Abstract
The health benefits of Mediterranean diet has long been reported and attributed to the consumption of virgin olive oil (VOO). Here, we evaluated the neuroprotective effect of VOO against Alzheimer’s disease by determining its effect on β-amyloid (Aβ)-induced cytotoxicity and oxidative stress, and explored the possibility that its hydroxycinnamic acids (Hc acids) content contribute significantly to this effect. SH-SY5Y cells treated with or without Aβ and with VOO or Hc acids (mixture of p-coumaric acid, ferulic acid, vanillic acid, and caffeic acid) were subjected to MTT assay and the results showed that both samples alleviated Aβ-induced cytotoxicity. Furthermore, both VOO and Hc acids decreased the reactive oxygen species level. Using western blot to determine the effect of these samples on Aβ-induced activation of pERK1/2, p38, and JNK MAPKs, results revealed that both VOO and Hc acids inhibited the activation of pERK1/2 and p-p38 MAPK, but not JNK. Moreover, VOO upregulated the glycolytic enzymes genes hexokinase (HK1), and phosphofructokinase (PFKM) expression which means that VOO enhanced the energy metabolism of the neurotypic cells, and therefore suggests another mechanism by which VOO could provide protection against Aβ-induced cytotoxicity. The findings in this study suggest that VOO has a neuroprotective effect, attributable to its hydroxycinnamic acids component, against Aβ-induced cytotoxicity and oxidative stress through the inhibition of the activation of MAPKs ERK and p38 and by enhancing the energy metabolism of the neurotypic cells.
Keywords: SH-SY5Y cells, Alzheimer’s disease, Picholine virgin olive oil, Hydroxycinnamic acids, Neuroprotection
Introduction
The role of oxidative stress in Alzheimer’s disease (AD) and in most neurodegenerative disorders has been the subject of many researches (Chen and Zhong 2014; Schroeter et al. 2001). Oxidative stress is the cytotoxic consequences of oxygen radials—superoxide anion (·O2), hydroxyl radical (OH), and hydrogen peroxide (H2O2), generated as by-products of normal and aberrant metabolic processes that utilize the molecular oxygen (O2) (Birben et al. 2012). The balance between reactive oxygen species (ROS) production and antioxidant defenses determines the degree of oxidative stress. Consequences of this stress include modifications to cellular proteins, lipids and DNA (Betteridge 2000). Neurons are extremely sensitive to attacks by destructive free radicals. In AD patients, there are lesions typically associated with attacks by free radicals causing damage to DNA, protein oxidation, lipid peroxidation, and advanced glycosylation end products. The neuronal damage caused by oxidative stress eventually leads to neuronal cell death. AD is the most common neurodegenerative disease characterized by the deposition of senile β-amyloid (Aβ) peptide plaques in the brain (Stadtman 2001; Butterfield et al. 2013). When Aβ is aggregated, it produces more free radicals in the presence of free radicals but is eliminated by free radical scavengers (Christen 2000). The MAPK pathway participates in number of cellular processes, and this includes stressful situations such as oxidative stress (Daniels et al. 2001). Aβ induces apoptosis by activating p38 and JNK MAPKs (Zhou et al. 2014; Tamagno et al. 2003). Moreover, a pERK1/2 level is regulated by amyloid precursor protein, whose proteolysis generates Aβ (Nizzari et al. 2007). As with most neurodegenerative diseases, AD is propagated by compromised cell survival, metabolic dysfunction, and increase in oxidative stress.
Japan and other first world countries have the highest life expectancy in the world but the increase in incidence of neurodegenerative disorders such, as AD, have lowered the quality of life, and the means to cure or prevent these aging-related disorders has become very important (Norton et al. 2014; Brookmeyer et al. 2007; Bartzoki 2011; Wang et al. 2014). The “nutritional approach” to combat diseases, including neurological disorders, has recently gained attention. It harnesses the natural anti-oxidants present in the diet to increase the resistance towards oxidative damage (Florent-Bechard et al. 2007; Butterfield et al. 2001; Dimitrios 2006). The consumption of virgin olive oil (VOO), a staple in the Mediterranean diet, for example, has been associated with a reduced risk for AD and other neurodegenerative age-related diseases (cognitive deficit and Parkinson’s disease) (Scarmeas et al. 2006; Perez-Lopez et al. 2009).
Moreover, regular consumption of VOO has been suggested to be useful in combating oxidative stress-related diseases. The low incidences of atherosclerosis, cardiovascular disease, and certain types of cancer have also been attributed to VOO consumption (Scarmeas et al. 2006; Cicerale et al. 2010). Studies devoted on the biologically active phenolic compounds naturally present in VOO have shown that it contains high amounts of phenolic compounds that has a strong antioxidant activity such as the main components tyrosol, hydroxytyrosol, luteolin, and apigenin, as well as the minor components hydroxycinnamic acids p-coumaric acid, vanillic acid, ferulic acid, and caffeic acid (Nieves Franco et al. 2014; Owen et al. 2000; Chen and Ho 1997). Hydroxycinnamic acids, caffeic, chlorogenic, ferulic and sinapic acids, have cytoprotective effect that is strongly correlated to their antioxidant activities in lipid membrane system (Zhang and Melton 2008). We have previously reported that extracts of purple sweet potato that contains high amounts of caffeoylquinic acid, a derivative of caffeic acid, imparts neuroprotection and contributes to the improvement of spatial learning and memory of SAMP8 mouse (Sasaki et al. 2013). Similarly, 3,4,5-tri-O-caffeoylquinic acid can also inhibit Aβ-mediated cellular toxicity on SH-SY5Y cells by promoting the expression of glycolytic enzymes phosphoglycerate mutase 1 (PGAM1) and glyceraldehyde-3-phosphate dehydrogenase (G3PDH) (Miyamae et al. 2011). More importantly, VOO phenolic components have been demonstrated to be generally readily bioavailable (Cicerale et al. 2010; D’Archivio et al. 2007). Data obtained from 97 studies investigating the extent of the absorption of polyphenols in adults show that hydroxycinnamic acids ferulic, caffeic, and vanillic acids are efficiently absorbed (Manach et al. 2005; Bourne and Rice-Evans 1998). The levels of phenolic compounds in olives differ widely among varieties and location and the main French olive variety, Picholine contains hydroxycinnamic acids in significant amounts and has higher polyphenols and tocopherol content compared to most VOO varieties (Ollivier et al. 2003; Aparicio and Luna 2002). Moreover, Picholine VOO, as we have recently reported, has anti-allergy effect attributed to its phenolic components (Isoda et al. 2012). Here, we evaluated the neuroprotective effect of VOO and determined if this can be attributed to its minor component, the hydroxycinnamic acids (p-coumaric acid, ferulic acid, vanillic acid, and caffeic acid). The underlying mechanism of their effects was also assessed.
