Abstract
The intestinal epithelial barrier is critical to limit potential harmful consequences from exposure to deleterious luminal contents on the organism. Although this barrier is functionally important along the entire gut, specific regional regulatory mechanisms involved in the maintenance of this barrier are poorly defined. Herein, we identified Gata4 as a crucial regulator of barrier integrity in the mouse proximal intestinal epithelium. Conditional deletion of Gata4 in the intestine led to a drastic increase in claudin-2 expression that was associated with an important increase of gut barrier permeability without causing overt spontaneous inflammation. Administration of indomethacin, a non-steroidal anti-inflammatory drug (NSAID) that causes enteritis, led to rapid and restricted proximal small intestinal injuries in Gata4 mutant mice as opposed to control mice. Comparative analysis of gene transcript profiles from indomethacin-challenged control and Gata4 mutant mice identified defects in epithelial cell survival, inflammatory cell recruitment and tissue repair mechanisms. Altogether, these observations identify Gata4 as a novel crucial regulator of the intestinal epithelial barrier and as a critical epithelial transcription factor implicated in the maintenance of proximal intestinal mucosal integrity after injury.
The small intestinal epithelium is a dynamic system that constantly and rapidly regenerates throughout individual life. The continuous production of intestinal epithelial cells is ensured by crypt based columnar stem cells capable of producing progenitors that will differentiate upon distinct integrated molecular programs responsible for the specification of the main cell lineages including enterocytes, goblet, enteroendocrine and Paneth cells1. Tight regulation of this process is crucial in order to ensure basic epithelium functions and the integrity of the intestinal barrier that protects against potential harmful luminal content2. Toward this end, the intestinal epithelium maintains a permissive mechanical barrier function strictly dependent on apical junctional complexes between epithelial cells that include tight junctions and adherens junctions3. Tight junctions localize to the apical end of the lateral surface of adjacent epithelial cells and consist of several transmembrane proteins such as occludin and claudins that mediate adhesion and barrier formation as well as paracellular diffusion4. The exact molecular composition and relative expression level of claudins can modulate the overall properties of tight junctions. While most claudins are recognized to maintain strong junctional integrity, several evidences support an opposite role for the claudin-2 member5. When overexpressed in Madin-Darby canine kidney (MDCK) epithelial cells, claudin-2 was able to increase tight junction paracellular cation permeability6. In addition, knockout mice for Clnd2 display decreased transepithelial conductance of the small intestinal epithelium7. Thus, claudin-2 is functionally associated with tight junction leakiness and its modulation upon biological processes or pathological conditions correlates well with decreased intestinal barrier integrity8,9.
A subset of transcriptional regulators has been reported to promote intestinal epithelial cell polarization and differentiation. Cdx2 was originally identified as a master regulator of this process in cultured cells10. With the use of conditional mouse knockout strategies, Cdx2 has been further established as being crucial for intestinal epithelial cell fate adoption as well as apical-basolateral polarity11,12. Both Hnf4α and Hnf1α were also identified to modulate cell polarization and differentiation both in culture13 and in the mouse intestinal epithelium14. Gata4, a member of zinc finger-containing GATA transcription factor family, regulates several intestinal epithelial genes in combination with Cdx2, Hnf4α and Hnf1α15,16. The expression of Gata4 in the intestine is mostly restricted to the proximal small intestinal epithelium and the generation of Gata4-conditional intestinal epithelial knockout mouse models elucidated some of its functions in this context. Indeed, Gata4 regulates specific gene networks along the anterior-caudal axis of the gut epithelium by activating jejunum specific genes17,18 and by repressing the ileal gene expression program19,20. Intestinal deletion of Gata4 also affects lipid metabolism17, induces bile acid absorption from the jejunum21 and was recently proposed to act as a mediator of the gut microbiota-dependent negative effects on jejunum bile acid absorption22. Gata4 was also recently shown to regulate intestinal epithelial expression of regenerating islet derived family members (REG)23 for which some members are involved in defense mechanisms and epithelial maintenance of the small intestinal epithelium24,25.
Here we investigated the potential role for Gata4 in regulating intestinal mucosal barrier integrity. Our findings support a crucial function for this transcriptional regulator in actively repressing the leaky tight junction claudin-2 component, maintaining gut barrier properties and also preventing acute mucosal injury in the proximal small intestine.
Results
Gata4 intestinal epithelial deletion impacts functional barrier integrity and regulation of claudin-2 expression
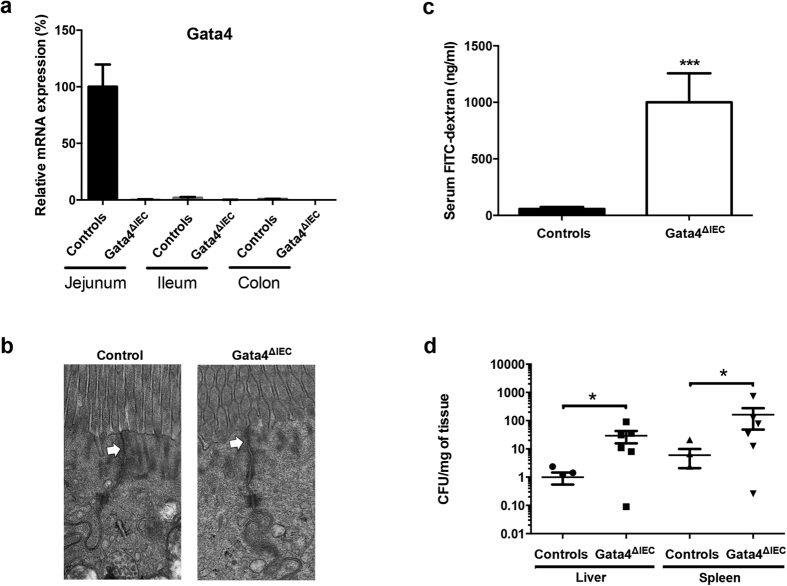
Gata4 intestinal epithelial conditional knockout mice were generated from previously characterized Gata4loxP allele mice26 with Villin-Cre transgenic mice that exclusively express Cre in the intestinal epithelium27. Gata4ΔIEC mice were significantly deleted for Gata4 gene transcript expression in the jejunum when compared to control littermates (Fig. 1a), while weak expression was recorded in the most distal portion of the ileum and no expression in the colon of control mice, as originally reported18. Gata4 deletion was also confirmed at the level of Gata4 protein expression, as visualized by immunofluorescence (data not shown)23. Since the overall expression of Gata4 was substantially more important in the proximal intestine than distally, we focused on the proximal region of the small intestine. Electron microscopy was first performed to monitor the overall integrity of enterocytes in absence of Gata4. Epithelial cells from control mice displayed typical junctional complexes between cells with sealed tight junction structures at the enterocytes apex (Fig. 1b). Although overall epithelial cell ultrastructures were similar among Gata4ΔIEC and control mice, tight junctions systematically appeared less well defined in Gata4ΔIEC mice when compared to controls (Fig. 1b). Since it was difficult to quantitatively evaluate potential tight junction defects based on ultrastructure analysis, intestinal permeability was next assessed after Gata4ΔIEC and control groups of mice were gavaged with FITC-labeled dextran. Detection of FITC concentrations in the serum of Gata4ΔIEC mice was significantly increased more than 18.2-fold (P < 0.001) when compared to littermate controls (Fig. 1c). To further understand the physiological impact of such increases in mucosal permeability and to determine whether it results in increased translocation of intestinal bacteria, mice were orally infected with the Salmonella typhimurium invasion-deficient strain SB103. Due to a mutation in the invA gene, this strain is unable to invade enterocytes28,29 and therefore, its translocation to the mucosa solely depends on other mechanisms (e. g., increased epithelial permeability). Oral infections of Gata4ΔIEC mice with Salmonella typhimurium SB103 led to significant increases in liver (29.3-fold; P < 0.05) and spleen (26.9-fold; P < 0.05) colonization when compared to infected controls (Fig. 1d). Taken together, these observations support the existence of intestinal barrier defects in the absence of Gata4.
