Abstract
Awareness of the body (i.e., interoceptive awareness) and self-referential thought represent two distinct, yet habitually integrated aspects of self. A recent neuroanatomical and processing model for depression and anxiety incorporates the connections between increased but low fidelity afferent interoceptive input with self-referential and belief-based states. A deeper understanding of how self-referential processes are integrated with interoceptive processes may ultimately aid in our understanding of altered, maladaptive views of the self – a shared experience of individuals with mood and anxiety disorders. Thus, the purpose of the current study was to examine how negative self-referential processing (i.e., brooding rumination) relates to interoception in the context of affective psychopathology. Undergraduate students (N = 82) completed an interoception task (heartbeat counting) in addition to self-reported measures of rumination and depression and anxiety symptoms. Results indicated an interaction effect of brooding rumination and interoceptive awareness on depression and anxiety-related distress. Specifically, high levels of brooding rumination coupled with low levels of interoceptive awareness were associated with the highest levels of depression and anxiety-related distress, whereas low levels of brooding rumination coupled with high levels of interoceptive awareness were associated with lower levels of depression and anxiety-related distress. The findings provide further support for the conceptualization of anxiety and depression as conditions involving the integration of interoceptive processes and negative self-referential processes.
Keywords: Interoceptive awareness, Rumination, Self-referential processing, Depression, Anxiety
1. Introduction
Two of the most commonly occurring emotional disorders, major depressive disorder (MDD) and generalized anxiety disorder (GAD), represent an enormous public health burden. Both disorders are similar in that impairment occurs within cognitive, affective, and somatic domains of functioning. Although significant progress in treating these disorders has been made, less than half of patients receiving a combination of medication and psychotherapy for MDD achieve remission (Casacalenda, Perry, & Looper, 2002). Similarly, only 50–60% of patients seeking treatment for GAD demonstrate clinically meaningful change (Borkovec & Ruscio, 2001). Thus, further integrative research is needed to identify aberrant mechanisms that can be targeted for more efficient treatment interventions (Sanislow et al., 2010).
The capacity to reflect on one's self (i.e., self-referential processing) is a defining characteristic of human beings and may represent the default state of the brain (Raichle et al., 2001). The neuroanatomy of self-referential processing has been linked primarily to activity in the medial prefrontal cortex (mPFC; Buckner, Andrews-Hanna, & Schacter, 2008), an area of the brain that is also implicated in the detection of emotionally salient stimuli (Morris et al., 1998; Phillips, Drevets, Rauch, & Lane, 2003) as well as determining whether beliefs are “acceptable” or “unacceptable” (Paulus & Stein, 2010). Activation in medial prefrontal regions has been observed in association with subjective reports and behavioral measures of mind wandering (Buckner et al., 2008; Christoff et al., 2009) which is akin to activity typically associated with the “default mode network” involved in self-regulation and monitoring of the internal milieu (Farb et al., 2007; Paulus & Stein, 2010). Indeed, the mPFC is often found active during conditions in which attention is internally directed, processing self-relevant thoughts and beliefs (Paulus & Stein, 2010). Thus, in the absence of task demands, neural activation tends to reflect an automatic tendency to engage in narrative, or evaluative cognitive processing (Mason et al., 2007; McKiernan, D'Angelo, Kaufman, & Binder, 2006). In its healthy forms, self-referential processing is critical for self-regulation, adaptive social cognition, and planning of personally relevant goals (Mennin & Fresco, 2013).
However, the human capacity for such higher-level self-consciousness may also be associated with dysfunction (Olatunji, Naragon-Gainey, & Wolitzky-Taylor, 2013), as a shared aspect of both anxiety and depression is the altered experience of the individual with respect to self (Paulus & Stein, 2010). Both affective psychopathologies exhibit the feature of negative self-referential processing (Northoff, 2007), though the content and temporal orientation of the negative thoughts may vary between depression and anxiety (Beckwé, Deroost, Koster, De Lissnyder, & De Raedt, 2014). Negative self-referential thought in depression characteristically takes on the form of rumination, which describes a focus on negative past events along with a tendency to respond to sad mood by passively and repetitively focusing on the causes and consequences of negative emotions (Nolen-Hoeksema & Morrow, 1993). Worry, a core feature of anxiety, focuses on possible negative events in the future and strategies to prevent such events from occurring (Borkovec & Inz, 1990). Though distinct, recent literature suggests that rumination and worry are transdiagnostic processes that cut across MDD, GAD, and comorbid patients (Kircanski, Thompson, Sorenson, Sherdell, & Gotlib, 2015). Thus, both rumination and worry represent dysfunctional forms of self-referential processing (Farb et al., 2015) and reflect cognitive processing that is temporally oriented toward the past or future – that is, away from present moment experience (Kircanski et al., 2015). Importantly, research has found that easily triggered self-evaluative processes to negative emotion may result in maladaptive cognitive reactivity, suggesting that the inability to disengage from such self-evaluative processes may ultimately be a hallmark of relapse risk (Farb, Anderson, Bloch, & Segal, 2011). It is this endemic reliance upon networks supporting temporally extended processing that may obscure the recruitment of networks implicated in more immediate, perceptual self-reflection (Farb et al., 2007).
Another important aspect of self is corporeal awareness (Berlucchi & Aglioti, 2010) or the “material self” (Craig, 2002). Research is beginning to uncover the importance of receiving, accessing, and appraising internal visceral signals, as such processing is critical for an organism's maintenance of desired physiological states, self-regulation, homeostasis, and in turn, survival (Craig, 2013; Farb et al., 2015; Paulus, 2007). The term “interoception” has been variously employed to reference the awareness of body signals (Mussgay, Klinkenberg, & Rüddel, 1999), physiological feedback from the whole body (Wiens, 2005), and afferent information that arises from anywhere and everywhere within the body (Cameron, 2001). Neuroanatomical evidence supports such broad definitions as research has indicated that a class of afferent fibers that monitor the physiological state of all internal organs of the body converge in the insular cortex (Craig, 2002; Paulus & Stein, 2006). The insula, a candidate brain area implicated in interoception (Critchley, Wiens, Rotshtein, Öhman, & Dolan, 2004), is critical for evaluating the potential impact of stimuli on the body (Paulus & Stein, 2006), including generation and regulation of affective responses and detection of emotionally salient stimuli (Paulus & Stein, 2010). However, other evidence suggests that the insula may not be solely necessary for interoception (Khalsa, Rudrauf, Feinstein, & Tranel, 2009), and that other body sensitive brain regions are engaged by interoceptive processing, including the somatosensory cortex and cerebellum (Cameron & Minoshima, 2002; Rapps, Van Oudenhove, Enck, & Aziz, 2008).
Various techniques have been developed to quantify interoceptive awareness in research settings. Efforts have focused on cardiovascular perception primarily because heartbeats are distinct autonomic events that are easily measured via non-invasive methods, and clearly associated with emotion (Barrett, Quigley, Bliss-Moreau, & Aronson, 2004; Herbert, Pollatos, Flor, Enck, & Schandry, 2010; Wiens, Mezzacappa, & Katkin, 2000). The most widely used method is heartbeat counting (Ehlers & Breuer, 1992; Schandry, 1981), which requires participants to silently count their heartbeats during various intervals of time. Performance is captured by an error score, which reflects the difference between the number of heartbeats reported by the individual compared to the actual number of heartbeats that occurred in a given time period as recorded by physiological monitoring equipment. “Good perceivers” on these tasks are individuals whose subjective reports align with their objective number of recorded heartbeats. Other non-invasive approaches to assessing interoception include various forms of respiratory load discrimination tasks (Zechman, Hall, & Hull, 1957), water load tasks (Herbert, Muth, Pollatos, & Herbert, 2012), and tasks that involve simply paying attention to interoceptive sensations of the heartbeat or breath (Farb, Segal, & Anderson, 2013; Simmons et al., 2013).
A growing body of research has shown that interoceptive awareness is implicated in decision making (Dunn, Evans, Makarova, White, & Clark, 2012; Dunn, Galton et al. 2010; Kirk, Downar, & Montague, 2011; Werner, Jung, Duschek, & Schandry, 2009), affect regulation (Füstös, Gramann, Herbert, & Pollatos, 2013; Sze, Gyurak, Yuan, & Levenson, 2010), and in other domains of cognitive and behavioral functioning (Herbert et al., 2010; Pollatos & Schandry, 2008; Pollatos, Schandry, Auer, & Kaufmann, 2007; Werner, Mannhart, Reyes Del Paso, & Duschek, 2014; Werner, Peres, Duschek, & Schandry, 2010). Specifically, Kirk et al. (2011) found that interoception drives increased rational decision-making while playing the ultimatum game, a two-person monetary exchange task. Füstös et al. (2013) provided electrophysiological evidence for the relevance of interoceptive awareness in emotion regulation; specifically, interoceptive awareness facilitated downregulation of affect-related arousal during a cognitive reappraisal task, suggesting that the more aware a person is of ongoing bodily processes, the more successful this person will be in regulating emotions in response to negative affect. However, another study found that individuals who demonstrated superior interoceptive awareness revealed greater arousal (i.e. greater P300 amplitudes) in response to a set of emotional stimuli (Pollatos, Kirsch, & Schandry, 2005). The finding that interoceptive awareness was associated with down-regulation of emotional arousal (Füstös et al., 2013), yet associated with enhanced arousal (Pollatos et al., 2005) suggests that individuals with greater interoceptive awareness may be able to respond more flexibly to task demands. Finally, previous work has also found that individuals who show greater interoceptive sensitivity spend less physical effort due to their better perception of bodily feedback coming from their cardiac system (Herbert, Ulbrich, & Schandry, 2007).
