Abstract
This study evaluated the ability of human osteoprotegerin gene-modified autologous periodontal ligament cells (PDLCs) in combination with cell transplantation to promote periodontal regeneration in beagle dogs. Adenovirus Ad5-hOPG-EGFP-transfected PDLCs and BME-10X collagen membranes were fabricated and used for periodontal repair. Buccal periodontal defects (mesiodistal width × depth: 5 × 5 mm) were created on the second, third, and fourth mandibular premolars in six normal beagle dogs, and the defects were histologically and histomorphometrically assessed for periodontal regeneration in the following four groups: (1) hOPG-PDLCs + BME-10X, (2) mock-PDLCs + BME-10X, (3) PDLCs + BME-10X, and (4) BME-10X. The radiographic and histological results suggested that hOPG-PDLCs significantly promoted periodontal defect repair. This study demonstrates the potential of hOPG-modified PDLCs for periodontal tissue regeneration.
Keywords: OPG, Periodontal tissue defects, Gene therapy, Tissue regeneration
Introduction
Periodontal disease, which is one of the most common oral chronic inflammatory infectious diseases, is initiated by the host’s immune response to bacterial biofilms that accumulate on tooth root surfaces (Page et al. 1978). If left untreated, periodontal disease can result in irreversible destruction of the periodontal supporting tissues, including alveolar bone, cementum and the periodontal ligament. Severe periodontal disease may increase a patient’s risk for chronic systemic disorders, such as diabetes (Mealey and Rethman 2003) rheumatoid arthritis (Mercado et al. 2000), adverse pregnancy outcomes (Dörtbudak et al. 2005) and cardiovascular complications (Beck et al. 1998). Consequently, periodontal disease significantly impacts the patient’s quality of life and financially constitutes a substantial public health burden worldwide.
Osteoprotegerin (OPG) and receptor activator of nuclear factor-κB ligand (RANKL), which are produced by osteoblasts, are the functional factors that directly control osteoclast generation and regulate bone metabolism (Silness and Löe 1964). The main function of RANKL is to inhibit the differentiation of osteoclasts and mature osteoclast activity and to induce apoptosis, which plays an important role in maintaining the balance between bone resorption and bone formation. In this study, we implanted hOPG gene-modified autologous periodontal ligament cells (PDLCs) with a BME-10X collagen membrane scaffold into artificial periodontal defects (class II furcation area) created in beagle dogs, and regeneration was assessed by histological observation and histomorphometric analysis.
Materials and methods
Materials
The following materials were used: hOPG recombinant adenovirus Ad5-hOPG-EGFP (The Benyuan Zhengyang Gene Technology Co., Ltd., Beijing, China); DMEM-LG medium (Gibco, Gaithersburg, MD, USA); HyClone fetal bovine serum (HyClone, Logan, UT, USA); trypsin (Sigma, St. Louis, MO, USA); medical tissue-guided regeneration collagen membrane BME-10X (Biomedical Engineering Research Institute, Chinese Academy of Medical Sciences, Tianjin, China); an Olympus inverted phase-contrast fluorescence microscope (with camera system) (Olympus, Tokyo, Japan); and a BLX-8-type high-frequency oral X-ray machine (Tianjie Electronic Co., Ltd., Zhengzhou, China); Canine (dog) Osteoprotegerin, OPG ELISA Kit (BIOTANG Inc., Lexington, MA, USA).
Animals
Six male beagle dogs, 6–8 months of age and weighing between 13 and 15 kg, were used in this study. The experimental protocol followed the institutional Animal Use and Care regulations of the Fujian Animal Management Committee.
Thirty-six class II furcation defects were randomly assigned to 4 treatment groups: hOPG-PDLCs + BME-10X (recombinant adenovirus Ad5-hOPG-EGFP transfected PDLCs + BME-10X membrane); mock-PDLCs + BME-10X (mock-transfected PDLCs + BME-10X membrane); PDLCs + BME-10X (untransfected PDLCs + BME-10X membrane); and BME-10X (BME-10X membrane only).
Immunocytochemical staining
The third-passage PDLCs were cultured until influence on the cover slips in a 6-well plate, and then fixed with 2 % paraformaldehyde for 30 min at room temperature. Vimentin staining and keratin staining were performed according to the protocol in the kit, and photographed under a microscope.
Preparation of hOPG-PDLCs
PDLCs were isolated from front teeth extracted during the furcation preparation and were cultured in DMEM. Third-passage PDLCs (1 × 105 cells/well) were seeded in a 6-well plate and cultured at 37 °C in a humidified 95 % air/5 % CO2 atmosphere. The hOPG transfection with recombinant adenovirus Ad5-hOPG-EGFP was performed according to the manufacturer’s protocol.
Enzyme-linked immunosorbent assay
After the recombinant adenovirus Ad5-hOPG-EGFP transfection, an enzyme-linked immunosorbent assay (ELISA) was used to assay the OPG expression of three groups: hOPG-PDLCs, Mock-PDLCs and PDLCs. After 72 h culture, OPG concentrations in cell-free supernatants were determined using (dog) OPG ELISA Kit according to the manufacturer’s protocols. The optical densities of each well were read at 450 nm.