Materials and methods
Quantifying hydroxycinnamic acids content and total polyphenol content of virgin olive oil
To analyze the actual concentration of the hydroxycinnamic acids present in VOO used in this study, a 2 g sample of Picholine virgin olive oil (PVOO) from Southern France was diluted in 1.5 mL of hexane prior to analysis. The solid phase extraction (SPE) cartridge was equilibrated with 6 mL methanol and 6 mL of hexane, and the sample was put on the cartridge. After two successive washes with 3 mL of hexane and once with 4 mL hexane/ethyl acetate, 90/10, v/v, the sample was eluted with 10 mL of methanol and the eluate evaporated by vacuum evaporation. The dry residue was reconstituted in 3 mL of methanol/water mixture, 50/50, v/v and filtered through Acrodisk (0.45 μm) prior to injection into the HPLC system. The flow of the mobile phase was 0.8 mL/min; its composition varied from 40 % methanol and 60 % of 100 mM formate buffer pH = 2.5 up to 100 % methanol in 15 min. The stationary phase was a Zorbax SB-phenyl column 5 μm, 4.6 × 250 mm. The UV absorption spectrum for HPLC–UV analysis was set in the range of 220–400 nm while the wavelength used for analysis was 295 nm. Stock solutions of caffeic, vanillic, p-coumaric and ferulic acid were prepared at 1 g/L in methanol/water, 50/50, v/v. The calibration range was created from the stock solutions in five concentrations (0.08–3 mg/L) for each of compounds using a mixture of solvents, methanol/water (1:1 ratio, v/v). Three quality control samples were prepared independently at concentrations of 0.15, 0.8, and 2 mg/L in the same solvent dilution. Standards for caffeic acid, p-coumaric acid, ferulic acid, vanillic acid, ammonium formate, formic acid, water, all of analytic or HPLC grade, were purchased from Sigma (St. Louis, MO, USA). SPE LC-Si cartridges (500 mg of sorbent) 3 mL was supplied by Supelco, Inc. (Bellefonte, PA, USA). Ammonium formate buffer was prepared by mixing ammonium formate and water to a concentration of 100 mM; the pH was adjusted to 2.5 with formic acid. HPLC Spectra System was equipped with a column ZORBAX SB-Phenyl 5 μm, 4.6 × 250 mm—Agilent ®, an AS3000 auto sampler, a P4000 pump and a UV detector DAD 6000LP THERMO® (Thermo Fisher Scientific, Waltham, MA, USA). The total polyphenol present in the oil sample was determined using the Folin Ciocalteu (Sigma-Aldrich, MO, USA) method.
VOO sample and sample preparation
The VOO used in this study was the commercially available VOO from the first cold pressing of the olives of the Picholine variety. Phenolic compounds p-coumaric acid (PCA), ferulic acid (FA), vanillic acid (VA), and caffeic acid (CA), collectively known as hydroxycinnamic acids (Hc acids), were used to determine the effect of these minor components on oxidative stress and were prepared by mixing the compounds at their equivalent concentration in 1/1000 v/v VOO, based on the HPLC analysis results: PCA at 0.495 μg/mL, VA at 0.227 μg/mL, FA 0.155 μg/mL, and CA at 0.047 μg/mL. Amyloid-β1-42 (Aβ) was purchased from Funakoshi (Tokyo, Japan) and used at a final concentration of 15 μM.
Cell culture
Human neurotypic SH-SY5Y cells obtained from American Type Culture Collection (ATCC; Manassas, VA, USA) were cultured in 100-mm petri dishes or in 96-well plates with a 1:1 (v/v) mixture of Dulbecco’s minimum essential medium (Sigma) and Ham’s F-12 nutrient mixture (Sigma) supplemented with 15 % fetal bovine serum (Sigma), and 1 % penicillin (5000 µg/mL)-streptomycin (5000 IU/mL) solution (ICN Biomedicals, Solon, OH, USA) at 37 °C in a 5 % CO2 incubator. For cell viability evaluation, cells were cultured in a serum-free Eagle’s minimum essential medium (Opti-MEM; Gibco-BRL/Invitrogen, Carlsbad, CA, USA). SH-SY5Y cells were observed and photographed using a Leica DFC300FX CCD camera (Leica, Wetzlar, Germany). The images were analyzed using a light microscope system (Leica).
Determination of the neuroprotective effect of VOO and Hc acids against amyloid β (Aβ) cytotoxicity
The neuroprotective effect of VOO was assessed using the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) method as previously reported (Sasaki et al. 2013). In brief, SH-SY5Y cells (2 × 104 cells/well) were seeded onto fibronectin-coated 96-well plates (BD BioCoat, Bedford, MA, USA) and cultured as described above for 24 h. The medium was then replaced with medium containing VOO at different concentrations (0 or control, 1/10,000, 1/5000, 1/2500, 1/1000, 1/500, 1/250, and 1/100) and after 1 h incubation, Aβ was added to a final concentration of 15 μM. The plates were then covered with aluminum foil and incubated further for 48 h at 37 °C in a 5 % CO2 incubator; MTT (5 mg/mL) was added at 10 μL per well at a final concentration of 0.45 mg/mL. Ten percent (10 %) sodium dodecyl sulphate (SDS) was then added at 100 μL per well and incubated further for 18 h (overnight) or until the formazan crystals have been completely dissolved. Powerscan HT (Dainippon Pharmaceuticals USA Corporation, Fort Lee, NJ, USA), a microplate reader, was used to obtain the absorbances at 570 nm. To correct the absobances, blanks containing only medium, MTT, and SDS were used to correct the absorbances.
Intracellular reactive oxygen species (ROS) assay
SH-SY5Y cells (2 × 105 cells/mL) were seeded in 96-well plates and were incubated in Opti-MEM with or without Aβ1-42, VOO (1/1000 v/v), or Hc acids mixture for 24 h. The cells were then incubated with 10 μM DCFH-DA at 37 °C for 1 h, washed with PBS, and the fluorescence intensity of dichlorofluorescin (DCF) was measured using a Powerscan HT (Dainippon Pharmaceuticals USA Corporation, NJ, USA) with a 485/538 nm filter set and 530 nm cutoff. The intracellular ROS level is proportional to the fluorescence of the standard (DCF).
Western blotting
SH-SY5Y cells at a density of (2 × 105 cells/mL) were seeded into 100-mm fibronectin-coated Petri dishes (BD BioCoat) and cultivated by the method described above. After overnight incubation, the medium was then replaced by medium with or without Aβ1-42, VOO (1/1000 v/v), or Hc acids mixture dissolved in OPTI-MEM. The plates were then incubated for 1 h or 24 h and the total protein extracted using RIPA buffer (Sigma) according to the manufacturer’s instructions. Protein sample (10 μg) was resolved in 10 % sodium dodecyl sulphate (SDS)-polyacrylamide gel electrophoresis, transferred to nitrocellulose membrane, and blotted with primary antibodies from Cell Signaling, Inc. (Danvers, MA, USA) (pERK1/2, ERK2, p-p38, p38, pJNK, and JNK) and Santa Cruz Biotechnology, Inc. (Santa Cruz, CA, USA) (GAPDH). The signal was visualized using LiCor Odyssey Infrared Imaging System after reaction with goat anti-mouse IRDye 680LT or goat anti-rabbit IRDye 800CW (LI-COR, Lincoln, NE, USA).