Previous research also suggests that the focus of attention on directly experienced sensations (i.e., visceral signals) may represent a critical aspect of well-being (Davidson, 2004; Watkins & Moulds, 2005). Evidence has shown that localized attention to body sensations enables subsequent gains in emotional and cognitive regulation by enhancing sensory information processing in the brain (Kerr, Sacchet, Lazar, Moore, & Jones, 2013). The notion that more experiential forms of attention are important for well-being is shared by previous theories which distinguish maladaptive verses adaptive modes of mind, or self-focus (Teasdale, 1999; Watkins & Teasdale, 2004). For example, Interacting Cognitive Subsystems (ICS) theory (Teasdale, 1999) proposes that direct experience of one's sensations (i.e., mindful experiencing-being mode) allows the integration of multiple elements of experience, which subsequently facilitates emotional well-being. Similarly, experiential forms of self-awareness appear to be more adaptive than analytical forms of self-reference in recovered depressed patients (Watkins & Teasdale, 2004). From this reasoning, attention to body signals in the present moment may disrupt forms of self-reference that underlie maladaptive cognitive reactivity (Farb et al., 2011).
However, greater interoceptive ability may also contribute to anxiety and panic related disorders when catastrophized (Farb et al., 2015). Although the exact relationship between interoceptive awareness and psychopathology remains unclear, the literature generally supports a positive association between cardiac awareness and anxiety (Domschke, Stevens, Pfleiderer, & Gerlach, 2010; Ehlers & Breuer, 1992; Paulus & Stein, 2010; Pollatos, Traut-Mattausch, & Schandry, 2009; Schandry, 1981), though results are not equivocal (Ehlers & Breuer, 1996; Van der Does, Antony, Ehlers, & Barsky, 2000). For example, one study found that patients with panic disorder, infrequent panickers, and individuals with other anxiety disorders reported greater cardiac awareness compared to healthy controls (Ehlers & Breuer, 1992). In a similar study with children, increased panic symptoms were associated with enhanced heartbeat perception (Eley, Stirling, Ehlers, Gregory, & Clark, 2004). Increased cardiac awareness has also been found in social phobia (Pineles & Mineka, 2005). Such findings have engendered the hypothesis that enhanced body awareness may be implicated in the etiology and maintenance of anxiety disorders (Mehling et al., 2012), though recent evidence suggests that hypervigilance to body symptoms is not necessarily a bottom-up dispositional tendency, but rather a metacognitive process related to threatening beliefs about somatic sensations (Yoris et al., 2015). Relatedly, it has been proposed that anxiety sensitivity (Reiss, Peterson, Gursky, & McNally, 1986), or the tendency to view body signals as threatening, is mediated through processes underlying the perception of heightened interoceptive prediction signals (Paulus & Stein, 2006); that is, anxiety-prone individuals may not have a heightened baseline interoceptive state, but an exaggerated expectation (i.e., belief) about future body states. Thus, whether greater interoceptive ability contributes to or detracts from well-being may depend on how such awareness is understood, and how to skillfully relate to body sensations remains a question for further inquiry (Farb et al., 2015).
Conversely, most studies report that heartbeat perception is less accurate in depressed individuals (Dunn, Dalgleish, Ogilvie, & Lawrence, 2007; Paulus & Stein, 2010); however, Dunn et al. (2007) noted that an increase from low to moderate depression severity resulted in less accurate heartbeat perception, whereas an increase from moderate to severe depression resulted in increased perception accuracy. Overall, there seems to be an interactive effect of depressive and anxious symptomatology on interoceptive sensitivity. Dunn, Stefanovitch et al. (2010) examined the interaction of scores on depression-specific anhedonia and anxiety-specific arousal symptom dimensions on interoceptive accuracy and found that as anhedonia symptoms increased, the relationship between arousal and interoceptive accuracy became less marked. Pollatos et al. (2009) also demonstrated a moderating effect of anxiety on the relationship of interoception and depression. Furman, Waugh, Bhattacharjee, Thompson, and Gotlib (2013) reported poorer heartbeat counting accuracy in a sample of depressed females without anxiety comorbidities. Finally, neuroimaging results provide evidence of abnormal insula activity in relation to depression symptoms in both clinical (Wiebking et al., 2010) and non-clinical (Wiebking et al., 2014) populations. In summary, accumulating evidence suggests abnormal somatic signaling and interoceptive dysfunction in depression (Harshaw, 2015).
A recent neuroanatomical and processing model (Paulus & Stein, 2010) for depression and anxiety incorporates the connections between increased but low fidelity afferent interoceptive input with self-referential and belief-based states. Specifically, Paulus and Stein (2010) propose that propositional states that refer to the state of the individual, which are processed in the mPFC, contribute significantly to the evaluation of anticipatory interoceptive signals; it is assumed that an exaggeration of the valence (i.e., positive or negative) relative to the individual's internal state plays a role in enhancing the aversive aspects of predictive body signals associated with potential negative outcomes. Furthermore, Paulus and Stein (2010) propose that individuals at risk for anxiety and depression exhibit a reduced signal to noise ratio of interoceptive afferents; for example, a heartbeat or sensation related to breathing interpreted with a negative valance, or belief-based process (e.g., “there is something wrong with my heart”) will increase the sympathetic nervous system response and result in withdrawal or avoidance behaviors. Consequently, low fidelity interoceptive afferents result in overactive top-down brain modulatory areas (e.g., mPFC) that engage constantly to differentially amplify or attenuate body signals. In summation, the model proposed by Paulus and Stein (2010) proposes that a change in one's internal state, due either to an increased attentional bias toward negative self-view (depression) or threat (anxiety), generates beliefs that are implicated in the interpretation of afferent internal body signals.
Thus, to better understand the dysfunctions observed in depression and anxiety with respect to self, the current study sought to examine the integration of interoceptive processes with self-referential processes in the context of self-reported depression and anxiety symptoms. In particular, the aim of the current study was to further elucidate the relationship between negative self-referential processing (i.e., rumination), interoceptive awareness, and depression and anxiety symptoms. Although previous studies have separately examined rumination and interoceptive awareness in the context psychopathology, research has yet to investigate the integration of brooding rumination and interoceptive awareness in the context of affective psychopathology. In conceptualizing the basic dysfunction in depression and anxiety as potentially overactive top-down modulatory processes (i.e., negative self-referential processing), which subsequently increase attentional bias (Paulus & Stein, 2010), we hypothesized that similar results would be obtained for anxiety and depression in the context of rumination. Results from Dunn, Stefanovitch et al. (2010), raise the possibility that depression symptoms attenuate the relationship between interoceptive accuracy and anxiety symptoms; we postulate that this may occur via rumination. Our hypothesis draws from other research raising the possibility that networks supporting temporally extended processing (e.g., rumination) may obscure the recruitment of networks implicated in more immediate, perceptual self-reflection (Farb et al., 2007), which may include the perception of body signals. Finally, this inability to focus attention on directly experienced sensations may negatively affect well-being (Davidson, 2004; Watkins & Moulds, 2005). Thus, we hypothesized an interaction effect of brooding rumination and interoceptive awareness on depression and anxiety-related symptoms. Specifically, we hypothesized that high levels of rumination coupled with low levels of interoceptive awareness would be associated with the highest levels of depression and anxiety symptoms, and that lower levels of rumination coupled with higher levels of interoceptive awareness would be associated with lower levels of depression and anxiety symptoms.
2. Methods
2.1. Participants
Data were obtained from 101 college students at a large mid-western university using an online research pool that compensated participation with course credits. Of these participants, 75 were female (74.3%) and the mean age was 19.63 years (SD = 2.04). The sample consisted of Caucasian (69.3%), African American (12.5%), Biracial (4.5%), Latino (3.4%), Asian (2.3%), and Other (6.8%) participants. All participants provided written informed consent. A power analysis for a linear regression with 3 predictive variables determined that this sample size was sufficient to reveal a medium effect size.
To ensure a broad range of mood symptoms, we allowed for the presence of self-reported clinical symptoms. Seven participants self-reported a current anxiety diagnosis. Two participants self-reported a current depression diagnosis, and two participants self-reported comorbid anxiety and depression diagnoses. One participant reported diabetes, and one participant reported high blood pressure. Self-reported medication use in the current sample included two participants taking benzodiazepines, seven participants taking anti-depressants, and four participants taking ADHD medication. Participants were excluded if they reported current or past cardiac or respiratory disease, including arrhythmia; no such cases were reported.