Preparation of the PDLC and BME-10X membrane composite
Prior to cell implantation, the sterilized collagen membrane was trimmed to 4 × 4 mm and pre-wetted with DMEM-LG in a Petri dish for 4 h. The PDLCs were seeded on the BME-10X membrane at a density of 1 × 106 cells per 4 × 4 mm.
Surgical procedure
The surgical procedures were performed under general anesthesia with ketamine and droperidol and local anesthesia with lidocaine hydrochloride. U-shaped defects measuring 5 mm apico-coronally, 4 mm mesiodistally and 3 mm buccolingually were surgically created in the mandibular second, third premolars and fourth premolars. The root surface facing the defect was planed to remove the periodontal ligament using Gracey curettes. 6 weeks after the defects were prepared, the full-thickness flap was evaluated to remove the granulation tissue, cementum and gutta-percha and to establish notches at the base of the defects using curettes. The e-PTFE membranes were trimmed to completely cover the defects and extend 2 mm beyond the margin. 12 weeks following the placement surgery, the animals were sacrificed to harvest the specimens. Subsequently, the specimens were fixed in 10 % buffered formalin and embedded in paraffin for morphological analysis.
Histomorphometric analysis
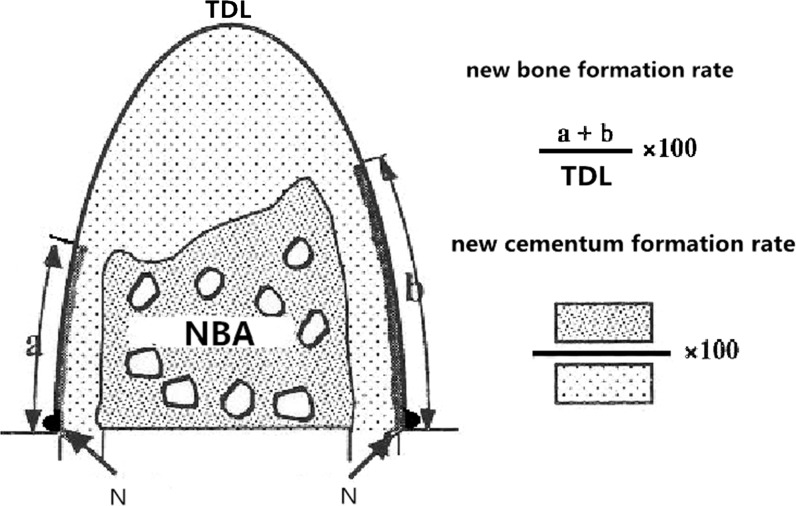
Five hematoxylin and eosin (HE)-stained sections were selected from each specimen for histomorphometric analysis using a software package (Image-Pro Plus 6.0). The histomorphometric measurements included NCL (newly formed cementum length), which represented the length of root surface that was covered by newly formed cementum on the mesial and distal roots; NBA (newly formed alveolar bone area), which represented the area that was filled with newly formed alveolar bone; total defect length (TFL), which represented the distance between the notches on the mesial and distal roots; and total defect area (TDA), which represented the TDA between the notches on the mesial and distal roots (Fig. 1).
Fig. 1.
Schematic diagram for histological processing. N, notch on the root surface. TDL total defect length. a, b Newly formed cementum length. TDA total defect area, NBA newly formed alveolar bone area
Statistical analysis
The means and standard deviations of each parameter were calculated for each group. Differences among groups were analyzed using one-way analysis of variance (ANOVA). All statistical procedures were performed using a software package (SPSS 17.0, Chicago, IL, USA).
Results
Histological observation of PDLCs
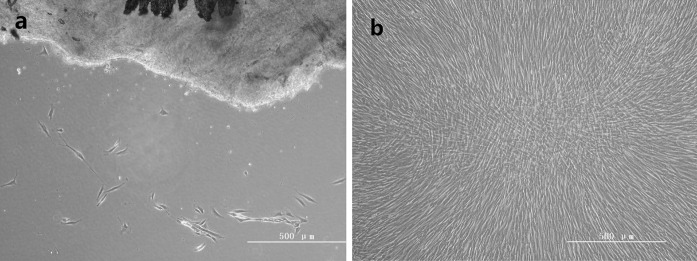
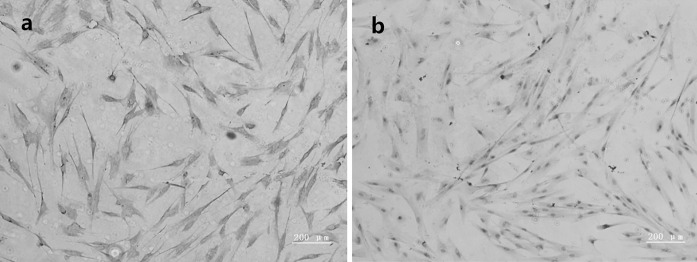
Immunohistochemical staining for vimentin and keratin were used to identify the histological origin of PDLCs (Fig. 2). PDLCs were spindle-shaped and expressed positive stain of vimentin and negative of keratin (Fig. 3).
Fig. 2.
Microscopic features of PDLCs. a Spindle-shaped PDLCs emigrate from periodontal tissue (HE, original magnification ×100). b Palisade-like arrangement of epithelioid cells (HE, original magnification ×100)
Fig. 3.
