Abstract
Granulocyte-macrophage colony-stimulating factor (GM-CSF) is known to be a potential vaccine adjuvant despite contradictory results from animal and human studies. The discrepancies may be due to the different doses and regimens of GM-CSF that were used, given that either mature or immature dendritic cells (DCs) could be induced under different conditions. To test the hypothesis that GM-CSF can be used as a novel adjuvant for a hepatitis B virus (HBV) therapeutic vaccine, we administered GM-CSF once per day for three days prior to vaccination with recombinant HBV vaccine (rHBVvac) in mice. We observed greater DC maturation in these pre-treated animals at day 3 as compared to day 1 or day 2 of daily GM-CSF administration. This strategy was further investigated for its ability to break the immune tolerance established in hepatitis B surface antigen-transgenic (HBsAg-Tg) animals. We found that the levels of induced anti-HBsAg antibodies were significantly higher in animals following three days of GM-CSF pre-treatment before rHBV vaccination after the third immunization. In addition to the increase in anti-HBsAg antibody levels, cell-mediated anti-HBsAg responses, including delayed-type hypersensitivity, T-cell proliferation, interferon-γ production, and cytotoxic T lymphocytes, were dramatically enhanced in the three-day GM-CSF pre-treated group. After adoptive transfers of CD8+ T cells from immunized animals, antigen-specific CD8+ T cells were observed in the livers of recipient HBsAg-Tg animals. Moreover, the three-day pre-treatments with GM-CSF prior to rHBVvac vaccination could significantly eliminate HBsAg-positive hepatocytes, suggesting beneficial therapeutic effects. Therefore, this protocol utilizing GM-CSF as an adjuvant in combination with the rHBVvac vaccine has the potential to become a novel immunotherapy for chronic hepatitis B patients.
Keywords: adjuvant, chronic hepatitis B, GM-CSF, immune-tolerance, therapeutic vaccine
Introduction
Chronic hepatitis B virus (HBV) infection is a global health problem that causes liver cirrhosis and hepatocellular carcinoma (HCC).1 Approximately two billion people have been infected with HBV, and more than 370 million are chronic HBV carriers (CHB).2,3 In the past 20 years, prophylactic HBV vaccination of children has decreased the prevalence of HBV. However, the treatment options for chronic HBV are limited, and few patients are cured.4,5 Treatments for CHB currently include subcutaneous injection of recombinant interferon-α (IFN-α) and five oral nucleoside analogues (NAs: lamivudine, telbivudine, adefovir, entecavir, and tenofovir). All the available treatments require a course lasting more than one year, with some of them causing undesirable side effects and drug-resistance issues. Notably, none of the therapies achieves cure in a substantial proportion of patients. Thus, a novel immunotherapy to improve the cure rate in chronic HBV patients is highly desirable.6,7
The host immune system plays a critical role in HBV infection. Both innate and adaptive immune responses affect the pathogenesis and outcome of CHB. Successful clearance of HBV requires the coordination of potent and antigen-specific immune responses, particularly the reactivation and appearance of HBV-specific CD8+ T cells.8 One of the obstacles to the reactivation of antigen-specific T cells in CHB is systemic immune tolerance and exhaustion.9 Thus, neither a prophylactic HBV vaccine alone nor a vaccine in combination with IFN-α or with antiviral compounds has been found to be better than current standard-of-care drugs.10 To overcome immune tolerance, an effective adjuvant may be needed. Several potential adjuvants have been examined in various systems, and the results have been reviewed.11,12,13
Granulocyte-macrophage colony-stimulating factor (GM-CSF), a cytokine produced by macrophages, T cells, NK cells, endothelial cells, and fibroblasts, has been used clinically as a hematopoietic growth factor for more than 20 years.14 It can increase neutrophil counts and induce the differentiation and maturation of myeloid dendritic cells (DCs) to improve the functions of antigen-presenting cells (APCs), as well as promote the development and perpetuation of Th1 responses.15,16,17 Given its immune modulatory functions, GM-CSF has been used as an adjuvant in several studies as an immunotherapy for the treatment of cancers and HIV infection.17,18 However, some recent studies suggest that the use of GM-CSF as a vaccine adjuvant could induce suppressive immune responses in certain cases. Although the mechanism remains uncertain, one possibility is the induction of immature status in DCs.18,19,20,21 This undesirable effect of using GM-CSF as an adjuvant could be a consequence of a too-short duration of maturation for DCs rather than becoming fully mature before being presented with HBV antigens.15
With this in mind, we investigated whether pre-treatment of animals with GM-CSF for more than one day could allow DCs to become fully mature such that subsequent HBV vaccination could elicit a substantially increased HBV-specific immune response.
In this study, we used an immune-tolerant HBV transgenic animal model to evaluate pre-treatment with GM-CSF daily for three days before vaccination with recombinant HBV vaccine (rHBVvac). The results showed that three-day pre-treatment with GM-CSF followed by rHBVvac (3 × GM-CSF + rHBVvac) induced robust DC maturation and differentiation, but one or two days of GM-CSF pre-treatment did not. This protocol was successfully used to break and overcome immune tolerance in hepatitis B surface antigen-transgenic (HBsAg-Tg) animals, resulting in significant cell-mediated responses. More importantly, this protocol elicited HBsAg-specific interferon-γ (IFN-γ)-producing CD8+ T cells and CTL responses, resulting in significant elimination of HBsAg-positive hepatocytes in the HBsAg-Tg animals. Therefore, three-day pre-treatment with GM-CSF followed by rHBVvac could provide a basis for the development of a therapeutic HBV vaccine for the treatment of chronic HBV infections.
Materials and methods
Animals, cell line, and reagents
Six- to eight-week-old C57BL/6 female mice (wild-type (WT) mice) were purchased from Shanghai SLAC Laboratory Animal Co. Ltd (Shanghai, China). Six- to eight-week-old HBsAg male and female transgenic mice (C57BL/6J-Tg(A1b1HBV)44Bri/J mice)22 were purchased from the Animal Center of the Shanghai Public Health Clinical Center (Shanghai, China). All animal protocols were approved by the Animal Welfare Committee of Fudan University, and the mice were kept under specific pathogen-free conditions.
Recombinant human GM-CSF and CHO cells expressing recombinant HBsAg vaccine (rHBVvac) were kindly provided by the China North Pharmaceutical Group Corporation (Shijiazhuang, China). Purified recombinant HBsAg was purchased from Shanghai Guikang Biotechnology, Ltd (Shanghai, China). Ovalbumin (OVA)-specific CTL peptide OVA257-264 (SIINFEKL; H-2b-restricted) and HBsAg-specific CTL epitope S208-215 (ILSPFLPL; H-2b-restricted) were synthesized by Scipeptide Biotechnology, Ltd (Shanghai, China).
Immunization
The HBsAg-Tg (Alb1HBV)44Bri/J mice were randomly divided into five groups (n = 5) according to the design shown in Figure 2a. Mice in the 3 × GM-CSF + rHBVvac group were subcutaneously injected with 10 μg of GM-CSF once per day for three days, then subcutaneously immunized with 1 μg of rHBVvac at the same site; Mice in the 30 GM-CSF + rHBVvac group were subcutaneously immunized with a mixture of 30 μg of GM-CSF and 1 μg of rHBVvac. The mice in the rHBVvac group were immunized with 1 μg of rHBVvac alone. The mice in the GM-CSF group were injected with 10 μg of GM-CSF alone. All mice were immunized on day 0 and boosted at weeks 2 and 4. For long-term evaluation of anti-HBsAg antibodies, animals were further boosted at week 12.
Figure 2.
