Abstract
Most prior work with positron emission tomography (PET) dopamine subtype 2/3 receptor (D2/3R) non-selective antagonist tracers suggests that obese (OB) individuals exhibit lower D2/3Rs when compared with normal weight (NW) individuals. A D3-preferring D2/3R agonist tracer, [11C](+)PHNO, has demonstrated that body mass index (BMI) was positively associated with D2/3R availability within striatal reward regions. To date, OB individuals have not been studied with [11C](+)PHNO. We assessed D2/3R availability in striatal and extrastriatal reward regions in 14 OB and 14 age- and gender-matched NW individuals with [11C](+)PHNO PET utilizing a high-resolution research tomograph. Additionally, in regions where group D2/3R differences were observed, secondary analyses of 42 individuals that constituted an overweight cohort was done to study the linear association between BMI and D2/3R availability in those respective regions. A group-by-brain region interaction effect (F7, 182=2.08, p=0.047) was observed. Post hoc analyses revealed that OB individuals exhibited higher tracer binding in D3-rich regions: the substantia nigra/ventral tegmental area (SN/VTA) (+20% p=0.02), ventral striatum (VST) (+14% p<0.01), and pallidum (+11% p=0.02). BMI was also positively associated with D2/3R availability in the SN/VTA (r=0.34, p=0.03), VST (r=0.36, p=0.02), and pallidum (r=0.30, p=0.05) across all subjects. These data suggest that individuals who are obese have higher D2/3R availability in brain reward regions densely populated with D3Rs, potentially identifying a novel pharmacologic target for the treatment of obesity.
INTRODUCTION
It has been estimated that over one-third of Americans are obese (OB) (Flegal et al, 2012) and thus at increased risk for developing obesity-related comorbidities such as coronary artery disease, hypertension, functional limitations, and type-2 diabetes (Field et al, 2001; Lakdawalla et al, 2004; Mokdad et al, 2003; Must et al, 1999; Sturm et al, 2004). Considerable preclinical and human research examining obesity has focused on dopamine 2/3 receptors (D2/3Rs) due to their expression within brain regions associated with reward and motivation such as the striatum and the substantia nigra/ventral tegmental area (SN/VTA). In rodents, D2R density was lower in the dorsal striatum of chronic high-fat-induced OB mice as compared with OB-resistant and low-fat-fed controls (Huang et al, 2006), implicating lower D2R availability similar to rodent stimulant administration paradigms (Culver et al, 2008; Dalley et al, 2007; Ujike et al, 1989; Yi and Johnson, 1990). Further work has investigated a selective D3-antagonist medication in an operant food self-administration paradigm and showed that a D3-antagonist attenuated lever presses and food intake in both lean and OB rats, providing preliminary evidence of D3R-related motivation to consume food (Thanos et al, 2008). This notion is consistent with clinical data implicating the D3R in populations abusing substances that influence nigrostriatal reward pathway function (Boileau et al, 2012; Erritzoe et al, 2014; Matuskey et al, 2014; Payer et al, 2014).
In humans, prior work utilizing positron emission tomography (PET) and D2/3R antagonist tracers has not yielded particularly well-defined results in obesity (for a comprehensive review of the PET dopaminergic and obesity/BMI literature, please see the work of Val-Laillet et al (2015). Studies employing [11C]raclopride, a D2/3R non-selective tracer, have demonstrated lower striatal D2/3R availability in both severely obese (body mass index (BMI)>39.9 kg/m2) (Wang et al, 2001) and OB (BMI 30.0–39.9 kg/m2) individuals (Haltia et al, 2007) when compared with normal-weight (NW) (BMI 18.5–24.9 kg/m2) individuals. Furthermore, within both severely obese (Wang et al, 2001) and OB (Haltia et al, 2007) individuals, a negative linear association between D2/3R availability and BMI was observed. This relationship did not extend to non-OB individuals, where no associations between striatal D2/3R availability and BMI have been found with [11C]raclopride (Caravaggio et al, 2013). Work done with the D2-high affinity tracer, [11C]N-methyl-benperidol (NMB), failed to show any striatal D2/3R availability alterations between NW and severely obese individuals (Eisenstein et al, 2013). Lastly, work has also been done with [11C]FLB457 and [18F]fallypride, D2/3R tracers capable of extrastriatal reward region receptor quantification. In NW individuals, a positive linear association between amygdalar D2/3R availability and BMI was observed with [11C]FLB457 (Yasuno et al, 2001), whereas work done with [18F]fallypride has suggested a negative linear association in the left amygdala and the left caudate in a wide-ranged BMI (range 19–35 kg/m2, mean 24.8 kg/m2) cohort (Kessler et al, 2014). Other regions investigated with [18F]fallypride have shown slightly higher D2/3R availability (not statistically significant) in the SN/VTA of OB as compared with NW females (Savage et al, 2014) and a positive linear association between D2/3R availability and BMI in the caudate and putamen of a large cohort of individuals with a wide BMI range (range 18–45 kg/m2) (Guo et al, 2014).
