Abstract
The HLA-I antigen processing machinery (APM) plays a crucial role in the anticancer immune response. The loss of surface expression of HLA-I molecules is particularly important as this enables tumor cells to evade recognition and lysis by cytotoxic T-lymphocytes. Transcriptional control of the APM genes is regulated by the nuclear factor kappa B (NF-κB). BCRFl is an Epstein–Barr virus homologue of human IL-10 (hIL-10) and is known as viral IL-10 (vIL-10). vIL-10 shares many immunosuppressive effects with hIL-10 but lacks the immunostimulatory effect of hIL-10. The aim of this study was to assess whether vIL-10 inhibits APM components (TAP-1, TAP-2, LMP-2, LMP-7 and HLA-I) through the NF-κB signaling pathway in nasopharyngeal carcinoma. This work demonstrated that vIL-10 inhibited NF-κB activation by blocking IKK phosphorylation and promoting the expression of IKB. TNF-α treatment led to a strong translocation of NF-κB p65, whereas pretreatment with vIL-10 before TNF-α treatment blocked NF-κB p65 translocation. vIL-10 also inhibited TNF-α-induced DNA-binding of NF-κB p65 in the nucleus. Furthermore, chromatin immunoprecipitation analysis demonstrated that NF-κB p65 could bind to the TAP-1, TAP-2, LMP-2, LMP-7 and HLA-I gene promoters, and after TNF-α stimulation, the down-regulation of TAP-1, TAP-2, LMP-2, LMP-7 and HLA-I transcription by vIL-10 correlated with the suppression of NF-κB in CNE-2 cells. Surprisingly, vIL-10 inhibits only TAP-1 and LMP-7 transcription in CNE-1 cells. Taken together, these results suggest that the inhibition of NF-κB activity may be an important mechanism for vIL-10 suppression of APM (TAP-1, TAP-2, LMP-2, LMP-7 and HLA-I) gene transcription in CNE-2 cells.
Keywords: Nasopharyngeal neoplasms, Nuclear factor Kappa B (NF-κB), Viral interleukin-10 (vIL-10), Antigen presentation
Introduction
Both viruses and tumors evade cytotoxic T lymphocyte-mediated host immunity by down-regulation of antigen presentation machineries. This effect can be achieved by either down-regulation of the transcription of antigen presentation genes or posttranslational inactivation of proteins involved in antigen presentation (Yang et al. 2003). Optimal cell surface expression of HLA molecules requires the coordinated expression of several genes, such as transporters associated with antigen processing (TAP)-1/2, low molecular weight peptide (LMP)-2/7, and tapasin, as well as HLA class I heavy chain and β2-microglobulin (β2M). In cases of both tumorigenesis and viral infection, the expression of these genes and the function of the encoded proteins are often impaired. Latent EBV infections are associated with lymphocyte and epithelial cell malignancies. Nasopharyngeal carcinoma (NPC) is the most frequent EBV-associated malignancy (Vujanovic et al. 2003). Current research is focused on how NPC escapes from EBV-specific immune destruction and developing novel strategies for immune intervention.
Human interleukin-10 (hIL-10) is mainly produced by activated lymphocytes, monocytes/macrophages, and other cell types (Takayama et al. 2003). hIL-10 inhibits macrophage and dendritic cell function and cytokine synthesis by activated T cells and NK cells by blocking the ability of such cells to act as antigen-presenting or costimulatory cells (Liu 2003). IL-10 can also exhibit T cell stimulatory properties, including enhancing thymocyte proliferation and CD8+ T cell generation, and exacerbating organ allograft rejection and graft-vs-host disease. The EBV homologue BCRFl (vIL-10) is approximately 85 % identical with hIL-10, with most differences found in the 20 N-terminal amino acids, and is expressed as a 17 kDa non-glycosylated polypeptide. vIL-10 shares many of the anti-inflammatory properties ascribed to mammalian IL-10 but lacks the immunostimulatory properties, such as augmentation of CTL proliferation and B cell expansion, and can induce local anergy to syngeneic or allogeneic tumors.
Previous studies have demonstrated that the down-regulation of TAP-1, TAP-2, LMP-2, LMP-7 and HLA-I in NPC induced immunosuppression (Ren et al. 2003). In vitro, vIL-10 can down-regulate the expression of TAP-1, TAP-2, LMP-2, LMP-7 and HLA-I in CNE-2 cells (data is not shown). The expression of TAP-1, TAP-2, LMP-2, LMP-7 and HLA-I involves the activation of NF-κB and its recruitment to the promoter elements proximal to the gene transcription start sites (Moschonas et al. 2003; Molinero et al. 2004).
The NF-κB proteins in mammalian cells consist of five members: p50, p52, p65, c-Rel, and RelB. These proteins form homodimers or heterodimers with other family members and positively regulate a number of cellular genes. NF-κB dimers are sequestered in the cytoplasm in an inactive form by association with an inhibitory IkB subunit. Following cellular activation, multiple kinase cascades lead to serine phosphorylation of IkB by IkB kinases (IKKs) and proteosome-mediated degradation, resulting in the release of an active NF-κB complex that translocates to the nucleus. In the nucleus, NF-κB transactivates genes containing specific consensus sequences in their transcriptional regulatory regions (Portis et al. 2001).
IL-10 inhibits the activation of NF-κB (Wang et al. 1995) and its nuclear localization (Lentsch et al. 1997), and induces IkB protein production (Driessler et al. 2004). Therefore, IL-10 can influence NF-κB down-stream gene transcription. Our data presented here provide evidence that vIL-10 can inhibit the activation, nuclear translocation and the DNA-binding affinity of NF-κB p65, and then inhibit the transcription of NF-κB targeted genes TAP-1, TAP-2, LMP-2, LMP-7 and HLA-I, down-regulating antigen presentation.
Materials and methods
Cell culture and treatment
CNE-1 (a gift from the SUN YAT-SEN University Cancer Center, Guangzhou, Guangdong Province) and CNE-2 (purchased from the experimental center of the XiangYa School of Medicine, Changsha, Hunan Province) are well-differentiated human NPC cell lines. The cells were cultured in RPMI-1640 medium (Sigma-Aldrich, St. Louis, MO, USA) containing 10 % fetal bovine serum (FBS) (Sigma-Aldrich), 100 U/ml penicillin and 100 μg/ml streptomycin. The cells were cultured at 37 °C in a humidified 5 % CO2 incubator. Cells in logarithmic growth phase were used in all of the experiments. Prior to treatment, cells were seeded in serum free medium overnight.
