Abstract
The objective of this study was to investigate the influence of the genes encoding the KIR receptors and their HLA ligands in the susceptibility of ocular toxoplasmosis. A total of 297 patients serologically-diagnosed with toxoplasmosis were selected and stratified according to the presence (n = 148) or absence (n = 149) of ocular scars/lesions due to toxoplasmosis. The group of patients with scars/lesions was further subdivided into two groups according to the type of ocular manifestation observed: primary (n = 120) or recurrent (n = 28). Genotyping was performed by PCR-SSOP. Statistical analyses were conducted using the Chi-square test, and odds ratio with a 95% confidence interval was also calculated to evaluate the risk association. The activating KIR3DS1 gene was associated with increased susceptibility for ocular toxoplasmosis. The activating KIR together with their HLA ligands (KIR3DS1-Bw4-80Ile and KIR2DS1+/C2++ KIR3DS1+/Bw4-80Ile+) were associated with increased susceptibility for ocular toxoplasmosis and its clinical manifestations. KIR-HLA inhibitory pairs -KIR2DL3/2DL3-C1/C1 and KIR2DL3/2DL3-C1- were associated with decreased susceptibility for ocular toxoplasmosis and its clinical forms, while the KIR3DS1−/KIR3DL1+/Bw4-80Ile+ combination was associated as a protective factor against the development of ocular toxoplasmosis and, in particular, against recurrent manifestations. Our data demonstrate that activating and inhibitory KIR genes may influence the development of ocular toxoplasmosis.
Ocular Toxoplasmosis, the most common form of posterior uveitis, results from Toxoplasma gondii infection1. The prevalence varies widely between different countries however, both the frequency and the severity of the resulting ocular manifestations are higher in Brazil than in many other parts of the world2,3. Eye injuries affect the retina and the choroid with local inflammatory reactions being observed in ocular tissues infected by T. gondii1.
The damage to ocular tissues led to the proposal of phenomena that may be related to pathogenic mechanisms of ocular toxoplasmosis, including autoimmune mechanisms1,4. Now it is known that an exaggerated T-helper 1 (Th-1) response, in particular by Th-17 cells, can cause tissue damage and contribute to the severity of ocular toxoplasmosis due to the production of interleukin-17 (IL-17), a potent inducer of inflammation5,6. In addition to Th-17, other sources of IL-17, including natural killer cells (NK), can contribute to the development of inflammatory conditions7.
Increases in the numbers of circulating proinflammatory monocytes and NK CD56dim cytotoxic cells and a decrease in immunoregulatory NK CD56bright cells have been identified in children congenitally infected with T. gondii who have active eye lesions. Furthermore, subsets of NK cells and CD8+ T cells play a crucial role as biomarkers of cicatricial lesion of the eye8. There is also evidence that NK cells have a predominantly proinflammatory profile in vitro during T. gondii infections, due to an increased production of interferon-gamma (IFN-γ) in patients with congenital ocular toxoplasmosis6.
The effector function of NK cells is regulated by a set of receptors named killer immunoglobulin-like receptors (KIR) expressed on the cell surface that recognize human leukocyte antigen (HLA) class I molecules of target cells9,10. KIR genes are responsible for coding the KIR receptors of NK cells. These genes comprise a family of 15 genes located on chromosome 19q13.4 characterized as inhibitors (KIR2DL1, -2DL2, -2DL3, -2DL5A,-2DL5B -3DL1, -3DL2, and -3DL3) or activators (KIR2DS1, -2DS2, -2DS3, -2DS4, -2DS5, -3DS1 and -2DL4,), and two pseudogenes (KIR2DP1 and -3DP1). Moreover, based on the content of the genes, KIR genotypes are divided into two groups designated AA and BX (BB and AB) that differ in the number and type of KIR genes11.
KIR genes have been described as risk or protective factors in different types of non-toxoplasmic uveitis and inflammatory ocular diseases. These diseases include Behcet’s uveitis12, uveitis in patients with spondyloarthropathies13,14 and Vogt-Koyanagi-Harada syndrome15,16, all of which are triggered by autoimmune processes. KIR genes are also associated with many other infectious diseases17,18,19. Additionally, both murine and human studies have shown that major histocompatibility complex (MHC) class I (called HLA class I in human) are associated with Toxoplasma susceptibility20,21,22,23,24,25,26.
NK cells have great importance in the control of T. gondii infection27 however, the role of KIR genes that encode the immune receptors of NK cells and can trigger local inflammation in the eye has not been elucidated in ocular toxoplasmosis yet. The objective of this study was to investigate the influence of the genes encoding the KIR receptors and their HLA ligands in the resistance or susceptibility to the development of ocular toxoplasmosis.
Results
General characteristics of patients with and without ocular manifestations of toxoplasmosis
The characteristics of the study population with respect to age, gender, clinical diagnosis and serological profile are shown in Table 1. The median ages were significantly different between the groups: A higher mean age was observed for the group of patients without ocular toxoplasmosis compared to the group of patients with ocular toxoplasmosis (P < 0.0001, t = 7.00), with the subgroup of patients with primary manifestations of ocular toxoplasmosis (P < 0.0001; t = 5.48) and with the subgroup of patients with the recurrent form of the disease (P < 0.0001; t = 7.51). A higher mean age was also observed for the subgroup of patients with primary manifestations than those who had recurrent manifestations (P = 0.002; t = 3.12).
Table 1. General characteristics and serological profile of patients with and without ocular toxoplasmosis and its primary or recurrent manifestations.
| Characteristic | Patients without ocular toxoplasmosis* (n = 149) | Patients with ocular toxoplasmosis** (n = 148) | Patients with primary manifestation (n = 120) | Patients with recurrent manifestation (n = 28) |
|---|---|---|---|---|
| Age (Mean ± SD) | 57.7 ± 16.9ª,b,c | 42.3 ± 20.6ª | 44.9 ± 20.9b,d | 31.8 ± 30.5c,d |
| Median | 60 | 37 | 46 | 30 |
| Gender N (%) | ||||
| Female | 76 (51.0) | 69 (46.6) | 55 (45.8) | 14 (50.0) |
| Male | 73 (49.0) | 79 (53.4) | 65 (54.2) | 14 (50.0) |
| Serological profile N (%) | ||||
| IgM−/IgG+ | 149 (100.0) | 143 (96.6) | 115 (95.8) | 28 (100.0) |
| IgM +/IgG+ | 0 (0.0) | 5 (3.4) | 5 (4.2) | 0 (0.0) |
| IgM +/IgG− | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
Clinical diagnosis: *Presence of ocular diseases other than toxoplasmosis as cataracts (17.5%), pterygium (4.0%), agerelated macular degeneration (23.0%), glaucoma (6.7%), retinal detachment (16.1%), optic neuropathy (3.4%), macular edema (4.6%), macular atrophy (2.6%), diabetic retinopathy (8.7%) and other ocular diseases (13.4%). **Presence of ocular scars/lesions due to toxoplasmosis. t = Student t test.
aP < 0.0001 t = 7.00 (Patients without ocular toxoplasmosis vs. Patients with ocular toxoplasmosis).
bP < 0.0001 t = 5.48 (Patients without ocular toxoplasmosis vs. Patients with primary manifestation).
cP < 0.0001 t = 7.51 (Patients without ocular toxoplasmosis vs. Patients with recurrent manifestation).
dP = 0.002 t = 3.12 (Patients with primary manifestation vs. Patients with recurrent manifestation).
