Abstract
Indoxyl sulfate (IS) is a digestive intermediate product that is a known indicator of chronic kidney disease. Its toxicity has also been suggested to accelerate chronic kidney disease. Recently, mesenchymal stem cells (MSCs) have been confirmed as a potential treatment in kidney regeneration. To determine the universal alteration in gene expression, we combined high-throughput microarray technology and in vitro culture of adipose-derived mesenchymal stem cells at different doses of IS (20, 40, 60 mg/l). We found that indoxyl sulfate has a remarkable interconnection with stem cell and calcium/calmodulin-dependent kinase pathways. In vitro results showed that indoxyl sulfate exerts anti-proliferation and anti-migration effects on ADMSCs. In addition, IS effects lead to increase in apoptotic cells and cells arrested at the G1 phase. Moreover, MEF2-A, MEF2-D and CACNA1S expression significantly decreased after indoxyl sulfate treatment. It can be speculated that following treatment with indoxyl sulfate, the function of ADMSCs is decreased and ADMSCs’ ability to support renal tubule regeneration in chronic kidney disease patients may be lower.
Keywords: Chronic kidney disease, Mesenchymal stem cell, Indoxyl sulfate, Kidney regeneration, L-type calcium channel
Introduction
Chronic kidney disease (CKD) has been documented as a public health dilemma because of the high cost treatment and health weakening issue (Tonelli and Riella 2014). In addition, chronic kidney disease has other major effects on the overall population. Several studies have suggested that all-cause mortality such as high-risk diabetics, hypertensive population, especially cardiovascular disease (CVD) events, are strongly affected by loss of kidney function (Nugent et al. 2011). The kidneys play a crucial role in excreting numerous types of body waste, such as metabolites and other biochemical substances. Once renal function is decreased, these substances accumulate in the body and form uremic toxins (Vanholder et al. 2003). Over 100 types of uremic toxins have been determined to play roles in various biological processes that result in uremic syndromes. These disorders have a closed relationship with cardiovascular malfunction (Duranton et al. 2012; Vanholder and De Smet 1999; Vanholder et al. 2003). Patients suffering from chronic kidney disease caused by indoxyl sulfate also have cardiovascular dysfunction (Barreto et al. 2009). Indoxyl sulfate has been detected in the plasma of patients who underwent renal replacement therapy and is associated with heart failure (Cao et al. 2014). In addition, indoxyl sulfate is thought to take part in the disease mechanism of atherosclerosis in dialysis patients; however, the molecular mechanistic and signal transduction pathways of indoxyl sulfate and chronic kidney disease still need investigation.
High-throughput microarray technology is an important investigational model that assists advances in functional genomics and biological systems to determine global changes in gene expression. This technology allows the measurement of thousands of gene expressions using a single microarray. Popular applications for this technology have led to the rapid advancement of microarray datasets that are available in the gene expression omnibus (GEO), which is maintained by the National Center for Biotechnology Information (NCBI). Consequently, we collected gene expression data from GSE34259 and used Metacore to analyze potential signaling in indoxyl sulfate-treated cells. A previous study also showed that indoxyl sulfate stimulates chronic kidney disease progression (Wu et al. 2011), however the details of the downstream signaling pathway remain largely unknown. Based on our bioinformatics analysis, we found that indoxyl sulfate-induced chronic kidney disease has a high connection to stem cell and calcium/calmodulin-dependent kinase (CaMK) signaling pathways.
By employing bioinformatics tools, we obtained outcomes showing that indoxyl sulfate-induced chronic kidney disease conceivably could involve the calcium/calmodulin-dependent kinase (CaMK) signal pathway. Chronic kidney disease patients usually develop cardiovascular disease at higher rates than patients with other types of conditions. Preclinical treatment revealed a correlation between vascular modifications and indoxyl sulfate concentrations in patients’ sera (Ardhanari et al. 2014; Barreto et al. 2009; Vanholder et al. 2005). Previous studies indicated that CaMK and cardiovascular diseases are correlated (Ai et al. 2005; Maier and Bers 2007). However, evidence-based investigations are needed to confirm the underlying mechanism in which indoxyl sulfate induces high cardiovascular risks and modest renal malfunctions. A previous study presented the correlation between voltage-dependent calcium channel (VGGCs) and cardiovascular diseases (Hansen 2014). However, the manner in which indoxyl sulfate modulates cardiovascular diseases through VGGC is still unknown. The L-type voltage-dependent calcium channel family (VGCCL) is very important in many physiological processes in various types of cells (Li and Xiong 2011) playing extremely critical roles in modulating cell cycle progression (MacFarlane and Sontheimer 2000), cell proliferation (Pardo 2004), cell migration (Becchetti and Arcangeli 2010), and apoptosis (Lang et al. 2005; Patel and Lazdunski 2004). Nifedipine, an L-type calcium channel blocker, has been suggested to decrease calcium currents, which might lead to reductions in cell growth and the differentiation of bone marrow Mesenchymal Stem Cells (MSCs) (Wen et al. 2012). Mesenchymal stem cells are prominent cells because of their multitude, harvesting proficiency, and low immune response, in which adipose-derived MSCs (ADMSCs) (Humphreys and Bonventre 2008; Salem and Thiemermann 2010) particularly show their specific distinctive in massive production and slightest encroachment (Gimble and Nuttall 2011). Accordingly, in the current investigation, we used ADMSCs as a prominent cell model to elucidate the molecular mechanistic in ongoing chronic kidney disease induced by indoxyl sulfate at different concentrations. Thus, an investigation of VGCCL’s effect on the cellular activities of ADMSCs in uremic patients is essential to interpret the fundamental mechanism of indoxyl sulfate with regard to ADMSCs. They could be new targets for the prospective treatment for renal failure.
We hypothesize that the accumulation of indoxyl sulfate during the different stages of chronic kidney disease progression might result in the functional incompetence of ADMSCs. This study aims to determine the effects of indoxyl sulfate in various chronic kidney disease stages on the performance of ADMSCs and assess the protein expressions of VGCCL, MEF2A, and MEF2D as a possible underlying mechanism for indoxyl sulfate metabolism in ADMSCs.
Materials and methods
Bioinformatics analysis
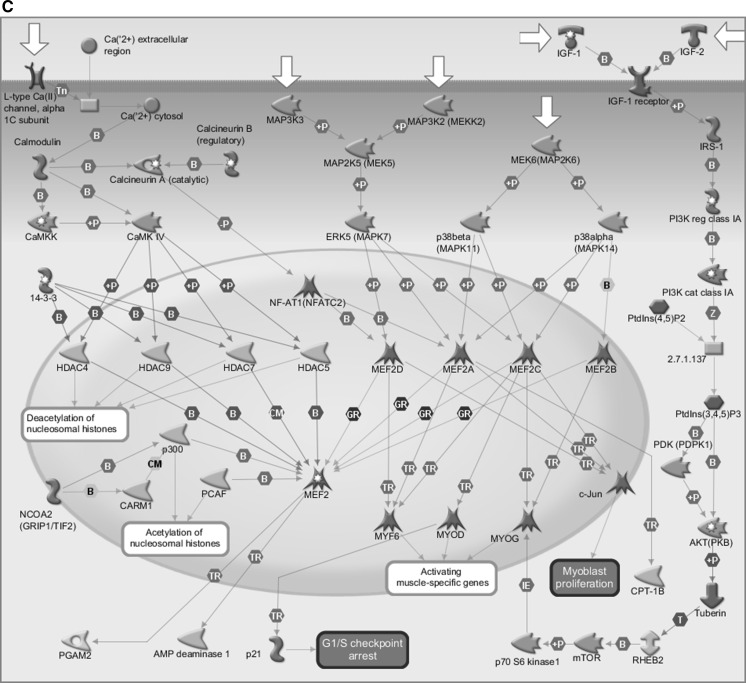
The underlying mechanism on how indoxyl sulfate promotes the onset of chronic kidney disease still unclear (Wu et al. 2011). We accordingly accumulated gene expression data from the gene expression omnibus (GEO) database (http://www.ncbi.nlm.nih.gov/geo/) under series accession number GSE34259. Each data set provides normalized signal data. By computing the fold change, the average expression in KCl control samples (n = 7) and the difference between average expression in indoxyl sulfate induced samples (n = 7), we could detect the dominant signal pathways responsible for indoxyl sulfate inducing chronic kidney disease. MetaCore (GeneGo, Inc, St. Joseph, MI, USA) was employed in investigating GO molecular functions and GeneGo maps (pathways) MetaCore builds biological networks from the input gene set, then the biological processes associated with each network were listed. Fold change >2.0 genes were uploaded into Metacore software by comparing the regular expression with indoxyl sulfate treatment and KCl control samples. Based on the data, “Cell cycle and its regulation” (p value = 4.320e−25, FDR = 2.679e−23), “Calcium signaling” (p value = 2.681e−10, FDR = 7.915e−10) and “Stem cells signaling” (p value = 1.421e−8, FDR = 3.671e−8) were supposed to take essential roles in indoxyl sulfate induced microarray data (Fig. 1a). Despite that, calcium signaling also encompasses various pathways (Fig. 1b) as “Development Role of HDAC and calcium/calmodulin-dependent kinase (CaMK) signaling” pathway, this is also accounted as an important pathway in indoxyl sulfate induced microarray data (p value = 4.413e−2). Significant difference was set at p value < 0.05 (Fig. 1c).
