Abstract
Rheumatoid arthritis is characterized by inflammation of the synovial membrane, which can result in joint destruction within the first few years after onset. Lipopolysaccharides (LPS) are glycolipids found in abundance on the outer membranes of all Gram-negative bacteria and incite a vigorous inflammatory response. We studied the potential involvement of mitogen-activated protein kinase serine/threonine kinases in LPS-induced growth of HS synovial cells. Various concentrations of LPS were applied to cultured HS cells and growth rate, as well as changes in the phosphorylation states of extracellular regulated kinase 1/2, Jun N-terminal kinase (JNK), and p38 were determined. As results, growth of LPS-treated HS cells was inhibited primarily by phosphorylated JNK and this phosphorylation was mediated by the LPS receptor. Our results suggest that LPS inhibits growth of HS cells primarily through the JNK pathway.
Keywords: LPS, MAPK, Rheumatoid arthritis
Introduction
Rheumatoid arthritis is characterized by inflammation of the synovial membrane, which can result in joint destruction within the first few years after onset (Zrioual et al. 2009). Inflammation and joint damage result in loss of physical function, which is a hallmark of progressive disease (Lattuada et al. 2015). Synovial cells produce inflammatory mediators, proteases, and angiogenic growth factors, such as prostaglandin E2 (PGE2), matrix metalloproteinases, and vascular endothelial growth factor (Rothenberg et al. 1988; Yin et al. 2015).
Lipopolysaccharides (LPS) are glycolipids found in abundance on the outer membranes of all Gram-negative bacteria and incite a vigorous inflammatory response (Muller-Decker et al. 2005). Nanogram quantities of LPS injected into the bloodstream of humans result in all of the physiological manifestations of septic shock (Martirosyan et al. 2013). LPS recognition is mediated by a receptor complex comprised of Toll-like receptor 4 (TLR4) and CD14. Recognition of LPS by this receptor leads to the production of cytokines and co-stimulatory molecules by antigen-presenting cells, such as dendritic cells and macrophages (Meyer et al. 2001). Activation of antigen-presenting cells and production of cytokine is a prerequisite for activating acquired immunity in mammals (Martirosyan et al. 2013).
Mitogen-activated protein kinase (MAPK) signaling pathways convert extracellular signals into specific cellular responses through a cascade of phosphorylation events (Xiong et al. 2015). Hence, tight regulation of these signaling pathways is essential for normal cell function, and deregulation leads to various diseases, including tumorigenesis (Urosevic et al. 2014). Three major MAPK families are deregulated in cancer, such as extracellular regulated kinase (ERK)1 and ERK2 (ERK1/2), p38 MAPK, and Jun N-terminal kinase (JNK). ERK1/2 is involved mainly in the themogenic response in non-transformed cells, whereas p38 MAPK and JNK mediate cellular stress and inflammatory responses (Wei et al. 2014).
Here, we used LPS to investigate molecular mechanisms in the HS synovial cell line. Our data reveal that LPS triggers growth through the JNK signaling pathway.
Materials and methods
All chemicals and reagents used in the study were purchased from Sigma Chemical Co. (St. Louis, MO, USA). LPS was purchased from Chemicon International (Temecula, CA, USA). Antibodies to IgG, TLR4, GAPDH, ERK1/2, JNK, phospho-ERK1/2, phospho-JNK, activating transcription factor-2 (ATF-2), and phospho-ATF-2 were purchased from Millipore (Billerica, MA, USA). Anisomycin was purchased from Santa Cruz. The synovial HS cell line was purchased from ScienCell Research Laboratories (Carlsbad, CA, USA). Cell cultures were performed at 38.5 °C in a 5 % CO2 incubator under humidified air.
Immunofluorescence
The HS cells were fixed in 3.7 % paraformaldehyde for 30 min at room temperature and permeabilized with 0.5 % Triton X-100 in phosphate-buffered saline (PBS) for 15 min, followed by blocking overnight at 4 °C in 1 % bovine serum albumin in PBS with 10 % goat serum. The samples were then stained with primary antibody. Primary antibody binding was detected with Alexa Fluor 488 Goat anti-rabbit IgG (H + L) secondary antibodies. Images were captured with a Nikon A1 confocal microscope (Tokyo, Japan). Experiments were performed in triplicate.
