Abstract
Acid ceramidases are enzymes with a vital role in metabolizing ceramide to sphingosine-1-phosphate that is an antiproliferative metabolite in the ceramide pathway. Inhibition of exogenous ceramides with ceramidase inhibitors lead to augmented ceramide levels in cells and in turn lead to cell cycle arrest and apoptosis. Our study aimed at targeting ceramide metabolic pathway to induce apoptosis in human breast cancer cell line (MCF7) and we examined the antiproliferative and apoptotic activities of ceranib-2, an inhibitor of human ceramidase, on this cell line as well ultrastructural and mophological changes. Methods used for our examinations in this study were the colorimetric MTT assay, Annexin V/Propidium iodide and JC-1 staining, transmission electron microscopy and confocal microscopy. Ceranib-2 effectively inhibited the viability of MCF7 cells in 24 h in a dose dependent manner leading to apoptosis via the mitochondrial pathway by reducing the potential of mitochondrial membrane. Additionally, significant changes on cell morphology and ultrastructure were observed on MCF7 cells exposed to ceranib-2 indicating apoptotic cell death. Collectively, our data demonstrate that ceranib-2 exerts a great potential to be an antineoplastic compound and that the mechanism of its action rely on its apoptosis inducing ability.
Keywords: Breast cancer, Ceranib-2, Confocal, Ultrastructure
Introduction
Ceramide, a bioactive sphingolipid, has a great significance in sphingolipid metabolism and plays a vital role as a pleiotropic cellular activator which may enable the induction of two mutually exclusive cellular functions, cell proliferation and cell death (Kus et al. 2015). Ceramide-mediated cell death has been the focus of various research in the last years (von Haefen et al. 2002). As tumor suppressor lipid ceramide is a regulatory compound for bioeffector functions like apoptosis and growth arrest (Ogretmen 2006). It has been thought for many years that endogenous ceramide production in response to several different stress stimuli is linked to senescence, growth arrest or cell death (Ogretmen and Hannun 2004; Futerman and Hannun 2004).
The fact that ceramide has an important role in the regulation of cell growth and in chemotherapy-induced cell death suggests novel therapeutic approaches based on elevating levels of endogenous ceramide actions. The treatment of human cancer cells with chemotherapeutic agents, such as daunorobicin, vincristine or gemcitabine, generally lead to ceramide generation by the de novo pathway (Ogretmen and Hannun 2004; Futerman and Hannun 2004). Endogenous levels of ceramide seems to be increased by targeting enzymes of ceramide clearance, thus causing increased cytotoxic responses in different cancer cells (Ogretmen and Hannun 2004; Fox et al. 2006). Strategies that manipulate the metabolism of sphingolipids, resulting in enhanced ceramide levels in cancer cells, have been launched out as unprecedented approaches towards cancer chemotherapy (Ogretmen and Hannun 2004; Reynolds et al. 2004).
Ceramides are intensely being examined as potential chemopreventive molecules. As suggested by evidence, primary and metastatic cancer cells have less endogenous ceramides than normal mucosa taken from the same patient. Thus, it can be inferred that it is possible for ceramide biosynthesis processes to be hindered in cancer cells (Struckhoff et al. 2004). Further, it is reported that radiation-resistant and multi-drug resistant tumor cells do not generate or accumulate ceramides suggesting an alteration in the sphingolipid pathway (Cai et al. 1997).