Western blot analysis
Cells were lysed on ice in 200 µl RIPA (Solarbio, Beijing, China) containing 1 mM phenylmethylsulfonyl fluoride (PMSF) (Sigma) for 30 min and then centrifuged at 12,000×g for 10 min. The supernatant was collected as whole cell lysates. Protein concentration was determined by BCA Assay Reagent (Beyotime, Haimen, China). In total, 50 μg of the protein from various cell preparations and rainbow molecular weight markers (GE Healthcare Bio-Sciences, Pittsburgh, PA, USA) were separated on SDS polyacrylamide gels (reducing condition, 60 V in stacking gel for 20 min and 120 V in separating gel for 1 h) and then electrotransferred onto a nitrocellulose membrane (200 mA for 2 h). The membranes were blocked with buffer containing 5 % non-fat milk in PBS with 0.05 % Tween-20 (PBST) for 2 h, and then incubated with different primary antibodies overnight at 4 °C. After a second wash, the membranes were incubated with goat anti-rabbit (Cell Signaling, Shanghai, China) or goat anti-mouse (Cell Signaling) horseradish peroxidase-conjugated secondary antibodies for 1 h at room temperature and developed with the enhanced chemiluminescence detection kit. The following antibodies were used for Western blot analysis: rabbit NF-κB p65 polyclonal antibody, rabbit phospho-NF-κB p65 polyclonal antibody, rabbit anti-IKKα polyclonal antibody, rabbit anti-IKKβ polyclonal antibody, rabbit phospho-IKKα/β polyclonal antibody, and mouse anti-phospho-IKKα polyclonal antibody (Cell Signaling). Generally, the primary antibodies were diluted in PBST by 1:1000 and the secondary antibodies (HRP conjugated goat anti rabbit or goat anti mouse antibodies (Santa Cruz Biotechnology Inc., Santa Cruz, CA, USA).) at 1:5000.
Indirect immunofluorescence assays
Cells were fixed with 4 % p-formaldehyde for 20 min, permeabilized with PBS containing 0.1 % triton for 20 min and blocked with 1 % BSA. Indirect immunofluorescence was performed using 1/100 dilutions of the following primary antibody: rabbit polyclonal IgG anti-NF-κB p65 antibody (Cell Signaling) overnight. Then, incubation was followed by a subsequent incubation with a FITC-labeled secondary sheep anti-rabbit antibody (Beckman Coulter, Brea, CA, USA). The subcellular distribution of these proteins was monitored by confocal microscopy using an Olympus (Tokyo, Japan) Fluoview 1100 microscope. Image analyses for co-immunolocalization were performed using the colocalization threshold plug-in of the Image-J program.
Electrophoretic mobility shift assay (EMSA)
An NF-κB EMSA was performed according to the manufacturer’s instructions (Beyotime). Briefly, 1 × 105 cells were washed in ice-cold phosphate-buffered saline, and the nuclear protein was extracted with the Nuclear and Cytoplasmic Protein Extraction Kit (Beyotime). Protein concentration was determined using the BCA Assay Reagent. A NF-κB consensus oligonucleotide (5′-AGT TGA GGG GAC TTT CCC AGG C-3′, 3′-TCA ACT CCC CTG AAA GGG TCC G-5′) was labeled with biotin-binding reactions containing 10 µl extract protein, 4 µl nuclease-free water, 4 µl EMSA/Gel-Shift buffer and 2 µl labeled probe for 2 h at 20 °C. Complexes were resolved on a 4 % polyacrylamide gel for electrophoresis and transferred to nitrocellulose membranes. The membranes were exposed to an ultraviolet lamp for 30 min and then developed on film.
Chromatin immunoprecipitation (ChIP) assays
ChIP experiments were performed in CNE-1/2 cells using the ChIP assay kit (Millipore, Billerica, MA, USA) (Erlejman et al. 2014). Briefly, proteins and DNA were cross-linked after CNE-1/2 cells were treated with 1 % formaldehyde for 10 min at 37 °C and neutralized with glycine added at a final concentration of 0.125 M. Cells were rinsed twice with cold PBS containing 2.5 ml Protease Inhibitor cocktail II (Abcam, Cambridge, MA, USA), scraped, and centrifuged at 7200×g for 5 min at 4 °C. Cells were washed with PBS and homogenized in SDS lysis buffer containing Inhibitor cocktail II. Chromatin was fragmented into 200–300 bp fragments by sonication, and the lysates were centrifuged at 2500×g for 10 min at 4 °C. The supernatants were mixed with 600 μl preheating Enzymatic Cocktail and heated for 10 min at 37 °C before EZ-Zyme stop buffer was added to the mixture, which was then incubated for 10 min over an ice-bath. The mixture was centrifuged at 12,000×g for 15 min at 4 °C, and the supernatants were collected. Immunoprecipitation was performed overnight at 4 °C. Samples were incubated with protein-G agarose for 1 h, and the immune complexes were collected by centrifugation (5000×g for 10 min at 4 °C), washed and extracted with eluant (10 μl 20 %SDS, 20 μl 1 M NaHCO3, 170 μl ddH2O). The cross-linking was reversed by heating with 5 M NaCl at 65 °C for 4 h. Chromatin-associated proteins were digested with proteinase K, and the samples were extracted with phenol/chloroform, followed by precipitation with ethanol. The pellets were lysed in nuclease-free water and subjected to polymerase chain reaction (PCR); the primer sequences were: glyceraldehyde-3-phosphate dehydrogenase (GAPDH) forward: 5′-TACTAGCGGTTTTACGGGCG-3′, reverse: 5′-TCG-AACAGGAGGAGCAGAGAGCGA-3′; TAP-1 forward: 5′-GGCAGACTCAGTTC-CTCTCAC-3′, reverse: 5′-CAGAACGGGTTGGGGATCAA-3′; TAP-2 forward: 5′-GGCCTGTACAGTGCGAACC-3′, reverse: 5′-GTCTCTCCCAACCTCGCTAC-3′; LMP-2 forward: 5′-CCACTTGCACCAGTTTTCCC-3′, reverse: 5′-AGATCTG-CCCCGAGACAAGT-3′; LMP-7 forward: 5′-TGGGAAACGTTGGTGTCCTT-3′, reverse: 5′-GGAAAGACATCGGACCGTCA-3′; HLA-I forward: 5′-CACAAGAC-CGAGGTGGAGAC-3′, reverse: 5′-GTTCCCACCACACTGTCACT-3′. The PCR conditions were as follows: 94 °C for 3 min, and then 28 cycles of 94 °C for 30 s, 55 °C for 30 s, and 72 °C for 30 s, and finally 72 °C for 5 min.