The D3-preferring D2/3R agonist PET tracer, [11C](+)PHNO, has been found to be especially reliable (due to an excellent specific-to-nonspecific binding ratio) at quantifying extrastriatal midbrain reward regions particularly essential for the synthesis and production of dopamine such as the SN/VTA, where D3Rs are predominant (Graff-Guerrero et al, 2008; Narendran et al, 2006; Searle et al, 2010; Tziortzi et al, 2011). In addition, [11C](+)PHNO has also been shown to bind to high-affinity, presumably ‘active', G-protein coupled forms of the D2R within the striatum (Shotbolt et al, 2012; Willeit et al, 2006). Prior work done with [11C](+)PHNO has indicated that BMI correlated positively with D2/3R availability in the ventral striatum (VST) (Caravaggio et al, 2013), a region of mixed D2Rs and D3Rs (Tziortzi et al, 2011), in a cohort with a relatively narrow non-OB BMI range (range 18.6–27.8 kg/m2, mean 23.4 kg/m2). Further work in a wide-BMI-ranged (range 21.5–36.5 kg/m2, mean 27.9 kg/m2) small cohort showed a positive association between D2/3R availability and BMI in the right dorsal caudate (Cosgrove et al, 2015).
The current study investigated, for the first time, differences in D2/3R availability between 14 OB and 14 age- and gender-matched NW individuals utilizing [11C](+)PHNO PET. Additionally, in regions where D2/3R differences were observed, a cohort of 14 overweight (OW) individuals was added to investigate linear associations in a sample representative of the United States population based on BMI (ie, ~33% NW, ~33% OW, and ~33% OB) (Flegal et al, 2012). Based on prior work (Caravaggio et al, 2013) and tracer-binding characteristics (Shotbolt et al, 2012; Tziortzi et al, 2011), we expected to observe higher [11C](+)PHNO tracer binding in important brain reward areas in OB compared with NW individuals in regions populated with D3Rs (ie, the SN/VTA) and in mixed striatal regions where high-affinity ‘active' forms of the D2R may exist (ie, VST) as well as positive linear relationships between tracer binding and BMI in those respective regions after the OW cohort was added. Overall, this work aims to extend the obesity literature by providing useful and informative data contributing to mechanistic understanding of the disease and identify potential novel pharmacologic targets for treatment.
MATERIALS AND METHODS
Research participants were recruited from the greater New Haven area by advertisement, word of mouth, and referral. Once determined initially eligible through telephone questionnaire, participants reported to the Yale PET Center or the Clinical Neuroscience Research Unit of the Connecticut Mental Health Center where they were consented and screened by members of the research team. As part of the screening process, study participants underwent comprehensive medical and psychiatric histories, physical examination, neurological and mental status exam, routine laboratory studies, electrocardiogram, and a semistructured (Sheehan et al, 1998) or a structured clinical interview (American Psychiatric Association. and American Psychiatric Association. Task Force on DSM-IV, 2000) to confirm study eligibility. Study participants were excluded based on evidence of a current or previous major psychiatric (eg, schizophrenia or bipolar disorder) or eating disorder (eg, binge eating disorder), current or history of serious medical or neurological illness (eg, past traumatic brain injury resulting in loss of consciousness), current pregnancy (as documented by urine human chorionic gonadotropin testing at screening and on the day of PET imaging), current or history of a substance abuse disorder, evidence of drug use on day of screening and day of imaging (as documented by urine toxicology studies), taking weight loss medications or medications known to influence the dopamine system (eg, methylphenidate or amphetamine), breastfeeding, or contraindications to magnetic resonance imaging.
Fourteen otherwise healthy OB (BMI>29.9 kg/m2) individuals were compared with 14 age-and gender-matched healthy NW (BMI 18.5–24.9 kg/m2) individuals. As part of the secondary analyses, 14 otherwise healthy OW (BMI 25–29.9 kg/m2) individuals were added to the analysis to examine the linear association between BMI and D2/3R availability in a large, broad-BMI-ranged cohort (see Tables 1 and 2 for means and standard deviations of demographics, injection parameters, and radioactivity data for all individuals studied). All participants with the exception of one OW participant were non-nicotine/tobacco users and some have been reported on elsewhere (Matuskey et al, 2015; Matuskey et al, 2016). Once research participants were eligible for the study, they were asked to abstain from all food and liquids (except for water) the night prior to presenting to the Yale PET Center for imaging until the scanning procedures were completed.
Table 1. Demographic, Injection, and Radioactivity Data for Normal Weight (NW) and Obese (OB) Participants.
| NW (n=14) | OB (n=14) | p-value | |
|---|---|---|---|
| Gender | 10 M, 4 F | 10 M, 4 F | – |
| Age (years) | 34.9 (10.2) | 37.0 (10.1) | 0.59 |
| BMI (kg/m2) | 22.3 (1.8) | 35.3 (4.5) | <0.01 |
| Specific activity (MBq/nmol) | 52.1 (26.3) | 53.8 (25.8) | 0.87 |
| Radioactive dose (MBq) | 358.3 (181.3) | 450.9 (125.6) | 0.14 |
| Cerebellar mean concentration (nM) | 0.0747 (0.0169) | 0.0672 (0.0224) | 0.34 |
| Injected mass (μg/kg) | 0.0267 (0.0055) | 0.0230 (0.0073) | 0.15 |
Bold values indicate statistical significance.