3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltertrazolium bromide (MTT) assay
Cells were grown in 96-well plates (1 × 103 cells/well) with LPS. Control cells were switched from RPMI1640 to DMEM medium containing 0.1 % dimethyl sulfoxide (DMSO). Various final concentrations of LPS (0, 10, 20, or 40 ng/ml) were added for 12, 24, 48, and 72 h. Then, 20 μL of MTT was added to each well to a final concentration of 0.5 %. After a 4 h incubation at 37 °C in the dark, 150 μL DMSO was added to each well for 10 min to dissolve the formazan crystals. Absorbance was measured at 490 nm using a microplate reader (EXL800; Bio-Tek, Winooski, VT, USA). All experiments were repeated three times. The viability of cells exposed to LPS was expressed as a percentage of population growth plus the standard error of the mean relative to that of untransfected control cells. Cell death caused by LPS was calculated as a percentage of inhibition as: % inhibition = (1 − mean experimental absorbance/mean control absorbance) × 100.
Flow cytometric analysis
Forty-eight hours after LPS addition, samples (1 × 106 cells) were washed with PBS. The cells were then labeled with Annexin V and Propidium Iodide (PI). Apoptotic rates were determined by flow cytometry and analyzed by Flowjo software. The percentage of the early apoptosis was calculated by Annexin V-positivity and PI-negativity, while the percentage of the late apoptosis was calculated by Annexin V- and PI-positivity.
siRNA assay
The cells were transfected with the specific TLR4 siRNA (sense- CGAUGAUAUUAUUGACUUA [dT]; antisense- [dT]UAAGUCAAUAAUAUCAUGG[dT][dT]) (Hsieh et al. 2014).
SiRNA transfection was performed according to the protocol supplied by Invitrogen (Carlsbad, CA, USA). Briefly, 1 x 105 cells were seeded into six-well plates containing an antibiotic-free medium and incubated overnight. For each well, 5 μL siRNA was mixed with 125 μL OPTI-MEM I. The mixture was then combined with a solution of 5 μL lipofectamine (Invitrogen) in 125 μL OPTI-MEM I. After a 20-min incubation period at room temperature, the mixture was applied to the cells in an appropriate volume of OPTI-MEM I so as to achieve a final concentration of 100 nM for each siRNA. After incubation for 6 h at 37 °C, RPMI-1640 supplemented with serum was added to the wells and cells were cultured for an additional 24 h at 37 °C before analysis.
Western blot
The HS cells were homogenized; the proteins were separated on 8–12 % sodium dodecyl sulfate–polyacrylamide gel electrophoresis and transferred to a immunoblot nitrocellulose membrane. The membranes were blocked with PBS containing 5 % fat-free milk and 0.1 % Tween 20 for 30 min at room temperature and then incubated with primary anti-Rac1 antibody for at least 1 h at room temperature or overnight at 4 °C. The membranes were washed three times with PBS containing 0.1 % Tween 20, incubated with peroxidase-conjugated secondary antibodies, and developed using an enhanced chemiluminescent reagent (Pierce, Rockford, IL, USA).
Statistical analysis
Differences between gene expression levels were detected using one-way analysis of variance followed by Newman–Keuls test using GraphPad Prism 5 software (GraphPad Software, Inc., La Jolla, CA, USA). Three replicates were included in the statistical model. A P value <0.05 was considered significant. Data are presented as mean ± standard deviation.