Distribution of KIR genes and KIR genotypes in patients with and without ocular toxoplasmosis
The distribution of genotype frequencies of KIR2DL2/3 and KIR3DL1/S1 were in Hardy–Weinberg equilibrium (P > 0.05) in this study population. However, KIR3DL1/S1 for the patient group that developed ocular toxoplasmosis was not in Hardy-Weinberg equilibrium (P = 0.03). KIR framework genes, KIR2DL4, KIR3DL2, KIR3DL3 and KIR3DP1, as expected were present in all samples, which are important internal controls to check the quality of genotyping.
The distribution of KIR gene frequencies and AA and BX genotype frequencies are shown in Table 2. An increased susceptibility for developing ocular toxoplasmosis (OR = 2.15; CI = 1.31–3.50; P = 0.003, Pc = 0.04) was observed for the KIR3DS1 activating gene. There was also a positive association between KIR3DS1 and recurrent manifestations of disease (OR = 3.25; CI = 1.42–7.44, P = 0.007; Pc = 0.1) when this patient group was compared to patients without ocular toxoplasmosis, although it was lost after applying the Bonferroni correction. On the other hand, the KIR2DS2 activating gene was associated with decreased susceptibility for ocular toxoplasmosis (OR = 0.55; CI = 0.31–0.97; P = 0.05; Pc = 0.80), but the significance was also lost after applying the Bonferroni correction. No significant difference was observed in the AA and BX genotype frequencies between all groups investigated in this study.
Table 2. Distribution of KIR genes and KIR genotypes in patients with and without ocular toxoplasmosis and its primary or recurrent manifestations.
| Genes | Patients without ocular toxoplasmosis (n = 149) | Patients with ocular toxoplasmosis (n = 148) | Patients with primary manifestation (n = 120) | Patients with recurrent manifestation (n = 28) |
|---|---|---|---|---|
| N (%) | N (%) | N (%) | N (%) | |
| KIR2DL1 | 149 (100) | 146 (98.6) | 118 (98.3) | 28 (100) |
| KIR2DL2 | 95 (63.8) | 101 (68.2) | 75 (62.5) | 23 (82.1) |
| KIR2DL3 | 131 (87.9) | 128 (86.5) | 102 (85.0) | 26 (92.9) |
| KIR2DL4 | 149 (100) | 148 (100) | 120 (100) | 28 (100) |
| KIR2DL5 | 85 (57.0) | 93 (62.8) | 70 (58.3) | 23 (82.1) |
| KIR2DP1 | 149 (100) | 146 (98.6) | 118 (98.3) | 28 (100) |
| KIR2DS1 | 63 (42.3) | 66 (44.6) | 51 (42.5) | 15 (53.6) |
| KIR2DS2 | 40 (26.8)a | 25 (16.9)a | 20 (16.6) | 5 (17.9) |
| KIR2DS3 | 54 (36.2) | 58 (39.2) | 42 (35.0) | 16 (57.1) |
| KIR2DS4 | 144 (96.6) | 141 (95.2) | 113 (94.2) | 28 (100) |
| KIR2DS5 | 56 (37.6) | 62 (41.9) | 49 (40.8) | 13 (46.4) |
| KIR3DL1 | 143 (96.0) | 145 (98.0) | 117 (97.5) | 28 (100) |
| KIR3DL2 | 149 (100) | 148 (100) | 120 (100) | 28 (100) |
| KIR3DL3 | 149 (100) | 148 (100) | 120 (100) | 28 (100) |
| KIR3DP1 | 149 (100) | 148 (100) | 120 (100) | 28 (100) |
| KIR3DS1 | 39 (26.1)b,c | 64 (43.2)b | 49 (40.8) | 15 (53.6)c |
| Genotypes | ||||
| AA | 41 (27.5) | 33 (22.3) | 27 (22.5) | 6 (21.4) |
| BX | 108 (72.4) | 115 (77.7) | 93 (80.0) | 22 (78.5) |
aOR = 0.55; CI = 0.31–0.97; P = 0.05; Pc = 0.80 (Patients without ocular toxoplasmosis vs. Patients with ocular toxoplasmosis).
bOR = 2.15; CI = 1.31–3.50; P = 0.003, Pc = 0.04 (Patients without ocular toxoplasmosis vs. Patients with ocular toxoplasmosis).
cOR = 3.25; CI = 1.42–7.44, P = 0.007; Pc = 0.1 (Patients without ocular toxoplasmosis vs. Patients with recurrent manifestation).
Distribution of the HLA class I KIR-ligands in in patients with and without ocular toxoplasmosis
Human leucocyte antigen frequencies was in Hardy-Weinberg equilibrium (P > 0.05) in all groups studied. The frequencies of the HLA class I ligands of KIR (A3 or A11, Bw4–80Ile and −80Thr, C1 and C2, in homozygosity and heterozygosity) were analyzed and were similar between groups (Table 3).
Table 3. Distribution of the HLA class I KIR-ligands in in patients with and without ocular toxoplasmosis and its primary or recurrent manifestations.
| HLA ligands* | Patients without ocular toxoplasmosis (n = 149) | Patients with ocular toxoplasmosis (n = 148) | Patients with primary manifestation (n = 120) | Patients with recurrent manifestation (n = 28) |
|---|---|---|---|---|
| N (%) | N (%) | N (%) | N (%) | |
| A3 and/or A11 | 31 (20.8) | 42 (28.4) | 32 (26.7) | 10 (35.7) |
| Bw4 | 113 (75.8) | 104 (70.3) | 84 (70.0) | 20 (71.4) |
| Bw4-80Ile | 93 (62.4) | 87 (58.8) | 72 (60.0) | 15 (53.6) |
| Bw4-80Thr | 46 (30.9) | 39 (26.4) | 29 (24.2) | 10 (35.7) |
| C1C2 | 62 (41.6) | 58 (39.2) | 46 (38.3) | 12 (42.9) |
| C1C1 | 26 (17.4) | 25 (16.9) | 19 (15.8) | 6 (21.4) |
| C2C2 | 61 (40.9) | 65 (43.9) | 55 (45.8) | 10 (35.7) |
Bw4 = HLA-A*23, *24, *32; HLA-B *13, *27, *44, *51, *52, *53, *57, *58. Bw4-80Ile = HLA-A*23, *24, *32; HLA-B*51, *52, *53, *57, *58. Bw4-80Thr = HLA-B *13, *27, *44. Group C1 = HLA-C*01, *03, *07, *08, *12, *14, *16. Group C2 = HLA-C*02, *04, *05, *06, *07, *15, *17, *18. *The same individual could express more than one pair KIR-HLA ligand.
Distribution of KIR and their respective HLA ligands in patients with and without ocular toxoplasmosis
Data on the distribution of KIR genes with their HLA class I ligands are listed in Table 4. The KIR3DS1-Bw4-80Ile pair was associated to increased susceptibility for developing ocular toxoplasmosis. The frequency was higher for patients with ocular toxoplasmosis (OR = 2.28; CI = 1.24–4.19; P = 0.007; Pc = 0.01), patients with primary manifestations (OR = 2.08; CI = 1.09–3.95; P = 0.02; Pc = 0.04) and patients with recurrent manifestations (OR = 3.24; CI = 1.28–8.89; P = 0.02; Pc = 0.04) than patients without ocular toxoplasmosis.