Figure 1. Conditional deletion of Gata4 in the mouse intestinal epithelium negatively impacts mucosal barrier integrity.
(a) Total RNA was isolated from the jejunum, ileum and colon of control and Gata4ΔIEC mice (n = 3–4 per group) and RT-qPCR was performed to quantify Gata4 gene transcripts. (b) Electron microscopic analysis of jejunum epithelial cells from control and Gata4ΔIEC mice. White arrows indicate apical tight junctions. (c) Intestinal permeability was assessed by measuring circulating FITC-dextran levels 4 h following gavage of control and Gata4ΔIEC mice (n = 8–10 per group). ***P < 0.001 (Student-t test). (d) Control and Gata4ΔIEC mice were orally infected with Salmonella typhimurium SB103 and bacterial counts were done in liver and spleen (n = 5–6 per group). *P < 0.05 (Mann-Whitney test).
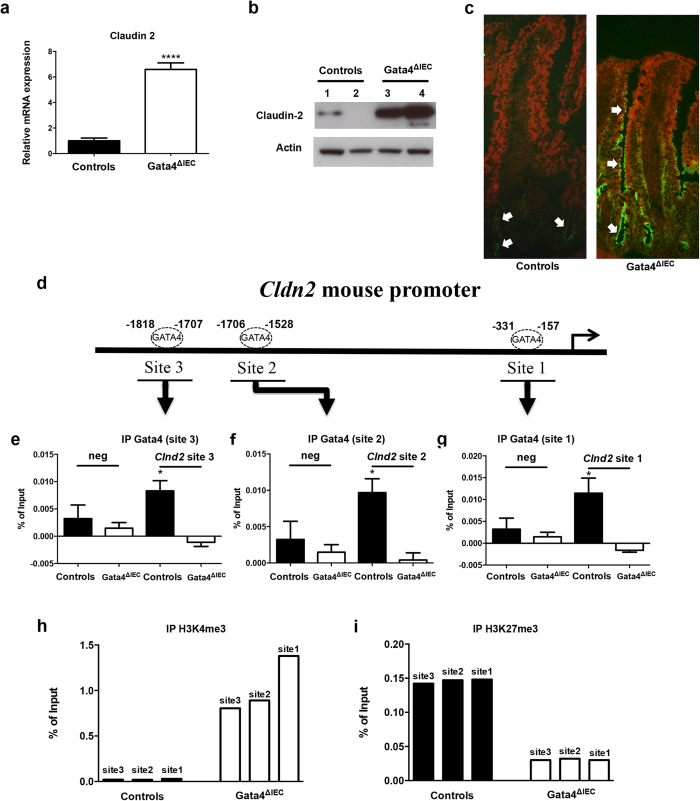
To explore by which mechanisms Gata4 could transcriptionally influence epithelial permeability, gene transcript expression of several molecules functionally involved in the formation of junctional complexes were quantified in Gata4ΔIEC and control mouse jejunum samples (Supplementary Table 1). From this analysis, claudin-2 was found to be the most drastically modulated transcript with a significant 6.6-fold increase (P < 0.0001) in Gata4ΔIEC mice in comparison to controls (Fig. 2a). This induction of expression was also reflected at the protein level as determined by Western blot on total jejunum extracts (Fig. 2b) and immunofluorescence on jejunum sections (Fig. 2c). Interestingly, claudin-2 was strongly detected at the apex of crypt and differentiated enterocytes in Gata4ΔIEC mice while mostly restricted to crypt epithelial cells in control mice (Fig. 2c). Gata4 was previously identified to positively regulate transcription of the human CLDN2 gene promoter30. Sequence analysis of the 5′-flanking region of the murine Clnd2 gene predicted three putative GATA elements for which one was located in the TSS vicinity of the promoter (Fig. 2d). ChIP experiments were then conducted on mouse jejunum isolated from control and Gata4ΔIEC mice. Antibody specific for Gata4 was able to precipitate mouse wild-type jejunum chromatin encompassing the three predicted GATA-binding sites of the murine Cldn2 gene but failed to precipitate mouse Gata4ΔIEC jejunum chromatin under the same conditions (Fig. 2e–g). To further monitor whether loss of Gata4 interaction with the Cldn2 gene could be functionally linked to active chromatin, histone modification methylation marks of the Cldn2 gene were quantified in the presence or absence of Gata4. Pull-down of H3K4me3, that labels active chromatin, showed a drastic enrichment of mouse Gata4ΔIEC jejunum chromatin in the vicinity of Cldn2 GATA interacting sites (Fig. 2h). Coincidentally, H3K27me3, a repressive histone modification marker showed a decreased association with Gata4ΔIEC jejunum chromatin surrounding Cldn2 GATA binding sites (Fig. 2i). Taken together, these observations support a direct role for Gata4 in repressing Cldn2 gene transcription in mouse jejunum epithelial cells.
Figure 2. Claudin-2 expression is derepressed in the jejunum of Gata4 mutant mice.
(a) Total RNA was isolated from the jejunum of control and Gata4ΔIEC mice (n = 3–4 per group) and RT-qPCR was performed to quantify claudin-2 gene transcripts. ****P < 0.0001 (Student-t test). (b) Western blot analysis was performed using a claudin-2 polyclonal antibody on total lysates prepared from control and Gata4ΔIEC mice. An actin polyclonal antibody was used as a loading control to monitor protein integrity. Cropped blots are displayed and full-length blots are included in the supplementary information. (c) Immunofluorescence detection of claudin-2 on jejunum sections prepared from control and Gata4ΔIEC mice. Arrows display typical labeling in the tight junctions. Original magnification: 10X. (d) Schematic representation of the Cldn2 gene with its predicted binding sites for GATA. (e–g) ChIP analysis of three paired biological samples (n = 3) obtained from the jejunum of control and Gata4ΔIEC mice. Data were obtained by qPCR and are expressed as the percent of total DNA input used for precipitation with an antibody against Gata4 relative to the DNA precipitated with normal goat-IgG. *P < 0.05 (ANOVA test). (h,i) ChIP analysis from the jejunum of control and Gata4ΔIEC mice. Data were obtained by qPCR and are expressed as the percent of total DNA input used for precipitation with an antibody against H3K4me3 (h) or H3K27me3 (i) relative to the DNA precipitated with normal IgG.