Results
Optimal LPS concentration and HS cell culture time to detect LPS-induced effects
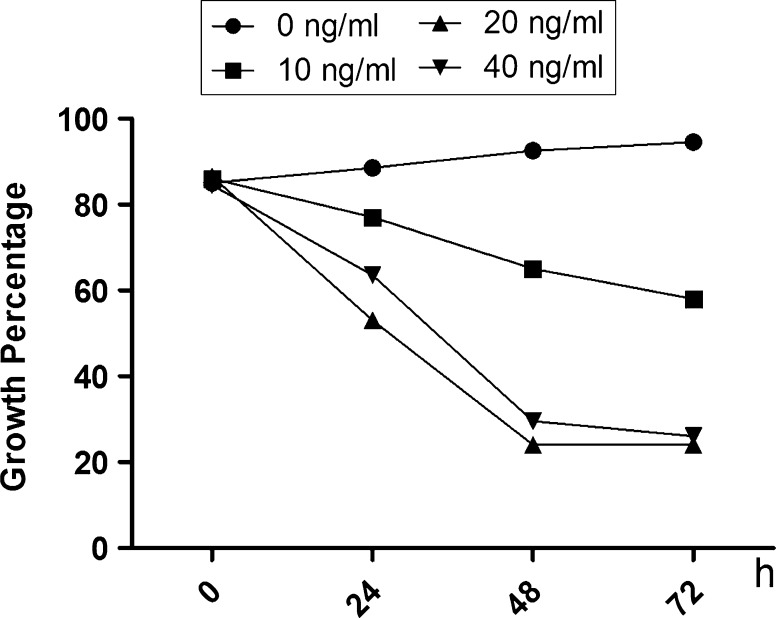
The optimal LPS concentration and HS cell culture duration to observe LPS-induced effects were determined. Figure 1 shows the growth percentages of HS cells at 0, 24, 48, 72, and 96 h after addition of LPS (0, 10, 20, or 40 ng/ml) to the cell culture media. The minimum growth rate occurred in cells exposed to 20 ng/ml LPS for 48 h.
Fig. 1.
Percentage of inhibited synovial HS cell growth following treatment with increasing concentrations (0, 10, 20, and 40 ng/ml) of lipopolysaccharide (LPS) for 0, 24, 48, and 72 h
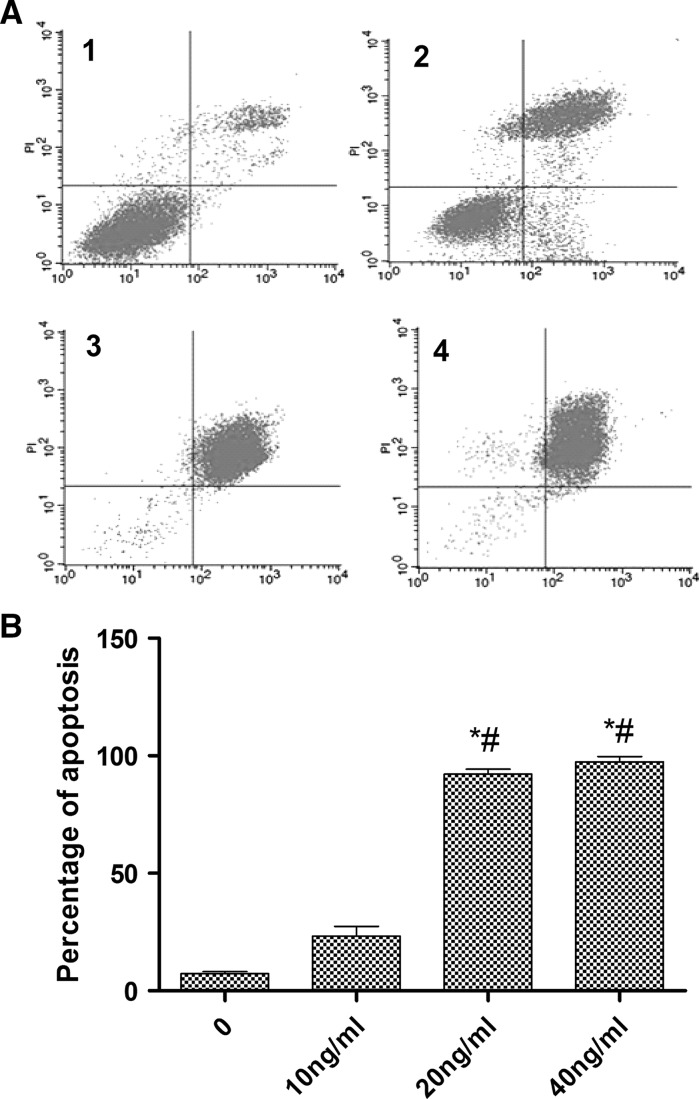
To identify the best concentration of LPS for HS cells apoptosis, flow cytometric analysis was used. The results showed that the best concentration was 20 ng/ml (Fig. 2a, b).