Table 4. Distribution of KIR and their respective HLA ligands in patients with and without ocular toxoplasmosis and its primary or recurrent manifestations.
| KIR - HLA ligands | Patients without ocular toxoplasmosis (n = 149) | Patients with ocular toxoplasmosis (n = 148) | Patients with primary manifestation (n = 120) | Patients with recurrent manifestation (n = 28) |
|---|---|---|---|---|
| N (%) | N (%) | N (%) | N (%) | |
| 2DL1-C2 | 123 (82.6) | 121 (81.8) | 99 (82.5) | 22 (78.6) |
| 2DL2-C1 | 55 (36.9) | 57 (38.5) | 41 (34.2) | 16 (57.1) |
| 2DL3-C1 | 79 (53.0) | 71 (48.0) | 55 (45.8) | 16 (57.1) |
| 3DL2-A3/A11 | 31 (20.8) | 42 (28.4) | 32 (26.7) | 10 (35.7) |
| 3DL1-Bw4 | 109 (71.2) | 101 (68.2) | 82 (68.3) | 19 (67.8) |
| 3DL1-Bw4-80Ile | 90 (60.4) | 81 (54.7) | 68 (56.6) | 13 (46.4) |
| 3DL1-Bw4-80Thr | 45 (30.2) | 38 (25.7) | 28 (23.3) | 10 (35.7) |
| 2DS1-C2 | 49 (32.9) | 55 (37.2) | 44 (36.7) | 11 (39.3) |
| 2DS2-C1 | 22 (14.8) | 16 (10.8) | 11 (9.2) | 5 (17.9) |
| 3DS1-Bw4-80Ile | 19 (12.8)a,b,c | 37 (25.0)a | 28 (23.3)b | 9 (32.1)c |
| 2DL1-C2C2 | 61 (40.9) | 63 (42.6) | 53 (44.2) | 10 (35.7) |
| 2DL2-C1C1 | 19 (12.8) | 20 (13.5) | 14 (21.4) | 6 (21.4) |
| 2DL3-C1C1 | 23 (15.4) | 23 (15.5) | 17 (14.2) | 6 (21.4) |
| 2DS1-C2C2 | 18 (12.1) | 31 (20.9) | 26 (21.7) | 5 (17.9) |
| 2DS2-C1C1 | 10 (6.7) | 4 (2.7) | 2 (1.7) | 2 (7.1) |
| 2DL2/2DL2-C1C2 | 6 (4.0) | 10 (6.8) | 8 (6.7) | 2 (7.1) |
| 2DL2/2DL3-C1C2 | 30 (20.1) | 27 (18.2) | 19 (15.8) | 8 (28.6) |
| 2DL3/2DL3-C1C2 | 56 (37.6)d,e,f | 21 (14.2)d | 19 (15.8)e | 2 (7.1)f |
| 2DL2/2DL2-C1C1 | 3 (2.0) | 2 (1.4) | 2 (1.7) | 0 (0.0) |
| 2DL2/2DL3-C1C1 | 16 (10.7) | 18 (12.2) | 12 (10.0) | 6 (21.4) |
| 2DL3/2DL3-C1C1 | 23 (15.4)g,h,i | 5 (3.4)g | 5 (4.2)h | 0 (0.0)i |
aOR = 2.28; CI = 1.24–4.19; P = 0.007; Pc = 0.01 (Patients without ocular toxoplasmosis vs. Patients with ocular toxoplasmosis).
bOR = 2.08; CI = 1.09–3.95; P = 0.02; Pc = 0.04 (Patients without ocular toxoplasmosis vs. Patients with primary manifestation).
cOR = 3.24; CI = 1.28–8.89; P = 0.02; Pc = 0.04 (Patients without ocular toxoplasmosis vs. Patients with recurrent manifestation).
dOR = 0.27; CI = 0.15–0.48; P = 0.000007; Pc = 0.00002 (Patients without ocular toxoplasmosis vs. Patients with ocular toxoplasmosis).
eOR = 0.31; CI = 0.17–0.56; P = 0.0001; Pc = 0.0003 (Patients without ocular toxoplasmosis vs. Patients with primary manifestation).
fOR = 0.12; CI = 0.29–0.59; P = 0.003; Pc = 0.009 (Patients without ocular toxoplasmosis vs. Patients with recurrent manifestation).
gOR = 0.19; CI = 0.07–0.51; P = 0.0005; Pc = 0.001 (Patients without ocular toxoplasmosis vs. Patients with ocular toxoplasmosis).
hOR = 0.23; CI = 0.08–0.64; P = 0.002; Pc = 0.006 (Patients without ocular toxoplasmosis vs. Patients with primary manifestation).
iOR = 0.09; CI = 0.05–0.91; P = 0.01; Pc = 0.05 (Patients without ocular toxoplasmosis vs. Patients with recurrent manifestation).
The KIR2DL3 inhibitory allele in the homozygous state and presence of its ligands whether homozygous or not (KIR2DL3/2DL3-C1/C1 and KIR2DL3/2DL3-C1) was associated with resistance to ocular toxoplasmosis when patients without ocular toxoplasmosis were compared with those with ocular toxoplasmosis (OR = 0.19; CI = 0.07–0.51; P = 0.0005; Pc = 0.001 and OR = 0.27; CI = 0.15–0.48; P = 0.000007; Pc = 0.00002 respectively), primary manifestations (OR = 0.23; CI = 0.08–0.64; P = 0.002; Pc = 0.006 and OR = 0.31; CI = 0.17–0.56; P = 0.0001; Pc = 0.0003 respectively) and recurrent manifestations (OR = 0.09; CI = 0.05–0.91; P = 0.01; Pc = 0.05 and OR = 0.12; CI = 0.29–0.59; P = 0.003; Pc = 0.009 respectively) (Table 4).
Frequencies of the number of KIR-HLA class I activating and inhibitory ligands in patients with and without ocular toxoplasmosis
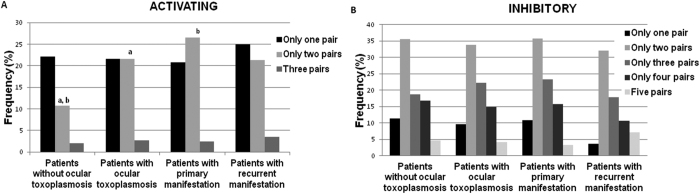
Figure 1 shows the influence of the number of KIR-HLA class I activating and inhibitory ligands on the development of ocular toxoplasmosis and its clinical manifestations. There were significant differences between patients with and without ocular toxoplasmosis (OR = 2.29; CI = 1.19–4.39; P = 0.01; Pc = 0.04) and patients with primary manifestations and without ocular toxoplasmosis (OR = 2.29; CI = 1.16–4.52; P = 0.01; Pc = 0.04) when only two pairs of activating ligands were present (Fig. 1A). Significant associations were not found in the analysis of pairs of inhibitory KIR-HLA class I ligands (Fig. 1B).
Figure 1.
Frequencies of pairs of KIR-HLA class I activating (A) and inhibitory (B) ligands in patients with and without ocular toxoplasmosis and its manifestation as primary or recurrent. aOR = 2.29; CI = 1.19–4.39; P = 0.01; Pc = 0.04 (Patients without ocular toxoplasmosis vs. Patients with ocular toxoplasmosis); bOR = 2.29; CI = 1.16–4.52; P = 0.01; Pc = 0.04 (Patients without ocular toxoplasmosis vs. Patients with primary manifestation).