Fig. 1.
a MetaCore pathway analysis indicated that cell cycle regulation (p value = 4.320e−25) and Calcium signaling (p value = 2.681e−10) and stem cell signaling (p value = 1.421e−8) and were significantly associated with IS treated microarray datasets. b Metacore pathway analysis indicated that Development_Role of HDAC and calcium/calmodulin-dependent kinase (CaMK) signaling pathways were significantly modulated after indoxyl sulfate treatment (p value = 4.413e−2). c CaMK signaling pathways were identified from MetaCore database (p < 0.05 means significant different in statistical analysis. B binding, CM covalent modifications, +P phosphorylation, T transformation, Tn transport, Z catalysis, TR transcription regulation, IE influence on expression, GR group relation, CM covalent modifications. Green arrow means positive/activation of process. Red arrow means negative/inhibition of process. Grey arrow means unspecified process. (Color figure online)
Culture of ADMSCs
Based on our bioinformatics analyses (Fig. 1a), we found that stem cell signaling (p value = 1.421e−8, FDR = 3.671e−8) plays an essential part in indoxyl sulfate-induced microarray datasets. MSCs are prominent cells because of their multitude, harvesting proficiency, and low immune response, in which ADMSCs (Humphreys and Bonventre 2008; Salem and Thiemermann 2010) particularly show their specific distinctive in massive production and slightest encroachment (Gimble and Nuttall 2011).
The ADMSCs were purchased from Promocell (Heidelberg, Germany). ADMSCs were cultured using DMEM (Gibco, Grand Island, NY, USA) with 10 U/ml penicillin, 100 µg/ml streptomycin (all from Sigma-Aldrich, St. Louis, MO, USA), and 10 % fetal bovine serum supplement (FBS; Gibco) at 37 °C in a humidified 5 % CO2 incubator. The medium was changed every 3 days thereafter. The cells were passaged using Trypsin–EDTA after the culture reached approximately 80 % (Sigma Aldrich). All subsequent experiments used the cells from the third passage.
Chemical and reagent preparation
A 250 mg aliquot of indoxyl sulfate was purchased from Sigma-Aldrich (I3875-250MG). Based on previous studies, we used different concentrations (20, 40, 60 mg/l) that represented the different stages of chronic kidney disease progression (Lin et al. 2011). Indoxyl sulfate was mixed with DMEM and applied to the ADMSCs. The following assays were then performed.
MTT proliferation assay
For this assay, 24-well plates were used for cell seeding at a clone density of 4 × 103 cells/well in 600 µl of the medium. Four FBS treatment groups were used: 20 mg/l indoxyl sulfate, 40 mg/l indoxyl sulfate, 60 mg/l indoxyl sulfate, and a pure basal medium as the control. The MTT assay was performed every 24 h. In brief, 40 µl of the 3-(4,5-dimethythiazol-2-y)-2,5-diphenyl tetrazolium bromide MTT (5 mg/ml) solution was added to each well and then kept at 37 °C and 5 % CO2 for 4 h. The MTT solution was then washed off, 450 µl of dimethyl sulfoxide was added to each well, and resuspended by pipetting to dissolve the formazan. All the steps were performed in the dark. The data were collected over 15 days with six repeats using an ELISA reader (Epoch, BioTek, Winooski, VT, USA) at a wavelength of 520 nm.
Apoptosis analysis
To induce cell apoptosis, the ADMSCs were cultured in a medium with or without indoxyl sulfate in 25 cm2 flasks. After 15 days, the ADMSCs were detached using trypsin and collected by centrifugation (Kubota Corp., Tokyo, Japan, Model 3740, ser. no. NY3285-B600) at 1000 rpm for 5 min. Next, the Cell Meter™ Annexin V Binding Apoptosis Assay Kit (AAT Bioquest, Sunnyvale, CA, USA, cat. no. 22824) was employed. The cells were washed with ice-cold PBS, mixed in 200 µl of the binding buffer, and incubated within 30 min in the dark at 4 °C with 2 µl of Annexin V and 2 µl of the PI solution. Then 200 µl of the binding buffer was added to the samples to increase the total volume. Finally, flow cytometry (BD, San Diego, CA, USA, Accuri™ C6, ser. no. 3038) was performed immediately with a counting threshold of 106 cells.
Cell cycle assessment
For the cell cycle assay, ADMSCs were grown in 25 cm2 flasks with or without indoxyl sulfate for 15 days. The cells were then dissociated using trypsin and collected by centrifugation (Kubota Corp., Model 3740, ser. no. NY3285-B600, Tokyo, Japan). Ice-cold PBS was used to wash the cells. Afterwards, the cells were centrifuged for 5 min at 1000×g, fixed with 2 ml ice-cold 70 % alcohol and 1 ml ice-cold PBS, and incubated at 40 °C for a minimum of 30 min. RNase (Takara, Shiga, Japan) was added to the samples within 30 min at 37 °C. The Annexin V-FITC Apoptosis Kit (AAT Bioquest, cat. no. 22824) was used. The DNA was labeled using 2 µl of the PI solution in the dark for 30 min at 37 °C. All samples were analyzed using flow cytometry (BD Accuri™ C6, ser. no. 3038) with a counting threshold of 106 cells. Each group was analyzed in triplicate.
In vitro scratch wound healing assays
To evaluate the effects of indoxyl sulfate on ADMSCs’ migration capacity, we conducted scratch wound healing assays. ADMSCs were seeded in 25 cm2 flasks with different concentrations of indoxyl sulfate for 15 days. Next, 106 cells were grown in each well of a 6-well plate. We used a pipette tip to scratch the cell layer and simulate physical trauma. All samples were washed with ice-cold PBS and maintained at 37 °C in a humidified 5 % CO2 incubator within for 12 h. The samples were then washed using PBS before adding a negative control (serum-free DMEM) and ADMSC growth medium with one of the different treatments, and the cells were incubated at 37 °C and 5 % CO2 for 12 h. We used a light microscope to capture images of the wound area both before incubation and after 12 h of incubation for comparison (Jie et al. 2010).
Transwell migration assay
ADMSC cultures were prepared with DMEM containing 10 % FBS at standard culture conditions. For this assay, the cells were harvested and resuspended in the DMEM medium with 0.2 % BSA at 106 cells/ml. We then placed Thincert™ 8 µm-pore 12-well cell culture inserts (Greiner Bio-One GmbH, Frickenhausen, Germany, cat. no. 662160) into the wells of a Cellstar® 12-well cell culture plate (Greiner Bio-One GmbH, cat. no. 662638). Then, 1000 µl of the culture serum-free DMEM medium with different concentrations of indoxyl sulfate (including controls) were added to each well for the lower part of the arrangement. For the upper part of the arrangement, 400 µl of the ADMSC cell suspensions were added followed by incubation at standard conditions for 3–24 h. On the second day, the medium in each well was replaced with 800 µl DMEM containing 8 µM of Calcein-AM (4 mM in anhydrous DMSO) (Sigma, cat. no. C1359) and incubated for 45 min. Afterwards, the media in the cell inserts were removed, and the cell inserts were transferred into a 24-well plate with each well containing 1000 µl of the pre-warmed Trypsin–EDTA. The plate was then incubated for 10 min and was gently agitated from time to time. Finally, 200 µl of the Trypsin–EDTA solution (now containing migratory cells) were placed in a black 96-well polystyrene plate (Greiner Bio-One GmbH, cat. no. 756076), and a fluorescence reading was taken at the 485 nm excitation and 520 nm emission wavelengths.
