Dear Editors
Cognitive impairment associated with schizophrenia (CIAS) is related to the functional outcome of the illness. Oxytocin (OT), a “social” neuropeptide, has recently been linked with “non-social” cognition, such as cognitive flexibility, spatial and episodic memory (Chini et al., 2014) and with immediate and short-term verbal memory in schizophrenia (Feifel et al., 2012). Working memory (WM) is a central cognitive deficit of schizophrenia and a target domain for the development of new CIAS treatments (Barch and Smith, 2008). In the present study, we investigated the effects of a single OT dose on WM. We included the Digit Span (DS) as secondary outcome measure in a study implemented in our laboratory (Averbeck et al., 2012). DS measures two WM components: a) information storage and maintenance (Digits Forward - DF) and b) information maintenance plus manipulation, i.e. the “executive component” of WM (Digits Backward - DB), which is more sensitive to WM deficits in schizophrenia (Kim et al., 2004). We also included Digit Symbol Coding (DSC), a processing speed test.
The design details can be found elsewhere (Averbeck et al., 2012). Briefly, we conducted a double-blind placebo-controlled, crossover study. 21 participants who met DSM-IV criteria for schizophrenia (mean illness duration 15.157±6.61 years, NART IQ 100.79±9.59, education years 12.19±2.7) and were male, 18–50 years old (mean age 38.19±8.04), clinically stable for a year prior to study entry (PANSS total score 58.86±15.13), on atypical (n=19) or low-doses of typical (n=2) antipsychotics (daily mean chlorpromazine equivalent 469.81±351.44) were included in the study and were randomly allocated to a single intranasal dose of 24 IU OT (Syntocinon, Novarits) or saline placebo [(n1=11 received placebo and n2=10 OT during the first visit – 7 or 8 days between the two visits (mean=7.33±0.48)]. There were no significant differences in demographic and clinical measures between n1 and n2 groups. Testing begun 50 min after OT/placebo administration. No adverse events occurred.
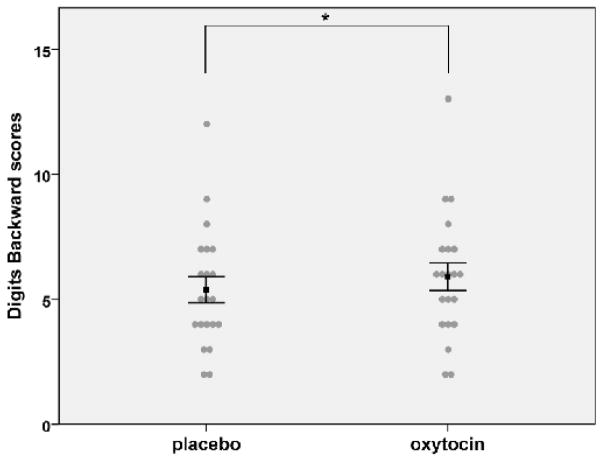
We used repeated measures ANOVA with drug as the within and order of drug administration as the between subject factor (alpha level=0.05). Effect sizes were measured using Cohen’s formula for one sample comparisons (Cohen’dz). We found a significant main effect of drug on DB [F (1, 19)=7.354, p=0.014] (DB under placebo=5.38±2.42 and OT=5.90±2.55). Post-hoc analysis (paired t-tests, p after Bonferroni correction=0.016) showed significant DB improvement under OT, irrespective of the visit OT was taken [t (20)=−2.75, p=0.012, Cohen’s dz= 0.6] (Figure 1). No significant order effect [F (1, 19) =0.814, p=0.378] or drug×order interaction [F (1, 19)=1.272, p=0.273] was found. No significant main effect of drug [F (1, 19)=0.188, p=0.669], order [F (1, 19)=0.968, p=0.338] or drug×order interaction [F (1, 19)=1.340, p=0.066] was found for DF (DF under placebo=9.95±2.56 and OT=9.81±2.87). No significant drug [F (1, 16)=0.238, p=0.632] or order effects [F (1, 16)=0.638, p=0.436] were found for DSC (DSC under placebo=50.94± 15.8 and OT=50.33±18.09). A significant drug×order interaction [F (1, 16)=11.067, p=0.004] was found. Post-hoc separate comparison of O/P and P/O groups on the DSC (paired t-tests) showed that the drug × time interaction was due to significant DSC improvement during the 2nd session in the O/P group [t (8)=3.25, p=0.012], while there was no significant difference between the two sessions in the P/O group [t (8)= −1.753, p=0.118]; this most likely reflects OT carryover effects from the 1st session in the O/P group and not practice effects. Additional post-hoc between-groups comparison of the data from the 1st session alone showed no significant OT effects on DSC [t (16)= 0.86, p=0.4].
