Abstract
Neuroimaging studies in stimulant use (eg, cocaine, methamphetamine) disorders show that diminished dopamine release by dopamine-elevating drugs is a potential marker of relapse and suggest that increasing dopamine at the D2/3 receptors may be therapeutically beneficial. In contrast, recent investigations indicate heightened D3 receptor levels in stimulant users prompting the view that D3 antagonism may help prevent relapse. Here we tested whether a ‘blunted' response to amphetamine in methamphetamine (MA) users extends to D3-rich brain areas. Fourteen MA users and 15 healthy controls completed two positron emission tomographic scans with a D3-preferring probe [11C]-(+)-PHNO at baseline and after amphetamine (0.4 mg/kg). Relative to healthy controls, MA users had greater decreases in [11C]-(+)-PHNO binding (increased dopamine release) after amphetamine in D3-rich substantia nigra (36 vs 20%, p=0.03) and globus pallidus (30 vs 17%, p=0.06), which correlated with self-reported ‘drug wanting'. We did not observe a ‘blunted' dopamine response to amphetamine in D2-rich striatum; however, drug use severity was negatively associated with amphetamine-induced striatal changes in [11C]-(+)-PHNO binding. Our study provides evidence that dopamine transmission in extrastriatal ‘D3-areas' is not blunted but rather increased in MA users. Together with our previous finding of elevated D3 receptor level in MA users, the current observation suggests that greater dopaminergic transmission at the D3 dopamine receptor may contribute to motivation to use drugs and argues in favor of D3 antagonism as a possible therapeutic tool to reduce craving and relapse in MA addiction.
INTRODUCTION
Methamphetamine (MA) is a widely abused psychostimulant that has been associated with neuroadaptive changes and/or neurotoxic damage to monoaminergic neurons (in particular, dopaminergic) in the mammalian brain. Human postmortem brain studies and preclinical data show decreased levels of dopaminergic markers, including dopamine (DA) itself, tyrosine hydroxylase, and dopamine transporter (DAT), in the brains exposed to MA (see Kish, 2014 for a review).
Brain imaging of dopaminergic markers in living MA (MA and dexamphetamine) users have for the most part been in line with preclinical and postmortem data by showing reduced DAT binding (see Kish, 2014 for a review), D2 receptors (see Boileau et al, 2012; Trifilieff and Martinez, 2014; Volkow et al, 2015 for reviews and references), and reduced amphetamine (with [123I]IBZM in light recreational users of dexamphetamine; Schrantee et al, 2015) and methylphenidate (with [11C]raclopride positron emission tomography (PET)) induced DA release (Wang et al, 2012). The results of the Wang et al, 2012 PET study, although limited to the left putamen, interestingly predicted clinical outcome (relapse rate), as has been shown in cocaine addiction (Martinez et al, 2011). Recently, we reported elevated [11C]-(+)-dihydrotetrabenazine binding, a vesicular monoamine transporter (VMAT2) probe sensitive to changes in vesicular DA, in the striatum of chronic MA users (Boileau et al, 2015a), suggesting low stored DA, consistent with postmortem observations (Wilson et al, 1996a). Together, clinical and preclinical studies suggest that chronic MA use in humans might be associated with a hypodopaminergic state, which could in theory be remedied by DA substitution medication (although no evidence yet exists from clinical trials).
In contrast, a recent body of work suggests that, unlike other measured dopaminergic markers, the D3 receptor might be upregulated in animal models (Le Foll et al, 2005; Neisewander et al, 2004) and in postmortem (Mash, 1997; Staley and Mash, 1996) and living brain of humans with stimulant (Boileau et al, 2012; Matuskey et al, 2014; Payer et al, 2014) and perhaps alcohol (Erritzoe et al, 2014) addiction (see (Boileau et al, 2015b) for a review). General interest in D3 receptor has developed in large part because of preferential expression of D3 in limbic brain areas associated with reward/motivation (eg, ventral striatum) and because animal models suggested that D3-selective antagonists decrease drug-seeking behavior. Together these studies raised the possibility that increased transmission at D3 receptors could underlie, in part, motivation to self-administer drugs and, by extension, some aspects of psychostimulant addiction and that D3 antagonists may have therapeutic values (Heidbreder and Newman, 2010; Le Foll et al, 2014). Nevertheless, the D3 antagonism strategy has not been tested in large-scale clinical trials in humans (although several experimental studies showed promise; Le Foll et al, 2014). It is still unclear whether heightened D3 levels observed in PET studies of stimulant addiction (Boileau et al, 2012; Matuskey et al, 2014; Payer et al, 2014) and postmortem brain of cocaine overdose fatalities (Mash, 1997; Staley and Mash, 1996) reflect a compensatory response to low DA levels although findings in preclinical reports (Levesque et al, 1995) and studies in Parkinson's disease (Boileau et al, 2009) of downregulated D3 receptors upon DA loss argue against this possibility.
At present, limited information is available on ‘synaptic DA' status in MA users, with data employing only D2-preferring probes that have limited ability to measure a signal in D3-rich brain areas. This literature deficiency is relevant given the potential importance of D3-rich areas in drug addiction and the possibility that DA level status and stimulated release might be different in D3 vs D2 brain areas (Drevets et al, 2001; Wilson et al, 1996a). The current study therefore employed [11C]-(+)-propyl-hexahydro-naphtho-oxazin ([11C]-(+)-PHNO), a D3-preferring radiotracer having ~20-fold selectivity for D3 over D2 and allowing estimation of both D3 and D2 receptor signal in a region-dependent manner, with binding in dorsal striatum (high D2/low D3 expression) reflecting primarily D2 receptor availability, and binding in hypothalamus and substantia nigra (SN) reflecting predominantly D3 availability. The ventral pallidum (VP) and globus pallidus (GP) are areas of mixed D2/D3 binding where the D3 fraction was estimated to represent 75 and 65%, respectively (Tziortzi et al, 2011). Furthermore, because of its presumed greater sensitivity than the more commonly used [11C]raclopride, [11C]-(+)-PHNO offers the advantage of detecting smaller changes in synaptic DA fluctuations and therefore may allow a better discrimination of between-group effects (Shotbolt et al, 2012a).
The specific aim of our study was to compare amphetamine-induced changes in [11C]-(+)-PHNO binding between MA-abusing and healthy control subjects. We hypothesized that amphetamine would induce smaller changes in [11C]-(+)-PHNO binding in D3 as well as D2 receptor-rich areas in MA users vs healthy controls.
