Abstract
Brain iron levels in patients of Parkinson’s disease (PD) are usually measured in postmortem samples or by MRI imaging including R2* and SWI. In this study we performed a meta-analysis to understand PD-associated iron changes in various brain regions, and to evaluate the accuracy of MRI detections comparing with postmortem results. Databases including Medline, Web of Science, CENTRAL and Embase were searched up to 19th November 2015. Ten brain regions were identified for analysis based on data extracted from thirty-three-articles. An increase in iron levels in substantia nigra of PD patients by postmortem, R2* or SWI measurements was observed. The postmortem and SWI measurements also suggested significant iron accumulation in putamen. Increased iron deposition was found in red nucleus as determined by both R2* and SWI, whereas no data were available in postmortem samples. Based on SWI, iron levels were increased significantly in the nucleus caudatus and globus pallidus. Of note, the analysis might be biased towards advanced disease and that the precise stage at which regions become involved could not be ascertained. Our analysis provides an overview of iron deposition in multiple brain regions of PD patients, and a comparison of outcomes from different methods detecting levels of iron.
Iron overload has been implicated in the pathology and pathogenesis of Parkinson’s disease (PD). The substantia nigra, where the selective loss of dopaminergic neurons occurs, is the primary region in the brain known to deposit iron. Additionally, aberrant iron concentrations have been observed in other brain regions such as red nuclei, globus pallidus and cortex of PD patients, despite of unknown pathology1,2,3. Spectroscopic analyses of postmortem brains display an increased iron levels in the substantia nigra, which has been suggested to correlate with the severity of PD2,4. In recent decades, advancements in imaging techniques, such as magnetic resonance imaging (MRI), have contributed to an enhanced understanding of the pathological progression and clinical diagnosis of PD. Consequently, iron load may be estimated in a non-invasive manner using R2/R2* relaxometry (with better results obtained using R2* 5,6,7) and, more recently, susceptibility-weighted imaging (SWI). Nonetheless, while largely consistent and reproducible results can be obtained in many experiments these techniques are not yet fully validated8.
In this study, we extracted results of iron analyses employing postmortem brains and R2* and SWI methods from the literature, and performed a systematical meta-analysis aiming to 1) confirm the iron overload observation in the substantia nigra, 2) explore other regions of the brain carrying different levels of iron, and 3) evaluate to what extent these two MRI methods correlate with the measurements of postmortem brains. Meanwhile, as detailed in the discussion section, several limitations are disclosed in an attempt to fully understand the scope of this meta-analysis, such that the disease severity was not differentiated due to insufficient information during data extraction that may affect outcomes of MRI imaging.
Results
Search Results
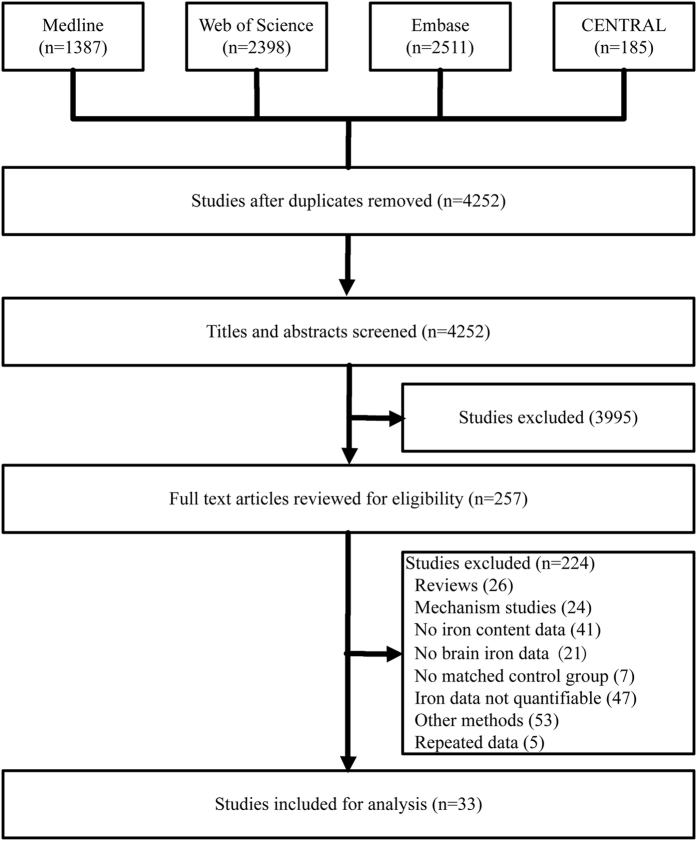
The initial search using the keywords as described in the method section returned a total of 4252 articles (Fig. 1). A subsequent screening of the titles and abstracts reduced the number to 257. Following an exhaustive examination of the contents, 224 articles were excluded according to the selection criteria detailed in the method section. Of the 33 articles being selected that report iron content (summarized in Table 1), 11 of them employed postmortem analyses2,4,9,10,11,12,13,14,15,16,17, 14 were measured by R2* 3,18,19,20,21,22,23,24,25,26,27,28,29,30 and 8 by MRI relaxometry SWI31,32,33,34,35,36,37,38. The disease comorbidity and diagnostic performance of the cohorts of these 33 studies are summarized in Table S1.
Figure 1. Flow chart describing the selection process of articles retrieved from initial literature search.
CENTRAL, Cochrane Central Register of Controlled Trials.
Table 1. Characteristics of the 33 studies included for meta-analyses.
