Abstract
15-Lipoxygenase-1 (15-Lox-1) as a member of fatty acid dioxygenases family has received considerable attention as an effector of cancer cell growth. The relevance of sodium butyrate on 15-Lox-1 pathway has not been determined in breast cancer. This study is aimed to investigate the possible involvement of 15-Lox-1 in the regulation of breast cancer cell growth by sodium butyrate. MTT assay was used to assess the cytotoxicity effect and Annexin-V-FITC staining was applied for detection of apoptosis using flow cytometry. The involvement of 15-Lox-1 was examined using 15-Lox-1 specific inhibitor and enzyme gene expression level and activity was further analyzed by Real-time PCR and measurement of 13(S)-HODE. The results revealed that sodium butyrate increased the expression of 15-Lox-1 and production of 13(S)HODE. 15-Lox-1 was also involved in the sodium butyrate-induced breast cancer cell cytotoxicity and apoptosis. This study provided more evidences on the positive effectiveness of 15-Lox-1/13(S)-HODE on controlling growth of breast cancer cells.
Keywords: Sodium butyrate, 15-Lipoxygenase-1, Apoptosis, Cell cytotoxicity
Introduction
Breast cancer imposes a considerable number of cancer-related mortality among women in the world (Lambrechts et al. 2011). Many scientific attempts are directed to find more efficient therapeutic approaches for managing breast cancer and reducing its related mortality (Fallahian et al. 2012; Giancotti 2006; Hoshyar et al. 2015). Aberrant expression of proteins responsible for cellular growth and apoptosis, is highly correlated to tumorigenesis (Burdick et al. 2006; Kasibhatla and Tseng 2003; Salimi et al. 2013) 15-lipoxygenase (15-Lox) belongs to the non-heam iron containing deoxygenase family, which is responsible for metabolism of polyunsaturated fatty acids. Two types of human 15-Lox isomers have been recognized: 15-lipoxygenase-1 (15-Lox-1) and 15-lipoxygenase-2 (15-Lox-2). 15-Lox-1 catalyzes the deoxygenation of linoleic acid and produces 13-S-hydroxyoctadecadienoic acid (13(S)-HODE) as its primary product (Brash 1999). The function of 15-Lox-1 is associated with cellular growth and differentiation and it has widespread tissue expression (Comba et al. 2010). 15-Lipoxygenase-2 (15-Lox-2) oxidizes arachidonic acid at the 15th carbon and produces 15-S-hydroxyeicosatetraenoic acid (15(S)HETE) subsequently. Unlike widespread expression of 15-Lox-1, the expression of 15-Lox-2 is restricted to a limited number of tissues including the prostate, skin, esophagus, and cornea (Suraneni et al. 2014). Multiple lines of evidences have elucidated the relationship of 15-Lox-1 and cancer cell growth and development. Down regulation of 15-Lox-1 in malignancies such as lung (Yuan et al. 2010), colon (Shureiqi et al. 1999), esophageal (Xu et al. 2003), pancreatic (Hennig et al. 2007) and breast (Jiang et al. 2006) cancer have been reported so far which was associated with low level of 13(S)-HODE and contributed to cancer progression (Tavakoli-Yaraki et al. 2013; Tavakoli Yaraki and Karami Tehrani 2013). However, the expression of 15-Lox-2 was down regulated or completely lost in prostate cancer therefore most investigations are devoted to peruse its role in prostate cancer development (Tang et al. 2009). It has been reported that 15-Lox-1 expression is regulated by methylation, acetylation or through binding of STATs (signal transducer and activator of transcription) to their binding sites in 15-Lox-1 promoter (Liu et al. 2015), however, the expression of 15-Lox-2 is not regulated by acetylation but other regulatory mechanisms, such as, regulation by glucocorticoid receptors are proposed instead (Feng et al. 2010). Acetylation is a well-described molecular mechanism mediating epigenetic modulation of genes. Acetylation of lysine residues at the N terminus of histone proteins neutralizes the positive charge and decreases the affinity of DNA to histones and results in subsequent relaxation of chromatin structure and more accessibility of the promoter of target genes to transcription factors. This process is reversed by histone deacetylases which leads to suppression of gene expression (Margueron et al. 2004). The relationship between deregulation of HDACs and cancer progression has been well-evidenced in the literature (Lane and Chabner 2009). In support of this, development of histone deacetylase inhibitors (HDACi) has received much attention due to their positive role on cellular pathways (Bolden et al. 2006). Sodium butyrate is a natural short-chain fatty acid and a well-known HDACi which affects cell proliferation, differentiation and apoptosis (Davie 2003). Several studies have shown that sodium butyrate regulates cancer cell growth via induction of apoptosis (Natoni et al. 2005). Different mechanisms are proposed to be involved in the sodium butyrate-elicited apoptosis including cell cycle arrest, inhibition of DNA double strand break repair, and oxidative stress (Cao et al. 2015; Paskova et al. 2013). However, the molecular mechanism underlying sodium butyrate-induced apoptosis has yet to be elucidated. Based on previous studies, 15-Lox-1 was notably down-regulated in human breast cancer samples and its main product 13(S)-HODE was reduced accordingly which was associated with cancer severity (Jiang et al. 2006). Therefore, activation of 15-Lox-1 is proposed to have beneficial effect on induction of apoptosis. Understanding the relationship between lipoxygenase and sodium butyrate anticancer effects may brighten the mechanistic details of sodium butyrate-induced apoptosis in breast cancer which might be further exploited in cancer therapy. Therefore, this study is aimed to investigate whether sodium butyrate can regulate cell proliferation through the 15-lipoxygenase-1 pathway. To aim this, tightly cohesive MCF-7 and triple negative highly metastatic MDA-MB-468 cells were selected and treated with different concentrations of sodium butyrate then the gene expression and activity of 15-Lox-1 was further assessed. The role of 15-Lox-1 in sodium butyrate-induced cell toxicity and apoptosis was evaluated using 15-Lox-1 specific inhibitor.