Table 2. Demographic, Injection, and Radioactivity Data for Overweight (OW) and Combined Cohort (All).
| Gender | Age (years) | BMI (kg/m2) | Specific activity (MBq/nmol) | Radioactive dose (MBq) | Cerebellar mean concentration (nM) | Injected mass (μg/kg) | |
|---|---|---|---|---|---|---|---|
| OW (n=14) | 13 M, 1 F | 36.7 (11.5) | 27.2 (1.3) | 54.7 (33.5) | 441.9 (197.1) | 0.0797 (0.0280) | 0.0283 (0.0117) |
| All (N=42) | 33 M, 9 F | 36.2 (10.5) | 28.3 (6.1) | 53.5 (28.4) | 417.0 (174.1) | 0.0738 (0.0235) | 0.0260 (0.0087) |
[11C](+)PHNO was prepared as previously reported (Gallezot et al, 2012). All PET scans were performed with the high-resolution research tomograph (Siemens/CTI, Knoxville, TN, USA), which acquired 207 slices separated by 1.2 mm with a reconstructed image resolution of ~3 mm. Scans were acquired over 120 min at rest. Prior to [11C](+)PHNO emission imaging, a transmission scan was obtained for attenuation correction.
Motion correction was based on an optical detector (Vicra; NDI Systems, Waterloo, Ontario, Canada). Dynamic PET imaging data were reconstructed with corrections for attenuation, normalization, scatter, randoms, deadtime, and motion employing the MOLAR algorithm (Carson et al, 2003) with a frame timing of 6 × 30 s; 3 × 1 min; 2 × 2 min; and 22 × 5 min.
PET data were used to construct a time–activity curve for the cerebellum, a region of minimal D2/3R binding, which was used as the reference region as in previous studies (Boileau et al, 2012; Ginovart et al, 2007; Matuskey et al, 2014, 2015, 2016; Mizrahi et al, 2011; Payer et al, 2014; Searle et al, 2010). A summed image (0–10 min after injection) was created from the motion-corrected PET data and registered to the participant's MR image, which was nonlinearly registered to an MR template in Montreal Neurological Institute (MNI) space. All transformations were performed with Bioimagesuite (version 2.5; http://www.bioimagesuite.com). Parametric images of non-displaceable tracer binding potentials (BPND), which are linearly proportional to the density of available D2/3Rs, were computed using a simplified reference tissue model (2-parameter version: SRTM2). This method has been previously validated for [11C](+)PHNO (Gallezot et al, 2014b; Wu and Carson, 2002) and utilized to optimize the statistical quality of the SRTM applied in prior studies by reducing noise of the functional images (Matuskey et al, 2014, 2015, 2016). Unbound concentration in tissue was estimated as the radioligand mass concentration in the cerebellum at 60–90 min post injection.
Structural magnetic resonance imaging (MRI) was performed on a 3-Tesla Trio system (Siemens Medical Solutions, Malvern, Pennsylvania) with a circularly polarized head coil for reasons of excluding individuals with anatomical abnormalities and anatomically coregistering with PET images. The dimension and voxel size of MR images were 256 × 256 × 176 voxels and 0.98 × 0.98 × 1.0 mm3, respectively.
Regions of interest (ROIs) included the amygdala, caudate, hypothalamus, pallidum, putamen, SN/VTA, thalamus, and VST. ROIs were based on the automated anatomical labeling template delineated in MNI space (Tzourio-Mazoyer et al, 2002), with the exception of the hypothalamus, SN/VTA, and VST. More specifically, the hypothalamus was manually delineated on the MRI template image, the template SN/VTA ROI was manually delineated using [11C](+)PHNO BPND images from a previous study (Gallezot et al, 2014b), and individual, hand-drawn VST delineations were performed in MNI space on each subject's MRI based on the guidelines described by Mawlawi et al (2001). ROIs were applied to the parametric BPND images to extract individual values.
In addition to ROI analyses, a voxel-wise analysis was performed on parametric BPND images with SPM12 (Wellcome Trust Centre for Neuroimaging, London, UK). Similar to the ROI methods, summed PET images were registered to participants' MR images before optimized unified segmentation (Ashburner and Friston, 2005) was used to determine nonlinear registrations into MNI standard space. Transformations were applied to parametric BPND images, resliced into 2 × 2 × 2 mm isotropic voxels and smoothed using a 2-mm FWHM Gaussian kernel. Group differences were assessed using a two-sample t-test at a voxel-level threshold of p<0.001 with a cluster extent threshold (k)>10 voxels.
Data were summarized descriptively and assessed for normality prior to analyses employing normality probability plots and Kolmogorov test statistics. Linear mixed models were used to examine the independent and joint effects of obesity (between-group factor) and ROIs (within-group) on BPND values. Between-group differences within each region were estimated to explain significant interactions. Within-group associations were accounted for by fitting three variance—covariance structures to the data (unstructured, compound symmetry, and heterogeneous compound symmetry) with an unstructured form fitting the data best according to the Bayesian Information Criterion. Secondary correlation analyses were not adjusted for multiple tests given the targeted nature of the analyses (ie, based on the between-group findings). All analyses were conducted using SAS version 9.3 (Cary, NC) or SPSS version 22 (Armonk, NY) and considered significant at the two-tailed α=0.05 threshold.