| Article | Healthy controls |
PD patients |
PD diagnosis | Detection | Method type | UPDRS score | UPDRS motor score | H-Y scale | Disease duration | Publication Quality Assessment | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | Agea | Gender (F/M) | n | Agea | Gender (F/M) | |||||||||
| Yu et al.17 | 10 | 84.6 ± 1.5 | 6/4 | 10 | 82.7 ± 1.7 | 4/6 | UK PD Brain Bank criteria | ICP | Postmortem | — | — | — | — | ****** |
| Loeffler et al.12 | 8 | 74.6 ± 7.6 | 4/4 | 14 | 74.9 ± 8.7 | 5/9 | Pathological examination | COL | Postmortem | — | — | — | — | **** |
| Griffiths et al.11 | 6 | 83.3 ± 2.1 | — | 6 | 83.6 ± 2.4 | — | Pathological examination | AA | Postmortem | — | — | — | — | ****** |
| Dexter et al.2 | 34 | 81.3 ± 1.5 | 21/13 | 27 | 74.9 ± 1.4 | 11/16 | Clinical and pathological examination | ICP | Postmortem | — | — | — | — | ***** |
| Riederer et al.4 | 4 | 73 (68–78) | 3/1 | 13 | 76 (68–82) | 7/6 | Pathological examination | SPH | Postmortem | — | — | — | — | ***** |
| Sofic et al.13 | 8 | 75.3 (66–86) | 4/4 | 8 | 71.3 ± 12.5 | 4/4 | Pathological examination | SPH | Postmortem | — | — | — | 7.5 ± 3.4 | ****** |
| Visanji et al.15 | 3 | 62.7 (47–78) | 1/2 | 3 | 69.3 (56–79) | 1/2 | Pathological examination | AA | Postmortem | — | — | — | 21 ± 3.8 | ***** |
| Wypijewska et al.16 | 29 | 61–85 | — | 17 | 61–85 | — | Clinical and pathological examination | MS | Postmortem | — | — | — | — | ****** |
| Galazka-Friedm et al.10 | 8 | 64 ± 6 | — | 6 | 70 ± 4 | — | Clinical and pathological examination | MS | Postmortem | — | — | 4–5 | 4–7 | ***** |
| Uitti et al.14 | 12 | 70 | 4/8 | 9 | 73 | 3/6 | Pathological examination | AA | Postmortem | — | — | — | — | **** |
| Chen et al.9 | 6 | — | — | 10 | — | — | — | AA | Postmortem | — | — | — | — | **** |
| Gorell et al.18 | 10 | 60.0 ± 8.7 | 5/5 | 13 | 65.2 ± 12.7 | 2/11 | Clinical diagnosis | 3T | R2* | — | — | 1.5–3.0 | 3–13 | ***** |
| Graham et al.19 | 25 | 64.0 ± 6.6 | 6/7 | 21 | 61.4 ± 7.3 | 10/11 | UK PD Brain Bank criteria | 1.5T | R2* | — | — | — | 11.1 ± 4.5 | ****** |
| Martin et al.20,b | 11 | 55.9 ± 7.3 | 4/7 | 22 | 61.9 ± 9.0 | 8/14 | Published criteria66 | 3T | R2* | — | 16.7 ± 7.1 | — | 3.2 ± 1.7 | ******* |
| 19 | 60.3 ± 8.4 | 6/13 | — | 16.9 ± 7.5 | — | 2.9 ± 1.6 | ||||||||
| Du et al.21 | 29 | 59.6 ± 6.7 | 17/12 | 40 | 60.7 ± 8.3 | 17/23 | Published criteria66 | 3T | R2* | — | 23.4 ± 15.2 | 1.8 ± 0.6 | 4.2 ± 4.7 | ****** |
| Bunzeck et al.22 | 20 | 66.0 ± 9.1 | 10/10 | 20 | 66.3 ± 9.0 | 9/11 | Queens Square Brain Bank criteria67 | 3T | R2* | 34.6 ± 17.4 | — | — | — | ****** |
| Lee et al.23 | 21 | 60.0 ± 6.1 | 9/12 | 29 | 59.1 ± 7.6 | 12/17 | UK PD Brain Bank criteria | 3T | R2* | — | 25.5 ± 9.2 | 2.05 ± 0.5 | 2.5 ± 1.9 | ******* |
| Lewis et al.3 | 23 | 59.9 ± 7.0 | 17/12 | 38 | 60.6 ± 8.0 | 17/23 | Published criteria66 | 3T | R2* | — | 23.8 ± 15.4 | 1.8 ± 0.6 | 4.4 ± 4.7 | ***** |
| Rossi et al.24 | 21 | 66 (58–80) | 17/4 | 37 | 69 (42–86) | 18/19 | Clinical diagnosis | 3T | R2* | — | — | — | — | ***** |
| Ulla et al.25 | 26 | 57.0 ± 8.5 | 17/9 | 27 | 60.2 ± 10.7 | 14/13 | PD Society Brain Bank68 | 1.5T | R2* | — | 12.1 ± 8.5 | 1.9 ± 0.7 | 5.7 ± 4.4 | ****** |
| Rossi et al.26 | 19 | 65 (58–80) | 15/4 | 25 | 73 (44–87) | 14/11 | Clinical diagnosis | 3T | R2* | — | — | — | — | ***** |
| Barbosa et al.27 | 30 | 64 ± 7 | 21/9 | 20 | 66 ± 8 | 8/12 | UK PD Brain Bank criteria | 3T | R2* | — | — | 2.3 ± 0.6 | 8.1 ± 4.2 | ****** |
| Murakami et al.30 | 21 | 69.7 ± 8.6 | 12/9 | 21 | 72.0 ± 7.5 | 12/9 | UK PD Brain Bank criteria | 3T | R2* | — | — | 2 (1–3) | 2.7 ± 2.3 | ***** |
| He et al.29 | 35 | 60.5 ± 6.5 | 14/21 | 44 | 58.0 ± 8.8 | 19/25 | UK PD Brain Bank criteria | 3T | R2* | — | 15.6 ± 6.2 | 1.4 ± 0.5 | 2.8 ± 1.6 | **** |
| Du et al.28 | 47 | 62.2 ± 8.8 | 24/23 | 47 | 65.8 ± 10.1 | 25/22 | UK PD Brain Bank criteria | 3T | R2* | 39.6 ± 24.8 | 21.8 + 15.2 | — | 5.5 ± 4.8 | ***** |
| Zhang et al.34 | 26 | 57.3 ± 11.6 | 12/14 | 40 | 58.7 ± 12.8 | 19/21 | UK PD Brain Bank criteria | 3T | SWI | — | 19.0 ± 7.8 | — | 3.6 ± 2.9− | ****** |
| Jin et al.35 | 45 | 55.4 ± 14.9 | 19/26 | 45 | 56.3 ± 10.9 | 14/31 | UK PD Brain Bank criteria | 3T | SWI | 15.1 ± 9.3 | 12.0 ± 7.1 | — | — | ****** |
| Wang et al.32 | 14 | 64.3 ± 12.7 | 7/7 | 20 | 67.2 ± 10.7 | 10/10 | Clinical diagnosis | 3T | SWI | — | — | — | 2.8 ± 2.8 | ****** |
| Wang et al.37 | 44 | 59.4 ± 11.8 | 23/21 | 16 | 63.3 ± 10.6 | 7/9 | UK PD Brain Bank criteria | 1.5T | SWI | — | — | — | 2.5 ± 1.7 | ***** |
| Han et al.31 | 20 | 55.9 ± 6.2 | 8/12 | 15 | 57.4 ± 7.1 | 8/7 | UK PD Brain Bank criteria | 3T | SWI | 23.0 ± 5.6 | — | 2.2 ± 0.5 | 2.5 ± 1.6 | ***** |
| Kim et al.36 | 25 | 56.2 ± 6.5 | 13/12 | 30 | 57.6 ± 6.8 | 11/19 | UK PD Brain Bank criteria | 3T | SWI | — | 24.5 ± 8.4 | 1.7 ± 0.5 | 1.7 ± 1.1 | ***** |
| Wu, et al.33 | 40 | 66.5 ± 6.0 | 18/22 | 54 | 65.6 ± 5.8 | 21/33 | UK PD Brain Bank criteria | 3T | SWI | — | — | ≥1.5 | — | **** |
| Huang, et al.38 | 19 | 65.0 ± 9.0 | — | 30 | 68.0 ± 9.0 | 6/24 | — | 3T | SWI | — | — | — | — | **** |
aData in this column are presented as mean ± SD or Range or Median (Range) or Mean (Range) or the detail ages; bIn this study the patient group with n = 22 is for mid-brain images including substantia nigra and red nucleus, and the one with n = 19 is for forebrain images including globus pallidus, putamen, nucleus caudatus, and white matter. UPDRS, Unified Parkinson’s Disease Rating Scale; H-Y, Hoehn and Yahr; ICP, inductively coupled plasma spectroscopy; COL, colorimetry; AA, atomic absorption; SPH, spectrophotometry; MS, Mössbauer spectroscopy.
Quality Assessment
Quality assessment by Newcastle-Ottawa Scale suggested four-stars or above out of a maximum of nine for all of the 33 publications. The detailed quality assessment is listed in Table 1.