MATERIALS AND METHODS
Subjects
Sixteen healthy controls and 16 chronic MA users signed consent and were enrolled to participate in an open-label amphetamine challenge PET study approved by the Centre for Addiction and Mental Health (CAMH) Research Ethics Board.
All participants underwent a comprehensive medical and psychiatric screening interview. MA users and controls were included if they were between the ages of 19 and 45 years and were free of significant medical conditions (as per medical history and standard laboratory tests) and current or previous DSM-IV Axis I disorders (excluding stimulant abuse/dependence in the MA group and nicotine dependence in both the groups). Study inclusion criteria for the MA group included: (1) self-reported use of MA as the primary drug of abuse; (2) meeting DSM-IV criteria for MA abuse or dependence; (3) testing positive for MA in scalp hair; and (4) no current (12 months) self-reported abuse of or dependence on drugs other than MA (except nicotine).
Image Acquisition and Amphetamine Challenge
On the day of the scan, all subjects were required to test negative on a urine drug screen (9-Drug Test Panel, BTNX, Markham, ON) and were asked to not smoke cigarettes or eat for at least 3 h prior to their appointment.
The scan session was comprised of two [11C]-(+)-PHNO scans performed (at least) 5 h apart. One scan occurred during resting baseline and the second scan was scheduled 2 h after the administration of an oral dose of dextro-amphetamine (0.4 mg/kg). Scan sessions included periodic assessments of mood and visual analog scales assessing measuring ‘drug-liking', ‘drug-wanting', ‘energetic', ‘mind-racing', ‘rush', ‘high', ‘euphoria', ‘anxious', and ‘excited'. Heart rate and blood pressure (HR/BP) were monitored at 15- min intervals, and blood was drawn to measure plasma amphetamine levels.
[11C]-(+)-PHNO synthesis and image acquisition protocols on the CPS-HRRT neuro-PET camera system (Siemens Medical Imaging, Knoxville, TN) are described in detail elsewhere (Boileau et al, 2012). Scans were initiated following bolus injection of [11C]-(+)-PHNO (scan parameters are reported in Table 1). Raw data were reconstructed by filtered-back projection. Standard spin echo proton-density weight magnetic resonance images were obtained (Signa 1.5T MRI scanner, General Electric Medical Systems, Milwaukee, WI) for region of interest (ROI) delineation.
Table 1. Subject Demographic Information.
| Control subjects (n=15), mean±SD | Methamphetamine users (n=14), mean±SD | Group difference p-value | |
|---|---|---|---|
| Age (years) | 28.53±5.18 | 27.57±5.96 | 0.64 |
| Gender | 13 (M) | 10 (M) | 0.29 |
| Ethnicity | 13 (W) | 12 (W) | 0.17 |
| Weight (kg) | 74.45±17.53 | 78.86±14.49 | 0.69 |
| Years of education | 16.73±2.71 | 12.57±2.68 | <0.01 |
| Premorbid IQ (NART) | 117.43±5.77 | 115.1±4.50 | 0.27 |
| Beck Depression Inventory | 1.93±2.19 | 5.36±6.45 | 0.06 |
| Nicotine smokers | 4 | 7 | 0.18 |
| Cigarettes/day | 2.93±2.25 | 7.43±1.51 | 0.03 |
| Cannabis (⩾1 week last month) | 3 | 8 | 0.05 |
| Alcohol misusea | 0 | 1 | 0.48 |
| [11C]-(+)-PHNO dose (mCi) | |||
| Baseline scan | 8.3±1.6 | 8.1±1.4 | 0.69 |
| Amphetamine scan | 8.3±1.4 | 8.1±1.9 | 0.78 |
| [11C]-(+)-PHNO mass (μg) | |||
| Baseline scan | 2.3±0.4 | 2.4±0.1 | 0.27 |
| Amphetamine scan | 2.2±0.4 | 2.3±0.3 | 0.43 |
| [11C]-(+)-PHNO Spec. Act. | |||
| Baseline scan | 1764±3363 | 819±151 | 0.30 |
| Amphetamine scan | 989±335 | 897±276 | 0.49 |
Abbreviations: NART, National Adult Reading Test; Spec. Act., [11C]-(+)-PHNO specific activity at the time of the injection.
The National Institute on Alcohol Abuse and Alcoholism defines Alcohol Misuse (ie, more than moderate alcohol use) as a pattern of drinking that exceeds drinking one drink per day in women and two drinks a day in men or drinking more than five alcoholic drinks on a single occasion for men and three drinks on a single occasion for women (in the past 30 days). p-values in italics represent significance at p<0.05.
ROI delineation and time activity curve analyses were performed using the in-house image analysis software for automated quantification of PET data (ROMI) (details in Rusjan et al, 2006). Bilateral subcompartments of the striatum, including sensorimotor striatum (SMST), associative striatum (AST), and limbic striatum (LST), were automatically segmented as described in Martinez et al (2003). The (whole) GP and midbrain SN were automatically segmented using the atlas of Kabani et al (1998). The automatically selected VP covered approximately five coronal slices starting at the interhemispheric anterior commissural connection and was defined laterally and medially as described in Tziortzi et al (2011). Cerebellar cortex (excluding vermis) served as reference region. [11C]-(+)-PHNO time activity curves were obtained from dynamic data, and specific binding (BPND) was estimated in each ROI using the simplified reference tissue method (SRTM) (Lammertsma and Hume, 1996). Parameter estimation was performed with PMOD (Version 2.8.5; PMOD Technologies, Zurich, Switzerland).
Estimation of Amphetamine Effect on [11C]-(+)-PHNO and Statistical Analysis
Amphetamine-induced changes in [11C]-(+)-PHNO BPND were calculated in each ROI as the difference between [11C]-(+)-PHNO BPND measured in the baseline condition and that measured in the amphetamine condition, expressed as a percentage of baseline as described in the equation below.
Group comparisons of [11C]-(+)-PHNO BPND and of Δ[11C]-(+)-PHNO BPND across ROIs were conducted using standard repeated-measures ANOVAs or ANCOVAs. When indicated, sphericity corrections were made with Greenhouse–Geisser adjustments. Least Significant Difference t-tests, Bonferroni corrected for planned comparisons, were applied to determine the significance of regional differences in BPND between groups. Relationships between continuous variables were analyzed with Pearson product moment correlation coefficient and Spearman's Rank test for categorical data.