Western blot analysis
ADMSC lysates were made using the RIPA lysis buffer (0.5 M Tris–HCl at pH 7.4, 10 % NP-40, 1.5 M NaCl, 10 mM EDTA, 2.5 % deoxycholic acid, 1 mM DTT, and phosphatase and protease inhibitors) and centrifuged at 2900×g and 4 °C for 20 min. The protein concentration was measured using the Bradford Assay (Bio-Rad, Richmond, CA, USA).
A detailed description of the theoretical and experimental study can be found in a previous publication (Zor and Seliger 1996). The mathematical equation was experimentally tested and found to yield a linear calibration curve over the entire protein concentration range (Zor and Seliger 1996; Ernst and Zor 2010).
Each protein sample (20 µg) was mixed with the loading buffer (125 mM Tris at pH 6.8, 4 % SDS, 20 % glycerol, 0.06 % bromophenol blue, 10 % beta-mercaptoethanol) to obtain a final volume of 15 µl. Next, the samples were heated at 95 °C for 10 min before they were separated by gel electrophoresis using either 6 % or 10 % SDS–polyacrylamide gel. The proteins were then transferred to polyvinylidene difluoride (PVDF) membranes (Thermo Fisher Scientific, Taipei, Taiwan) and blocked using the blocking buffer. The blots were then incubated overnight with the primary antibodies in 5 % non-fat milk at 4 °C. Following the manufacturer’s instructions, the primary antibodies (polyclonal antibodies) against phospho-MEF2A (Ser408)(1:1000, Cell Signaling Technology, Danvers, MA, USA), against phosphor-MEF2D (Ser444)(1:1000, GeneTex, Irvine, CA, USA), against CACNA1S (1:1000, GeneTex), and against actin (1:1000, GeneTex) were first incubated with the antigen at a 1 µg: 1 µg ratio at room temperature for 2 h as a pre-absorption control. The membranes were then washed three times with TBST for 15 min and were incubated with the relevant HRP-conjugated secondary antibody in 5 % non-fat milk for 2 h at room temperature while avoiding light. Finally, the membranes were washed three times with TBST for 20 min, and the signals were read using the SuperSignal® West Dura Extended Duration ECL Substrate for 8 min with GeneSnap v. 6.08 software.
Statistical analysis
The experiments were performed in triplicate and were displayed as the mean ± standard deviation (SD). A one-way ANOVA was used to compare multiple groups with appropriate post hoc tests, and the Student’s t test was used to compare the two groups (StatView 5.01; SAS Institute, Cary, NC, USA). A p value of ≤0.05 indicated a significant difference.
Results
Bioinformatics analysis results
To identify pathways relevant to indoxyl sulfate-induced disease, we utilized Metacore GeneGo software to map a list of differentially expressed genes in indoxyl sulfate induced microarray data. MetaCore software utilizes a proprietary, manually curated database associated with protein–DNA, protein–protein, protein compound interactions, and metabolism-associated signaling pathways. After comparing the average expressions in indoxyl sulfate treatment groups and KCl control samples, we uploaded genes with a fold change greater than 2.0 into the Metacore software. The data showed that the cell cycle and its regulation (p value = 4.320e−25, FDR = 2.679e−23), calcium signaling (p value = 2.681e−10, FDR = 7.915e−10), and stem cell signaling (p value = 1.421e−8, FDR = 3.671e−8) might play important roles in indoxyl sulfate-induced microarray data (Fig. 1a). Calcium signaling contains many pathways; however, after MetaCore analysis, we found that the development role of HDAC and CaMK signaling might be key players in indoxyl sulfate-induced microarray data (p value = 4.413e−2). A p value of <0.05 was generally considered significant (Fig. 1b).
Two families of transcription factors take part in the mammalian differentiation process: myocyte enhancer factor 2 (MEF2), which consists of MEF2A, MEF2B, MEF2C, and MEF2D. These transcription factors could shape homo- and heterodimers that constitutively unite to the promoters or enhancers of the majority of muscle-specific genes. Furthermore, MRF and MEF2 members could physically interact with each other to supportively trigger various muscle-specific genes (McKinsey et al. 2002; Xu and Wu 2000). The MEF2 activity is contingent on the complex regulation of binding protein p300 (p300); K(lysine) acetyltransferase 2B (PCAF); the nuclear factor of activated T-cells; nuclear receptor coactivator 2 [NCOA2 (GRIP1/TIF2)]; cytoplasmic; calcineurin-dependent 2 [NF-xAT1(NFATC2)]; MYOD; 14–3–3; mitogen-activated protein kinase 7 [ERK5 (MAPK7)]; and histone deacetylases 4, 5, 7, and 9 (HDAC4, HDAC5, HDAC7, HDAC9). The MAP kinase cascades and calcium signaling in turn regulate these kinds of proteins.
Extensive bioinformatics data strongly suggest that indoxyl sulfate plays a crucial role in various signal pathways and processes, including stem cell signaling, the role of HDAC, and CaMK signaling pathways via MEF2A, MEF2D, and CACNA1S signaling (Fig. 1c). Nevertheless, indoxyl sulfate and CaMK signaling pathways mechanisms have not been verified in human stem cell. Accordingly, ADMSCs were selected as the experiment model to investigate the molecular machinery of indoxyl sulfate-induced chronic kidney disease.
MTT proliferation assay results
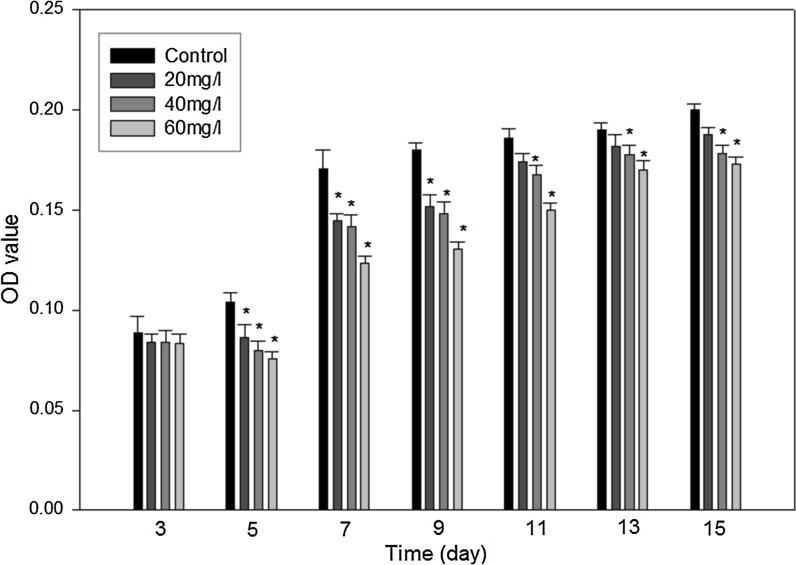
In our experiments, we elucidated the influences of indoxyl sulfate on the proliferation of ADMSCs using an MTT assay. Subsequently, we found that indoxyl sulfate displayed dose-dependent inhibition profiles on the growth of ADMSCs (Fig. 2). The results showed that 20 mg/l of indoxyl sulfate had a slightly inhibitory effect in comparison with other treatments. The viability of the cells in 60 mg/l of indoxyl sulfate was significantly lower than the cell viability in the other treatments.
Fig. 2.