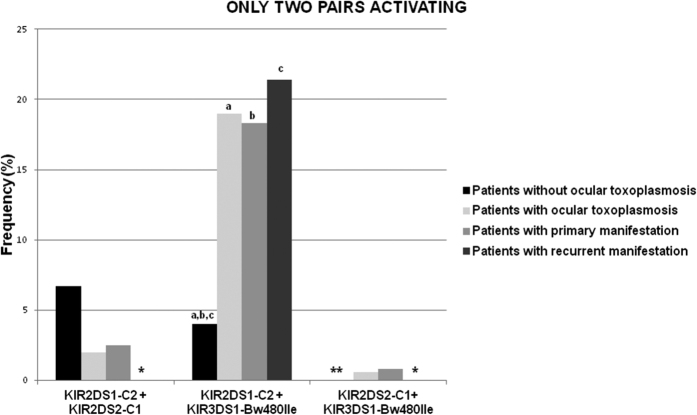
Subsequently, discrimination of the two activator pairs (KIR-HLA class I) for the association mentioned above was investigated. The combination involving KIR2DS1 and KIR3DS1 in the presence of their respective ligands (KIR2DS1+/C2++ KIR3DS1+/Bw4–80Ile+) is responsible for increasing the risk of developing ocular toxoplasmosis (OR = 5:56; CI = 2.22–13.88; P = 0.0001; Pc = 0.0004). The combination is also responsible for its primary (OR = 5.35; CI = 2.09–13.68; P = 0.0002; Pc = 0.0008) and recurrent clinical forms (OR = 6.50; CI = 1.92–21.96; P = 0.004; Pc = 0.01) compared to patients without ocular toxoplasmosis (Fig. 2).
Figure 2. Distribution of the frequencies of the possible combinations of only two KIR activating genes in the presence of their HLA Class I ligands in patients with and without ocular toxoplasmosis and its manifestations as primary or recurrent.
* and ** represents respectively that patients with recurrent manifestations and patients without ocular toxoplasmosis presented results of 0% for two activating pairs. aOR = 5.56; CI = 2.22–13.88; P = 0.0001; Pc = 0.0004 (Patients without ocular toxoplasmosis vs. Patients with ocular toxoplasmosis); bOR = 5.35; CI = 2.09–13.68; P = 0.0002; Pc = 0.0008 (Patients without ocular toxoplasmosis vs. Patients with primary manifestations); cOR = 6.50; CI = 1.92–21.96; P = 0.004; Pc = 0.01 (Patients without ocular toxoplasmosis vs. Patients with recurrent manifestations).
Distribution of activating KIR plus inhibitory KIR and their respective ligands in patients with and without ocular toxoplasmosis
The correlation between the distribution of activating and inhibitory KIR and their respective HLA ligands was analyzed (Table 5). A decreased risk of developing ocular toxoplasmosis (OR = 0.52; CI = 0.32–0.84; P = 0.009; Pc = 0.02) and recurrent manifestations of the disease (OR = 0.13; CI = 0.03–0.45; P = 0.0003; Pc = 0.0009) was observed for the KIR3DS1−/KIR3DL1+/Bw4–80Ile+ combination (KIR2DL1 and the Bw4-80Ile ligand in the absence of KIR3DS1) when compared to patients without ocular toxoplasmosis. This correlation was also observed when patients with recurrent manifestations were compared to patients with primary manifestations (OR = 0.20; CI = 0.05–0.70; P = 0.006; Pc = 0.01).
Table 5. Distribution of activating KIR plus inhibitory KIR and their respective ligands in patients with and without ocular toxoplasmosis and its primary or recurrent manifestations.
| KIR - HLA ligands | Patients without toxoplasmic retinochoroiditis (n = 149) | Patients with toxoplasmic retinochoroiditis (n = 148) | Patients with primary manifestation (n = 120) | Patients with recurrent manifestation (n = 28) |
|---|---|---|---|---|
| N (%) | N (%) | N (%) | N (%) | |
| KIR-C1 | ||||
| 2DS2+/2DL2−/C1+ | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 2DS2−/2DL2+/C1+ | 33 (22.1) | 41 (27.7) | 30 (25.0) | 11 (39.3) |
| 2DS2+/2DL3−/C1+ | 9 (6.0) | 12 (8.1) | 10 (8.3) | 2 (7.1) |
| 2DS2−/2DL3+/C1+ | 66 (44.3) | 67 (45.3) | 54 (54.0) | 13 (46.4) |
| 2DS2+/2DL2−/2DL3−/C1+ | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 2DS2−/2DL2−/2DL3+/C1+ | 33 (22.1) | 26 (17.6) | 24 (20.0) | 2 (7.1) |
| 2DS2+/2DL2−/2DL3+/C1+ | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 2DS2−/2DL2+/2DL3+/C1+ | 33 (22.1) | 41 (27.1) | 30 (25.0) | 11 (39.3) |
| 2DS2+/2DL2+/2DL3-/C1+ | 9 (6.0) | 12 (8.1) | 10 (8.3) | 2 (7.1) |
| 2DS2+/2DL2+/2DL3+/C1+ | 13 (8.7) | 4 (2.7) | 4 (3.3) | 0 (0.0) |
| KIR-C2 | ||||
| 2DS1+/2DL1−/C2+ | 0 (0.0) | 2 (1.4) | 2 (1.7) | 0 (0.0) |
| 2DS1−/2DL1+/C2+ | 74 (49.7) | 67 (45.3) | 56 (46.7) | 11 (39.3) |
| 2DS1+/2DL1+/C2+ | 49 (32.9) | 54 (36.5) | 43 (35.8) | 11 (39.3) |
| KIR-BW4-80Ile | ||||
| 3DS1+/3DL1+/BW4−80Ile+ | 32 (21.5) | 53 (35.8) | 42 (35.0) | 11 (39.3) |
| 3DS1+/3DL1−/BW4−80Ile+ | 1 (0.7) | 3 (2.0) | 3 (2.5) | 0 (0.0) |
| 3DS1−/3DL1+/BW4−80Ile+ | 71 (47.7)a,b | 48 (32.4)a | 45 (37.5)c | 3 (10.7)b,c |
aOR = 0.52; CI = 0.32–0.84; P = 0.009; Pc = 0.02 (Patients without ocular toxoplasmosis vs. Patientes with ocular toxoplasmosis).
bOR = 0.13; CI = 0.03–0.45; P = 0.0003; Pc = 0.0009 (Patients without ocular toxoplasmosis vs. Patientes with recurrent manifestation).
cOR = 0.20; CI = 0.05–0.70; P = 0.006; Pc = 0.01 (Patients with primary manifestation vs. Patients with recurrent manifestation).
Discussion
Possible causes of ocular manifestations of toxoplasmosis have not been fully elucidated, but it is believed that factors related both to the parasite and the host contribute to the development of this disease4,28. Therefore, the study of the impact of genes on infections, such as toxoplasmosis, is extremely important because it provides information about the contribution of the host’s genetic factors on the development of this type of disease. We have previously shown that MICA polymorphisms are not associated with the development of ocular toxoplasmosis29. Using the same samples, the current study found that KIR receptors genes and their HLA ligands are associated with the development of ocular lesions resulting from T. gondii infection. To the best of our knowledge, this is the first study of KIR genes and HLA ligands in the immunopathology of ocular toxoplasmosis.
The difference in the mean ages of the patient groups of this study was carefully discussed previously29. Briefly, T. gondii infection can occur at any time of life, and although most cases of ocular toxoplasmosis occur due to infections acquired after birth, a significant number of patients can acquire the disease congenitally and the resulting scars trend to be persistent1. It has also been demonstrated that the risk of recurrence is higher in the year following the first infection than in future years30,31. As no distinction was made between congenital and acquired disease in the analysis of the characteristics of eye injuries, this may be one of the possible explanations for the lower mean age observed for patients who developed ocular toxoplasmosis, including those who present with recurrent signs of the disease. Furthermore, other eye diseases, those without scars/lesions due to toxoplasmosis, are prevalent in older patients32.
