Graphical Abstract
Keywords: cellular oxidative stress, gold nanoparticle, green fluorescent protein, chemically modified protein, hydrogen peroxide
Hydrogen peroxide is a product of cellular respiration and is one of the most important reactive oxygen species endogenously generated by cells.[1] H2O2 plays fundamental roles in multiple physiological and pathological processes, with the controlled generation of H2O2 essential to maintaining homeostasis and hence cell survival.[2] An overproduction of H2O2 results in oxidative stress that causes a range of diseases, including neurodegeneration,[3] diabetes,[4] and cancer.[5] The importance of H2O2 in biological processes necessitates the development of real-time sensing platforms for H2O2. Recently, fluorescent probes have been designed for H2O2 sensing, including small molecule fluorescent sensors,[6] synthetic cell-penetrating peptides,[7] genetically encoded fluorescent proteins,[8] and nanozymes.[9] However, the transient nature of ROS and the similarity of H2O2 with other ROS in terms of size and oxidative properties make peroxide sensing challenging.
Hybrid nanomaterials integrating synthetic nanomaterials with biomolecules including proteins and nucleic acids are important tools for chemical and biosensing.[10] These systems incorporate key elements of biomolecular function with the unique physical structural attributes of nanomaterials.[11] A substantial body of research exists on nucleic acid and peptide-functionalized nanomaterials, with protein-based systems rapidly emerging.[12] These systems generally use native proteins for sensing applications, providing recognition elements (e.g. antibodies) as well as enzymatic function. Genetic engineering of proteins provides a tool for creating NP-protein sensors with improved sensing selectivity and stability,[13] however the complicated genetic fusion process and limited functional group options for proteins provide limitations to this approach.
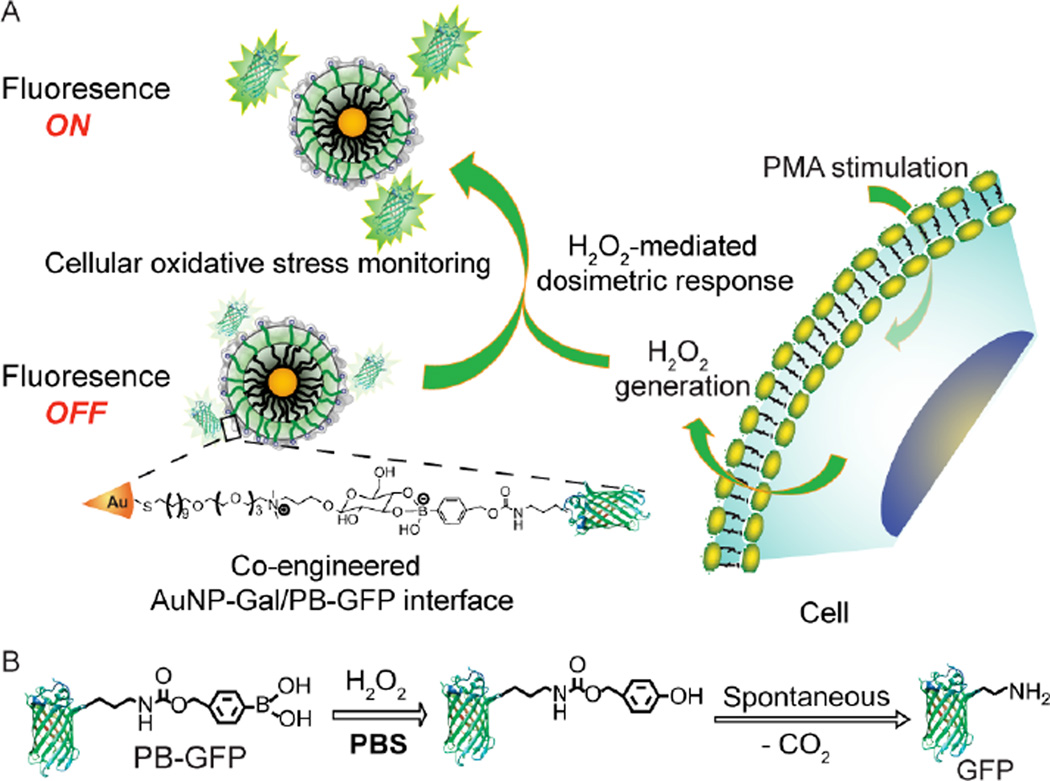
Chemical modification of proteins is an appealing alternative for endowing proteins with useful non-natural functionality and tunable properties.[14] We reasoned that integrating the strategies of chemically modified proteins with nanoparticle surface engineering would yield hybrid nanomaterials with synergetic functionality. We report here the creation of H2O2-responsive NP-protein conjugates and their ability to selectively monitor endogenous H2O2 production in live cells. In this study, we modify green fluorescent protein (GFP) with boronate functionality at the primary amine of lysine residues to provide a family of phenylboronate-functionalized GFPs (PB-GFPs). These proteins react with galactose-functionalized gold nanoparticles (AuNP-Gal) via boronate ester formation (Figure 1). By tuning the extent of boronate modification of GFP through convenient chemical modifications, we demonstrate that the conjugation of PB-GFP with AuNP-Gal quenches the fluorescence of GFP depending on the number of boronate moieties conjugated to GFP. The PB-GFP/AuNP-Gal functionalization is peroxide-responsive: H2O2 catalyzes the bioorthogonal oxidation of boronate.[14, 1a] This oxidation disassembles the complex with concomitant restoration of GFP fluorescence (Figure 1), providing selective GFP-based on-off H2O2 detection for real time and in situ cellular oxidative stress monitoring.