MTT assay results of ADMSCs cultured in the IS-treated group and control group (n = 6). *p < 0.05 versus control group
Apoptosis analysis results
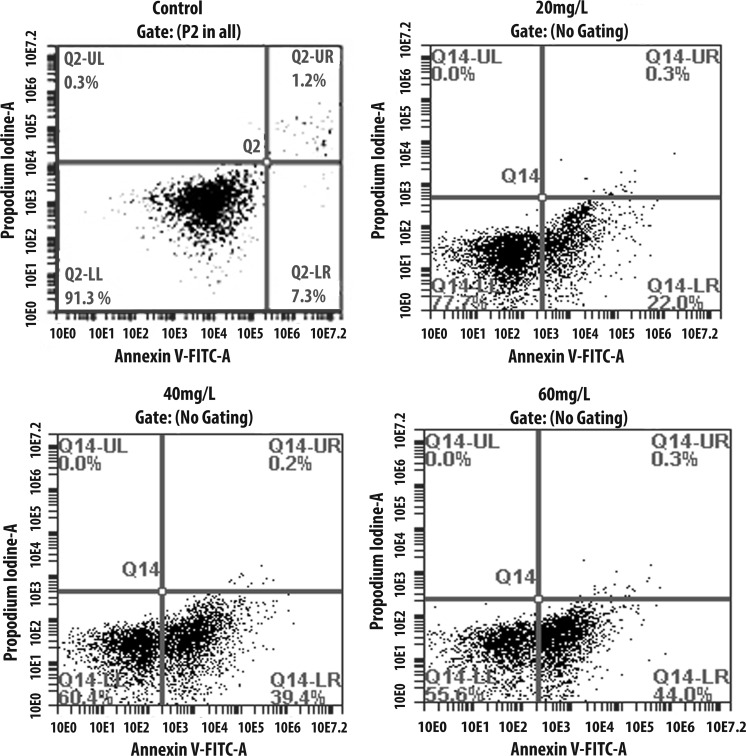
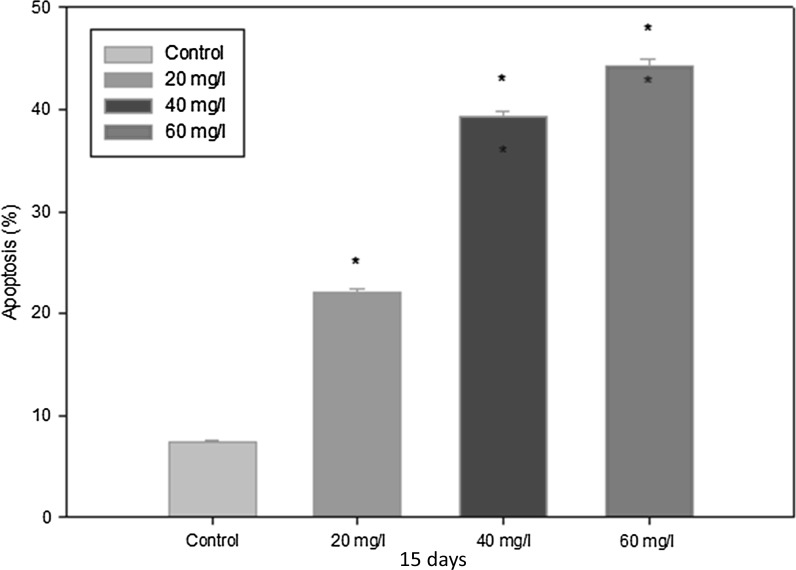
Indoxyl sulfate significantly increased the apoptosis of ADMSCs in a dose-dependent manner. We detected the induction of ADMSC apoptosis in the presence of different concentrations of indoxyl sulfate (Figs. 3, 4): 20 mg/l showed 19.5–20.2 %, 40 mg/l showed 38–39.4 %, and 60 mg/l showed 41–44 % compared to untreated ADMSCs, which showed 7–7.3 %. These results demonstrated that the presence of indoxyl sulfate increased the amount of apoptosis in ADMSCs. Similar to our previous results with MTT, the amount of apoptotic cells was directly proportional to the indoxyl sulfate levels.
Fig. 3.
Representative apoptosis results of ADMSCs by flow cytometer. Viable cells were stained by Annexin V−/PI− (LL quadrant). The Annexin V+/PI− cells were in the early process of apoptosis (LR quadrant), whereas the Annexin V+/PI+ cells had lost cell membrane integrity and entered the late phase of apoptosis (UR quadrant). Necrotic cells indicated in Annexin V−/PI+ staining (UL quadrant)
Fig. 4.
Apoptotic rate analysis of different groups. The results are presented as the ratio of apoptosis induced by 20, 40, 60 mg/l IS compared with corresponding control cells (mean ± SD, n = 6 each, *p < 0.05 vs. control group)
Cell cycle assessment results
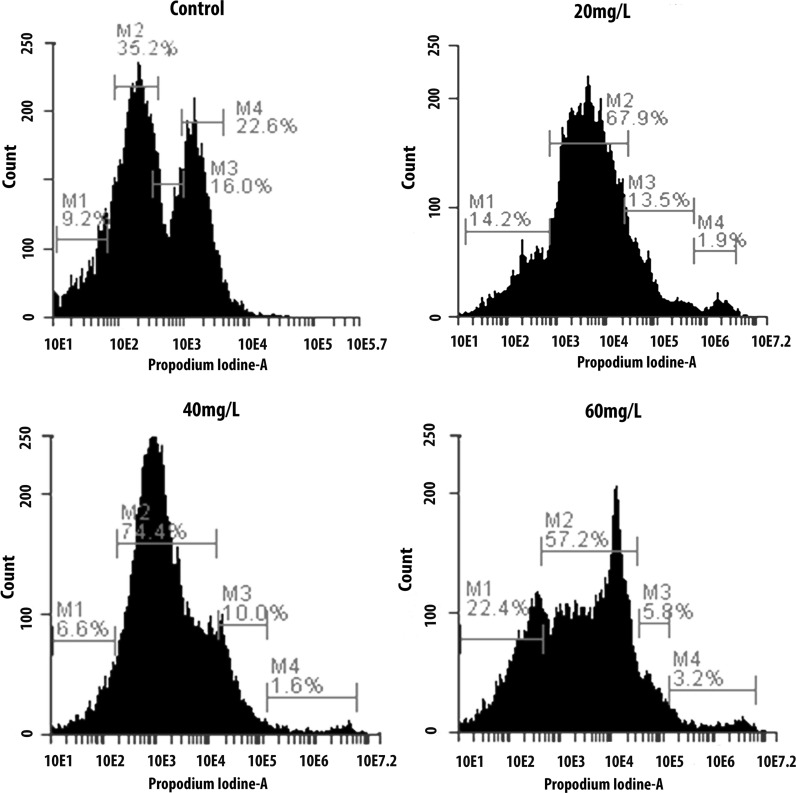
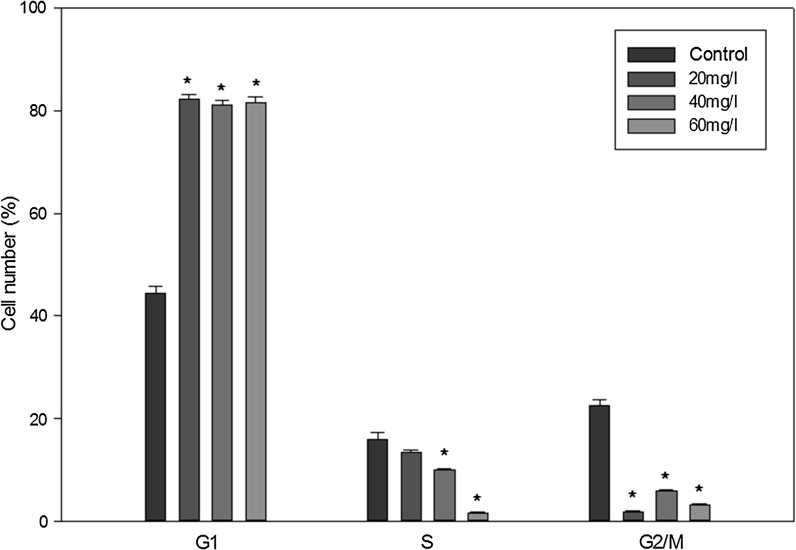
Using bioinformatics analysis (Fig. 1a), we found that the cell cycle and its regulation (p value = 4.320e−25, FDR = 2.679e−23) might play a crucial part in the indoxyl sulfate-induced microarray dataset. Therefore, we employed flow cytometry to explore the effect of indoxyl sulfate on ADMSCs. In this study, we also assessed the influences of indoxyl sulfate on cell cycle arrest using flow cytometry after 15 days of treatment (Figs. 5, 6). Our data showed an increment in the proportion of cells arrested in the G1 phase (p < 0.05; 43 % in the indoxyl sulfate-free condition, 82.5 % in the indoxyl sulfate 20 mg/l condition, 80 % in the indoxyl sulfate 40 mg/l condition, and 81.6 % in the indoxyl sulfate 60 mg/l condition). The data also showed a significant decrease in the proportion of cells entering the S and M phases for cells treated with different indoxyl sulfate concentrations (p < 0.05) compared to the untreated control condition.