Immunocytochemical staining on the primary PDLCs. a Positive staining for vimentin (×100); b negative staining for keratin (×100)
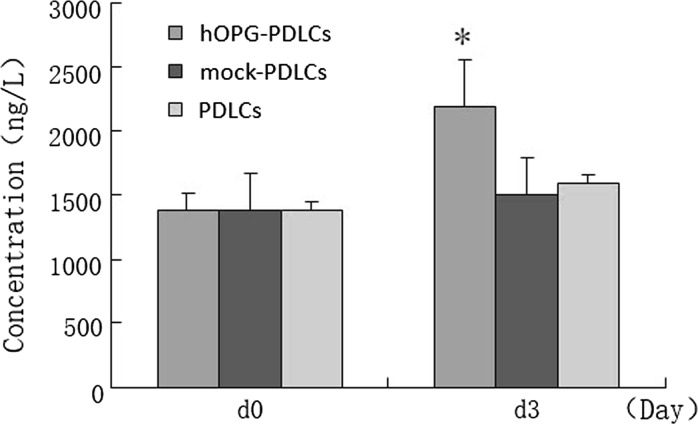
OPG expression
72 h after the transfection, OPG concentration of hOPG-PDLCs increased significantly than that in mock-PDLCs and PDLCs groups. There were no significant increase of OPG concentration in mock-PDLCs or PDLCs groups from d0 to d3 (Fig. 4).
Fig. 4.
OPG expression in cell-free supernatants
Clinical findings
6 weeks after the defects were created, the flap was raised again to expose the surgically created defects. Granulation tissue filled the defects, and bone regeneration was not observed after removing the granulation tissue. 4 weeks after the placement surgery, the sutures and e-PTFE membranes fell off naturally, and the wound healed well. Approximately 1–2 mm of gingival recession was observed in some defect areas.
Radiographic results
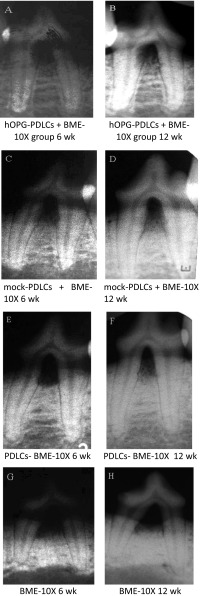
Dental radiographs were used to assess the bone formation of the defects. 6 weeks after the placement surgery, new bone formation was observed in all groups except for the BME-10X group. The differences in bone height among groups were not significant (Fig. 5A, C, E, G).
Fig. 5.
Bone formation of the defects
Compared with the radiographs obtained at 6 weeks, the 12 weeks radiographs displayed newly formed bone of high attenuation that almost filled the furcation defects in the hOPG-PDLCs + BME-10X group (Fig. 5A, B); in contrast, newly formed bone of low attenuation was observed in the mock-PDLCs + BME-10X group (Fig. 5C, D). Little newly formed alveolar bone was observed in the BME-10X group (Fig. 5E, F), and periapical lesions were not observed in any of the four groups (Fig. 5A–H).
Histological observation of the furcation
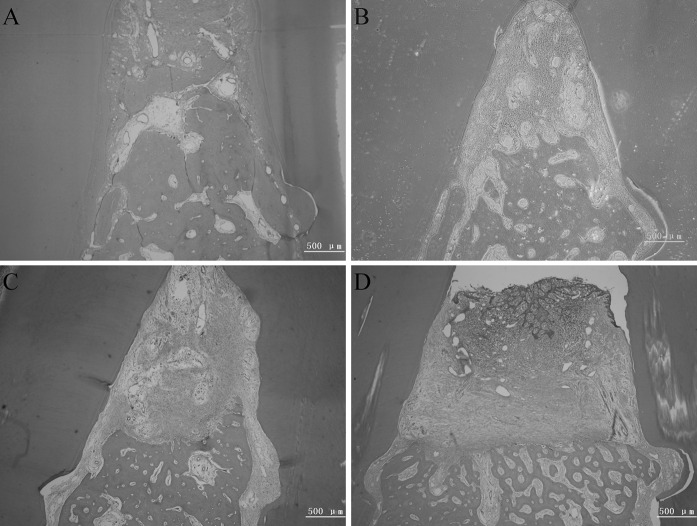
At 12 weeks, the newly formed cementum, alveolar bone and periodontium was distributed non-uniformly in all groups (Fig. 6). In sites that received hOPG-PDLCs + BME-10X, newly formed alveolar bone and cementum-like tissue almost filled the defect (Fig. 6A). No long junctional epithelium, adhesion or root absorption were observed (Fig. 6A–D). Sites that were treated with mock-PDLCs + BME-10X and PDLCs + BME-10X displayed considerable newly formed cementum and alveolar bone and a small amount of connective tissue (Fig. 6B, C). In addition, the BME-10X group showed a small amount of tissue regeneration, and the scaffolds were completely absorbed (Fig. 6D).
Fig. 6.