Fig. 2.
Detection of the apoptosis status of HS cells using the Annexin V/PI assay. a Annexin V/PI. 1 0 ng/ml, 2 10 ng/ml, 3 20 ng/ml, 4 40 ng/ml. b Percentage of HS apoptosis induced by different concentrations of LPS (X-axis - Annexin V, Y-axis - PI)
LPS inhibits growth of HS cells via the JNK pathway
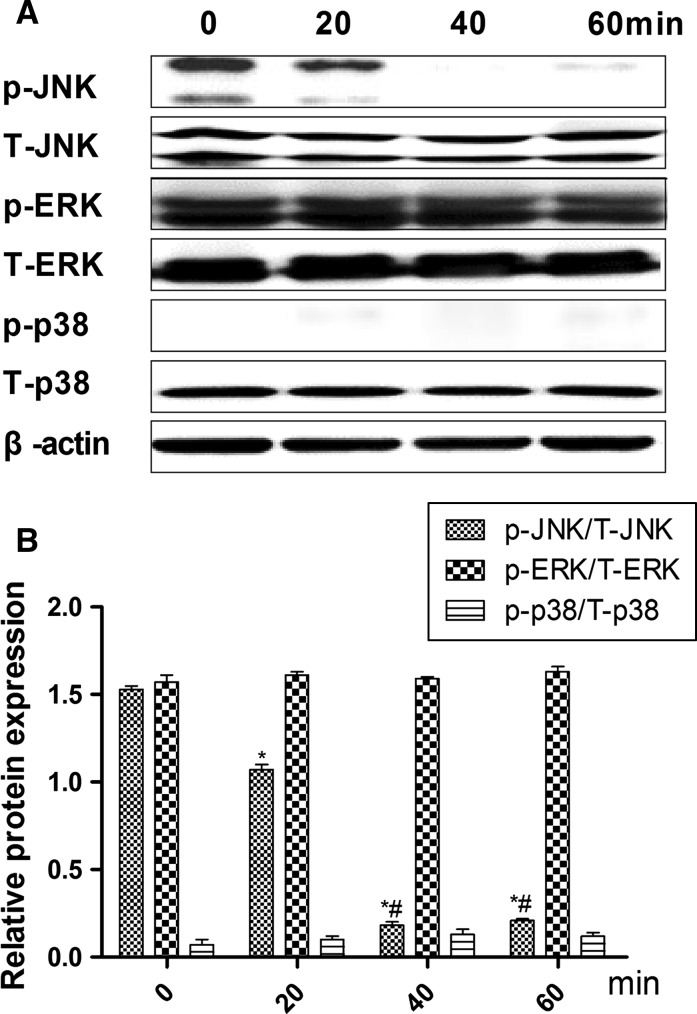
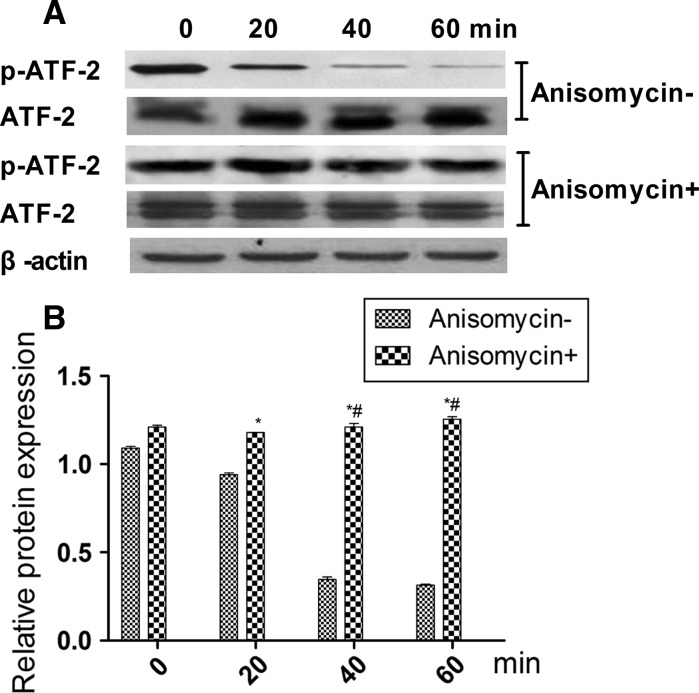
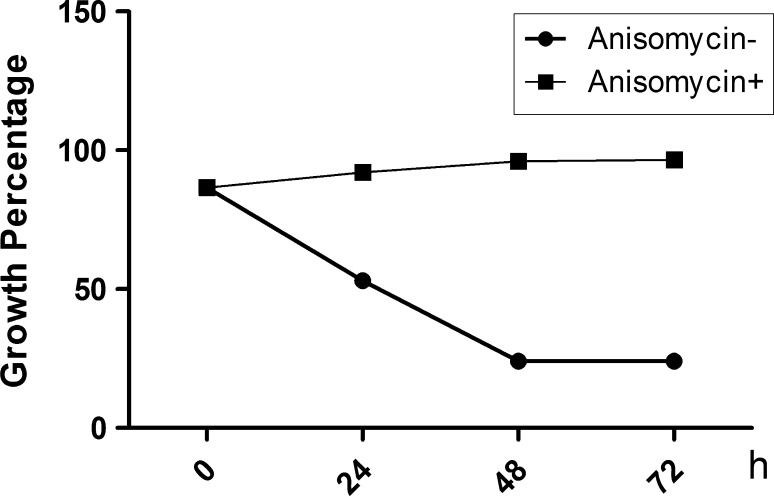
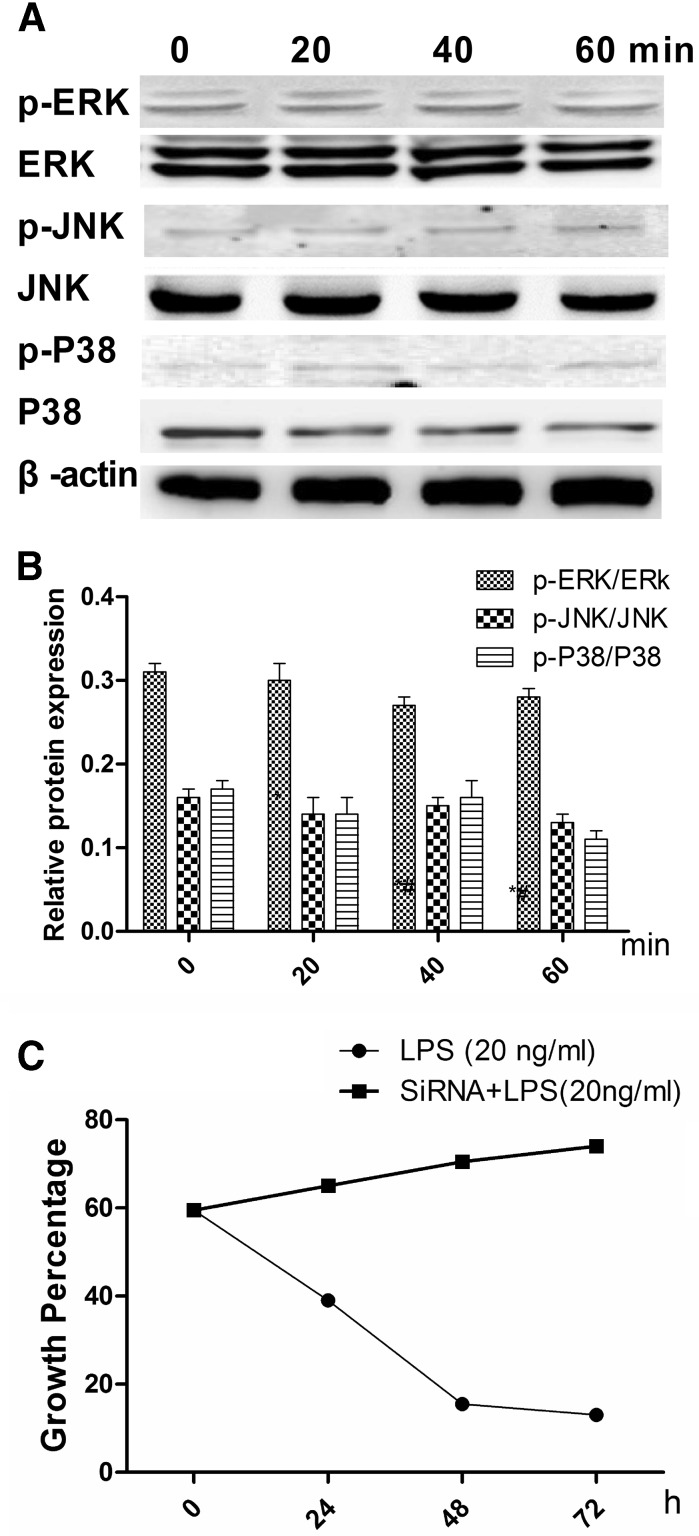
To determine which pathway mediates LPS-inhibited HS cell growth, the phosphorylation states of MAPKs, ERK1/2, JNK, and p38 were detected after 0, 20, 40, and 60 min (Fig. 3a) of treatment with 20 ng/ml LPS. The resulting decrease in JNK phosphorylation