RESULTS
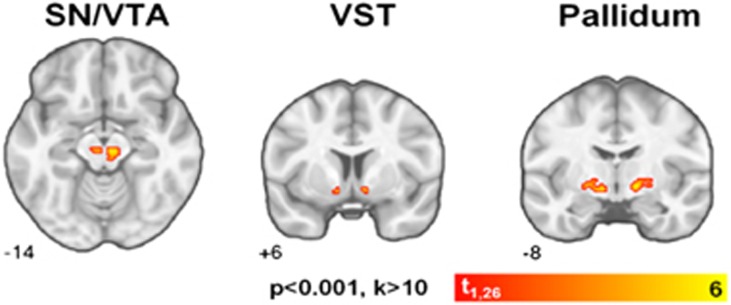
We observed a main effect of obesity (F1,26=6.11, p=0.020) demonstrating that OB and NW individuals differed in overall D2/3R availability as well as a group-by-brain region interaction effect (F7,182=2.08, p=0.047) between OB and NW individuals. Post hoc analyses revealed that OB participants exhibited higher D2/3R availability compared with NW individuals in the SN/VTA (+20% F1,182=5.13, p=0.025), the VST (+14% F,182=8.14, p=0.005), and the pallidum (+11% F1,182=5.30, p=0.022). Group-by-brain interaction results persisted after independently adjusting for potential confounding effects of age (F7,182=2.06, p=0.050), gender (F7,182=2.08, p=0.048), specific activity at time of injection (F7,182=2.08, p=0.048), and injected mass (F7,182=2.07, p=0.048). Post hoc results were unaffected by these confounding variables similar to the unadjusted models. Further, there were no differences observed between groups in mean cerebellar mass concentration of unbound [11C](+)PHNO, thus providing additional evidence that these results were not driven by injected mass. Of the three regional findings, the VST was the only brain region that survived correction for multiple comparisons utilizing the Bonferroni method (adjusted α threshold=0.05/8=0.006). Mean BPND values for both OB and NW individuals along with relative group differences are shown in Table 3. Voxel-wise analyses confirmed significant ROI findings of higher D2/3R availability in OB as compared with NW individuals in the SN/VTA, VST, and pallidum (Table 4 and Figure 1).
Table 3. Regional [11C](+)PHNO BPND (with SD) and Relative Differences Between Normal Weight (NW) and Obese (OB) Participants.
| Amygdala | Caudate | Hypothalamus | Pallidum | Putamen | SN/VTA | Thalamus | VST | |
|---|---|---|---|---|---|---|---|---|
| NW (n=14) | 0.27 (0.08) | 1.88 (0.32) | 1.24 (0.42) | 3.37 (0.39) | 2.52 (0.35) | 1.85 (0.36) | 0.35 (0.09) | 4.12 (0.52) |
| OB (n=14) | 0.30 (0.07) | 1.98 (0.42) | 1.27 (0.24) | 3.73 (0.47) | 2.73 (0.41) | 2.21 (0.42) | 0.37 (0.07) | 4.73 (0.58) |
| ΔOB % | +13 | +5 | +2 | +11 | +8 | +20 | +6 | +14 |
| p-Value | 0.22 | 0.47 | 0.81 | 0.02 | 0.14 | 0.02 | 0.54 | <0.01 |
Bold values indicate statistical significance.
Table 4. Details of BMI-Related Clusters Identified by Whole-Brain Voxel-Wise Analysis: Spatial Extent (k) in Voxels, MNI Coordinates (x, y, z) and t-score (t) of Peak Value.
| Region | k | x | y | z | t |
|---|---|---|---|---|---|
| L/R midbrain (SN/VTA) | 74 | 6 | −22 | −14 | 5.42 |
| R pallidum | 61 | 14 | −6 | −6 | 6.22 |
| L pallidum | 46 | −8 | −8 | −6 | 5.22 |
| L VST | 13 | −10 | 6 | −10 | 4.67 |
| R VST | 11 | 8 | 6 | −8 | 5.34 |
Figure 1.
Voxel-wise analyses of obese relative to normal weight [11C](+)PHNO BPND in the substantia nigra/ventral tegmental area (SN/VTA), the ventral striatum (VST), and the pallidum. Whole-brain results displayed at uncorrected p<0.001 and k>10; coronal coordinates in MNI space.