Evidence indicates that treating cancer cells in vitro with exogenous ceramides constantly bring about cycle arrest, senescence, differentiation, apoptosis or autophagy (Radin 2003; Ogretmen and Hannun 2004; Bansode et al. 2011). In addition to this ceramide response, enzymes, as well, are involved in the regulation of cancer cell growth and/or survival. For instance, several enzymes such as ceramidases that reduce ceramide levels, lead to anti-apoptotic, prosurvival and/or resistance to chemotherapy in various cancer cells (Kolesnick 2002). Acid ceramidase is an enzyme that metabolize ceramides to sphingosine-1-phosphate which has antiproliferative activity and promotes apoptotic cell death (Beckham et al. 2013; Brizuela et al. 2014). This enzyme is overexpressed in prostate and breast cancers (Sanger et al. 2015). Acid ceramidase has been reported to exert a potential target for drugs for cancer treatment (Vethakanraj et al. 2015). Inhibitors of ceramidases were reported to induce apoptosis and cause cell cycle arrest (Bhabak et al. 2013). Therefore, pharmaceutical/preclinical research and later clinical trials of cancer cases may include ceramide and sphingolipids (Sleiman et al. 2013) as well ceramidases. In the treatments with ceramidase inhibitors or exogenous ceramides cell cycle arrest and apoptosis are induced by increasing the ceramide concentration in the cells of breast (Sanger et al. 2015) and prostate cancers (Beckham et al. 2012). Such data triggered the discovery of acid ceramidases, a possible future target for anticancer therapy.
It is possible to find a large number of acid ceramidase inhibitors in the market of which N-oleolyl ethanolamine, B13, D-MAPP, D-NMAPPD have all been affirmed to induce apoptosis in colon, prostate, glioma, melanoma and breast cancer cell lines (Bhabak et al. 2013). It is also known that ceranib-2, which is a new type of acid ceramidase inhibitor considerably induced apoptosis in different cells such as human ovarian adenocarcinoma cells (Draper et al. 2011), and rat fibroblast cancer cells (Vejselova et al. 2014). But, the morphological and ultrastructural changes it causes in different cancer cell lines remain unknown. In this study we have examined the apoptotic activity of ceranib-2 in the human breast cancer cell line (MCF7) and evaluated the ability of ceranib-2, as a novel human ceramidase inhibitor to have antiproliferative and proapoptotic activities on human breast carcinoma cells by causing ultrastructural and morphological changes.
Materials and methods
Materials
Ceranib-2 was obtained from Cayman Chemical (Ann Arbor, MI, USA). Fetal bovine serum, penicillin streptomycin, dimethyl sulfoxide (DMSO), and 3-(4,5-dimethylthiazol-2-yl)-2,5 diphenyl-2H-tetrazolium bromide (MTT) were obtained from Sigma-Aldrich (St. Louis, MO, USA), and Roswell Park Memorial Institute medium (RPMI-1640) was obtained from GIBCO (Grand Island, NY, USA). MCF7 cells were from the American Type Culture Collection (Manassas, VA, USA).
Cell culture
In this study human breast adenocarcinoma MCF7 cells were used. MCF7 cells were grown in RPMI medium with penicillin–streptomycin (100 units/mL–100 μg/mL) and 10 % fetal bovine serum (v/v) at 37 °C under 5 % CO2 in humidified incubator conditions. Each third day cells were passaged and confluent cells were used as test cell line.
MTT cytotoxicity assay
MCF7 cells were plated into 96-well plates (1 × 103 cells/well). Different concentrations of ceranib-2 (in DMSO) ranging from 5 to 100 μM were added in each well and cells were incubated for 24 h at 37 °C in a humidified incubator with 5 % of CO2 in air. Following incubation period 20 μL of MTT solution (5 mg/mL) was added into each well followed by further incubation for 2 h in incubator. Growth media were aspirated and replaced to 200 μL of DMSO per well. Plates were read on an ELISA reader (EL × 808, BioTek, Winooski, VT, USA) at a wavelength of 540 nm (n = 3) (Kus et al. 2013; Kabadere et al. 2014).
Annexin V-FITC/PI evaluation
The apoptotic effects of ceranib-2 in MCF7 cells was examined by flow cytometry. MCF7 cells were exposed to 13 μM of ceranib-2 for 24 h. The treated cells were collected separately then washed twice with PBS by centrifugation. Collected cells were incubated with Annexin V/propidium iodide in dark at room temperature for 15 min and then analysed by flow cytometry (Akalin Ciftci et al. 2015).