Fig. 5.
Cell cycle distribution analysis by flow cytometry. Cells were incubated in different concentration of IS. Ordinate represents cell number while abscissa depicts DNA content. The M1 area denotes Sub G1 phase, the first sharp peak M2 stand for G0/G1 phase cells, and the M3 area indicates S phase cells and the following lower peak M4 shows G2/M phase cells (n = 4). The numbers below each M-area of each figure indicated the percentage of cells in each phase
Fig. 6.
Flow cytometry analysis results of the cell cycle between different groups (n = 4). The results are presented as the ratio of cells in different phases of cell cycle induced by 20, 40, 60 mg/l IS with corresponding control cells (mean ± SD, n = 6 each, *p < 0.05 vs. control group)
Migration wound healing analysis results
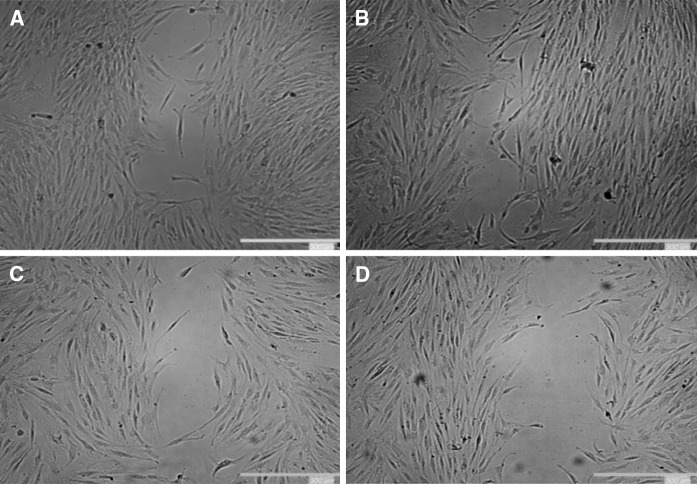
To further analyze the effect of indoxyl sulfate on ADMSCs’ biological functions, its effect on ADMSC migration was studied. As shown in Fig. 7, cell migration was significantly reduced in uremic MSCs compared to the control cells. The effect was also dose-dependent; as indoxyl sulfate levels increased, the cell migration efficiency decreased.
Fig. 7.
Representative images of wound-healing assays. ADMSCs were incubated with different indoxyl sulfate concentration groups. a Control, b 20 mg/l, c 40 mg/l, d 60 mg/l of IS. When the cells are confluent, wound-healing assays were performed as described in the “Materials and methods” section
Transwell migration assay results
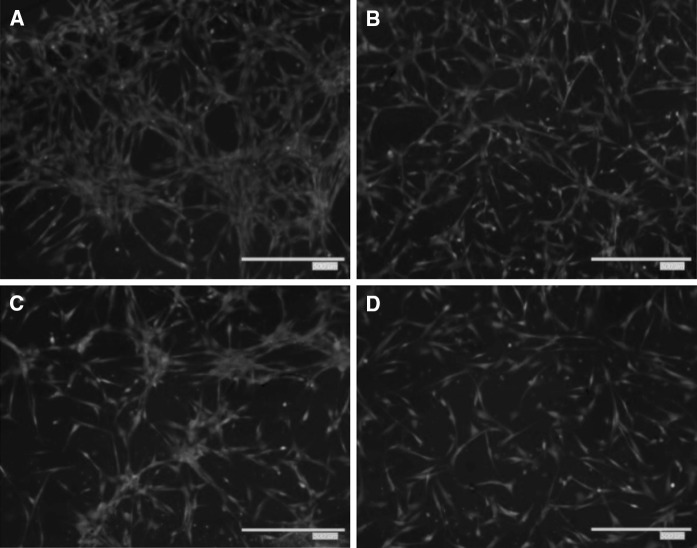
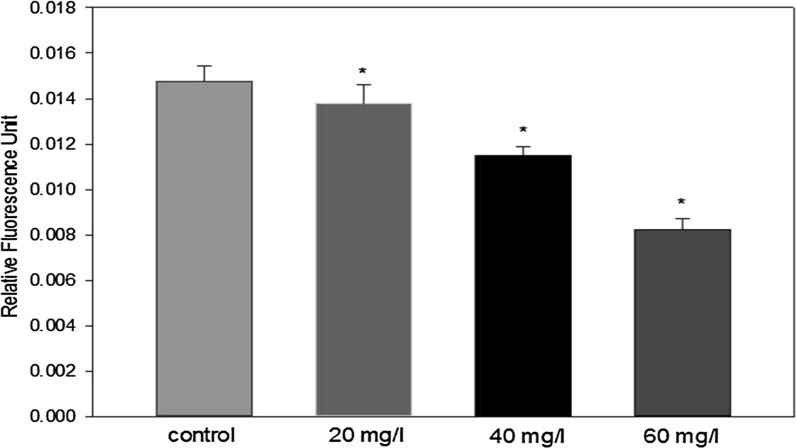
We tested whether indoxyl sulfate diminishes ADMSC migration using transwell migration assays. A significant decline in migration was detected in uremic ADMSCs relative to the control MSCs 24 h after incubation (Figs. 8, 9). This result is similar to the previous result obtained from the wound healing assay and the overall trend of reduced cell proliferation with increased indoxyl sulfate levels.
Fig. 8.
Representative images of transwell migration assay. ADMSCs were seeded on upper transwell migration chamber and the serum-free DMEM medium was added in the lower part for 24 h; (a–d control, 20, 40, 60 mg/l of IS). The cells migrated to the lower chamber and were stained with Calcein-AM as mentioned in “Materials and methods” section. The images of migrated cells were captured after stained with Calcein-AM
Fig. 9.
Effect of different IS concentrations on ADMSCs migration evidenced by the relative fluorescence activity of ADMSCs (n = 7). *p < 0.05. ADMSCs migration was negatively affected by IS in a dose-dependent manner. All groups with IS were characterized by a significantly decreased cell migration ability
Effects of indoxyl sulfate on MEF2 protein expression levels results
These outcomes prove our hypothesis that indoxyl sulfate reduces the proliferation and migration of ADMSCs, induces apoptosis, causes G1 cell cycle arrest, and could eventually lead to decreased kidney regeneration. However, the exact mechanisms of indoxyl sulfate with regard to the biological functions of ADMSCs is still unclear. Because MEF2 plays an important role in mediating diverse cellular progresses, we conducted western blot assays to clarify the protein expression levels of MEF2A and MEF2D.
Our results show that indoxyl sulfate inhibits MEF2A and MEF2D expressions as measured by western blot analysis. MEF2A and MEF2D protein expressions were reduced in a dose-dependent manner. We found that indoxyl sulfate substantially decreased the MEF2A protein expression in ADMSCs treated with 40 and 60 mg/l of indoxyl sulfate, while cells treated with 20 mg/l of indoxyl sulfate showed a slight decrease. As with MEF2A, the MEF2D protein expression of ADMSCs treated with different indoxyl sulfate concentrations was slightly reduced. However, our results showed a stronger expression of MEF2D in comparison with MEF2A (Fig. 10). This might imply that MEF2D in ADMSCs is more common and plays a more crucial role in chronic kidney disease than does MEF2A. Taken together, these results suggest that indoxyl sulfate inhibits the expression of MEF2 family transcription factors in the different stages of chronic kidney disease.