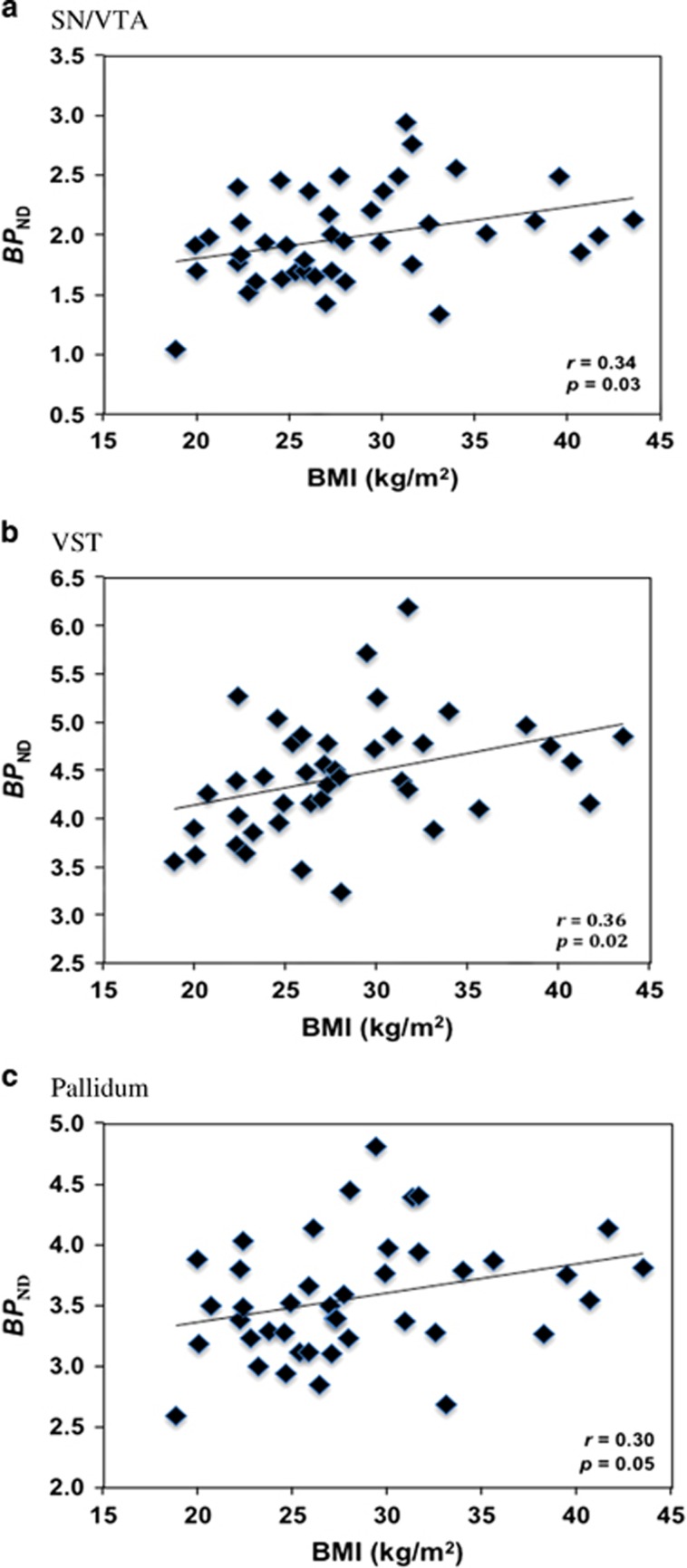
The secondary analyses employing a larger cohort to include OW individuals in the above-mentioned significant regions indicated that BMI was positively associated with D2/3R availability in the SN/VTA (r=0.34, p=0.029; Figure 2a), the VST (r=0.36, p=0.018; Figure 2b), and the pallidum (r=0.30, p=0.051; Figure 2c) across all subjects. Although these regions were targeted based on our between-group comparisons, none of these associations survived correction for multiple comparisons using Bonferroni correction (adjusted α threshold=0.05/3=0.017). That stated, the associations persisted after independently adjusting for age (SN/VTA: r=0.32, p=0.043; VST: r=0.37, p=0.018; pallidum: r=0.31, p=0.045), gender (SN/VTA: r=0.34, p=0.029; VST: r=0.37, p=0.017; pallidum: r=0.30, p=0.060), specific activity at time of injection (SN/VTA: r=0.34, p=0.031; VST: r=0.36, p=0.020; pallidum: r=0.31, p=0.049), injected mass (SN/VTA: r=0.32, p=0.043; VST: r=0.39, p=0.011; pallidum: r=0.29, p=0.071), and mean cerebellar mass concentration of unbound [11C](+)PHNO (SN/VTA: r=0.32, p=0.041; VST: r=0.39, p=0.013; pallidum: r=0.27, p=0.087).
Figure 2.
Unadjusted correlations between body mass index (BMI) and [11C](+)PHNO BPND in the substantia nigra/ventral tegmental area (SN/VTA) (a), in the ventral striatum (VST) (b), and in the pallidum (c).