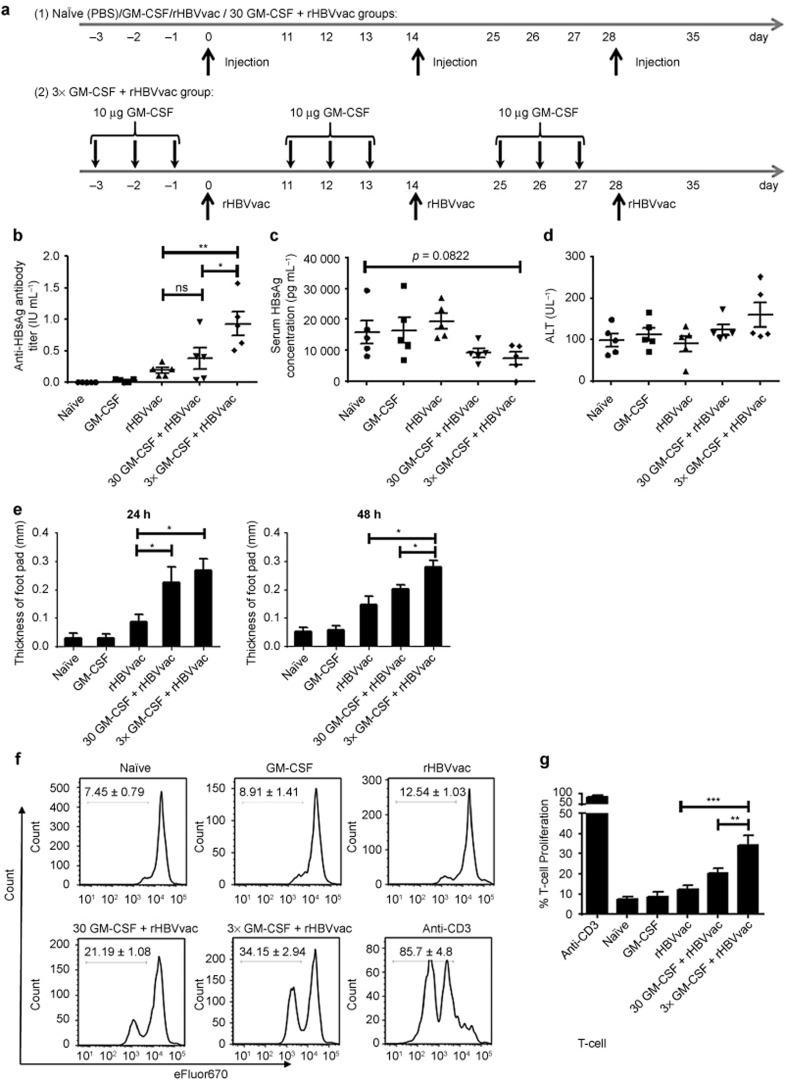
GM-CSF as a rHBV vaccine adjuvant breaks the immune tolerance of HBsAg-Tg mice. (a) Schematic illustration of the immunization regimen. HBsAg-Tg mice were immunized with GM-CSF (10 μg or 30 μg) and rHBV (1 μg) as shown. Mice were immunized on days 0, 14, and 28. (b) Total serum anti-HBsAg IgG was analyzed on the seventh day after third immunization. Mean antibody titers are expressed as IU mL−1 (ns > 0.05, **p < 0.01). (c) Serum HBsAg was analyzed on the seventh day after third immunization. (d) Alanine aminotransferase (ALT) activity in the serum was analyzed on the seventh day after the third immunization. (e) Mice were challenged with rHBVvac on the seventh day after the third immunization, and DTH was measured 24 and 48 h later (ns > 0.05, *p < 0.05, **p < 0.01). (f) Representative eFluor670 plots of the proliferating T cells are shown; splenic T cells were isolated on day 14 after the third immunization and labeled with eFluor670, cultured with rHBsAg (10 μg mL−1) for three days and stained for CD3. (g) The percentage increase in T-cell proliferation is summarized (*p < 0.05). There were five mice in each group. Data shown are representative of three independent experiments.
Enzyme-linked immunosorbent assay (ELISA)
On the seventh day after the final immunization, serum samples were obtained and assayed with an HBsAg ELISA kit (KH-T-01* Shanghai Kehua Bio-engineering Co., Ltd., Shanghai, China) or an anti-HBsAg-specific IgG ELISA kit (KH-T-02*). The optical density (OD) at 450/620 nm was measured with a xMark Microplate spectrophotometer (Bio-Rad Laboratories, Mississauga, Canada). The HBsAg levels were expressed as picogram per milliliter (pg mL−1), and the anti-HBsAg titers were expressed in international units per milliliter (IU mL−1). The standard curves were generated from measurements of HBsAg or HBsAb standards that were obtained from Beijing Kinghaw Biological Pharmacy Enterprise Co. Ltd. (Beijing, China). All serum samples were assayed in triplicate.
Delayed-type hypersensitivity (DTH) assay
On the seventh day after the third immunization, all groups were challenged with rHBsAg (10 μg/10 μL) in the right footpad as a test and saline solution in the left footpad as a negative control. After 24 and 48 h, the thickness of each footpad was measured with a micrometer and calculated using the following formula: footpad thickness = thickness of right footpad − thickness of left footpad.
In vivo cytotoxic lysis assay
To prepare target cells for in vivo CTL, syngeneic naïve C57BL/6 splenocytes were divided into two parts. One part was labeled with 20 μM of (5-(and 6)-carboxyfluorescein diacetate succinimidyl ester) (CFSE) and pulsed with 10−6 M of the HBsAg-derived peptide S208-215 (defined as CFSEhigh target cells). The other part was labeled with 1 μM of CFSE and pulsed with 10−6 M of the OVA-derived peptide OVA257-264 (defined as CFSElow cells) as a non-HBV target control. A mixture of CFSEhigh and CFSElow cells in a 1:1 ratio was adoptively transferred intravenously into immunized recipient mice at 2 × 107 cells per mouse on the 14th day after the final immunization. Eight hours later, recipient mice were euthanized and splenocytes isolated, and then the CFSE fluorescence intensities were measured with a BD FACS LSR Fortessa flow cytometer (BD Biosciences, San Jose, CA, USA). The specific lysis percent was calculated with the following formula: percentage specific lysis = [1 − (ratio unprimed/ratio primed) × 100].
Intracellular cytokine staining
On the 14th day after the final immunization, single-splenocyte suspensions were prepared. The splenocytes were cultured in round-bottom 96-well polypropylene plates (1 × 106 cells per well) in a 100-μL volume, then stimulated with HBsAg (10 μg mL−1) or S208-215 (10 μg mL−1) for 24 h at 37 °C and 5% CO2 in the presence of anti-CD28 (0.1 μg mL−1). The positive control was stimulated by phorbol 12-myristate 13-acetate (PMA) and ionomycin. In the last 6 h of incubation, 3 μg mL−1 brefeldin A (protein transport inhibitor, BD Biosciences, San Diego, CA, USA) was added into the culture medium. After culture for 24 h, the stimulated cells were pre-stained with specific monoclonal antibodies for cell surface markers (including CD3, CD4, or CD8) for 20 min at room temperature. For the detection of intracellular IFN-γ, IL-4, and IL-17A, splenocytes were fixed with 4% paraformaldehyde for 10 min and permeabilized with 0.1% saponin (Sigma-Aldrich, St. Louis, MO, USA) for 8 min. Then, the appropriate fluorescently labeled anti-mouse monoclonal antibodies (eBioscience, San Diego, CA, USA) were added on ice for 1 h. After a final wash, cells were collected and detected with a BD Fortessa flow cytometer (BD Biosciences, San Diego, CA, USA). Data were analyzed with FlowJo software (Tree Star, Ashland, OR, USA).
T-cell proliferation assay
On the 14th day after the final immunization, single-splenocyte suspensions were prepared from mice from different groups and labeled with eFluor670 Proliferation Dye (eBioscience, San Diego, CA, USA). Next, 5 × 105 cells were cultured in flat-bottom 96-well polypropylene plates in a 100-μL volume, followed by stimulation with HBsAg (10 µg mL−1) or S208-215 (10 μg mL−1) at 37 °C and 5% CO2 in the presence of anti-CD28 (0.1 µg mL−1). After cultivation for four days, the responding T-cell proliferation rate was determined by analyzing the eFluor670 Proliferation Dye dilution.
Treatment of DC cell lines
DC2.4 cells were plated at 2 × 106 mL−1 in 12-well cell culture plates. GM-CSF was added at 1 μg mL−1. After 72 h, the cells were analyzed by fluorescence-activated cell sorting (FACS). Fluorescently labeled monoclonal antibodies (mAbs) recognizing CD11c, CD11b, CD3, CD40, CD80, CD86, and MHC-II and their respective isotype controls were purchased from eBioscience (San Diego, CA, USA).