2.2. Procedure
Following consenting procedures, participants' weight, height, and blood pressure were measured. Participants then completed a series of questionnaires (described below), in addition to other measures not included in the present analysis.
Participants were fitted with non-polarizable Ag-AgCl adhesive disposable electrodes (Biopac Systems Inc., Santa Barbara, California, USA) attached to the right mid-clavicle and lower left rib cage for electrocardiogram (ECG) measurement. Two additional non-polarizable Ag-AgCl electrodes positioned on the medial phalanx of the index and middle fingers recorded skin conductance. Participants were instructed to remain as still as possible during recording to minimize the occurrence of motion artifacts. Baseline measures of ECG and skin conductance were collected for 3 min prior to experimental task initiation. Participants then completed the heartbeat perception task. ECG was recorded using a BIOPAC MP150 unit (Biopac Systems Inc., Santa Barbara, California, USA) acquiring data at 250 Hz, connected to a PC running Acqknowledge software. A live-feed camera during experimental procedures ensured participant compliance.
2.3. Interoceptive awareness
Interoceptive awareness was assessed using a heartbeat counting task similar to the one developed by Schandry (1981), which has been used widely throughout the literature (e.g., Herbert et al., 2007, 2010; Pollatos et al., 2007). Participants were instructed to silently count the number of heartbeats they perceived during various intervals of time (25, 35, and 55 s), each repeated twice for a total of 6 trials, with a 30 s rest period given between each trial. A 12 s practice trial preceded experimental trials. Participants were instructed to “concentrate on heart activity without feeling for a pulse or altering the breath” and to report the number of heartbeats they perceived for each trial. Each trial began with an 800 Hz, 100 ms tone, indicating that the participant should be prepared to start perceiving their heart activity. A 1000 Hz, 50 ms start tone was presented three seconds later, and the same auditory cue signified the end of each trial. Participants were not informed about the length of each interval, nor about their performance accuracy. Participants also completed 3 trials of time perception to determine whether performance on the heartbeat tracking task was attributable to ability to estimate time (Dunn, Dalgleish, & Lawrence, 2006). Lengths of the 3 time intervals were 23, 40, and 56 s. Participants were also asked to provide an estimate of their resting heart rate.
Interoceptive accuracy scores on the heartbeat counting task were calculated by taking the absolute value of the recorded heartbeats minus the estimated heartbeats, divided by the recorded number of heartbeats, and then multiplying by 100 to express this as a percentage, for each trial (ABS [actual − estimated] ÷ actual] × 100). Performance was averaged across all six trials and this average was subtracted from 100 to express that the higher the perception score, the better the accuracy. Heartbeats were calculated using an automated program within AcqKnowledge 4.0 (Biopac Systems Inc., Santa Barbara, California, USA). Heartbeat counts were manually estimated in the event that the amount of noise was too high for automated methods.
2.4. Rumination
Rumination was measured using the Ruminative Response Scale (RRS). The RSS a 22 item subscale of the Response Styles Questionnaire (RSQ; Nolen-Hoeksema & Morrow, 1991) that assesses people's tendencies to ruminate when distressed. Participants rate their agreement with statements on a Likert-type scale ranging from 1 (almost never) to 4 (almost always). The items describe responses to depressed mood that are self-focused, symptom-focused, and focused on the possible consequences and causes of their mood. The RSS features two subscales that capture distinguishable components of rumination: reflective pondering and brooding. Notably, the brooding scale is less confounded with depression and more useful in gaining a finer understanding of the relationship between rumination and depression (Armey et al., 2009; Treynor, Gonzalez, & Nolen-Hoeksema, 2003). The RSS has demonstrated high internal consistency (Cronbach's alpha ~0.90) as well as acceptable convergent and predictive validity (Butler & Nolen-Hoeksema, 1994; Nolen-Hoeksema & Morrow, 1991). RSS total score internal consistency for the current study was 0.92 and brooding subscale internal consistency was 0.77.
2.5. Depression and anxiety-related symptomatology
Depression and anxiety symptoms were assessed using the short form of the Mood and Anxiety Symptom Questionnaire (MASQ-S; Watson & Clark, 1991). The MASQ-S is a 62 item self-report questionnaire that measures depression-specific anhedonia symptoms (22 items), anxiety-specific hyper-arousal symptoms (17 items), and general distress symptoms common to anxiety and depression (23 items). Participants indicate on a 5 point scale, ranging from 1 (not at all) to 5 (extremely), how much they have experienced each symptom during the past week as well as the day of testing. The MASQ-S demonstrated good convergent and discriminant validity, reliability, and a stable factor structure in student, community, and adult substance abuse patient samples (Watson et al., 1995b, 1995a). MASQ-S total score internal consistency for the current study was 0.89. Internal consistency was also calculated for each subscale (general distress: = 0.92; anxious arousal = 0.77; anhedonic depression = 0.76).
3. Results
3.1. Descriptive statistics and correlations for mood and interoception measures
Overall, interoceptive accuracy evidenced a negative association with self-reported depression and anxiety symptoms. Specifically, correlational analyses revealed significant associations between interoceptive accuracy and MASQ-S dimensions of anhedonic depression, anxious arousal, general distress symptoms. Interoceptive accuracy was not significantly associated with rumination. Table 1 summarizes descriptive statistics and correlations for mood and interoception measures. Table 2 reports intercorrelations among MASQ-S dimensions.
Table 1.
Sample descriptives and correlations for mood and interoception indicators.
| Indicator | Mean | SD | Correlation with IA |
|---|---|---|---|
| Heartbeat counting accuracy | 63.04 | 17.28 | – |
| MASQ-S – total score | 126.42 | 29.9 | −0.38b |
| MASQ-S – general distress | 44.92 | 14.40 | −0.38b |
| MASQ-S – anxious arousal | 25.73 | 7.56 | −0.23a |
| MASQ-S – anhedonia | 55.77 | 13.32 | −0.32b |
| RSQ-RSS - brooding | 10.03 | 3.55 | −0.18 |
| RSQ-RSS – pondering | 8.97 | 3.34 | −0.08 |
| Time perception % accuracy | 61.57 | 9.34 | −0.42b |
| Body mass index | 26.70 | 6.16 | −0.08 |
| Resting HR (bpm) | 75.39 | 10.13 | −0.25a |
| Resting HR estimate (bpm) | 56.78 | 24.01 | 0.31b |
| HR variability (SDNN) | 69.66 | 27.54 | 0.18 |
| Physical activity (min/week) | 244.18 | 181.52 | 0.19 |
| Blood pressure (Systolic) | 118.90 | 12.18 | −0.00 |
| Blood pressure (Diastolic) | 69.56 | 8.58 | 0.09 |
| Skin conductance (spontaneous fluctuations/min) | 3.38 | 4.04 | 0.04 |
Note: MASQ-S = Mood and Anxiety Symptom Questionnaire – Short Form; RSQ-RSS = Response Styles Questionnaire – Ruminative Response Scale; IA = Interoceptive Awareness (indexed by heartbeat counting accuracy); bpm = beats per minute; HR = heart rate; SDNN = Standard deviation of normal-to-normal R-R intervals.
Correlation is significant at the 0.05 level (2-tailed).
Correlation is significant at the 0.01 level (2-tailed).
Table 2.
Intercorrelations among MASQ-S dimensions.
| 1 | 2 | 3 | |
|---|---|---|---|
| MASQ-S – general distress (1) | – | ||
| MASQ-S – anxious arousal (2) | 0.70a | – | |
| MASQ-S – anhedonia (3) | 0.60a | 0.44a | – |
Note: MASQ-S = Mood and Anxiety Symptom Questionnaire – Short Form.
Correlation is significant at the 0.01 level (2-tailed).
3.2. Examination of potential confound variables
We examined the extent to which interoceptive accuracy was related to potential confound variables such as body mass index (BMI), blood pressure, skin conductance, heart rate variability, exercise, resting heart rate, estimates about resting heart rate, self-reported current depression or anxiety diagnosis, self-reported prescribed psychotropic drug use, and ability to estimate time. Interoceptive accuracy was not significantly associated with BMI, blood pressure, skin conductance, HRV, minutes of exercise per week, depression or anxiety diagnoses, or prescribed psychotropic drug use. Slower resting heart rate, estimates about resting heart rate, and time accuracy were all significantly associated with interoceptive accuracy (see Table 1). As such, we conducted an additional regression analysis with estimate of resting heart rate, resting heart rate, and time estimation accuracy as covariates. After controlling for these factors, the regression results remained consistent as described below.