Figure 1.
Effects of oxytocin on Digits Backward score
Oxytocin significantly increased mean Digits Backward score. *Denotes significant difference from placebo at p<0.016. Error bars represent ±1 standard error
A single OT dose improved the “executive component” of WM. WM is highly dependent on dopaminergic neurotransmission in the prefrontal cortex (PFC), which is dysregulated in schizophrenia. OT’s involvement in WM is possible, since OT receptors have been identified in the PFC in animal studies (Smeltzer et al., 2006) and OT alters central dopaminergic responses (Baskerville et al., 2010). Additionally, GABA dysregulation is implicated in WM impairment in schizophrenia (Lewis et al., 2005) and OT enhances GABAergic neurotransmission (Huber et al., 2005).
In a previous study in schizophrenia, repeated OT administration showed no effects on WM, as measured by Letter Number Sequencing task (LNS) (Feifel et al., 2012). Possible explanations for the different results between our study and that of Feifel et al. (2012) may include the different dose and duration of OT treatment. Animal studies suggest an inverted U-shaped dose-response curve of OT and also differences between the effects of single and chronic administration of OT (Chini et al., 2014). Additionally, LNS and DS have different psychometric properties with measures of processing speed and visual spatial WM contributing to LNS performance (Crowe, 2000).
Our results suggest that OT may have the potential to improve WM in schizophrenia and given the association between cognition and the functional outcome of schizophrenia, replication and extension of our findings is warranted in larger trials with repeated OT administration.
References
- Averbeck BB, Bobin T, Evans S, Shergill SS. Emotion recognition and oxytocin in patients with schizophrenia. Psychol Med. 2012;42(2):259–266. doi: 10.1017/S0033291711001413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barch DM, Smith E. The cognitive neuroscience of working memory: relevance to CNTRICS and schizophrenia. Biol Psychiatry. 2008;64(1):11–17. doi: 10.1016/j.biopsych.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baskerville TA, Douglas AJ. Dopamine and oxytocin interactions underlying behaviors: Potential contributions to behavioral disorders. CNS Neurosci Ther. 2010;16(3):e92–123. doi: 10.1111/j.1755-5949.2010.00154.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chini B, Leonzino M, Braida D, Sala M. Learning about oxytocin: Pharmacologic and behavioural issues. Biol Psychiatry. 2014;76(5):360–366. doi: 10.1016/j.biopsych.2013.08.029. [DOI] [PubMed] [Google Scholar]
- Crowe SF. Does the letter number sequencing task measure anything more than digit span? Assessment. 2000;7(2):113–117. doi: 10.1177/107319110000700202. [DOI] [PubMed] [Google Scholar]
- Feifel D, Macdonald K, Cobb P, Minassian A. Adjunctive intranasal oxytocin improves verbal memory in people with schizophrenia. Schizophr Res. 2012;139(1–3):207–210. doi: 10.1016/j.schres.2012.05.018. [DOI] [PubMed] [Google Scholar]
- Huber D, Veinante P, Stoop R. Vasopressin and oxytocin excite distinct neuronal populations in the central amygdala. Science. 2005;308(5719):245–248. doi: 10.1126/science.1105636. [DOI] [PubMed] [Google Scholar]
- Kim J, Glahn DC, Nuechterlein KH, Cannon TD. Maintenance and manipulation of information in schizophrenia: further evidence for impairment in the central executive component of working memory. Schizophr Res. 2004;68(2–3):173–187. doi: 10.1016/S0920-9964(03)00150-6. [DOI] [PubMed] [Google Scholar]
- Lewis DA, Hashimoto T, Volk DW. Cortical inhibitory neurons and schizophrenia. Nature Rev Neurosci. 2005;6(4):312–324. doi: 10.1038/nrn1648. [DOI] [PubMed] [Google Scholar]
- Smeltzer MD, Curtis JT, Aragona T, Brandon BJ, Wang Z. Dopamine, oxytocin, and vasopressin receptor binding in the medial prefrontal cortex of monogamous and promiscuous voles. Neurosci Lett. 2006;394(2):146–151. doi: 10.1016/j.neulet.2005.10.019. [DOI] [PubMed] [Google Scholar]