Reverse transcription PCR
RNA extraction and first-strand cDNA synthesis were performed according to the manufacturer’s instructions (Takara, Dalian, China). For semi-quantitative RT-PCR and real-time PCR, the primers used were as follows: the primers for TAP-1 (163 bp product) were 5′-GCAGCCTATTTAGGTTCGGGA-3′ (forward) and 5′-ATAGATCCCGTCACCCACGA-3′ (reverse), for TAP-2 (158 bp product) were 5′-GCGGGACAGAAACAACGTCT-3′ (forward) and 5′-TGAGCAATCACCAGCACTGT-3′ (reverse), for LMP-2 (105 bp product) were 5′-GGCGTTGTGATGGGTTCTGA-3′ (forward) and 5′-GAGTGCACAGTAGATGCGCT-3′ (reverse), for LMP-7 (166 bp product) were 5′-CCTCACAACTCACCACCTCG-3′ (forward) and 5′-GGCCTCATCTACCCAGCAAC-3′ (reverse); and for HLA-I (179 bp product) were 5′-CCACTCACAGACTGACCGAG-3′ (forward) and 5′-CCTCGTTCAGGGCGATGTAA-3′ (reverse); and for β-actin (163 bp product) were 5′-CTCACCATGGATGATGATATCGC-3′ (forward) and 5′-AGGAATCCTTCTGACCCATGC-3′ (reverse). After RT-PCR, the amplicons were electrophoresed in 2 % agarose gels, stained by ethidium bromide and viewed under ultraviolet illumination.
Statistical analysis
All data are presented as the means ± SEMs. Two-sample comparisons were performed using Student’s t-tests.
Results
vIL-10 inhibits NF-κB-dependent transcription in CNE-1 and CNE-2 cells
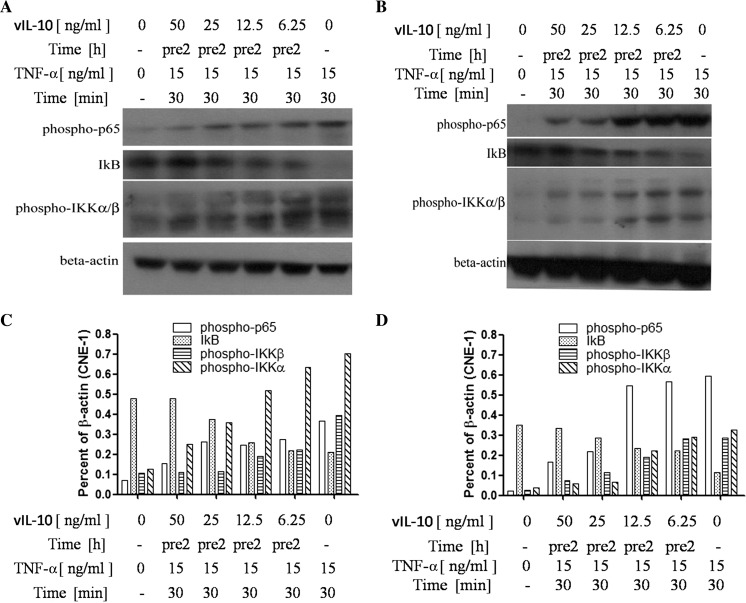
Cultured CNE-1/2 cells were incubated with vIL-10 at different concentrations for 2 h and then incubated with 15 ng/ml TNF-α for 30 min (Wang et al. 1995). NF-κB p-p65, which is the activated form of NF-κB p65, was examined by Western blot analysis. vIL-10 inhibited TNF-α-induced NF-κB p-p65 expression in a dose-dependent manner in CNE-1 and CNE-2 cells (Fig. 1a, c and b, d). The NF-κB inhibitor IκB interacts and sequesters p65, among other proteins of the NF-κB complex, in the cytoplasm, preventing its nuclear translocation and the transcription of target genes. The degradation of IκB is necessary for the translocation of NF-κB to the nucleus and subsequent binding to DNA. IKK plays a role in the phosphorylation and degradation of IκB (Clarke et al. 1998; Hovsepian et al. 2013). We next examined IκB and the phosphorylation of IKKa/β in these cells by Western blot analysis. Figure 1a, c and b, d demonstrated that vIL-10 significantly inhibited IKK phosphorylation and promoted the expression of IKB. These findings suggest that vIL-10 may have an inhibitory role in NF-κB signaling pathways.
Fig. 1.
The activity changes of the NF-κB signaling pathway in NPC cells after treatment with vIL-10. a The CNE-1 cells were treated with TNF-α (15 ng/ml) for 30 min or untreated (−). Alternatively, the cells were treated with vIL-10 for 2 h (pre2) before TNF-α treatment. The cells were lysed for Western blot analysis as described in the “Materials and methods” section. b CNE-2 cells were treated in the same manner as a, and the results are presented. c The results in a were semi-quantified by ImageJ. d The results in b were semi-quantified by ImageJ
vIL-10 inhibits NF-κB nuclear translocation in CNE-1 and CNE-2 cells
In most cells, NF-κB transcription complexes are present in a latent, inactive state in the cytoplasm where they are bound to IκB (inhibitory κB). Many stimuli can rapidly activate NF-κB complexes by freeing them from their inhibitor and enabling them to translocate into the nucleus. The most common form of NF-κB is a heterodimer of p65/p50, and the expression of most NF-κB-regulated genes is activated by p65, whereas p50 modulates the expression levels (Naschberger et al. 2004). We hypothesized that NF-κB nuclear translocation could be modulated by vIL-10.