JC-1 assay for evaluation of mitochondrial membrane potential
In order to examine the changes in the mitochondrial membrane polarization cell-permeant JC-1 dye was used. In the mitochondria with intact membrane, JC-1 dye easily enters and remains in the form of aggregates. Unlikely, in case of lost/decreased mitochondrial membrane potential it remains as monomers in the cytosol. Here in, MCF7 cells were incubated with 13 μM of ceranib-2 in six-well plates for 24 h. Untreated and ceranib-2 treated cells were collected and centrifuged at 1200× rpm for 5 min at room temperature. The supernatant was replaced by JC-1 working solution (0.5 mL/tube) and cells were resuspended and then incubated in a humidified CO2 incubator at 37 °C for 15 min. MCF7 cells were washed twice with assay buffer (1×) at room temperature and pellets were vortexed in 0.5 mL of assay buffer and then analyzed by flow cytometry. A Mitochondrial Membrane Potential Detection Kit (BD Pharmingen, Franklin Lakes, NJ, USA) was used (Liu et al. 2007a, b).
Confocal microscopic analysis for morphological changes
MCF7 cells exposed to IC50 concentration (13 μM) of ceranib-2 and untreated cells were washed in phosphate buffered saline (Invitrogen, Carlsbad, CA, USA) and fixed in glutaraldehyde/paraformaldehyde (2 %) at room temperature then cells were washed again stained with phalloidin (Sigma, St. Louis, MO, USA) and acridine orange (Sigma). Stained cells were evaluated under confocal microscope (Leica ICS-SP5 II, Wetzlar, Germany) with the supplied software (Leica Confocal Software Version 2.00, Leica) (Vejselova et al. 2014).
Transmission electron microscopic examination of MCF7 cells
Changes in the ultrastructure of ceranib-2 treated MCF7 cells were evaluated by transmission electron microscopy (TEM). For this manner untreated and ceranib-2 treated MCF7 cells were fixed in glutaraldehyde (2.5 %), post-fixed in osmium tetroxide and dehydrated in a graded ethanol. Dehydrated cells were embedded in Epon 812 epoxy and sectioned on ultramicrotome (Leica EMUC6). Thin-sections (100 nm) were stained in lead citrate and uranyl acetate, respectively, and examined by TEM (FEI Tecnai BioTWIN, Limmen, the Nederland) (Kus et al. 2015).
Statistical analysis
For statistical evaluation was performed using one-way variance analysis for multiple comparisons of SPSS 11.5 for Windows.
Results
The different concentrations of ceranib-2 caused dose-dependent growth inhibition on MCF7 cells for 24 h. Increasing concentrations of ceranib-2 effectively inhibited the growth of the treated cells (Table 1). The IC50 value of ceranib-2 on MCF7 cells for 24 h was found to be 13 µM.
Table 1.
Percentages of viability of MCF7 cells exposed to different concentrations of ceranib-2 for 24 h
| Applied ceranib-2 doses (µM) | Percentage of viability | Standard deviation (±) |
|---|---|---|
| 0 | 100.00 | 0.00 |
| 10 | 52.30 | 0.89 |
| 20 | 32.89 | 1.01 |
| 30 | 24.23 | 1.00 |
| 40 | 23.11 | 0.55 |
| 50 | 21.44 | 0.99 |
| 60 | 18.92 | 1.23 |
| 70 | 7.12 | 1.56 |
| 80 | 4.82 | 0.78 |
| 90 | 8.73 | 1.47 |
| 100 | 8.03 | 1.03 |
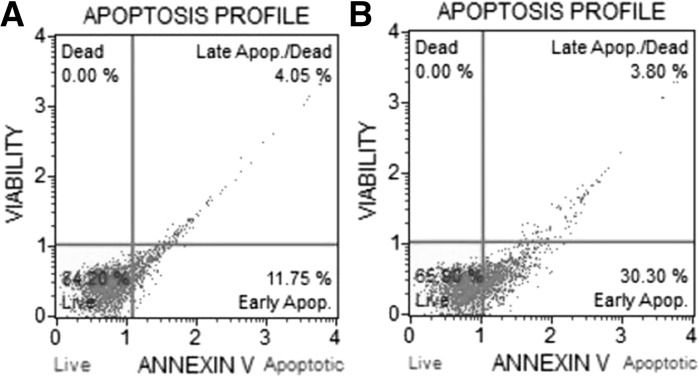
Apoptotic profiles of untreated and ceranib-2 treated MCF7 cells was determined by annexin V/PI staining. The percentage of viable cells in untreated and ceranib-2 treated MCF7 cells was found to be 84.20 and 65.90, respectively. The percentage of total apoptotic cells in untreated cell group was found to be 15.80 that includes 11.75 % early and 4.05 late apoptotic/dead cells. In ceranib-2 treated MCF7 cells 34.10 % of the population underwent apoptosis of which 30.3 % were early apoptotic and 3.80 % late apoptotic/dead cells (Fig. 1a, b).