RESULTS
Participant Demographic and Drug Use History
Two MA users were excluded: one for claustrophobia and the other for providing a urine sample positive for MA at the time of the scan. The data from one control was lost to [11C]-(+)-PHNO-induced nausea. The final sample size was 14 MA users and 15 controls. Part of the baseline data from some controls (12) and MA users (10) has been reported previously (Boileau et al, 2012). Groups were matched with respect to age, gender, and ethnicity. MA users self-reported marginally greater depressive symptoms (Beck Depression Inventory) without being clinically depressed. The MA user group also had more moderate cannabis smokers and reported smoking more nicotine cigarettes daily but did not report drinking more alcohol (Table 1).
MA users had been using MA for an average of ~5 years. The typical dose of MA per occasion at the time of the scan was ~0.3 g. Forty percent of the sample smoked crystal MA vs 60% who preferred intranasal or oral administration. Ten of the 14 users had been abstinent for >10 days at the time of the scan (10–90 days), whereas 4 reported using MA ~7 days before the scan. Hair analysis not only confirmed use of MA in all MA users (and none in controls) but also revealed the presence of other drugs in the hair of the MA users; particularly cocaine metabolites (Table 2).
Table 2. Drug Use Characteristics and Co-Used Substances.
| Methamphetamine users (n=14) | |
|---|---|
| Years of MA use | 5±3 (2–11) |
| Frequency of use (days a week) | 2±1 (1–5) |
| Estimated typical dose (mg) | 310±174 (100–500) |
| aBinges in the last 30 days (n) | 5±3 (0–10) |
| Route of administration | 6 (40%) smoked; 8 (60%) oral/intranasal |
| Days since last MA use | 20±21 (6–90) |
| Severity of Dependence Scaleb | 4±2 (2–8) |
| MA/amphetamine in hair (n, %) | 14, 100% |
| Cocaine/cocaine metabolites in hair (n, %) | 10, 71% |
| MDMA/MDA/MDEA in hair (n, %) | 8, 57% |
| Morphine/codeine in hair (n, %) | 6, 42% |
Abbreviations: MA, methamphetamine; MDA, 3,4-methylenedioxyamphetamine; MDEA, 3,4-methylenedioxyethylamphetamine; MDMA, 3,4-methylenedioxy-methamphetamine.
Period of 2–3 days of use.
PET [11C]-(+)-PHNO BPND
Seven subjects had scans performed on separate days (during the same week). The order of administration was reversed (ie: amphetamine session first) for one control and one MA user who received scans on separate days.
VP values in one control could not be estimated owing to poor segmentation of the VP. A repeated-measure ANOVA without the VP was conducted; group differences in the VP were assessed using a separate ANOVA (on [11C]-(+)-PHNO BPND) and a univariate test (on percentage difference in [11C]-(+)-PHNO BPND). Regional [11C]-(+)-PHNO BPND in the amphetamine and baseline conditions together with amphetamine-induced Δ[11C]-(+)-PHNO BPND (%) are reported in Table 3.
Table 3. Mean Regional [11C]-(+)-PHNO BPND in Methamphetamine (MA) Users and Healthy Controls at Baseline, After Amphetamine and Relative Changes in [11C]-(+)-PHNO BPND (Δ[11C]-(+)-PHNO BPND) After Oral Amphetamine (0.3 mg/kg Given 2 h Prior to Tracer Injection).
|
Healthy control subjects (n=15) |
Methamphetamine users (n=14) |
|||||
|---|---|---|---|---|---|---|
| Baseline (mean±SD) | Amphetamine (mean±SD) | ΔBPND (%) (mean±SD) | Baseline (mean±SD) | Amphetamine (mean±SD) | ΔBPND (%) (mean±SD) | |
| AST | 2.36±0.28 | 2.01±0.31 | 14±8 | 2.47±0.72 | 2.07±0.45 | 14±10 |
| LST | 2.98±0.46 | 2.23±0.12 | 24±17 | 3.00±0.65 | 2.41±0.12 | 23±17 |
| SMST | 2.61±0.37 | 1.99±0.44 | 23±8 | 2.57±0.58 | 2.04±0.48 | 20±11 |
| GP | 2.94±0.46 | 2.41±0.46 | 17±13 | 3.36±0.49a | 2.33±0.67 | 30±21b |
| VP | 4.19±0.61 | 2.89±0.66 | 30±19 | 4.60±0.89 | 3.45±0.79 | 25±9 |
| SN | 1.15±0.28 | 0.91±0.29 | 20±21 | 1.82±0.66a | 1.09±0.32 | 36±17c |
Abbreviations: AST, associative striatum; GP, globus pallidus; LST, limbic striatum; SMST, sensorimotor striatum; SN, substantia nigra; VP, ventral pallidum.
Indicating a significant difference in baseline [11C]-(+)-PHNO BPND (p<0.05) between methamphetamine users and healthy controls.
Indicating a non-significant trend in ΔBPND (p=0.06) between methamphetamine users and healthy controls.
Indicating a significant difference in ΔBPND (p<0.05) between methamphetamine users and healthy controls.
The repeated-measure ANOVA (condition (amphetamine vs baseline) × ROI (SN, GP, AST, LST, SMST) × group (controls vs MA users)) investigating differences in [11C]-(+)-PHNO BPND indicated a main effect of condition (F(1, 27)=180.73, p<0.001), suggesting that amphetamine led to a significant decrease in [11C]-(+)-PHNO BPND across ROIs. This effect corresponded to an overall decrease in [11C]-(+)-PHNO BPND of 23% (p<0.0001). The ANOVA also yielded a significant group × ROI × condition interaction (F(4, 108)=4.444, p=0.002) driven by greater baseline [11C]-(+)-PHNO BPND values in the SN and GP of MA users relative to controls (SN: +59%, p=0.001; GP: +14%, p=0.02). This finding at baseline in an overlapping group of individuals was presented in our previous publication (Boileau et al, 2012).
Taking into account cigarettes per day and cannabis use as covariates (as both variables were significantly different between groups and could impact [11C]-(+)-PHNO BPND) did not change this effect (main effect of condition: F(1,25)=28.598, p<0.001; three-way interaction: F(4, 100)=3.592, p=0.009, pairwise comparison SN: 65%, p=0.002; GP: 13% p=0.067).
The repeated-measure ANOVA in the VP revealed a main effect of condition (F(1, 26)=85.932, p<0.001) and a marginal effect of group ([11C]-(+)-PHNO BPND MA users>[11C]-(+)-PHNO BPND controls) (F(1, 26)=3.786, p=0.06) but no group × condition interaction. Taking into account cigarettes per day and recent cannabis use did not change the effect of condition but the marginal effect of group disappeared (p=0.22).