Impaired intestinal mucosal barrier integrity in Gata4 ΔIEC mice does not lead to spontaneous intestinal inflammation
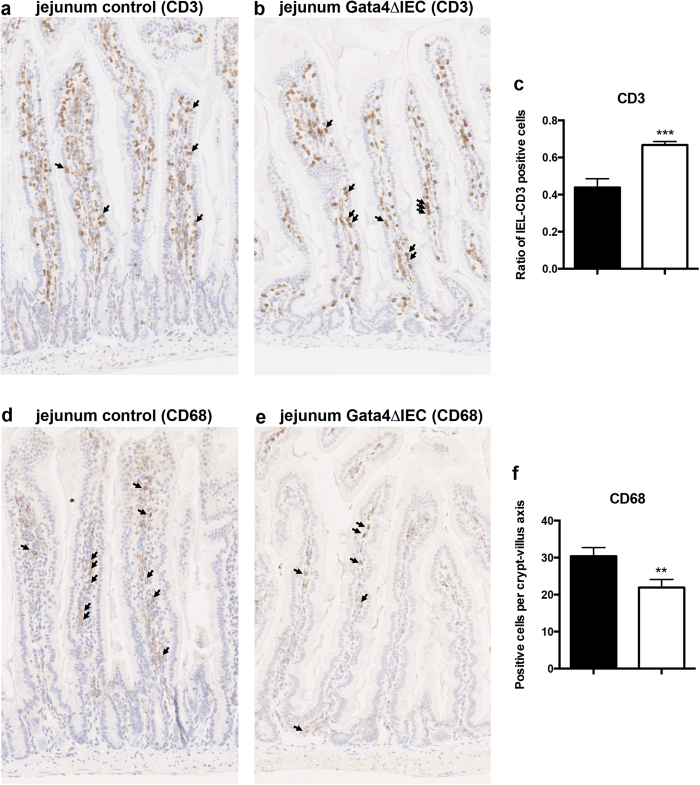
Histological observations of the small intestine from Gata4ΔIEC mice did not reveal signs of inflammation as late as 1 year of age (Fig. 3a,b). A mouse inflammatory response qPCR array performed on jejunum extracts did not indicate important inflammation related changes when Gata4ΔIEC mice were compared to controls (Supplementary Table 2). A careful examination of the jejunum distribution of inflammatory cells was next compared between Gata4ΔIEC and control mice. While no significant modification in the total number of CD3 positive cells per villus was noted between Gata4ΔIEC and control mice, a modest but significant increase in the ratio of CD3-positive intraepithelial lymphocytes (IELs) (1.4 fold; P < 0.0001) was observed in the mucosa of Gata4ΔIEC mice when compared to controls (Fig. 3a–c). A modest but significant decrease in the number of CD68-labeled macrophages per villus (1.5 fold; P < 0.001) was also observed in the jejunum of Gata4ΔIEC mice when compared to controls (Fig. 3d,e). These observations suggest that increased barrier permeability in non-inflammatory challenged Gata4ΔIEC mice modestly affected their jejunum mucosal immunity and do not spontaneously lead to intestinal inflammation.
Figure 3. Immune cells detection in the jejunum of Gata4 mutant mice.
T lymphocytes were visualized by immunohistochemistry against CD3 in control (a) and Gata4ΔIEC (b) mice. (c) The ratio of intra-epithelial lymphocytes (IEL) per total number of CD3 positive cells was averaged from 5 different crypt-villus axes of a total of 3 different mice per group. ***P < 0.001 (Student-t test). Macrophages were visualized by immunohistochemistry against CD68 in control (d) and Gata4ΔIEC (e) mice. (f) The total number of CD68 positive cells was averaged from 5 different crypt-villus axes of a total of 3 different mice per group. **P < 0.01 (Student-t test). Original magnification 10X.
Gata4 ΔIEC mice are severely sensitive to indomethacin-induced small intestinal injury response
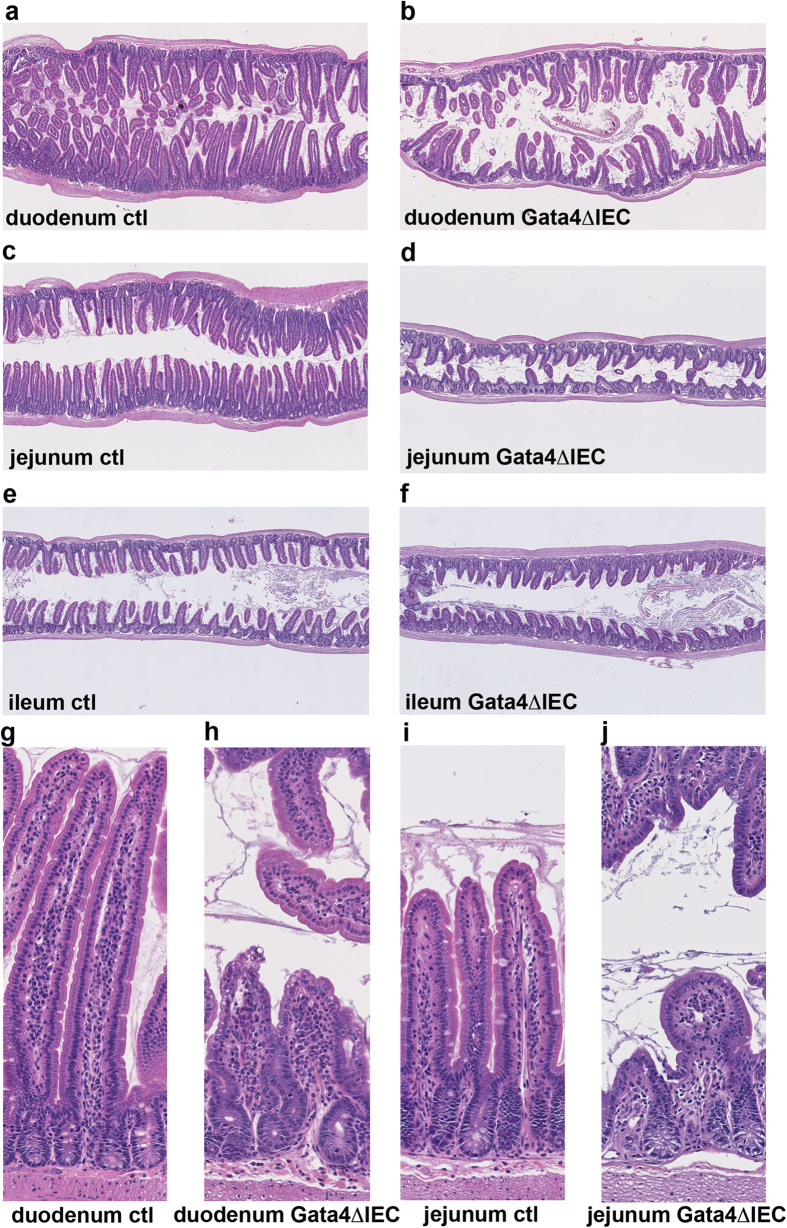
Indomethacin-induced small intestinal inflammation model was next used to investigate whether absence of jejunum Gata4 expression could sensitize mice to injury insults. Control and Gata4ΔIEC mice were injected with a single dose of indomethacin (10 mg/kg) and rapidly sacrificed 24 h after treatment due to the severity of Gata4ΔIEC mice response. Histological assessment of the small intestinal mucosa following this treatment revealed drastic villi regression in the duodenum of Gata4ΔIEC mice (Fig. 4b,h) as opposed to controls (Fig. 4a,g). This effect was also observed in the jejunum of Gata4ΔIEC mice (Fig. 4d,j) when compared to controls (Fig. 4c,i). Interestingly, ileum histology was similar between Gata4ΔIEC (Fig. 4f) and control (Fig. 4e) treated groups. We next investigated whether these changes in intestinal epithelium integrity can relate to proliferation defects of progenitor crypt epithelial cells. PCNA labeling on intestinal sections of Gata4ΔIEC and control indomethacin-treated mice was relatively constant among the crypts of both control (Supplementary Fig. 1a) and Gata4ΔIEC (Supplementary Fig. 1b) treated mice, without an overall significant change in the ratio of labeled cells per number of crypt cells (Supplementary Fig. 1c). This observation suggests that indomethacin-induced small intestinal mucosal injuries in absence of Gata4 were not originating from alterations of the epithelial cell proliferative pools but rather defects in epithelial cell viability or adherence.