Quantitative real-time PCR analysis
To elucidate the possible mechanism behind the neuroprotective effect of VOO, the expression of glycolytic enzymes genes were determined. SH-SY5Y cells (2 × 105 cells/mL) were seeded into 100-mm fibronectin-coated Petri dishes (BD BioCoat) and cultivated by the method described above. After overnight incubation, the medium was then replaced by medium with or without Aβ1-42, VOO (1/1000 v/v), or Hc acids mixture dissolved in OPTI-MEM. The plates were then incubated for 4 h after which the total RNA was extracted using ISOGEN kit (Nippon Gene, Tokyo, Japan) and quantified using a Nanodrop 2000 spectrophotometer (Nanodrop Technologies, Wilmington, DE, USA) following the manufacturers’ instructions. The RNAs were then used as templates for reverse transcription polymerase chain reaction (RT-PCR) using the Superscript III reverse transcriptase kit (Invitrogen, Carlsbad, CA, USA) following the manufacturer’s instructions. Specific TaqMan probes for phosphofructokinase (PFKM), hexokinase (HK1), and pyruvate kinase (PKLR) (Applied Biosystems, Foster City, CA, USA) were used and quantitative real-time polymerase chain reaction (rt-PCR) analysis was performed with a 7500 Fast Real-Time PCR system using TaqMan Universal PCR mix at the following thermal cycling protocol: 95 °C for 10 min followed by 40 cycles of 95 °C for 15 s and 60 °C for 1 min. GAPDH was used as the internal control. Data were analyzed using 7500 Fast System SDS Software 1.3.1. (Applied Biosystems).
Statistical analysis
All the results were expressed as mean ± standard error deviation (SD), and the statistical evaluation performed using the Student’s t test when two value sets (control vs treatment) were compared. P < 0.05 was considered to be statistically significant.
Results and discussion
Randomized controlled studies on the virgin olive oil’s (VOO) effect on health, revealed that consumption of VOO could reduce oxidative damage due to its richness in oleic acid and so in general the phenolic compounds (Christen 2000). VOO showed the presence of phenolic compounds in significant amounts (determined by the method DPPH) and the presence of several polyphenols belonging to the hydroxycinnamic group. Since we want to evaluate how the minor components—hydroxycinnamic acids (Hc acids) contribute to this effect, first we determined the concentration of Hc acids present in VOO, using HPLC and solid-phase extraction.
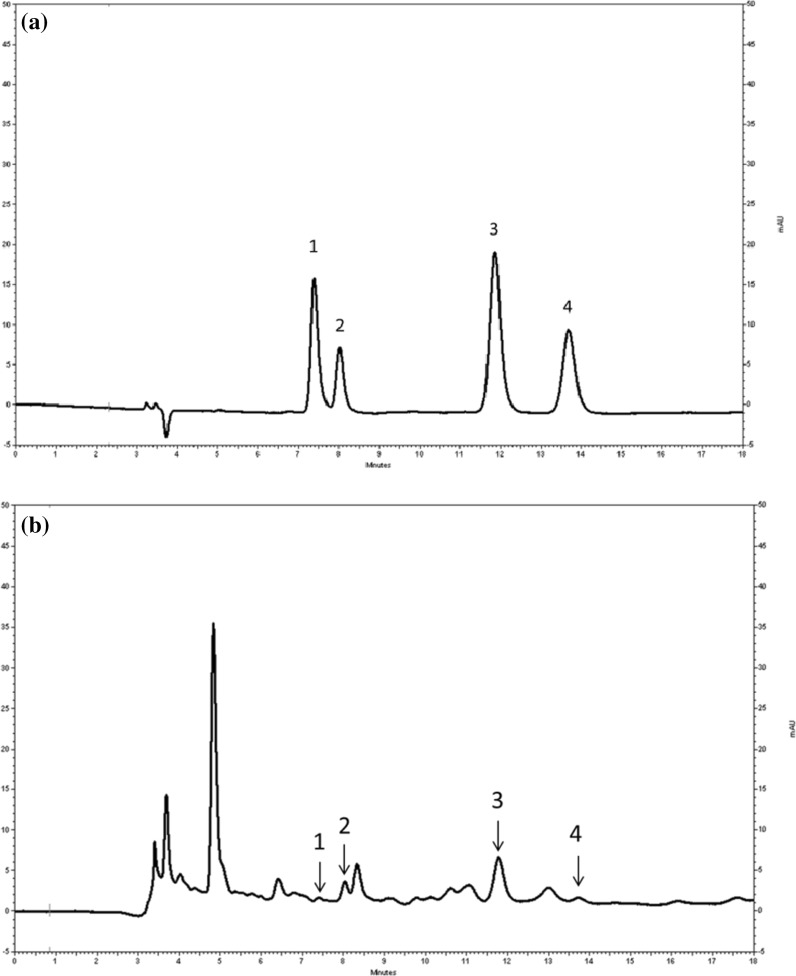
The range points of p-coumaric acid, ferulic acid, vanilic acid, and caffeic acid obtained by HPLC are presented in Fig. 1a, b, and revealed that the major Hc acids present were p-coumaric acid (0.544 mg/kg VOO), vanillic acid (0.250 mg/kg VOO), ferulic acid (0.171 mg/kg VOO), and caffeic acid (0.052 mg/kg VOO). The retention times and the LOD were: caffeic (7.3 min, 0.003 mg/L), vanillic (8.0 min, 0.01 mg/L), p-coumaric acid (11.9 min, 0.003 mg/L), and ferulic acid (13.7 min, 0.004 mg/L). Figure 1b shows the sample solid-phase extraction (SPE) of VOO with the major Hc acids content: caffeic (1), vanillic (2), p-coumaric (3), ferulic (4) acid. The total polyphenol present in the oil sample was in the order of 254 ± 5.9 mg/kg and since the percentage of caffeic acid is 0.02 % ferulic acid: 0.07 %, vanillic acid: 0.1 %, p-coumaric acid: 0.21 %.
Fig. 1.