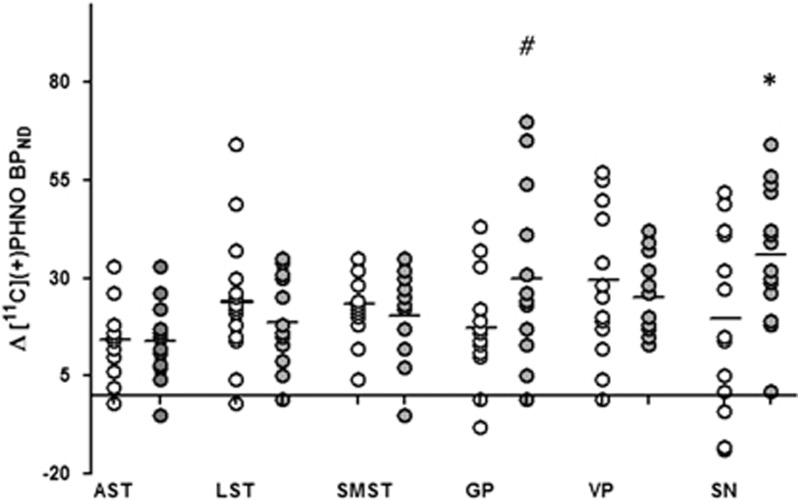
Testing whether amphetamine-induced Δ[11C]-(+)-PHNO BPND (%) were different across groups (repeated-measures ANOVA: ROI (SN, GP, AST, LST, SMST) × group (controls vs MA users)) revealed a significant interaction (F(4, 108)=3.12, p=0.02) which indicated that, relative to controls, MA users had significant, in the SN, and marginally significant, in the GP, greater decreases in [11C]-(+)-PHNO BPND after amphetamine (Figure 1) (SN: controls, 20±21% MA users, 36±17% p=0.03; GP: controls, 17±13% MA users, 30±21% p=0.06). In MA users (but not in controls), greater amphetamine-induced displacement (ΔBPND, %) was found to correlate with higher levels of baseline binding in the SN (r=0.64, p=0.015) and AST (r=0.69, p=0.007). An ANCOVA controlling for cigarettes per day and cannabis use again yielded a significant group × ROI interaction (F(4, 100)=3.59, p=0.009). Pairwise contrasts revealed that MA use was associated with significantly greater decreases in [11C]-(+)-PHNO BPND in the SN (p=0.003); effects were marginal in the GP (p=0.07). We did not observe group difference in response to amphetamine in the D2-rich dorsal striatum or VP (p>0.05). There were no differences in regional volume or cerebellar time activity curves (area under the curve) between groups (p>0.05; time activity curves for groups and conditions are represented in Supplementary Figure S2). Taking into account interscan interval did not affect any of the results. In line with previous reports (Shotbolt et al, 2012b), amphetamine-induced percent change in [11C]-(+)-PHNO BPND were smaller for studies completed on separate days but this effect was not significant (eg, dorsal striatum same day=−18%, different day=−16%, SN same day=−27%, different day=−19% p>0.05). Similarly percent change in [11C]-(+)-PHNO BPND in the two cases (one MA user and one HC) who completed the amphetamine scan first were smaller relative to the cases who had baseline scans first (respectively, −12 vs −17% in the dorsal striatum and −13 vs −29% in the SN).
Figure 1.
Scattergram of relative changes in regional [11C]-(+)-PHNO BPND (Δ[11C]-(+)-PHNO BPND) induced by an oral dose of amphetamine (0.4 mg/kg given 2 h before the tracer injection). Changes in [11C]-(+)-PHNO BPND correspond to the difference between [11C]-(+)-PHNO BPND during baseline and amphetamine conditions, expressed as a percentage of baseline (see equation (1)). AST, associative striatum; GP, globus pallidus; LST, limbic striatum; SMST, sensorimotor striatum; SN, substantia nigra; VP, ventral pallidum. *p=0.03, #p=0.06, methamphetamine users (closed circles) vs healthy controls (open circles).
A voxel-wise approach (SPM8) echoed results of our ROI analysis identifying no differences in amphetamine-induced changes in the striatum of MA users vs HC. Instead we found that changes in [11C]-(+)-PHNO BPND after amphetamine led to smaller, less significant clusters of significant voxel in HC relative to MA users in an area corresponding to the midbrain (SN/VTA) (see Supplementary Figure S1).
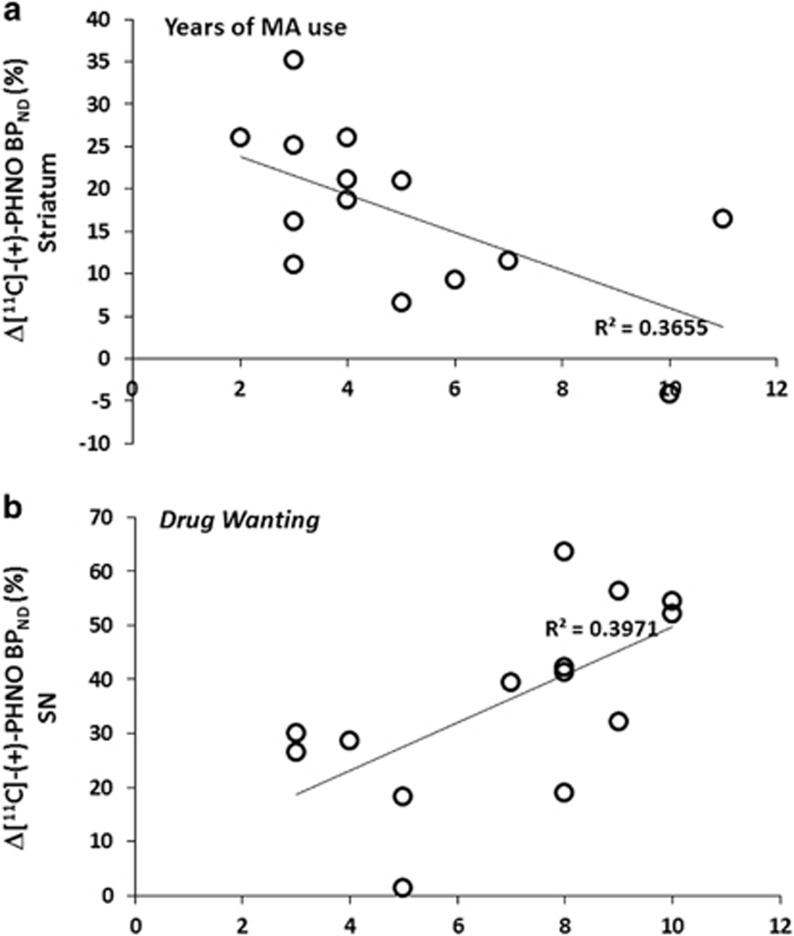
We tested for relationships between amphetamine-induced changes in [11C]-(+)-PHNO BPND (Δ[11C]-(+)-PHNO BPND) in the MA users with drug use pattern and self-report effects of amphetamine. In the MA group, smaller decreases in [11C]-(+)-PHNO binding in the striatum (implying less DA release) was associated with years of MA use (full striatum with years of use: r=−0.60, p=0.02; Figure 2a) and frequency of use (full striatum with days a week of MA use: r=−0.62, p=0.017). There was no relationship between Δ[11C]-(+)-PHNO BPND and days of abstinence. Greater decreases in amphetamine-induced [11C]-(+)-PHNO binding were related to overall greater self-reported effects of amphetamine (full striatum with ARCI: r=+0.74, p=0.006) as well as greater alertness (full striatum with VAS energetic: r=+0.56, p=0.039, VAS mind racing: r=+0.64, p=0.015) and greater self-reported ‘drug wanting' (SN with VAS drug-wanting: r=+0.63, p=0.016) (Figure 2b).