Figure 4. Indomethacin severely impairs proximal small intestinal integrity of Gata4 mutant mice.
Control (a,c,e,g,i) and Gata4ΔIEC (b,d,f,h,j) mice were sacrificed 24 h after indomethacin injection and sections from the duodenum (a,b,g,h), the jejunum (c,d,i,j) and the ileum (e,f) were stained with H&E. Images are representative of at least 3 individuals per group. Original magnification: 2.5X (a–f) and 10X (g–j).
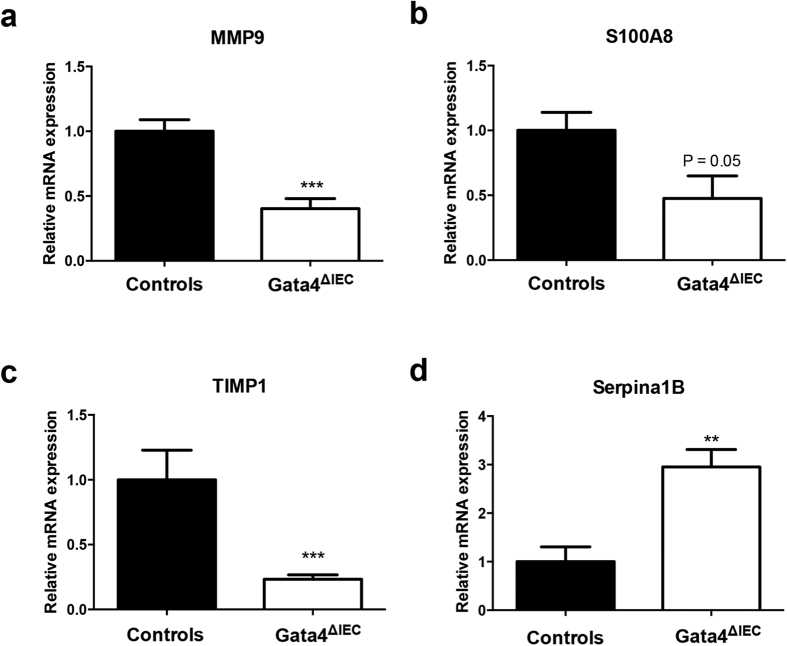
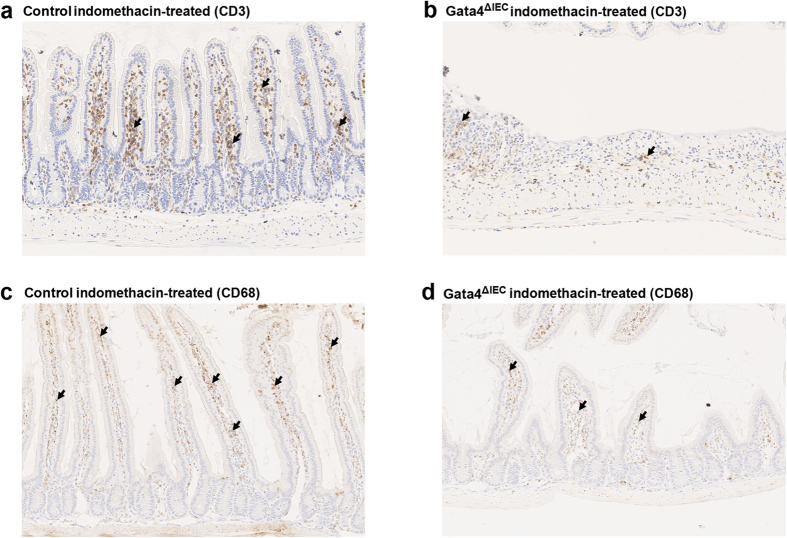
In order to clarify the nature of the signals linked with increased mucosal injury in the absence of Gata4, a gene expression profiling was next performed. This analysis was done in biological triplicate with RNA isolated from the jejunum of indomethacin-treated control and indomethacin-treated Gata4ΔIEC mice. The Illumina Mouse WG-6 v2.0 Expression Bead chip that contains more than 45,200 transcripts from the mouse genome was utilized to screen for mRNA expression variations. A statistical analysis (P value ≤0.05) predicted 309 unique and mapped transcripts being significantly modulated between control and Gata4ΔIEC indomethacin-treated mice (differential ratio ≥2.0; Supplementary Table 3). To gain insight into how these modifications could be classified as biological meaning, we used the Ingenuity Pathway Analysis (IPA) software. This analysis identified the haematological system and cellular movement as being the top network functions affected in the Gata4ΔIEC indomethacin-treated mice. Disorders in nutrition (40 molecules; P value range between 8E-03 and 5E-09) and in inflammatory response (64 molecules; P value range between 9E-03 and 3E-07) were identified as the top affected diseases and biological functions. Death of epithelial cells was also predicted to be increased (26 molecules; P value of 8E-06) (Supplementary Fig. 2) while immune cell trafficking (34 molecules; P value of 2E-04) was predicted to be decreased (Supplementary Fig. 3). These predicted changes in expression were next assessed for some candidate genes clustered among these networks of biological functions. A qRT-PCR analysis confirmed that matrix metallopeptidase 9 (MMP9) (2.4-fold reduction; P < 0.001) (Fig. 5a), S100 calcium binding protein A8 (S100A8) (2.1-fold reduction; P = 0.05) (Fig. 5b), tissue inhibitor of metalloproteinase 1 (TIMP1) (4.7-fold reduction; P < 0.001) (Fig. 5c) and Serpina1B (3.4-fold induction; P < 0.01) (Fig. 5d) gene transcripts were significantly modulated in the jejunum of Gata4ΔIEC indomethacin-treated mice when compared to control indomethacin-treated mice. In accordance to the predicted decrease in blood cells movement, the number of CD3-positive T cells was lower in the jejunum of Gata4ΔIEC indomethacin-treated mice (Fig. 6b) when compared to control treated mice (Fig. 6a). A similar observation was made for CD68-positive macrophages (Fig. 6c,d).