Major hydroxycinnamic acids present in virgin olive oil (VOO). a Chromatogram of the range point to 0.5 mg/L acid of 1 caffeic, 2 vanillic, 3 p-coumaric, 4 ferulic acid determined using HPLC; b sample solid-phase extraction (SPE) of VOO with the major hydroxycinnamic acids: caffeic (1), vanillic (2), p-coumaric (3), ferulic (4) acid
To determine the effect of VOO on the proliferation of SH-SY5Y cells, cells treated with different dilutions of VOO were subjected to MTT assay. The results showed that low concentrations of 1/10,000–1/1000 (Fig. 2a) had proliferative effect on SH-SY5Y cells while 1/500, 1/250, and 1/100 had cytotoxic effect (not shown). Choosing 1/1000 v/v for the succeeding experiments, the protective effect of 1/1000 v/v VOO on SH-SY5Y cells exposed to β-amyloid (Aβ) was determined using the MTT assay. Results showed that treatment with Aβ alone proved to be cytotoxic to the cells and decreased cell proliferation by more than 30 %, but this effect was alleviated by VOO treatment by around 80 % (Fig. 2b). The morphology of the cells observed under the microscope further confirmed the cytotoxicity of Aβ and the neuroprotective effect of the oil on SH-SY5Y cells (Fig. 2c).
Fig. 2.
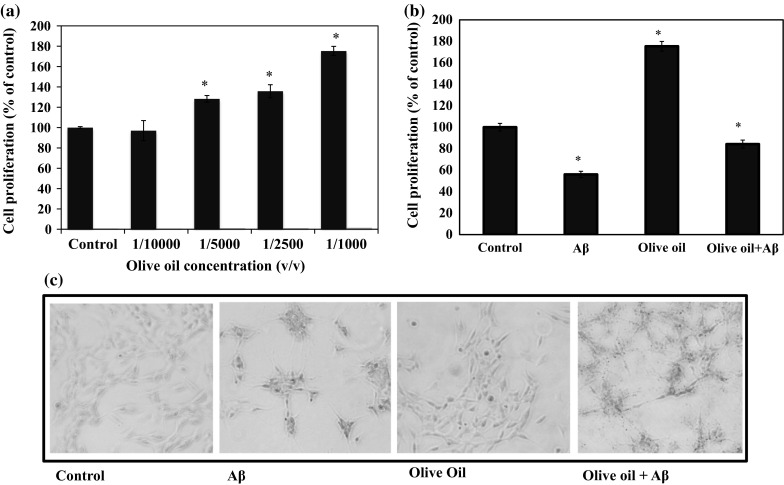
Effect of virgin olive oil (VOO) on human neurotypic SH-SY5Y cell proliferation. SH-SY5Y cells were treated with different dilutions of VOO (a) or VOO with or without β-amyloid1-42 (Aβ) (b) prior to determination of cell proliferation using MTT Assay (a, b). Photographs of the cells at ×100 magnification obtained using Leica DFC300FX CCD camera (c). For the MTT assay, the growth rates were estimated relative to those of the untreated cells (control) and blanks containing only the growth medium, MTT, and SDS were used to correct the absorbances. Results represent the mean ± SD of triplicate determinations. *Statistically significant (P < 0.05) difference between control and treated cells (control vs each sample)
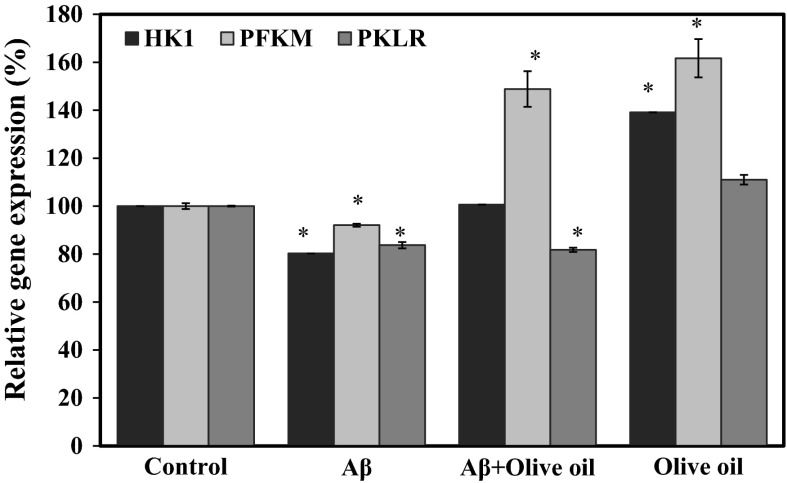
The brain utilizes glucose as primary substrate for its activities but cannot store it, and depends on systemic circulation for glucose supply for transport across the blood–brain barrier. Increasing glucose availability has been shown to improve memory in AD patients (Cunnane et al. 2011; Craft et al. 1996) and that is why AD is also considered as a metabolic neurodegenerative disease and manifests invariant glucose hypometabolism, possibly due to impairments of insulin signaling and altered thiamine metabolism (Chen and Zhong 2013). Therefore, therapies for AD targeting energy metabolism are considered to hold great promise. Previously we have reported that purple sweet potato (PSP) promotes the expression of anti-oxidant- and energy metabolism-associated proteins, imparting neuroprotective effect on SH-SY5Y cells, and that was mostly due to the high caffeoylquinic acid content of PSP (Sasaki et al. 2013). Caffeoylquinic acid, upon hydrolysis, produces caffeic acid and quinic acid. The effect of VOO on energy metabolism was determined by quantifying the mRNA expression of glycolytic enzymes genes phosphofructokinase (PFKM), hexokinase (HK1), and pyruvate kinase (PKLR). Treatment with Aβ decreased the expressions of PFKM, HK1, and PKLR genes in SH-SY5Y cells by 10–20 % but this effect was attenuated by VOO treatment by increasing the expression of PFKM by 50 % in cells with or without Aβ treatment (Fig. 3). No significant change in PKLR gene expression was observed following VOO treatment.
Fig. 3.
Virgin olive oil (VOO) regulated the mRNA expressions of glycolytic enzymes hexokinase (HK1), phosphofructokinase (PFKM), and pyruvate kinase (PKLR) in SH-SY5Y cells. SH-SY5Y cells were pre-treated with VOO at 1/1000 v/v and incubated for 24 h prior to treatment with or without β-amyloid1-42 (Aβ): Specific TaqMan probes for HK1, PFKM, and PKLR genes (Applied Biosystems, Foster City, CA, USA) were used and quantitative real-time polymerase chain reaction (rt-PCR) analysis was performed with a 7500 Fast Real-Time PCR system using TaqMan Universal PCR mix at the following thermal cycling protocol: 95 °C for 10 min followed by 40 cycles of 95 °C for 15 s and 60 °C for 1 min. GAPDH was used as the internal control. Data were analyzed using 7500 Fast System SDS Software 1.3.1. (Applied Biosystems). *Statistically significant (P < 0.05) difference between control and treated cells
Foods rich in caffeic acid, or its derivatives, have been reported to increase ATP production and cell proliferation (Sasaki et al. 2013). To find out if the neuroprotective effect of VOO can be attributed to the Hc acids, the effect of Hc acids (in the same concentration present in 1/1000 v/v VOO based on actual analysis results presented in Fig. 1a, b) was also determined. Treatment with Aβ decreased the cell proliferation by more than 30 % but treatment with Hc acids, like VOO, increased the cell proliferation by 20 % compared to the control, suggesting an alleviation of the cytotoxicity caused by Aβ (Fig. 4a). The cytotoxic effect of Aβ was also apparent in Aβ-treated cells as seen under the microscope (Fig. 4b). This is not surprising as cytoprotective effect of flavonoids and Hc acids has been reported for SH-SY5Y cells exposed to H2O2 (Zhang and Melton 2008), which like Aβ can cause oxidative stress and is cytotoxic at high concentrations. The MTT assay is a direct measurement of the mitochondrial activity related to the number of viable cells, and thus, is broadly used to measure the in vitro cytotoxic effects of drugs on cell lines (Van Merloo et al. 2011).