Fig. 1.
Annexin/propidium iodide analysis results of MCF7 cells. a Apoptosis profile of untreated MCF7 cells. b Profile of apoptosis of 13 µM ceranib-2 treated MCF7 cells for 24 h
Flow cytometry measurements for evaluation of polarization of mitochondrial membrane potential revealed that 75.8 % of the population of untreated MCF7 cells were with intact/polarized mitochondrial membrane and 23.5 % of this population was with depolarized membranes of mitochondria. Unlikely in ceranib-2 treated cells percentage of cells with reduced mitochondrial membrane was increased to 64.2 (Fig. 2a, b).
Fig. 2.
Mitochondrial membrane potential analysis of MCF7 cells. a Untreated MCF7 cells: P2 = Cells with polarized mitochondrial membrane (75.8 %), P3 = Percentage of cells with depolarized mitochondrial membrane (23.5 %). b MCF7 cells exposed to 13 µM of ceranib-2 for 24 h. P2 = Cells with polarized mitochondrial membrane (34.4 %), P3 = Percentage of cells with depolarized mitochondrial membrane (64.2 %)
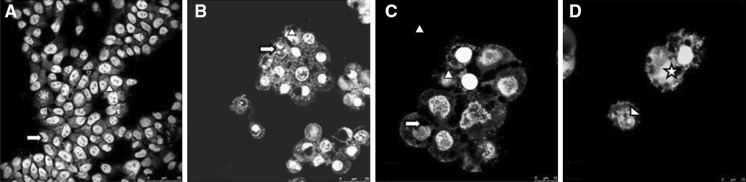
To investigate the characteristic sparks of apoptosis in the morphology of MCF7 cells acridin orange/phalloidin stained cells were observed under confocal microscope. A significant change in morphology was observed after ceranib-2 treatment whereas such changes were not observed in the control group (Fig. 3a). Early apoptotic cells were found to contain highly condensed chromatin, fragmented nuclei, cell shrinkage (Fig. 3b–d) whereas late apoptotic cells and necrotic cells fluoresced orange (Fig. 3d).
Fig. 3.
Confocal images of acridin orange and phalloidin stained MCF7 cells. a Control cells (×20); arrow normal nucleus, arrowhead normal cytoskeleton. b Ceranib-2 (13 µM) treated cells for 24 h (×40); arrow condensed and fragmented chromatin, arrowhead shrinked cell; c (×40): arrow hole in cytoskeleton, arrowhead shrinked and fragmented cell. d (×40): asterix damaged cell with hole in cytoskeleton and arrowhead condensed nucleus
The state of apoptosis and damages in organels in ceranib-2 treated MCF7 cells were detected by transmission electron microscope with high resolution. In control cells mitochondria were with a normal morphology (Fig. 4a). MCF7 cells exposed to ceranib-2 found to have highly swollen morphology in mitochondria. Loss of cristae, membrane blebbing and shrinkage were observed ultrastructural changes (Fig. 4b).
Fig. 4.
Electron micrographs of MCF7 cells. a Untreated MCF7 cells (×6000); asterix cell membrane, arrow nuclear membrane and arrowhead mitochondria. b (×8000): Ceranib-2 (13 µM) treated MCF7 cells for 24 h; arrow loss of cristae and deformed mitochondria
Discussion
The most prevalent cancer in woman which ends up with dead is breast cancer (Siegel et al. 2014). It is reported that nearly 30 % of the women who suffer from breast cancer developed metastases and eventually this disease caused their death. MCF7 cell line derived from a patient with metastatic breast cancer in 1970 has been regarded as an outstanding model system for the study of breast cancer for it pertains to the vulnerability of the cells to apoptosis (Yang et al. 2006). There are a good number of studies that suggest that cancer formation, progression and metastasis are related with the changes in apoptotic pathways (Bold et al. 1997). Therefore, recently it is focused on finding an anticancer therapy by the agents with ability to induce apoptosis in cancer cells (Kamesaki 1998).