3.3. Regression analysis
We tested a regression model that examined interoceptive accuracy (indexed by heartbeat counting), brooding rumination, and their interaction as predictors of self-reported depression and anxiety symptoms for each MASQ-S dimension. Two outliers were removed (Z-scores > 3 SDs from mean), and two participants had missing data. Nine participants' data were unusable due to high amounts of noise such that heartbeats could not be calculated by either automated or manual methods. Six participants' data from the heartbeat counting task were removed due to non-compliance with the research protocol (e.g., taking pulse, eating, or using electronic devices during procedures). The final sample size following exclusions was 82. Removed individuals were excluded from all following analyses. On average, the removed individuals scored significantly higher (M = 54.27, SD = 19.28) than included individuals (M = 44.77, SD = 14.41) on the MASQ-S general distress dimension; of these individuals, 1 self-reported a depression diagnosis and antidepressant use, 1 self-reported a diagnosis of ADHD, and 1 self-reported a diagnosis of ADHD with medication use. Greater interoceptive accuracy was associated with lower scores on the general distress dimension, and greater brooding rumination was associated with higher scores on the general distress dimension, r = 0.54, p = 0.000. For the general distress dimension, adding the interaction of interoceptive accuracy and brooding rumination significantly improved model fit. Table 3 describes the regression coefficients and model associated with the general distress dimension. Interoceptive accuracy was not associated with anxious arousal; however, greater interoceptive accuracy was associated with lower scores on the dimension of anhedonic depression. Greater brooding rumination was also associated with higher scores on the anxious arousal (r = 0.40, p = 0.000) and anhedonic depression (r = 0.36, p 0.001) dimensions. The addition of the interaction of interoceptive accuracy and brooding rumination did not improve the fit of either model predicting anxious arousal or anhedonic depression, though the interaction term evidenced an association at the level of a non-significant trend for the model predicting anxious arousal. Tables 4 and 5 describe the regression coefficients and model associated with anxious arousal and anhedonic depression, respectively.
Table 3.
Linear regression model predicting MASQ-S general distress.
| Step | Predictor | Regression coefficients |
Regression model |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
| B | pr | t | p | R 2 | F Change | df | p | Cohen's f2 | ||
| 1 | IA | −0.25 | −0.35 | −3.30 | 0.001 | |||||
| Brooding rumination | 2.09 | 0.54 | 5.69 | 0.000 | 0.38 | 25.77 | 2, 79 | 0.000 | 0.65 | |
| 2 | IA × Brooding rumination | −0.06 | −0.31 | −2.82 | 0.006 | 0.43 | 7.98 | 1, 78 | 0.006 | 0.10 |
Note. Both Step 1 predictors are centered; B = unstandardized regression coefficient (i.e., beta value); pr = partial correlation between predictor and dependent measure; R2 = Variance explained by the model (e.g., 0.40 = 40%); IA = Interoceptive Awareness (indexed by heartbeat counting accuracy).
Table 4.
Linear regression model predicting MASQ-S anxious arousal.
| Step | Predictor | Regression coefficients |
Regression model |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
| B | pr | t | p | R 2 | F Change | df | p | Cohen's f2 | ||
| 1 | IA | −0.07 | −0.18 | −1.6 | 0.113 | |||||
| Brooding rumination | 0.85 | 0.4 | 3.81 | 0.000 | 0.20 | 9.96 | 2, 79 | 0.000 | 0.25 | |
| 2 | IA × Brooding rumination | −0.03 | −0.21 | −1.92 | 0.058 | 0.24 | 3.69 | 1, 78 | 0.058 | 0.05 |
Note. Both Step 1 predictors are centered; B = unstandardized regression coefficient (i.e., beta value); pr = partial correlation between predictor and dependent measure; R2 = Variance explained by the model (e.g., 0.40 = 40%); IA = Interoceptive Awareness (indexed by heartbeat counting accuracy).
Table 5.
Linear regression model predicting MASQ-S anhedonic depression.
| Step | Predictor | Regression coefficients |
Regression model |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
| B | pr | t | p | R 2 | F Change | df | p | Cohen's f2 | ||
| 1 | IA | −0.20 | −0.27 | −2.52 | 0.014 | |||||
| Brooding rumination | 1.31 | 0.36 | 3.4 | 0.001 | 0.22 | 10.82 | 2, 79 | 0.001 | 0.28 | |
| 2 | IA × Brooding rumination | −0.03 | −0.16 | −1.46 | 0.148 | 0.24 | 2.1 | 1, 78 | 0.148 | 0.03 |
Note. Both Step 1 predictors are centered; B = unstandardized regression coefficient (i.e., beta value); pr = partial correlation between predictor and dependent measure; R2 = Variance explained by the model (e.g., 0.40 = 40%); IA = Interoceptive Awareness (indexed by heartbeat counting accuracy).
3.4. Simple slopes analysis
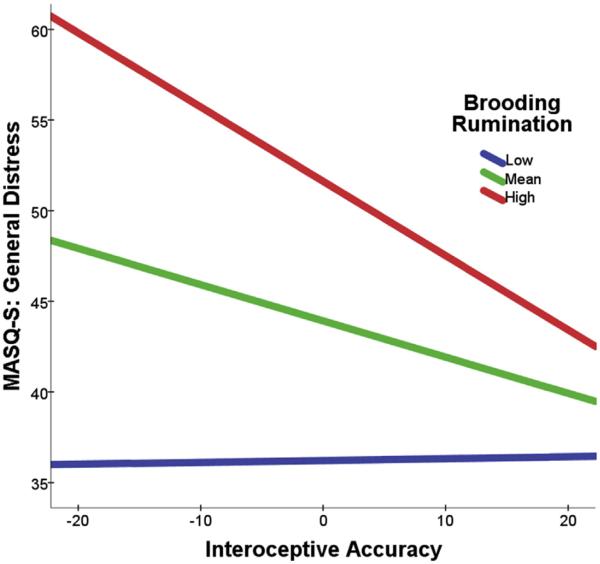
To further examine the form of the significant interaction in the model for general distress, we conducted simple slope analyses for the association between interoceptive accuracy and general distress at low (−1 SD below the mean), moderate (mean), and high (+1 SD above the mean) levels of brooding rumination. Notably, high levels of brooding rumination coupled with low levels of interoceptive accuracy were associated with the highest levels of depression and anxiety-related distress, whereas lower levels of brooding rumination coupled with higher levels of interoceptive accuracy were associated with lower levels of depression and anxiety-related distress. The simple slopes tests revealed a significant negative association between interoceptive accuracy and general distress; the strength of this association was greater for high levels of brooding rumination, β = −0.41, t(77) = −4.37, p < 0.001, than for moderate levels of brooding rumination β = −0.19, t(77) = −2.96, p < 0.01. At low levels of brooding rumination, the slope was not significantly different from zero, β = 0.01, t(77) = 0.10, p = 0.92. The interaction is illustrated in Fig. 1.
Fig. 1.
Decomposed Interaction of IA and Brooding Rumination on MASQ-S: General Distress. Note: Brooding rumination and interoceptive accuracy are mean-centered.
4. Discussion
The present study examined the relationship between negative self-referential processing (i.e., rumination), interoceptive awareness, and self-reported depression and anxiety symptoms. We hypothesized an interaction effect of brooding rumination and interoceptive awareness on depression and anxiety-related symptoms. Specifically, we hypothesized that high levels of rumination coupled with low levels of interoceptive awareness would be associated with the highest levels of depression and anxiety symptoms, and that lower levels of rumination coupled with higher levels of interoceptive awareness would be associated with lower levels of depression and anxiety symptoms. The hypothesis was supported for the statistical model predicting MASQ-S general distress. The results did not support an interaction effect of brooding rumination and interoceptive awareness on symptom dimensions of anxious arousal or anhedonic depression; however, greater interoceptive awareness was associated with lower scores on the dimension of anhedonic depression and greater brooding rumination was associated with higher scores on both the anxious arousal and anhedonic depression dimensions.
The findings provide further support for the conceptualization of anxiety and depression as conditions involving an integration of interoceptive processes with self-referential processes (Paulus & Stein, 2010). In corroboration with previous work (Nolen-Hoeksema & Morrow, 1993), results indicated that rumination was positively associated with self-reported depression and anxiety symptoms, whereas increased interoceptive awareness evidenced a negative association with depression and anxiety symptoms. The finding that interoceptive awareness was inversely related to anhedonia symptoms replicates previous work (Dunn, Stefanovitch et al., 2010; Furman et al., 2013). The inverse association between interoceptive awareness and anxious arousal appears contrary to results obtained in previous studies that evidence a positive association between interoception and anxiety (e.g., Domschke et al., 2010; Ehlers & Breuer, 1992; Pollatos et al., 2009); however, it is important to note that these prior studies included clinically diagnosed samples, whereas the current study used a convenient sample with general measures of self-reported depression and anxiety symptoms, which may account for the different findings. Another explanation may be that arousal only predicts greater interoception in individuals who are not anhedonic (Dunn, Stefanovitch et al., 2010). Given recent work demonstrating the significance of metacognitive interoception in the interpretation of body signals (Yoris et al., 2015), it is also possible that, overall, the current sample did not view their body signals as threatening, although future work should aim to measure meta-cognitive interoception in addition to interoceptive awareness.