Fig. 4.
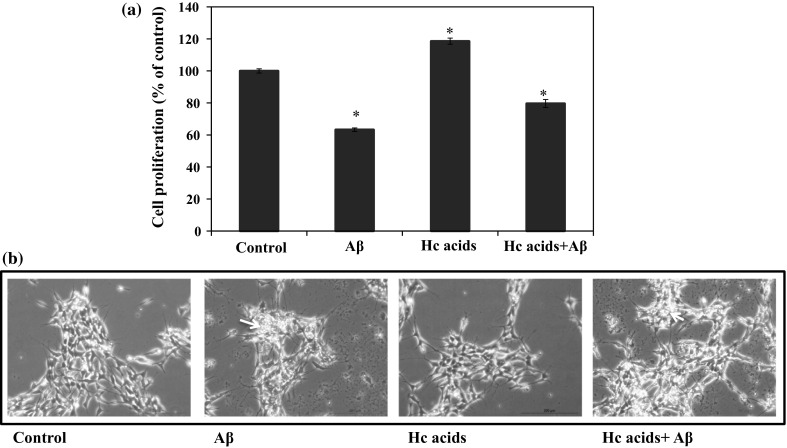
Cell proliferation of human neurotypic SH-SY5Y cells following treatment with mixture of hydroxycinnamic acids (Hc acids) caffeic, vanillic, p-coumaric, and ferulic acid. SH-SY5Y cells were pre-treated without (control) or with the samples (Hc acids) and incubated for 24 h prior to treatment without or with β-amyloid (Aβ)1-42: MTT Assay (a) and photographs of the cells at ×100 magnification using Leica DFC300FX CCD camera (b). The growth rates were estimated relative to those of the untreated cells (control) and blanks containing only the growth medium, MTT, and SDS were used to correct the absorbances. Results represent the mean ± SD of triplicate determinations. *Statistically significant (P < 0.05) difference between control and treated cells
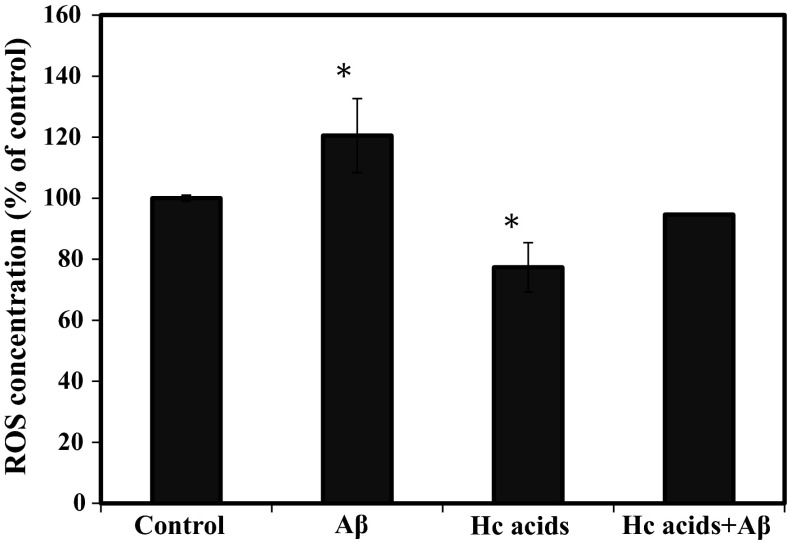
The formation of ROS is an early cellular response to the presence of Aβ and plays a significant role in effecting cellular pathogenesis. To evaluate the effect of Hc acids in countering the effect of Aβ in increasing ROS levels in the cells, SH-SY5Y cells were exposed to Aβ and the ROS levels were measured following treatment without or with Hc acids. Aβ significantly increased the ROS levels to 120 % but this effect can be abrogated by treatment with Hc acids as shown by the decreased ROS level of Hc acids + Aβ treatment that is almost similar to the control (untreated samples) (Fig. 5). Oxidative stress has been linked to aging, development of cancer, and neurological disorders such as AD (Finkel and Holbrook 2000). In AD, the 40–42 amino acid peptide Aβ accumulates and forms plaques in the brain, playing an important role in normal aging and in the pathogenesis of AD. Brain with AD is described to have lipid peroxidation, high concentration of free radicals and is under an intense oxidative stress (Butterfield 2002). Aβ is aggregated and in the presence of free radicals, produces more free radicals (Christen 2000).
Fig. 5.
Reactive oxygen species (ROS) production in human neurotypic SH-SY5Y cells following treatment with virgin olive oil (VOO) or mixture of hydroxycinnamic acids (Hc acids) caffeic, vanillic, p-coumaric, and ferulic acid. SH-SY5Y cells (2 × 105 cells/mL) were seeded in 96-well plates and were incubated in Opti-MEM with or without Aβ1-42, VOO (1/1000 v/v), or Hc acids mixture for 24 h. The intracellular ROS was determined as described in the Materials and Methods. The intracellular ROS level is proportional to the fluorescence of the standard (DCF) and expressed as percentage (%) of control (untreated). *Statistically significant (P < 0.05) difference between control and treated cells
ROS encompass a variety of diverse chemical species including superoxide anions, hydroxyl radicals, and hydrogen peroxide. The stress caused by ROS production is mainly counteracted by an intricate antioxidant system that includes the enzymatic scavengers superoxide dismutase or SOD, catalase, and glutathione peroxidase (Finkel and Holbrook 2000). Aβ increases ROS levels and in this study, it was used to induce oxidative stress in neurotypic SH-SY5Y cells.