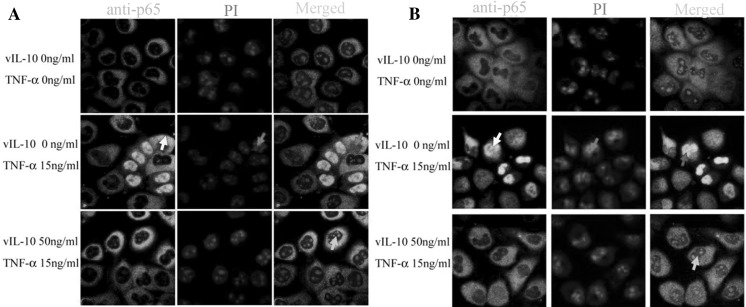
To test that premise, we divided cells into three groups: cells without treatment (control group), cells treated with TNF-α, and cells treated with TNF-α and vIL-10. After the cells were treated with TNF-α or TNF-α and vIL-10, the nuclear localization of NF-κB p65 was analyzed by confocal microscopy. In the control group, NF-κB p65 stayed in the cytoplasm of CNE-1 and CNE-2 cells, and significant nuclear accumulation was not observed (Fig. 2a, b, first line) while NF-κB p65 was significantly activated (white arrow) and accumulated in the nucleus (red arrow) after 30 min of stimulation with 15 ng/ml TNF-α (Fig. 2a, b, middle line). Meanwhile, vIL-10 affected the nuclear import rate of NF-κB p65 stimulated by TNF-α. The cells were incubated with 50 ng/ml vIL-10 for 2 h then 15 ng/ml TNF-α was added for 30 min. The nuclear translocation of NF-κB p65 decreased compared to cells stimulated by TNF-α only (yellow arrows, Fig. 2a, b, lower line). This observation suggested that vIL-10 could inhibit NF-κB p65 nuclear translocation in CNE-1 and CNE-2 cells.
Fig. 2.
vIL-10 influences NF-κB p65 nuclear translocation stimulated by TNF-α. CNE-1 (a) or CNE-2 (b) cells were treated with TNF-α for 30 min, or alternatively pre-treated with vIL-10 for 2 h. The cells were fixed with 4 % p-formaldehyde for 20 min then used for indirect immunofluorescence assays. White arrows indicate nuclear translocated p65. Red arrows indicate the nucleus. Yellow arrows indicate the merged nucleus and stained p65
vIL-10 inhibits DNA-binding of NF-κB
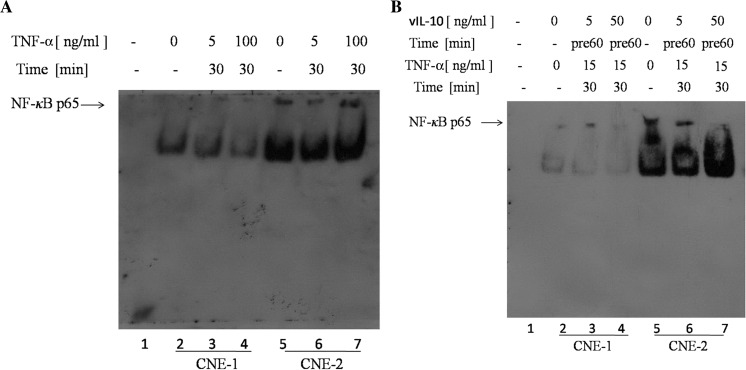
To assess whether vIL-10 inhibited NF-κB-dependent transcription through the suppression of DNA-binding of this transcription factor, EMSAs were performed. For these assays, we used biotin, an oligonucleotide probe with binding sites that bind p65/p50 heterodimers. Nuclear extracts were prepared from cells treated with TNF-α alone or treated with TNF-α in the presence of an vIL-10 pretreatment, and nuclear proteins were analyzed by EMSA. Constitutive binding of NF-κB complexes was not seen in negative control samples (Fig. 3a, b, lane 1). Stimulation for 30 min with TNF-α in 0, 5 and 100 ng/ml slightly induced DNA-binding of NF-κB p65 in CNE-1 cells (Fig. 3a, lane 2, 3, 4) while the same stimulation with TNF-α could strongly induce DNA-binding of NF-κB p65 in CNE-2 cells (Fig. 3a, lane 5, 6, 7). The DNA-binding of NF-κB p65 was proportional to the concentration of TNF-α in CNE-2 cells. However, pretreatment with vIL-10 followed by TNF-α stimulation significantly inhibited TNF-α-induced DNA-binding of NF-κB p65 in CNE-1 and CNE-2 (Fig. 3b, lane 2, 3, 4, 5, 6, 7), and the DNA-binding of NF-κB p65 was inversely proportional to the concentration of vIL-10. This observation suggested that vIL-10 could inhibit the DNA-binding of NF-κB p65.
Fig. 3.