Figure 1.
Chemically engineered AuNP/PB-GFP interface for H2O2 sensing. A) Schematic illustrating the interfacing of AuNP-Gal with PB-GFP, and phorbol 12-myristate-13-acetate (PMA) stimulated in situ H2O2 generation modulated GFP release from the AuNP/PB-GFP interface. B) Reaction mechanism of PB-GFP with H2O2 catalyzed bioorthogonal PB cleavage.
Boronate-functionalized GFP was prepared through the reaction of GFP with 4-nitrophenyl 4-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)benzyl carbonate (NBC) at different molar ratios (see experimental details in Supporting Information). The number of phenylboronic acid (PB) conjugated to GFP was determined using matrix-assisted laser desorption/ionization-time of flight (MALDI-TOF) mass spectroscopy (Figure S1, Supporting Information). In this study, GFP conjugated with 5, 9, and 20 PB per protein molecule, designated as PB5-GFP, PB9-GFP, and PB20-GFP, respectively, were complexed with gold nanoparticles (2 nm core) functionalized with galactose ligands (AuNP-Gal) that were prepared through the place-exchange reaction of 1-pentanethiol protected gold nanoparticles (AuNP-C5) and galactose ligands (Supporting Information).15 The AuNPs were characterized using transmission electron microscopy (TEM) and dynamic light scattering (DLS) analysis (Figure S2, Supporting Information). In addition, GFP without PB conjugation (GFP) was used as a negative control to evaluate the effect of PB conjugation on enhancing protein-NP interaction.
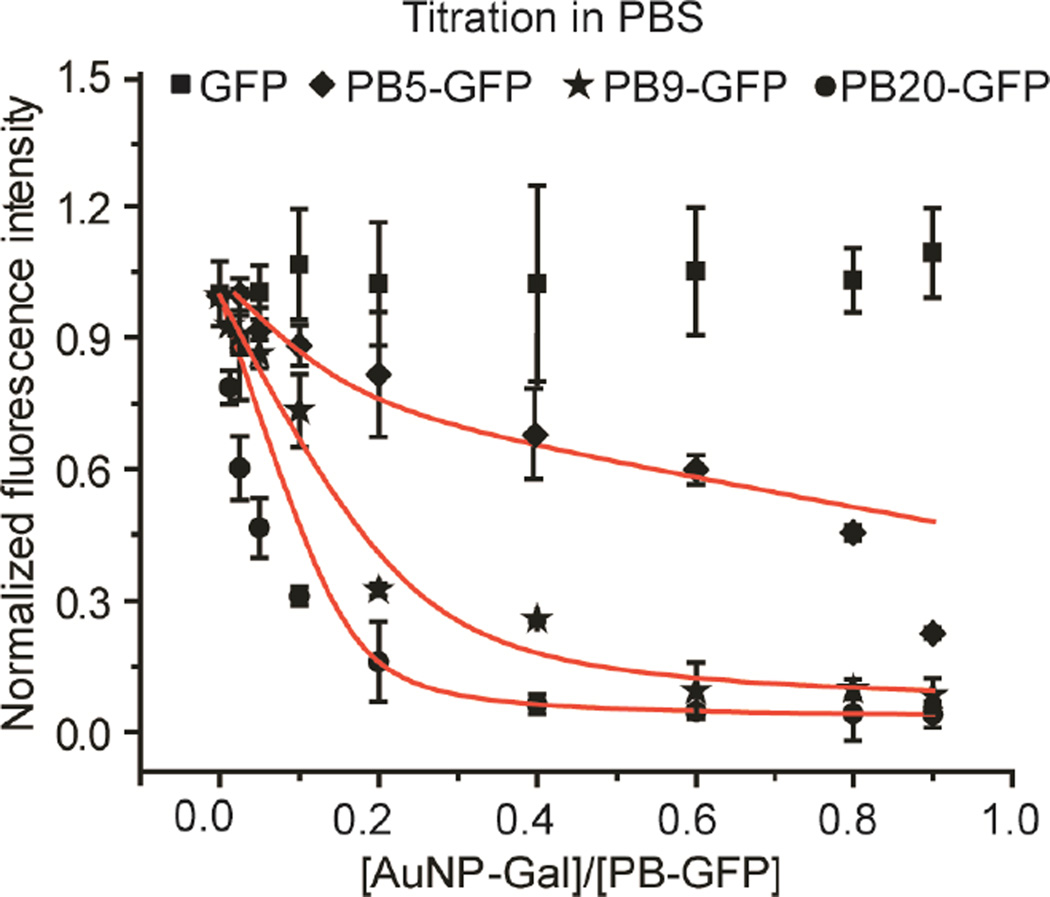
The attachment between AuNP-Gal and PB-GFP was quantified by titrating GFP with AuNP and measuring the GFP fluorescence intensity change. The formation of a stable AuNP/PB-GFP interface efficiently quenches GFP fluorescence, providing straightforward observation of binding. As shown in Figure 2, the fluorescence of GFP solution (100 nM protein in PBS) shows little change with the addition of AuNP, indicating little binding between GFP and AuNP, in the presence of high concentration of salt (137 mM NaCl). In contrast, PB-GFP fluorescence was quenched with the addition of AuNP, depending on the number of PB units conjugated to GFP and the amount of AuNP added to PB-GFP. For example, PB20-GFP fluorescence was quenched up to 90% at an NP to