Fig. 10.
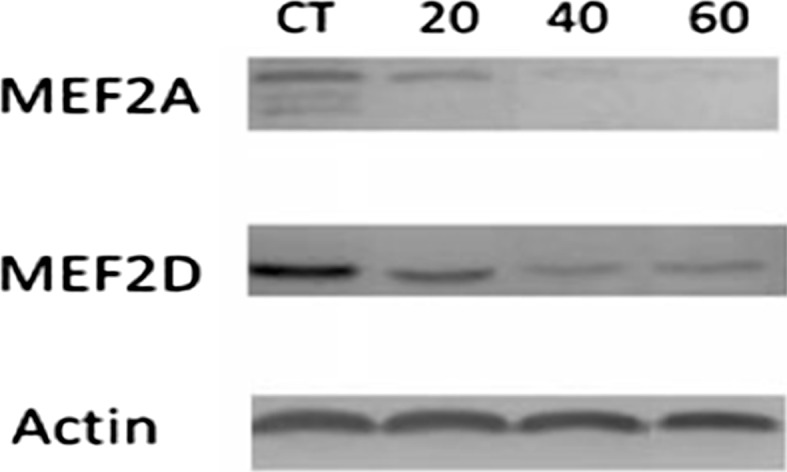
Western blotting analysis of MEF2A and MEF2B under control and uremic ADMSCs conditions. CT control, 20: 20, 40: 40 and 60: 60 mg/l of IS. Actin was employed as loading protein control
Next, to determine the relationship between the L-type calcium channel and the different stages of chronic kidney disease, we examined the expression of the α1S subunit (CACNA1S) L-type calcium channel in ADMSCs treated with different concentrations of indoxyl sulfate. After each treatment, a western blot was performed for the α1S subunit (CACNA1S) protein. Our results demonstrated that the expression of the α1S subunit (CACNA1S) protein significantly decreased compared to the controls in a dose-dependent manner (Fig. 11). Indoxyl sulfate substantially decreased the CACNA1S protein expression of ADMSCs treated with 40 and 60 mg/l of indoxyl sulfate, while cells treated with 20 mg/l of IS showed a slight decrease. These observations suggest that indoxyl sulfate might block the α1S subunit of VGCCL, which could lead to the promotion of chronic kidney disease. Indoxyl sulfate down-regulates MEF2A, MEF2D, and CACNA1S protein expressions in vitro.
Fig. 11.
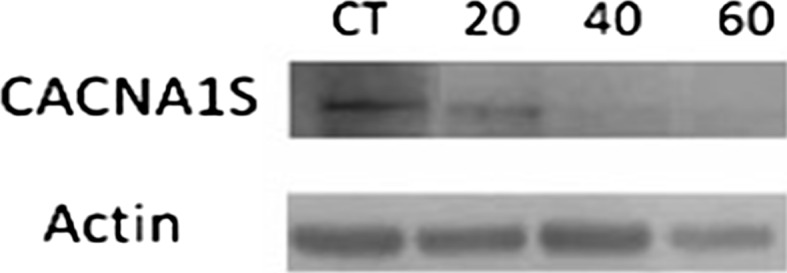
Expression of α-subunit L-type calcium channel upon the treatment of IS. (Upper panel) Representative blots showing the expression of α-subunit L-type calcium channel in ADMSCs upon the treatment in the presence of different concentrations of IS. Actin was used as protein loading control
Discussion
Stem cells’ potential benefits in regenerative medicine have been known for over a decade because of their roles in regeneration and tissue repair (Stocum 2001). MSC treatments for acute myocardial infarction, skeletal muscle regeneration, and acute renal failure (ARF) have provided promising results (Crop et al. 2009; Ferrari et al. 1998; Poulsom et al. 2001; Semedo et al. 2007). Furthermore, the positive outcomes obtained from MSC treatments have led to a focus on kidney regeneration. Surprisingly, the current study revealed that indoxyl sulfate induces the functional incompetence of ADMSCs in the different stages of chronic kidney disease through the EMF2 protein family and VGCCL. Previous evidence demonstrated that indoxyl sulfate suppresses endothelial cell proliferation, wound repair (Dou et al. 2004), and other metabolic activities in a time- and dose-dependent manner (Ying et al. 2010). A recent study showed that indoxyl sulfate stimulates chronic kidney disease progression (Lin et al. 2011), and one study indicated that indoxyl sulfate may cause heart diseases (Barreto et al. 2009). However, a detailed mechanism of CaMK signaling in indoxyl sulfate-induced pathways is still largely unknown. Thus, we collected gene expression data from GSE34259 and utilized Metacore software to reanalyze potential signaling in indoxyl sulfate-induced human cells. We found that CaMK signaling is the most significant pathway for indoxyl sulfate regulation. The goal of this study is to determine the target proteins involved in indoxyl sulfate-induced chronic kidney disease and the consequent effects of this malfunction on the cardiac system. Data regarding the signal transduction pathways of two target proteins (MEF2A and MEF2D) that are involved in CaMK signaling also represent a novel finding obtained by this research. Our results indicated that accumulation of indoxyl sulfate, which is the most important protein-bound compound in chronic kidney disease, considerably induces the functional incompetence of human ADMSCs.
A recent study reported that indoxyl sulfate decreased bone marrow-derived stromal cell proliferation capacities after 48 h of treatment (Noh et al. 2011). Our results extend this finding by monitoring the influences of indoxyl sulfate on ADMSCs with a longer treatment period (15 days). We found that the dose-dependent manner and the proliferation capacity significantly decreased in the presence of uremic indoxyl sulfate concentrations when compared to the control condition. On the other hand, we observed an increase in the proportion of apoptotic cells using flow cytometry after 15 days of treatment, which also confirmed the inhibitory effect of indoxyl sulfate on cell proliferation. In addition, our results show that indoxyl sulfate inhibited the cell cycle progression and caused the arrest of the ADMSC cell cycle in the G1 phase. The expressions of the p21 and p27 proteins, which are involved in regulating cell cycle progression (Gorenne et al. 2006), were reduced by the presence of indoxyl sulfate (Mozar et al. 2011). Here, we found that the protein expressions of MEF2A and MEF2D were increased in comparison with the control samples. Han and Prywes (1995) suggested that MEF2 was an important downstream effector involved in mitogenic signaling pathways through the serum-inducible expression of C-JUN. This could help explain why the cell cycle arrest of ADMSCs increased under uremic conditions. Decreased cell proliferation, apoptosis induction, cell cycle arrest, and compromised migratory capacity are some characteristics of stem cell incompetence. Taken together, our results support the hypothesis that the accumulation of indoxyl sulfate in chronic kidney disease stages substantially impairs the function of ADMSCs.
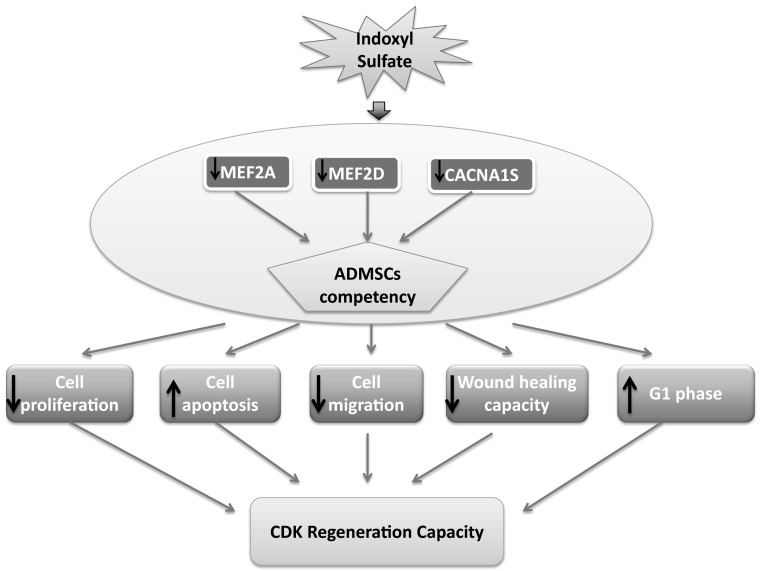
Many studies have demonstrated that the mRNA of the α1 subunit VGCCL is expressed in undifferentiated hMSCs (Graf et al. 2005; Heubach et al. 2004; Zahanich et al. 2005). Thus, we aimed to conduct a western blot analysis to investigate the protein expression levels of the α1S (CACNA1S) subunit VGCCL under different uremic conditions. Our results show that the expression of CACNA1S is highly decreased compared to that of the control condition. These observations showed the same trend as those seen for MEF2A and MEF2D protein expression. Furthermore, a recent study suggested that the MEF2 family of transcription factors plays an important role in regulating the genes responsible for cell proliferation, differentiation, and death by linking to calcium-dependent signaling pathways. Hence, the MEF2 protein family could associate with L-type calcium channels to regulate cell behavior and enhance wound healing (Fig. 12). This information might help researchers to interpret or elucidate the important fundamental and molecular mechanisms leading to changes in ADMSCs induced by indoxyl sulfate.