was low, however, after 20 min there were no changes for ERK or p38 (Fig. 3b), suggesting that JNK plays a key role in LPS-inhibited growth of HS cells. To verify this result, the JNK-specific phosphorylation inhibitor anisomycin was used to silence JNK expression. As results, ATF-2 phosphorylation was low (Fig. 4), and the combination of anisomycin and LPS (20 ng/ml) did not accelerate growth of HS cells (Fig. 5).
Fig. 3.
Effects of 20 ng/ml lipopolysaccharide (LPS) on activation of mitogen-activated protein kinase (MAPK) in synovial HS cells. a Expression of total and phosphorylated forms of the extracellular regulated kinase (ERK), Jun N-terminal kinase (JNK), and p38 proteins. b Relative protein expression (phosphorylated/total) of ERK, JNK, and p38 by Western blot. Results are mean ± standard deviation (n = 5). *Significantly different from the 0 min group (P < 0.05). #Significantly different from the 20 min group (P < 0.05)
Fig. 4.
Effects of activating transcription factor-2 (ATF-2) and phosphorylation of ATF-2 after Jun N-terminal kinase (JNK) was silenced with anisomycin (selleckchem). a Protein expression levels of ATF-2 and phosphorylated ATF-2. b Relative ATF-2 protein expression (phosphorylated/total) level by Western blot. Results are mean ± standard deviation (n = 5). *Significantly different from the 0 min group (P < 0.05). #Significantly different from the 20 min group (P < 0.05)
Fig. 5.