Representative photomicrographs of periodontal tissue regeneration. The mock-PDLCs + BME-10X group, the hOPG-PDLCs + BME-10X group, and the PDLCs + BME-10X group showed more newly formed alveolar bone, periodontium and new cementum-like tissue. a hOPG-PDLCs + BME-10X, b mock-PDLCs + BME-10X, c PDLCs + BME-10X, and d BME-10X
Histomorphometric analysis
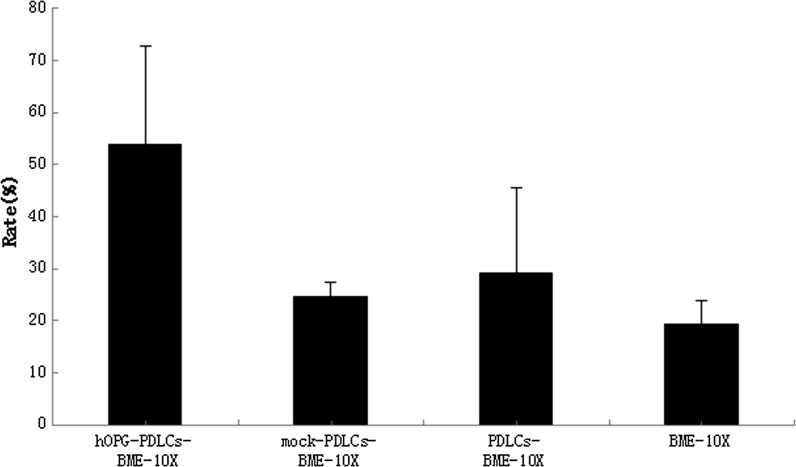
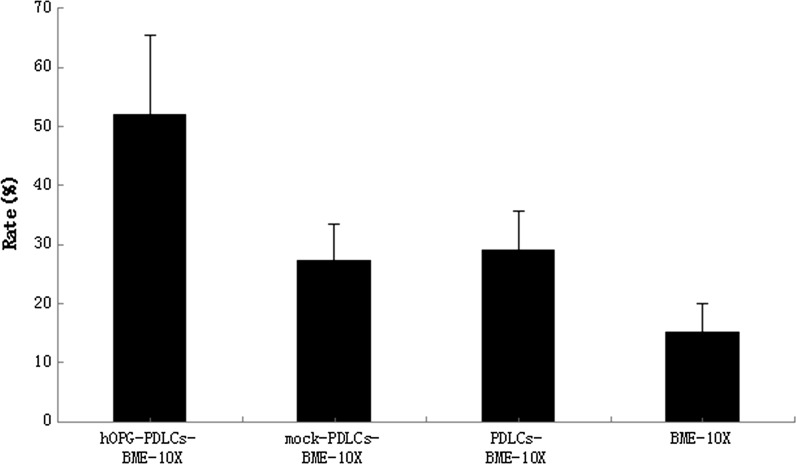
Table 1 and Figure 11 shows that the new cementum formation rate in the hOPG-PDLCs + BME-10X group was higher than that in the other three groups (P < 0.05). No significant differences were observed among the other three groups. In addition, the new bone formation rates of the hOPG-PDLCs + BME-10X group, the mock-PDLCs + BME-10X group, and the PDLCs + BME-10X group were higher than that in the BME-10X group, and this difference was significant (P < 0.05) (Table 1, Fig. 12). The new bone formation rate of the hOPG-PDLCs + BME-10X group was higher than those observed in the mock-PDLCs + BME-10X group and PDLCs + BME-10X group (P < 0.05) (Table 1, Fig. 12).
Table 1.
New cementum formation rate and new bone formation rate (%)
| Group | New cementum formation rate (%) | New bone formation rate (%) |
|---|---|---|
| hOPG-PDLCs + BME-10X group | 53.97 ± 18.93 | 51.91 ± 13.65△ |
| Mock-PDLCs + BME-10X group | 24.68 ± 2.62* | 27.28 ± 6.26#△ |
| PDLCs + BME-10X group | 29.22 ± 16.37* | 29.06 ± 6.63#△ |
| BME-10X group | 19.35 ± 4.31* | 15.19 ± 4.89# |
* P < 0.05; Student’s t test: mock-PDLCs + BME-10X group versus hOPG-PDLCs + BME-10X group; PDLCs + BME-10X group versus hOPG-PDLCs + BME-10X group; BME-10X group versus hOPG-PDLCs + BME-10X group
# P < 0.05; Student’s t test: mock-PDLCs + BME-10X group versus hOPG-PDLCs + BME-10X group; PDLCs + BME-10X group versus hOPG-PDLCs + BME-10X group; BME-10X group versus hOPG-PDLCs + BME-10X group
△ P < 0.05; Student’s t test: hOPG-PDLCs + BME-10X group versus BME-10X group; mock-PDLCs + BME-10X group versus BME-10X group; PDLCs + BME-10X group versus BME-10X group
Fig. 11.
New cementum formation rate
Fig. 12.
New bone formation rate
Bundle bone adjacent to the periodontal ligament and Haversian system were observed in the new alveolar bone (Figs. 7, 8). The new periodontal tissue included the new alveolar bone, new cementum, new periodontal ligament and vessels (Figs. 9, 10).
Fig. 7.