One possible explanation for the interaction finding is that individuals with low fidelity interoceptive afferents recruit overactive top-down modulatory areas (e.g., mPFC) that underlie negative self-referential processing; individuals reporting the highest levels of anxiety and depression symptoms may exhibit a reduced signal to noise ratio of interoceptive afferents (Paulus & Stein, 2010). However, given the correlational nature of the present study, this explanation remains speculative. In future research, it will be important to investigate this effect using fMRI to test this hypothesis. Current fMRI evidence supports two distinct, but habitually integrated modes of self-reference: extended self-reference (i.e., narrative focus; NF), which refers to the self as experienced across time, and momentary self-reference (i.e., experiential focus; EF) centered on present moment experience (Farb et al., 2007). Functional connectivity analyses conducted by Farb et al. (2007) demonstrated a strong coupling between the right insula (associated with EF) and the mPFC (associated with NF) that became uncoupled as a result of attention training (i.e., mindfulness meditation). Other research has also documented afferent and efferent connections between the insula and mPFC (Paulus & Stein, 2010). As suggested by Farb et al. (2007), the development of moment-to-moment self-reference (including interoceptive awareness) may facilitate a shift away from neural processes devoted to more abstract, narrative forms of self-reference. Whether this is attributable to an increase of the signal to noise ratio of interoceptive afferents and a decrease of overactive top-down brain modulatory areas (Paulus & Stein, 2010) remains unclear. Future fMRI research may also want to consider whether there is an optimal signal to noise ratio of interoceptive afferents.
Although the current study focuses on a specific theoretical model which hypothesizes interactions between interoceptive and self-referential processes, it is helpful to consider other theoretical frameworks. For example, it is well-documented that rumination and worry are associated with cognitive inflexibility and deficits in attention switching (Nolen-Hoeksema, S., Wisco, B. E., & Lyubomirsky, S., 2008). Rumination has also been linked to deficits in attentional control (Koster, De Lissnyder, Derakshan, & De Raedt, 2011) and retrieval of information from memory (Nolen-Hoeksema, Wisco, & Lyubomirsky, 2008). Thus, in the case of heartbeat counting tasks, which require ongoing monitoring, or attention, the potential role of attentional biases and deficits should be considered. From a cognitive processing perspective, various attentional control deficits may result in poor interoception, as well as high amounts of distress. Because rumination occupies central executive resources (Watkins & Brown, 2002), impairments to concentration and memory resulting from rumination (Nolen-Hoeksema et al., 2008) may also affect performance on heartbeat counting tasks. As such, an alternative interpretation of the interaction effect found in the current study may be that individuals who exhibit high rumination also have difficulty utilizing the attentional and working memory resources necessary to perform accurately on the task, and may have the poorest outcomes in terms of distress. Such considerations warrant further investigation of additional or alternative causal mechanisms that might account for the results of the current study.
Although the current study was not designed to address issues of causality, the results may provide guidance for a model predicting an interaction between interoceptive and self-referential processing. However, before a more convincing causal interpretation can be offered, future investigations utilizing a longitudinal design must be conducted to investigate whether rumination impairs interoception function, or vice versa, or whether interoception and rumination may be influenced by a third process such as attentional control, cognitive inflexibility, or deficits in retrieving information from memory. Rumination induction procedures (e.g., Watkins & Brown, 2002) may be particularly useful in examining whether rumination impairs interoception function, while manipulating interoceptive awareness (e.g., Ainley, Maister, Brokfeld, Farmer, & Tsakiris, 2013) may be useful in addressing causality in the opposite direction. Future studies may also want to include measures of various executive functions (e.g., attentional control, cognitive flexibility) to assess the degree to which such processes affect rumination and interoceptive ability.
Awareness of the breath or body sensations as a method of focusing attention in the present moment is emphasized in many mindfulness-based interventions. In fact, it has been proposed that the development of interoceptive awareness and focal attention to bodily sensations in early mindfulness training is one of the primary ways in which such practices promote cognitive change (Farb et al., 2015; Kerr et al., 2013) via the reduction of evaluative self-referential processing and enhancement of present-moment experience (Tang, Hölzel, & Posner, 2015). Accordingly, many imaging studies have shown IA-specific functional plasticity in insular regions (Craig, 2009; Farb et al., 2007, 2013), suggesting the possibility that enhanced insula activity reflects an amplified awareness of present-moment experience (Tang et al., 2015). A possible mechanism by which interoceptive awareness may serve to mitigate the association between affective symptoms and rumination is via the deliberate switching of attention away from ruminative tendencies and toward body signals. This hypothesis follows directly from theories proposing that experiential forms of self-awareness appear to be more adaptive than analytical forms of self-reference (Teasdale, 1999; Watkins & Teasdale, 2004).
As such, the disengagement from ruminative forms of self-reference via attention to interoceptive sensation has important clinical implications for mood and anxiety disorders (e.g., Farb et al., 2007). Interoceptive training, or increasing awareness of directly experienced sensations may allow individuals to respond more flexibly, which may aid in decision making, and emotion and affect regulation. However, given the theoretical model proposed by Paulus and Stein (2010), along with the interaction finding of the current study, it may be equally important to address belief-based states as they relate to the interpretation of body signals. Specifically, targeting interoceptive sensitivity while ignoring beliefs regarding body signals (which may be catastrophic) may contribute to psychopathology; thus, of prime importance is to train individuals to skillfully relate to bodily sensations by enhancing interoceptive awareness in the context of a regulatory framework (Farb et al., 2015). Many body-based contemplative practices, including mindfulness-based practices (see Farb et al., 2015), appear suited for such a purpose. Further explorations of causality will enhance and clarify these clinical implications.
Although heartbeat counting tasks are generally well validated and reliable (Cronbach's α 0.69–90; Dunn et al., 2007), questions remain as to whether these tasks represent the “gold standards” of interoceptive awareness measurement (Knapp-Kline & Kline, 2005; Knoll & Hodapp, 1992; Ring, Brener, Knapp, & Mailloux, 2015). For example, in a recent study conducted by Ring et al. (2015), findings demonstrated that significant increases in the accuracy of heartbeat counting tasks were mediated by non-sensory processes rather than by training participants to detect heartbeat sensations; specifically, performance improved solely on the basis of beliefs about heart rate, leading the authors to conclude that data generated by heartbeat counting tasks are uninterpretable without additional control measures such as beliefs about heart rate. The current findings revealed correlations between performance on the heartbeat counting task and estimates of resting heart rate, actual resting heart rate, and time estimation accuracy; however, results remained consistent after controlling for such factors. Nonetheless, it is important to consider the correlative nature of these findings. First, in previous studies (e.g., Dunn et al., 2006), a correlation between time estimation and heartbeat counting performance was viewed as evidence that performance on the heartbeat counting could be attributed to ability to estimate time. However, the relationship between heartbeat counting and time estimation may be explained by recent research suggesting that sense of time in the range of seconds is directly related to activity in the insular cortex, which is also a primary area for interoception (Meissner & Wittmann, 2011). Second, habitually good heartbeat perceivers demonstrate lower resting heart rate, perhaps because a lower heart rate is accompanied by greater stroke volume when cardiac output is constant (Schandry, Bestler, & Montoya, 1993). Finally, as raised by Ring et al. (2015), it is possible that individuals in the current study relied on belief information when unable to gather physiological information during the heartbeat counting task. Given that all MASQ-S scales exhibited a negative correlation with heartbeat counting performance, the presence of self-reported affective symptoms may have resulted in an increased reliance on belief information when completing the heartbeat counting task. However, future work will want to include more in-depth measures of “metacognitive interoception” (Yoris et al., 2015), or beliefs about interoceptive signals, to obtain a deeper understanding of how beliefs may affect performance.
Given the limitations raised by Ring et al. (2015), future research may want focus on the development of interoception tasks that are less confounded by non-sensory processes. One study found that performance on a heartbeat counting task correlated with sensitivity for gastric signals using a water load test, providing evidence for interoceptive coherence across visceral modalities (Herbert et al., 2012). Other investigators have operationalized interoceptive awareness in terms of respiratory sensitivity or accuracy, typically using various forms of the respiratory load task (Zechman et al., 1957). These tasks require participants to detect when resistance has been introduced into a tube through which they are breathing (Davenport, Chan, Zhang, & Chou, 2007; Zhao, Martin, & Davenport, 2002) or to discriminate among various resistive loads introduced into the airway (Webster & Colrain, 2000). Recent neuroanatomical and neurophysiological evidence suggests a correlation between heartbeat perception and respiratory sensitivity. Research has indicated that a class of afferent fibers that monitor the physiological state of all internal organs of the body converge in the insular cortex (Craig, 2002; Paulus & Stein, 2006). Indeed, evidence from other studies has demonstrated activation in the anterior insular cortex during heartbeat perception (Critchley et al., 2004), bladder and stomach distension, sexual arousal, sensual touch, and pain (Craig, 2009). Findings by Herbert et al. (2012) also suggest that interoceptive ability extends across visceral modalities. Taken together, a battery of tasks may represent a more reliable measurement of interoceptive awareness, as opposed to reliance on a single, potentially confounded heartbeat counting paradigm.