protein ratio of 0.2, while 18% and 70% GFP emission quenching was observed for PB5-GFP and PB9-GFP respectively under the same conditions. This enhanced quenching indicates PB20-GFP protein has a stronger binding affinity with AuNP than that of PB5-GFP and PB9-GFP. The affinity and the Stern-Volmer constant of AuNP-Gal and PB20-GFP are substantially higher than PB5-GFP and PB9-GFP, as determined by the GFP fluorescence titration curve (Table S1 and Figure S3, Supporting Information)
Figure 2.
Fluorescence titration of PB-GFP with AuNP-Gal. 100 nM protein was mixed with increased amounts of AuNP-Gal in PBS, and after incubation for 15 min, the fluorescence intensity of GFP was recorded. GFP fluorescence (λex = 475 nm, λem = 510 nm) in the presence of AuNP-Gal was normalized to that without AuNP-Gal addition. Each titration experiment was performed in three replicates. The red solid lines represent the best curve fitting using the previously reported method.[16]
We previously demonstrated that PB modified proteins are ROS-responsive, as hydrogen peroxide can cleave the boronate from the conjugated proteins through a bioorthogonal oxidization reaction.[14, 1a] We confirmed the ROS-responsive nature of PB-GFP by treating PB20-GFP protein with H2O2, and characterizing PB cleavage using MALDI-TOF. As shown in Figure S4 (Supporting Information), MALDI-TOF analysis indicated that the treatment of PB20-GFP (30 μM protein) with 30 mM H2O2 resulted in a loss of fourteen PB groups from PB20-GFP. The disassembly of the AuNP/PB-GFP by H2O2 was then followed using fluorescence spectroscopy. As shown in Figure 3, the attachment of AuNP (25 nM) to PB20-GFP (100 nM protein in PBS) quenched GFP fluorescence by 90%. Treatment of the complex with 1 mM H2O2 for 30 min resulted in essentially complete restoration of GFP fluorescence. As shown in Figure S5 (Supporting Information), the fluorescence of AuNP/PB20-GFP complex (100 nM GFP and 25 nM AuNP-Gal) increased shortly after adding 1 mM H2O2, no further GFP fluorescence changed was observed after 25 minutes of incubation, indicating PB20-GFP protein was completely released from AuNP. The fast and dynamic process of the H2O2-regulated AuNP/PB20-GFP interface prompted us to study its potential application as an H2O2 assay.