Materials and methods
Chemical reagents and materials
RPMI 1640, trypsin/EDTA, NaCl/Pi, penicillin and streptomycin were purchased from Gibco (Rockville, MD, USA). PD146176, the annexin-V-FITC apoptosis detection kit, MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide] and dimethylsulfoxide and sodium butyrate were obtained from Sigma-Aldrich (Munich, Germany). 13(S)HODE ELISA kit was provided by Assay Design (Ann Arbor, MI, USA). Trizol and High Capacity RNA-to-cDNA Kit were purchased from Invitrogen (Grand Island, NY, USA). The SYBR Green kit was obtained from Qiagen (Hilden, Germany).
Cell culture
The human breast cancer cell lines, MCF-7 and MB-MDA-468 were obtained from the Pasteur Institute of Iran. The cells were cultured in RPMI 1640 medium containing 10 % (v/v) fetal bovine serum, 100 U/ml of penicillin and 100 µg/ml of streptomycin and subsequently were maintained at 37 °C with an atmosphere of 5 % CO2 and 100 % humidity. For collecting and passaging the cells, cells were exposed to trypsin at the confluence of 70–100 %. Trypsin facilitates the cell separation by affecting focal adhesion of cells. The collected cells were used freshly or were frozen and stored at −80 °C for further experiments.
Cell viability assay
For determination of sodium butyrate toxicity on cells, MTT assay was selected as a method of choice. MCF-7 and MB-MDA-468 cells were seeded in 96-well plates at 5 × 103 cells/well in RPMI 1640 (supplemented with 10 % fetal bovine serum, 100 U/ml of penicillin and 100 µg/ml of streptomycin) in 5 % CO2 at 37 °C until nearly confluent. Cells were treated with 0.5, 1 and 5 mM of sodium butyrate and incubated for 24, 48 and 72 h. To evaluate the viability of cells, 20 µl of MTT (5 mg/ml in PBS) was added to each well then incubated for 4 h at 37 °C, the supernatant of each well was removed and formazan crystals were dissolved in 200 µl of dimethylsulfoxide. The absorbance values were read using a microplate reader (Bio-Rad, Hercules, CA, USA) at 570 nm. In all experiments treated groups were compared with vehicle control groups (DMSO) and the percentage of cell viability was calculated, respectively. Data are representative of at least three experiments. The viability of cells toward treatment by sodium butyrate (0.5, 1 and 5 mM) for 24, 48, and 72 h were measured and the involvement of 15-Lox-1 was assessed following measurement of cell viability in the presence and absence of PD146176 (0.5 μM) for 48 h, respectively. For indicating the role of 15-Lox, cells were pretreated with PD146176 (0.5 μM) as an specific 15-Lox inhibitor then exposed to sodium butyrate (5 mM) for 48 h for further assessments.
ELISA measurement of 13(S)HODE Level
An 13(S)HODE immunoassay kit were used to measure the concentration of 13(S)HODE (Assay Design, Ann Arbor, MI, USA) according to the manufacturer’s instructions. Briefly, 3 × 105 cells were seeded per each well and treated with sodium butyrate at the final concentrations of 0.5, 1 and 5 mM for 48 h, lysis buffer was used for lysing the cells and protein concentration was determined by Bradford’s Method. 0.2 M HCl were applied to acidify the medium and organic phase of each sample was extracted using saturated ethyl acetate. 100 µl of extracted samples were taken and placed in with anti-13(S)HODE antibodies pre-coated plates followed by administration of 100 µl of 13(S)HODE-horseradish peroxi-2 dase (HRP) conjugate (1:1000), the plates were incubated for 2 h, washed, and incubated for extra 2 h in the presence of conjugate and 13(S)HODE-enzyme conjugate substrate (which is PNPP for conjugated enzyme). The measurement of 13(S)HODE absorbance was performed using microplate reader (Bio-Rad) at 405 nm.
Assessment of apoptosis and cell death
Detection of apoptosis was conducted using Annexin-V-FITC kit and flow cytometry according to the manufacturer’s specifications. Briefly, treated and untreated cells were seeded in 6 well plates then harvested at the density of 1 × 106 cell/ml and washed with PBS. 500 µl of 1× binding buffer were used to re-suspend cell pellets, 5 µl of annexin-V-FITC and, 5 µl of PI were added to each sample followed by gentle vortex. After incubation for 10 min at room temperature, cells were analyzed by FACSCalibur flowcytometer (BectonDickinson, SanJose, CA, USA). Analysis was further performed using the supplied software in the instrument (BD CellQuest software).
Reverse transcription-PCR
For determining the effect of sodium butyrate on 15-Lox-1 expression, analysis of 15-Lox-1 mRNA expression was evaluated using quantitative real-time RT-PCR. Total RNA of cells were extracted by Trizol (Invitrogen, Grand Island, NY, USA) according to the manufacturer’s instructions. RNA integrity was determined by agarose gel electrophoresis. Then cDNA was synthesize from 1 µg of total RNA with single-stranded cDNA using high capacity RNA-to-cDNA Kit according to the manufacturer’s protocol (Invitrogen) which was targeted as a template for analysis of 15-Lox-1 using the SYBR Green kit (Qiagen) in an ABI 7500 Sequence Detection System (Applied Biosystems, Foster City, CA, USA). The level of relative gene expression were normalized to the GAPDH expression level (housekeeping gene) and evaluated by the 2−ΔΔCt analysis method. The primer sets applied were: 15-lox-1: Forward: 5′-gaccgagggtttcctgtctc-3′ and Reverse: 5′-tgtctccagcgttgcatcc-3′, glyceraldehydes-3-phosphatedehydrogenase (GAPDH): Forward: 5′-aatgaccccttcattgacctc-3′, Reverse: 5′-agttgtcatggatgaccttgg-3′.