Regarding the distribution of KIR genes, after Bonferroni correction only the KIR3DS1 activating gene was associated with increased risk of developing ocular toxoplasmosis with the other associations being lost. The Bonferroni correction decreases the chance of a significant difference by chance alone, making the data more robust33. The association observed for the KIR3DS1 with ocular toxoplasmosis can be explained by the absence of KIR3DL1, because 3DS1 and 3DL1 segregate as alleles of a single locus. Thus, the presence of KIR3DS1, and consequently the absence of KIR3DL1, can create an increased potential for the activation of NK cells owing to decreases in the ratio of inhibitory/activating receptors. Previous studies have shown involvement of the KIR3DS1/L1 alleles in various types of non-toxoplasmic uveitis and inflammatory eye diseases triggered by autoimmune factors. Levinson et al.15 observed that individuals with the KIR3DS1 gene in their haplotype have an increased risk of developing Vogt-Koyanagi-Harada syndrome, while the presence of KIR3DL1 was associated to protection against the development of the disease. A similar result was observed by Moon et al.14 for the development of uveitis related to ankylosing spondylitis: KIR3DS1 was associated as a risk factor and KIR3DL1 was associated as a protective factor.
In this study, KIR3DL1/S1 for the patient group that developed the ocular toxoplasmosis were not in Hardy-Weinberg equilibrium. However, some authors claim that this equilibrium should only be investigated in the control group, because it represents the general population34,35. Thus, the high frequency of KIR3DS1 might be changing the distribution of these alleles in individuals with ocular toxoplasmosis, resulting in a deviation from the Hardy-Weinberg equilibrium. Considering that numerous precautions were adopted to prevent bias in this study, we can safely say that the 3DS1 gene exerts a real influence on the development of ocular toxoplasmosis, even though KIR3DL1/S1 were not in Hardy-Weinberg equilibrium.
The function of KIR genes in immune response is highly dependent on the HLA molecules expressed on the target cell surface. Therefore, KIR receptors influence susceptibility for or protection against certain illnesses by means of a balance in activation and inhibition signals that regulate the NK cell effector function9,10. The recognition of specific HLA ligands by inhibiting KIR is well established36. There is clear evidence that HLA-Bw4-80Ile is powerfully recognized by KIR3DL1, but there is still controversy as to whether its homologue, KIR3DS1, interacts with the same ligand, as only indirect evidence was found37,38. There is also a hierarchy of inhibition related to KIR2DL receptors in which KIR2DL3-C1 has lower inhibitory potential than KIR2DL2-C1 and KIR2DL1-C239.
In this study, where the KIR genes were analyzed in the presence of their respective ligands (KIR-HLA), the KIR3DS1-Bw4-80Ile pair was associated with the development of ocular toxoplasmosis irrespective of the type of clinical manifestation (primary or recurrent); while KIR2DL3/2DL3-C1/C1 and KIR2DL3/2DL3-C1 were associated with protection against the development of ocular toxoplasmosis and its clinical manifestations. In accord with our results, other studies found an association involving the KIR3DS1-Bw4-80Ile pair in other human diseases40,41,42,43, suggested an interaction between these molecules. KIR2DL3 and KIR2DL2 are considered alleles, as are KIR3DL1 and KIR3DS1. Although the KIR2DL2-C1 interaction is stronger than the KIR2DL3-C1 interaction, the inhibitory signal generated by the absence of KIR2DL2 (KIR2DL3/2DL3-C1/C1 and KIR2DL3/2DL3-C1) appears to be sufficient to inhibit the effector function of NK cells and protect against elevated inflammation and tissue damage. It has been shown that abnormal expressions of inhibitory receptors of NK cells, including the KIR2DL3 receptor, may be associated with the development of Behcet’s disease44.
It is important to highlight the results observed for the number of pairs of KIR-HLA ligands and the correlation between the distribution of activating KIR and their respective HLA ligands. A higher frequency of only two pairs of ligands was observed in patients with ocular toxoplasmosis and in patients with primary manifestations compared to patients without ocular toxoplasmosis. The combination responsible for this association was KIR2DS1+/C2++ KIR3DS1+/Bw4-80Ile+, although the KIR2DS1 and KIR3DS1 genes are not in linkage disequilibrium as observed among patients with ocular toxoplasmosis (Δ′ = 0.06; P = 0.68) and patients without ocular toxoplasmosis (Δ′ = 0.15; P = 0.45). The combination of one pair (inhibitory) in the absence of the other pair (activating) was analyzed. It was possible to observe that the KIR3DS1−/KIR3DL1+/Bw4-80Ile+ combination decreases the risk of developing ocular toxoplasmosis and its recurrent clinical forms.
These results may suggest that the activating function mediated by KIR2DS1 plus KIR3DS1 and their respective HLA ligands is, in fact, an important factor interfering in ocular toxoplasmosis, since such a combination may affect the balance of inhibiting/activating signals. NK cells are activated when there is an increase of activation signals, even if there is a combination of strong or weak inhibitory signals45. Yet, the absence of KIR3DS1 in the presence of its inhibitor homologue, KIR3DL1, was indicative of the inhibitory or protective role played by NK cells, which perhaps avoids subsequent immune responses that may trigger inflammation and autoimmunity. In support of our findings, Levinson et al.46 demonstrated both negative and positive associations mediated by KIR-HLA pairs in another ocular inflammatory disease, birdshot chorioretinopathy.
NK, CD4 and CD8 T cells, and type 1 cytokines, such as IFN-γ and IL-2, play a protective role in T. gondii infection. IL-12 stimulates NK cells to produce IFN-γ and to promote the development of Th-1 cells that produce IFN-γ, a cytokine involved in the activation of macrophages, the main phagocytes in chronic inflammation47,48. In the eye, although the immune response is usually suppressed to prevent tissue damage, there is experimental evidence that T. gondii infection promotes the production of factors such as IFN-γ that suppress the immune privilege of this organ49. This possibly leads to increased severity of lesions with marked necrosis or inflammation of the retina and the choroid50,51.
The immune response can determine the development of eye injuries resulting from T. gondii infection, and the mechanisms involved may be associated with both the pathogenesis and protective effects that control tissue damage. It has been shown that increases in the frequency of circulating NK cells and proinflammatory monocytes in children infected by T. gondii, particularly in those with active ocular lesions, are indicative of a strong and persistent proinflammatory response. Moreover, subsets of NK cells and CD8+ T cells act as biomarkers for cicatricial lesions of the eye8. It has also been demonstrated that NK cells show increased production of IFN-γ in patients with congenital ocular toxoplasmosis6. Furthermore, the high imunopathogenicity responsible for tissue damage and deterioration of ocular toxoplasmosis may be due to the presence of a potent inducer of inflammation, IL-175,6, which is also produced by NK cells7. On the other hand, ocular toxoplasmosis may be linked to autoimmunity1,4.
The current study investigated the KIR-HLA ligand as a risk factor in ocular toxoplasmosis and these results may improve to understanding of the immunopathogenic mechanism involving NK cells in ocular manifestations related to toxoplasmosis. However, others studies should be performed such as histological analyses of the ocular tissue affected by T. gondii and NK cytotoxicity assays to better understanding the role of NK cells and the expression of KIR in the immunopathogenesis of ocular toxoplasmosis.