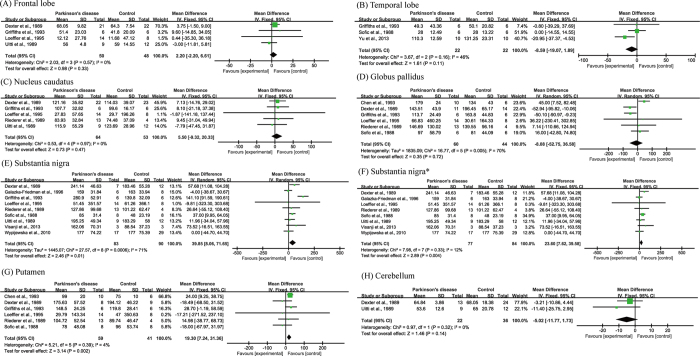
Postmortem comparison of iron concentration in defined brain regions
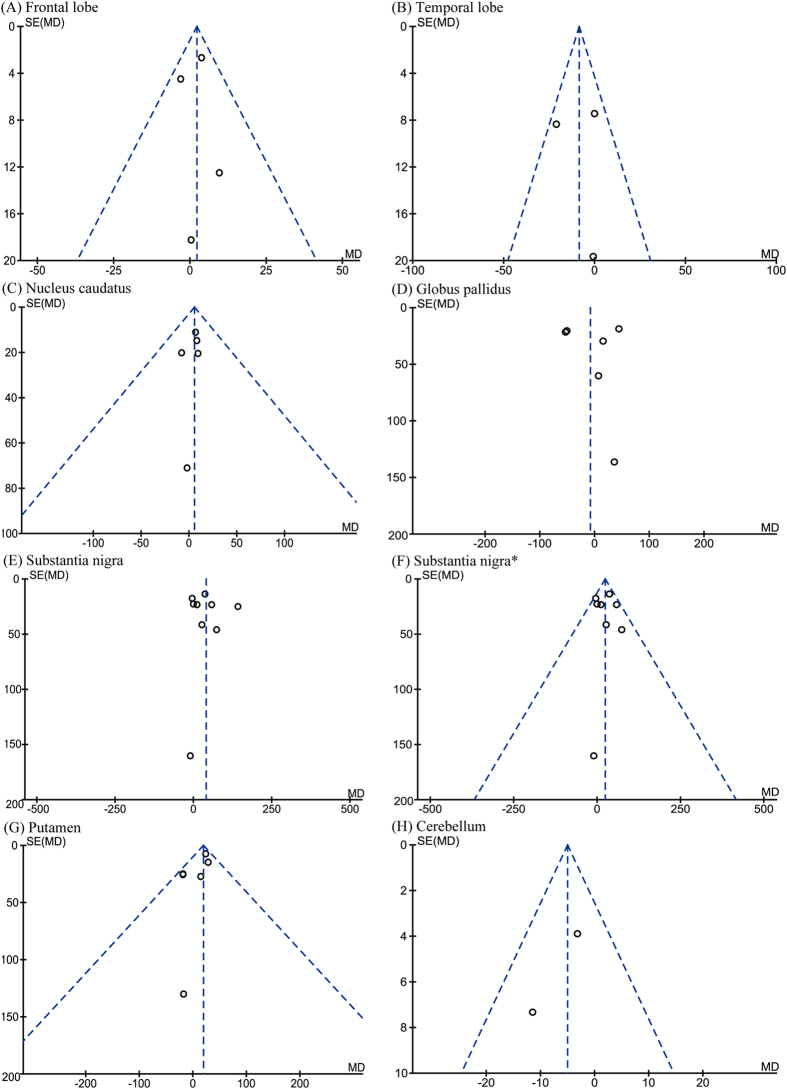
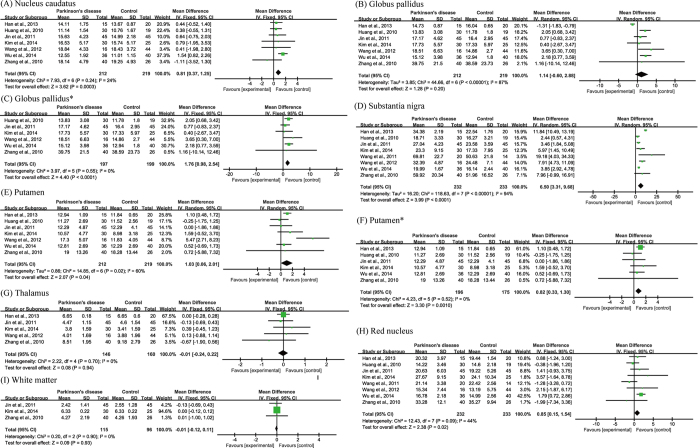
Eleven of the manuscripts examined iron concentration in seven regions of postmortem brains. The numbers of subjects for each region were 98 (frontal lobe), 44 (temporal lobe), 117 (nucleus caudatus), 104 (globus pallidus), 173 (substantia nigra), 100 (putamen), and 58 (cerebellum). Although iron concentration was significantly increased in the substantia nigra of PD patients (WMD = 39.85, 95% CI, 8.06–71.65, p = 0.01; Fig. 2E), significant heterogeneity was detected in these cohorts (I2 = 71%; p = 0.0006). Subsequent sensitivity analysis suggested that such heterogeneity was attributed to the study of Griffiths et al.11. Further analysis that eliminated this study (I2 = 12%; p = 0.33) also showed a significant increase of iron concentration in the substantia nigra (WMD = 23.60, 95% CI = 7.62–39.58, p = 0.004; Fig. 2F). Additionally, increased iron levels were observed in the putamen of PD subjects (WMD = 19.30, 95% CI = 7.24–31.36, p = 0.002, I2 = 4%; Fig. 2G). No significant differences were observed in other brain regions (Fig. 2). The funnel plots analyzing publication bias appeared to be symmetric by visual inspection (Fig. 3).
Figure 2. Statistical summaries and forest plots of studies comparing iron concentrations by postmortem analysis.
(D,E) Pooled using random-effects models. The others were pooled using fixed-effects models. *Analyzed after heterogeneity was removed.
Figure 3. Funnel plots that examine possible publication bias in the studies by postmortem analysis.
*Analyzed after heterogeneity was removed.