Statistical analysis
Non-parametric one-way analysis of variance (ANOVA) with post hoc Dennet’s test and turkey’s multiple comparison test was selected for analysis and data were compared using software GraphPad Prism. All experiments were performed in triplicate and repeated three times at least. Data are presented as mean ± SD and differences were considered significant for P < 0.05, P < 0.01 and P < 0.001. The statistical differences were determined by asterisk and shown as *P < 0.05, **P < 0.01, ***P < 0.001 in the corresponding figures.
Results
15-Lox-1 inhibition attenuates sodium butyrate-induced cell toxicity in breast cancer cells
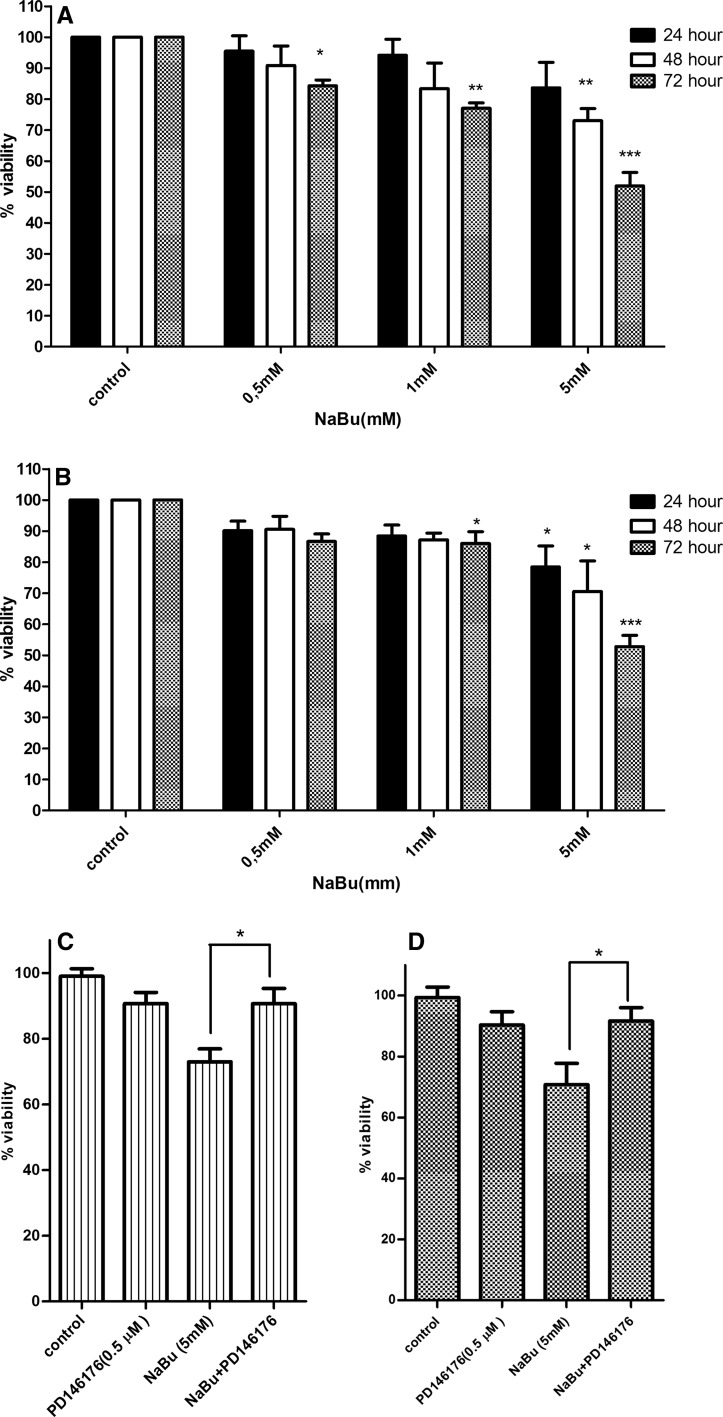
To investigate the cytotoxic effect of sodium butyrate on growth rate of MCF-7 and MDA-MB-468 breast cancer cells, MTT assay was carried out. PD146176 was selected as specific 15-Lox-1 inhibitor to evaluate the role of 15-Lox-1 (Sendobry et al. 1997). The inhibitory effect of PD146176 on 15-Lox-1 has been proven previously and as it was shown in our previous study that PD146176, alone, did not influence cell viability in its mentioned concentration (Tavakoli-Yaraki et al. 2013). As indicated in Fig. 1a, b, the viability of cells was decreased significantly following treatment with sodium butyrate (0.5, 1, and 5 mM) for 24, 48, and 72 h). Our results have shown that the percentage of viable cells was reduced following treatment with sodium butyrate in a dose and time-dependent manner. Accordingly, treatment of MCF-7 cells with 5 mM of sodium butyrate resulted in the reduction of 25 and 48 % of cell viability after 48 and 72 h, respectively (Fig. 1a). Treatment of MDA-MB-468 cells with 5 mM of sodium butyrate reduced cell viability about 28 and 45 % after 48 and 72 h (Fig. 1b).The inhibition of 15-Lox-1 significantly decreased the cytotoxic effect of sodium butyrate in MCF-7 (P < 0.05) (Fig. 1c) and MDA-MB-468 cells (P < 0.05) (Fig. 1d). The abrogation of sodium butyrate cytotoxic effect following 15-Lox-1 inhibition emphasized on the possible role of 15-Lox-1 in the pathway of sodium butyrate-induced cytotoxicity.