Figure 5. Expression of inflammatory modulators after indomethacin treatment of Gata4 mutant mice.
Total RNA was isolated from the jejunum of control and Gata4ΔIEC mice that were sacrificed after indomethacin injection (n = 4–10 per group). RT-qPCR was performed to quantify MMP9 (a), S100A8 (b), TIMP1 (c) and Serpina1B (d) gene transcripts. **P < 0.01; ***P < 0.001 (Student-t test).
Figure 6. Macrophages and T-lymphocytes mucosal distribution after indomethacin treatment of Gata4 mutant mice.
T lymphocytes were visualized by immunohistochemistry against CD3 in control (a) and Gata4ΔIEC (b) indomethacin-treated mice. Macrophages were visualized by immunohistochemistry against CD68 in control (c) and Gata4ΔIEC (d) indomethacin-treated mice (black arrows). The images were representative of 3 different mice per group. Original magnification: 10X.
Discussion
The intestinal epithelial barrier integrity is critical to prevent the translocation of luminal components to the mucosa and to maintain the physiological and immunological homeostasis of the gut. In homeostatic conditions, pathogenic or environmental insults to the intestinal epithelium would stimulate the mucosal immune system and not necessarily lead to disease31,32. However, a leaky gut barrier might both facilitate some level of constitutive inflammation and exacerbates the pro-inflammatory effects of the insults, given the increased exposure of the immune system to intruding luminal content that includes intestinal bacteria and their products. Our observations show that even though Gata4 deficiency results in a compromised intestinal epithelial barrier, the small intestine remains free of overt inflammation. At first glance, this could appear contradictory. Indeed, several genetically engineered mouse models for which gut epithelial barrier function has been altered displayed defects in their mucosal immune response33,34,35. However, one commonality among those models is that amplified immune response was mainly localized to the gut distal part. One possible explanation for these observations is the significant lower bacterial load in the jejunum because of higher antimicrobial activities when compared to the gut distal part36. This could explain why Gata4 mutant mice lack significant spontaneous inflammatory symptoms in the absence of nutritional or environmental stresses similar to what is observed for specific proximal gut disorders such as celiac disease37.
The defect of barrier integrity as observed in the Gata4 mutant mice is mechanistically linked to the direct interaction between Gata4 and Clnd2 transcription in the jejunum. Claudin-2 is well accepted to act by itself as a mediator of leaky gut barrier during intestinal inflammation9 as well as during exposure to microbial products38. Gata4 was previously shown to stimulate transcription of a Clnd2 promoter construct under artificial co-transfection assays in cultured cells30. In contrast, our data indicate that deletion of Gata4 leads to the activation of Cldn2 promoter in the jejunum and a subsequent increase in claudin-2 expression. Gata4 can act either as an activator or a repressor of gene transcription, depending on the nature of contextually-recruited co-regulators. Friend of Gata-1 (Fog-1) acts as a co-repressor of Gata4 in the small intestine20 and negatively regulates the expression of several genes including some members of the REG family23. Whether Fog1 is functionally involved during Gata4 dependent repression of Clnd2 remains to be investigated, but our observations support a novel and global repressive role for Gata4 in claudin-2 expression and barrier integrity in the context of an in vivo physiological system. How the increase in claudin-2 expression promotes mucosal permeability to FITC-dextran and Salmonella in Gata4 mutant mice remains unclear. Claudin-2 forms a channel that is selectively permeable to small cations but not for molecules such as FITC-dextran or pathogens9. Whether the increase in claudin-2 expression alters other tight junction components or modifies the pattern of tight junction strands of intestinal epithelial cells in Gata4 mutants are possible. Since no reduction in tight junction gene transcripts expression was observed in the jejunum of Gata4 mutants and since ZO-1 protein distribution remains similar under these conditions (Supplementary Fig. 4), we postulate that the increase of claudin-2 and resulting changes in combination and mixing ratios of claudin molecules could influence the overall tightness of tight junction strands39. In addition, increased gut permeability in these mice could also be caused by apoptosis resulting in epithelial cells loss.
NSAIDs are well described to cause damages to the human gastrointestinal tract but the exact mechanisms involved are not yet completely understood40. Indomethacin treatment in mice often results in damage restricted to the more distal regions of the small intestine and will manifest after a relatively long period of time. Our findings that Gata4 mutant mice become rapidly and highly sensitive to the deleterious effects of indomethacin in the proximal small intestine are, to our knowledge, unique and provide the opportunity to investigate in more detail the overall nature of the mechanisms involved during NSAID induced mucosal injuries. One interesting clue as to how Gata4 might be involved in protecting the jejunum from such effects relates to the NSAID’s potential to preferentially cause enteropathy by combination with bile41 and changes in the gut microbiota42. Intriguingly, bile acid absorption is induced in the jejunum of Gata4 mutant mice21 and gut microbiota can inhibit bile acid reabsorption through Gata422.
Although Gata4 mutant mice did not show important spontaneous inflammation, their jejunum displayed minor but significant increases in IELs content. IELs are composed of a mixed population of lymphocytes and are believed to play an important role in protecting the epithelial barrier43. Increase of their mucosal recruitment to a leaky barrier would make sense in this context. However, our data indicate that the jejunum of Gata4 mutant mice is more prone to tissue damage following indomethacin treatment. It is tempting to speculate that subclasses of activated IELs might participate into the initial steps of deleterious damaging effects caused by indomethacin as it is observed in the case of celiac disease43. However, the transcriptome analysis coupled to the immunolocalization of immune cells in the jejunum of short-term indomethacin-treated Gata4 mutant mice supports that the recruitment of immune cells and the inflammatory response are inhibited under these conditions. S100A8, abundantly expressed in immune cells of myeloid origin and thought to be functionally involved during epithelial wound healing of several tissues44,45, was found to be decreased in injured mutant mice. MMP-9, an endopeptidase involved in wound healing through regulating the turnover of matrix proteins46, was also found decreased under these conditions. It is intriguing that MMP-9 was found to be increased during the healing of indomethacin-induced small intestinal damage in rats and that administration of MMP inhibitors significantly impaired the healing of ulceration during this treatment47. Serpina1, described as a potent blocker of hematopoietic stem cell mobilization48, was found to be increased in injured mutant mice. It is also plausible that the jejunal epithelium of Gata4 mutant mice be intrinsically less competent for epithelial restitution. In support of this, we recently reported that Reg1, a crucial factor for the maintenance of the villous structure of the small intestine25, was spontaneously reduced in the jejunum of Gata4 mutant mice23.
Our study identifies Gata4 as an epithelial transcriptional regulator crucial for the maintenance of physiological barrier integrity, as well as for the protection of the jejunal mucosa against epithelial injury. Few model systems are currently available to define the epithelial intrinsic contribution involved in proximal gut protection against inflammatory stimuli. For instance, specific transgenic mouse models with modified MHC class II molecules have been generated and showed to recapitulate some aspects of celiac disease pathogenesis when exposed to dietary gluten43. Upon our analyses, Gata4 deleted mice did not display classical signs of celiac disease associated signature, such as for instance, modification in the expression of IL-15 or activating natural killer receptor NKG2D gene transcripts (data not shown). Our findings open up on exploring whether Gata4 and its regulatory network are involved in the pathogenesis and/or the protection against environmental damage and inflammatory diseases of the proximal gastrointestinal tract.