MRI comparison of iron concentration in defined brain regions
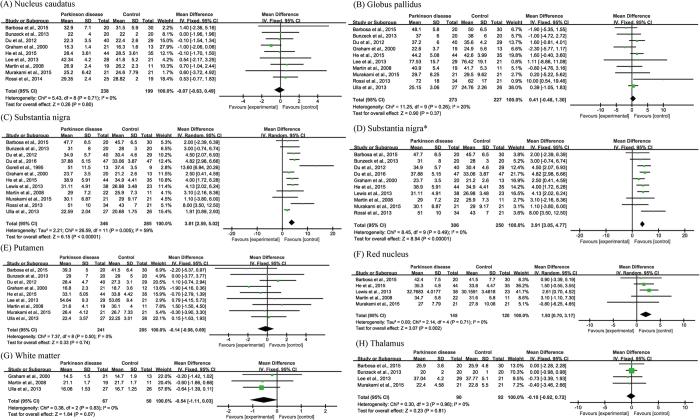
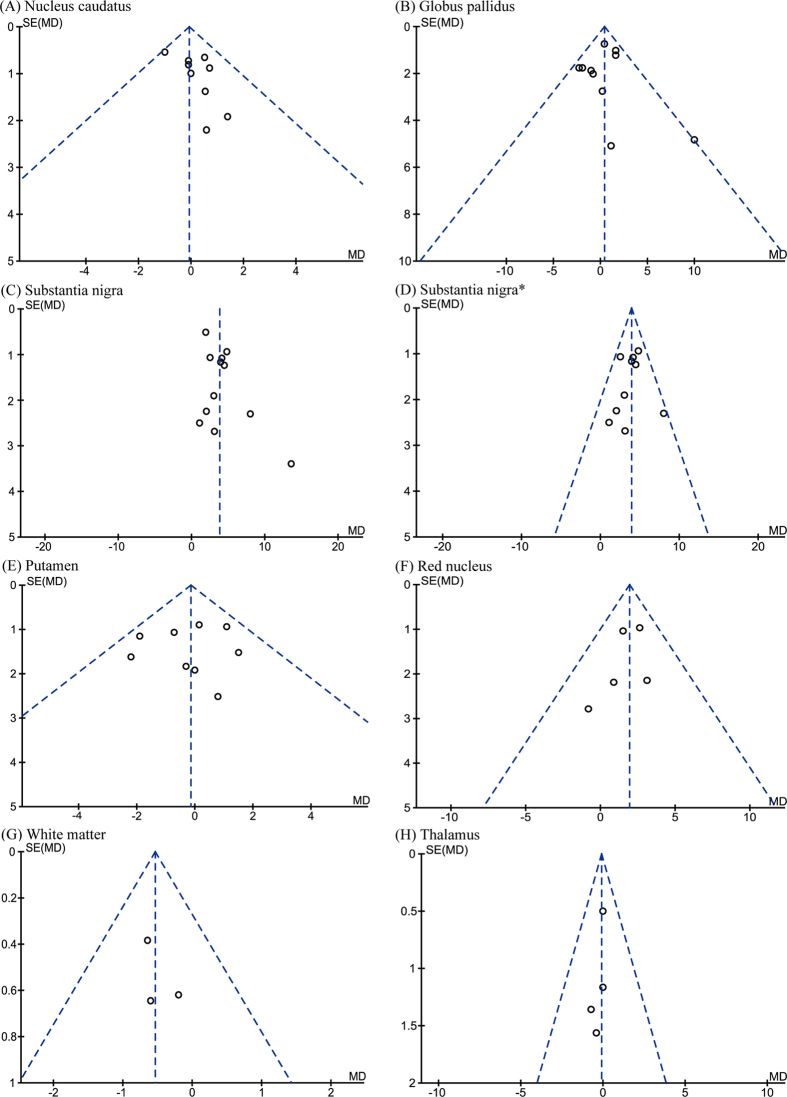
Fourteen articles were included in the R2* subgroup of meta-analyses in seven brain regions. The total subject numbers were 437 (nucleus candatus), 500 (globus pallidus), 631 (substantia nigra), 446 (putamen), 265 (red nucleus), 117 (white matter) and 182 (thalamus). In the substantia nigra of PD subjects, iron content was elevated (WMD = 3.81, 95% CI = 2.59–5.02, p < 0.00001) despite of a relatively high heterogeneity (I2 = 59%, p = 0.005; Fig. 4C). Results of a sensitivity analysis ascribed the heterogeneity to the studies of Ulla et al.25 and Gorell et al.18, as exclusion of them eliminated the heterogeneity (I2 = 0%, p = 0.49 ; Fig. 4D). Subsequent meta-analysis again demonstrated a significant increase of iron concentration in the substantia nigra (WMD = 3.91, 95% CI = 3.05–4.77, p < 0.00001; Fig. 4D). Iron concentration was significantly increased in the red nucleus (WMD = 1.93, 95% CI = 0.70–3.17, p = 0.002, I2 = 0%; Fig. 4F), but not in other brain regions (Fig. 4). The publication biases were acceptable as determined by funnel plots (Fig. 5).
Figure 4. Statistical summaries and forest plots of studies comparing iron concentrations by MRI R2* relaxometry.
(C) Pooled using random-effects models. The others were pooled using fixed-effects models. *Analyzed after heterogeneity was removed.
Figure 5. Funnel plots that examine possible publication bias in the studies by R2*.
*Analyzed after heterogeneity was removed.
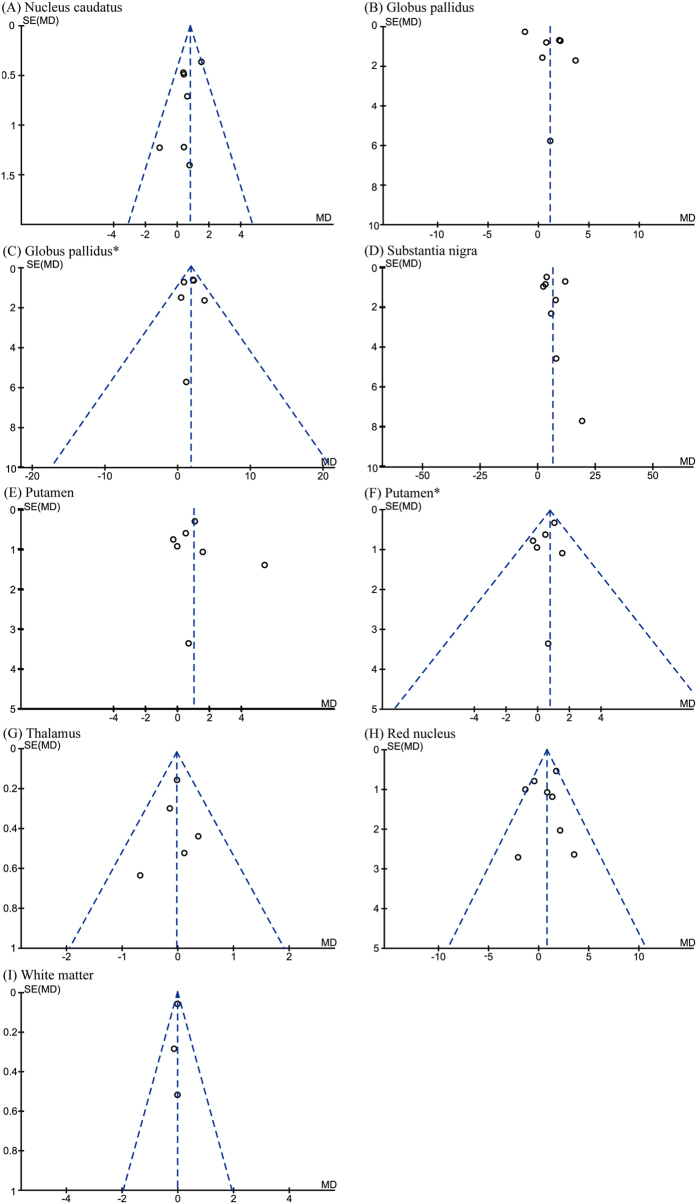
Eight articles were included in the SWI subgroup of meta-analyses in seven brain regions. The total subject numbers were 431 (nucleus caudatus), 431 (globus pallidus), 431 (putamen), 306 (thalamus), 465 (substantia nigra), 465 (red nucleus) and 211 (white matter). A significant increase in iron concentration was observed in the substantia nigra (WMD = 6.5, 95% CI = 3.31–9.68, p < 0.0001) with high heterogeneity (I2 = 94%, p < 0.0001; Fig. 6D). Significant increases in iron concentration were also shown in the nucleus caudatus (WMD = 0.81, 95% CI = 0.37–1.25, p = 0.0003, I2 = 24%; Fig. 6A), putamen (WMD = 1.03, 95% CI = 0.06–2.01, p = 0.04, I2 = 60%; Fig. 6E), and red nucleus (WMD = 0.85, 95% CI = 0.15–1.54, p = 0.02, I2 = 44%; Fig. 6H). When the article of Wang et al.37 was removed based on sensitivity analysis, we still observed an increase of iron concentration in the putamen (WMD = 0.82, 95% CI = 0.33–1.30, p = 0.001, I2 = 0%; Fig. 6F). Significant heterogeneity (I2 = 87%, p < 0.00001) was detected in the globus pallidus group (Fig. 6B), which was attributed to Han et al.31 as determined by a sensitivity analysis. Meta-analysis after exclusion of this paper showed a significant increase of iron concentration in the globus pallidus (WMD = 1.76, 95% CI = 0.98–2.54, p < 0.0001, I2 = 0%; Fig. 6C). The publication biases were acceptable as determined by funnel plots (Fig. 7).