Figure 3.
Fluorescence intensity of PB20-GFP, and AuNP-Gal/PB20-GFP complexes before and after addition of H2O2. The AuNP-Gal/PB20-GFP conjugates were prepared by incubating PB20-GFP (100 nM) with a fixed PB20-GFP to AuNP-Gal ratio (4:1) for 15 min. All GFP fluorescence values were presented relative to the PB20-GFP control. Error bar represents the standard deviation of three independent studies. Photograph showing fluorescence of PB20-GFP, AuNP-Gal/PB20-GFP complexes, and AuNP-Gal/PB20-GFP complexes upon addition of H2O2 under ultraviolet light.
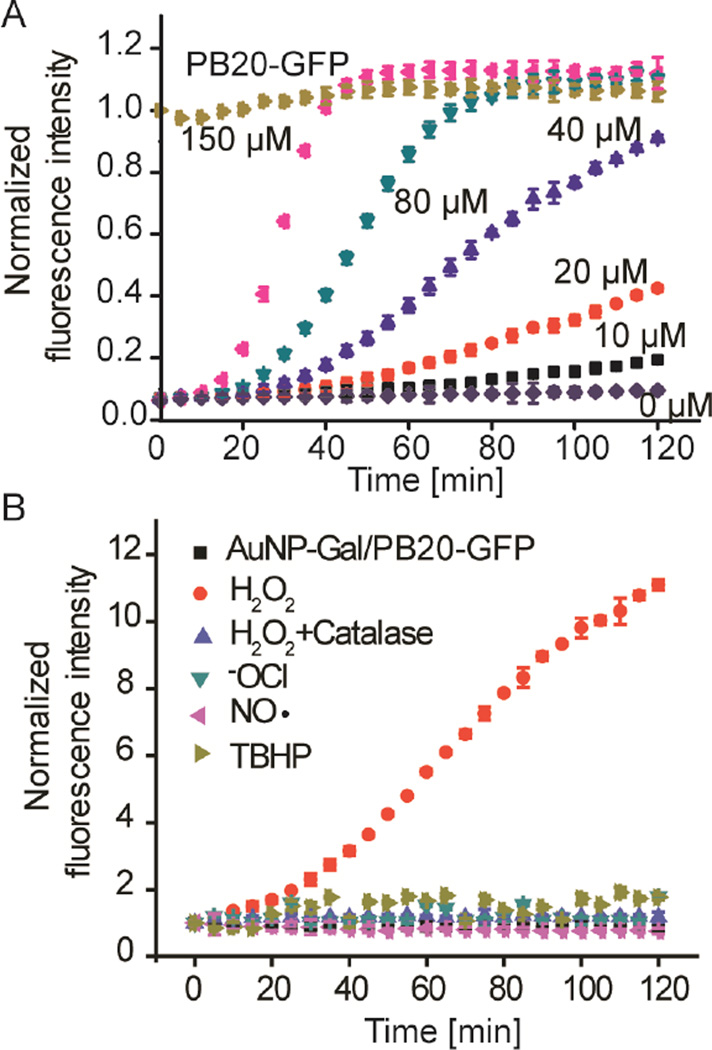
Novel sensing platforms that discriminate different types of ROS and are capable of real time monitoring of ROS generation are important to study the physiological role of ROS and develop new disease diagnostic tools.[17] We evaluated the capability of the AuNP/PB20-GFP complex for sensitive and selective sensing of H2O2. As shown in Figure 4A, treatment of AuNP/PB20-GFP complex (100 nM PB20-GFP and 25 nM AuNP-Gal mixed in PBS containing 10% fetal bovine serum) with 20 μM H2O2, restored GFP fluorescence gradually within 2 h of incubation. Fast restoration of GFP fluorescence was observed for AuNP/ PB20-GFP complex treated with a higher concentration of H2O2. For example, 150 μM H2O2 restored GFP fluorescence to more than 90% after 40 minutes of incubation. The response of the AuNP/ PB20-GFP complex is very selective toward H2O2 (Figure 4B). The addition of a variety of biologically relevant ROS, including hypochlorite (ClO-), H2O2, tert-butyl hydroperoxide (TBHP), nitroxide radical (NO·), and H2O2 in the presence of catalase (which acts to degrade H2O2), generated no perceptible response from the complex.