Fig. 1.
The effect of sodium butyrate in the presence and absence of 15-Lox-1 inhibitor on breast cancer cell growth. MCF-7 (a) and MDA-MB-468 (b) cells were treated with sodium butyrate (0.5, 1, and 5 mM) for 24, 48, and 72 h. The percentages of viable cells were determined using MTT assay. Cell growth inhibition occurred in a dose and time-dependent manner in both cell lines. The viability of cells at each concentration and time was compared to the viability of control cells in its matched concentration and time. 15-Lox-1 inhibitor attenuated cell growth inhibition induced by sodium butyrate in both MCF-7 (c) and MDA-MB-468 (d) cells. Data represent the mean ± SD of three separate experiments. Statistical differences between treated and untreated groups were analyzed by ANOVA (*P < 0.05; **P < 0.01; ***P < 0.001)
Inhibition of 15-Lox-1 diminshed the induction of apoptosis triggered by sodium butyrate in the breast cancer cells
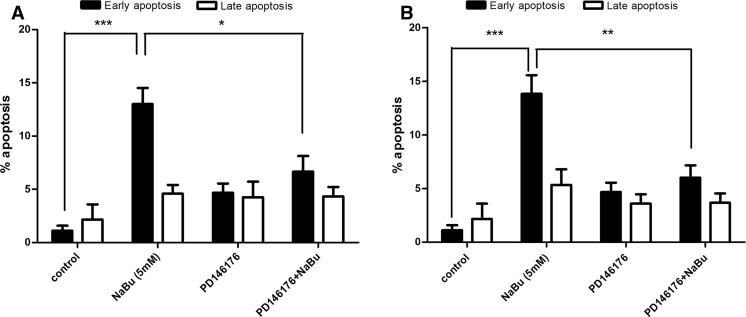
Induction of apoptosis was determined using annexin V and PI double staining method and further analyzed by flow cytometry with BD CellQuest software. In this regards, annexin V-positive, PI negative cells are known as early apoptotic cells while annexin V-positive, PI positive were recognized as late apoptotic cells. MCF-7 and MDA-MB-468 cells were treated with 5 mM of sodium butyrate for 48 h, and then analyzed by Flow cytometry. Controls were annexin V-negative and PI-negative cells. Following treatment with sodium butyrate, a significant increase was observed in the percentage of apoptotic cells at early stage in MCF-7 (P < 0.001) and MDA-MB-468 (P < 0.001) which is shown in Fig. 2a (MCF-7), 2b (MDA-MB-468). PD146176 was applied as a specific 15-Lox-1 inhibitor in order to assess the role of 15-Lox-1 in sodium butyrate- induced apoptosis. Cell were pretreated with PD146176 (0.5 μM) following treatment with sodium butyrate and the status of apoptosis were further analysis by flow cytometry. According to our results, inhibition of 15-Lox-1 reduced the sodium butyrate-elicited apoptosis in both cell lines MCF-7 (P < 0.05) and MDA-MB-468 (P < 0.01) as indicated in Fig. 2a, b, respectively.
Fig. 2.
The effect of sodium butyrate and 15-Lox-1 inhibition on induction of apoptosis in breast cancer cell lines. MCF-7 (a) and MDA-MB-468 (b) cells were treated with sodium butyrate (5 mM) for 48 h in the presence and absence of PD146176 (0.5 μM) and apoptosis was detected by flow cytometric analysis using annexin V and PI staining. The percentage of early apoptotic cells was increased significantly in both cells and PD146176 attenuated the effects of sodium butyrate on the induction of apoptosis in both cell lines. Data represent the mean ± SD of three separate experiments. Statistical differences between treated and untreated cells were analyzed by ANOVA (*P < 0.05; **P < 0.01; ***P < 0.001)
13-S-hydroxyoctadecadienoic acid increased following treatment by sodium butyrate in the breast cancer cell lines
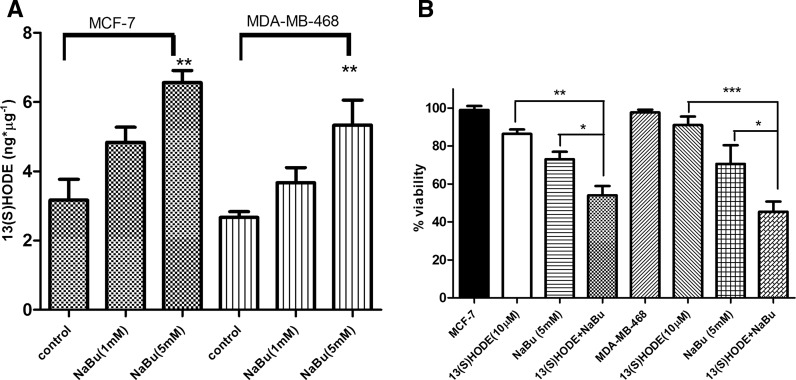
To determine the effect of sodium butyrate on 15-Lox-1 activity, the intracellular level of 13(S)-HODE was measured in response to sodium butyrate-treated cells. Cells were treated with 1 and 5 mM of sodium butyrate for 48 h and the level of 13(S)-HODE was measured using ELISA assay, accordingly. The level of 13(S)-HODE was significantly elevated in treated MCF-7 (P < 0.01) and MDA-MB-468 (P < 0.01) compared to untreated cells (Fig. 3a). Based on our previous study, 13(S)-HODE induced apoptosis and cell death in MCF-7 and MDA-MB-231 (Tavakoli Yaraki and Karami Tehrani 2013). However, to further characterize the involvement of 13(S)-HODE in sodium butyrate-induced breast cancer cell death, cells were treated with 13(S)-HODE (5 μM) alone and sodium butyrate (5 mM) + 13(S)-HODE (5 μM) for 48 h and the rate of viable cells were determined using the MTT assay. Our data have shown the synergistic effect of 13(S)-HODE on sodium butyrate-induced cell death (Fig. 3b).