Methods
Animals
Gata4loxP26 and 12.4KbVilCre27 mice were used to generate 12.4KbVilCre/Gata4+/+ (control) and 12.4KbVilCre/Gata4loxP/loxP (Gata4ΔIEC) mice on a pure C57BL/6J background. Mice were kept under pathogen free conditions and were tested negative for Helicobacter, Pasteurella and murine norovirus. Some of the mice were injected a single dose of indomethacin (Sigma-Aldrich Canada Co., Oakville, ON) (10 mg/kg body weight) intraperitoneally and based on the observed severity phenotype for mutant mice, sacrificed 24 h later for tissue samples. Mice were treated in accordance with a protocol reviewed and approved by the Institutional Animal Research Review Committee of the Université de Sherbrooke (approval ID number 102-10B). The study followed the standards and policies of the Canadian Council on Animal Care in sciences.
RNA isolation and qPCR analysis
Total RNA was isolated from jejunum, ileum and colon mouse biopsies and subjected to a DNase treatment according to the manufacturer’s instructions (Totally RNA kit, Life Technologies Inc., Burlington, ON). Reverse transcription and quantitative PCR (qPCR) were performed as described previously14,49 or were performed by the RNomics Platform at the Université de Sherbrooke (Sherbrooke, QC). Target expression was quantified relatively to porphobilinogen deaminase (PBGD) expression. Primer sequences used for qPCR are listed in Supplementary Table S4.
Electron microscopy
Mouse jejunum segments were prepared as reported before49. Ultramicrotome-prepared thin sections were contrasted with lead citrate and uranyl acetate and then observed on a Jeol 100 CX transmission electron microscope.
Intestinal permeability in vivo
Permeability was assessed with the fluorescent isothiocyanate (FITC)-labeled dextran method as described previously50. Mice were oral gavage with 60 mg/100g body weight of FITC-dextran (FD4, average molecular weight of 3,000–5,000, Sigma-Aldrich Canada Co., Oakville, ON) and sacrificed after 4 h. FITC concentration in the serum was quantified with a BioTek Synergy HT spectrometer plate reader (Winooski, VT) with excitation of the fluorophore at 492 nm and emission at 525 nm. Serum from mice not administered with FITC-dextran was used to determine the background.
Bacterial strain and mouse infections
Salmonella enterica serovar Typhimurium strain SB103 (invA) was grown overnight at 37 °C in LB supplemented with 100 μg/mL streptomycin. Inoculum was prepared in sterile HEPES 100 mM, NaCl 0.9%, pH 8.0. Mice were infected orally with 5 × 107 bacteria as previously described29, and sacrificed after 3 days. For bacterial counts, tissues were homogenized, followed by plating of serial dilutions in LB plates containing 100 μg/mL streptomycin. All infections experiments were done in duplicate with a total of 5–6 mice per group.
Immunostaining
Immunofluorescence and immunohistochemistry staining was performed as previously described51. Non-specific binding was blocked and antibodies were diluted in PBS/Triton 0.05% solution containing 2% BSA (Sigma-Aldrich Canada Co., Oakville, ON). The following antibodies were used at the indicated dilutions: anti-PCNA (1:1000, Abcam), anti-CD3 (1:200, Dako), anti-CD68 (1:500, Aviva), FITC-conjugated anti-rabbit IgG (1:300, Santa Cruz), Alexa 568-conjugated anti-mouse (1:400, Invitrogen). For claudin-2 and ZO-1 immunodetection, 5 μm thick OCT cryosections were fixed in 100% methanol for 10 min at −20 °C and further processed for incubations with antibodies. Immunohistochemistry staining (DAB kit, Dako) was performed following the manufacturer’s protocol.
Immunoblot analysis
Total protein extracts and western blots were performed as described previously51. The following antibodies were used: anti-claudin-2 (#51-6100, 1:500) (Invitrogen, Life Technologies Inc., Burlington, ON) and anti-β-actin (MAB1501R, 1:10,000) (EMD Millipore, Etobicoke, ON).
Chromatin immunoprecipitation assays
Chromatin immunoprecipitation assays (ChIP) were performed with the EZ-ChIP assay kit (EMD Millipore, Etobicoke, ON), according to the manufacturer’s instructions. The jejunum was harvested from mice, cut opened in fragments and incubated in 1% formaldehyde for 15 min at 20 °C. Jejunum fragments were then rinsed twice with ice cold PBS/Glycine buffer and mucosal isolation was performed by scraping. Enriched mucosal fractions were weighed and a total of 40 mg was used for each condition. Mucosal fractions were chemically crosslinked by the addition of formaldehyde (1% final concentration) for 10 min at 20 °C, then lysed and sonicated to solubilize and shear crosslinked DNA to an average length of 200 base pairs. The cell extract was pre-cleared with 40 μl Protein G magnetic beads (Life Technologies Inc, Burlington, ON) for 1 h and then, incubated at 4 °C overnight with 40 μl Protein G magnetic beads and corresponding antibodies: 4 μg of an isogenic immunoglobulin (Santa Cruz Biotechnology Inc., Santa Cruz, CA), 4 μg of GATA-4 affinity-purified polyclonal antibody (#SC-1237, Santa Cruz Biotechnology Inc., Santa Cruz, CA), 4 μg of anti-Histone H3 (tri methyl K4) purified polyclonal antibody (#ab8580, Abcam, Toronto, ON) or 4 μg of anti-trimethyl-Histone H3 (Lys27) purified polyclonal antibody (#07-449, EMD Millipore, Etobicoke, ON). Beads were washed twice with the provided buffers. Bound complexes were eluted from the beads by heating at 65 °C with occasional vortexing, and crosslinking was reversed by overnight incubation at 65 °C. A portion of the whole-cell extract DNA from the sonication step was also treated for crosslink reversal for input. Immunoprecipitated DNA and whole-cell extract DNA (1% input) were treated with proteinase K and RNaseA and then purified. Purified DNA was used as template for qPCR with a LightCycler apparatus V2.0 (Roche Diagnostics, Laval, QC). Sets of primers used to amplify GATA containing regions for Cldn2 gene promoter and for a negative region from the Il1β gene promoter are listed in Supplementary Table S5. Calculation of enrichment was performed using the 2-ΔΔCt method that normalized ChIP DNA to input DNA and included signals obtained from both wild-type and Gata4ΔIEC mouse jejunum extracts23.
DNA microarray and analysis
Probes for hybridization with Illumina BeadChips were generated from isolated jejunum RNA of three independent mice from both Gata4ΔIEC and control groups after 24 h of indomethacin treatment. The Illumina MouseWG-6 v2.0 Expression BeadChips were screened with the six generated probes via the McGill University and Génome Québec Innovation Center ( http://genomequebec.mcgill.ca). FlexArray version 1.6.1 was used for data analysis ( http://genomequebec.mcgill.ca/FlexArray). Genes were then filtered for up- or down-regulation of expression of a minimum of 2.0-fold and gene signature datasets analyzed by the Ingenuity Pathway Analysis tool ( www.ingenuity.com).