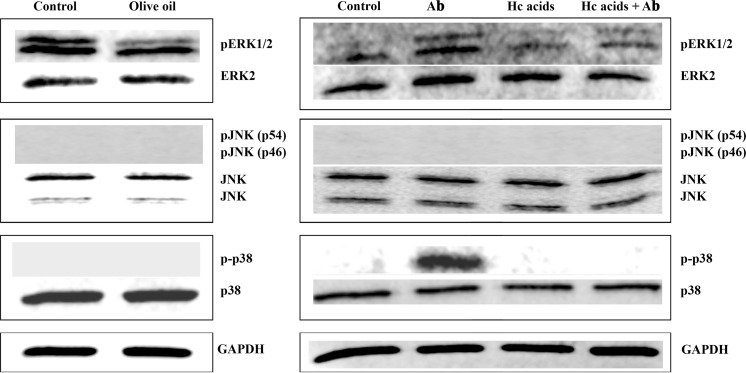
Survival of cells undergoing severe oxidative stress is dependent on its ability to resist or adapt to the stress, and to repair or replace the damaged molecules. Cells have adapted to acute stress through a number of stress response mechanisms. Among the main stress signaling pathways activated are the mitogen-activated protein kinase (MAPK) pathways. The MAPK pathways (extracellular signal-regulated kinase (ERK), c-Jun N-terminal kinase/stress activated protein kinase (JNK/SAPK) and p38 pathways) are the central mediators that propagate extracellular signals from the membrane to the nucleus. To verify the anti-oxidant effect of VOO and Hc acids, their effect on oxidative stress-associated MAPK proteins—ERK1/2, JNK, and p38 in SH-SY5Y cells was determined. Initial test treated cells with VOO, which reduced the levels of phosphorylated ERK1/2 but had no observed effects on p38 and JNK activation (Fig. 6). Checking whether the Hc acids will have the same influence on MAPKs revealed that like VOO, the Hc acids could also lower the levels of phosphorylated ERK1/2. Treatment with Aβ increased the levels of phospho-p38 and phosphor-ERK1/2 which was not observed when cells were treated with the Hc acids mixture (Fig. 6, right panel). While ERK is primarily activated by mitogenic stimuli, cellular stressors such as oxidative stress generally activate JNK/SAPK and p38. Although there are reports that ERK signaling pathway exerts a pro-survival influence during oxidant injury (Daniels et al. 2011), an increase in the activation of ERK is observed when there is an increase in the levels of the Aβ precursor protein (Nizzari et al. 2007). JNK and p38 activation is usually linked to Aβ–induced apoptosis (Giovannini et al. 2002; Finkel and Holbrook 2000). In this study, the level of phosphorylated ERK1/2 was decreased by both the VOO and Hc acids mixture. And though Aβ activated p38, VOO or Hc acids were able to counter this effect by inhibiting p38 phosphorylation (Fig. 6). In primary striatal neurons under oxidative stress, for example, attenuation of the activation of ERK1/2 and JNK by flavonoids protect against neuronal cell death (Schroeter et al. 2001). In cancer cells, p38 is activated by p53 by generating ROS (Bragado et al. 2007). The inhibition of p38 activation, therefore, correlates with decreased ROS levels. JNK expression appeared to be not affected by either VOO or Hc acids treatment which suggests that JNK most likely does not play a role in the neuroprotective effect of VOO or Hc acids.
Fig. 6.
Effect of virgin olive oil or mixture of hydroxycinnamic acids (Hc acids) caffeic, vanillic, p-coumaric, ferulic acid on the expression of activated and non-activated MAPK proteins ERK1/2, p38, and JNK in SH-SY5Y cells. SH-SY5Y cells (2 × 105 cells/mL) were treated with the samples and incubated for 24 h prior to treatment with or without β-amyloid (Aβ)1-42 (control). Protein samples (10 μg) were resolved in 10 % SDS-polyacrylamide gel by polyacrylamide gel electrophoresis, transferred to nitrocellulose membrane, and blotted with primary antibodies from Cell Signaling, Inc. (pERK1/2, ERK2, pJNK, and JNK). The signal was visualized using LiCor Odyssey Infrared Imaging System after reaction with goat anti-mouse IRDye 680LT or goat anti-rabbit IRDye 800CW (LI-COR)
In neurodegenerative diseases, therapies targeting basic mitochondrial processes, such as energy metabolism are believed to hold great promise as impairment of cellular energy metabolism is toxic to cells (Lin and Beal 2006). In AD, abnormal parietotemporal metabolism has been reported to involve a metabolic shift from glycolytic to oxidative metabolism and this impairment of glucose degradation may be the basis for synoptic dysfunction underlying the impairment observed in AD (Vlassenko and Raichle 2014). Results of the determination of the influence of VOO on the overall metabolism of SH-SY5Y cells showed that VOO clearly improved the cellular energy metabolism by promoting the expression of the glycolytic enzymes’ genes (Fig. 6). An increase in neuronal cells’ metabolism is associated with neuronal protection and therapies that lessen cognitive dysfunction that target energy metabolism and adaptive stress responses have been reported to be effective in animal models and in preliminary clinical studies (Kapogiannis and Mattson 2011). Furthermore, glycolysis stimulation contributes to the cells ability to resist stress (Nciri et al. 2013). Cells with increased glycolytic activity can scavenge endogenous ROS. VOO and Hc acids appeared to neutralize the effect of ROS and acted as the radical scavenger or substitute for superoxide dismutase in protecting the cells from oxidative stress. In our previous reports, natural compounds such as caffeoylquinic acids can attenuate Aβ cytotoxicity by promoting the energy production and upregulation of glycolytic enzymes expression (Sasaki et al. 2013; Miyamae et al. 2011). Whether the improved metabolism is the main cause of the decrease in ROS levels or a result was not determined.
The overall neuroprotective effect of VOO can be attributed to all its components, both the major and minor compounds, but here, we have shown that even the minor flavonoid components, the Hc acids, can contribute immensely to the effect of VOO. Reports on the ability of polyphenols and phenolic acids to cross the blood–brain barrier (BBB), however, vary. Lardeau and Poquet (2013) demonstrated that (natural) phenolic acids metabolites (e.g. caffeic acid) exhibit a very low rate of permeation across blood brain barrier. On the other hand, the report of Zhang et al. (2014) states that although Hc acids do not localize in the brain, in the case of ferulic acid, it can increase the levels of erythropoietin in the brain and blood which contributes to its neuroprotective effect (Zhang et al. 2014).
In this study, the Hc acids played an important role in the neuroprotective effect of VOO by the virtue of its antioxidant effect which was evident in the decreased ROS levels, leading to the alleviation of Aβ-induced oxidative stress in SH-SY5Y cells. The decrease in oxidative stress was a result of the inhibition of the activation of ERK1/2 and p38 MAPKs. In addition, we have previously reported that caffeic acid derivatives upregulated the expression of the genes of glycolytic enzymes, which alleviated the cytotoxic effect of Aβ on treated SH-SY5Y cells. In this study, VOO also increased the expression of glycolytic enzymes’ genes.
Our results have demonstrated that VOO has neuroprotective effect against Aβ toxicity in SH-SH5Y cells and this effect can be attributed to its hydroxycinnamic acids content. More generally, our findings highlight the need to conduct more studies that explore the use of hydroxycinnamic acids-rich foods, other than VOO, as promising therapeutic strategy in the management of oxidative stress-associated neuronal disorders such as AD.
Compliance with ethical standards
Conflict of interest
The authors declare no conflict of interests.