Figure 6. Statistical summaries and forest plots of studies comparing iron concentrations by SWI relaxometry.
(B,D,E) Pooled using random-effects models. The others were pooled using fixed-effects models. *Analyzed after heterogeneity was removed.
Figure 7. Funnel plots that examine possible publication bias in the studies by SWI.
*Analyzed after heterogeneity was removed.
Structure by structure analyses of results from individual studies and meta-analyses
It is known that inferences can be particularly prone to Type-I error in studies based on a small number of papers, especially with a small sample size39. Therefore, we herein elaborated on the results reported in each study combining the results of meta-analyses and the methodological factors that could have contributed to discrepancies in a brain structure-based fashion.
Substantia nigra
As expected, an elevation of iron concentration was found in the substantia nigra in all the three types of measurements (Table 2). This was in line with the majority of the 29 articles we analyzed. Except for the three that did not show a change in postmortem samples10,12,16, the other 26 articles reported a trend toward or a statistically significant increase in iron content in the substantia nigra regardless of the type of measurement (postmortem, SWI or R2*). As a note, three postmortem iron analyses12,14,16 indicated that the pars compacta and reticulata were not discriminated during the measurement, while the other six studies did not state the relevant information to make this determination.
Table 2. A summary of changes in brain iron levels of PD patients based on the current meta-analysis.
| Postmortem |
R2* |
SWI |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Change | p | n | Change | p | n | Change | p | n | |
| Substantia nigra | ↑ | 0.01 | 173 | ↑ | <10−5 | 631 | ↑ | <10−4 | 465 |
| ↑a | 0.004 | 161 | ↑a | <10−5 | 556 | ||||
| Putamen | ↑ | 0.002 | 100 | — | 0.74 | 446 | ↑ | 0.04 | 431 |
| ↑a | 0.001 | 371 | |||||||
| Globus pallidus | — | 0.72 | 104 | — | 0.37 | 500 | — | 0.20 | 431 |
| ↑a | <10−4 | 396 | |||||||
| Nucleus caudatus | — | 0.47 | 117 | — | 0.80 | 437 | ↑ | 0.0003 | 431 |
| Frontal lobe | — | 0.33 | 98 | NA | NA | NA | NA | NA | NA |
| Temporal lobe | — | 0.11 | 44 | NA | NA | NA | NA | NA | NA |
| Cerebellum | — | 0.14 | 58 | NA | NA | NA | NA | NA | NA |
| Red nucleus | NA | NA | NA | ↑ | 0.002 | 265 | ↑ | 0.02 | 465 |
| Thalamus | NA | NA | NA | — | 0.81 | 182 | — | 0.94 | 306 |
| White matter | NA | NA | NA | — | 0.07 | 117 | — | 0.93 | 211 |
↑Increased iron level in PD.
—No change of iron level in PD.
NA, no data available.
aAnalyzed after heterogeneity was removed.
Putamen
Both postmortem and SWI meta-analyses showed an iron overload in PD patients. However, when individual articles describing postmortem samples were analyzed, we found that only one study reported a significant increase in iron content9, while the other five were completely negative with mixed trends2,4,11,12,13. Although the results of our meta-analysis suggested a significant increase in iron content in the putamen of PD patients in postmortem samples, caution should be taken in the interpretation of these results as one positive study9 dominated the other five negative ones in the analysis (Fig. 2G). For SWI, an iron overload was suggested in the putamen based on both random and fixed effects models. Results of two independent studies showed elevated iron content in this structure31,37, whereas the other five were not significantly different33,34,35,36,38. One of the positive studies37 was removed following a sensitivity analysis, and the remaining one31 drove half of the total effect size thereafter in the fixed effects model (Fig. 6F). Taken together, additional studies are needed to confirm iron accumulation in the putamen.
Globus pallidus
For SWI, results of six studies suggested a trend toward, or a significant, increase in the level of iron33,34,35,36,37,38, while one showed a decrease in iron content31, which was later removed based on a sensitivity analysis. The subsequent meta-analysis returned a significant increase of iron content in the globus pallidus. However, results of either postmortem or R2* meta-analyses did not display significant difference, which was in line with the mixed trends of changes in individual studies.
Nucleus caudatus
Similar to globus pallidus, both postmortem and R2* meta-analyses returned no significant difference with mixed trends in iron content in the individual studies. Results of pooled SWI analysis showed a significant increase of iron content in PD patients. There were six studies that showed a significant31,37 or a trend of increase33,34,35,36 in iron levels in the nucleus caudatus while only one study suggested a trend of decrease34.
Frontal lobe, temporal lobe and cerebellum
Although postmortem results of these structures were available, the pooled sample sizes were small (98, 44 and 58, respectively). All the four studies on frontal lobe2,11,12,14 and two on cerebellum2,14 reported negative results. Although one article reported a significant decrease of iron levels in the temporal lobe17, two studies showed no change11,13. Further studies were needed to clarify iron levels in these structures.
Red nucleus
No available studies using postmortem samples fit our criteria. Results of R2* and SWI pooled analyses suggested an increase of iron levels in the red nucleus. For the R2* analyses, four studies reported a significant increase3 or an increasing trend20,27,29, whereas one showed a decreasing trend30. For the SWI analyses, seven out of eight studies reported no remarkable changes, among which three showed a decreasing trend32,34,38 and four an increasing trend in iron content32,36,37,38. In comparison, the study that showed significantly elevated iron content in PD patients33 drove roughly half of the total effect size (Fig. 6H). Noteworthy, two PD groups (advanced and mild disease stage) were included in this study that had the same control group33. The advanced PD group was chosen for the current analysis to compare with postmortem samples that are usually obtained at late stage PD. When the mild group was included, results of SWI meta-analyses were not affected except in the red nucleus. There was no significant increase of iron content detected (Figs S1 and S2), suggesting that the severity of PD might be a factor affecting iron deposits in the red nucleus. As a note, the mild stage in this study33 was Hoehn and Yahr scale <1.5, which appeared milder than normally defined.
Thalamus and white matter
No qualified study using postmortem samples was available. Results of both R2* and SWI meta-analyses suggested no association of iron levels with PD in the thalamus and white matter of the brain. Furthermore, all of the selected individual studies31,34,35,36,37 returned negative results.
Discussion
Iron dysregulation is frequently associated with neurodegenerative disorders, including Huntington disease, Alzheimer’s disease, amyotrophic lateral sclerosis, and frontotemporal lobar degeneration40,41. Nonetheless, it remains unclear whether such defect is a cause or a consequence of neurodegeneration. A large body of evidence suggests abnormal iron levels in the brains of PD patients and a role for iron dysregulation in PD pathogenesis42,43,44. Our study represents the first meta-analysis that systematically assesses iron levels in various brain regions of PD patients by postmortem measurements and by MRI (R2* and SWI). Our analysis confirms a perturbed iron homeostasis in the substantia nigra and suggests that an increase in iron levels may also occur in the putamen and red nucleus (Table 2).
Some caveats in regard to the scope of this meta-analysis must be taken into account. First, in the postmortem analyses different iron quantification methods (SPH, AA, COL, ICP and MS) have been used. The differential sensitivity and specificity of these methods may contribute to an elevated heterogeneity. Second, disease stage and age may be two influencing factors when evaluating iron concentration in the brain40,45,46, which unfortunately is not addressed in the current study due to incomplete information and limited sample size. For example, the inclusion of a sub-group of mild-stage PD patients results in a loss of significance in iron levels in the red nucleus of SWI meta-analysis.