Additional Information
How to cite this article: Lepage, D. et al. Gata4 is critical to maintain gut barrier function and mucosal integrity following epithelial injury. Sci. Rep. 6, 36776; doi: 10.1038/srep36776 (2016).
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Material
Acknowledgments
The authors thank Pr. S.A. Duncan (Medical College of Wisconsin, Milwaukee, USA) for providing the Gata4loxP mouse line, the McGill University and Génome Québec Innovation Centre for DNA microarray services, the RNomics Platform at the Université de Sherbrooke for qPCR services and the Electron Microscopy & Histology Research Core of the FMSS at the Université de Sherbrooke for histology and phenotyping services. This work was supported by the Natural Sciences and Engineering Research Council of Canada grant number 262094 (FB). JB and JA were recipients of a FRQS fellowship. CA, NP, AM, FPG and FB are members of the FRQS-funded ⟪Centre de Recherche du CHUS⟫.
Footnotes
Author Contributions D.L., É.B., C.J., S.T., J.A. and J.M.B. designed and performed the experiments, and analysed the results. F.P.G., C.A., A.M., N.P. and F.B. designed and supervised the work. F.B. wrote the manuscript.
References
- Tan D. W. & Barker N. Intestinal stem cells and their defining niche. Current topics in developmental biology 107, 77–107, doi: 10.1016/B978-0-12-416022-4.00003-2 (2014). [DOI] [PubMed] [Google Scholar]
- Turner J. R. Intestinal mucosal barrier function in health and disease. Nature reviews. Immunology 9, 799–809, doi: 10.1038/nri2653 (2009). [DOI] [PubMed] [Google Scholar]
- Laukoetter M. G., Bruewer M. & Nusrat A. Regulation of the intestinal epithelial barrier by the apical junctional complex. Current opinion in gastroenterology 22, 85–89, doi: 10.1097/01.mog.0000203864.48255.4f (2006). [DOI] [PubMed] [Google Scholar]
- Van Itallie C. M. & Anderson J. M. Claudins and epithelial paracellular transport. Annu Rev Physiol 68, 403–429 (2006). [DOI] [PubMed] [Google Scholar]
- Tamura A. & Tsukita S. Paracellular barrier and channel functions of TJ claudins in organizing biological systems: advances in the field of barriology revealed in knockout mice. Seminars in cell & developmental biology 36, 177–185, doi: 10.1016/j.semcdb.2014.09.019 (2014). [DOI] [PubMed] [Google Scholar]
- Amasheh S. et al. Claudin-2 expression induces cation-selective channels in tight junctions of epithelial cells. Journal of cell science 115, 4969–4976 (2002). [DOI] [PubMed] [Google Scholar]
- Tamura A. et al. Loss of claudin-15, but not claudin-2, causes Na+ deficiency and glucose malabsorption in mouse small intestine. Gastroenterology 140, 913–923, doi: 10.1053/j.gastro.2010.08.006 (2011). [DOI] [PubMed] [Google Scholar]
- Zeissig S. et al. Changes in expression and distribution of claudin 2, 5 and 8 lead to discontinuous tight junctions and barrier dysfunction in active Crohn’s disease. Gut 56, 61–72, doi: 10.1136/gut.2006.094375 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luettig J., Rosenthal R., Barmeyer C. & Schulzke J. D. Claudin-2 as a mediator of leaky gut barrier during intestinal inflammation. Tissue barriers 3, e977176, doi: 10.4161/21688370.2014.977176 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suh E. & Traber P. G. An intestine-specific homeobox gene regulates proliferation and differentiation. Molecular and cellular biology 16, 619–625 (1996). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao N. & Kaestner K. H. Cdx2 regulates endo-lysosomal function and epithelial cell polarity. Genes & development 24, 1295–1305, doi: 10.1101/gad.1921510 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao N., White P. & Kaestner K. H. Establishment of intestinal identity and epithelial-mesenchymal signaling by Cdx2. Developmental cell 16, 588–599, doi: 10.1016/j.devcel.2009.02.010 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lussier C. R., Babeu J. P., Auclair B. A., Perreault N. & Boudreau F. Hepatocyte nuclear factor-4alpha promotes differentiation of intestinal epithelial cells in a coculture system. American journal of physiology. Gastrointestinal and liver physiology 294, G418–G428, doi: 10.1152/ajpgi.00418.2007 (2008). [DOI] [PubMed] [Google Scholar]
- Babeu J. P., Darsigny M., Lussier C. R. & Boudreau F. Hepatocyte nuclear factor 4alpha contributes to an intestinal epithelial phenotype in vitro and plays a partial role in mouse intestinal epithelium differentiation. American journal of physiology. Gastrointestinal and liver physiology 297, G124–G134, doi: 10.1152/ajpgi.90690.2008 (2009). [DOI] [PubMed] [Google Scholar]
- Boudreau F. et al. Hepatocyte nuclear factor-1 alpha, GATA-4, and caudal related homeodomain protein Cdx2 interact functionally to modulate intestinal gene transcription. Implication for the developmental regulation of the sucrase-isomaltase gene. The Journal of biological chemistry 277, 31909–31917, doi: 10.1074/jbc.M204622200 (2002). [DOI] [PubMed] [Google Scholar]
- Sumi K. et al. Cooperative interaction between hepatocyte nuclear factor 4 alpha and GATA transcription factors regulates ATP-binding cassette sterol transporters ABCG5 and ABCG8. Molecular and cellular biology 27, 4248–4260, doi: 10.1128/MCB.01894-06 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Battle M. A. et al. GATA4 is essential for jejunal function in mice. Gastroenterology 135, 1676–1686 e1671, doi: 10.1053/j.gastro.2008.07.074 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosse T. et al. Gata4 is essential for the maintenance of jejunal-ileal identities in the adult mouse small intestine. Molecular and cellular biology 26, 9060–9070, doi: 10.1128/MCB.00124-06 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aronson B. E. et al. GATA4 represses an ileal program of gene expression in the proximal small intestine by inhibiting the acetylation of histone H3, lysine 27. Biochimica et biophysica acta 1839, 1273–1282, doi: 10.1016/j.bbagrm.2014.05.018 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beuling E. et al. GATA4 mediates gene repression in the mature mouse small intestine through interactions with friend of GATA (FOG) cofactors. Developmental biology 322, 179–189, doi: 10.1016/j.ydbio.2008.07.022 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beuling E. et al. Conditional Gata4 deletion in mice induces bile acid absorption in the proximal small intestine. Gut 59, 888–895, doi: 10.1136/gut.2009.204990 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Out C. et al. Gut microbiota inhibit Asbt-dependent intestinal bile acid reabsorption via Gata4. Journal of hepatology 63, 697–704, doi: 10.1016/j.jhep.2015.04.030 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lepage D. et al. Identification of GATA-4 as a novel transcriptional regulatory component of regenerating islet-derived family members. Biochimica et biophysica acta , doi: 10.1016/j.bbagrm.2015.10.011 (2015). [DOI] [PubMed] [Google Scholar]