DISCUSSION
To the best of our knowledge, the current study is the first to utilize the D3-preferring D2/3R agonist tracer [11C](+)PHNO to assess both striatal and extrastriatal D2/3R availability differences between otherwise healthy OB and age- and gender-matched NW individuals. Specifically, this work demonstrated that OB individuals exhibited higher D2/3Rs in both extrastriatal (ie, SN/VTA and pallidum) and striatal (ie, VST) regions associated with reward and motivation with the latter finding being the first published difference in any addiction-like condition with [11C](+)PHNO in the striatum. These results were unaffected by adjustment for potential confounding factors such as age (Ishibashi et al, 2009; Kim et al, 2011; Matuskey et al, 2016; Nakajima et al, 2015; Volkow et al, 2000) or tracer injection parameters, and were confirmed with a whole-brain voxel-wise analysis. Moreover, after adding a cohort of OW individuals, we observed a positive linear association between BMI and D2/3R availability in the aforementioned regions that also persisted after adjusting for age, gender, and tracer injection parameters.
Prior work with [11C](+)PHNO has demonstrated in non-OB individuals a positive linear association between BMI and tracer binding in the VST (Caravaggio et al, 2013). Our work extends those findings into an OB population when BMI categorical cohorts were combined. Other work with [11C](+)PHNO has also yielded a positive linear association between BMI and right dorsal caudate tracer binding in a wide-BMI-ranged cohort of 12 individuals (Cosgrove et al, 2015). The small cohort studied, as well as the unilateral nature of the finding in the dorsal caudate, warrants caution however. Additionally, the current work, one that includes the largest cohort to date examining D2/3R alterations imaged with [11C](+)PHNO as related to BMI, did not observe any receptor differences between OB and NW individuals in the dorsal striatum.
Much prior PET and obesity research has utilized D2/3R antagonist tracers. Like our current work, studies that used [18F]fallypride have demonstrated positive associations between BMI and striatal D2/3R availability (although in the dorsal and not the VST) in a large, wide-BMI-ranged cohort (Guo et al, 2014) as well as non-statistically significant higher tracer binding in the SN/VTA of OB as compared with NW females (Savage et al, 2014). That stated, our work is not consistent with studies that used other D2/3R antagonist tracers. For instance, work with [11C]raclopride has demonstrated reductions in striatal D2/3R availability in both severely obese (Wang et al, 2001) and OB (Haltia et al, 2007) individuals when compared with NW controls. Complicating the picture further, [11C]raclopride findings are not consistent with studies that utilized [11C]FLB457, which demonstrated BMI was positively associated with amygdalar D2/3R availability (Yasuno et al, 2001) or [11C]NMB, where no association between BMI and striatal D2/3R availability was found (Eisenstein et al, 2013). Such lack of agreement across the obesity and PET dopamine literature is complex and several factors could be responsible including differences in tracers and research participants. To that end, the radiotracer employed, [11C](+)PHNO, is responsible for at least part of these observed differences and worth further commentary as it has unique properties.
As previously mentioned, [11C](+)PHNO is a D2/3R agonist tracer that has demonstrated higher binding in D3R-predominant extrastriatal regions compared with D2R-predominant dorsal striatal regions (Graff-Guerrero et al, 2008; Narendran et al, 2006; Searle et al, 2010; Tziortzi et al, 2011). Such a preference might explain why the current study demonstrated higher tracer binding in regions densely populated with D3Rs (ie, the SN/VTA and the pallidum) and in the mixed D2/3R area of the VST when compared with studies that utilized D2/3R antagonist tracers that predominantly estimate D2Rs. These distinctions suggest that the D3R subtype and the D2R subtype may be regulated differently with respect to obesity, a finding also observed in the stimulant-use-disorders literature (Boileau et al, 2012; Lee et al, 2009; Martinez et al, 2004; Matuskey et al, 2014; Payer et al, 2014; Volkow et al, 1990, 2001). Further, the D3R has been implicated to be positively associated with behavioral impulsiveness and risky decision making, thus our findings of higher D3R availability in OB may be explained by underlying trait impulsivity (Payer et al, 2014). As such, these data suggest, albeit speculatively, that the D3R may be a novel pharmacologic target for the treatment of obesity. In fact, a prior study has demonstrated preliminary evidence that a D3R antagonist, when compared with placebo, may attenuate motivational attractiveness to palatable food cues in otherwise healthy OW and OB individuals (Mogg et al, 2012). However, a follow-up study done by the same group using the same D3R antagonist compound failed to show any evidence of changes in neural activation in response to food images on fMRI (Dodds et al, 2012), thus demonstrating the need for clarity in further studies involving D3R antagonist compounds in obesity.
In addition to providing an estimate of D3R sites, [11C](+)PHNO, as an agonist tracer, could bind to high-affinity G-protein-coupled forms of the D2R within the striatum that are presumed to be ‘active' (Shotbolt et al, 2012; Willeit et al, 2006). Although debate still exists whether this can be reliably measured in vivo (Seeman, 2012; Skinbjerg et al, 2012), in theory, only agonist tracers like [11C](+)PHNO would prefer these sites as D2/3R antagonist tracers typically bind with equal preference to both high- and low-affinity forms of the D2/3R. Therefore, it is possible that these differential binding characteristics could also help explain our VST findings such that the regulation of ‘active' high-affinity forms vs ‘inactive' low-affinity forms of the D2/3R may be altered in obesity. Our finding in the VST is also consistent with the previous [11C](+)PHNO work in non-OB individuals (Caravaggio et al, 2013) and preclinical research that posits the nucleus accumbens, a central component of the VST, plays a key role in the formation/development of the OB phenotype (Davis et al, 2008; Geiger et al, 2009; Hryhorczuk et al, 2016; Rada et al, 2010; Valenza et al, 2015). This regional specificity, along with tracer properties, may help explain why differences were not found in the dorsal striatum. Evidence does exist that obesity may be mediated by the dorsal striatum through work with preclinical research (Huang et al, 2006), functional connectivity (Contreras-Rodriguez et al, 2015), and other PET studies done in humans utilizing antagonist tracers (Haltia et al, 2007; Wang et al, 2001). However, our lack of findings here are not surprising considering [11C](+)PHNO has only recently been found to be significantly different in the dorsal striatum of humans with any addiction-like phenotype (Worhunsky et al, 2016), suggesting decreased sensitivity in this region.