Other limitations of the current study should be noted. First, the sample was comprised of a convenient population of primarily female, Caucasian, college students, in which the presence of self-reported clinical symptoms was allowed. Future work should seek to replicate the findings in more diverse populations, as well as clinically diagnosed populations, before strong conclusions are drawn. Second, only one aspect of interoception was examined in the current study, as we operationalized interoceptive awareness as accuracy on a heartbeat counting paradigm. Future work should test this effect among other forms of interoception (e.g., interoceptive sensitivity, interoceptive coherence, metacognitive interoception; Farb et al., 2015). Third, the current study did not include a measure of worry, which may be more relevant for anxiety than rumination measures. Fourth, we chose to include individuals with self-reported clinical diagnoses and medication use, which may introduce possible confounds. Finally, the exclusion of data points in the current study resulted in loss of power, and the individuals excluded may not be missing at random (e.g., excluded participants seemed to have higher distress scores); thus, estimates presented in the results may contain bias and the sample may not be representative. In future research, it will also be important to utilize more behavioral-based measures of rumination due to the limitations of self-report measures. Given that the RSS is a trait measure of rumination, it is unclear in the current study what state levels of rumination were during the interoception task.
In summary, the present study evidenced an interaction effect of brooding rumination and interoceptive awareness on self-reported depression and anxiety-related symptomatology, such that high levels of brooding rumination coupled with low levels of interoceptive accuracy were associated with the highest levels of depression and anxiety-related distress, whereas lower levels of brooding rumination coupled with higher levels of interoceptive accuracy were associated with lower levels of depression and anxiety-related distress. Specifically, analyses revealed a significant negative association between interoceptive accuracy and general distress; the strength of this association was greater for high levels of brooding rumination, than for moderate levels of brooding rumination. The findings provide further support for the conceptualization of anxiety and depression as conditions involving an interaction between interoceptive processes and negative self-referential processes.
Footnotes
Conflict of interest statement The authors declare that there are no conflicts of interest.
References
- Ainley V, Maister L, Brokfeld J, Farmer H, Tsakiris M. More of myself: Manipulating interoceptive awareness by heightened attention to bodily and narrative aspects of the self. Consciousness and cognition. 2013;22(4):1231–1238. doi: 10.1016/j.concog.2013.08.004. [DOI] [PubMed] [Google Scholar]
- Armey MF, Fresco DM, Moore MT, Mennin DS, Turk CL, Heimberg RG, et al. Brooding and pondering: Isolating the active ingredients of depressive rumination with exploratory factor analysis and structural equation modeling. Assessment. 2009;16:315–327. doi: 10.1177/1073191109340388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett LF, Quigley KS, Bliss-Moreau E, Aronson KR. Interoceptive sensitivity and self-reports of emotional experience. Journal of personality and social psychology. 2004;87(5):684. doi: 10.1037/0022-3514.87.5.684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckwé M, Deroost N, Koster EH, De Lissnyder E, De Raedt R. Worrying and rumination are both associated with reduced cognitive control. Psychological Research. 2014;78(5):651–660. doi: 10.1007/s00426-013-0517-5. [DOI] [PubMed] [Google Scholar]
- Berlucchi G, Aglioti SM. The body in the brain revisited. Experimental Brain Research. 2010;200(1):25–35. doi: 10.1007/s00221-009-1970-7. [DOI] [PubMed] [Google Scholar]
- Borkovec TD, Inz J. The nature of worry in generalized anxiety disorder: A predominance of thought activity. Behaviour Research and Therapy. 1990;28(2):153–158. doi: 10.1016/0005-7967(90)90027-g. [DOI] [PubMed] [Google Scholar]
- Borkovec TD, Ruscio AM. Psychotherapy for generalized anxiety disorder. Journal of Clinical Psychiatry. 2001;62:37–45. [PubMed] [Google Scholar]
- Buckner RL, Andrews-Hanna JR, Schacter DL. The brain's default network. Annals of the New York Academy of Sciences. 2008;1124(1):1–38. doi: 10.1196/annals.1440.011. [DOI] [PubMed] [Google Scholar]
- Butler LD, Nolen-Hoeksema S. Gender differences in responses to depressed mood in a college sample. Sex Roles. 1994;30(5–6):331–346. [Google Scholar]
- Cameron OG. Interoception: The inside story—a model for psychosomatic processes. Psychosomatic medicine. 2001;63(5):697–710. doi: 10.1097/00006842-200109000-00001. [DOI] [PubMed] [Google Scholar]
- Cameron OG, Minoshima S. Regional brain activation due to pharmacologically induced adrenergic interoceptive stimulation in humans. Psychosomatic medicine. 2002;64(6):851–861. doi: 10.1097/01.psy.0000038939.33335.32. [DOI] [PubMed] [Google Scholar]
- Casacalenda N, Perry JC, Looper K. Remission in major depressive disorder: A comparison of pharmacotherapy, psychotherapy, and control conditions. American Journal of Psychiatry. 2002;159(8):1354–1360. doi: 10.1176/appi.ajp.159.8.1354. [DOI] [PubMed] [Google Scholar]
- Christoff K, Gordon AM, Smallwood J, Smith R, Schooler JW, Posner MI. Experience sampling during fMRI reveals default network and executive system contributions to mind wandering. Proceedings of the National Academy of Sciences of the United States of America. 2009;106(21):8719–8724. doi: 10.1073/pnas.0900234106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig AD. How do you feel? Interoception: The sense of the physiological condition of the body. Nature Reviews Neuroscience. 2002;3(8):655–666. doi: 10.1038/nrn894. [DOI] [PubMed] [Google Scholar]
- Craig AD. How do you feel—now? the anterior insula and human awareness. Nature Reviews Neuroscience. 2009;10(1):59–70. doi: 10.1038/nrn2555. [DOI] [PubMed] [Google Scholar]
- Craig AD. An interoceptive neuroanatomical perspective on feelings, energy, and effort. Behavioral and Brain Sciences. 2013;36(6):685–686. doi: 10.1017/S0140525X13001489. [DOI] [PubMed] [Google Scholar]
- Critchley HD, Wiens S, Rotshtein P, Öhman A, Dolan RJ. Neural systems supporting interoceptive awareness. Nature neuroscience. 2004;7(2):189–195. doi: 10.1038/nn1176. [DOI] [PubMed] [Google Scholar]
- Davenport PW, Chan P-YS, Zhang W, Chou Y-L. Detection threshold for inspiratory resistive loads and respiratory-related evoked potentials. Journal of Applied Physiology. 2007;102(1):276–285. doi: 10.1152/japplphysiol.01436.2005. [DOI] [PubMed] [Google Scholar]
- Davidson RJ. Well-being and affective style: Neural substrates and bio-behavioural correlates. Philosophical Transactions-Royal Society of London Series B Biological Sciences. 2004;359(1449):1395–1412. doi: 10.1098/rstb.2004.1510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domschke K, Stevens S, Pfleiderer B, Gerlach AL. Interoceptive sensitivity in anxiety and anxiety disorders: An overview and integration of neurobiological findings. Clinical Psychology Review. 2010;30(1):1–11. doi: 10.1016/j.cpr.2009.08.008. http://dx.doi.org/10.1016/j.cpr.2009.08.008. [DOI] [PubMed] [Google Scholar]
- Dunn BD, Dalgleish T, Lawrence AD. The somatic marker hypothesis: A critical evaluation. Neuroscience & Biobehavioral Reviews. 2006;30(2):239–271. doi: 10.1016/j.neubiorev.2005.07.001. [DOI] [PubMed] [Google Scholar]
- Dunn BD, Dalgleish T, Ogilvie AD, Lawrence AD. Heartbeat perception in depression. Behaviour Research and Therapy. 2007;45(8):1921–1930. doi: 10.1016/j.brat.2006.09.008. http://dx.doi.org/10.1016/j.brat.2006.09.008. [DOI] [PubMed] [Google Scholar]
- Dunn BD, Evans D, Makarova D, White J, Clark L. Gut feelings and the reaction to perceived inequity: The interplay between bodily responses, regulation, and perception shapes the rejection of unfair offers on the ultimatum game. Cognitive, Affective, & Behavioral Neuroscience. 2012;12(3):419–429. doi: 10.3758/s13415-012-0092-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn BD, Galton HC, Morgan R, Evans D, Oliver C, Meyer M, et al. Listening to your heart how interoception shapes emotion experience and intuitive decision making. Psychological Science. 2010;21(12):1835–1844. doi: 10.1177/0956797610389191. [DOI] [PubMed] [Google Scholar]