Figure 2.
Correlation plot indicating a relationship between relative changes in regional [11C]-(+)-PHNO BPND (Δ[11C]-(+)-PHNO BPND) induced by an oral dose of amphetamine (0.4 mg/kg given 2 h before the tracer injection) and (a) years of methamphetamine use and (b) self-reported ‘drug-wanting' after a priming dose of amphetamine.
DISCUSSION
Contrary to our hypothesis and in contradistinction to some previous work showing that stimulant use disorder is associated with reduced amphetamine or methylphenidate-induced DA release (the so called ‘blunted response') (Narendran and Martinez, 2008; Schrantee et al, 2015; Trifilieff and Martinez, 2014; Volkow et al, 2015; Wang et al, 2012), here we find instead that, in a group of MA-preferring polystimulant users, DA release (as indexed by decreased amphetamine-stimulated [11C]-(+)-PHNO PET binding) is within the normal range in striatum and is actually increased in the D3-rich SN and (marginally in) GP relative to controls. Of potential clinical relevance, greater DA release in the SN was associated with higher self-reported drug-wanting, suggesting that exaggerated transmission at the D3 receptor level could increase risk for relapse, therefore providing further evidence for testing the D3 antagonism strategy in human stimulant users (Heidbreder and Newman, 2010; Le Foll et al, 2014).
Enhanced Amphetamine-Induced DA Release in D3 Brain Areas
The main novel finding of the current study is that amphetamine-stimulated release of DA is greater, in particular in the DA cell body regions, in MA users (who also have greater D3 receptor levels—this finding is discussed in Boileau et al, 2012) relative to HC. This finding is rather surprising in light of the ‘generally accepted' ‘blunted theory' of addiction—which includes the finding of smaller DA release not only in the striatum but also in the extrastriatal regions (Narendran et al, 2014) of stimulants (Martinez et al, 2011; Narendran and Martinez, 2008; Schrantee et al, 2015; Trifilieff and Martinez, 2014; Wang et al, 2012) as well as opiate and alcohol users (Trifilieff and Martinez, 2014). However, it is in line with some (but see Richtand, 2006) preclinical findings suggesting that intermittent repeated exposure to stimulants is associated with a greater dopaminergic response to drugs (ie: sensitization) (Robinson and Berridge, 2001) and that heightened D3 receptor may be critically involved (Guillin et al, 2001). In this regard, elevation in D3 receptor level in rodents has been shown to accompany sensitization to DA-elevating drugs after repeated stimulation of DA (D1) receptors (Guillin et al, 2001) and inversely DA depletion has been related to downregulation of the D3 receptor (Boileau et al, 2009; Levesque et al, 1995). In line with studies linking D3 receptor upregulation and dopaminergic sensitization, our group reported in a sample of pathological gamblers a correlation between elevated D3 receptor level in SN and exaggerated limbic striatal dopaminergic response to an amphetamine challenge (Boileau et al, 2014). In the present study, enhanced DA response to an amphetamine challenge in MA users is in fact observed in areas with elevated baseline D3 receptor levels and the two outcome measures are positively correlated with each other. How the D3 might modulate DA transmission is not yet entirely clear. Pharmacological studies conducted with D3 antagonist ligands (including the striatum SB-277011-A with 70-fold affinity for D3 over D2) suggest that prolonged transmission at the D3 receptor could provoke increases in synaptic DA levels via modulation of DAT activity (Castro-Hernandez et al, 2015; Zapata et al, 2007). Specifically, some data suggest that subchronic (Joyce et al, 2004) (vs acute see (Zapata et al, 2007)) exposure to D3 agonists decreases DAT activity and that MA-induced deficits in DAT, a rather constant finding in many human MA studies, could be in part D3 mediated (Baladi et al, 2014).
The Lack of Blunted DA Response in the Striatum in MA Users
Previous studies investigating synaptic DA levels in stimulant users with [11C]raclopride or [123I]IBZM have, for the most part, shown that cocaine users and perhaps, to a lesser extent, MA users, have smaller or no changes in [11C]raclopride binding after IV or oral administration of amphetamine or methylphenidate, findings which have been interpreted as a blunted DA release (Martinez et al, 2011; Narendran and Martinez, 2008; Schrantee et al, 2015; Trifilieff and Martinez, 2014; Wang et al, 2012). These data have further been strengthened by the observation in cocaine users of smaller changes in striatal [11C]raclopride binding after a DA-depletion paradigm (with alpha-methyl-para-tyrosine) (Martinez et al, 2009) and with findings of reduced VMAT2 levels (interpreted as a loss of presynaptic DA terminal) in some cocaine users (Narendran et al, 2012; Wilson et al, 1996b). In the case of MA, it was assumed, based on postmortem brain data (Wilson et al, 1996a) indicating a severe loss of tissue levels of DA in MA (but not in cocaine (Wilson et al, 1996b) users), that changes in [11C]raclopride binding after methylphenidate or amphetamine would likely reflect this ‘blunted' response. However, more recent in vivo data employing the VMAT2 probe [11C]DTBZ suggest that a MA-induced DA deficiency in the humans might only be short-lived (Boileau et al, 2015a). One study investigating this question in a group of 15 MA users finds a small effect (slightly blunted DA release employing methylphenidate-induced changes in PET [11C]raclopride binding) but limited to a small area in the left putamen (Wang et al, 2012)—a ‘marginal' finding not inconsistent with our observation that using a tracer highly sensitive to changes in synaptic DA levels (Shotbolt et al, 2012a) we do not find that MA users have a blunted striatal response to amphetamine.