References
- Aparicio R, Luna G. Characterisation of monovarietal virgin olive oils. Eur J Lipid Sci Technol. 2002;104:614–627. doi: 10.1002/1438-9312(200210)104:9/10<614::AID-EJLT614>3.0.CO;2-L. [DOI] [Google Scholar]
- Bartzoki G. Alzheimer’s disease as homeostatic response to age-related myelin breakdown. Neurobiol Aging. 2011;32:1341–1372. doi: 10.1016/j.neurobiolaging.2009.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betteridge JD. What is oxidative stress? Metabolism. 2000;49:3–8. doi: 10.1016/S0026-0495(00)80077-3. [DOI] [PubMed] [Google Scholar]
- Birben E, Sahiner U, Sackesen C, Erzurum S, Kalayci D. Oxidative stress and antioxidant defense. WAO J. 2012;5:9–19. doi: 10.1097/WOX.0b013e3182439613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourne LC, Rice-Evans CA. Urinary detection of hydroxycinnamates and flavonoids in humans after high dietary intake of fruit. Free Radic Res. 1998;28:429–438. doi: 10.3109/10715769809070812. [DOI] [PubMed] [Google Scholar]
- Bragado P, Armesilla A, Silva A, Porras A. Apoptosis by cisplatin requires p53-mediated p38α activation through ROS generation. Apoptosis. 2007;12:1733–1742. doi: 10.1007/s10495-007-0082-8. [DOI] [PubMed] [Google Scholar]
- Brookmeyer R, Johnson E, Ziegler-Graham K, Arrighi HM. Forecasting the global burden of Alzheimer’s disease. Alzheimer’s Dement. 2007;3:186–191. doi: 10.1016/j.jalz.2007.04.381. [DOI] [PubMed] [Google Scholar]
- Butterfield DA. Amyloid β-peptide (1-42)-induced oxidative stress and neurotoxicity: implications for neurodegeneration in Alzheimer’s disease brain. Free Radic Res. 2002;36:1307–1313. doi: 10.1080/1071576021000049890. [DOI] [PubMed] [Google Scholar]
- Butterfield DA, Drake J, Pocernich C, Castegna A. Evidence of oxidative damage in Alzheimer’s disease brain: central role for amyloid beta-peptide. Trends Mol Med. 2001;7:548–554. doi: 10.1016/S1471-4914(01)02173-6. [DOI] [PubMed] [Google Scholar]
- Butterfield DA, Swomley AM, Sultana R. Amyloid β-peptide (1–42)-induced oxidative stress in Alzheimer disease: importance in disease pathogenesis and progression. Antiox Redox Sign. 2013;19:823–835. doi: 10.1089/ars.2012.5027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Ho C. Antioxidant activities of caffeic acid and its related hydroxycinnamic acid compounds. J Agric Food Chem. 1997;45:2374–2378. doi: 10.1021/jf970055t. [DOI] [Google Scholar]
- Chen Z, Zhong C. Decoding Alzheimer’s disease from perturbed cerebral glucose metabolism: implications for diagnostic and therapeutic strategies. Prog Neurobiol. 2013;108:21–43. doi: 10.1016/j.pneurobio.2013.06.004. [DOI] [PubMed] [Google Scholar]
- Chen Z, Zhong C. Oxidative stress in Alzheimer’s disease. Neurosci Bull. 2014;30:271–281. doi: 10.1007/s12264-013-1423-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christen Y. Oxidative stress and Alzheimer disease. Am J Clin Nutr. 2000;71:621s–629s. doi: 10.1093/ajcn/71.2.621s. [DOI] [PubMed] [Google Scholar]
- Cicerale S, Lucas L, Keast R. Biological activities of phenolic compounds present in virgin olive oil. Int J Mol Sci. 2010;11:458–479. doi: 10.3390/ijms11020458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craft S, Newcomer J, Kanne S, Dagogo-Jack S, Cryer P, Sheline Y, Luby J, dagogo-Jack A, Anderson A. Memory improvement following induced hyperinsulinemia in Alzheimer’s disease. Aging. 1996;17:123–130. doi: 10.1016/0197-4580(95)02002-0. [DOI] [PubMed] [Google Scholar]
- Cunnane S, Nugent S, Roy M, Courchesne-Loyer A, Croteau E, Tremblay S, Castellano A, et al. Brain fuel metabolism, aging, and Alzheimer’s disease. Nutrition. 2011;27:3–20. doi: 10.1016/j.nut.2010.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniels WM, Hendricks J, Salie R, Taljaard JJ. The role of MAP-kinase superfamily in beta-amyloid toxicity. Metab Brain Dis. 2001;16:175–185. doi: 10.1023/A:1012541011123. [DOI] [PubMed] [Google Scholar]
- D’Archivio M, Filesi C, Di Benedetto R, Gargiulo R, Giovannini C, Masella R. Polyphenols, dietary sources and bioavailability. Annali dell’Istituto Superiori Sanita. 2007;43:348–361. [PubMed] [Google Scholar]
- Dimitrios B. Sources of natural phenolic antioxidants. Trends Food Sci Technol. 2006;2006:505–512. doi: 10.1016/j.tifs.2006.04.004. [DOI] [Google Scholar]
- Finkel T, Holbrook N. Oxidants, oxidative stress and the biology of ageing. Nature (London) 2000;408:239–247. doi: 10.1038/35041687. [DOI] [PubMed] [Google Scholar]
- Florent-Bechard S, Malaplate-Armand C, Koziel V, Kriem B, Olivier JL, Pillot T, Oster T. Towards a nutritional approach for prevention of Alzheimer’s disease: biochemical and cellular aspects. J Neurol Sci. 2007;262:27–36. doi: 10.1016/j.jns.2007.06.046. [DOI] [PubMed] [Google Scholar]
- Giovannini MG, Scali C, Prosperi C, Bellucci A, Vannucchi MG, Rosi S, Pepeu G, Casamenti F. β-amyloid-induced inflammation and cholinergic hypofunction in the rat brain in vivo: involvement of the p38MAPK pathway. Neurobiol Dis. 2002;11:257–274. doi: 10.1006/nbdi.2002.0538. [DOI] [PubMed] [Google Scholar]
- Isoda H, Motojima H, Margout D, Neves M, Han J, Nakajima M, Larroque M. Antiallergic effect of Picholine olive oil-in-water emulsion through β-hexosaminidase release inhibition and characterization of their physicochemical properties. J Agric Food Chem. 2012;60:7851–7858. doi: 10.1021/jf3016078. [DOI] [PubMed] [Google Scholar]