Apoptosis has morphological and ultrastructural sparks of which cell shrinkage, excess of cell membrane activity also peripheral heterochromatin condensation, fragmentation of nucleus and cytoplasm as apoptotic bodies are significant (Eranshaw 1995). Above mentioned sparks are compatible with our confocal microscopy and electron microscopy results of MCF7 cells exposed to ceranib-2.
Cell shrinkage, chromatin condensation and nuclear fragmentation (karyorrhexis) are some of the morphological hallmarks of apoptosis (Vethakanraj et al. 2015). Similar morphological changes were observed in MCF7 cell line after 24 h of ceranib-2 treatment (Fig. 3c, d). These hallmarks were interpreted as apoptotic activity of ceranib-2 in MCF7 cells.
Another significant hallmark of apoptosis is loss of membrane symmetry that means moving of phosphatidyl serine from the inner membrane to the outer side. This event can easily be detected by annexin V and define the apoptotic population in a given cell population. Similarly, in this study we found that a good percentage of MCF7 cells exposed to ceranib-2 underwent apoptosis.
Ceramide induced programmed cell death in chemotherapy and radiotherapy has provided a new comprehension for mechanism of action in cancer treatment. Ceramidase enzyme metabolize ceramide to sphingosine-1-phosphate that is an antiproliferative metabolite in the ceramide pathway (Beckham et al. 2013). Ceramidase inhibitors cause augmented ceramide levels in the cells and in turn lead to cell cycle arrest and apoptosis by inhibition of exogenous ceramides (Liu et al. 2007a, b).
Different types of acid ceramidase inhibitors such as N-oleoyl ethanolamine, B-13, DMAPP, DM102, ceranib-2 have been shown to promote apoptosis in many cancer (5RP7) as prostate, glioma, breast and rat fibroblast cancer (5RP7) (Bhabak et al. 2013; Vejselova et al. 2014). Ceranib-2 a novel acid ceramidase inhibitor, and the apoptotic activity of ceranib-2 has been demonstrated in the 5RP7 cell line (Vejselova et al. 2014) and the MCF7 cell line (Vethakanraj et al. 2015) our MTT data demonstrated that ceranib-2 caused mitochondrial dysfunction and loss in mitochondrial membrane potential. Additionally, our data showed that ceranib-2 can induce death of MCF7 cells initially leading to mitochondrial dysfunction. Similarly we found that the mitochondrial membrane potential of ceranib-2 treated MCF7 cells was decreased. In addition, it was reported that endogenously produced or exogenously applied ceramide can lead to production of free radicals and hydrogen peroxide at the mitochondria thus disrupting the mitochondrial membrane potential (Garcia-Ruiz et al. 1997). This may have occurred in ceranib-2 treated MCF7 cells in our study.
Production of reactive oxygen species, ATP shortening, decrease in the inner mitochondrial membrane potential and inhibition of electron transport chain activity are reported as hallmarks for mitochondrial dysfunction (Rivzi et al. 2011). In addition to this the release of cytochrome c through opened permeabilization transition pore complex is reported to occur after a decrease in mitochondrial membrane potential (Struckhoff et al. 2004). Our findings clearly revealed that mitochondrial membrane polarization was remarkably decreased in MCF7 cells exposed to ceranib-2 for 24 h. This demonstrates the involvement of mitochondria in apoptosis caused by ceranib-2. It was reported for ceramide that it promoted apoptosis via activation of mitochondria (Ghafourifar et al. 1999). The percentage of cells with depolarized mitochondrial membrane potential was augmented remarkably in ceranib-2 treated MCF7 cells (Fig. 3b).