It is well recognized that iron overload contributes to oxidative stress through Fenton reaction, promoting the death of dopaminergic neurons in the substantia nigra47. Such iron accumulation is known to be associated with increased ferritin and neuromelanin iron loads48,49, as well as increased expression of divalent metal transporter 1 that may contribute to PD pathogenesis via its capacity of transporting ferrous iron47. Furthermore, aggregation of α-synuclein can be accelerated when bound with free iron50. However, it remains unclear whether iron deposit triggers or accelerates neurodegeneration, or if they are a secondary event due to neuronal degeneration. Therefore, it is important to determine the timing of iron deposit in substantia nigra during the pathogenesis of PD. Because postmortem measurements are usually made in a very late stage of PD, future longitudinal studies of iron contents are warranted47. Consistent results obtained from postmortem, R2*, and SWI measurements suggest that longitudinal evaluation of iron content in the substantia nigra can be appropriately made by MRI methods.
It appears that the MRI methods of R2* and SWI do not completely match the postmortem results, presumably the latter being the standard. Iron deposit is detected by SWI in the globus pallidus and nucleus caudatus, but these are inconsistent with the postmortem observations. Results from R2* studies also suggest an inconsistency in the putamen as both postmortem and SWI effects show an iron overload. Loss of striatal dopamine in PD is most prominent in sub-regions of the putamen51, which may be associated with an increase in iron levels. However, this may be a weak argument considering that the postmortem iron increase in this structure is driven by a single study as noted in the Results. It has previously been proposed that SWI is more specific and precise than other methods to estimate brain iron content52. Our results suggest that both methods have weakness in measuring iron content. The iron signal determined by R2* may be disrupted by calcification53 and lipid content54, and the output value is a weighted summation of magnetic properties from both local and surrounding tissues28. Intrinsic defects of SWI include a difficulty in distinguishing diamagnetic and paramagnetic susceptibility owning to the convoluting effect of the dipole fields55. There are also limitations of MRI per se, such that myelin, especially small myelinated fibers, cannot be easily distinguishable from iron deposition46, and the phase value of MRI reflects not only non-heme iron deposited in the tissue but also the heme iron in hemosiderin or in circulating blood56. Microbleeds may also be a confounding factor especially when brain iron content is estimated in older adults57. Given the MRI phase’s nonlocal behavior, one should pay attention to the signal interference of adjacent structures. For example, the red nucleus lies adjacent to substantia nigra in the midbrain and is likely high in iron levels due to its proximity58. In other words, the differences detected in iron levels in the red nucleus may arise from the adjacent substantia nigra, instead of from the structure itself. Increased iron levels in red nucleus are associated with levodopa-induced dyskinesia of PD3. Future postmortem studies are warranted to confirm iron deposit in this structure. This is also the case for the putamen and globus pallidus, due to their relative proximity. Recently, quantitative susceptibility mapping (QSM), a potentially superior method to measuring iron content in vivo, has been applied to measure PD-related iron deposition and progression28. By this method, Guan et al.59 have recently reported a distinct pattern of iron accumulation according to disease stage, with iron spreading from the substantia nigra in early stages to the substantia nigra, red nucleus and globus pallidus in later stages. This could explain the aforementioned discrepancy in the red nucleus when the mild PD group is included, as well as provide a potential explanation for inconsistent findings between neuropathology and MRI techniques.
In conclusion, the current meta-analysis corroborates iron overload in substantia nigra and suggests such iron homeostasis defect in the putamen (by postmortem and SWI, but not R2*) and the red nucleus (by R2* and SWI; no data by postmortem) of PD patients. Both the R2* or SWI techniques may not authentically reflect iron changes in brain regions other than substantia nigra. Our results offer a comprehensive understanding of iron loads in different brain regions in association with PD, and contribute to the evaluation of measuring accuracy of iron concentration by MRI methods.
Methods
Literature Search Strategy
Literature related to iron and Parkinson’s disease were searched in four databases including Medline via PubMed, Web of Science, the Cochrane Central Register of Controlled Trials (CENTRAL) and Embase via OVID, dated till 19th November 2015. The keywords for iron and Parkinson’s disease are “iron” or “Fe” and “Parkinson disease”, “Parkinson’s disease”, “Parkinsons disease” or “Parkinsonian”, respectively.
Study Selection
Based on the keywords, titles and abstracts of the identified publications were screened. Following an exhaustive examination of the literature contents, articles were included according to our selection criteria: population (idiopathic PD patients), comparators (individuals free of neurological disorders), outcome measurement (iron content in brain regions), and language (articles written in English or Chinese). Review articles, qualitative and semi-quantitative studies were excluded.
Data Extraction
The literature search and data extraction were conducted by two researchers (Qing-Qing Zhuang and Jian-Yong Wang) independently. In the case of a dispute, a third investigator was included to discuss and reach an agreement. The following data was extracted: sample size, age, sex, PD diagnosis, iron detection methods, the type of samples, clinical scores, and iron content or R2* value or phase value in brain regions. Assessment of the detailed information was listed in Table 1. As shown in this table, the disease severity (Hoehn and Yahr scale) was not provided by all the included studies and the provided else information was also varied in forms including UPDRS score, UPDRS motor score, and/or disease duration. Therefore, we did not include the disease severity as a source of variance in the analysis.
Iron quantification methods employed in the postmortem study of brain samples included spectrophotometry (SPH), atomic absorption (AA), colorimetry (COL), inductively coupled plasma spectroscopy (ICP) and Mössbauer spectroscopy (MS). To be consistent in brain weights, a conversion of dry weight to wet weight was applied based on a dry/wet ratio as suggested in previous studies60,61. The SWI signal phase is orientation-dependent and nonlocal55. As a result, the phase value appears to be either positively or negatively correlated with iron concentration depending on the orientation relative to the Bo field62. Thus, a conversion from SWI phase value to iron concentration was applied based on formulas suggested in previous studies35,36; that is, concentration = 397.72 × (phase value) + 3.4097 (extracted from Fig. 1 of ref. 36) for the studies of positive setting31,34,36, and concentration = −128.23 × (phase value) + 3.1897 (extracted from Fig. 2 of ref. 35) for the studies of negative setting32,33,35,37,38.
Quality Assessment
The Newcastle-Ottawa Scale63 was employed to assess the quality of the chosen studies. This tool classified studies in three broad perspectives: selection of the study groups, comparability of the groups, and ascertainment of either exposure or outcome of interest for the studies. Semi-quantitative measurement using a star system assesses the quality of study. The highest quality studies can get a maximum of nine stars.
Statistical Analysis
Eleven postmortem analysis and 22 MRI analysis articles were eventually selected for our meta-analysis. Means, standard deviations (or standard errors), and the number of samples were extracted in each study. Meta-analyses were conducted within the studies of the same brain region after sorting into their respective quantitative groups of postmortem analysis, R2* and SWI. In the case that the same data appeared in multiple studies, the data were used only once. All of the analyses were performed using Review Manager 5.2 for Windows (http://ims.cochrane.org/recman). A two-tailed p value <0.05 was considered statistically significant. Weighted mean difference (WMD) was regarded as an effect size. Q-statistics and I2 were used for assessing the heterogeneity64,65. A random effects model was applied when heterogeneity was found by Q-statistics or when I2 > 50%. A fixed effects model was applied otherwise.
Additional Information
How to cite this article: Wang, J.-Y. et al. Meta-analysis of brain iron levels of Parkinson’s disease patients determined by postmortem and MRI measurements. Sci. Rep. 6, 36669; doi: 10.1038/srep36669 (2016).
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Material
Acknowledgments
The authors appreciate Drs Jennifer Harr and Wen-Hsing Cheng for critical help improving readability and accuracy of the manuscript. This work was supported by funding from Zhejiang Provincial Natural Science Foundation (LY16H250003, LY16H260003, and LR13H020002), National Natural Science Foundation of China (81571087), and Wenzhou Science and Technology Bureau (Y20150005).