Percentage of inhibited synovial HS cell growth after Jun N-terminal kinase (JNK) was silenced with anisomycin
Silencing the TLR4 LPS receptor HS cells by siRNA
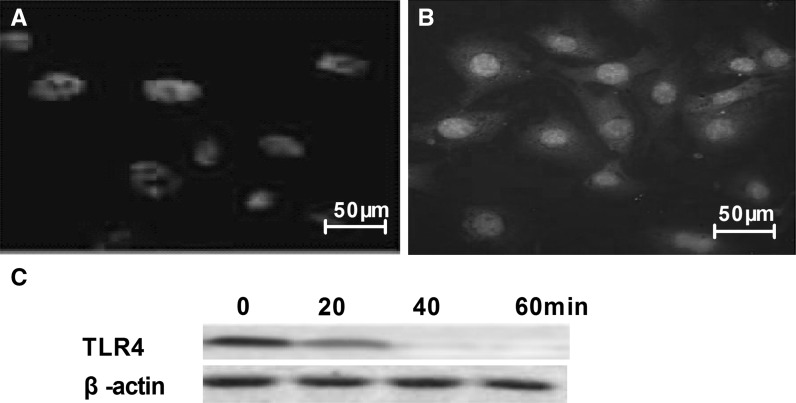
Immunocytochemistry was used to detect the expression status of the TLR4 LPS receptor in HS cells. Figure 6 shows that the TLR4 LPS receptor exists in HS cells.
Fig. 6.
The TLR4 lipopolysaccharide (LPS) receptor is expressed in cultured synovial HS cells. a Negative group. b TLR4. c TLR4 expression level after TLR4 siRNA added
TLR4 was silenced to verify that LPS acts through TLR4. Figure 7a shows that JNK phosphorylation was not significantly modified (Fig. 7b). The percentages of HS cells growing after 0, 24, 48, and 72 h were higher in the siRNA group than those in the other groups (LPS was added to each group, Fig. 7c).
Fig. 7.
Effects of silencing the TLR4 lipopolysaccharide (LPS) receptor by siRNA on mitogen-activated protein kinase (MAPK) activation in synovial HS cells. a Expression levels of the total and phosphorylated forms of the extracellular regulated kinase (ERK)1/2, Jun N-terminal kinase (JNK), and p38 proteins. b Relative expression (phosphorylated/total) of the ERK, JNK, and p38 proteins by Western blot. Results are mean ± standard deviation (n = 5). c Growth percentage of HS cells after 0, 24, 48, and 72 h (20 ng/ml LPS and siRNA + 20 ng/ml LPS)
Discussion
LPS is the main component of the outer cell wall of Gram-negative bacteria and is ranked among the most biologically active compounds (Im et al. 2012). LPS evokes dramatic systemic effects in eukaryotic organisms, which can culminate in endotoxic shock syndrome, as a consequence of activation of the complement and innate immune systems (Desaki et al. 2006).
In this study, we found that LPS inhibited growth of HS cells by inhibiting the JNK branch of the MAPK pathway. The optimal LPS concentration to inhibit growth was 20 ng/ml. We also demonstrated that this LPS effect was mediated through its TLR4 receptor, which is highly expressed in HS cells. siRNA blocked LPS-mediated growth, providing further support for the role of the TLR4 receptor.
To gain further insight into the mechanism by which LPS inhibited growth of HS cells, we evaluated signaling pathway activities downstream of TLR4. MAPKs are a superfamily of serine/threonine kinases that include ERK, JNK, and p38 (Reus et al. 2014). These kinases are primarily involved in activating nuclear transcription factors that control cell proliferation, differentiation, and apoptosis (Delhanty et al. 2006). Our results suggest that LPS inhibited growth of HS cells via the JNK signaling pathway and not through ERK or p38. We found that a 20–60 min LPS exposure was required to inhibit JNK phosphorylation; therefore, the inhibitory effect was time-dependent.
Overall, our results suggest that LPS inhibited growth of HS cells mainly through a JNK-dependent pathway. Thus, our findings suggest that LPS could be useful for inhibiting HS cells. Further studies are necessary to verify our findings before clinical application is considered.