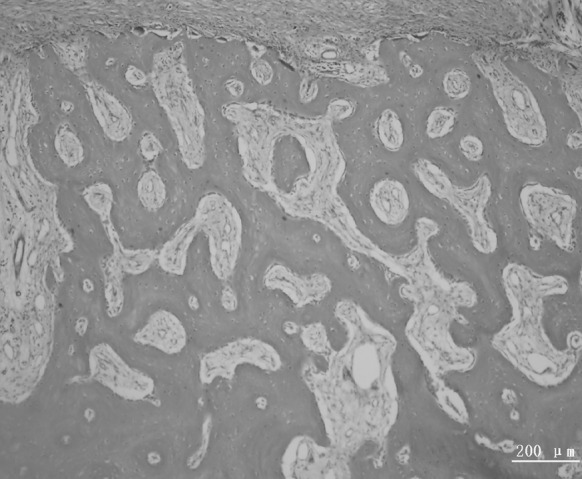
A representative photomicrograph showing bundle bone adjacent to the periodontal ligament
Fig. 8.
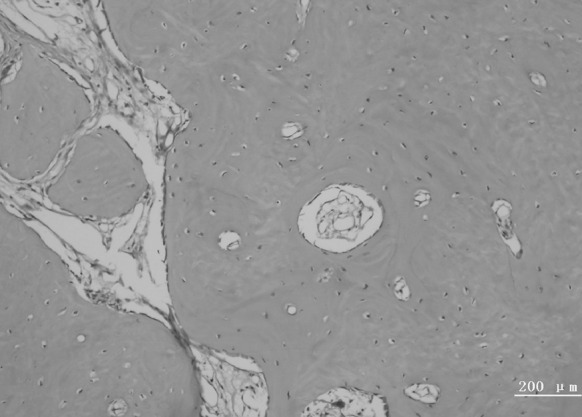
A representative photomicrograph showing the Haversian system of new alveolar bone
Fig. 9.
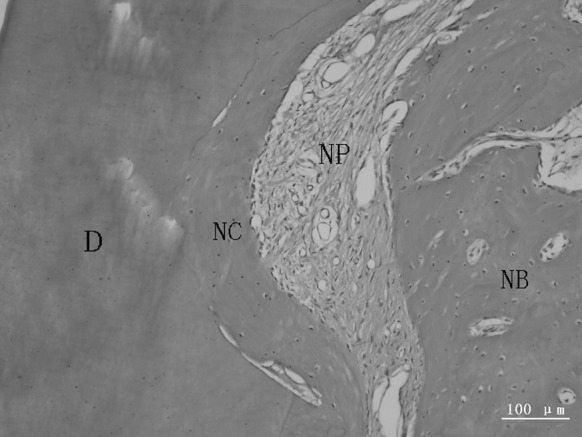
A representative photomicrograph showing new periodontal tissue. The obliquely oriented periodontal ligament contained new vessels. D dentin, NB new alveolar bone, NC new cementum, NP new periodontal ligament
Fig. 10.
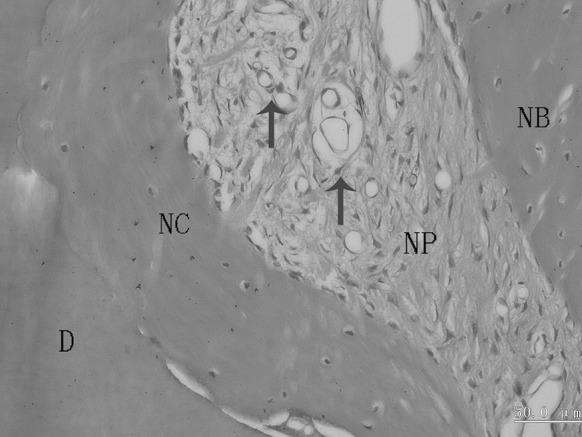
A representative photomicrograph showing a newly formed periodontal ligament. The arrows indicate vessels in the obliquely oriented periodontal ligament. D dentin, NB new alveolar bone, NC new cementum, NP new periodontal ligament
Discussion
Periodontal disease is a common oral disease with a variety of manifestations, the causative agents of which include plaque and biofilm (Ribeiro et al. 2005). Bacterial metabolites, the host inflammatory immune response and other risk factors contribute to periodontal defection (Eke et al. 2012). Without treatment, the loss of alveolar tissue and the loosening of teeth caused by disease progression eventually lead to tooth loss. Periodontitis has become the primary reason for tooth loss in adults (Nibali et al. 2013). The purpose of periodontal treatment is to prevent disease or terminate disease progression, with the ultimate goal of periodontal tissue regeneration. Furcation, a process underlying periodontal disease, is difficult to treat due to the complicated anatomical structure. In particular, the existing treatments, which include initial periodontal therapy, flap surgery, guided tissue regeneration and guided bone regeneration, cannot provide thorough regeneration to undo furcation.
Periodontal regenerative therapy has been developed as a new technology based on tissue engineering and involves seed cells, signaling molecules and scaffolds. Adenoviral vectors are the most commonly used vectors due to their efficient transduction and expression (Villar and Cochran 2010). Previous studies have confirmed the safety and efficiency of adenoviral vectors without any dose-limiting toxicities and adverse reactions (Liu 2006). This study used genetic engineering combined with cell transplantation technology to promote periodontal tissue regeneration in beagle dogs. A scaffold constructed with adenovirus Ad5-hOPG-EGFP-transfected PDLCs obtained from beagle dogs and BME-10X collagen membrane was implanted into the furcation areas, and no deaths or toxic reactions caused by the recombinant adenovirus were observed during the experiment.