Figure 4.
H2O2 concentration-dependent and selective modulation of the AuNP-Gal/PB20-GFP interface. A) The fluorescence response of AuNP-Gal/PB20-GFP (100 nM PB20-GFP) upon the addition of increasing concentrations of H2O2 in PBS containing 10% FBS. The fluorescence response was presented relative to the PB20-GFP control. B) Time-dependent fluorescence response of AuNP-Gal/ PB20-GFP (100 nM PB20-GFP) upon addition of H2O2 (50 μM) or other ROS (100 μM) as indicated in PBS containing 10% FBS. Catalase (0.5 mg/mL) was added to verify the role of H2O2 in modulating AuNP/PB20-GFP interface. The fluorescence response was presented relative to the AuNP-Gal/ PB20-GFP controls.
We next explored the utility of AuNP/PB20-GFP complex for real time monitoring of cellular oxidative stress. We first verified the use of the AuNP/PB20-GFP interface for H2O2 sensing in cell culture medium. To this end, human T lymphocyte Jurkat cells were spiked with different concentrations of H2O2, followed by an incubation with AuNP/PB20-GFP complex (100 nM PB20-GFP and 25 nM AuNP-Gal) and PB20-GFP fluorescence measurement. As shown in Figure 5A, Jurkat cells alone had no effect on disassembling the AuNP/PB20-GFP complex during the 2h of incubation, indicating the high stability of AuNP/PB20-GFP interface in biological media. The addition of 2.5 μM H2O2 resulted in a noticeable increase in fluorescence within 2h, while the GFP fluorescence was 2.5 times higher for AuNP/PB20-GFP complex spiked with 40 μM H2O2 (Figure 5A). Additionally, the GFP fluorescence is linearly correlated to the concentration of H2O2 spiked to the cell culture medium in the range of 2.5 to 40 μM (Figure S6, Supporting Information).
Figure 5.
Detection of H2O2 using the AuNP-Gal/PB20-GFP in cellular environment. (a) Time-dependent fluorescence response of AuNP-Gal/PB20-GFP complex (100 nM of PB20-GFP) added to Jurkat cells spiked with different concentrations of H2O2. The fluorescence response was presented relative to the AuNP-Gal/PB20-GFP controls. (b) The fluorescence response of AuNP-Gal/PB20-GFP (100 nM of PB20-GFP) incubated with Jurkat cells stimulated with PMA (0.5 μM), or incubated with catalase (0.5 mg/mL) and PMA (0.5 μM) simultaneously. All fluorescence values were presented relative to the AuNP-Gal/PB20-GFP controls. Error bar represents the standard deviation of three independent studies.
We next studied the capability of the AuNP/PB20-GFP interface to monitor endogenous H2O2 generated by Jurkat cells in the presence of the ROS stimulator, phorbol 12-myristate-13-acetate (PMA).[7, 14] PMA treatment activates NAPDH oxidase and induces the generation of superoxide, which is converted to ROS species including H2O2.[7] As shown in Figure 5B, Jurkat cells pretreated with 0.5 μM PMA before AuNP/PB20-GFP complex (100 nM PB20-GFP and 25 nM AuNP-Gal) incubation resulted in a significant enhancement of GFP fluorescence, compared to the cells without PMA stimulation. In addition, when 0.5 mg/mL catalase was simultaneously added to PMA-stimulated Jurkat cells, no GFP fluorescence enhancement was observed due to consumption of the generated peroxide. By calibrating the GFP fluorescence of PMA-stimulated Jurkat cells to that of the AuNP/PB20-GFP complex in the presence of exogenous H2O2 after 120 minutes incubation, we estimated that the PMA-stimulation generated H2O2 at a rate of 0.49 nmol/104 cells/h, consistent with previous reports.[7]
In summary, we have co-engineered an NP-protein complex to provide an ROS-responsive system for real time H2O2 sensing and monitoring of cellular oxidative stress. This complex was created and modulated by the complexation between galactose-decorated gold nanoparticles and boronate modified protein. The assembled AuNP/PB-GFP interface is highly stable under a physiological environment, with GFP fluorescence generated by H2O2, providing timely biological dose estimates. The responsiveness of this system demonstrates the utility of co-engineering for creating functional and responsive synthetic-biological hybrid nanomaterials, a strategy that can be applied to many biological and physiological challenges.