Ultrastructural analyses of LNCaP cells treated with 25 and 50 μM of ceranib-2 for 24 h showed chromatin condensation, mitochondrial dysfunction and damaged cell integrity (Kus et al. 2015). Similarly our ultrastructural analyses resulted in findings that showed MCF7 cells exposed to 13 μM ceranib-2 for 24 h to have a modified ultrastructure. Observed ultrastructural changes in ceranib-2 treated cells were loss of cristae that indicates mitochondrial dysfunction and damaged and condensed nuclei. Ultrastructural changes provided an excessive swollen morphology in mitochondria in ceranib-2 treated cells when compared to untreated MCF7 cells, the mitochondria of ceranib-2 treated cells were swollen. Sun et al. (2007) have reported that this swelling may occur only if the mitochondrial membrane potential is lost or cytochrome c is released outside the mitochondria. Similarly, we stated that the apoptosis pathway was activated by ceranib-2 treatment and that the cells tented to undergo apoptosis via reducing mitochondrial membrane potential. Similarly, in our previous study ceranib-2 caused mitochondrial dysfunction in NIH/3T3 and 5RP7 cells (Vejselova et al. 2014).
On the basis of our findings (growth inhibition, apoptosis induction, morphological and ultrastructural changes) it can be concluded that ceranib-2 has a great potential to be cytotoxic in MCF7 cell line and cause programmed cell death/apoptosis and thus may be useful in designing antineoplastic therapeutics after further investigations.
Acknowledgments
We kindly thanks the Scientific and Technological Research Council of Turkey (TUBITAK) for the financial support that was provided for Djanan Vejselova and the Anadolu University Scientific Research Project Unit (Project No: 1505F234).
Compliance with ethical standards
Conflict of interest
Authors claim no conflicts of interest.
References
- Akalin Ciftci G, Iscan A, Kutlu M. Escin reduces cell proliferation and induces apoptosis on glioma and lung adenocarcinoma cells. Cytotechnology. 2015 doi: 10.1007/s10616-015-9877-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bansode RR, Ahmedna M, Svoboda KR, Losso JN. Coupling in vitro and in vivo paradigm reveals a dose dependent inhibition of angiogenesis followed by initiation of autophagy by C6-ceramide. Int J Biol Sci. 2011;7:629–644. doi: 10.7150/ijbs.7.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckham TH, Lu P, Cheng JC, Zhao D, Turner LS, Zhang X, Hoffman S, Armeson KE, Liu A, Marrison T, Hannun YA, Liu X. Acid ceramidase-mediated production of sphingosine-1 phosphate promotes prostate cancer invasion through upregulation of cathepsin B. Int J Cancer. 2012;131:2034–2043. doi: 10.1002/ijc.27480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckham TH, Cheng JC, Lu P, Marrison ST, Norris JS, Liu X. Acid ceramidase promotes nuclear export of PTEN through sphingosine 1-phosphate mediated Akt signaling. PlosOne. 2013;8:765–793. doi: 10.1371/journal.pone.0076593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhabak KP, Kleuser B, Huwiler A, Arenz C. Effective inhibition of acid and neutral ceramidases by novel B-13 and LCL-464 analogues. Bioorg Med Chem. 2013;21:874–882. doi: 10.1016/j.bmc.2012.12.014. [DOI] [PubMed] [Google Scholar]
- Bold RJ, Termuhlen PM, McConkey DJ. Apoptosis cancer and cancer therapy. Surg Oncol. 1997;6:133–142. doi: 10.1016/S0960-7404(97)00015-7. [DOI] [PubMed] [Google Scholar]