Histology and immunohistochemistry (IHC)
Seven days after the third immunization, livers from immunized HBsAg-Tg mice were fixed and paraffin-embedded, then cut into 4-µm sections. The tissue sections were stained with hematoxylin-eosin (H&E) for histopathological evaluation. For IHC staining, unstained paraffin-embedded tissue section slides were immunostained with anti-HBsAg mAb, anti-mouse CD8 mAb, or anti-IFN-γ mAb. The secondary antibody incubation was performed with horseradish peroxidase-conjugated goat anti-mouse IgG. The images were captured with a light microscope. The integral OD for HBsAg staining was analyzed with Image-Pro Plus (Media Cybernetics, Inc., Silver Spring, MD, USA) software.
Adoptive transfer of immunized CD8+ T cells
Fourteen days after the third immunization, single-splenocyte suspensions were prepared from spleens and labeled with 15 µM CFSE. CD8+ T cells were isolated and purified with a MagCellect Mouse CD8+ T cell Isolation kit (R&D Systems, Inc., Minneapolis, MN, USA) according to the manufacturer's protocol. The purity of each cell preparation was at least 90%. The labeled CD8+ T cells were adoptively transferred intravenously into normal HBsAg-Tg mice or normal C57BL/6 mice (WT) at 5 × 106 cells per recipient mouse.
Statistical analysis
Statistics were performed using GraphPad Prism Software 6.0 (GraphPad, La Jolla, CA, USA) and are presented as means with standard error of the mean (SEM). Pairwise differences were analyzed with an unpaired Student's t-test. A value of p ≤ 0.05 was considered significant.
Results
GM-CSF has a time-sensitive effect on dendritic cell maturation and differentiation
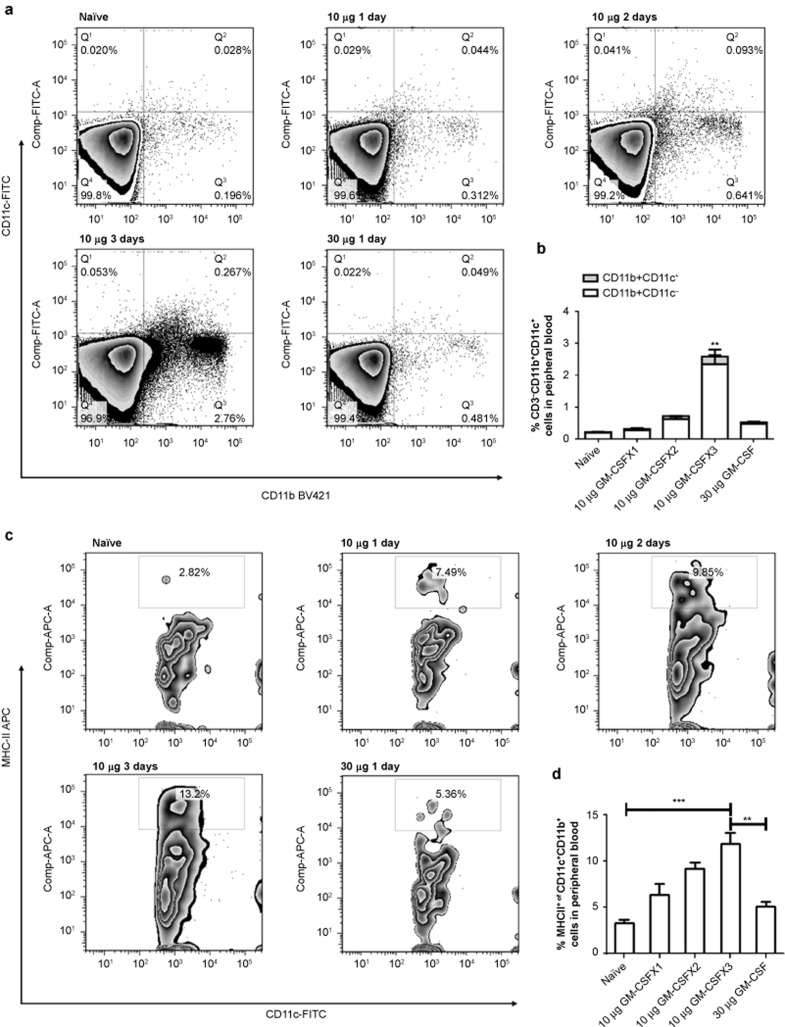
The induction of mature dendritic cells from human peripheral blood mononuclear cells requires several days of in vitro culturing with GM-CSF, and the DC maturation process requires continued simulation for more than one day.23,24 This requirement for prolonged stimulation has also been demonstrated in in vivo experiments.49 To examine whether DC maturation in HBV transgenic mice also requires continuous stimulation, animals were subcutaneously injected with 10 μg of GM-CSF daily for one, two, or three days, or with a single dose of 30 μg for one day. Cells from peripheral blood, spleen, and lymph nodes were isolated on day 4. FACS analysis was performed to determine the percentage of cells with a myeloid DC phenotype. Three days of daily injections of GM-CSF increased the proportions of both CD11b+CD11c+ and CD11b+CD11c− DCs in peripheral blood over time, reaching six-fold higher levels than in the peripheral blood of animals with one or two days of GM-CSF administration (p < 0.01) (Figure 1a and 1b). Maturation of these DCs, as indicated by MHC-II levels, was also significantly greater than the maturation of DCs with one- or two-day treatment (Figure 1c and 1d). Interestingly, mice injected with a single dose of GM-CSF that was equivalent to the sum of the three daily doses (30 μg) did not achieve the same levels of CD11b+CD11c+ and MHC-II expression, suggesting that one dose with a higher concentration of GM-CSF did not provide adequate support for DC differentiation and maturation. This is in good agreement with the results from in vitro DC maturation experiments using GM-CSF stimulation. Evidently, the DCs from the tolerant animals required prolonged GM-CSF stimulation for at least three days to achieve substantially increased levels of differentiation and maturation.
Figure 1.
GM-CSF stimulation exerts time-sensitive effects on dendritic cells in vivo. HBV transgenic mice were subcutaneously injected with 10 μg of GM-CSF daily for one, two, and three days or with 30 μg of GM-CSF for one day. Peripheral blood cells were isolated on day 4. FACS analysis was performed to determine the percentage of DCs with a myeloid phenotype by staining with CD11c-FITC, CD11b-PB, and MHC-II-PE. The percentages of double-stained cells were calculated. The CD11c+CD11b+ cells among peripheral blood cells (Figure 1a and 1b) were immunostained for MHC-II (Figure 1c and 1d) and analyzed by FACS. Data are shown as mean ± SEM (n = 3) and represent one of the three independent experiments. *p <0.05, **p <0.01, and ***p <0.005 (unpaired Student's t-test).
GM-CSF promotes HBV vaccination to break immune tolerance in HBsAg-Tg mice
To determine whether three daily treatments with GM-CSF could induce HBV vaccination to break the immune tolerance that is established in HBsAg-Tg mice, we designed five treatment regimens with five animals in each group as illustrated in Table 1 and Figure 2a. These were: naïve group; GM-CSF-only group receiving a single dose of 10 μg of GM-CSF on days 0, 14, and 28; rHBVvac-only group receiving 1 μg of rHBVvac vaccination on days 0, 14, and 28; 30GM-CSF+rHBVvac group receiving 30 μg of GM-CSF and 1 μg of rHBVvac vaccine at the same location as the GM-CSF injection on days 0, 14, and 28; 3×GM-CSF+rHBVvac group receiving 10 μg of GM-CSF daily treatment three times followed by 1 μg of rHBVvac vaccination at the same location repeated on days 0, 14, and 28. Levels of serum HBsAg and IgG antibodies against HBsAg were analyzed by quantitative ELISA seven days after the third immunization. After the three-day treatment with GM-CSF followed by HBV vaccination (3×GM-CSF+rHBVvac) that was given three times at two-week intervals, the mean titer of total serum anti-HBsAg IgG was increased four-fold compared to the rHBVvac group (p < 0.005) and two-fold over that of the group receiving 30 μg of GM-CSF+rHBVvac (p < 0.05, Figure 2b). By contrast, the amount of HBsAg in serum was maintained at a low level (7366 ± 2133 pg mL−1, n = 5) compared to the naïve group (15 863 ± 3708 pg mL−1, n = 5) or rHBVvac group (14 108 ± 3822 pg mL−1, n = 5, Figure 2c). Along with the enhanced humoral response in the 3×GM-CSF+HBVvac group, alanine aminotransferase (ALT) levels were also augmented (Figure 2d), indicating the occurrence of a cell-mediated immune response. These data demonstrate that the immune tolerance of HBV transgenic mice was broken and overcome by the administration of 3×GM-CSF+rHBVvac.