Another potential explanation for dissimilarities between this study and other studies that employed antagonist tracers may be due to endogenous dopamine competition. Without utilizing a pharmacologic intervention such as the tyrosine-hydroxylase inhibitor α-methly-para-tyrosine (AMPT) to deplete tonic dopamine (Abi-Dargham et al, 2000; Caravaggio et al, 2014; Laruelle et al, 1997; Martinez et al, 2009), our outcome measure, BPND, reflects tracer bound to receptors not currently occupied by the endogenous neurotransmitter (ie, dopamine) and therefore measures receptor availability (Innis et al, 2007). [11C](+)PHNO has been shown to be substantially more sensitive to endogenous dopamine competition (ie, increased tracer displacement resulting in lower BPND) compared with the antagonist tracers used in prior studies (Cropley et al, 2008; Gallezot et al, 2014a; Ginovart et al, 2006; Moerlein et al, 1997; Shotbolt et al, 2012; Willeit et al, 2008); thus, our receptor availability findings could also be due to OB individuals exhibiting lower levels of tonic dopamine. In fact, this concept is consistent with prior reports that have shown diet-induced OB and OB-prone rats exhibited lower levels of tonic dopamine or less dopaminergic-mediated behaviors (ie, conditioned place preference or amphetamine-induced locomotor activity) compared with standard chow fed or OB-resistant controls (Geiger et al, 2009; Hryhorczuk et al, 2016; Rada et al, 2010).
There are several potential limitations of the current study worth discussing. First, this was a retrospective study, and we did not collect information such as percent adiposity, eating and exercising behavior questionnaires, impulsivity measures, and nutritional intake that would be potentially useful to relate to our PET measures. Future prospective studies may include collecting those measures as well as investigating exercise interventions to examine D2/3R transformations as BMI decreases. In fact, one study using [18F]fallypride has recently demonstrated that exercise intervention for 8 weeks, compared with psychoeducation, normalized lower striatal D2/3Rs in humans with methamphetamine-use disorder (Robertson et al, 2015). Second, although research participants were gender- and age-matched in the primary between-group comparisons, the cohort for the secondary correlational analyses was heavily dominated by males (33 males vs 9 females); thus, further correlational analyses confirming our findings are warranted in a gender-balanced cohort. Lastly, as previously mentioned, BPND only reflects PET tracer binding to available receptors and since [11C](+)PHNO is susceptible to endogenous dopamine fluctuations, it is not possible to reconcile whether our findings in OB individuals are driven by an upregulation of D2/3Rs or accounted for by lower levels of endogenous dopamine. Future studies employing an AMPT tonic dopamine depletion paradigm may be in order to resolve the underlying effect obesity is having on brain neurochemistry.
In summary, this study, to the best of our knowledge, is the first to examine both striatal and extrastriatal D2/3R availability differences between otherwise healthy OB and age-and gender-matched NW individuals utilizing the D3-preferring D2/3R agonist tracer [11C](+)PHNO. Our data suggest that D2/3Rs are higher in OB compared with NW individuals in regions densely populated with D3Rs, thus implicating the D3R as a potential pharmacologic target for the treatment of obesity. These data also implicate the same nigrostriatal pathway alterations in obesity that have been previously observed with [11C](+)PHNO in cocaine (Matuskey et al, 2014; Payer et al, 2014), methamphetamine (Boileau et al, 2012), and alcohol (Erritzoe et al, 2014) abusers, indicating excessive food consumption might alter reward pathways in similar ways as do substances of abuse. The direct role of the D3R in addiction is not currently clear (Boileau et al, 2015). A full discussion is beyond the scope of the current work because of the dynamic interactions of D3Rs that include dopamine autoreceptor properties and heterodimers that have diverse direct and indirect effects on GABA, adenosine, and glutamate systems (Casado-Anguera et al, 2016; Fiorentini et al, 2015; Leggio et al, 2015; Sokoloff et al, 2013). That being stated, one speculative mechanism to explain these phenomena could be that initial responses to ingestion of an addictive substance (ie, drug of abuse or high-fat palatable food) causes increases of dopamine which leads to a desensitization of postsynaptic striatal D2Rs and a compensatory presynaptic D3R upregulation in the SN/VTA on either autoreceptors or postsynaptic GABA neurons, both of which have negative control on dopamine neurons (Sokoloff et al, 2013).