Fig. 3.
13(S)-HODE was elevated in response to sodium butyrate in the breast cancer cell lines. MCF-7 and MDA-MB-468 cells were treated with sodium butyrate (1 and 5 mM) for 48 h and the production of 13(S)-HODE was measured using ELISA. The level of 13(S)HODE was increased following treatment with sodium butyrate in both cell lines (a). The combination of 13(S)-HODE and sodium butyrate induced cell cytotoxicity synergistically in MCF-7 and MDA-MB-468 cells (b). Data represent the mean ± SD of three separate experiments. Statistical differences between groups were analyzed by ANOVA (*P < 0.05; **P < 0.01; ***P < 0.001)
Sodium butyrate increased the 15-Lox-1 gene expression in breast cancer cells
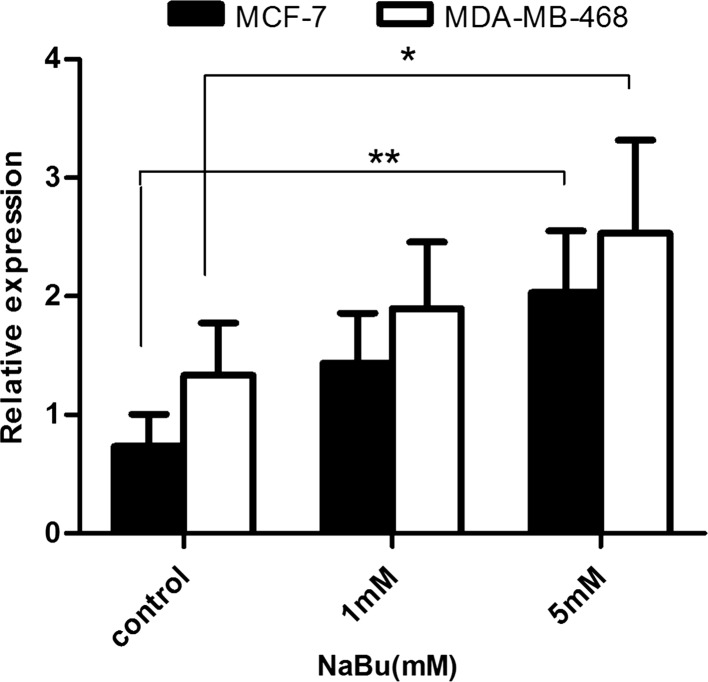
Cells were treated with increasing concentrations of sodium butyrate for 48 h and the level of 15-Lox-1 gene expression was assessed using Real-time PCR. Our results demonstrated that sodium butyrate increased the expression level of 15-Lox-1 in both cell lines significantly, which is in line with the elevation of 15-Lox-1 activity in sodium butyrate-treated cells (Fig. 4).
Fig. 4.
The expression of 15-Lox-1 was increased in response to sodium butyrate in breast cancer cells. Cells were treated with sodium butyrate (1 and 5 mM) for 48 and relative gene expression level of 15-Lox-1 was evaluated. The expression level of 15-Lox-1 was significantly increased following treatment with sodium butyrate. The data represent mean ± SD of three separate experiments. Statistical differences between sodium butyrate-treated and untreated groups were analyzed by ANOVA (*P < 0.05; **P < 0.01)
Discussion
15-Lox-1 belongs to the lipoxygenase family which mediates oxidative metabolism of poly unsaturated fatty acids (Brash 1999). 15-Lox-2, as another isoform of 15-Lox family, mediates production of 15(S)HETE and its expression is restricted to special tissues such as prostate and its regulation is under regulation of glucocorticoid receptors (Feng et al. 2010; Tang et al. 2009). Evidences have revealed that 15-Lox-1 was down regulated in colon (Shureiqi et al. 1999), breast (Jiang et al. 2006), lung (Yuan et al. 2010), pancreas (Hennig et al. 2007) and esophagus (Xu et al. 2003) cancers. The low level of 15-Lox-1 was associated with reduction in the level of 13(S)-HODE as 15-Lox-1 main product. The expression of 15-Lox-1 is under direct control of epigenetic modifications including acetylation and methylation (Liu et al. 2015). It has been shown that suberoylanilide hydroxamic acid (SAHA) induced the expression of 15-Lox-1 in colorectal cancer cells. The increased expression of 15-Lox-1 was accompanied by elevation of 13(S)-HODE, inhibition of growth and induction of apoptosis in the mentioned cells (Hsi et al. 2004). Acetylation is a well-studied molecular mechanism mediating epigenetic modulation of genes depending on the balance between activity of histone deacetylase and histone acetyl transferase (Haberland et al. 2009).