Inhibition of DNA-binding NF-κB p65 by vIL-10. a, b lane 1: negative control; a CNE-1 cells were treated with TNF-α alone (lane 2: 0 ng/ml, lane 3: 5 ng/ml, 30 min, lane 4: 100 ng/ml, 30 min); CNE-2 cells were treated with TNF-α alone (lane 5: 0 ng/ml, lane 6: 5 ng/ml, 30 min; lane 7: 100 ng/ml, 30 min). b CNE-1 (lane 2) and CNE-2 (lane 5) were treated without TNF-α or vIL-10; CNE-1 cells were stimulated with TNF-α (15 ng/ml, 30 min) in the presence of vIL-10 (lane 3, 4: 5, 50 ng/ml) as a 30 min pretreatment; CNE-2 cells were stimulated with TNF-α (15 ng/ml, 30 min) in the presence of vIL-10 (lane 6, 7: 5, 50 ng/ml) as a 60 min pretreatment
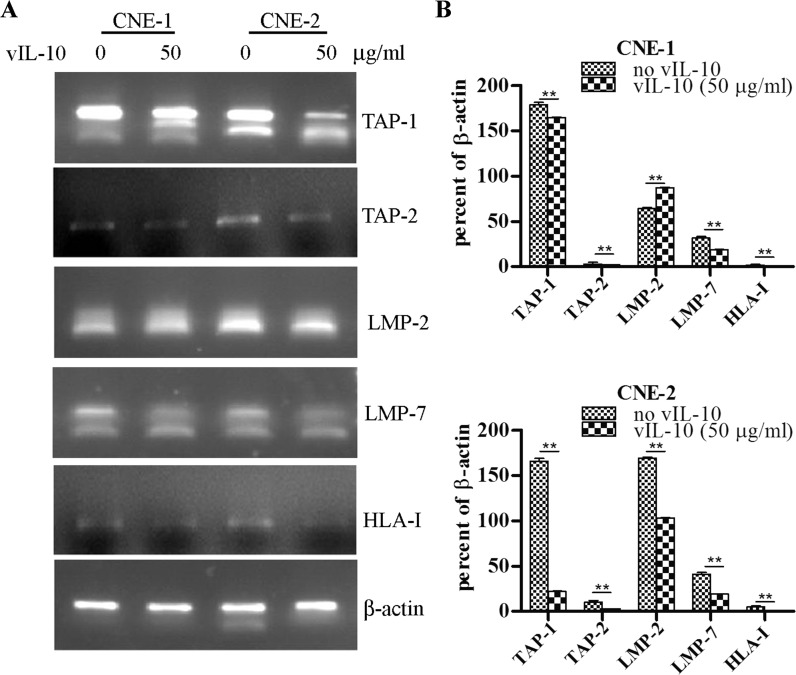
vIL-10 inhibits the transcription of TAP-1, TAP-2, LMP-2, LMP-7 and HLA-I genes regulated by NF-κB p65 in CNE-2 cells
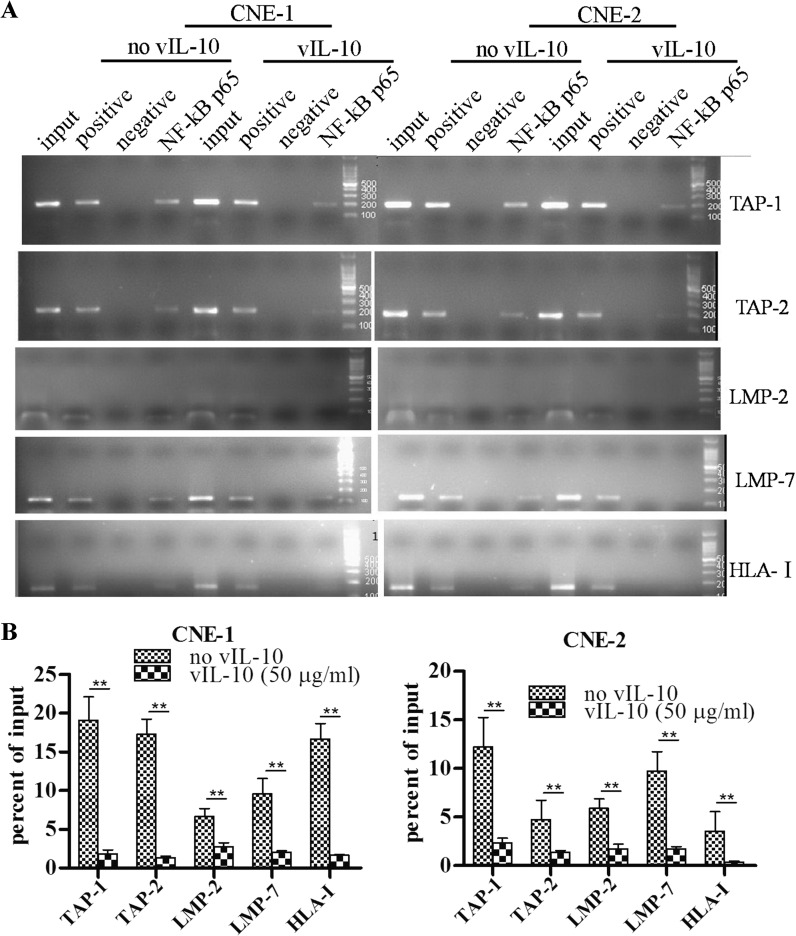
In the NF-κB signaling pathway, NF-κB p65 can translocate into the nucleus and bind to DNA after activation. p65-containing complexes bind with high affinity to the consensus DNA sequences 5′-GGGPuNNPyPyCC-3′ or 5′-GGGPuNPyPyCC-3′ leading to the activation of transcription. NF-κB is a key regulator of immune cell function. It regulates the expression of genes for the transporter associated with antigen processing (TAP-1), the proteasome subunit latent membrane protein 1 (LMP-1) and the MHC class II invariant chain, proteins with essential functions for antigen presentation (Neurath et al. 1998). The data shown in Figs. 1, 2 and 3 suggested that vIL-10 could inhibit activation, nuclear translocation and DNA-binding of NF-κB. Therefore, we assessed whether vIL-10 could down-regulate the expression of TAP-1, TAP-2, LMP-2, LMP-7 and HLA-I genes through the NF-κB signaling pathway. ChIP experiments were performed. Chromatin from CNE-1 and CNE-2 cells treated with TNF-α or TNF-α and vIL-10 was used for immunoprecipitation with an anti-p65 antibody, and precipitated DNA encompassing the TAP-1, TAP-2, LMP-2, LMP-7 and HLA-I gene promoter NF-κB binding sites were assayed by PCR. The results (Fig. 4) showed that NF-κB p65 could bind the TAP-1, TAP-2, LMP-2, LMP-7 and HLA-I gene promoters with or without vIL-10 treatment in CNE-1 and CNE-2. The recruitment of NF-κB p65 to TAP-1, TAP-2, LMP-2, LMP-7 and HLA-I gene promoters in the cells incubated with vIL-10 were further assayed. As shown in Fig. 4, the recruitment of NF-κB p65 to TAP-1, TAP-2, LMP-2, LMP-7 and HLA-I gene promoters decreased after treatment with 50 ng/ml vIL-10 in both CNE-1 and CNE-2. Taken together, these observations suggest that NF-κB p65 participated in vIL-10—inhibited TAP-1, TAP-2, LMP-2, LMP-7 and HLA-I expression in CNE-1 and CNE-2 cells. TNF-α upregulated the expression of TAP-1, TAP-2, LMP-2, LMP-7 and HLA-I by RT-PCR assay (Fig. 5), and vIL-10 pre-treatment significantly reduced the expression of these genes (Fig. 5).
Fig. 4.
The ChIP results of TAP-1, TAP-2, LMP-2, LMP-7 and HLA-I promoter analysis with NF-κB p65. a, b CNE-1 (a, left) or CNE-2 (a, right) cells were treated with TNF-α for 30 min, or alternatively pre-treated with vIL-10 for 2 h. The cells were lysed for ChIP. The precipitated DNA was amplified by PCR as described in the “Materials and methods” section. c, d The experiments in a and b were repeated three times and semi-quantified. ** P < 0.05
Fig. 5.