Differences in the amount of daily MA used in the subjects of the different studies could explain inconsistent findings. In this regard, cases in the study by Wang et al (2012) used MA for 13 years on average and were using 1.2 g per day at the time of the scan. In contrast, our sample of MA users was using for an average of 5 years and were taking 0.3 g a day. In light of the relationship found between years of use and changes in [11C]-(+)-PHNO binding (such that the more chronic the use the lesser the changes in [11C]-(+)-PHNO binding), it could be argued that our failure to find a blunted DA signal (but instead a greater dopaminergic response to amphetamine) could be related to severity of the MA use disorder. In this regard, preclinical studies have shown that, in contrast to chronic high-dose exposure to MA, a regimen of repeated intermittent lower doses is associated with dopaminergic sensitization. Arguing against this explanation, however, is the recent report of Schrantee et al (2015) who find in their [123I]IBZM study a robust blunting of DA release after amphetamine in a group of non-addicted, ‘light' dexamphetamine users.
Alternatively, the difference in striatal DA response between chronic MA and cocaine exposure might be at least partly explained by differential adaptations in dopaminergic system. In this regard, whereas loss of DAT has been replicated in postmortem brain studies of MA users by independent groups and in six brain-imaging studies (see Kish, 2014 for a review), the data concerning DAT loss in cocaine users are negative (showing even elevation of DAT in early abstinence)/inconclusive and/or suggests a much less robust effect (see Narendran and Martinez, 2008 for a review). Given the role of DAT in removing DA after release, it is plausible that lower levels of DAT in MA vs cocaine addiction may lead to greater amphetamine-induced DA occupancies (recorded with PET) in MA users, ie, an apparent lack of ‘blunting'. Indeed, a recent study suggests that in MA users lower DAT levels are associated with increased methylphenidate-induced DA signal (Volkow et al, 2015); heterozygote DAT KO mice (+/−), unlike homozygotes (−/−) (Jones et al, 1998), had increased amphetamine-induced DA response in the striatum compared wth the wild type (+/+) (Ji and Dluzen, 2008); low dose of amphetamine as used in our human neuroimaging studies might depend more on DAT blocking (vs vesicular depletion) to increase extracellular DA (Siciliano et al, 2014).
Another possible explanation for our inability to find a ‘blunted' DA response in the striatum could be the use of an agonist tracer [11C]-(+)-PHNO (which measures the high-affinity state of the receptor; ie: D2/3 receptors coupled to G-proteins) as compared with the antagonist tracer [11C]raclopride used in previous studies (which does not discern between high- and low-affinity states). In principle, a greater proportion of D2/3 receptors in the low-affinity functional state, which can be measured with [11C]raclopride but not [11C]-(+)-PHNO, would lead to a smaller DA occupancy and a perceived blunted DA response as detected by [11C]raclopride. Although no differences in DA receptor in high- vs low-affinity states ([11C]NPA/[11C]raclopride) have been noted in cocaine use disorder (Narendran and Martinez, 2008), this possibility cannot be ruled out in users of MA. Given the on-going debate on the selectivity of functional D2 receptor status by the current agonist tracers including [11C]-(+)-PHNO and the validity of the dichotomy of ‘high' vs ‘low' D2 status in vivo (Seeman, 2012; Skinbjerg et al, 2012; van Wieringen et al, 2013), the significance of possible differences in functional status of striatal D2/D3 receptors in MA users in the interpretation of our finding is not entirely clear.
Clinical Relevance and Limitations
Any significance of our study needs to be interpreted in light of many limitations, including the following: the study was not placebo controlled and therefore increased DA release in MA users could be the result of a conditioned DA release to drug cues; the study was, for the most part, not counterbalanced with most of the scans conducted on the same day; however, we can rule out tracer mass carryover effect given the between-subject design and >5-h interval between scans. Most users in our studies reported being M- preferring users but also tested positive in the hair for cocaine (though not in urine); therefore, it is unclear if some past use of cocaine contributed to the finding. This study was not performed with arterial sampling, and given the finding of specific [11C]-(+)-PHNO binding in the cerebellum, we cannot rule out the possibility that the true effect of amphetamine on [11C]-(+)-PHNO binding may have been underestimated and that difference in D2/3 receptor levels and therefore of specific binding in the cerebellum of MA users vs HC could have influenced the finding. However, analysis of cerebellar time activity curves suggested no differences between conditions and across groups (see Supplementary Figure S1). Generic caveats related to [11C]-(+)-PHNO imaging are given in (Boileau et al, 2012).
The clinical implications of our finding and function of the D3 receptor in the SN still need to be clarified. In theory, increased neurotransmission at the D3 receptor (in the SN and GP), which receive afferent ventral striatum projections (Haber et al, 2000), could modify output to the LST affecting addiction-relevant behaviors. Perhaps increased DA release in somatodendritic field could be explained by diminished D2 autoreceptor inhibition (D2 receptor desensitization, leading to more easily depolarized neurons) owing to chronic stimulation during binge drug use (Calipari et al, 2014). In the current study, we found that greater changes in [11C]-(+)-PHNO binding (enhanced DA release) correlated with drug-wanting, suggesting that D3 receptor activation could contribute to craving and motivation to use drugs. Our findings are in line with preclinical work suggesting that D3 receptor antagonists block drug-seeking behaviors, self-administration, and cue- and stress- induced drug reinstatement (a model of relapse) (for a review, see Le Foll et al, 2014) and suggest that increased D3 receptor activity might contribute to the development of MA addiction.
FUNDING AND DISCLOSURE
This project is funded in part by the National Institute of Health (NIDA) R01DA025096 (to SJK and IB). The authors declare no conflict of interest.
Acknowledgments
We thank Alvina Ng, Jeannie Fong, Armando Garcia, Winston Stableford, and Min Wong for excellent technical assistance.