Fig. 12.
Schematic summary of IS induced ADMSCs functionally incompetence via MEF2A, MEF2D and CACNA1S. The malfunction of ADMSCs subsequently resulted in declined the capacity of stem cells proliferation, migration and wound healing power. Meanwhile, cell apoptosis process has been progressively escalated concomitant with cells blocked at G1 phase. These features eventually hold back the regeneration capacity of chronic kidney disease patients
In conclusion, our study demonstrated that VGCCL is related to the functional activities of ADMSCs in chronic kidney disease patients. Indoxyl sulfate induces the functional incompetence of ADMSCs in the different stages of chronic kidney disease through the EMF2 protein family and VGCCL. Moreover, the α1S subunit might be a key player in controlling VGCCL. These findings provide important new insights and a novel treatment for chronic kidney disease patients. On the other hand, these novel observations could draw research attention to a mechanism that could result in ADMSC dysfunction under uremic conditions and utilize the prospective functions of Ca2+ channels as a target for controlling MSCs in kidney regeneration. The current work could provide a better approach for the treatment of chronic kidney disease, thereby improving the quality of life of patients with chronic kidney disease.
Acknowledgments
Computational analyses and data mining were performed using the system provided by the Bioinformatics Core at the National Cheng Kung University, which is supported by the National Science Council, Taiwan. We thank the National Science Council (NSC) of the Executive Yuan, Taiwan [NSC 101-2320-B-034-001; NSC 102-2325-B-400-005; NSC 101-2311-B-400-005-MY3; NSC 104-2320-B-034-003] for their support and the Grant Funding. We also thank Ministry of Science and Technology for the Grant MOST103-2325-B006-012 and 104-2917-I-006-002. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. We also would like to thank Dr. Yung-Kai Lin for his technical support.
Abbreviations
- ADMSCs
Adipose-derived mesenchymal stem cells
- IS
Indoxyl sulfate
- CKD
Chronic kidney disease
Footnotes
Duyen Thi Do and Nam Nhut Phan have contributed equally to this work.
References
- Ai X, Curran JW, Shannon TR, Bers DM, Pogwizd SM. Ca2+/calmodulin-dependent protein kinase modulates cardiac ryanodine receptor phosphorylation and sarcoplasmic reticulum Ca2+ leak in heart failure. Circ Res. 2005;97:1314–1322. doi: 10.1161/01.RES.0000194329.41863.89. [DOI] [PubMed] [Google Scholar]
- Ardhanari S, Alpert MA, Aggarwal K. Cardiovascular disease in chronic kidney disease: risk factors, pathogenesis, and prevention. Adv Perit Dial. 2014;30:40–53. [PubMed] [Google Scholar]
- Barreto FC, Barreto DV, Liabeuf S, Meert N, Glorieux G, Temmar M, Choukroun G, Vanholder R, Massy ZA, European Uremic Toxin Work Group (EUTox) Serum indoxyl sulfate is associated with vascular disease and mortality in chronic kidney disease patients. Clin J Am Soc Nephrol. 2009;4:1551–1558. doi: 10.2215/CJN.03980609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becchetti A, Arcangeli A (eds) (2010) Integrins and ion channels in cell migration: implications for neuronal development, wound healing and metastatic spread. In: Integrins and ion channels. Springer, New York, pp 107–123 [DOI] [PubMed]
- Cao X-S, Chen J, Zou JZ, Zhong YH, Teng J, Ji J, Chen ZW, Liu ZH, Shen B, Nie YX, Lv WL, Xiang FF, Tan X, Ding XQ. Association of indoxyl sulfate with heart failure among patients on hemodialysis. Clin J Am Soc Nephrol. 2014;10:111–119. doi: 10.2215/CJN.04730514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crop MJ, Baan CC, Korevaar SS, Ijzermans JN, Alwayn IP, Weimar W, Hoogduijn MJ. Donor-derived mesenchymal stem cells suppress alloreactivity of kidney transplant patients. Transplantation. 2009;87:896–906. doi: 10.1097/TP.0b013e31819b3d72. [DOI] [PubMed] [Google Scholar]
- Dou L, Bertrand E, Cerini C, Faure V, Sampol J, Vanholder R, Berland Y, Brunet P. The uremic solutes p-cresol and indoxyl sulfate inhibit endothelial proliferation and wound repair. Kidney Int. 2004;65:442–451. doi: 10.1111/j.1523-1755.2004.00399.x. [DOI] [PubMed] [Google Scholar]
- Duranton F, Cohen G, De Smet R, Rodriguez M, Jankowski J, Vanholder R, Argiles A, European Uremic Toxin Work Group Normal and pathologic concentrations of uremic toxins. J Am Soc Nephrol. 2012;23:1258–1270. doi: 10.1681/ASN.2011121175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernst O, Zor T. Linearization of the bradford protein assay. J Vis Exp. 2010;38:1918. doi: 10.3791/1918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrari G, Angelis D, Coletta M, Paolucci E, Stornaiuolo A, Cossu G, Mavilio F. Muscle regeneration by bone marrow-derived myogenic progenitors. Science. 1998;279:1528–1530. doi: 10.1126/science.279.5356.1528. [DOI] [PubMed] [Google Scholar]
- Gimble JM, Nuttall ME. Adipose-derived stromal/stem cells (ASC) in regenerative medicine: pharmaceutical applications. Curr Pharm Des. 2011;17:332–339. doi: 10.2174/138161211795164220. [DOI] [PubMed] [Google Scholar]
- Gorenne I, Kavurma M, Scott S, Bennett M. Vascular smooth muscle cell senescence in atherosclerosis. Cardiovasc Res. 2006;72:9–17. doi: 10.1016/j.cardiores.2006.06.004. [DOI] [PubMed] [Google Scholar]
- Graf EM, et al. Tissue distribution of a human Cav 1.2 α1 subunit splice variant with a 75bp insertion. Cell Calcium. 2005;38:11–21. doi: 10.1016/j.ceca.2005.03.005. [DOI] [PubMed] [Google Scholar]
- Han T-H, Prywes R. Regulatory role of MEF2D in serum induction of the c-jun promoter. Mol Cell Biol. 1995;15:2907–2915. doi: 10.1128/MCB.15.6.2907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen PB. Functional importance of T-type voltage-gated calcium channels in the cardiovascular and renal system-news from the world of knock-out mice. Am J Physiol Regul Integr Comp Physiol. 2014;308:R227–R237. doi: 10.1152/ajpregu.00276.2014. [DOI] [PubMed] [Google Scholar]
- Heubach JF, Graf EM, Leutheuser J, Bock M, Balana B, Zahanich I, Christ T, Boxberger S, Wettwer E, Ravens U. Electrophysiological properties of human mesenchymal stem cells. J Physiol. 2004;554:659–672. doi: 10.1113/jphysiol.2003.055806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphreys BD, Bonventre JV. Mesenchymal stem cells in acute kidney injury. Annu Rev Med. 2008;59:311–325. doi: 10.1146/annurev.med.59.061506.154239. [DOI] [PubMed] [Google Scholar]