The change of transcription of TAP-1, TAP-2, LMP-2, LMP-7 and HLA-I. a CNE-1 or CNE-2 cells were treated with TNF-α for 30 min, or alternatively they were pre-treated with vIL-10 for 2 h. The expression of TAP-1, TAP-2, LMP-2, LMP-7 and HLA-I were assessed by RT-PCR. b The experiments in a were repeated three times and semi-quantified. ** P < 0.05
Discussion
NF-κB plays a significant role in moderating the immunization and inflammation reaction. The activity of NF-κB is moderated by the inhibitory molecule IKB that resides inside the cytoplasm. IKB contains factors, such as IΚBα, IΚBβ, IΚBε, IΚBγ and bcl-3, which have six or seven ankyrin repetitive sequences (Whiteside and Israël 1997). IKB kinase (IKK) can phosphorylate two Ser terminal residues on IΚBa and IΚBβ N during cytokine inducement. Under a static state, NF-κB is a dimer (p65/p50), mainly residing inside the cytoplasm. p65 has a transcriptional regulation action zone, whereas p50 does not (Driessler et al. 2004). Exogenous stimulation activates the IKK upstream kinase through signal transmission. Activated IKK phosphorylates its substrate IKB, and p65 is phosphorylated, starting nuclear transcription regulation. Phosphorylated NF-κB p65 (activated form) can directly bind to certain factors inside the nucleus or gene promoters, triggering a sequence of physiologic or pathologic effects.
We investigated the effect of vIL-10 on TNF-α-mediated NF-κB pathway proteins in CNE-1 and CNE-2 cells. We found that vIL-10 can inhibit the NF-κBp-p65 and p-IKKα/β kinase activation by observing the change of NF-κB signaling protein using Western blot analysis. In addition, NF-κB activity inhibiting protein (IKB) increases with the increase in vIL-10 concentration, which implies that vIL-10 can increase IKB expression or inhibit IKB degradation. These results indicate that vIL-10 can inhibit NF-κB activity, and this inhibition is concentration dependent. Furthermore, using an immunofluorescence method, we determined that the amount of NF-κB p65 nuclear translocation in nasopharyngeal carcinoma cells affected by vIL-10 decreased or even disappeared. This finding indicates that vIL-10 can inhibit the activity of NF-κB p65 nuclear translocation in nasopharyngeal carcinoma cells. EMSA assays demonstrated that vIL-10 can significantly inhibit the combination activity of NF-κB with DNA, and this inhibition is concentration dependent in both CNE-1 and CNE-2 cells.
Schottelius et al. (1999) disclosed that IL-10 could inhibit IKBa degradation through inhibiting IKK activity, preventing IKK degradation, and blocking NF-κB nuclear translocation. They found that TNF-α pretreated cells treated with IL-10 can inhibit IKBa degradation at 15 min, but this effect was lost at 30 min. IKBa reassembled after degradation, indicating that IL-10 can only inhibit IKBa degradation temporarily. The findings showed that, to some extent, the inhibition of NF-κB by IL-10 is achieved by inhibiting IKBa degradation. However, Schottelius was unsure how IL-10 inhibited NF-κB DNA binding activity. Having extended TNF-α stimulating time, IL-10 can still inhibit NF-κB combination activity while this inhibition is not related to IKK activity decrease or NF-κB nuclear translocation. The presence of the IKB protein in the cytoplasm and nucleus was assayed by Western blot analysis. Analysis of nuclear extracts failed to demonstrate an accumulation of IkB proteins following IL-10 treatment, suggesting that the inhibitory effect of IL-10 on NF-κB DNA binding is not mediated by increased nuclear levels of these inhibitory molecules. Therefore, Schottelius believed that this observation must occur through a different mechanism. Raychaudhuri et al. (2000) discovered that by specifically inducing nuclear translocation of the p50/p50 homodimer and inhibiting IKK activity, IL-10 exerted a dual inhibitory function in the NF-κB pathway (Varfolomeev et al. 2006). Researchers also found, from Northern blot assays, that IL-10 could not inhibit the activity of LPS-induced NF-κB binding to DNA. Furthermore, the expression of the NF-κB transcription factor p65 was not affected by IL-10, nor did IL-10 impact IKB mRNA expression (Dokter et al. 1996). Clarke et al. (1998) found that IL-10 could not accelerate IKBa/IKBβ degradation or inhibit NF-κB nuclear translocation. Clarke et al. (1998) speculated that there must be some other mechanism besides IKB degradation and DNA combination. A comparison of the activity of vIL-10 and results from other groups focused on human IL-10 suggests that vIL-10 may be similar to human IL-10, but there are many differences. This finding indicated that vIL-10 may have a potential therapeutic effect clinically.
The results, which demonstrate that vIL-10 inhibits the expression ChIP of NF-κB through the NF-κB signaling pathway, demonstrate that NF-κB p65 can bind to the promoter of TAP-1, TAP-2, LMP-2, LMP-7 or HLA-I after entering the nucleus of nasopharyngeal carcinoma cells. There are few reports concerning the transcriptional regulation of NF-κB to TAPs and LMPs. The NF-κB binding site in the bidirectional promoter domain of TAP-1 and LMP-2 can bind with activated NF-κB, which moderates TAP-1 and LMP-2 gene transcription (Gobin et al. 1998). Through gene fingerprint technology and site-directed mutagenesis, Wright et al. (1995) discovered that the Sp1-GC box shared by both TAP-1 and LMP-2 is much closer to the TAP-1 gene, and TNF-α can assist TAP-1 and LMP-2 gene expression by inducing the NF-κB pathway. The ATG codon is found in several promoter domains located at position 120 bp of the TAP-1 gene, in an external promoter located −427 bp upstream, and in the LMP-2 transcription origin position −40 bp. Therefore, both the TAP-1 and LMP-2 genes are moderated by both the Sp1-GC box and NF-κB. LMP-7 and LMP-2, located at the MHC-II gene and close to the TAP gene, are less important than TAPs during antigen presentation because cells that lack the LMP gene can also present antigens. Pai et al. (2002) discovered that inside Burkitt’s lymphoma and nasopharyngeal carcinoma cells, EB virus antigen LMP-1 could assist in the expression of TAP-1 and TAP-2 through its C-terminal activity region (CTAR1 and CTAR1). This effect was lost after the C-terminal activity region was removed or mutated. They further discovered that LMP-1 could activate NF-κB through the C-terminal activity region, assist in RelB nuclear translocation, and perform transcriptional regulation. Either NF-κB’s inhibitors or the RelB antibody may inhibit the induction of LMP-1. Therefore, they concluded that LMP-1 could increase the antigen presentation of both TAP-1 and TAP-2 through NF-κB. Moschonas et al. (2003) discovered that the CD40 ligand could induce antigen presentation and related gene expression (TAP-1, TAP-2, LMP-2, LMP-10 and tapasin). These gene promoters all contained NF-κB binding regions. The CD40 ligand could recruit p65 into the nucleus and bind to the gene promoter domain to initiate the gene expression. NF-κB was the key moderator of the expression of TAP-1 and LMP-2. We found that vIL-10 can significantly inhibit NF-κB p65’s binding to the gene promoters of target genes TAP-1, TAP-2, LMP-2 and LMP-7 in CNE-2. However, vIL-10 only inhibits TAP-1 and LMP-7 in CNE-1. CNE-1 is a highly differentiated nasopharyngeal carcinoma cell line, and CNE-2 is poorly differentiated or undifferentiated. CNE-1 and CNE-2 may have different biological activities. CNE-2 represents the immunization of the majority of nasopharyngeal carcinoma patients because poorly differentiated nasopharyngeal carcinoma is seen more commonly in clinics.