Footnotes
Supplementary Information accompanies the paper on the Neuropsychopharmacology website (http://www.nature.com/npp)
Supplementary Material
References
- Baladi MG, Newman AH, Nielsen SM, Hanson GR, Fleckenstein AE (2014). Dopamine D(3) receptors contribute to methamphetamine-induced alterations in dopaminergic neuronal function: role of hyperthermia. Eur J Pharmacol 732: 105–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boileau I, Guttman M, Rusjan P, Adams JR, Houle S, Tong J et al (2009). Decreased binding of the D3 dopamine receptor-preferring ligand [11C]-(+)-PHNO in drug-naive Parkinson's disease. Brain 132: 1366–1375. [DOI] [PubMed] [Google Scholar]
- Boileau I, McCluskey T, Tong J, Furukawa Y, Houle S, Kish SJ (2015. a). Rapid recovery of vesicular dopamine levels in methamphetamine users in early abstinence. Neuropsychopharmacology 41: 1179–1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boileau I, Nakajima S, Payer D (2015. b). Imaging the D3 dopamine receptor across behavioral and drug addictions: positron emission tomography studies with [(11)C]-(+)-PHNO. Eur Neuropsychopharmacol 25: 1410–1420. [DOI] [PubMed] [Google Scholar]
- Boileau I, Payer D, Chugani B, Lobo DS, Houle S, Wilson AA et al (2014). In vivo evidence for greater amphetamine-induced dopamine release in pathological gambling: a positron emission tomography study with [C]-(+)-PHNO. Mol Psychiatry 19: 1305–1313. [DOI] [PubMed] [Google Scholar]
- Boileau I, Payer D, Houle S, Behzadi A, Rusjan PM, Tong J et al (2012). Higher binding of the dopamine D3 receptor-preferring ligand [11C]-(+)-propyl-hexahydro-naphtho-oxazin in methamphetamine polydrug users: a positron emission tomography study. J Neurosci 32: 1353–1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calipari ES, Sun H, Eldeeb K, Luessen DJ, Feng X, Howlett AC et al (2014). Amphetamine self-administration attenuates dopamine D2 autoreceptor function. Neuropsychopharmacology 39: 1833–1842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castro-Hernandez J, Afonso-Oramas D, Cruz-Muros I, Salas-Hernandez J, Barroso-Chinea P, Moratalla R et al (2015). Prolonged treatment with pramipexole promotes physical interaction of striatal dopamine D3 autoreceptors with dopamine transporters to reduce dopamine uptake. Neurobiol Dis 74: 325–335. [DOI] [PubMed] [Google Scholar]
- Drevets WC, Gautier C, Price JC, Kupfer DJ, Kinahan PE, Grace AA et al (2001). Amphetamine-induced dopamine release in human ventral striatum correlates with euphoria. Biol Psychiatry 49: 81. [DOI] [PubMed] [Google Scholar]
- Erritzoe D, Tziortzi A, Bargiela D, Colasanti A, Searle GE, Gunn RN et al (2014). In vivo imaging of cerebral dopamine D3 receptors in alcoholism. Neuropsychopharmacology 39: 1703–1712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gossop M, Darke S, Griffiths P, Hando J, Powis B, Hall W et al (1995). The Severity of Dependence Scale (SDS): psychometric properties of the SDS in English and Australian samples of heroin, cocaine and amphetamine users. Addiction 90: 607–614. [DOI] [PubMed] [Google Scholar]
- Guillin O, Diaz J, Carroll P, Griffon N, Schwartz JC, Sokoloff P (2001). BDNF controls dopamine D3 receptor expression and triggers behavioural sensitization. Nature 411: 86–89. [DOI] [PubMed] [Google Scholar]
- Haber SN, Fudge JL, McFarland NR (2000). Striatonigrostriatal pathways in primates form an ascending spiral from the shell to the dorsolateral striatum. J Neurosci 20: 2369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heidbreder CA, Newman AH (2010). Current perspectives on selective dopamine D(3) receptor antagonists as pharmacotherapeutics for addictions and related disorders. Ann NY Acad Sci 1187: 4–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji J, Dluzen DE (2008). Sex differences in striatal dopaminergic function within heterozygous mutant dopamine transporter knock-out mice. J Neural Transm 115: 809–817. [DOI] [PubMed] [Google Scholar]
- Jones SR, Gainetdinov RR, Wightman RM, Caron MG (1998). Mechanisms of amphetamine action revealed in mice lacking the dopamine transporter. J Neurosci 18: 1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joyce JN, Woolsey C, Ryoo H, Borwege S, Hagner D (2004). Low dose pramipexole is neuroprotective in the MPTP mouse model of Parkinson's disease, and downregulates the dopamine transporter via the D3 receptor. BMC Biol 2: 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabani NJ, MacDonald D, Holmes CJ, Evans AC (1998). 3D anatomical atlas of the human brain. Neuroimage 7: S717. [Google Scholar]
- Kish SJ. Chapter 08: The pathology of methamphetamine use in the human brain. In: Madras B, Kuhar M (eds). The Effects of Drug Abuse on the Human Nervous System. Elsevier Inc: Oxford, UK; (2014, pp 203–298. [Google Scholar]
- Lammertsma AA, Hume SP (1996). Simplified reference tissue model for PET receptor studies. Neuroimage 4: 153. [DOI] [PubMed] [Google Scholar]
- Le Foll B, Collo G, Rabiner EA, Boileau I, Merlo Pich E, Sokoloff P (2014). Dopamine D3 receptor ligands for drug addiction treatment: update on recent findings. Progr Brain Res 211: 255–275. [DOI] [PubMed] [Google Scholar]
- Le Foll B, Diaz J, Sokoloff P (2005). A single cocaine exposure increases BDNF and D3 receptor expressions:implications for drug-conditioning. Neuroreport 16: 175–178. [DOI] [PubMed] [Google Scholar]
- Levesque D, Martres MP, Diaz J, Griffon N, Lammers CH, Sokoloff P et al (1995). A paradoxical regulation of the dopamine D3 receptor expression suggests the involvement of an anterograde factor from dopamine neurons. Proc Natl Acad Sci USA 92: 1719–1723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez D, Carpenter KM, Liu F, Slifstein M, Broft A, Friedman AC et al (2011). Imaging dopamine transmission in cocaine dependence: link between neurochemistry and response to treatment. Am J Psychiatry 168: 634–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez D, Greene K, Broft A, Kumar D, Liu F, Narendran R et al (2009). Lower level of endogenous dopamine in patients with cocaine dependence: findings from PET imaging of D(2)/D(3) receptors following acute dopamine depletion. Am J Psychiatry 166: 1170–1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez D, Slifstein M, Broft A, Mawlawi O, Hwang DR, Huang Y et al (2003). Imaging human mesolimbic dopamine transmission with positron emission tomography. Part II: amphetamine-induced dopamine release in the functional subdivisions of the striatum. J Cereb Blood Flow Metab 23: 285. [DOI] [PubMed] [Google Scholar]