Table 1. Immunization groups.
| Group | GM-CSF (μg) | rHBVvac (μg) |
|---|---|---|
| Naïve | / | / |
| GM-CSF | 10 μg | / |
| rHBVvac | / | 1 μg |
| 30GM-CSF+rHBVvac | 30 μg | 1 μg |
| 3×GM-CSF+rHBVvac | 10 μg daily for three days | 1 μg |
The DTH reaction in skin can be used as an in vivo assay for measuring T-cell responses to immunization. The effects of treatment with GM-CSF followed by rHBVvac on DTH were evaluated by challenging with an injection of 1 μg of rHBsAg in the foot pads of treated mice on day 7 after the third immunization. Footpad thickness was measured at 24 h and 48 h after challenge. At both time points, DTH reactions against rHBsAg reached their highest levels in the 3×GM-CSF+rHBVvac group (Figure 2e), although the animals that received 30GM-CSF+rHBVvac also demonstrated higher levels of DTH. Consistently, the proliferative capacity of antigen-specific T cells was also significantly enhanced after 3×GM-CSF+rHBVvac (Figure 2f and 2g). Given that this GM-CSF adjuvant protocol resulted in both higher humoral immune responses and stronger cell-mediated immune responses in HBsAg-Tg mice, its potential for therapeutic vaccination was indicated.
3×GM-CSF+rHBVvac immunization induces strong Tc1 immune responses and HBs-specific CTLs
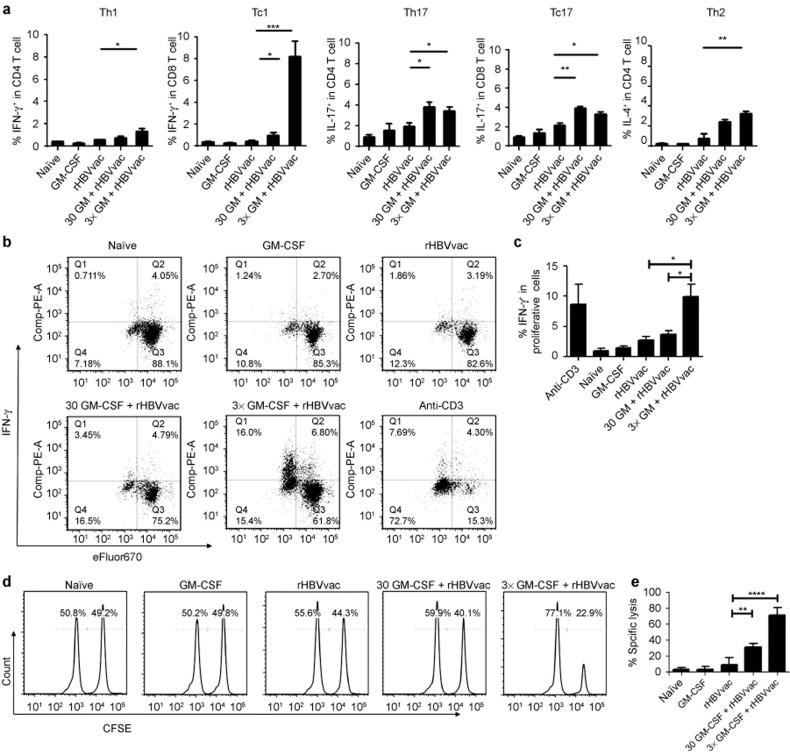
We next sought to determine whether the GM-CSF/rHBVvac regimen for therapeutic vaccination resulted in strong antigen-specific Th1 and cytolytic responses. We analyzed these responses by flow cytometry for intracellular cytokines and with an in vivo CTL assay on day 14 after the third immunization. As shown in Figure 3a, the regimen of 3×GM-CSF+rHBVvac induced Th1 (CD4+ IFN-γ+), Th2 (CD4+IL-4+), Th17 (CD4+IL-17+), Tc1 (CD8+ IFN-γ+), and Tc17 (CD8+IL-17+) responses to a certain degree. Strikingly, the Tc1 cell response was very robust and was increased significantly in the 3×GM-CSF+rHBVvac group compared to the other groups (p < 0.005, Figure 3a). This impressive Tc1 clonal expansion was consistent with the results of in vitro antigen-specific stimulation of these isolated CD8+ T cells (eFluor670low IFN-γ+, Figure 3b).
Figure 3.
3×GM-CSF+rHBVvac immunization induced a strong Tc1 immune response and enhanced HBs-specific CTLs. (a) Th1, Tc1, Th17, Tc17, and Th2 cell induction in response to vaccination. Splenic T cells were isolated on day 14 after the third immunization, stimulated with rHBsAg (10 μg mL−1) for 12 h in culture and stained intracellularly for IFN-γ, IL-4, and IL-17a in CD4+ or CD8+ T cells. (b) IFN-γ+ intracellular staining of CD8+ eFluor 670-labeled T cells after culture with rHBsAg (10 μg mL−1) for three days. (c) The percentage of IFN-γ+ CD8+ eFluor 670low T cells is summarized (d). In vivo cytotoxic lysis assays were performed on the 14th day after the third immunization. (e) The percentage of specific lysis is summarized. The data are shown as mean ± SEM (n = 5) from three independent experiments. *p < 0.05 and **p < 0.01 (unpaired Student's t-test).
Because antigen-specific CTL responses are a crucial factor for the development of a therapeutic HBV vaccine, we performed an in vivo cytotoxic assay. We observed that the 3×GM-CSF+rHBVvac group demonstrated a dramatically enhanced HBsAg-specific CTL response, reaching 70%. In comparison, the responses of the 30GM-CSF+rHBVvac and rHBVvac groups were, respectively, ∼30% and ∼10% (Figure 3d and 3e). Taken together, these results demonstrate that the protocol comprising three daily treatments with GM-CSF followed by HBV vaccination induced both a robust Tc1 response and HBsAg-specific CTLs in HBsAg-Tg mice, indicating that a strong anti-HBsAg response was elicited in these otherwise tolerized mice.
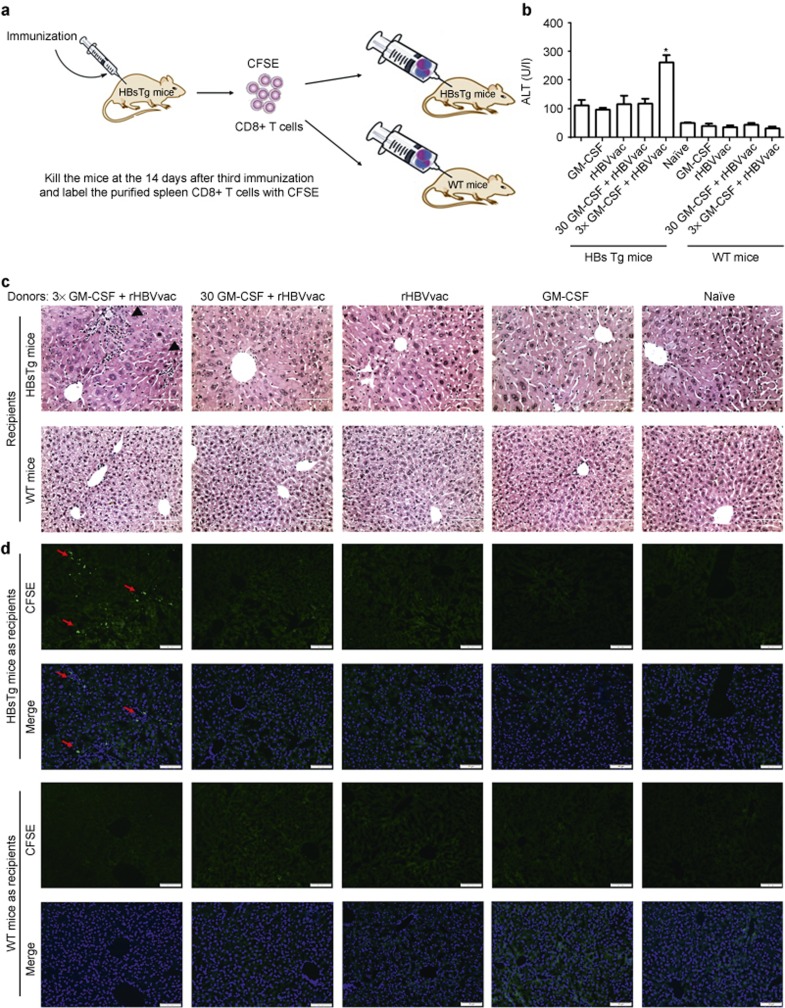
HBV-specific CTLs in the livers of HBV transgenic mice have been reported to be key for eliminating established HBV infection.25 To demonstrate that HBsAg-specific CTLs were induced by our protocol, we isolated CD8+ T cells from immunized mice on day 14 after the third immunization, labeled them with CFSE, then adoptively transferred them intravenously into untreated HBsAg-Tg mice or WT C57BL/6 mice at 5 × 106 cells per recipient (Figure 4a). Three days later, we observed that the CD8+ T cells from donors immunized with 3×GM-CSF+rHBVvac resulted in significantly elevated levels of serum ATL in HBsAg-Tg mice but not in WT animals (Figure 4b). This suggested a specific immune response in the livers of HBV-positive recipient mice only. Consistently, the infiltration of CFSE-labeled CD8+ T cells in the livers of recipient HBsAg-Tg mice was also observed only in recipients of cells from 3×GM-CSF+rHBVvac group donors, not from the other groups (Figure 4c and 4d).