In conclusion, the results of this study show that activating and inhibitory KIR in the presence of their respective HLA ligands may have influence on the development of ocular toxoplasmosis and its clinical form in this population. In particular this is seen with the strong presence of activating signals as risk factors (KIR3DS1, KIR3DS1-Bw4-80Ile and KIR3DS1+/Bw4-80Ile++ KIR2DS1+/C2+) and inhibitory signals as protective factors (KIR2DL3/2DL3-C1, KIR2DL3/2DL3-C1/C1 and KIR3DS1-/KIR3DL1+/Bw4-80Ile+).
Methods
The design of this study aimed to follow, as close as possible, the criteria recommended by STrengthening the REporting of Genetic Association Studies (STREGA)52.
Ethics Information
This study was approved by the Research Ethics Committee of the Medicine School in São José do Rio Preto (#1980/2009) and all individuals who agreed to participate signed informed consent forms. The experiments were carried out in accordance with the approved relevant guidelines and regulations.
Patient selection
A total of 297 unrelated patients from the Retinopathy Outpatient Service of Hospital de Base of the Medicine School in São José do Rio Preto (HB-FUNFARME) and Medical Outpatient Clinic (AME) in São José do Rio Preto participated in this study.
The study subjects have been described previously29. Patients were grouped according to the presence of ocular scars/lesions due to toxoplasmosis (n = 148; 79 men and 69 women; mean age: 42.3 ± 20.6 years) or to the presence of ocular diseases not related to toxoplasmosis (n = 149; 73 men and 76 women; mean age: 57.7 ± 16.9 years). The group of patients with scars/lesions due to toxoplasmosis was further subdivided into two groups according to the type of ocular manifestation observed during a follow up period of at least two years: primary manifestations (n = 120; 65 men and 55 women; mean age: 44.9 ± 20.9 years) and recurrent manifestations characterized by the presence of satellite lesions53 (n = 28; 14 men and 14 women; mean age: 31.8 ± 30.5 years) (Table 1).
All individuals who participated in this study were monitored and evaluated in respect to clinical symptoms, serology for T. gondii and epidemiological data. Besides, although patients self-reported themselves as European descent, mixed African and European descent, and African descent, due to high miscegenation of the Brazilian population they were defined as a population of mixed ethnicity54.
In this study, in order to avoid bias in the results, all patients were selected after clinical examination using the same criteria. Additionally, the probability of variations in the allele frequencies due to ethnic background was minimized by matching patients with ocular toxoplasmosis and patients without ocular toxoplasmosis from similar ethnic backgrounds. Furthermore, gender and residence in the same geographical areas were carefully matched during group selection.
The number of patients enrolled is sufficient to demonstrate whether there is an association between ocular toxoplasmosis and KIR genes with statistical power of more than 90% and it was chosen according to frequency of KIR genes recorded in Allele*Frequencies database (http://www.allelefrequencies.net) observed in a population located in the southeast region of Brazil and defined as a population of mixed ethnicity.
Inclusion/exclusion criteria
The inclusion criteria of patients with ocular toxoplasmosis were positive laboratory diagnosis of toxoplasmosis, the presence of ocular scars/lesions due to toxoplasmosis and live in municipalities in the northwest region of the State of Sao Paulo (located in the southeast region of Brazil, between 20°49'13″S and 49°22′47″W). The inclusion criteria of patients without ocular toxoplasmosis were positive laboratory diagnosis of toxoplasmosis but without ocular scars/lesions due to toxoplasmosis and living in the same geographical region as the patients with ocular toxoplasmosis. All patients were clinically evaluated by two experienced physicians.
The exclusion criteria were: patients with other infectious and parasitic diseases, patients with any type of mental disability, patients with blood dyscrasia and using oral anticoagulants and related patients.
Laboratory diagnosis
Blood samples were collected into tubes without anticoagulant to obtain serum. Anti-T. gondii antibodies were detected by immunosorbent assay (ELISA) according to the manufacturer’s instructions (ETI-TOXOK-M reverse PLUS; DiaSorin S.p.A. Italy and ETI-TOXO-G PLUS; DiaSorin S.p.A. Italy). The microplates were read using Epoch™ equipment using the Gen5™ 2.0 software (BioTek, Winooski, Vermont, USA). The samples were tested in duplicate and in cases of indeterminate results, the samples were retested in duplicate. Positive and negative controls were included in all reactions.
Clinical diagnosis
All patients were clinically assessed using an indirect binocular ophthalmoscope (Binocular Ophthalmoscope ID10, Topcon Corporation, USA). The evaluation of visual acuity followed the logMAR Early Treatment Diabetic Retinopathy Study chart (ETDRS) criteria55. Intraocular pressure was measured by Goldmann applanation tonometry, and stereoscopic biomicroscopy was performed using a 78-diopter lens (Volk) and a slit lamp and all they were classified according to the ETDRS criteria55.
As no invasive test was performed, the ocular toxoplasmosis diagnostic criteria used were the same as in the clinical practice: injury identified by ophthalmoscopy associated with positive serology for T. gondii. This is therefore a presumptive diagnosis.
KIR and HLA genotyping
Blood samples were also collected into tubes containing EDTA anticoagulant for DNA extraction. DNA of all patients was extracted using the commercial kit for silica column extraction (QIAamp® DNA Blood Mini Kit, QIAGEN, the Netherlands) following the manufacturer’s instructions. All DNA samples were subjected to an evaluation of concentration and purity using the ratio of the absorbance at optical densities (OD) of 260 and 280 nm with Epoch™ equipment (BioTek, Winooski, Vermont, USA). KIR and HLA-A, -B and -C were genotyped according to manufacturer’s instructions by Polymerase chain reaction-sequence specific oligonucleotide probe (PCR-SSOP) protocols with Luminex® technology (One Lambda Inc., Canoga Park, CA, USA). This technique uses PCR-amplified DNA with specific biotinylated primers. The amplified product is hybridized by complementary DNA probes conjugated to fluorescently coded microspheres, with detection using R-Phycoerythrin-conjugated Streptavidin (SAPE). The data were interpreted using a computer program (HLA Fusion, 2.0 Research, One Lambda).
KIR2DL1 and KIR2DS1 bind to HLA molecules from the C2 group, which include the HLA-C*02, *04, *05, *06, *07,*15, *17, and *18 specificities. KIR2DL2, KIR2DL3 and KIR2DS2 interact with HLA molecules from the C1 group, among them: HLA-C*01, *03, *07, *08, *12, *13, *14 and *16. KIR3DL2 binds to HLA-A*03 or -A*11 specificities and KIR3DL1 recognizes HLA-Bw4 epitopes (HLA-A*23, *24, *25, *32; HLA-B*13, *27, *44, *51, *52, *53, *57, *58). HLA-Bw4 molecules were divided into two groups based on whether isoleucine or threonine was present at position 80 (Bw4-80Ile and Bw4-80Thr). KIR3DS1 binds to Bw4-80Ile molecules. HLA-KIR ligand specificities were considered according to Carr et al.37, Thananchai et al.56 and Kulkarni et al.57.
Two types of KIR genotypes have been described based on the content of the genes: AA and BX (BB and AB) (defined according to http://www.allelefrequencies.net). Individual genotypes were determined to be AA when the genes KIR2DL1, KIR2DL3, KIR2DL4, KIR2DS4, KIR3DL1, KIR3DL2, KIR3DL3, KIR2DP1 and KIR3DP1 were present. The presence of one or more of the following genes: KIR2DL5, KIR2DS1, KIR2DS2, KIR2DS3, KIR2DS5 and KIR3DS1 characterized the BX genotype.