- Cash H. L., Whitham C. V., Behrendt C. L. & Hooper L. V. Symbiotic bacteria direct expression of an intestinal bactericidal lectin. Science 313, 1126–1130, doi: 10.1126/science.1127119 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ose T. et al. Reg I-knockout mice reveal its role in regulation of cell growth that is required in generation and maintenance of the villous structure of small intestine. Oncogene 26, 349–359, doi: 10.1038/sj.onc.1209799 (2007). [DOI] [PubMed] [Google Scholar]
- Watt A. J., Battle M. A., Li J. & Duncan S. A. GATA4 is essential for formation of the proepicardium and regulates cardiogenesis. Proceedings of the National Academy of Sciences of the United States of America 101, 12573–12578, doi: 10.1073/pnas.0400752101 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madison B. B. et al. Cis elements of the villin gene control expression in restricted domains of the vertical (crypt) and horizontal (duodenum, cecum) axes of the intestine. The Journal of biological chemistry 277, 33275–33283 (2002). [DOI] [PubMed] [Google Scholar]
- Galan J. E. & Curtiss R. 3rd. Distribution of the invA, -B, -C, and -D genes of Salmonella typhimurium among other Salmonella serovars: invA mutants of Salmonella typhi are deficient for entry into mammalian cells. Infection and immunity 59, 2901–2908 (1991). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romain G. et al. Enterohepatic bacterial infections dysregulate the FGF15-FGFR4 endocrine axis. BMC microbiology 13, 238, doi: 10.1186/1471-2180-13-238 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escaffit F., Boudreau F. & Beaulieu J. F. Differential expression of claudin-2 along the human intestine: Implication of GATA-4 in the maintenance of claudin-2 in differentiating cells. Journal of cellular physiology 203, 15–26, doi: 10.1002/jcp.20189 (2005). [DOI] [PubMed] [Google Scholar]
- Su L. et al. Targeted epithelial tight junction dysfunction causes immune activation and contributes to development of experimental colitis. Gastroenterology 136, 551–563, doi: 10.1053/j.gastro.2008.10.081 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pastorelli L., De Salvo C., Mercado J. R., Vecchi M. & Pizarro T. T. Central role of the gut epithelial barrier in the pathogenesis of chronic intestinal inflammation: lessons learned from animal models and human genetics. Frontiers in immunology 4, 280, doi: 10.3389/fimmu.2013.00280 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vijay-Kumar M. et al. Deletion of TLR5 results in spontaneous colitis in mice. The Journal of clinical investigation 117, 3909–3921, doi: 10.1172/JCI33084 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nenci A. et al. Epithelial NEMO links innate immunity to chronic intestinal inflammation. Nature 446, 557–561, doi: 10.1038/nature05698 (2007). [DOI] [PubMed] [Google Scholar]
- Kajino-Sakamoto R. et al. Enterocyte-derived TAK1 signaling prevents epithelium apoptosis and the development of ileitis and colitis. Journal of immunology 181, 1143–1152 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donaldson G. P., Lee S. M. & Mazmanian S. K. Gut biogeography of the bacterial microbiota. Nature reviews. Microbiology 14, 20–32, doi: 10.1038/nrmicro3552 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verdu E. F., Galipeau H. J. & Jabri B. Novel players in coeliac disease pathogenesis: role of the gut microbiota. Nature reviews. Gastroenterology & hepatology 12, 497–506, doi: 10.1038/nrgastro.2015.90 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X. et al. Microbial products induce claudin-2 to compromise gut epithelial barrier function. PloS One 8, e68547, doi: 10.1371/journal.pone.0068547 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuse M., Furuse K., Sasaki H. & Tsukita S. Conversion of zonulae occludentes from tight to leaky strand type by introducing claudin-2 into Madin-Darby canine kidney I cells. The Journal of cell biology 153, 263–272 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace J. L. NSAID gastropathy and enteropathy: distinct pathogenesis likely necessitates distinct prevention strategies. British journal of pharmacology 165, 67–74, doi: 10.1111/j.1476-5381.2011.01509.x (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y., Dial E. J., Doyen R. & Lichtenberger L. M. Effect of indomethacin on bile acid-phospholipid interactions: implication for small intestinal injury induced by nonsteroidal anti-inflammatory drugs. American journal of physiology. Gastrointestinal and liver physiology 298, G722–G731, doi: 10.1152/ajpgi.00387.2009 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace J. L. et al. Proton pump inhibitors exacerbate NSAID-induced small intestinal injury by inducing dysbiosis. Gastroenterology 141, 1314–1322, 1322 e1311-1315, doi: 10.1053/j.gastro.2011.06.075 (2011). [DOI] [PubMed] [Google Scholar]
- Meresse B., Malamut G. & Cerf-Bensussan N. Celiac disease: an immunological jigsaw. Immunity 36, 907–919, doi: 10.1016/j.immuni.2012.06.006 (2012). [DOI] [PubMed] [Google Scholar]
- Dessing M. C. et al. The calcium-binding protein complex S100A8/A9 has a crucial role in controlling macrophage-mediated renal repair following ischemia/reperfusion. Kidney international 87, 85–94, doi: 10.1038/ki.2014.216 (2015). [DOI] [PubMed] [Google Scholar]
- Kerkhoff C. et al. Novel insights into the role of S100A8/A9 in skin biology. Experimental dermatology 21, 822–826, doi: 10.1111/j.1600-0625.2012.01571.x (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mott J. D. & Werb Z. Regulation of matrix biology by matrix metalloproteinases. Current opinion in cell biology 16, 558–564, doi: 10.1016/j.ceb.2004.07.010 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gyenge M., Amagase K., Kunimi S., Matsuoka R. & Takeuchi K. Roles of pro-angiogenic and anti-angiogenic factors as well as matrix metalloproteinases in healing of NSAID-induced small intestinal ulcers in rats. Life sciences 93, 441–447, doi: 10.1016/j.lfs.2013.07.021 (2013). [DOI] [PubMed] [Google Scholar]
- van Pel M. et al. Serpina1 is a potent inhibitor of IL-8-induced hematopoietic stem cell mobilization. Proceedings of the National Academy of Sciences of the United States of America 103, 1469–1474, doi: 10.1073/pnas.0510192103 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boudreau F. et al. Loss of cathepsin L activity promotes claudin-1 overexpression and intestinal neoplasia. FASEB journal : official publication of the Federation of American Societies for Experimental Biology 21, 3853–3865, doi: 10.1096/fj.07-8113com (2007). [DOI] [PubMed] [Google Scholar]
- Coulombe G. et al. Epithelial tyrosine phosphatase SHP-2 protects against intestinal inflammation in mice. Molecular and cellular biology 33, 2275–2284, doi: 10.1128/MCB.00043-13 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darsigny M. et al. Loss of hepatocyte-nuclear-factor-4alpha affects colonic ion transport and causes chronic inflammation resembling inflammatory bowel disease in mice. PloS One 4, e7609, doi: 10.1371/journal.pone.0007609 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.