- Brizuela L, Martin C, Jeannot P, Ader I, Gstalder C, Andrieu G, Bocquet M, Laffosse JM, Gomez-Brouchet A, Malavaud B, Sabbadini RA, Cuvillier O. Osteoblast-derived sphingosine 1-phosphate to induce proliferation and confer resistance to therapeutics to bone metastasis-derived prostate cancer cells. Mol Oncol. 2014;8:1181–1195. doi: 10.1016/j.molonc.2014.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai Z, Bettaieb A, Mahdani NE, Legres LG, Stancou R, Masliah J, Chouaib S. Alteration of the sphingomyelin/ceramide pathway is associated with the resistance of human breast carcinoma MCF7 cells to tumor necrosis factor-alpha-mediated cytotoxicity. J Biol Chem. 1997;272:6918–6926. doi: 10.1074/jbc.272.11.6918. [DOI] [PubMed] [Google Scholar]
- Draper JM, Xia Z, Smith RA, Zhung Y, Wang W, Smith CD. Discovery and evaluation of inhibitors of human ceramidase. Mol Cancer Ther. 2011;10:2052–2261. doi: 10.1158/1535-7163.MCT-11-0365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eranshaw WC. Nuclear changes in apoptosis. Curr Opin Cell Biol. 1995;7:337–343. doi: 10.1016/0955-0674(95)80088-3. [DOI] [PubMed] [Google Scholar]
- Fox TE, Finnegan CM, Blumenthal R, Kester M. The clinical potential of sphingolipid based therapeutics. Cell Mol Life Sci. 2006;63:1017–1023. doi: 10.1007/s00018-005-5543-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Futerman AH, Hannun YA. The complex life of simple sphingolipids. EMBO Rep. 2004;5:777–782. doi: 10.1038/sj.embor.7400208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Ruiz C, Colell A, Mari M, Morales A, Fernandez-Checa JC. Direct effect of ceramide on the mitochondrial electron transport chain leads to generation of reactive oxygen species. Role of mitochondrial glutathione. J Biol Chem. 1997;272:11369–11377. doi: 10.1074/jbc.272.17.11369. [DOI] [PubMed] [Google Scholar]
- Ghafourifar P, Klein SD, Schucht O, Schenk U, Pruschy M, Rocha S, Richter C. Mitochondrial nitric-oxide synthase stimulation causes cytochrome c release from isolated mitochondria. Evidence for intramitochondrial peroxynitrite formation. J Biol Chem. 1999;274:6080–6084. doi: 10.1074/jbc.274.10.6080. [DOI] [PubMed] [Google Scholar]
- Kabadere S, Kuş G, Uyar R, Oztopcu-Vatan P. Licofelone abolishes survival of carcinogenic fibroblasts by inducing apoptosis. Drug Chem Toxicol. 2014;37:1–7. doi: 10.3109/01480545.2013.806525. [DOI] [PubMed] [Google Scholar]
- Kamesaki H. Mechanisms involved in chemotherapy-induced apoptosis and their implications in cancer chemotherapy. Int J Hematol. 1998;68:29–43. doi: 10.1016/S0925-5710(98)00038-3. [DOI] [PubMed] [Google Scholar]
- Kolesnick R. The therapeutic potential of modulating the ceramide/sphingomyelin pathway. J Clin Invest. 2002;110:3–8. doi: 10.1172/JCI0216127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kus G, Oztopcu-Vatan P, Uyar R, Kabadere S. Cytotoxic and apoptotic functions of licofelone on rat glioma cells. Acta Biol Hungarica. 2013;64:438–452. doi: 10.1556/ABiol.64.2013.4.4. [DOI] [PubMed] [Google Scholar]
- Kus G, Kabadere S, Uyar R, Kutlu HM. Induction of apoptosis in prostate cancer cells by the novel ceramidase inhibitor ceranib-2. In Vitro Cell Dev Biol Animal. 2015;51:1056–1063. doi: 10.1007/s11626-015-9932-9. [DOI] [PubMed] [Google Scholar]
- Liu T, Hannafon B, Gill L, Kelly W, Benbrook D. Flex-Hets differentially induce apoptosis in cancer over normal cells by directly targeting mitochondria. Mol Cancer Ther. 2007;6:1814–1822. doi: 10.1158/1535-7163.MCT-06-0279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Elojeimy S, Turner LS, Mahdy AEM, Zeidan YH, Bielawski J, Dong JY, El-Zawahry AM, Guo GW, Hannun YA, Holman DH, Rubinchik S, Szulc Z, Keane TE, Tavassol M, Norris JS. Acid ceramidase inhibition: a novel target for cancer therapy. Front Biosci. 2007;13:2293–2298. doi: 10.2741/2843. [DOI] [PubMed] [Google Scholar]