Statistical analysis
KIR, HLA and KIR-HLA frequencies were obtained by direct counting. The comparisons of the frequencies of HLA ligands, KIR genes, KIR AA and BX genotypes and KIR with or without ligands between groups of patients were performed with the Chi-square test with Yates’ correction or, when necessary, Fisher’s exact test using the program Graph Pad Instat (http://www.graphpad.com/quickcalcs/contingency1.cfm). Odds ratio (OR) with a 95% confidence interval (95% CI) was also calculated to evaluate the risk association. The mean ages were compared using the t-test. Differences with P-values < 0.05 corrected by the Bonferroni inequality method for multiple comparisons (Pc) were considered statistically significant. A Hardy-Weinberg equilibrium fit was performed by calculating expected genotype frequencies and comparing that with the observed values for KIR2DL2/3, KIR3DL1/S1, and the HLA alleles using ARLEQUIN software, version 3.1. (http://cmpg.unibe.ch/software/arlequin3).
Additional Information
How to cite this article: Ayo, C. M. et al. Ocular toxoplasmosis: susceptibility in respect to the genes encoding the KIR receptors and their HLA class I ligands. Sci. Rep. 6, 36632; doi: 10.1038/srep36632 (2016).
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Acknowledgments
The authors are grateful to all of the volunteers who participated in this study and to Regional Blood Bank of Sao Jose do Rio Preto for their assistance (Denise, Mirela, Otávia and Dr. Octávio). Thanks to David Hewitt for his help with the English version and to Professor Stephen Henry from Auckland University of the Technology for providing library access. This study was supported by Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP) [grant numbers: 2013/06580-9, 2013/10050-5, 2013/25650-8, 2009/17540-2, 2013/15879-8] and by Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) [grant number: 473579/2009-0]. The opinions, assumptions, and conclusions or recommendations expressed in this material are the responsibility of the authors and do not necessarily reflect the views of FAPESP. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Footnotes
Author Contributions Conceived and designed the experiments: C.M.A., L.C.M. and C.C.B.M. Head of the FAMERP Toxoplasma Research Group: C.C.B.M. Performed the experiments: C.M.A., F.H.M. and A.P.S.C. Performed the inclusion of patients, sample collection, and developed the clinical evaluation and clinical analyses: F.B.F., A.P.B. and R.C.S., M.P. Analysed the data: C.M.A. and L.C.M. Wrote the paper: C.M.A. and L.C.M.
References
- Maenz M. et al. Ocular toxoplasmosis past, present and new aspects of an old disease. Prog Retin Eye Res. 39, 77–106 (2014). [DOI] [PubMed] [Google Scholar]
- Portela R. W. et al. A multihousehold study reveals a positive correlation between age, severity of ocular toxoplasmosis, and levels of glycoinositolphospholipid-specific immunoglobulin A. J Infect Dis. 190, 175–183 (2004). [DOI] [PubMed] [Google Scholar]
- Gilbert R. E. et al. Ocular sequelae of congenital toxoplasmosis in Brazil compared with Europe. PLoS Negl Trop Dis 2, e277 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pleyer U., Schlüter D. & Mänz M. Ocular toxoplasmosis: recent aspects of pathophysiology and clinical implications. Ophthalmic Res. 52, 116–123 (2014). [DOI] [PubMed] [Google Scholar]
- Dutra M. S. et al. Association of a NOD2 gene polymorphism and T-helper 17 cells with presumed ocular toxoplasmosis. J Infect Dis. 207, 152–163 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carneiro A. C. et al. Cytokine signatures associated with early onset, active lesions and late cicatricial events of retinochoroidal commitment in infants with congenital toxoplasmosis. J Infect Dis. pii:jiw041 (2016). [DOI] [PubMed] [Google Scholar]
- Passos S. T. et al. IL-6 promotes NK cell production of IL-17 during toxoplasmosis. J Immunol. 184, 1776–1783 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machado A. S. et al. Biomarker analysis revealed distinct profiles of innate and adaptive immunity in infants with ocular lesions of congenital toxoplasmosis. Mediators Inflamm. 2014, ID 910621 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanier L. L. NK cell receptors. Annu Rev Immunol. 16, 359–393 (1998). [DOI] [PubMed] [Google Scholar]
- Moretta A., Bottino C., Mingari M. C., Biassoni R. & Moretta L. What is a natural killer cell? Nat Immunol. 3, 6–8 (2002). [DOI] [PubMed] [Google Scholar]
- Middleton D. & Gonzelez F. The extensive polymorphism of KIR genes. Immunology. 129, 8–19 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S. J. et al. Targeted resequencing of candidate genes reveals novel variants associated with severe Behçet’s uveitis. Exp Mol Med. 45, e49 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levinson R. D., Martin T. M. & Luo L. Killer Cell Immunoglobulin-like Receptors in HLA-B27-Associated Acute Anterior Uveitis, with and without Axial Spondyloarthropathy. Invest Ophthalmol Vis Sci. 51, 1505–1510 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon S. J. et al. Diversity of killer cell immunoglobulin-like receptor genes in uveitis associated with autoimmune diseases: ankylosing spondylitis and Behçet disease. Ocul Immunol Inflamm. 21, 135–143 (2013). [DOI] [PubMed] [Google Scholar]
- Levinson R. D., Okada A. A., Ashouri E., Keino H. & Rajalingam R. Killer cell immunoglobulin-like receptor gene-cluster 3DS1-2DL5-2DS1-2DS5 predisposes susceptibility to Vogt-Koyanagi-Harada syndrome in Japanese individuals. Hum Immunol. 71, 192–194 (2010). [DOI] [PubMed] [Google Scholar]
- Sheereen A. et al. A study of KIR genes and HLA-C in Vogt-Koyanagi-Harada disease in Saudi Arabia. Mol Vis. 17, 3523–3528 (2011). [PMC free article] [PubMed] [Google Scholar]
- Taniguchi M. & Kawabata M. KIR3DL1/S1 genotypes and KIR2DS4 allelic variants in the AB KIR genotypes are associated with Plasmodium-positive individuals in malaria infection. Immunogenetics. 61, 717–730 (2009). [DOI] [PubMed] [Google Scholar]
- Marangon A. V. et al. KIR genes and their human leukocyte antigen ligands in the progression to cirrhosis in patients with chronic hepatitis C. Hum Immunol. 72, 1074–1078 (2011). [DOI] [PubMed] [Google Scholar]
- Ayo C. M. et al. Killer Cell Immunoglobulin-like Receptors and Their HLA Ligands are Related with the Immunopathology of Chagas Disease. PLoS Negl Trop Dis. 9, e0003753 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohno S., O’Connor G. R. & Kimura S. J. HLA antigens and toxoplasmic retinochoroiditis. Tohoku J Exp Med. 123, 91–94 (1977). [DOI] [PubMed] [Google Scholar]
- Brown C. R., David C. S., Khare S. J. & McLeod R. Effects of human class I transgenes on Toxoplasma gondii cyst formation. J Immunol. 152, 4537–4541 (1994). [PubMed] [Google Scholar]