Downregulation of HLA-I has been observed in many tumors, at a rate of 16–50 %, which is closely associated with tumor immunoevasion. In many tumors, the downregulation of HLA-I expression is related to pathologic characteristics, poor prognosis and disease-free survival time. HLA-I has three main promoter regions: enhancer A, interference simulator reaction region (ISRE), and enhancer B (SXY). Enhancer A is located between −201 and −159, including two regions: I, which can bind to NF-κB (Dejardin et al. 1998), and II. Enhancer B is located between −120 and −61 relative to the transcription initiation site. In vitro, enhancer B binds to AP-1, but it is unclear whether AP-1 moderates MHC-I’s expression in vivo (Romano et al. 1996). The NF-κB family includes p65 (RelA), p50, c-Rel, p52, and RelB. They form either dimers or trimers. These polymers bind to the target gene locus points of 10 bp and start transcription regulation. In most cases, an NF-κB dimer is bound to its inhibitory protein IKB inside the cytoplasm and is in a static status. An activated simulator can quickly activate NF-κB, making it effective by entering the cytoplasm. p65/p50 is the most commonly seen NF-κB dimer (Symeonidou et al. 2010). Dejardin et al. (1998) discovered that NF-κB/IκB can moderate MHC-I expression in breast cancers. NF-κB complexes that contain p65 or RelB bind to the κB region of the MHC-I promoter and activate MHC-I transcription. The results in the present study shows that vIL-10 can significantly inhibit the expression of the HLA-I gene in CNE-2 cells but have little impact in CNE-1 cells. The possible reasons are as follows (1) NF-κB contains dimers, such as p65/p50, p50/c-Rel, p50/p50 and p65/p65, which have different effects. The most common p65 for ChIP was used in the test. It is possible that p65/p50 is not the primary element of transcriptional regulation in CNE-1 nasopharyngeal carcinoma cells, but other elements are (Wright et al. 1995; Pai et al. 2002). (2) The HLA-I gene contains several loci and promoters. The promoter in the test may not contain NF-κB’s action zone. Therefore, the negative result does not necessarily mean that NF-κB has no transcriptional effect on the HLA-I gene. (3) NF-κB expression has organizational and cellular distinctiveness. The MHC-I gene’s transcription level depends on NF-κB expression, NF-κB combinational ability, and the moderate ability of different NF-κB dimers. (4) There are other signaling pathways in CNE-1 cells that moderate HLA-I expression.
In conclusion, the results in the present study suggest that the inhibition of NF-κB activity may be an important mechanism for vIL-10 suppression of APM (TAP-1, TAP-2, LMP-2, LMP-7 and HLA-I) gene transcription in CNE-2 cells. The role of vIL-10 and hIL-10 is more or less different, which may indicate a possible therapeutic role for vIL-10 in immune-related diseases.
Acknowledgments
This study was supported by National Natural Science Foundation in 2012 (No: 81260312), Research project fund of Healthy Science and Technology of Yunnan Province in 2011 and 2012 (Nos: 2011WS0068, 2012WS0035), Project of the basic research on the application of Science and Technology Department of Yunnan Province and technology Kunming Medical University in 2014 (No: 2014FZ037), and Key medical project of Xishan District of Kunming City Science and technology bureau in 2014.
Footnotes
Yan-xin Ren and Jie Yang have contributed equally to this work.
References
- Clarke CJ, Hales A, Hunt A, Foxwell BM. IL-10- mediated suppression of NF-alpha production is independent of its ability to inhibit NF kappa B activity. Eur J Immunol. 1998;28:1719–1726. doi: 10.1002/(SICI)1521-4141(199805)28:05<1719::AID-IMMU1719>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- Dejardin E, Deregowski V, Greimers R, Cai Z, Chouaib S, Merville MP, Bours V. Regulation of major histocompatibility complex class I expression by NF-kappaB-related proteins in breast cancer cells. Oncogene. 1998;16:3299–3307. doi: 10.1038/sj.onc.1201879. [DOI] [PubMed] [Google Scholar]
- Dokter WH, Koopmans SB, Vellenga E. Effects of IL-10 and IL-4 on LPS-induced transcription factors (AP-1, NF-IL6 and NF-kappa B) which are involved in IL-6 regulation. Leukemia. 1996;10:1308–1316. [PubMed] [Google Scholar]
- Driessler F, Venstrom K, Sabat R, Asadullah K, Schottelius AJ. Molecular mechanisms of interleukin-10-mediated inhibition of NF-kappaB activity: a role for p50. Clin Exp Immunol. 2004;135:64–73. doi: 10.1111/j.1365-2249.2004.02342.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erlejman AG, De Leo SA, Mazaira GI, Molinari AM, Camisay MF, Fontana VA, Piwien-Pilipuk MB, Galigniana MD. NF-κB transcriptional activity is modulated by FK506-binding proteins FKBP51 and FKBP52: a role for peptidyl-prolyl isomerase activity. J Biol Chem. 2014;289:26263–26276. doi: 10.1074/jbc.M114.582882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gobin SJ, Keijsers V, van Zutphen M, van den Elsen PJ. The role of enhancer A in the locus-specific transactivation of classical and nonclassical HLA class I genes by nuclear factor kappa B. J Immunol. 1998;161:2276–2283. [PubMed] [Google Scholar]
- Hovsepian E, Penas F, Siffo S, Mirkin GA, Goren NB. IL-10 inhibits the NF-κB and ERK/MAPK-mediated production of pro-inflammatory mediators by up-regulation of SOCS-3 in Trypanosoma cruzi-infected cardiomyocytes. PLoS One. 2013;8:e79445. doi: 10.1371/journal.pone.0079445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lentsch AB, Shanley TP, Sarma V, Ward PA. In vivo suppression of NF-kappa B and preservation of I kappa B alpha by interleukin-10 and interleukin-13. J Clin Invest. 1997;100:2443–2448. doi: 10.1172/JCI119786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, de Waal Malefyt R, Briere F, Parham C, Bridon JM, Banchereau J, Moore KW, Xu J. The EBV IL-10 homologue is a selective agonist with impaired binding to the IL-10 receptor. J Immunol. 2003;158:604–613. [PubMed] [Google Scholar]