OPG, a member of tumor necrosis factor (TNF) receptor superfamily, is the natural soluble receptor for RANKL (Sasaki and Kusano 2006). Osteoblasts are involved in the differentiation of osteoclast cells and secrete RANKL, which can directly promote osteoclast differentiation and activity via binding to mature osteoclasts via nuclear factor-κB (RANK) (Taubman et al. 2005). OPG-binding RANKL and TNF-related apoptosis-inducing ligand (TRAIL) can inhibit the binding of RANKL and RANK and indirectly decrease the differentiation and activity of osteoclasts (Takayanagi 2005). In addition, OPG plays an important role in maintaining the balance between bone resorption and formation; in particular, OPG reduces bone resorption by inhibiting the formation of the F-actin ring in mature osteoclasts or inducing osteoclast apoptosis by interfering with the interaction between stromal cells and osteoclasts (Lacey et al. 1998; Yasuda et al. 1998; Lacey et al. 2000). Therefore, RANKL and OPG are indispensable regulatory molecules for osteoclastogenesis.
During destruction of the alveolar bone, the balance of OPG/RANKL expression is disturbed by microbial invasion (Belibasakis et al. 2005) and periodontal disease (Teng et al. 2000; Lacey et al. 1998), resulting in the increased expression of RANKL and the enhanced formation and activation of osteoclasts. Thus, increasing studies have focused on how OPG and RANKL are regulated in periodontal disease. Bostanci et al. investigated RANKL and OPG mRNA levels in patients with gingivitis, chronic periodontitis and aggressive periodontitis, and their results showed that patients with chronic periodontitis and aggressive periodontitis had higher RANKL levels and RANKL/OPG ratios than healthy individuals. When chronic periodontitis patients were compared to those receiving immunosuppressant therapy, osteoprotegerin, but not RANKL, expression was stronger in the latter group (Bostanci et al. 2007). Crotti et al. analyzed the expression of RANKL and OPG in granulation tissue from alveolar bone absorption sites and the gingival tissue of patients with periodontal disease, and they noted that RANKL expression in periodontal disease was significantly higher than in non-periodontal subjects, while OPG expression was much lower. OPG expression was positive in endothelial cells, and RANKL expression was positive in thymus-dependent cells and macrophages (Crotti et al. 2003). Liu et al. compared the gene expression levels of RANKL and OPG among patients with moderate and advanced periodontitis and healthy subjects using semi-quantitative RT-PCR. The highest level of RANKL mRNA was observed in the advanced periodontitis patients, and the highest OPG mRNA level was observed in the healthy group. These data suggest that an increased ratio of RANKL/OPG may be associated with the activation of osteoclastic bone resorption in periodontitis (Liu 2006).
The increased RANKL/OPG ratio could be regarded as a bioindicator of periodontitis (Bostanci et al. 2007), as this ratio increases steadily after treatment and suggests active bone resorption and possible recurrence of periodontitis. Thus, additional adjuvant treatment strategies are required to control the progression of periodontitis (Belibasakis and Bostanci 2012). The inflammatory cascade of periodontitis eventually leads to osteoclast formation and bone loss through the RANK/RANKL/OPG axis. Cochran suggested that an expected therapeutic target should decrease the RANKL/OPG ratio and reduce bone loss (Cochran 2008). Therefore, some scholars have attempted to reduce alveolar bone loss by increasing OPG secretion; inhibiting RANKL expression; and blocking the proliferation, differentiation and maturation of osteoclasts. Shiba et al. (2000) first determined that the proliferative ability and the expression of OPG correlated with periodontal ligament aging and then identified that enhancing OPG expression and inhibiting RANKL may be an effective method to reduce alveolar bone and tooth loss. Chen et al. (2008) transferred the OPG gene into periodontal tissue with an HVJ envelope-vector delivery system containing the mouse OPG gene, and this treatment resulted in significant inhibition of osteoclastogenesis and alveolar bone resorption in lipopolysaccharide (LPS)-induced experimental periodontal disease. In Jin’s study, 32 rats with experimental ligature-induced periodontitis received human OPG-Fc fusion protein (10 mg/kg) for 6 weeks. Their results showed significant preservation of alveolar bone volume among OPG-Fc-treated animals, suggesting that RANKL inhibition may represent an important therapeutic strategy for preventing progressive alveolar bone resorption. Hence, OPG had been accepted by many scholars as playing a role in periodontal tissue regeneration (Jin et al. 2007). Zhang et al. selected 24 anterior teeth from beagle dogs to construct a periodontitis model using a high-sugar diet and silk ligature. OPG gene-modified bone marrow stromal cells (BMSCs) were implanted into the defects to observe their effect on tissue repair. After 6 weeks, X-ray and histological results indicated that the OPG gene-modified BMSCs significantly promoted repair of the periodontal bone defects (Zhang et al. 2004).
PDLCs were selected as seed cells for cell transplantation because they are the optimal cells for periodontal tissues. Indeed, PDLCs can influence osteoclast formation through the expression of RANKL and OPG (Hasegawa et al. 2002; Kanzaki et al. 2001). PDLCs express OPG and RANKL under physiological conditions but express more OPG than RANKL in the absence of external stimuli, which is not conducive to osteoclast formation. Nevertheless, periodontal pathogens could promote RANKL expression and inhibit OPG expression. PDLCs may also mediate bone metabolism via the OPG/RANKL system. OPG, which is secreted by PDLCs located between the alveolar bone and cementum, may play a certain defensive role in the RANKL-mediated bone resorption of periodontitis.