- Meenken C. et al. HLA typing in congenital toxoplasmosis. Br J Ophthalmol. 79, 494–497 (1995). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan T. G. et al. Identification of T. gondii epitopes, adjuvants, and host genetic factors that influence protection of mice and humans. Vaccine. 28, 3977–3989 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cong H. et al. Towards an immunosense vaccine to prevent toxoplasmosis: protective Toxoplasma gondii epitopes restricted by HLA-A*0201. Vaccine. 29, 754–762 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardona N. I., Moncada D. M. & Gómez-Marin J. E. A rational approach to select immunogenic peptides that induce IFN-γ response against Toxoplasma gondii in human leukocytes. Immunobiology. 220, 1337–1342 (2015). [DOI] [PubMed] [Google Scholar]
- McMurtrey C. et al. Toxoplasma gondii peptide ligands open the gate of the HLA class I binding groove. Elife. 5, e12556 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller C. M., Boulter N. R., Ikin R. J. & Smith N. C. The immunobiology of the innate response to Toxoplasma gondii. Int J Parasitol. 39, 23–39 (2009). [DOI] [PubMed] [Google Scholar]
- Rothova A. Ocular manifestations of toxoplasmosis. Curr Opin Ophthalmol. 14, 384–388 (2003). [DOI] [PubMed] [Google Scholar]
- Ayo C. M. et al. MHC class I chain-related gene A polymorphisms and linkage disequilibrium with HLA-B and HLA-C alleles in ocular toxoplasmosis. PLoS One. 10, e0144534 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland G. N. Ocular toxoplasmosis: a global reassessment. Part I: epidemiology and course of disease. Am J Ophthalmol. 136, 973–988 (2003). [DOI] [PubMed] [Google Scholar]
- Aleixo A. L., Curi A. L., Benchimol E. I. & Amendoeira M. R. Toxoplasmic retinochoroiditis: clinical characteristics and visual outcome in a prospective study. PLoS Negl Trop Dis. 10, e0004685 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg E. A. & Sperazza L. C. The visually impaired patient. Am Fam Physician. 77, 1431–1436 (2008). [PubMed] [Google Scholar]
- McDonald J. H. Handbook of Biological Statistics (Sparky House Publishing, Baltimore, Maryland, 2014). [Google Scholar]
- Xu J., Turner A., Little J., Bleecker E. R. & Meyers D. A. Positive results in association studies are associated with departure from Hardy-Weinberg equilibrium: hint for genotyping error? Hum Genet. 111, 573–574 (2002). [DOI] [PubMed] [Google Scholar]
- Li M. & Li C. Assessing departure from Hardy-Weinberg equilibrium in the presence of disease association. Genet Epidemiol. 32, 589–599 (2008). [DOI] [PubMed] [Google Scholar]
- Moretta L. & Moretta A. Unravelling natural killer cell function: triggering and inhibitory human NK receptors. EMBO J. 23, 255–259 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr W. H., Pando M. J. & Parham P. KIR3DL1 polymorphisms that affect NK cell inhibition by HLA-Bw4 ligand. J Immunol. 175, 5222–5229 (2005). [DOI] [PubMed] [Google Scholar]
- Alter G. et al. Differential natural killer cell-mediated inhibition of HIV-1 replication based on distinct KIR/HLA subtypes. J Exp Med. 204, 3027–3036 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bashirova A. A., Martin M. P., McVicar D. W. & Carrington M. The killer immunoglobulin like receptor gene cluster: tuning the genome for defence. Annu Rev Genomics Hum Genet. 7, 277–300 (2006). [DOI] [PubMed] [Google Scholar]
- Martin M. P. et al. Epistatic interaction between KIR3DS1 and HLA-B delays the progression to AIDS. Nat Genet. 31, 429–434 (2002). [DOI] [PubMed] [Google Scholar]
- López-Vázquez A. et al. Protective effect of the HLA-Bw4I80 epitope and the killer cell immunoglobulin-like receptor 3DS1 gene against the development of hepatocellular carcinoma in patients with hepatitis C virus infection. J Infect Dis. 192, 162–165 (2005). [DOI] [PubMed] [Google Scholar]
- Gagne K. et al. Donor KIR3DL1/3DS1 gene and recipiment Bw4 KIR ligand as prognostic markers for outcome in unrelated hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 15, 1366–1375 (2009). [DOI] [PubMed] [Google Scholar]
- Jiang Y. et al. KIR3DS1/L1 and HLA-Bw4-80I are associated with HIV disease progression among HIV typical progressors and long-term nonprogressors. BMC Infect Dis. 13, 405 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeno M. et al. Abnormal killer inhibitory receptor expression on natural killer cells in patients with Behçet’s disease. Rheumatol Int. 24, 212–216 (2004). [DOI] [PubMed] [Google Scholar]
- Levinson R. D. Killer immunoglobulin-like receptor genes in uveitis. Ocul Immunol Inflamm. 19, 192–201 (2011). [DOI] [PubMed] [Google Scholar]
- Levinson R. D., Du Z. & Luo L. Combination of KIR and HLA gene variants augments the risk of developing birdshot chorioretinopathy in HLA-A*29-positive individuals. Genes Immun. 9, 249–258 (2008). [DOI] [PubMed] [Google Scholar]
- Hunter C. A., Suzuki Y., Subauste C. S. & Remington J. S. Cells and cytokines in resistance to Toxoplasma gondii. Curr Top Microbiol Immunol. 219, 113–125 (1996). [DOI] [PubMed] [Google Scholar]
- Yap G. S. & Sher A. Cell-mediated immunity to Toxoplasma gondii: initiation, regulation and effector function. Immunobiology. 201, 240–247 (1999). [DOI] [PubMed] [Google Scholar]
- Gazzinelli R. T., Brézin A., Li Q., Nussenblatt R. B. & Chan C. C. Toxoplasma gondii: acquired ocular toxoplasmosis in the murine model, protective role of TNF-alpha and IFN-gamma. Exp Parasitol. 78, 217–229 (1994). [DOI] [PubMed] [Google Scholar]
- Lemaitre C., Thillaye-Goldenberg B., Naud M. C. & de Kozak Y. The effects of intraocular injection of interleukin- 13 on endotoxin- induced uveitis in rats. Invest Ophthalmol Vis Sci. 42, 2022–2030 (2001). [PubMed] [Google Scholar]
- Lu F., Huang S. & Kasper L. H. CD4 + T cells in the pathogenesis of murine ocular toxoplasmosis. Infect Immun. 72, 4966–4972 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little J. et al. Strengthening the reporting of genetic association studies (STREGA): an extension of the STROBE statement. PLoS Med. 6, e1000022 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosch-Driessen L. E., Berendschot T. T., Ongkosuwito J. V. & Rothova A. Ocular toxoplasmosis: clinical features and prognosis of 154 patients. Ophthalmology. 109, 869–878 (2002). [DOI] [PubMed] [Google Scholar]
- Parra F. C. et al. Color and genomic ancestry in Brazilians. Proc Nat Aca Sci. 100, 177–182 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Photocoagulation for diabetic macular edema. Early Treatment Diabetic Retinopathy Study report number 1. Early Treatment Diabetic Retinopathy Study research group. Arch Ophthalmol. 103, 796–806 (1985). [PubMed] [Google Scholar]
- Thananchai H. et al. Allele-specific and peptide-dependent interactions between KIR3DL1 and HLA-A and HLA-B. J Immunol. 178, 33–37 (2007). [DOI] [PubMed] [Google Scholar]
- Kulkarni S., Martin M. P. & Carrington M. The Yin and Yang of HLA and KIR in human disease. Semin Immunol. 20, 343–352 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]