Figure 4.
Adoptive transfer of immunized CD8+ T cells. (a) Fourteen days after the third immunization, CD8+ T cells were purified from donor mice and labeled with CFSE and then adoptively transferred into HBsTg mice or WT mice. (b) The ALT levels in serum were detected on day 3 after adoptive transfer. (c) Livers of recipient mice were obtained on day 3 after the adoptive cell transfer and stained with H&E. Bar = 50 μm. (d) Fluorescence microscopy of livers from recipient mice. Green indicates the transferred CFSE-labeled CD8+ T cells and blue indicates DAPI-stained nuclei. Bar = 200 μm. Data are shown as means ± SEM (n = 3) and represent one of two independent experiments. *p < 0.05 (unpaired Student's t-test).
Treatment with 3×GM-CSF+rHBVvac eliminates HBsAg-positive hepatocytes in vivo and induces CD8+ T-cell infiltration into the livers of HBsAg-Tg mice
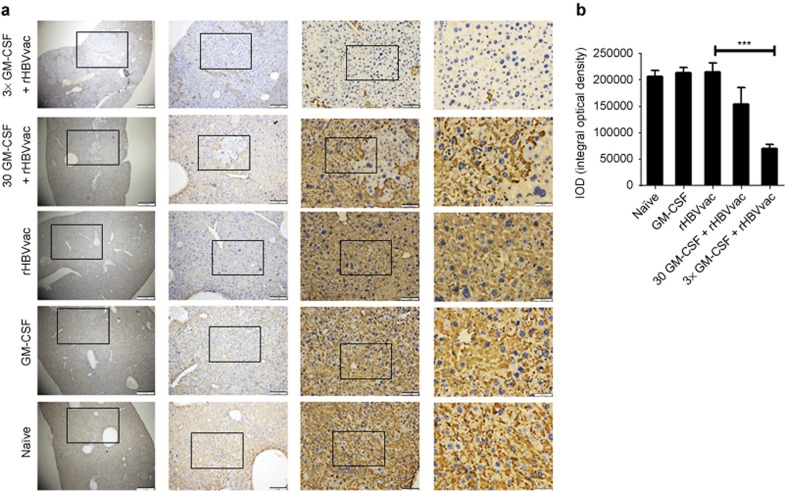
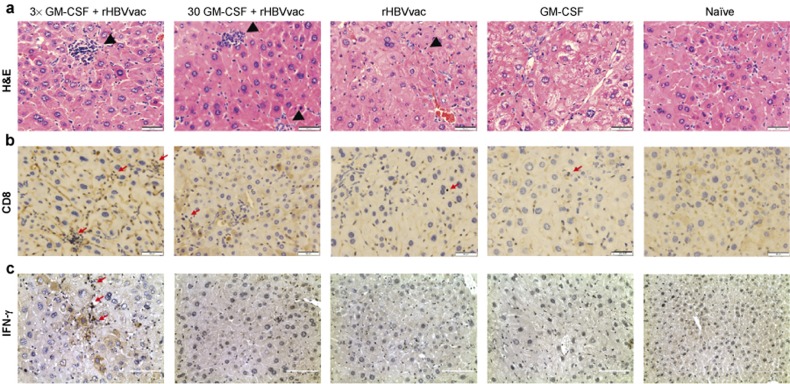
The elimination of virus-infected hepatocytes is the key indication for the cure of chronic HBV infection. To test the efficacy of our protocol, we stained liver sections of immunized HBsAg-Tg mice with an anti-HBsAg antibody on day 14 after the third immunization. The number of HBsAg-positive hepatocytes was dramatically reduced in HBsAg-Tg mice that had been treated with 3×GM-CSF+rHBVvac compared to the other groups (p < 0.005, Figure 5). The reduction was accompanied by lymphocyte infiltration into the livers. This infiltration was associated with IFN-γ+CD8+ T cells, particularly in the 3×GM-CSF+rHBVvac group and to a lesser degree in 30GM-CSF+rHBVvac group but not in the rHBVvac group (Figure 6b and 6c). No changes in liver morphology or pathological changes were observed in the group treated with 3×GM-CSF+rHBVvac, indicating that the induced infiltration of CD8+ T cells was not overly pathogenic. On the contrary, inflammation and abnormalities of “swelling” hepatocytes with larger nuclei were observed in the other groups but not in the 3×GM-CSF+rHBVvac group, indicating that the cell nuclei had been restored to normal. These results suggested that the elimination of HBsAg-positive hepatocytes was likely occurring due to the infiltration of antigen-specific IFN-γ producing CD8+ T cells, leaving the hepatocytes with normal nuclear morphologies.
Figure 5.
Specific immunostaining of HBsAg in HBV transgenic mice on the 14th day after the third immunization. (a) Microscopic examination of liver sections with specific immunostaining of HBsAg; objective amplification from left to right is 40×, 100×, 200×, and 400×. (b) The integral optical density for HBsAg staining in each group. Data are expressed as mean ± SEM (n = 5) and represent one of the three independent experiments (***p < 0.001).
Figure 6.
Histopathology of HBV transgenic mouse livers. (a) Livers were obtained on day 14 after the third immunization, fixed, sectioned, and stained with H&E. Bar = 50 μm. (b) CD8-specific immunostaining of liver on day 14 after the final immunization. Bar = 50 μm. Specific immunostaining of HBsAg on day 14 after the final immunization. Bar = 50 μm. (c) IFN-γ-specific immunostaining on day 14 after the final immunization. Bar = 100 μm.
Immunization with 3×GM-CSF+rHBVvac induced durable and high levels of anti-HBsAg antibodies and reduced the levels of HBsAg in HBsAg-Tg mice
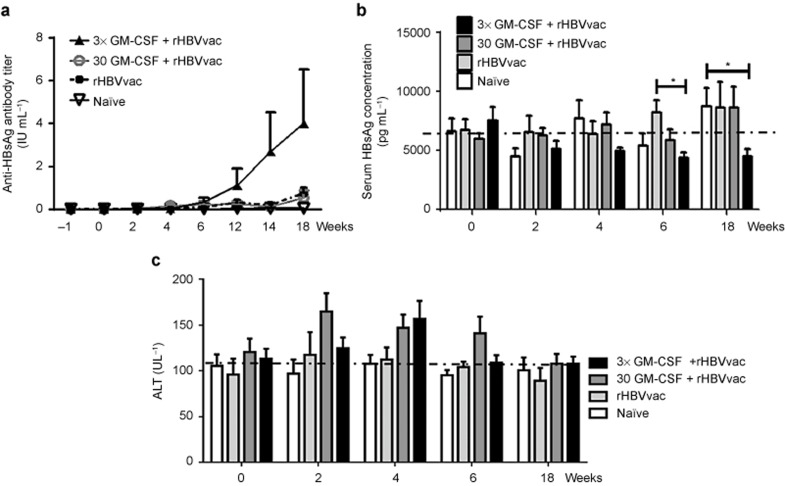
Vaccine-induced breaking of immune tolerance might be of short duration. To test the long-term effects of the GM-CSF+rHBVvac regimen, we immunized HBsAg-Tg mice four times instead of 3 and examined the serological changes after 18 weeks. As depicted in Figure 7, the levels of serum anti-HBsAg IgG in the 3×GM-CSF+rHBVvac group were significantly increased after the fourth immunization, and at week 18, the levels were consistently higher at week 18 compared with the other groups (Figure 7a). The levels of serum HBsAg were decreased compared to the other groups and remained at low levels from the second immunization through the end of the study at week 18 (Figure 7b). We also observed that the levels of ALT in serum remained at base levels in the group treated with 3×GM-CSF+rHBVvac but were transiently increased at weeks 2 and 4 (Figure 7c). These results suggested that the regimen of three daily treatments with GM-CSF followed by HBV vaccination (3×GM-CSF+rHBVvac) administered four times at two-week intervals not only can break immune tolerance but can also result in a long-term antigen-specific immune response in HBsAg-Tg mice.