Histone deacetylase (HDAC) inhibitors have emerged as anticancer candidates due to their well-established role on several cellular pathways (Bolden et al. 2006). Sodium butyrate is a naturally occurring fatty acid recognized as an HDAC inhibitor which can affect cell growth through regulation of cell proliferation, differentiation and death (Davie 2003). The molecular mechanism of cell death which is induced by sodium butyrate has yet to be elucidated. It has been shown that in both MCF-7 and non-cancerous human embryonic kidney 293 (HEK293) cells sodium butyrate induced cell cycle arrest by inhibiting the repair of double strand breaks in the cells which resulted in cell death (Li et al. 2015). Also it has been proposed that up regulation of TRPM2 (Transient receptor potential cation channel, subfamily M, member 2) expression in bladder cancer cells is involved in the sodium butyrate-induced apoptosis (Cao et al. 2015). Interestingly, energy metabolism of breast cancer cells can be affected by sodium butyrate however sodium butyrate manipulates metabolic pathways differently through attenuation of glycolysis and glucose 6-phosphate dehydrogenase activity in MCF-7 and activation of glucose 6-phosphate dehydrogenase and consumption of oxygen in MDA-MB-231 (Rodrigues et al. 2013). The effect of HDAC inhibitors on the regulation of 15-Lox-1 pathway has been exhibited in colorectal cancer (Hsi et al. 2004). Breast cancer is considered as a remarkable cause of cancer-related mortality among women in the world and many efforts are focused on improving therapeutic strategies. To the best of our knowledge data on the relevance of HDAC inhibitors and 15-Lox-1 pathway in breast cancer is lacking. This led us to study the effect of sodium butyrate on breast cancer cell growth and explore the possible involvement of 15-Lox-1 in the pathway that sodium butyrate applies to regulate breast cancer cell growth. Our results have shown that the percentage of viable cells was reduced following treatment with increasing concentration of sodium butyrate which is consistent with the effect of sodium butyrate on tongue (Li et al. 2014), pancreas (Natoni et al. 2005), and prostate (Paskova et al. 2013). Accordingly, the sodium butyrate-induced cell death was significantly abrogated upon pretreatment with 15-Lox-1 inhibitor in MCF-7 and MDA-MB-468. Moreover, treatment with sodium butyrate resulted in an increased rate of early apoptotic cells which was consistent with the study on pancreatic cancer cell lines that were sensitized to apoptosis in response to sodium butyrate (Natoni et al. 2005). Similarly, the percentages of early apoptotic cells treated with sodium butyrate were reduced following exposure to 15-Lox-1 inhibitor. The similar results were reported previously on the involvement of 15-Lox-1 in the HDAC inhibitor-induced apoptosis in colorectal cancer (Hsi et al. 2004). Moreover, treatment of cells with sodium butyrate resulted in a significant increase in the gene expression of 15-Lox-1 in both cell lines. To get more insight into the effect of sodium butyrate on 15-Lox-1, the level of 13(S)-HODE as its main product was further evaluated. It has been revealed that 13(S)-HODE was notably elevated in cells upon exposure to sodium butyrate. Additionally, 13(S)-HODE synergized with sodium butyrate to reduce viable breast cancer cells which can further confirmed that sodium butyrate-elicited cell death might be mechanistically relevant to 15-Lox-1/13(S)-HODE pathway. Our results provided evidences that regulation of breast cancer cell growth by sodium butyrate is associated with induction of 15-Lox-1 and production of 13(S)-HODE in MCF-7 and MDA-MB-468. Notably, no significant differences were observed regarding the sensitivity of estrogen receptor positive (MCF-7) and negative (MDA-MB-468) cells to sodium butyrate. It can be suggested that sodium butyrate might exert its effect on breast cancer cell growth through 15-Lox-1 in an estrogen independent manner. To get more insight about the role of estrogen receptor, more studies are required.
The pro or anti tumorigenic role of 15-Lox-1 pathway is discussed controversially in the literature. It has been shown that 15-Lox-1 mediates invasion of tumor cells to the lymphatic vessels and further facilitates the metastasis of cells in mammary carcinoma (Kerjaschki et al. 2011). However, Jiang et al. (2006) have shown that the expression of 15-Lox was reduced in patients with breast cancer. They have demonstrated that the expression of 15-Lox was related to the degree and severity of breast cancer. According to their study, the lower expression of 15-Lox was detected in patients with developed metastasis and local recurrence or those who died as a result of breast cancer (Jiang et al. 2006). The oncogenic role of 15-Lox-1 was confirmed by up regulation of mitogen activated protein kinase (MAPK) and reduction of PPAR-γ (Hsi et al. 2002). Based on evidences, in colorectal and lung cancer, 15-Lox-1 is down regulated in patients compared to controls (Shureiqi et al. 1999; Yuan et al. 2010). Accordingly, 15-Lox-1 might act as a tumor suppressor in colorectal and lung cancer. These inconsistencies might be related to the production of several lipid mediators and engagement of different signaling pathways downstream of 15-Lox-1 which are required to be unraveled in future studies. In conclusion, our study attempts to provide evidences that up regulation of 15-Lox-1 and elevation of 13(S)-HODE might be involved in the regulation of breast cancer cell growth following treatment with sodium butyrate. Our current findings alongside with our previous data on the importance of 15-Lox-1/13(S)HODE pathways emphasize on the importance of this pathway as a promising molecular pathway for regulation of breast cancer cell growth, however, this avenue of research is required to be investigated more comprehensively.
Acknowledgments
This work was financially supported by Iran University of Medical Sciences (Grant Number: 93-01-30-24624).
Compliance with ethical standards
Conflict of interest
The authors declare that there is no conflict of interest.