Footnotes
Author Contributions J.Y.W. and Q.Q.Z. performed the data collection, extraction and analyses, L.B.Z., H.Z., T.L., R.L. and S.F.C. contributed to partial data extraction and interpretation, X.Z., C.P.H. and J.H.Z. designed and supervised the study, J.Y.W., Q.Q.Z. and J.H.Z. wrote the manuscript. All authors read and approved the final manuscript.
References
- Dexter D. T. et al. Alterations in the levels of iron, ferritin and other trace metals in Parkinson’s disease and other neurodegenerative diseases affecting the basal ganglia. Brain 114 (Pt 4), 1953–1975 (1991). [DOI] [PubMed] [Google Scholar]
- Dexter D. T. et al. Increased nigral iron content and alterations in other metal ions occurring in brain in Parkinson’s disease. J Neurochem 52, 1830–1836 (1989). [DOI] [PubMed] [Google Scholar]
- Lewis M. M. et al. Higher iron in the red nucleus marks Parkinson’s dyskinesia. Neurobiol Aging 34, 1497–1503 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riederer P. et al. Transition metals, ferritin, glutathione, and ascorbic acid in parkinsonian brains. J Neurochem 52, 515–520 (1989). [DOI] [PubMed] [Google Scholar]
- Hardy P. A. et al. Correlation of R2 with total iron concentration in the brains of rhesus monkeys. J Magn Reson Imaging 21, 118–127 (2005). [DOI] [PubMed] [Google Scholar]
- Langkammer C. et al. Quantitative MR Imaging of Brain Iron: A Postmortem Validation Study. Radiology 257, 455–462 (2010). [DOI] [PubMed] [Google Scholar]
- Ordidge R. J., Gorell J. M., Deniau J. C., Knight R. A. & Helpern J. A. Assessment of relative brain iron concentrations using T2-weighted and T2*-weighted MRI at 3 Tesla. Magn Reson Med 32, 335–341 (1994). [DOI] [PubMed] [Google Scholar]
- Pyatigorskaya N., Gallea C., Garcia-Lorenzo D., Vidailhet M. & Lehericy S. A review of the use of magnetic resonance imaging in Parkinson’s disease. Ther Adv Neurol Disord 7, 12–26 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J. C. et al. MR of human postmortem brain tissue: correlative study between T2 and assays of iron and ferritin in Parkinson and Huntington disease. Am J Neuroradiol 14, 275–281 (1993). [PMC free article] [PubMed] [Google Scholar]
- Galazka-Friedman J. et al. Iron in parkinsonian and control substantia nigra–a Mossbauer spectroscopy study. Mov Disord 11, 8–16 (1996). [DOI] [PubMed] [Google Scholar]
- Griffiths P. D. & Crossman A. R. Distribution of iron in the basal ganglia and neocortex in postmortem tissue in Parkinson’s disease and Alzheimer’s disease. Dementia 4, 61–65 (1993). [DOI] [PubMed] [Google Scholar]
- Loeffler D. A. et al. Transferrin and iron in normal, Alzheimer’s disease, and Parkinson’s disease brain regions. J Neurochem 65, 710–724 (1995). [DOI] [PubMed] [Google Scholar]
- Sofic E. et al. Increased iron (III) and total iron content in post mortem substantia nigra of parkinsonian brain. J Neural Transm 74, 199–205 (1988). [DOI] [PubMed] [Google Scholar]
- Uitti R. J. et al. Regional metal concentrations in Parkinson’s disease, other chronic neurological diseases, and control brains. Can J Neurol Sci 16, 310–314 (1989). [DOI] [PubMed] [Google Scholar]
- Visanji N. P. et al. Iron deficiency in parkinsonism: region-specific iron dysregulation in Parkinson’s disease and multiple system atrophy. J Parkinsons Dis 3, 523–537 (2013). [DOI] [PubMed] [Google Scholar]
- Wypijewska A. et al. Iron and reactive oxygen species activity in parkinsonian substantia nigra. Parkinsonism Relat Disord 16, 329–333 (2010). [DOI] [PubMed] [Google Scholar]
- Yu X. et al. Decreased iron levels in the temporal cortex in postmortem human brains with Parkinson disease. Neurology 80, 492–495 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorell J. M. et al. Increased iron-related MRI contrast in the substantia nigra in Parkinson’s disease. Neurology 45, 1138–1143 (1995). [DOI] [PubMed] [Google Scholar]
- Graham J. M., Paley M. N., Grunewald R. A., Hoggard N. & Griffiths P. D. Brain iron deposition in Parkinson’s disease imaged using the PRIME magnetic resonance sequence. Brain 123 Pt 12, 2423–2431 (2000). [DOI] [PubMed] [Google Scholar]
- Martin W. R. W., Wieler M. & Gee M. Midbrain iron content in early Parkinson disease - A potential biomarker of disease status. Neurology 70, 1411–1417 (2008). [DOI] [PubMed] [Google Scholar]
- Du G. et al. Serum iron and ferritin level in idiopathic Parkinson. Pak J Biol Sci 15, 1094–1097 (2012). [DOI] [PubMed] [Google Scholar]
- Bunzeck N. et al. Motor phenotype and magnetic resonance measures of basal ganglia iron levels in Parkinson’s disease. Parkinsonism Relat Disord 19, 1136–1142 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee M. F. et al. N-acetylcysteine (NAC) inhibits cell growth by mediating the EGFR/Akt/HMG box-containing protein 1 (HBP1) signaling pathway in invasive oral cancer. Oral Oncol 49, 129–135 (2013). [DOI] [PubMed] [Google Scholar]
- Rossi M., Ruottinen H., Soimakallio S., Elovaara I. & Dastidar P. Clinical MRI for iron detection in Parkinson’s disease. Clin Imaging 37, 631–636 (2013). [DOI] [PubMed] [Google Scholar]
- Ulla M. et al. Is R2* a new MRI biomarker for the progression of Parkinson’s disease? A longitudinal follow-up. PLoS One 8, e57904 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi M. E., Ruottinen H., Saunamaki T., Elovaara I. & Dastidar P. Imaging brain iron and diffusion patterns: a follow-up study of Parkinson’s disease in the initial stages. Acad Radiol 21, 64–71 (2014). [DOI] [PubMed] [Google Scholar]
- Barbosa J. H. O. et al. Quantifying brain iron deposition in patients with Parkinson’s disease using quantitative susceptibility mapping, R2 and R2. Magn Reson Imaging 33, 559–565 (2015). [DOI] [PubMed] [Google Scholar]
- Du G. et al. Quantitative susceptibility mapping of the midbrain in Parkinson’s disease. Mov Disord 31, 317–324 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- He N. et al. Region-specific disturbed iron distribution in early idiopathic Parkinson’s disease measured by quantitative susceptibility mapping. Hum Brain Mapp 36, 4407–4420 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murakami Y. et al. Usefulness of quantitative susceptibility mapping for the diagnosis of Parkinson disease. Am J Neuroradiol 36, 1102–1108 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han Y. H. et al. Topographical differences of brain iron deposition between progressive supranuclear palsy and parkinsonian variant multiple system atrophy. J Neurol Sci 325, 29–35 (2013). [DOI] [PubMed] [Google Scholar]
- Wang C. et al. Application of quantitative measurement on midbrain in Parkinson disease with MR susceptibility-weighted imaging. Chin J Med Imaging Technol 27, 1129–1133 (2011). [Google Scholar]
- Wu S. F. et al. Assessment of cerebral iron content in patients with Parkinson’s disease by the susceptibility-weighted MRI. Eur Rev Med Pharmacol Sci 18, 2605–2608 (2014). [PubMed] [Google Scholar]