- Mash DC (1997). D3 receptor binding in human brain during cocaine overdose. Mol Psychiatry 2: 5–6. [PubMed] [Google Scholar]
- Matuskey D, Gallezot JD, Pittman B, Williams W, Wanyiri J, Gaiser E et al (2014). Dopamine D(3) receptor alterations in cocaine-dependent humans imaged with [(1)(1)C](+)PHNO. Drug Alcohol Depend 139: 100–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narendran R, Lopresti BJ, Martinez D, Mason NS, Himes M, May MA et al (2012). In vivo evidence for low striatal vesicular monoamine transporter 2 (VMAT2) availability in cocaine abusers. Am J Psychiatry 169: 55–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narendran R, Martinez D (2008). Cocaine abuse and sensitization of striatal dopamine transmission: a critical review of the preclinical and clinical imaging literature. Synapse 62: 851–869. [DOI] [PubMed] [Google Scholar]
- Narendran R, Mason NS, Paris J, Himes ML, Douaihy AB, Frankle WG (2014). Decreased prefrontal cortical dopamine transmission in alcoholism. Am J Psychiatry 171: 881–888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neisewander JL, Fuchs RA, Tran-Nguyen LT, Weber SM, Coffey GP, Joyce JN (2004). Increases in dopamine D3 receptor binding in rats receiving a cocaine challenge at various time points after cocaine self-administration: implications for cocaine-seeking behavior. Neuropsychopharmacology 29: 1479–1487. [DOI] [PubMed] [Google Scholar]
- Payer DE, Behzadi A, Kish SJ, Houle S, Wilson AA, Rusjan PM et al (2014). Heightened D3 dopamine receptor levels in cocaine dependence and contributions to the addiction behavioral phenotype: a positron emission tomography study with [C]-(+)-PHNO. Neuropsychopharmacology 39: 311–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richtand NM (2006). Behavioral sensitization, alternative splicing, and d3 dopamine receptor-mediated inhibitory function. Neuropsychopharmacology 31: 2368–2375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC (2001). Incentive-sensitization and addiction. Addiction 96: 103. [DOI] [PubMed] [Google Scholar]
- Rusjan P, Mamo D, Ginovart N, Hussey D, Vitcu I, Yasuno F et al (2006). An automated method for the extraction of regional data from PET images. Psychiatry Res 147: 79–89. [DOI] [PubMed] [Google Scholar]
- Schrantee A, Vaclavu L, Heijtel DF, Caan MW, Gsell W, Lucassen PJ et al (2015). Dopaminergic system dysfunction in recreational dexamphetamine users. Neuropsychopharmacology 40: 1172–1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeman P (2012). Dopamine agonist radioligand binds to both D2High and D2Low receptors, explaining why alterations in D2High are not detected in human brain scans. Synapse 66: 88–93. [DOI] [PubMed] [Google Scholar]
- Shotbolt P, Tziortzi AC, Searle GE, Colasanti A, van der Aart J, Abanades S et al (2012. a). Within-subject comparison of [(11)C]-(+)-PHNO and [(11)C]raclopride sensitivity to acute amphetamine challenge in healthy humans. J Cereb Blood Flow Metab 32: 127–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shotbolt P, Tziortzi AC, Searle GE, Colasanti A, van der Aart J, Abanades S et al (2012. b). Within-subject comparison of [(11)C]-(+)-PHNO and [(11)C]raclopride sensitivity to acute amphetamine challenge in healthy humans. J Cereb Blood Flow Metab 32: 127–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siciliano CA, Calipari ES, Ferris MJ, Jones SR (2014). Biphasic mechanisms of amphetamine action at the dopamine terminal. J Neurosci 34: 5575–5582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skinbjerg M, Sibley DR, Javitch JA, Abi-Dargham A (2012). Imaging the high-affinity state of the dopamine D2 receptor in vivo: fact or fiction? Biochem Pharmacol 83: 193–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staley JK, Mash DC (1996). Adaptive increase in D3 dopamine receptors in the brain reward circuits of human cocaine fatalities. J Neurosci 16: 6100–6106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trifilieff P, Martinez D (2014). Imaging addiction: D2 receptors and dopamine signaling in the striatum as biomarkers for impulsivity. Neuropharmacology 76 Pt B: 498–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tziortzi AC, Searle GE, Tzimopoulou S, Salinas C, Beaver JD, Jenkinson M et al (2011). Imaging dopamine receptors in humans with [11C]-(+)-PHNO: dissection of D3 signal and anatomy. Neuroimage 54: 264–277. [DOI] [PubMed] [Google Scholar]
- van Wieringen JP, Booij J, Shalgunov V, Elsinga P, Michel MC (2013). Agonist high- and low-affinity states of dopamine D(2) receptors: methods of detection and clinical implications. Naunyn Schmiedebergs Arch Pharmacol 386: 135–154. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Smith L, Fowler JS, Telang F, Logan J et al (2015). Recovery of dopamine transporters with methamphetamine detoxification is not linked to changes in dopamine release. Neuroimage 121: 20–28. [DOI] [PubMed] [Google Scholar]
- Wang GJ, Smith L, Volkow N, Telang F, Logan J, Tomasi D et al (2012). Decreased dopamine activity predicts relapse in methamphetamine abusers. Mol Psychiatry 17: 918–925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson JM, Kalasinsky KS, Levey AI, Bergeron C, Reiber G, Anthony RM et al (1996. a). Striatal dopamine nerve terminal markers in human, chronic methamphetamine users. Nat Med 2: 699–703. [DOI] [PubMed] [Google Scholar]
- Wilson JM, Levey AI, Bergeron C, Kalasinsky K, Ang L, Peretti F et al (1996. b). Striatal dopamine, dopamine transporter, and vesicular monoamine transporter in chronic cocaine users. Ann Neurol 40: 428–439. [DOI] [PubMed] [Google Scholar]
- Zapata A, Kivell B, Han Y, Javitch JA, Bolan EA, Kuraguntla D et al (2007). Regulation of dopamine transporter function and cell surface expression by D3 dopamine receptors. J Biol Chem 282: 35842–35854. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.