Supplementary Material
Acknowledgments
This work was supported by grants from the NIH (GM077173) to V. M. R., and National Science Foundation (DMR 1452122) to Q. X.
Footnotes
Supporting Information
Supporting Information is available from the Wiley Online Library or from the author.
Contributor Information
Ying Jiang, Department of Chemistry, University of Massachusetts Amherst, 710 North Pleasant Street, Amherst, MA 01003, USA.
Dr. Ming Wang, Department of Biomedical Engineering, Tufts University, 4 Colby Street, Medford, Massachusetts, 02115, USA
Joseph Hardie, Department of Chemistry, University of Massachusetts Amherst, 710 North Pleasant Street, Amherst, MA 01003, USA.
Gulen Yesilbag Tonga, Department of Chemistry, University of Massachusetts Amherst, 710 North Pleasant Street, Amherst, MA 01003, USA.
Moumita Ray, Department of Chemistry, University of Massachusetts Amherst, 710 North Pleasant Street, Amherst, MA 01003, USA.
Qiaobing Xu, Department of Biomedical Engineering, Tufts University, 4 Colby Street, Medford, Massachusetts, 02115, USA
Vincent M. Rotello, Department of Chemistry, University of Massachusetts Amherst, 710 North Pleasant Street, Amherst, MA 01003, USA.
References
- 1.a) Lippert AR, Van de Bittner GC, Chang CJ. Acc. Chem. Res. 2011;44:793. doi: 10.1021/ar200126t. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Nathan C, Cunningham-Bussel A. Nat. Rev. Immunol. 2013;13:349. doi: 10.1038/nri3423. [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Finkel T. Curr. Opin. Cell Biol. 2003;15:247. doi: 10.1016/s0955-0674(03)00002-4. [DOI] [PubMed] [Google Scholar]
- 2.a) Rhee SG. Science. 2006;312:1882. doi: 10.1126/science.1130481. [DOI] [PubMed] [Google Scholar]; b) Shim MS, Xia Y. Angew. Chem. Int. Ed. 2013;52:6926. doi: 10.1002/anie.201209633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.a) Mattson MP. Nature. 2004;430:631. doi: 10.1038/nature02621. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Lin MT, Beal MF. Nature. 2006;443:787. doi: 10.1038/nature05292. [DOI] [PubMed] [Google Scholar]
- 4.a) Houstis N, Rosen ED, Lander ES. Nature. 2006;440:944. doi: 10.1038/nature04634. [DOI] [PubMed] [Google Scholar]; b) Pop-Busui R, Sima A, Stevens M. Metab. Res. Rev. 2006;22:257. doi: 10.1002/dmrr.625. [DOI] [PubMed] [Google Scholar]
- 5.a) Fruehauf JP, Meysken FL. Clin. Cancer Res. 2007;448:789. doi: 10.1158/1078-0432.CCR-06-2082. [DOI] [PubMed] [Google Scholar]; b) Finkel T, Serrano M, Blasco MA. Nature. 2007;448:767. doi: 10.1038/nature05985. [DOI] [PubMed] [Google Scholar]; c) Ishikawa K, Takenaga K, Akimoto M, Koshikawa N, Yamaguchi A, Imanishi H, Nakada K, Honma Y, Hayashi J. Science. 2008;320:661. doi: 10.1126/science.1156906. [DOI] [PubMed] [Google Scholar]
- 6.a) Miller EW, Tulyathan O, Isacoff EY, Chang CJ. Nat. Chem. Biol. 2007;3:263. doi: 10.1038/nchembio871. [DOI] [PubMed] [Google Scholar]; b) Dickinson BC, Huynh C, Chang CJ. J. Am. Chem. Soc. 2010;132:5906. doi: 10.1021/ja1014103. [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Abo M, Urano Y, Hanaoka K, Terai T, Komatsu T, Nagano T. J. Am. Chem. Soc. 2011;133:10629. doi: 10.1021/ja203521e. [DOI] [PubMed] [Google Scholar]; d) Zhang R, Zhao J, Han G, Liu Z, Liu C, Zhang C, Liu B, Jiang C, Liu R, Zhao T, Han M-Y, Zhang Z. J. Am. Chem. Soc. 2016;138:3769. doi: 10.1021/jacs.5b12848. [DOI] [PubMed] [Google Scholar]; e) Jiang C, Liu R, Han G, Zhang Z. Chem. Commun. 2013;49:6647. doi: 10.1039/c3cc43410a. [DOI] [PubMed] [Google Scholar]; f) Zhang W, Li P, Yang F, Hu X, Sun C, Zhang W, Chen D, Tang B. J. Am. Chem. Soc. 2013;135:14956. doi: 10.1021/ja408524j. [DOI] [PubMed] [Google Scholar]