- Dunn BD, Stefanovitch I, Evans D, Oliver C, Hawkins A, Dalgleish T. Can you feel the beat? Interoceptive awareness is an interactive function of anxiety- and depression-specific symptom dimensions. Behaviour Research and Therapy. 2010;48(11):1133–1138. doi: 10.1016/j.brat.2010.07.006. http://dx.doi.org/10.1016/j.brat.2010.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehlers A, Breuer P. Increased cardiac awareness in panic disorder. Journal of Abnormal Psychology. 1992;101(3):371–382. doi: 10.1037//0021-843x.101.3.371. http://dx.doi.org/10.1037/0021-843X.101.3.371. [DOI] [PubMed] [Google Scholar]
- Ehlers A, Breuer P. How good are patients with panic disorder at perceiving their heartbeats? Biological psychology. 1996;42(1):165–182. doi: 10.1016/0301-0511(95)05153-8. [DOI] [PubMed] [Google Scholar]
- Eley TC, Stirling L, Ehlers A, Gregory AM, Clark DM. Heart-beat perception, panic/somatic symptoms and anxiety sensitivity in children. Behaviour Research and Therapy. 2004;42(4):439–448. doi: 10.1016/S0005-7967(03)00152-9. [DOI] [PubMed] [Google Scholar]
- Farb NA, Anderson AK, Bloch RT, Segal ZV. Mood-linked responses in medial prefrontal cortex predict relapse in patients with recurrent unipolar depression. Biological Psychiatry. 2011;70(4):366–372. doi: 10.1016/j.biopsych.2011.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farb NAS, Daubenmier JJ, Price CJ, Gard T, Kerr C, Dunn B, et al. Interoception, contemplative practice, and health. Frontiers in Psychology. 2015;6(763) doi: 10.3389/fpsyg.2015.00763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farb NAS, Segal ZV, Anderson AK. Mindfulness meditation training alters cortical representations of interoceptive attention. Social Cognitive and Affective Neuroscience. 2013;8(1):15–26. doi: 10.1093/scan/nss066. http://dx.doi.org/10.1093/scan/nss066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farb NAS, Segal ZV, Mayberg H, Bean J, McKeon D, Fatima Z, et al. Attending to the present: Mindfulness meditation reveals distinct neural modes of self-reference. Social Cognitive and Affective Neuroscience. 2007;2(4):313–322. doi: 10.1093/scan/nsm030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furman DJ, Waugh CE, Bhattacharjee K, Thompson RJ, Gotlib IH. Interoceptive awareness, positive affect, and decision making in major depressive disorder. Journal of Affective Disorders. 2013;151(2):780–785. doi: 10.1016/j.jad.2013.06.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Füstös J, Gramann K, Herbert BM, Pollatos O. On the embodiment of emotion regulation: Interoceptive awareness facilitates reappraisal. Social Cognitive and Affective Neuroscience. 2013;8(8):911–917. doi: 10.1093/scan/nss089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harshaw C. Interoceptive dysfunction: Toward an integrated framework for understanding somatic and affective disturbance in depression. Psychological Bulletin. 2015;141(2):311–363. doi: 10.1037/a0038101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbert BM, Muth ER, Pollatos O, Herbert C. Interoception across Modalities: On the relationship between cardiac awareness and the sensitivity for gastric functions. PLoS One. 2012;7(5):e36646. doi: 10.1371/journal.pone.0036646. http://dx.doi.org/10.1371/journal.pone.0036646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbert BM, Pollatos O, Flor H, Enck P, Schandry R. Cardiac awareness and autonomic cardiac reactivity during emotional picture viewing and mental stress. Psychophysiology. 2010;47(2):342–354. doi: 10.1111/j.1469-8986.2009.00931.x. [DOI] [PubMed] [Google Scholar]
- Herbert BM, Ulbrich P, Schandry R. Interoceptive sensitivity and physical effort: Implications for the self-control of physical load in everyday life. Psychophysiology. 2007;44(2):194–202. doi: 10.1111/j.1469-8986.2007.00493.x. [DOI] [PubMed] [Google Scholar]
- Kerr CE, Sacchet MD, Lazar SW, Moore CI, Jones SR. Mindfulness starts with the body: Somatosensory attention and top-down modulation of cortical alpha rhythms in mindfulness meditation. Frontiers in Human Neuroscience. 2013;7:12. doi: 10.3389/fnhum.2013.00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khalsa SS, Rudrauf D, Feinstein JS, Tranel D. The pathways of interoceptive awareness. Nature Neuroscience. 2009;12(12):1494–1496. doi: 10.1038/nn.2411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kircanski K, Thompson RJ, Sorenson JE, Sherdell L, Gotlib IH. Rumination and worry in daily life examining the naturalistic validity of theoretical constructs. Clinical Psychological Science. 2015;3(6):926–939. doi: 10.1177/2167702614566603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirk U, Downar J, Montague PR. Interoception drives increased rational decision-making in meditators playing the ultimatum game. Frontiers in Human Neuroscience. 2011;5(49) doi: 10.3389/fnins.2011.00049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knapp-Kline K, Kline JP. Heart rate, heart rate variability, and heartbeat detection with the method of constant stimuli: Slow and steady wins the race. Biological Psychology. 2005;69(3):387–396. doi: 10.1016/j.biopsycho.2004.09.002. [DOI] [PubMed] [Google Scholar]
- Knoll JF, Hodapp V. A comparison between two methods for assessing heartbeat perception. Psychophysiology. 1992;29(2):218–222. doi: 10.1111/j.1469-8986.1992.tb01689.x. http://dx.doi.org/10.1111/j.1469-8986.1992.tb01689.x. [DOI] [PubMed] [Google Scholar]
- Koster EH, De Lissnyder E, Derakshan N, De Raedt R. Understanding depressive rumination from a cognitive science perspective: The impaired disengagement hypothesis. Clinical Psychology Review. 2011;31(1):138–145. doi: 10.1016/j.cpr.2010.08.005. [DOI] [PubMed] [Google Scholar]
- Mason MF, Norton MI, Van Horn JD, Wegner DM, Grafton ST, Macrae CN. Wandering minds: The default network and stimulus-independent thought. Science. 2007;315(5810):393–395. doi: 10.1126/science.1131295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKiernan KA, D'Angelo BR, Kaufman JN, Binder JR. Interrupting the “stream of consciousness”: An fMRI investigation. Neuroimage. 2006;29(4):1185–1191. doi: 10.1016/j.neuroimage.2005.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehling WE, Price C, Daubenmier JJ, Acree M, Bartmess E, Stewart A. The multidimensional assessment of interoceptive awareness (MAIA) PLoS One. 2012;7(11):e48230. doi: 10.1371/journal.pone.0048230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meissner K, Wittmann M. Body signals, cardiac awareness, and the perception of time. Biological Psychology. 2011;86(3):289–297. doi: 10.1016/j.biopsycho.2011.01.001. [DOI] [PubMed] [Google Scholar]
- Mennin DS, Fresco DM. What, me worry and ruminate about DSM-5 and RDoC? The importance of targeting negative self-referential processing. Clinical Psychology: Science and Practice. 2013;20(3):258–267. doi: 10.1111/cpsp.12038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris JS, Friston KJ, Büchel C, Frith CD, Young AW, Calder AJ, et al. A neuromodulatory role for the human amygdala in processing emotional facial expressions. Brain. 1998;121(1):47–57. doi: 10.1093/brain/121.1.47. [DOI] [PubMed] [Google Scholar]
- Mussgay L, Klinkenberg N, Rüddel H. Heart beat perception in patients with depressive, somatoform, and personality disorders. Journal of Psychophysiology. 1999;13(1):27–36. [Google Scholar]
- Nolen-Hoeksema S, Morrow J. A prospective study of depression and posttraumatic stress symptoms after a natural disaster: The 1989 Loma Prieta Earthquake. Journal of personality and Social Psychology. 1991;61(1):115–121. doi: 10.1037//0022-3514.61.1.115. [DOI] [PubMed] [Google Scholar]
- Nolen-Hoeksema S, Morrow J. Effects of rumination and distraction on naturally occurring depressed mood. Cognition & Emotion. 1993;7(6):561–570. [Google Scholar]
- Nolen-Hoeksema S, Wisco BE, Lyubomirsky S. Rethinking rumination. Perspectives on Psychological Science. 2008;3(5):400–424. doi: 10.1111/j.1745-6924.2008.00088.x. [DOI] [PubMed] [Google Scholar]
- Northoff G. Psychopathology and pathophysiology of the self in depression—neuropsychiatric hypothesis. Journal of Affective Disorders. 2007;104(1):1–14. doi: 10.1016/j.jad.2007.02.012. [DOI] [PubMed] [Google Scholar]
- Olatunji BO, Naragon-Gainey K, Wolitzky-Taylor KB. Specificity of rumination in anxiety and depression: A multimodal meta-analysis. Clinical Psychology: Science and Practice. 2013;20(3):225–257. [Google Scholar]
- Paulus MP. Decision-making dysfunctions in psychiatry—altered homeostatic processing? Science. 2007;318(5850):602–606. doi: 10.1126/science.1142997. [DOI] [PubMed] [Google Scholar]