References
- Delhanty PJ, van der Eerden BC, van der Velde M et al (2006) Ghrelin and unacylated ghrelin stimulate human osteoblast growth via mitogen-activated protein kinase (MAPK)/phosphoinositide 3-kinase (PI3 K) pathways in the absence of GHS-R1a. J Endocrinol 188:37–47 [DOI] [PubMed]
- Desaki Y, Miya A, Venkatesh B, et al. Bacterial lipopolysaccharides induce defense responses associated with programmed cell death in rice cells. Plant Cell Physiol. 2006;47:1530–1540. doi: 10.1093/pcp/pcl019. [DOI] [PubMed] [Google Scholar]
- Hsieh YY, Shen CH, Huang WS. Resistin-induced stromal cell-derived factor-1 expression through Toll-like receptor 4 and activation of p38 MAPK/NFkappaB signaling pathway in gastric cancer cells. J Biomed Sci. 2014;21:59. doi: 10.1186/1423-0127-21-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Im HJ, Park NH, Kwon YJ. Bacterial lipopolysaccharides induce steroid sulfatase expression and cell migration through IL-6 pathway in human prostate cancer cells. Biomol Ther (Seoul) 2012;20:556–561. doi: 10.4062/biomolther.2012.20.6.556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lattuada D, Casnici C, Crotta K. Proapoptotic activity of a monomeric smac mimetic on human fibroblast-like synoviocytes from patients with rheumatoid arthritis. Inflammation. 2015;38:102–109. doi: 10.1007/s10753-014-0012-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martirosyan A, Ohne Y, Degos C. Lipopolysaccharides with acylation defects potentiate TLR4 signaling and shape T cell responses. PLoS ONE. 2013;8:e55117. doi: 10.1371/journal.pone.0055117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer A, Puhler A, Niehaus K. The lipopolysaccharides of the phytopathogen Xanthomonas campestris pv. campestris induce an oxidative burst reaction in cell cultures of Nicotiana tabacum. Planta. 2001;213:214–222. doi: 10.1007/s004250000493. [DOI] [PubMed] [Google Scholar]
- Muller-Decker K, Manegold g, Butz H. Inhibition of cell proliferation by bacterial lipopolysaccharides in TLR4-positive epithelial cells: independence of nitric oxide and cytokine release. J Invest Dermatol. 2005;124:553–561. doi: 10.1111/j.0022-202X.2004.23598.x. [DOI] [PubMed] [Google Scholar]
- Reus GZ, Vieira FG, Abelaira HM. MAPK signaling correlates with the antidepressant effects of ketamine. J Psychiatr Res. 2014;55:15–21. doi: 10.1016/j.jpsychires.2014.04.010. [DOI] [PubMed] [Google Scholar]
- Rothenberg RJ, England D, Qureshi N. Stimulation of rabbit synoviocyte prostaglandin E2 synthesis by lipopolysaccharides and their subunit structures. Arthritis Rheum. 1988;31:238–247. doi: 10.1002/art.1780310212. [DOI] [PubMed] [Google Scholar]
- Urosevic J, Nebreda AR, Gomis RR. MAPK signaling control of colon cancer metastasis. Cell Cycle. 2014;13:2641–2642. doi: 10.4161/15384101.2014.946374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei C, Liu X, Long D. Molecular cloning and expression analysis of mulberry MAPK gene family. Plant Physiol Biochem. 2014;77:108–116. doi: 10.1016/j.plaphy.2014.02.002. [DOI] [PubMed] [Google Scholar]
- Xiong H, Xu Y, Tan G. Glycyrrhizin ameliorates imiquimod-induced psoriasis-like skin lesions in BALB/c mice and inhibits TNF-alpha-induced ICAM-1 expression via NF-kappaB/MAPK in HaCaT cells. Cell Physiol Biochem. 2015;35:1335–1346. doi: 10.1159/000373955. [DOI] [PubMed] [Google Scholar]
- Yin G, Wang Y, Cen XM. Lipid peroxidation-mediated inflammation promotes cell apoptosis through activation of NF-kappaB pathway in rheumatoid arthritis synovial cells. Mediators Inflamm. 2015;2015:460310. doi: 10.1155/2015/460310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zrioual S, Ecochard R, Tournadre A. Genome-wide comparison between IL-17A- and IL-17F-induced effects in human rheumatoid arthritis synoviocytes. J Immunol. 2009;182:3112–3120. doi: 10.4049/jimmunol.0801967. [DOI] [PubMed] [Google Scholar]