- Ogretmen B. Minireview: sphingolipids in cancer: regulation of pathogenesis and therapy. FEBS Lett. 2006;580:5467–5476. doi: 10.1016/j.febslet.2006.08.052. [DOI] [PubMed] [Google Scholar]
- Ogretmen B, Hannun YA. Biologically active sphingolipids in cancer pathogenesis and treatment. Nat Rev Cancer. 2004;4:604–616. doi: 10.1038/nrc1411. [DOI] [PubMed] [Google Scholar]
- Radin NS. Killing tumours by ceramide-induced apoptosis: a critique of available drugs. Biochem J. 2003;371:243–256. doi: 10.1042/bj20021878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds CP, Maurer BJ, Kolesnik RN. Ceramide synthesis and metabolism as a target for cancer therapy. Cancer Lett. 2004;206:169–180. doi: 10.1016/j.canlet.2003.08.034. [DOI] [PubMed] [Google Scholar]
- Rivzi F, Heimann T, Herrneiter A, O’brien WJ. Mitochondrial dysfunction links ceramide activated HRK expression and cell death. PlosOne. 2011;6:e18137. doi: 10.1371/journal.pone.0018137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger N, Ruckhäberle E, Györffy B, Engels K, Heinrich T, Fehm T, Graf A, Holtrich U, Becker S, Karn T. Acid ceramidase is associated with an improved prognosis in both DCIS and invasive breast cancer. Mol Oncol. 2015;9:58–67. doi: 10.1016/j.molonc.2014.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel R, Ma J, Zou Z, Ahmedin J. Cancer statistics. CA Cancer J Clin. 2014;64:9–29. doi: 10.3322/caac.21208. [DOI] [PubMed] [Google Scholar]
- Sleiman RH, Esmerian MO, Kobeissy H, Dbaibo G. P53 and ceramide as collaborators in the stress response. Int J Mol Sci. 2013;14:4982–5012. doi: 10.3390/ijms14034982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Struckhoff AP, Bittman R, Burow ME, Clean S, Elliott S, Hammond T, Tang Y, Beckman BS. Novel ceramide analogs as a potential chemotherapeutic agents in breast cancer. J Pharmacol Exp Ther. 2004;309:523–531. doi: 10.1124/jpet.103.062760. [DOI] [PubMed] [Google Scholar]
- Sun MG, Williams J, Munoz-Pinedo C, Perkins GA, Brown JM, Ellisman MH, Green DR, Frey TG. Correlated three-dimensional light and electron microscopy reveals transformation of mitochondria during apoptosis. Nat Cell Biol. 2007;9:1057–1065. doi: 10.1038/ncb1630. [DOI] [PubMed] [Google Scholar]
- Vejselova D, Kutlu HM, Kus G, Kabadere S, Uyar R. Cytotoxic and apoptotic effects of ceranib-2 offering potential for a new antineoplastic agent in the treatment of cancer cells. Turk J Biol. 2014;38:916–921. doi: 10.3906/biy-1405-36. [DOI] [Google Scholar]
- Vethakanraj HS, Babu TA, Sudarsanan GB, Duraisamy PK, Kumar SA. Targeting ceramide metabolic pathway induces apoptosis in human breast cancer cell lines. Biochem Biophys Res Commun. 2015;464:833–839. doi: 10.1016/j.bbrc.2015.07.047. [DOI] [PubMed] [Google Scholar]
- von Haefen C, Wieder T, Gillissen B, Stärck L, Graupner V, Dörken B, Daniel PT. Ceramide induces mitochondrial activation and apoptosis via a Bax-dependent pathway in human carcinoma cells. Oncogene. 2002;21:4009–4019. doi: 10.1038/sj.onc.1205497. [DOI] [PubMed] [Google Scholar]
- Yang HL, Chen CS, Chang WH, Lu FJ, Lai YC, Chan CC, et al. Growth inhibition and induction of apoptosis in MCF7 breast cancer cells by Antrodia camphorata. Cancer Lett. 2006;231:215–227. doi: 10.1016/j.canlet.2005.02.004. [DOI] [PubMed] [Google Scholar]