- Molinero LL, Fuertes MB, Girart MV, Fainboim L, Rabinovich GA, Costas MA, Zwirner NW. NF-kappa B regulates expression of the MHC class I-related chain A gene in activated T lymphocytes. J Immunol. 2004;173:5583–5590. doi: 10.4049/jimmunol.173.9.5583. [DOI] [PubMed] [Google Scholar]
- Moschonas A, Kouraki M, Knox PG, Thymiakou E, Kardassis D, Eliopoulos AG. CD40 induces antigen transporter and immunoproteasome gene expression in carcinomas via the coordinated action of NF-kappaB and of NF-kappaB-mediated de novo synthesis of IRF-1. Mol Cell Biol. 2003;28:6208–6222. doi: 10.1128/MCB.00611-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naschberger E, Werner T, Vicente AB, Guenzi E, Töpolt K, Leubert R, Lubeseder-Martellato C, Nelson PJ, Stürzl M. Nuclear factor-kappaB motif and interferon-alpha-stimulated response element co-operate in the activation of guanylate-binding protein-1 expression by inflammatory cytokines in endothelial cells. Biochem J. 2004;379:409–420. doi: 10.1042/bj20031873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neurath MF, Becker C, Barbulescu K. Role of NF-kappaB in immune and inflammatory responses in the gut. Gut. 1998;43:856–860. doi: 10.1136/gut.43.6.856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pai S, O’Sullivan BJ, Cooper L, Thomas R, Khanna R. RelB nuclear translocation mediated by C-terminal activator regions of Epstein-Barr virus-encoded latent membrane protein 1 and its effect on antigen-presenting function in B cells. J Virol. 2002;76:1914–1921. doi: 10.1128/JVI.76.4.1914-1921.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portis T, Harding JC, Ratner L. The contribution of NF-kappa B activity to spontaneous proliferation and resistance to apoptosis in human T-cell leukemia virus type 1 Tax —induced tumors. Blood. 2001;98:1200–1208. doi: 10.1182/blood.V98.4.1200. [DOI] [PubMed] [Google Scholar]
- Raychaudhuri B, Fisher CJ, Farver CF, Malur A, Drazba J, Kavuru MS, Thomassen MJ. Interleukin10 (IL-10)-mediated inhibition of inflammatory cytokine production by human alveolar macrophages. Cytokine. 2000;12:1348–1355. doi: 10.1006/cyto.2000.0721. [DOI] [PubMed] [Google Scholar]
- Ren YX, Yang J, Zhang LJ, Sun RM, Zhao LF, Zhang M, Chen Y, Ma J, Qiao K, Sun QM, Long HT, Huang YC, Li XJ. Downregulation of expression of transporters associated with antigen processing 1 and 2 and human leukocyte antigen I and its effect on immunity in nasopharyngeal carcinoma patients. Mol Clin Oncol. 2003;2:51–58. doi: 10.3892/mco.2013.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romano MF, Lamberti A, Petrella A, Bisogni R, Tassone PF, Formisano S, Venuta S, Turco MC. IL-10 inhibits nuclear factor-kappa B/Rel nuclear activity in CD3-stimulated human peripheral T lymphocytes. J Immunol. 1996;156:2119–2123. [PubMed] [Google Scholar]
- Schottelius AJ, Mayo MW, Sartor RB, Baldwin AS., Jr Interleukin-10 signaling blocks inhibitor of kappaB kinase activity and nuclear factor kappaB DNA binding. J Biol Chem. 1999;274:31868–31874. doi: 10.1074/jbc.274.45.31868. [DOI] [PubMed] [Google Scholar]
- Symeonidou I, Kourelis A, Frydas I, Karagouni E, Anogeianaki A, Hatzistilianou M, Frydas S. Modulation of NF-kappaΒ signalling pathways by parasites. J Biol Regul Homeost Agents. 2010;24:471–479. [PubMed] [Google Scholar]
- Takayama T, Morelli AE, Onai N, Hirao M, Matsushima K, Tahara H, Thomson AW. Mammalian and viral IL-10 enhance C–C chemokine receptor 5 but down-regulate C–C chemokine receptor 7 expression by myeloid dendritic cells: impact on chemotactic responses and in vivo homing ability. J Immunol. 2003;166:7136–7143. doi: 10.4049/jimmunol.166.12.7136. [DOI] [PubMed] [Google Scholar]
- Varfolomeev E, Wayson SM, Dixit VM, Fairbrother WJ, Vucic D. The inhibitor of apoptosis protein fusion c-IAP2.MALT1 stimulates NF-kappaB activation independently of TRAF1 AND TRAF2. J Biol Chem. 2006;281:29022–29029. doi: 10.1074/jbc.M605116200. [DOI] [PubMed] [Google Scholar]
- Vujanovic L, Whiteside TL, Potter DM, Chu J, Ferrone S, Butterfield LH. Regulation of antigen presentation machinery in human dendritic cells by recombinant adenovirus. Cancer Immunol Immunother. 2003;58:121–133. doi: 10.1007/s00262-008-0533-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang P, Wu P, Siegel MI, Egan RW, Billah MM. Interleukin (IL)-10 inhibits nuclear factor kappa B (NF kappa B) activation in human monocytes. IL-10 and IL-4 suppress cytokine synthesis by different mechanisms. J Biol Chem. 1995;270:9558–9563. doi: 10.1074/jbc.270.16.9558. [DOI] [PubMed] [Google Scholar]
- Whiteside ST, Israël A. I kappa B proteins: structure, function and regulation. Semin Cancer Biol. 1997;8:75–82. doi: 10.1006/scbi.1997.0058. [DOI] [PubMed] [Google Scholar]
- Wright KL, White LC, Kelly A, Beck S, Trowsdale J, Ting JP. Coordinate regulation of the human TAP1 and LMP2 genes from a shared bidirectional promoter. J Exp Med. 1995;181:1459–1471. doi: 10.1084/jem.181.4.1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang T, McNally BA, Ferrone S, Liu Y, Zheng P. A single-nucleotide deletion leads to rapid degradation of TAP-1 mRNA in a melanoma cell line. J Biol Chem. 2003;278:15291–15296. doi: 10.1074/jbc.M300954200. [DOI] [PubMed] [Google Scholar]