- Kapogiannis D, Mattson MP. Disrupted energy metabolism and neuronal circuit dysfunction in cognitive impairment and Alzheimer’s disease. Lancet Neurol. 2011;10:187–198. doi: 10.1016/S1474-4422(10)70277-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lardeau A, Poquet L. Phenolic metabolites derived from coffee consumption are unlikely to cross the blood–brain barrier. J Pharma Biomed Anal. 2013;766:134–138. doi: 10.1016/j.jpba.2012.12.016. [DOI] [PubMed] [Google Scholar]
- Lin MT, Beal MF. Mitochondrial dysfunction and oxidative stress in neurodegenerative diseases. Nature (London) 2006;443:787–795. doi: 10.1038/nature05292. [DOI] [PubMed] [Google Scholar]
- Manach C, Williamson G, Morand C, Scalbert A, Remesy C. Bioavailability and bioefficacy of polyphenols in humans. I. Review of 97 bioavailability studies. Am J Clin Nutr. 2005;81:230S–242S. doi: 10.1093/ajcn/81.1.230S. [DOI] [PubMed] [Google Scholar]
- Miyamae Y, Han J, Sasaki K, Terakawa M, Isoda H. 3,4,5-tri-Ο-caffeoylquinic acid inhibits amyloid β-mediated cellular toxicity on SH-SY5Y cells through the upregulation of PGAM1 and G3PDH. Cytotechnology. 2011;63:191–200. doi: 10.1007/s10616-011-9341-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nciri R, Desmoulin F, Saleh Allagui M, El Feki A, Vincent C, Croute F. Neuroprotective effect of chronic exposure of SH-SY5Y to low lithium concentration involve glycolysis stimulation, extracellular pyruvate accumulation and resistance to oxidative stress. Int J Neuropsychopharmacol. 2013;16:365–376. doi: 10.1017/S1461145712000132. [DOI] [PubMed] [Google Scholar]
- Nieves Franco M, Galeano-Diaz T, Lopez O, Fernandez-Bolanos JG, Sanchez J, De Miguel C, Victoria Gil M, Martin-Vertedor D. Phenolic compounds and antioxidant capacity of virgin olive oil. Food Chem. 2014;163:289–298. doi: 10.1016/j.foodchem.2014.04.091. [DOI] [PubMed] [Google Scholar]
- Nizzari M, Venezia V, Repetto E, Caorsi V, Magrassi R, Gagliani MC, Carlo P, Florio T, Schettini G, Tacchetti C, Russo T, Diaspro A, Russo C. Amyloid precursor protein and presenilin1 interact with the adaptor GRB2 and modulate ERK1,2 signaling. J Biol Chem. 2007;282:13833–13844. doi: 10.1074/jbc.M610146200. [DOI] [PubMed] [Google Scholar]
- Norton S, Matthews FE, Barnes DE, Yaffe K, Brayne C. Potential for primary prevention of Alzheimer’s disease: an analysis of population-based data. Lancet Neurol. 2014;13:788–794. doi: 10.1016/S1474-4422(14)70136-X. [DOI] [PubMed] [Google Scholar]
- Ollivier D, Artaud J, Pinatel C, Durbec JP, Guerere M. Triacylglycerol and fatty acid compositions of French virgin olive oils. Characterization by chemometrics. J Agric Food Chem. 2003;51:5723–5731. doi: 10.1021/jf034365p. [DOI] [PubMed] [Google Scholar]
- Owen RW, Giacosa A, Hull WE, Haubner R, Spiegelhalder B, Bartsch H. The antioxidant/anticancer potential of phenolic compounds isolated from olive oil. Eur J Cancer. 2000;36:1235–1247. doi: 10.1016/S0959-8049(00)00103-9. [DOI] [PubMed] [Google Scholar]
- Perez-Lopez FR, Chedraui P, Haya J, Cuadros JL. Effects of the Mediterranean diet on longevity and age-related morbid conditions. Maturitas. 2009;64:67–79. doi: 10.1016/j.maturitas.2009.07.013. [DOI] [PubMed] [Google Scholar]
- Sasaki K, Han J, Shimozono H, Villareal MO, Isoda H. Caffeoylquinic acid-rich purple sweet potato extract, with or without anthocyanin, imparts neuroprotection and contributes to the improvement of spatial learning and memory of SAMP8 Mouse. J Agric Food Chem. 2013;61:5037–5045. doi: 10.1021/jf3041484. [DOI] [PubMed] [Google Scholar]
- Scarmeas N, Stern Y, Tang MX, Mayeux R, Luchsinger JA. Mediterranean diet and risk for Alzheimer’s disease. Ann Neurol. 2006;59:912–921. doi: 10.1002/ana.20854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeter H, Spencer JPE, Rice-Evans C, Williams RJ. Flavonoids protect neurons from oxidized low-density lipoprotein-induced apoptosis involving JNK, c-jun and caspase-3. Biochem J. 2001;358:547–557. doi: 10.1042/bj3580547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stadtman ER. Protein oxidation in aging and age-related diseases. Ann N Y Acad Sci. 2001;928:22–38. doi: 10.1111/j.1749-6632.2001.tb05632.x. [DOI] [PubMed] [Google Scholar]
- Tamagno E, Robino G, Obbili A, Bardini P, Aragno M, Parola M, Danni O. H2O2 and 4-hydroxynonenal mediate amyloid β-induced neuronal apoptosis by activating jnks and p38mapk. Exp Neurol. 2003;180:144–155. doi: 10.1016/S0014-4886(02)00059-6. [DOI] [PubMed] [Google Scholar]
- Van Merloo J, Kaspers GJL, Cloos J. Cell sensitivity assays: the MTT assay. Methods Mol Biol. 2011;731:237–245. doi: 10.1007/978-1-61779-080-5_20. [DOI] [PubMed] [Google Scholar]
- Vlassenko AG, Raichle ME. Brain aerobic glycolysis functions and Alzheimer’s disease. Clin Transl Imaging. 2014;3:27–37. doi: 10.1007/s40336-014-0094-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Wang W, Li L, Perry G, Lee H, Zhu X. Oxidative stress and mitochondrial dysfunction in Alzheimer’s disease. Biochim Biophys Acta. 2014;1842:1240–1247. doi: 10.1016/j.bbadis.2013.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Melton LD. Cytoprotective effects of polyphenolics on H2O2-induced cell death in SH-SY5Y cells in relation to their antioxidant activities. Eur Food Res Technol. 2008;228:123–131. doi: 10.1007/s00217-008-0915-x. [DOI] [Google Scholar]
- Zhang L, Hongsheng W, Wang T, Jiang N, Yu P, Chong Y, Fu F. Ferulic acid ameliorates nerve injury induced by cerebral ischemia in rats. Exp Ther Med. 2014;9:972–976. doi: 10.3892/etm.2014.2157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou F, Xu Y, Hou XY. MLK3-MKK3/6-P38MAPK cascades following N-methyl-d-aspartate receptor activation contributes to amyloid-β peptide-induced apoptosis in SH-SY5Y cells. J Neurosci Res. 2014;92:808–817. doi: 10.1002/jnr.23354. [DOI] [PubMed] [Google Scholar]