Figure 7.
Serology changes observed over 18 weeks in HBV transgenic mice that were immunized at 0, 2, 4, and 12 weeks. (a) Total serum anti-HBsAg IgG was analyzed at −1, 0, 2, 4, 6, 12, 14, and 18 weeks. Mean titers are expressed as immunization IU mL−1. (b) Serum HBsAg was analyzed at 0, 4, 6, and 18 weeks. (c) ALT activity in the serum was analyzed at 0, 2, 4, 6, and 18 weeks. Data are expressed as mean ± SEM (n = 6).
Discussion
In this study, we demonstrated that treatment with GM-CSF daily for three days prior to HBV vaccination could induce significant DC maturation and subsequent cell-mediated immune responses; levels of IFN-γ producing and HBsAg-specific cytotoxic CD8+ T cells were robustly enhanced (p < 0.05). In addition, significant elimination of HBsAg-positive hepatocytes in HBsAg-Tg mice, an immune-tolerogenic animal model, was observed. These results suggest great potential for the treatment chronic HBV infection with this novel immune therapy, as HBV-specific cytotoxic CD8+ T cells are known to play a key role in viral clearance in chronically HBV-infected individuals.26,27,28
Chronic HBV infection often leads to systemic immune tolerance or immune exhaustion via two possible mechanisms.9,29 The first is by the induction of innate immune tolerance via down-regulation of the frequency and functions of innate immune cells such as NK, NKT, and DCs.30,31,32 The second mechanism is by the induction of inferior specific immune responses including deficiency of HBV-specific CTLs and low levels of antiviral cytokine production.29,33,34 For a successful immunotherapeutic approach, one should aim to break and overcome both of these immune tolerance mechanisms to mount an effective counterattack. We propose that our protocol of sustained stimulation via three daily pre-treatments with GM-CSF before HBV vaccination induced significantly higher levels of DC maturation compared to pre-treatment for one or two days, enabling DCs to present antigen more effectively to the adaptive immune system. As a result, not only were anti-HBsAg antibodies induced, but robust IFN-γ-producing and HBsAg-specific cytotoxic CD8+ T-cell responses were also elicited in the tolerized animals. This manifestation of cell-mediated immunity was apparently able to exert control over hepatocytes expressing HBsAg in HBsAg-Tg animals.
GM-CSF is an important hematopoietic growth factor, immune modulator, and dendritic cell potentiator.35 A number of clinical studies using GM-CSF as a vaccine adjuvant for the treatment of cancers have been performed. Unfortunately, contradictory results were observed: some studies showed the stimulation of immune responses,36,37 whereas others showed the suppression of immune responses.18,19 The reasons underlying such a discrepancy are not completely understood. However, it is known that the maturation of bone marrow-derived monocytes into DCs normally takes several days. Once the DCs have matured, they effectively take up and process antigen and thus promote anti-viral immunity.38,39,40 We therefore reasoned that the duration of GM-CSF stimulation might be a critical variable.
To drive bone marrow-derived dendritic cells into mature DCs in vitro, GM-CSF can be added every other day for one week. Several reports showed that the goal of immune stimulation in vivo was attained by the maintenance of elevated levels of GM-CSF. This induced the differentiation of circulating inflammatory monocytes into inflammatory DCs and resulted in effective immune responses.41,42,43,44 Insufficient stimulation with GM-CSF, on the other hand, can result in the production of immature DCs, or so-called tolerogenic DCs (tolDCs), that lead to tolerogenic responses in vivo.45,46 Thus, correct use of GM-CSF is the key to inducing effective immunity.47 We anticipated that multiple administrations of GM-CSF could enhance immune responses and might break and overcome immune tolerance in chronic HBV infection. To test this hypothesis, we first examined the total number of CD11b+CD11c+ DCs in peripheral blood and found enhanced expression levels of MHC-II, CD40, CD80, and CD86 after the administration of GM-CSF subcutaneously at the same site once daily for one, two, or three days. As expected, DC maturation was significantly enhanced after three days of GM-CSF administration and led to a dramatic increase in antigen-specific CMI when the HBV vaccine was given on day 4. A similar result was reported with a long-lasting polyethylene glycol-modified GM-CSF causing the expansion of CD11b+CD11c+ dendritic cells in vivo.35,42,48 To delineate the possible cumulative effects of sequential stimulation, a comparison was made with 30 μg of GM-CSF given to mice as a single injection. Although the effects were much greater than those from one or two low (10 μg) daily doses, its inferiority in terms of breaking immune tolerance was evidenced when the levels of induced anti-HBsAg antibodies, CTLs and HBsAg-positive hepatocyte clearance were compared. These results suggested that the effects of GM-CSF on DC maturation are time-dependent in vivo. However, further investigation is required to understand the molecular mechanisms underlying the various immune responses to different regimens.
Although we observed the significant elimination of HBsAg-positive hepatocytes with the optimized protocol (approximately 90% reduction), it did not reduce the levels of HBsAg in the serum to the same degree. This discrepancy may be due to the fact that a few HBsAg-positive hepatocytes could produce and maintain detectable amounts of HBsAg in the serum, even if a large proportion of HBsAg-positive hepatocytes are cleared.12,49 In this regard, HBV-specific CD8+ T cells are the key effector cells that mediate the clearance of HBsAg-positive hepatocytes from the liver.50 IFN-γ- and TNF-α-producing CD8+ T cells have been demonstrated to contribute to the antiviral immune response through cytolytic and non-cytolytic pathways in the HBV-transgenic mouse model.51,52,53 In our study, 3×GM-CSF+rHBVvac significantly enhanced IFN-γ secretion by CD8+ T cells (Tc1) and induced a strong specific CTL response (Figure 2) resulting in the elimination of a major proportion of HBsAg-positive hepatocytes (Figure 5). The local concentration of IFN-γ-producing HBV-specific CD8+ T cells in liver has been demonstrated as being key to the functional restoration of anti-HBV-specific immunity in persistent HBV infection.54,55 IFN-γ also promotes a non-cytopathic mechanism of HBV elimination that can limit liver damage during the immune-clearance of HBV.56 Consistent with this, we observed infiltration of IFN-γ-producing CD8+ T cells in the livers of HBsAg-Tg mice in the 3×GM-CSF+rHBVvac treated group (Figure 6).
T-cell-mediated cytolytic killing of hepatocytes sometimes causes liver tissue damage, which is detectable through the elevation of serum ALT levels. Although we observed elevated ALT levels at week 1 after the last immunization with the 3×GM-CSF+rHBVvac regimen in HBsAg-Tg mice, they gradually declined to basal levels (Figure 7). Transience ALT elevation may be attributable to the immune clearance of infected hepatocytes.57,58 Interestingly, the levels of perforin and granzyme B were not significantly increased (data not shown), indicating the effects on the liver are limited and might be tolerable in man. Consistently, histological analysis of the liver during the same period of time revealed no major morphological changes. Similar results were also observed in previous studies.59,60 Therefore, we can conclude that small-scale antigen-specific killing of the hepatocytes may cause little or no damage to the liver.60
In summary, we found that an enhanced regimen of GM-CSF as an HBV vaccine adjuvant could promote the breaking of immune tolerance in HBsAg-Tg mice. The strong humoral and cellular immune responses induced by 3×GM-CSF+rHBVvac might lead to the development of a therapeutic protocol to overcome immune tolerance and eliminate the HBsAg-positive hepatocytes of CHB. As both GM-CSF and the rHBV vaccines have demonstrated to be safe for clinical usage for over 20 years, such a regimen can easily be translated into clinical trials to test this novel immunotherapy in CHB patients. If successful, the combination of GM-CSF with HBV vaccination could provide a valuable option for treating chronic HBV patients and increase the cure rate of the disease.