- Jie KE, Zaikova MA, Bergevoet MW, Westerweel PE, Rastmanesh M, Blankestijn PJ, Boer WH, Braam B, Verhaar MC. Progenitor cells and vascular function are impaired in patients with chronic kidney disease. Nephrol Dial Transplant. 2010;25:1875–1882. doi: 10.1093/ndt/gfp749. [DOI] [PubMed] [Google Scholar]
- Lang F, Föller M, Lang KS, Lang PA, Ritter M, Gulbins E, Vereninov A, Huber SM. Ion channels in cell proliferation and apoptotic cell death. J Membr Biol. 2005;205:147–157. doi: 10.1007/s00232-005-0780-5. [DOI] [PubMed] [Google Scholar]
- Li M, Xiong Z-G. Ion channels as targets for cancer therapyI. Int J Physiol Pathophysiol Pharm. 2011;3:156–166. [PMC free article] [PubMed] [Google Scholar]
- Lin CJ, Chen HH, Pan CF, Chuang CK, Wang TJ, Sun FJ, Wu CJ. p-Cresylsulfate and indoxyl sulfate level at different stages of chronic kidney disease. J Clin Lab Anal. 2011;25:191–197. doi: 10.1002/jcla.20456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacFarlane SN, Sontheimer H. Changes in ion channel expression accompany cell cycle progression of spinal cord astrocytes. Glia. 2000;30:39–48. doi: 10.1002/(SICI)1098-1136(200003)30:1<39::AID-GLIA5>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- Maier LS, Bers DM. Role of Ca2+/calmodulin-dependent protein kinase (CaMK) in excitation–contraction coupling in the heart. Cardiovasc Res. 2007;73:631–640. doi: 10.1016/j.cardiores.2006.11.005. [DOI] [PubMed] [Google Scholar]
- McKinsey TA, Zhang CL, Olson EN. MEF2: a calcium-dependent regulator of cell division, differentiation and death. Trends Biochem Sci. 2002;27:40–47. doi: 10.1016/S0968-0004(01)02031-X. [DOI] [PubMed] [Google Scholar]
- Mozar A, Louvet L, Morlière P, Godin C, Boudot C, Kamel S, Drüeke TB, Massy ZA. Uremic toxin indoxyl sulfate inhibits human vascular smooth muscle cell proliferation. Ther Apher Dial. 2011;15:135–139. doi: 10.1111/j.1744-9987.2010.00885.x. [DOI] [PubMed] [Google Scholar]
- Noh H, Yu MR, Kim HJ, Jeon JS, Kwon SH, Jin SY, Lee J, Jang J, Park JO, Ziyadeh F, Han DC, Lee HB. Uremia induces functional incompetence of bone marrow-derived stromal cells. Nephrol Dial Transplant. 2011 doi: 10.1093/ndt/gfr267. [DOI] [PubMed] [Google Scholar]
- Nugent RA, Fathima SF, Feigl AB, Chyung D. The burden of chronic kidney disease on developing nations: a 21st century challenge in global health. Nephron Clin Pract. 2011;118:c269–c277. doi: 10.1159/000321382. [DOI] [PubMed] [Google Scholar]
- Pardo LA. Voltage-gated potassium channels in cell proliferation. Physiology. 2004;19:285–292. doi: 10.1152/physiol.00011.2004. [DOI] [PubMed] [Google Scholar]
- Patel AJ, Lazdunski M. The 2P-domain K+ channels: role in apoptosis and tumorigenesis. Pflügers Arch. 2004;448:261–273. doi: 10.1007/s00424-004-1255-8. [DOI] [PubMed] [Google Scholar]
- Poulsom R, Forbes SJ, Hodivala-Dilke K, Ryan E, Wyles S, Navaratnarasah S, Jeffery R, Hunt T, Alison M, Cook T, Pusey C, Wright NA. Bone marrow contributes to renal parenchymal turnover and regeneration. J Pathol. 2001;195:229–235. doi: 10.1002/path.976. [DOI] [PubMed] [Google Scholar]
- Salem HK, Thiemermann C. Mesenchymal stromal cells: current understanding and clinical status. Stem Cells. 2010;28:585–596. doi: 10.1002/stem.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semedo P, Wang PM, Andreucci TH, Cenedeze MA, Teixeira VP, Reis MA, Pacheco-Silva A, Câmara NO (2007) Mesenchymal stem cells ameliorate tissue damages triggered by renal ischemia and reperfusion injury. Transplant Proc 39:421–423. doi:10.1016/j.transproceed.2007.01.036 [DOI] [PubMed]
- Stocum DL. Stem cells in regenerative biology and medicine. Wound Repair Regen. 2001;9:429–442. doi: 10.1046/j.1524-475x.2001.00429.x. [DOI] [PubMed] [Google Scholar]
- Tonelli M, Riella MC. Chronic kidney disease and the aging population. Kidney Int. 2014;85:487–491. doi: 10.1038/ki.2013.467. [DOI] [PubMed] [Google Scholar]
- Vanholder R, De Smet R. Pathophysiologic effects of uremic retention solutes. J Am Soc Nephrol. 1999;10:1815–1823. doi: 10.1681/ASN.V1081815. [DOI] [PubMed] [Google Scholar]
- Vanholder R, De Smet R, Glorieux G, Argilés A, Baurmeister U, Brunet P, Clark W, Cohen G, De Deyn PP, Deppisch R, Descamps-Latscha B, Henle T, Jörres A, Lemke HD, Massy ZA, Passlick-Deetjen J, Rodriguez M, Stegmayr B, Stenvinkel P, Tetta C, Wanner C, Zidek W, European Uremic Toxin Work Group (EUTox) Review on uremic toxins: classification, concentration, and interindividual variability. Kidney Int. 2003;63:1934–1943. doi: 10.1046/j.1523-1755.2003.00924.x. [DOI] [PubMed] [Google Scholar]
- Vanholder R, Massy Z, Argiles A, Spasovski G, Verbeke F, Lameire N, European Uremic Toxin Work Group Chronic kidney disease as cause of cardiovascular morbidity and mortality. Nephrol Dial Transplant. 2005;20:1048–1056. doi: 10.1093/ndt/gfh813. [DOI] [PubMed] [Google Scholar]
- Wen L, Wang Y, Wang H, Kong L, Zhang L, Chen X, Ding Y. L-type calcium channels play a crucial role in the proliferation and osteogenic differentiation of bone marrow mesenchymal stem cells. Biochem Biophys Res Commun. 2012;424:439–445. doi: 10.1016/j.bbrc.2012.06.128. [DOI] [PubMed] [Google Scholar]
- Wu I-W, Hsu KH, Lee CC, Sun CY, Hsu HJ, Tsai CJ, Tzen CY, Wang YC, Lin CY, Wu MS. p-Cresyl sulphate and indoxyl sulphate predict progression of chronic kidney disease. Nephrol Dial Transplant. 2011;26:938–947. doi: 10.1093/ndt/gfq580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Q, Wu Z. The insulin-like growth factor-phosphatidylinositol 3-kinase-Akt signaling pathway regulates myogenin expression in normal myogenic cells but not in rhabdomyosarcoma-derived RD cells. J Biol Chem. 2000;275:36750–36757. doi: 10.1074/jbc.M005030200. [DOI] [PubMed] [Google Scholar]
- Ying Y, Yang K, Liu Y, Chen QJ, Shen WF, Lu L, Zhang RY. A uremic solute, P-cresol, inhibits the proliferation of endothelial progenitor cells via the p38 pathway. Circ J. 2010;75:2252–2259. doi: 10.1253/circj.CJ-11-0046. [DOI] [PubMed] [Google Scholar]
- Zahanich I, Graf EM, Heubach JF, Hempel U, Boxberger S, Ravens U. Molecular and functional expression of voltage-operated calcium channels during osteogenic differentiation of human mesenchymal stem cells. J Bone Miner Res. 2005;20:1637–1646. doi: 10.1359/JBMR.050521. [DOI] [PubMed] [Google Scholar]
- Zor T, Seliger Z. Linearization of the Bradford protein assay increases its sensitivity: theoretical and experimental studies. Anal Biochem. 1996;236:302–308. doi: 10.1006/abio.1996.0171. [DOI] [PubMed] [Google Scholar]