar]
- Norppa H, Bonassi S, Hansteen I-L, Hagmar L, Strömberg U, Rössner P, Boffetta P, Lindholm C, Gundy S, Lazutka J, Cebulska-Wasilewska A, Fabiánová E, Šrám RJ, Knudsen LE, Barale R, Fucic A (2006) Chromosomal aberrations and SCEs as biomarkers of cancer risk. Mutat Res/Fundam Mol Mech Mutagen 600:37–45. doi:10.1016/j.mrfmmm.2006.05.030 [DOI] [PubMed]
- OECD (1997) Guideline for the testing of chemicals. In Vitro Mammalian Chromosome Aberration Test. 1–10
- Rannug U, Bramstedt H, Nilsson U. The presence of genotoxic and bioactive components in indigo dyed fabrics-a possible health risk? Mutat Res. 1992;282:219–225. doi: 10.1016/0165-7992(92)90099-4. [DOI] [PubMed] [Google Scholar]
- Rocha L, Carvalho C, Martins S, Braga F, Carnide V. Morpho-agronomic charecterization and variation of indigo precursors in woad (Isatis tinctoria) accessions. Plant Genet Res. 2011;9:206–209. doi: 10.1017/S1479262111000499. [DOI] [Google Scholar]
- Sarıkaya R, Selvi M, Akkaya N, Acar M, Erkoç F. Effects of food dyes in different concentrations on percentage of survival in drosophila melanogaster (mwh x flr) SDÜ Fen Derg. 2010;5(1):38–46. [Google Scholar]
- Sarıkaya R, Selvi M, Erkoç F. Evaluation of potential genotoxicity of five food dyes using the somatic mutation and recombination test. Chemosphere. 2012;88:974–979. doi: 10.1016/j.chemosphere.2012.03.032. [DOI] [PubMed] [Google Scholar]
- Schneider K, Hafner C, Jäger I. Mutagenicity of textile dye products. J Appl Toxicol. 2004;24:83–91. doi: 10.1002/jat.953. [DOI] [PubMed] [Google Scholar]
- Stoker KG, Cooke DT, Hill DJ. An improved method for the large-scale processing of woad (Isatis tinctoria) for possible commercial production of woad indigo. J Agric Eng Res. 1998;71:315–320. doi: 10.1006/jaer.1998.0329. [DOI] [Google Scholar]
- Timocin T, Ila HB, Dordu T, Husunet MT, Tazehkand MN, Valipour E, Topaktas M. Assessment of in vitro genotoxic and cytotoxic effects of flurbiprofen on human cultured lymphocytes. Drug Chem Toxicol. 2016;39:338–343. doi: 10.3109/01480545.2015.1121276. [DOI] [PubMed] [Google Scholar]
- Timoroğlu İ, Yüzbaşıoğlu D, Ünal F, Yılmaz S, Aksoy H, Çelik M. Assessment of the genotoxic effects of organophosphorus insecticides phorate and trichlorfon in human lymphocytes. Environ Toxicol. 2014;29:577–587. doi: 10.1002/tox.21783. [DOI] [PubMed] [Google Scholar]
- Vourema A, John P, Keskitalo M, Marken F. Electrochemical determination of plant-derived leuco-indigo after chemical reduction by glucose. J Appl Electrochem. 2008;38:1683–1690. doi: 10.1007/s10800-008-9617-0. [DOI] [Google Scholar]
- Zhen Y, Rensen VS, Jin Y, Suo Z, Wi A. Indirubin-30-monoxime inhibits autophosphorylation o FGFR1and stimulates ERK1/2 activity via p38 MAPK. Oncogene. 2007;26:6372–6385. doi: 10.1038/sj.onc.1210473. [DOI] [PubMed] [Google Scholar]
- Zou P, Koh LH. Determination of indican, isatin, indirubin and indigotin in Isatis indigotica by liquid chromatography/electrospray ionization tandem mass spectrometry. Rapid Comm Mass Spectrom. 2007;21:1239–1246. doi: 10.1002/rcm.2954. [DOI] [PubMed] [Google Scholar]