Acknowledgments
This work was supported in part by the MOST National 863 Project of China (2012AA02A407) and the National Science and Technology Major Program of Infectious Diseases (2013ZX10002001) to Bin Wang. We thank Dr. Douglas Lowrie for his editing and proofreading of the manuscript. We also thank Dr. Jane Q.L. Yu, Mr. Zhonghuai He, and Mr. Xianghua Shi for their assistance with this work.
Footnotes
Supplementary Information accompanies the paper on Cellular & Molecular Immunology website: http://www.nature.com/cmi.
Supplementary Information
References
- Walter SR, Thein HH, Gidding HF, Amin J, Law MG, George J et al. Risk factors for hepatocellular carcinoma in a cohort infected with hepatitis B or C. J Gastroenterol Hepatol 2011; 26: 1757–1764. [DOI] [PubMed] [Google Scholar]
- Hahne SJ, Veldhuijzen IK, Wiessing L, Lim TA, Salminen M, Laar M. Infection with hepatitis B and C virus in Europe: a systematic review of prevalence and cost-effectiveness of screening. BMC Infect Dis 2013; 13: 181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boonstra A, Woltman AM, Janssen HL. Immunology of hepatitis B and hepatitis C virus infections. Best Pract Res Clin Gastroenterol 2008; 22: 1049–1061. [DOI] [PubMed] [Google Scholar]
- Chen DS. Hepatitis B vaccination: the key towards elimination and eradication of hepatitis B. J Hepatol 2009; 50: 805–816. [DOI] [PubMed] [Google Scholar]
- Michel ML, Tiollais P. Hepatitis B vaccines: protective efficacy and therapeutic potential. Pathol Biol (Paris) 2010; 58: 288–295. [DOI] [PubMed] [Google Scholar]
- Brooks J, Gelson W, Rushbrook SM. Therapeutic advances in the management of chronic hepatitis B infection. Ther Adv Chronic Dis 2013; 4: 157–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papatheodoridis GV, Manolakopoulos S, Archimandritis AJ. Current treatment indications and strategies in chronic hepatitis B virus infection. World J Gastroenterol 2008; 14: 6902–6910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lan P, Zhang C, Han Q, Zhang J, Tian Z. Therapeutic recovery of hepatitis B virus (HBV)-induced hepatocyte-intrinsic immune defect reverses systemic adaptive immune tolerance. Hepatology 2013; 58: 73–85. [DOI] [PubMed] [Google Scholar]
- Stoop JN, van der Molen RG, Baan CC, van der Laan LJ, Kuipers EJ, Kusters JG et al. Regulatory T cells contribute to the impaired immune response in patients with chronic hepatitis B virus infection. Hepatology 2005; 41: 771–778. [DOI] [PubMed] [Google Scholar]
- Heintges T, Petry W, Kaldewey M, Erhardt A, Wend UC, Gerlich WH et al. Combination therapy of active HBsAg vaccination and interferon-alpha in interferon-alpha nonresponders with chronic hepatitis B. Dig Dis Sci 2001; 46: 901–906. [DOI] [PubMed] [Google Scholar]
- Zhang W, Wang J, Su B, Li R, Ding Z, Kang Y et al. Cimetidine augments Th1/Th2 dual polarized immune responses to recombinant HBV antigens. Vaccine 2011; 29: 4862–4868. [DOI] [PubMed] [Google Scholar]
- Zou Q, Yao X, Feng J, Yin Z, Flavell R, Hu Y et al. Praziquantel facilitates IFN-gamma-producing CD8+ T cells (Tc1) and IL-17-producing CD8+ T cells (Tc17) responses to DNA vaccination in mice. PLoS One 2011; 6: e25525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu B, Zou Q, Hu Y, Wang B. Interleukin-22 as a molecular adjuvant facilitates IL-17-producing CD8 T cell responses against a HBV DNA vaccine in mice. Hum Vaccin Immunother 2013; 9: 2133–2141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metcalf D. The molecular biology and functions of the granulocyte-macrophage colony-stimulating factors. Blood 1986; 67: 257–267. [PubMed] [Google Scholar]
- van de Laar L, Coffer PJ, Woltman AM. Regulation of dendritic cell development by GM-CSF: molecular control and implications for immune homeostasis and therapy. Blood 2012; 119: 3383–3393. [DOI] [PubMed] [Google Scholar]
- Zhan Y, Xu Y, Lew AM. The regulation of the development and function of dendritic cell subsets by GM-CSF: more than a hematopoietic growth factor. Mol Immunol 2012; 52: 30–37. [DOI] [PubMed] [Google Scholar]
- Waller EK. The role of sargramostim (rhGM-CSF) as immunotherapy. Oncologist 2007; 12: 22–26. [DOI] [PubMed] [Google Scholar]
- Spearman P, Kalams S, Elizaga M, Metch B, Chiu YL, Allen M et al. Safety and immunogenicity of a CTL multiepitope peptide vaccine for HIV with or without GM-CSF in a phase I trial. Vaccine 2009; 27: 243–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilla L, Patuzzo R, Rivoltini L, Maio M, Pennacchioli E, Lamaj E et al. A phase II trial of vaccination with autologous, tumor-derived heat-shock protein peptide complexes Gp96, in combination with GM-CSF and interferon-alpha in metastatic melanoma patients. Cancer Immunol Immunother 2006; 55: 958–968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parmiani G, Castelli C, Pilla L, Santinami M, Colombo MP, Rivoltini L. Opposite immune functions of GM-CSF administered as vaccine adjuvant in cancer patients. Ann Oncol 2007; 18: 226–232. [DOI] [PubMed] [Google Scholar]
- Marshall JL, Gulley JL, Arlen PM, Beetham PK, Tsang KY, Slack R et al. Phase I study of sequential vaccinations with fowlpox-CEA(6D)-TRICOM alone and sequentially with vaccinia-CEA(6D)-TRICOM, with and without granulocyte-macrophage colony-stimulating factor, in patients with carcinoembryonic antigen-expressing carcinomas. J Clin Oncol 2005; 23: 720–731. [DOI] [PubMed] [Google Scholar]
- Babinet C, Farza H, Morello D, Hadchouel M, Pourcel C. Specific expression of hepatitis B surface antigen (HBsAg) in transgenic mice. Science 1985; 230: 1160–1163. [DOI] [PubMed] [Google Scholar]
- Romani N, Gruner S, Brang D, Kampgen E, Lenz A, Trockenbacher B et al. Proliferating dendritic cell progenitors in human blood. J Exp Med 1994; 180: 83–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palucka K, Banchereau J. Cancer immunotherapy via dendritic cells. Nat Rev Cancer 2012; 12: 265–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu C, Shu J, Jin X. Therapeutic vaccine for hepatitis B virus. J Immunol Tech Infect Dis 2012; 1: 1. [Google Scholar]
- Thimme R, Wieland S, Steiger C, Ghrayeb J, Reimann KA, Purcell RH et al. CD8(+) T cells mediate viral clearance and disease pathogenesis during acute hepatitis B virus infection. J Virol 2003; 77: 68–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rehermann B, Lau D, Hoofnagle JH, Chisari FV. Cytotoxic T lymphocyte responsiveness after resolution of chronic hepatitis B virus infection. J Clin Invest 1996; 97: 1655–1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maini MK, Boni C, Lee CK, Larrubia JR, Reignat S, Ogg GS et al. The role of virus-specific CD8(+) cells in liver damage and viral control during persistent hepatitis B virus infection. J Exp Med 2000; 191: 1269–1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kakimi K, Isogawa M, Chung J, Sette A, Chisari FV. Immunogenicity and tolerogenicity of hepatitis B virus structural and nonstructural proteins: implications for immunotherapy of persistent viral infections. J Virol 2002; 76: 8609–8620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Molen RG, Sprengers D, Binda RS, de Jong EC, Niesters HG, Kusters JG et al. Functional impairment of myeloid and plasmacytoid dendritic cells of patients with chronic hepatitis B. Hepatology 2004; 40: 738–746. [DOI] [PubMed] [Google Scholar]