- Paulus MP, Stein MB. An insular view of anxiety. Biological psychiatry. 2006;60(4):383–387. doi: 10.1016/j.biopsych.2006.03.042. [DOI] [PubMed] [Google Scholar]
- Paulus MP, Stein MB. Interoception in anxiety and depression. Brain Structure and Function. 2010;214(5–6):451–463. doi: 10.1007/s00429-010-0258-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips ML, Drevets WC, Rauch SL, Lane R. Neurobiology of emotion perception I: The neural basis of normal emotion perception. Biological Psychiatry. 2003;54(5):504–514. doi: 10.1016/s0006-3223(03)00168-9. [DOI] [PubMed] [Google Scholar]
- Pineles SL, Mineka S. Attentional biases to internal and external sources of potential threat in social anxiety. Journal of Abnormal Psychology. 2005;114(2):314–318. doi: 10.1037/0021-843X.114.2.314. [DOI] [PubMed] [Google Scholar]
- Pollatos O, Kirsch W, Schandry R. On the relationship between interoceptive awareness, emotional experience, and brain processes. Cognitive Brain Research. 2005;25(3):948–962. doi: 10.1016/j.cogbrainres.2005.09.019. [DOI] [PubMed] [Google Scholar]
- Pollatos O, Schandry R. Emotional processing and emotional memory are modulated by interoceptive awareness. Cognition & Emotion. 2008;22(2):272–287. [Google Scholar]
- Pollatos O, Schandry R, Auer DP, Kaufmann C. Brain structures mediating cardiovascular arousal and interoceptive awareness. Brain Research. 2007;1141:178–187. doi: 10.1016/j.brainres.2007.01.026. [DOI] [PubMed] [Google Scholar]
- Pollatos O, Traut-Mattausch E, Schandry R. Differential effects of anxiety and depression on interoceptive accuracy. Depression and Anxiety. 2009;26(2):167–173. doi: 10.1002/da.20504. [DOI] [PubMed] [Google Scholar]
- Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL. A default mode of brain function. Proceedings of the National Academy of Sciences. 2001;98(2):676–682. doi: 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapps N, Van Oudenhove L, Enck P, Aziz Q. Brain imaging of visceral functions in healthy volunteers and IBS patients. Journal of Psychosomatic Research. 2008;64(6):599–604. doi: 10.1016/j.jpsychores.2008.02.018. [DOI] [PubMed] [Google Scholar]
- Reiss S, Peterson RA, Gursky DM, McNally RJ. Anxiety sensitivity, anxiety frequency and the prediction of fearfulness. Behaviour Research and Therapy. 1986;24(1):1–8. doi: 10.1016/0005-7967(86)90143-9. [DOI] [PubMed] [Google Scholar]
- Ring C, Brener J, Knapp K, Mailloux J. Effects of heartbeat feedback on beliefs about heart rate and heartbeat counting: A cautionary tale about interoceptive awareness. Biological Psychology. 2015;104:193–198. doi: 10.1016/j.biopsycho.2014.12.010. [DOI] [PubMed] [Google Scholar]
- Sanislow CA, Pine DS, Quinn KJ, Kozak MJ, Garvey MA, Heinssen RK, et al. Developing constructs for psychopathology research: Research domain criteria. Journal of Abnormal Psychology. 2010;119(4):631–639. doi: 10.1037/a0020909. [DOI] [PubMed] [Google Scholar]
- Schandry R. Heart beat perception and emotional experience. Psychophysiology. 1981;18(4):483–488. doi: 10.1111/j.1469-8986.1981.tb02486.x. [DOI] [PubMed] [Google Scholar]
- Schandry R, Bestler M, Montoya P. On the relation between cardiodynamics and heartbeat perception. Psychophysiology. 1993;30(5):467–474. doi: 10.1111/j.1469-8986.1993.tb02070.x. [DOI] [PubMed] [Google Scholar]
- Simmons WK, Avery JA, Barcalow JC, Bodurka J, Drevets WC, Bellgowan P. Keeping the body in mind: Insula functional organization and functional connectivity integrate interoceptive, exteroceptive, and emotional awareness. Human brain mapping. 2013;34(11):2944–2958. doi: 10.1002/hbm.22113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sze JA, Gyurak A, Yuan JW, Levenson RW. Coherence between emotional experience and physiology: Does body awareness training have an impact? Emotion. 2010;10(6):803–814. doi: 10.1037/a0020146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang Y-Y, Hölzel BK, Posner MI. The neuroscience of mindfulness meditation. Nature Reviews Neuroscience. 2015;16(4):213–225. doi: 10.1038/nrn3916. [DOI] [PubMed] [Google Scholar]
- Teasdale JD. Emotional processing, three modes of mind and the prevention of relapse in depression. Behaviour research and therapy. 1999;37:S53–S77. doi: 10.1016/s0005-7967(99)00050-9. [DOI] [PubMed] [Google Scholar]
- Treynor W, Gonzalez R, Nolen-Hoeksema S. Rumination reconsidered: A psychometric analysis. Cognitive Therapy and Research. 2003;27(3):247–259. [Google Scholar]
- Van der Does AW, Antony MM, Ehlers A, Barsky AJ. Heartbeat perception in panic disorder: A reanalysis. Behaviour Research and Therapy. 2000;38(1):47–62. doi: 10.1016/s0005-7967(98)00184-3. [DOI] [PubMed] [Google Scholar]
- Watkins E, Brown RG. Rumination and executive function in depression: An experimental study. Journal of Neurology, Neurosurgery & Psychiatry. 2002;72(3):400–402. doi: 10.1136/jnnp.72.3.400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watkins E, Moulds M. Distinct modes of ruminative self-focus: Impact of abstract versus concrete rumination on problem solving in depression. Emotion. 2005;5(3):319–328. doi: 10.1037/1528-3542.5.3.319. [DOI] [PubMed] [Google Scholar]
- Watkins E, Teasdale JD. Adaptive and maladaptive self-focus in depression. Journal of Affective Disorders. 2004;82(1):1–8. doi: 10.1016/j.jad.2003.10.006. [DOI] [PubMed] [Google Scholar]
- Watson D, Clark LA. The mood and anxiety symptom questionnaire (MASQ) University of Iowa. Unpublished manuscript; Iowa City: 1991. [Google Scholar]
- Watson D, Clark LA, Weber K, Assenheimer JS, Strauss ME, McCormick RA. Testing a tripartite model: II. Exploring the symptom structure of anxiety and depression in student, adult, and patient samples. Journal of Abnormal Psychology. 1995a;104(1):15–25. doi: 10.1037//0021-843x.104.1.15. [DOI] [PubMed] [Google Scholar]
- Watson D, Weber K, Assenheimer JS, Clark LA, Strauss ME, McCormick RA. Testing a tripartite model: I. Evaluating the convergent and discriminant validity of anxiety and depression symptom scales. Journal of Abnormal Psychology. 1995b;104(1):3–14. doi: 10.1037//0021-843x.104.1.3. [DOI] [PubMed] [Google Scholar]
- Webster KE, Colrain IM. The relationship between respiratory-related evoked potentials and the perception of inspiratory resistive loads. Psychophysiology. 2000;37(6):831–841. [PubMed] [Google Scholar]
- Werner NS, Jung K, Duschek S, Schandry R. Enhanced cardiac perception is associated with benefits in decision-making. Psychophysiology. 2009;46(6):1123–1129. doi: 10.1111/j.1469-8986.2009.00855.x. [DOI] [PubMed] [Google Scholar]
- Werner NS, Mannhart T, Reyes Del Paso GA, Duschek S. Attention interference for emotional stimuli in cardiac interoceptive awareness. Psychophysiology. 2014;51(6):573–578. doi: 10.1111/psyp.12200. [DOI] [PubMed] [Google Scholar]
- Werner NS, Peres I, Duschek S, Schandry R. Implicit memory for emotional words is modulated by cardiac perception. Biological Psychology. 2010;85(3):370–376. doi: 10.1016/j.biopsycho.2010.08.008. [DOI] [PubMed] [Google Scholar]
- Wiebking C, Bauer A, de Greck M, Duncan NW, Tempelmann C, Northoff G. Abnormal body perception and neural activity in the insula in depression: An fMRI study of the depressed “material me”. The World Journal of Biological Psychiatry. 2010;11(3):538–549. doi: 10.3109/15622970903563794. [DOI] [PubMed] [Google Scholar]
- Wiebking C, Duncan NW, Tiret B, Hayes DJ, Marjaǹska M, Doyon J, et al. GABA in the insula—a predictor of the neural response to interoceptive awareness. Neuroimage. 2014;86:10–18. doi: 10.1016/j.neuroimage.2013.04.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiens S. Interoception in emotional experience. Current Opinion in Neurology. 2005;18(4):442–447. doi: 10.1097/01.wco.0000168079.92106.99. [DOI] [PubMed] [Google Scholar]
- Wiens S, Mezzacappa ES, Katkin ES. Heartbeat detection and the experience of emotions. Cognition & Emotion. 2000;14(3):417–427. [Google Scholar]
- Yoris A, Esteves S, Couto B, Melloni M, Kichic R, Cetkovich M, et al. The roles of interoceptive sensitivity and metacognitive interoception in panic. Behavioral and Brain Functions. 2015;8:11–14. doi: 10.1186/s12993-015-0058-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zechman F, Hall FG, Hull WE. Effects of graded resistance to tracheal air flow in man. Journal of Applied Physiology. 1957;10(3):356–362. doi: 10.1152/jappl.1957.10.3.356. [DOI] [PubMed] [Google Scholar]
- Zhao W, Martin AD, Davenport PW. Detection of inspiratory resistive loads in double-lung transplant recipients. Journal of Applied Phywsiology. 2002;93(5):1779–1785. doi: 10.1152/japplphysiol.00210.2002. [DOI] [PubMed] [Google Scholar]