However, few reports have addressed the combination of PDLCs with OPG in periodontal therapy. In our study, the percentage of new cementum area and the new alveolar length in furcation defects of the hOPG-PDLCs + BME-10X group were significantly greater than those in the other three groups (P < 0.05). The alveolar bone height also increased significantly after treatment, and new cementum-like tissue covered most of the furcation area. No long junctional epithelium, adhesion and root absorption were observed, providing further evidence that the increased OPG content could effectively promote periodontal tissue regeneration, especially alveolar bone regeneration. Thus, hOPG-transfected PDLCs may be used as seed cells for periodontal tissue regeneration, and gene therapy combined with tissue engineering opens up broad prospects for periodontal treatment. However, the clinical application of extrinsic OPG protein will need to overcome certain limitations, related to the in vivo degradation, short biological half-life, toxicity and immune response generated to OPG protein, which remain to be solved in future studies.
Acknowledgments
This study was supported by the International Cooperation Research and Develop Project of Nanjing Health Bureau (No. 201303051), “Six Talent Peaks” of High Level Talent Selection and Training Project of Jiangsu Province (No. 2013-SWYY-006), Research Fund for the Doctoral Program of Higher Education of China (No. 20093518110002) and Key Project of Science and Technology Bureau of Jiangsu Province (No. BL2013002).
References
- Beck JD, Offenbacher S, Williams R, Gibbs P, Garcia R. Periodontitis: a risk factor for coronary heart disease? Ann Periodontol. 1998;3:127–141. doi: 10.1902/annals.1998.3.1.127. [DOI] [PubMed] [Google Scholar]
- Belibasakis GN, Bostanci N. The RANKL-OPG system in clinical periodontology. J Clin Periodontol. 2012;39:239–248. doi: 10.1111/j.1600-051X.2011.01810.x. [DOI] [PubMed] [Google Scholar]
- Belibasakis GN, Johansson A, Wang Y, Chen C, Kalfas S, Lerner U. The cytolethal distending toxin induces receptor activator of NF-κB ligand expression in human gingival fibroblasts and periodontal ligament cells. Infect Immun. 2005;73:342–351. doi: 10.1128/IAI.73.1.342-351.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bostanci N, İlgenli T, Emingil G, Afacan B, Han B, Töz H, Atilla G, Hughes FJ, Belibasakis GN. Gingival crevicular fluid levels of RANKL and OPG in periodontal diseases: implications of their relative ratio. J Clin Periodontol. 2007;34:370–376. doi: 10.1111/j.1600-051X.2007.01061.x. [DOI] [PubMed] [Google Scholar]
- Chen R, Kanzaki H, Chiba M, Nishimura M, Kanzaki R, Igarashi K. Local osteoprotegerin gene transfer to periodontal tissue inhibits lipopolysaccharide-induced alveolar bone resorption. J Periodontal Res. 2008;43:237–245. doi: 10.1111/j.1600-0765.2007.01021.x. [DOI] [PubMed] [Google Scholar]
- Cochran DL. Inflammation and bone loss in periodontal disease. J Periodontol. 2008;79:1569–1576. doi: 10.1902/jop.2008.080233. [DOI] [PubMed] [Google Scholar]
- Crotti T, Smith MD, Hirsch R, Soukoulis S, Weedon H, Capone M, Ahern MJ, Haynes D. Receptor activator NF κB ligand (RANKL) and osteoprotegerin (OPG) protein expression in periodontitis. J Periodontal Res. 2003;38:380–387. doi: 10.1034/j.1600-0765.2003.00615.x. [DOI] [PubMed] [Google Scholar]
- Dörtbudak O, Eberhardt R, Ulm M, Persson GR. Periodontitis, a marker of risk in pregnancy for preterm birth. J Clin Periodontol. 2005;32:45–52. doi: 10.1111/j.1600-051X.2004.00630.x. [DOI] [PubMed] [Google Scholar]
- Eke P, Dye B, Wei L, Thornton-Evans G, Genco R. Prevalence of periodontitis in adults in the United States: 2009 and 2010. J Dent Res. 2012;91:914–920. doi: 10.1177/0022034512457373. [DOI] [PubMed] [Google Scholar]
- Hasegawa T, Yoshimura Y, Kikuiri T, Yawaka Y, Takeyama S, Matsumoto A, Oguchi H, Shirakawa T. Expression of receptor activator of NF-kappa B ligand and osteoprotegerin in culture of human periodontal ligament cells. J Periodontal Res. 2002;37:405–411. doi: 10.1034/j.1600-0765.2002.01603.x. [DOI] [PubMed] [Google Scholar]
- Jin Q, Cirelli JA, Park CH, Sugai JV, Taba M, Jr, Kostenuik PJ, Giannobile WV. RANKL inhibition through osteoprotegerin blocks bone loss in experimental periodontitis. J Periodontol. 2007;78:1300–1308. doi: 10.1902/jop.2007.070073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanzaki H, Chiba M, Shimizu Y, Mitani H. Dual regulation of osteoclast differentiation by periodontal ligament cells through RANKL stimulation and OPG inhibition. J Dent Res. 2001;80:887–891. doi: 10.1177/00220345010800030801. [DOI] [PubMed] [Google Scholar]