- Woltman AM, Op den Brouw ML, Biesta PJ, Shi CC, Janssen HL. Hepatitis B virus lacks immune activating capacity, but actively inhibits plasmacytoid dendritic cell function. PLoS One 2011; 6: e15324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan XZ, Wang M, Li HW, Zhuang H, Xu D, Wang FS. Decreased frequency and function of circulating plasmocytoid dendritic cells (pDC) in hepatitis B virus infected humans. J Clin Immunol 2004; 24: 637–646. [DOI] [PubMed] [Google Scholar]
- Han Q, Lan P, Zhang J, Zhang C, Tian Z. Reversal of hepatitis B virus-induced systemic immune tolerance by intrinsic innate immune stimulation. J Gastroenterol Hepatol 2013; 28: 132–137. [DOI] [PubMed] [Google Scholar]
- Carey I, D'Antiga L, Bansal S, Longhi MS, Ma Y, Mesa IR et al. Immune and viral profile from tolerance to hepatitis B surface antigen clearance: a longitudinal study of vertically hepatitis B virus-infected children on combined therapy. J Virol 2011; 85: 2416–2428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Y, Liu CH, Roberts AI, Das J, Xu G, Ren G et al. Granulocyte-macrophage colony-stimulating factor (GM-CSF) and T-cell responses: what we do and don't know. Cell Res 2006; 16: 126–133. [DOI] [PubMed] [Google Scholar]
- Barouch DH, Santra S, Tenner-Racz K, Racz P, Kuroda MJ, Schmitz JE et al. Potent CD4+ T cell responses elicited by a bicistronic HIV-1 DNA vaccine expressing gp120 and GM-CSF. J Immunol 2002; 168: 562–568. [DOI] [PubMed] [Google Scholar]
- Ogawa T, Kusumoto M, Kuroki S, Nagata S, Yamanaka N, Kawano R et al. [Adjuvant GM-CSF cytokine gene therapy for breast cancer]. Gan To Kagaku Ryoho 2001; 28: 1512–1514. [PubMed] [Google Scholar]
- Steinman RM. Dendritic cells: understanding immunogenicity. Eur J Immunol 2007; 37: S53–S60. [DOI] [PubMed] [Google Scholar]
- Fajardo-Moser M, Berzel S, Moll H. Mechanisms of dendritic cell-based vaccination against infection. Int J Med Microbiol 2008; 298: 11–20. [DOI] [PubMed] [Google Scholar]
- Dudek AM, Martin S, Garg AD, Agostinis P. Immature, semi-mature, and fully mature dendritic cells: toward a DC-cancer cells interface that augments anticancer immunity. Front Immunol 2013; 4: 438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreider BL, Phillips PD, Prystowsky MB, Shirsat N, Pierce JH, Tushinski R et al. Induction of the granulocyte-macrophage colony-stimulating factor (CSF) receptor by granulocyte CSF increases the differentiative options of a murine hematopoietic progenitor cell. Mol Cell Biol 1990; 10: 4846–4853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conti L, Gessani S. GM-CSF in the generation of dendritic cells from human blood monocyte precursors: recent advances. Immunobiology 2008; 213: 859–870. [DOI] [PubMed] [Google Scholar]
- Gosselin EJ, Wardwell K, Rigby WF, Guyre PM. Induction of MHC class II on human polymorphonuclear neutrophils by granulocyte/macrophage colony-stimulating factor, IFN-gamma, and IL-3. J Immunol 1993; 151: 1482–1490. [PubMed] [Google Scholar]
- Disis ML, Bernhard H, Shiota FM, Hand SL, Gralow JR, Huseby ES et al. Granulocyte-macrophage colony-stimulating factor: an effective adjuvant for protein and peptide-based vaccines. Blood 1996; 88: 202–210. [PubMed] [Google Scholar]
- Torres-Aguilar H, Blank M, Jara LJ, Shoenfeld Y. Tolerogenic dendritic cells in autoimmune diseases: crucial players in induction and prevention of autoimmunity. Autoimmun Rev 2010; 10: 8–17. [DOI] [PubMed] [Google Scholar]
- Lutz MB, Kukutsch NA, Menges M, Rossner S, Schuler G. Culture of bone marrow cells in GM-CSF plus high doses of lipopolysaccharide generates exclusively immature dendritic cells which induce alloantigen-specific CD4 T cell anergy in vitro. Eur J Immunol 2000; 30: 1048–1052. [DOI] [PubMed] [Google Scholar]
- Wei WC, Su YH, Chen SS, Sheu JH, Yang NS. GM-CSF plays a key role in zymosan-stimulated human dendritic cells for activation of Th1 and Th17 cells. Cytokine 2011; 55: 79–89. [DOI] [PubMed] [Google Scholar]
- Daro E, Pulendran B, Brasel K, Teepe M, Pettit D, Lynch DH et al. Polyethylene glycol-modified GM-CSF expands CD11b(high)CD11c(high) but notCD11b(low)CD11c(high) murine dendritic cells in vivo: a comparative analysis with Flt3 ligand. J Immunol 2000; 165: 49–58. [DOI] [PubMed] [Google Scholar]
- Nair S, Perrillo RP. Serum alanine aminotransferase flares during interferon treatment of chronic hepatitis B: is sustained clearance of HBV DNA dependent on levels of pretreatment viremia? Hepatology 2001; 34: 1021–1026. [DOI] [PubMed] [Google Scholar]
- Yang PL, Althage A, Chung J, Maier H, Wieland S, Isogawa M et al. Immune effectors required for hepatitis B virus clearance. Proc Natl Acad Sci USA 2010; 107: 798–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips S, Chokshi S, Riva A, Evans A, Williams R, Naoumov NV. CD8(+) T cell control of hepatitis B virus replication: direct comparison between cytolytic and noncytolytic functions. J Immunol 2010; 184: 287–295. [DOI] [PubMed] [Google Scholar]
- Ando K, Guidotti LG, Wirth S, Ishikawa T, Missale G, Moriyama T et al. Class I-restricted cytotoxic T lymphocytes are directly cytopathic for their target cells in vivo. J Immunol 1994; 152: 3245–3253. [PubMed] [Google Scholar]
- Xie X, Geng S, Liu H, Li C, Yang Y, Wang B. Cimetidine synergizes with Praziquantel to enhance the immune response of HBV DNA vaccine via activating cytotoxic CD8(+) T cell. Hum Vaccin Immunother 2014; 10: 1688–1699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Burg SH, Arens R, Melief CJ. Immunotherapy for persistent viral infections and associated disease. Trends Immunol 2011; 32: 97–103. [DOI] [PubMed] [Google Scholar]
- Schurich A, Khanna P, Lopes AR, Han KJ, Peppa D, Micco L et al. Role of the coinhibitory receptor cytotoxic T lymphocyte antigen-4 on apoptosis-Prone CD8 T cells in persistent hepatitis B virus infection. Hepatology 2011; 53: 1494–1503. [DOI] [PubMed] [Google Scholar]
- Chisari FV, Isogawa M, Wieland SF. Pathogenesis of hepatitis B virus infection. Pathol Biol (Paris) 2010; 58: 258–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth E, Pircher H. IFN-γ promotes Fas ligand-and perforin-mediated liver cell destruction by cytotoxic CD8 T cells. J Immunol 2004; 172:1588–1594. [DOI] [PubMed] [Google Scholar]
- Spanaus KS, Schlapbach R, Fontana A. TNF-alpha and IFN-gamma render microglia sensitive to Fas ligand-induced apoptosis by induction of Fas expression and down-regulation of Bcl–2 and Bcl-xL. Eur J Immunol 1998; 28: 4398–4408. [DOI] [PubMed] [Google Scholar]
- Zou Q, Yao X, Feng J, Yin Z, Flavell R, Hu Y et al. Praziquantel facilitates IFN-γ-producing CD8+ T cells (Tc1) and IL-17-producing CD8+ T cells (Tc17) responses to DNA vaccination in mice. PLoS One 2011; 6: e25525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertoletti A, Kennedy PT. The immune tolerant phase of chronic HBV infection: new perspectives on an old concept. Cell Mol Immunol 2015; 12: 258–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.