- 7.Weinstain R, Savariar EN, Felsen CN, Tsien RY. J. Am. Chem. Soc. 2014;136:874. doi: 10.1021/ja411547j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.a) Ermakova YG, Bilan DS, Matlashov ME, Mishina NM, Markvicheva KN, Subach OM, Subach FV, Bogeski I, Hoth M, Enikolopov G, Belousov VV. Nat. Commun. 2014;5:5222. doi: 10.1038/ncomms6222. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Belousov VV, Fradkov AF, Lukyanov KA, Staroverov DB, Shakhbazov KS, Terskikh AV, Lukyanov S. Nat. Methods. 2006;3:281. doi: 10.1038/nmeth866. [DOI] [PubMed] [Google Scholar]
- 9.a) Liu B, Sun Z, Huang PJ, Liu J. J. Am. Chem. Soc. 2015;137:1290–1295. doi: 10.1021/ja511444e. [DOI] [PubMed] [Google Scholar]; b) Ornatska M, Sharpe E, Andreescu D, Andreescu S. Anal. Chem. 2011;83:4273. doi: 10.1021/ac200697y. [DOI] [PubMed] [Google Scholar]
- 10.a) Jiang Y, Zhao H, Lin Y, Zhu N, Ma Y, Mao LQ. Angew. Chem. Int. Ed. 2010;49:4800. doi: 10.1002/anie.201001057. [DOI] [PubMed] [Google Scholar]; b) Saha K, Agasti SS, Kim C, Li X, Rotello VM. Chem. Rev. 2012;112:2739. doi: 10.1021/cr2001178. [DOI] [PMC free article] [PubMed] [Google Scholar]; c) You CC, Miranda OR, Gider B, Ghosh PS, Kim IB, Erdogan B, Krovi SA, Bunz UHF, Rotello VM. Nat Nanotechnol. 2007;2:318. doi: 10.1038/nnano.2007.99. [DOI] [PubMed] [Google Scholar]; d) Cutler JI, Auyeung E, Mirkin CA. J. Am. Chem. Soc. 2012;134:1376. doi: 10.1021/ja209351u. [DOI] [PubMed] [Google Scholar]
- 11.Rana S, Yeh YC, Rotello VM. Curr. Opin. Chem. Biol. 2010;14:828. doi: 10.1016/j.cbpa.2010.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rana S, Le NDB, Mout R, Saha K, Tonga GY, Bain RES, Miranda OR, Rotello CM, Rotello VM. Nat. Nanotechnol. 2015;10:65. doi: 10.1038/nnano.2014.285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mout R, Tonga GY, Ray M, Moyano DF, Xing Y, Rotello VM. Nanoscale. 2014;6:8873. doi: 10.1039/c4nr02097a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang M, Sun S, Neufeld CI, Perez-Ramirez B, Xu Q. Angew. Chem. Int. Ed. 2014;53:13444. doi: 10.1002/anie.201407234. [DOI] [PubMed] [Google Scholar]
- 15.a) Elci SG, Moyano DF, Rana S, Tonga G, Philips R, Bunz U, Rotello VM. Chem. Sci. 2013;4:2076. [Google Scholar]; b) Brust M, Walker M, Bethell D, Schiffrin DJ, Whyman R. J. Chem. Soc. Chem. Commun. 1994:801. [Google Scholar]
- 16.You CC, De M, Han G, Rotello VM. J. Am. Chem. Soc. 2005;127:12873. doi: 10.1021/ja0512881. [DOI] [PubMed] [Google Scholar]
- 17.a) Broaders KE, Grandhe S, Frechet JMJ. J. Am. Chem. Soc. 2011;133:756. doi: 10.1021/ja110468v. [DOI] [PubMed] [Google Scholar]; b) de Gracia Lux C, Joshi-Barr S, Nguyen T, Mahmoud E, Schopf E, Fomina N, Almutairi A. J. Am. Chem. Soc. 2012;134:15758. doi: 10.1021/ja303372u. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.