References
- Bolden JE, Peart MJ, Johnstone RW. Anticancer activities of histone deacetylase inhibitors. Nat Rev Drug Discov. 2006;5:769–784. doi: 10.1038/nrd2133. [DOI] [PubMed] [Google Scholar]
- Brash AR. Lipoxygenases: occurrence, functions, catalysis, and acquisition of substrate. J Biol Chem. 1999;274:23679–23682. doi: 10.1074/jbc.274.34.23679. [DOI] [PubMed] [Google Scholar]
- Burdick AD, Kim DJ, Peraza MA, Gonzalez FJ, Peters JM. The role of peroxisome proliferator-activated receptor-beta/delta in epithelial cell growth and differentiation. Cell Signal. 2006;18:9–20. doi: 10.1016/j.cellsig.2005.07.009. [DOI] [PubMed] [Google Scholar]
- Cao QF, Qian SB, Wang N, Zhang L, Wang WM, Shen HB. TRPM2 mediates histone deacetylase inhibition-induced apoptosis in bladder cancer cells. Cancer Biother Radiopharm. 2015;30:87–93. doi: 10.1089/cbr.2014.1697. [DOI] [PubMed] [Google Scholar]
- Comba A, Maestri DM, Berra MA, Garcia CP, Das UN, Eynard AR, Pasqualini ME. Effect of omega-3 and omega-9 fatty acid rich oils on lipoxygenases and cyclooxygenases enzymes and on the growth of a mammary adenocarcinoma model. Lipids Health Dis. 2010;9:112. doi: 10.1186/1476-511X-9-112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davie JR. Inhibition of histone deacetylase activity by butyrate. J Nutr. 2003;133:2485s–2493s. doi: 10.1093/jn/133.7.2485S. [DOI] [PubMed] [Google Scholar]
- Fallahian F, Karami-Tehrani F, Salami S. Induction of apoptosis by type Ibeta protein kinase G in the human breast cancer cell lines MCF-7 and MDA-MB-468. Cell Biochem Funct. 2012;30:183–190. doi: 10.1002/cbf.1831. [DOI] [PubMed] [Google Scholar]
- Feng Y, Bai X, Yang Q, Wu H, Wang D. Downregulation of 15-lipoxygenase 2 by glucocorticoid receptor in prostate cancer cells. Int J Oncol. 2010;36:1541–1549. doi: 10.3892/ijo_00000568. [DOI] [PubMed] [Google Scholar]
- Giancotti V. Breast cancer markers. Cancer Lett. 2006;243:145–159. doi: 10.1016/j.canlet.2006.01.035. [DOI] [PubMed] [Google Scholar]
- Haberland M, Montgomery RL, Olson EN. The many roles of histone deacetylases in development and physiology: implications for disease and therapy. Nat Rev Genet. 2009;10:32–42. doi: 10.1038/nrg2485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hennig R, Kehl T, Noor S, Ding XZ, Rao SM, Bergmann F, Furstenberger G, Buchler MW, Friess H, Krieg P, Adrian TE. 15-lipoxygenase-1 production is lost in pancreatic cancer and overexpression of the gene inhibits tumor cell growth. Neoplasia. 2007;9:917–926. doi: 10.1593/neo.07565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoshyar R, Mahboob Z, Zarban A. The antioxidant and chemical properties of Berberis vulgaris and its cytotoxic effect on human breast carcinoma cells. Cytotechnology. 2015 doi: 10.1007/s10616-015-9880-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsi LC, Wilson LC, Eling TE. Opposing effects of 15-lipoxygenase-1 and -2 metabolites on MAPK signaling in prostate alteration in peroxisome proliferator-activated receptor gamma. J Biol Chem. 2002;277:40549–40556. doi: 10.1074/jbc.M203522200. [DOI] [PubMed] [Google Scholar]
- Hsi LC, Xi X, Lotan R, Shureiqi I, Lippman SM. The histone deacetylase inhibitor suberoylanilide hydroxamic acid induces apoptosis via induction of 15-lipoxygenase-1 in colorectal cancer cells. Cancer Res. 2004;64:8778–8781. doi: 10.1158/0008-5472.CAN-04-1867. [DOI] [PubMed] [Google Scholar]
- Jiang WG, Watkins G, Douglas-Jones A, Mansel RE. Reduction of isoforms of 15-lipoxygenase (15-LOX)-1 and 15-LOX-2 in human breast cancer. Prostaglandins Leukot Essent Fat Acids. 2006;74:235–245. doi: 10.1016/j.plefa.2006.01.009. [DOI] [PubMed] [Google Scholar]
- Kasibhatla S, Tseng B. Why target apoptosis in cancer treatment? Mol Cancer Ther. 2003;2:573–580. [PubMed] [Google Scholar]
- Kerjaschki D, Bago-Horvath Z, Rudas M, Sexl V, Schneckenleithner C, Wolbank S, Bartel G, Krieger S, Kalt R, Hantusch B, Keller T, Nagy-Bojarszky K, Huttary N, Raab I, Lackner K, Krautgasser K, Schachner H, Kaserer K, Rezar S, Madlener S, Vonach C, Davidovits A, Nosaka H, Hammerle M, Viola K, Dolznig H, Schreiber M, Nader A, Mikulits W, Gnant M, Hirakawa S, Detmar M, Alitalo K, Nijman S, Offner F, Maier TJ, Steinhilber D, Krupitza G. Lipoxygenase mediates invasion of intrametastatic lymphatic vessels and propagates lymph node metastasis of human mammary carcinoma xenografts in mouse. J clin Invest. 2011;121:2000–2012. doi: 10.1172/JCI44751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambrechts S, Decloedt J, Neven P. Breast cancer prevention: lifestyle changes and chemoprevention. Acta Clin Belg. 2011;66:283–292. doi: 10.2143/ACB.66.4.2062570. [DOI] [PubMed] [Google Scholar]