- Lacey D, Timms E, Tan H-L, Kelley M, Dunstan C, Burgess T, Elliott R, Colombero A, Elliott G, Scully S. Osteoprotegerin ligand is a cytokine that regulates osteoclast differentiation and activation. Cell. 1998;93:165–176. doi: 10.1016/S0092-8674(00)81569-X. [DOI] [PubMed] [Google Scholar]
- Lacey DL, Tan HL, Lu J, Kaufman S, Van G, Qiu W, Rattan A, Scully S, Fletcher F, Juan T. Osteoprotegerin ligand modulates murine osteoclast survival in vitro and in vivo. Am J Pathol. 2000;157:435–448. doi: 10.1016/S0002-9440(10)64556-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu XY. Targeting gene-virotherapy of cancer and its prosperity. Cell Res. 2006;16:879–886. doi: 10.1038/sj.cr.7310108. [DOI] [PubMed] [Google Scholar]
- Mealey BL, Rethman MP. Periodontal disease and diabetes mellitus. Bidirectional relationship. Dent Today. 2003;22:107–113. [PubMed] [Google Scholar]
- Mercado F, Marshall RI, Klestov AC, Bartold PM. Is there a relationship between rheumatoid arthritis and periodontal disease? J Clin Periodontol. 2000;27:267–272. doi: 10.1034/j.1600-051x.2000.027004267.x. [DOI] [PubMed] [Google Scholar]
- Nibali L, Farias B, Vajgel A, Tu Y, Donos N. Tooth loss in aggressive periodontitis: a systematic review. J Dent Res. 2013;92:868–875. doi: 10.1177/0022034513501878. [DOI] [PubMed] [Google Scholar]
- Page RC, Engel LD, Narayanan AS, Clagett JA. Chronic inflammatory gingival and periodontal disease. JAMA. 1978;240:545–550. doi: 10.1001/jama.1978.03290060047012. [DOI] [PubMed] [Google Scholar]
- Ribeiro FS, Pontes AEF, Zuza EP, da Silva VC, Lia RCC, Junior EM, Sheikh Z, Abdallah M-N, Hamdan N, Javaid M. Position paper: periodontal regeneration. J Periodontol. 2005;76:1601–1622. doi: 10.1902/jop.2005.76.9.1601. [DOI] [PubMed] [Google Scholar]
- Sasaki N, Kusano E. Bone and bone related biochemical examinations. Bone and collagen related metabolites. Measurement and clinical role of OPG. Clin Calcium. 2006;16:956–962. [PubMed] [Google Scholar]
- Shiba H, Nakanishi K, Sakata M, Fujita T, Uchida Y, Kurihara H. Effects of ageing on proliferative ability, and the expressions of secreted protein, acidic and rich in cysteine (SPARC) and osteoprotegerin (osteoclastogenesis inhibitory factor) in cultures of human periodontal ligament cells. Mech Ageing Dev. 2000;117:69–77. doi: 10.1016/S0047-6374(00)00140-8. [DOI] [PubMed] [Google Scholar]
- Silness J, Löe H. Periodontal disease in pregnancy II. Correlation between oral hygiene and periodontal condition. Acta Odontol. 1964;22:121–135. doi: 10.3109/00016356408993968. [DOI] [PubMed] [Google Scholar]
- Takayanagi H. Inflammatory bone destruction and osteoimmunology. J Periodontal Res. 2005;40:287–293. doi: 10.1111/j.1600-0765.2005.00814.x. [DOI] [PubMed] [Google Scholar]
- Taubman MA, Valverde P, Han X, Kawai T. Immune response: the key to bone resorption in periodontal disease. J Periodontol. 2005;76:2033–2041. doi: 10.1902/jop.2005.76.11-S.2033. [DOI] [PubMed] [Google Scholar]
- Teng Y-TA, Nguyen H, Gao X, Kong Y-Y, Gorczynski RM, Singh B, Ellen RP, Penninger JM. Functional human T-cell immunity and osteoprotegerin ligand control alveolar bone destruction in periodontal infection. J Clin Investig. 2000;106:R59. doi: 10.1172/JCI10763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villar CC, Cochran DL. Regeneration of periodontal tissues: guided tissue regeneration. Dent Clin North Am. 2010;54:73–92. doi: 10.1016/j.cden.2009.08.011. [DOI] [PubMed] [Google Scholar]
- Yasuda H, Shima N, Nakagawa N, Mochizuki S-I, Yano K, Fujise N, Sato Y, Goto M, Yamaguchi K, Kuriyama M. Identity of osteoclastogenesis inhibitory factor (OCIF) and osteoprotegerin (OPG): a mechanism by which OPG/OCIF inhibits osteoclastogenesis in vitro. Endocrinology. 1998;139:1329–1337. doi: 10.1210/endo.139.3.5837. [DOI] [PubMed] [Google Scholar]
- Zhang D, Yang Y, Li X, Fu M. The expression of osteoprotegerin and the receptor activator of nuclear factor kappa B ligand in human periodontal ligament cells cultured with and without 1α, 25-dihydroxyvitamin D. Arch Oral Biol. 2004;49:71–76. doi: 10.1016/S0003-9969(03)00201-2. [DOI] [PubMed] [Google Scholar]