- Zhang J. et al. Characterizing iron deposition in Parkinson’s disease using susceptibility-weighted imaging: an in vivo MR study. Brain Res 1330, 124–130 (2010). [DOI] [PubMed] [Google Scholar]
- Jin L. et al. Decreased serum ceruloplasmin levels characteristically aggravate nigral iron deposition in Parkinson’s disease. Brain 134, 50–58 (2011). [DOI] [PubMed] [Google Scholar]
- Kim T. H. & Lee J. H. Serum uric acid and nigral iron deposition in Parkinson’s disease: A pilot study. Mov Disord 29, S88–S88 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y. et al. Different iron-deposition patterns of multiple system atrophy with predominant parkinsonism and idiopathetic Parkinson diseases demonstrated by phase-corrected susceptibility-weighted imaging. Am J Neuroradiol 33, 266–273 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang X. M., Sun B., Xue Y. J. & Duan Q. Susceptibility-weighted imaging in detecting brain iron accumulation of Parkinson’s disease. Zhonghua Yi Xue Za Zhi 90, 3054–3058 (2010). [PubMed] [Google Scholar]
- Manor O. & Zucker D. M. Small sample inference for the fixed effects in the mixed linear model. Comput Stat Data An 46, 801–817 (2004). [Google Scholar]
- Ward R. J., Zucca F. A., Duyn J. H., Crichton R. R. & Zecca L. The role of iron in brain ageing and neurodegenerative disorders. Lancet Neurol 13, 1045–1060 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biasiotto G., Di Lorenzo D., Archetti S. & Zanella I. Iron and Neurodegeneration: Is Ferritinophagy the Link? Mol Neurobiol 53, 5542–5574 (2015). [DOI] [PubMed] [Google Scholar]
- Oshiro S., Morioka M. S. & Kikuchi M. Dysregulation of iron metabolism in Alzheimer’s disease, Parkinson’s disease, and amyotrophic lateral sclerosis. Adv Pharmacol Sci 2011, 378278 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry G. et al. The role of iron and copper in the aetiology of neurodegenerative disorders: therapeutic implications. CNS Drugs 16, 339–352 (2002). [DOI] [PubMed] [Google Scholar]
- Sayre L. M., Perry G., Atwood C. S. & Smith M. A. The role of metals in neurodegenerative diseases. Cell Mol Biol (Noisy-le-grand) 46, 731–741 (2000). [PubMed] [Google Scholar]
- Zecca L., Youdim M. B., Riederer P., Connor J. R. & Crichton R. R. Iron, brain ageing and neurodegenerative disorders. Nat Rev Neurosci 5, 863–873 (2004). [DOI] [PubMed] [Google Scholar]
- Daugherty A. & Raz N. Age-related differences in iron content of subcortical nuclei observed in vivo: A meta-analysis. Neuroimage 70, 113–121 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadzhieva M., Kirches E. & Mawrin C. Review: iron metabolism and the role of iron in neurodegenerative disorders. Neuropathol Appl Neurobiol 40, 240–257 (2014). [DOI] [PubMed] [Google Scholar]
- Ben-Shachar D., Riederer P. & Youdim M. B. Iron-melanin interaction and lipid peroxidation: implications for Parkinson’s disease. J Neurochem 57, 1609–1614 (1991). [DOI] [PubMed] [Google Scholar]
- Jellinger K., Paulus W., Grundke-Iqbal I., Riederer P. & Youdim M. B. Brain iron and ferritin in Parkinson’s and Alzheimer’s diseases. J Neural Transm Park Dis Dement Sect 2, 327–340 (1990). [DOI] [PubMed] [Google Scholar]
- Wolozin B. & Golts N. Iron and Parkinson’s disease. Neuroscientist 8, 22–32 (2002). [DOI] [PubMed] [Google Scholar]
- Kish S. J., Shannak K. & Hornykiewicz O. Uneven pattern of dopamine loss in the striatum of patients with idiopathic Parkinson’s disease. Pathophysiologic and clinical implications. New Engl J Med 318, 876–880 (1988). [DOI] [PubMed] [Google Scholar]
- Ogg R. J., Langston J. W., Haacke E. M., Steen R. G. & Taylor J. S. The correlation between phase shifts in gradient-echo MR images and regional brain iron concentration. Magn Reson Imaging 17, 1141–1148 (1999). [DOI] [PubMed] [Google Scholar]
- Naderi S., Colakoglu Z. & Luleci G. Calcification of basal ganglia associated with pontine calcification in four cases: a radiologic and genetic study. Clin Neurol Neurosurg 95, 155–157 (1993). [DOI] [PubMed] [Google Scholar]
- He X. & Yablonskiy D. A. Biophysical mechanisms of phase contrast in gradient echo MRI. Proc Natl Acad Sci USA 106, 13558–13563 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C., Li W., Tong K. A., Yeom K. W. & Kuzminski S. Susceptibility-weighted imaging and quantitative susceptibility mapping in the brain. J Magn Reson Imaging 42, 23–41 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson C. M. et al. Brain T2 relaxation times correlate with regional cerebral blood volume. MAGMA 18, 3–6 (2005). [DOI] [PubMed] [Google Scholar]
- Penke L. et al. Brain iron deposits are associated with general cognitive ability and cognitive aging. Neurobiol Aging 33, 510–517 e512 (2012). [DOI] [PubMed] [Google Scholar]
- Drayer B. et al. MRI of brain iron. Am J Roentgenol 147, 103–110 (1986). [DOI] [PubMed] [Google Scholar]
- Guan X. et al. Regionally progressive accumulation of iron in Parkinson’s disease as measured by quantitative susceptibility mapping. NMR Biomed, doi: 10.1002/nbm.3489 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrag M., Mueller C., Oyoyo U., Smith M. A. & Kirsch W. M. Iron, zinc and copper in the Alzheimer’s disease brain: a quantitative meta-analysis. Some insight on the influence of citation bias on scientific opinion. Prog Neurobiol 94, 296–306 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- House M. J. et al. Correlation of proton transverse relaxation rates (R2) with iron concentrations in postmortem brain tissue from alzheimer’s disease patients. Magn Reson Med 57, 172–180 (2007). [DOI] [PubMed] [Google Scholar]
- Yablonskiy D. A. & Haacke E. M. Theory of NMR signal behavior in magnetically inhomogeneous tissues: the static dephasing regime. Magn Reson Med 32, 749–763 (1994). [DOI] [PubMed] [Google Scholar]
- Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol 25, 603–605 (2010). [DOI] [PubMed] [Google Scholar]
- Cochran W. G. The combination of estimates from different experiments. Biometrics 10, 101–129 (1954). [Google Scholar]
- Higgins J. P. T. & Thompson S. G. Quantifying heterogeneity in a meta-analysis. Stat Med 21, 1539–1558 (2002). [DOI] [PubMed] [Google Scholar]
- Calne D. B., Snow B. J. & Lee C. Criteria for diagnosing Parkinson’s disease. Ann Neurol 32 Suppl, S125–S127 (1992). [DOI] [PubMed] [Google Scholar]
- Hughes A. J., Daniel S. E., Blankson S. & Lees A. J. A clinicopathologic study of 100 cases of Parkinson’s disease. Arch Neurol 50, 140–148 (1993). [DOI] [PubMed] [Google Scholar]
- Gibb W. R. & Lees A. J. The relevance of the Lewy body to the pathogenesis of idiopathic Parkinson’s disease. J Neurol Neurosurg Psychiatry 51, 745–752 (1988). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.