- Lane AA, Chabner BA. Histone deacetylase inhibitors in cancer therapy. J Clin Oncol. 2009;27:5459–5468. doi: 10.1200/JCO.2009.22.1291. [DOI] [PubMed] [Google Scholar]
- Li Z, Wang Y, Yuan C, Zhu Y, Qiu J, Zhang W, Qi B, Wu H, Ye J, Jiang H, Yang J, Cheng J. oncogenic roles of Bmi1 and its therapeutic inhibition by histone deacetylase inhibitor in tongue cancer. Lab Invest. 2014;94:1431–1445. doi: 10.1038/labinvest.2014.123. [DOI] [PubMed] [Google Scholar]
- Li L, Sun Y, Liu J, Wu X, Chen L, Ma L, Wu P. Histone deacetylase inhibitor sodium butyrate suppresses DNA double strand break repair induced by etoposide more effectively in MCF-7 cells than in HEK293 cells. BMC Biochem. 2015;16:2. doi: 10.1186/s12858-014-0030-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C, Schain F, Han H, Xu D, Andersson-Sand H, Forsell P, Claesson HE, Bjorkholm M, Sjoberg J. Epigenetic and transcriptional control of the 15-lipoxygenase-1 gene in a Hodgkin lymphoma cell line. Exp Cell Res. 2015;318:169–176. doi: 10.1016/j.yexcr.2011.10.017. [DOI] [PubMed] [Google Scholar]
- Margueron R, Duong V, Castet A, Cavailles V. Histone deacetylase inhibition and estrogen signalling in human breast cancer cells. Biochem Pharmacol. 2004;68:1239–1246. doi: 10.1016/j.bcp.2004.04.031. [DOI] [PubMed] [Google Scholar]
- Natoni F, Diolordi L, Santoni C, Gilardini Montani MS. Sodium butyrate sensitises human pancreatic cancer cells to both the intrinsic and the extrinsic apoptotic pathways. Biochim Biophys Acta. 2005;1745:318–329. doi: 10.1016/j.bbamcr.2005.07.003. [DOI] [PubMed] [Google Scholar]
- Paskova L, Smesny Trtkova K, Fialova B, Benedikova A, Langova K, Kolar Z. Different effect of sodium butyrate on cancer and normal prostate cells. Toxicol In Vitro. 2013;27:1489–1495. doi: 10.1016/j.tiv.2013.03.002. [DOI] [PubMed] [Google Scholar]
- Rodrigues MF, Carvalho E, Pezzuto P, Rumjanek FD, Amoedo ND. Reciprocal modulation of histone deacetylase inhibitors sodium butyrate and trichostatin a on the energy metabolism of breast cancer cells. J Cell Biochem. 2013;116:797–808. doi: 10.1002/jcb.25036. [DOI] [PubMed] [Google Scholar]
- Salimi V, Tavakoli-Yaraki M, Mahmoodi M, Shahabi S, Gharagozlou MJ, Shokri F, Mokhtari-Azad T. The oncolytic effect of respiratory syncytial virus (RSV) in human skin cancer cell line, A431. Iran Red Crescent Med J. 2013;15:62–67. doi: 10.5812/ircmj.4722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sendobry SM, Cornicelli JA, Welch K, Bocan T, Tait B, Trivedi BK, Colbry N, Dyer RD, Feinmark SJ, Daugherty A. Attenuation of diet-induced atherosclerosis in rabbits with a highly selective 15-lipoxygenase inhibitor lacking significant antioxidant properties. Br J Pharmacol. 1997;120:1199–1206. doi: 10.1038/sj.bjp.0701007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shureiqi I, Wojno KJ, Poore JA, Reddy RG, Moussalli MJ, Spindler SA, Greenson JK, Normolle D, Hasan AA, Lawrence TS, Brenner DE. Decreased 13-S-hydroxyoctadecadienoic acid levels and 15-lipoxygenase-1 expression in human colon cancers. Carcinogenesis. 1999;20:1985–1995. doi: 10.1093/carcin/20.10.1985. [DOI] [PubMed] [Google Scholar]
- Suraneni MV, Moore JR, Zhang D, Badeaux M, Macaluso MD, Giovanni J, Kusewitt D, Tang DG. Tumor-suppressive functions of 15-Lipoxygenase-2 and RB1CC1 in prostate cancer. Cell Cycle. 2014;13:1798–1810. doi: 10.4161/cc.28757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang Y, Wang MT, Chen Y, Yang D, Che M, Honn KV, Akers GD, Johnson SR, Nie D. Downregulation of vascular endothelial growth factor and induction of tumor dormancy by 15-lipoxygenase-2 in prostate cancer. Int J Cancer. 2009;124:1545–1551. doi: 10.1002/ijc.24118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tavakoli Yaraki M, Karami Tehrani F. Apoptosis Induced by 13-S-hydroxyoctadecadienoic acid in the breast cancer cell lines, MCF-7 and MDA-MB-231. IJBMS. 2013;16:653–659. [PMC free article] [PubMed] [Google Scholar]
- Tavakoli-Yaraki M, Karami-Tehrani F, Salimi V, Sirati-Sabet M. Induction of apoptosis by Trichostatin A in human breast cancer cell lines: involvement of 15-Lox-1. Tumour Biol. 2013;34:241–249. doi: 10.1007/s13277-012-0544-7. [DOI] [PubMed] [Google Scholar]
- Xu XC, Shappell SB, Liang Z, Song S, Menter D, Subbarayan V, Iyengar S, Tang DG, Lippman SM. Reduced 15S-lipoxygenase-2 expression in esophageal cancer specimens and cells and upregulation in vitro by the cyclooxygenase-2 inhibitor, NS398. Neoplasia. 2003;5:121–127. doi: 10.1016/S1476-5586(03)80003-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan H, Li MY, Ma LT, Hsin MK, Mok TS, Underwood MJ, Chen GG. 15-Lipoxygenases and its metabolites 15(S)-HETE and 13(S)-HODE in the development of non-small cell lung cancer. Thorax. 2010;65:321–326. doi: 10.1136/thx.2009.122747. [DOI] [PubMed] [Google Scholar]