FUNDING AND DISCLOSURE
This work was supported by the National Institute on Drug Abuse (NIDA) (K24 DA017899, 1R03DA027456-01, K02DA031750 to RTM; K12DA00167, JH; P20 DA027844 to RTM, MNP, and REC), the National Institute of Mental Health (NIMH; T32 MH019961 to DM/RTM), NARSAD Young Investigator Award Grant (M132018 to DM), the Yale PET Center, and the Yale Center for Clinical Investigation (YCCI) Pilot Projects Utilizing Core Technologies. This work was also made possible by CTSA Grant Number UL1 TR000142 from the National Center for Advancing Translational Science (NCATS), a component of the National Institutes of Health (NIH). MNP has received financial support or compensation for the following: MNP has been consulted for and advised Lundbeck, Ironwood, Shire, INSYS, Lakelight Therapeutics and Rivermend Health; has received research support from the National Institutes of Health, Veteran's Administration, Mohegan Sun Casino, the National Center for Responsible Gaming, and Pfizer; has participated in surveys, mailings or telephone consultations related to drug addiction, impulse control disorders or other health topics; has consulted for gambling entities, law offices and the federal public defender's office in issues related to impulse control disorders; provides clinical care in the Connecticut Department of Mental Health and Addiction Services Problem Gambling Services Program; has performed grant reviews for the National Institutes of Health and other agencies; has edited journals; has given academic lectures in grand rounds, CME events, and other clinical or scientific venues; and has written books or book chapters for publishers of mental health texts. The remaining authors declare no conflict of interest.
Acknowledgments
We thank the staff of the Yale PET Center, the Clinical Neuroscience Research Unit at the Connecticut Mental Health Center, the Hospital Research Unit of the Yale Center for Clinical Investigation at Yale-New Haven Hospital, the Yale Magnetic Resonance Research Center, and especially Nina Levine, Julie Holub, Jane Wanyiri, RN, Samantha Massaro, Elisa Hidalgo, Peter T Morgan, MD, PhD, and Margaret Dion-Marovitz, RN, MSN.
References
- Abi-Dargham A, Rodenhiser J, Printz D, Zea-Ponce Y, Gil R, Kegeles LS et al (2000). Increased baseline occupancy of D2 receptors by dopamine in schizophrenia. Proc Natl Acad Sci USA 97: 8104–8109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Psychiatric Association, American Psychiatric Association. Task Force on DSM-IV (2000) Diagnostic and Statistical Manual of Mental Disorders: DSM-IV-TR, 4th edn. American Psychiatric Association: Washington, DC. xxxvii, p 943. [Google Scholar]
- Ashburner J, Friston KJ (2005). Unified segmentation. Neuroimage 26: 839–851. [DOI] [PubMed] [Google Scholar]
- Boileau I, Nakajima S, Payer D (2015). Imaging the D3 dopamine receptor across behavioral and drug addictions: positron emission tomography studies with [(11)C]-(+)-PHNO. Eur Neuropsychopharmacol 25: 1410–1420. [DOI] [PubMed] [Google Scholar]
- Boileau I, Payer D, Houle S, Behzadi A, Rusjan PM, Tong J et al (2012). Higher binding of the dopamine D3 receptor-preferring ligand [11C]-(+)-propyl-hexahydro-naphtho-oxazin in methamphetamine polydrug users: a positron emission tomography study. J Neurosci 32: 1353–1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caravaggio F, Nakajima S, Borlido C, Remington G, Gerretsen P, Wilson A et al (2014). Estimating endogenous dopamine levels at D and D receptors in humans using the agonist radiotracer [C]-(+)-PHNO. Neuropsychopharmacology 39: 2769–2776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caravaggio F, Raitsin S, Gerretsen P, Nakajima S, Wilson A, Graff-Guerrero A (2013). Ventral striatum binding of a dopamine D receptor agonist but not antagonist predicts normal body mass index. Biol Psychiatry 77: 196–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carson RE, Barker WC, Liow J-S, Johnson CA (2003). Design of a motion compensation OSEM list-mode algorithm for resolution-recovery reconstruction of the HRRT. In: Conference Record, IEEE Nuclear Science Symposium and Medical Imaging Conference; Portland, OR, pp 3281–3285.
- Casado-Anguera V, Bonaventura J, Moreno E, Navarro G, Cortes A, Ferre S et al (2016). Evidence for the heterotetrameric structure of the adenosine A2A-dopamine D2 receptor complex. Biochem Soc Trans 44: 595–600. [DOI] [PubMed] [Google Scholar]
- Contreras-Rodriguez O, Martin-Perez C, Vilar-Lopez R, Verdejo-Garcia A (2015). Ventral and dorsal striatum networks in obesity: link to food craving and weight gain. Biol Psychiatry pii: S0006-3223(15)00997-X. doi:10.1016/j.biopsych.2015.11.020. [DOI] [PubMed] [Google Scholar]
- Cosgrove KP, Veldhuizen MG, Sandiego CM, Morris ED, Small DM (2015). Opposing relationships of BMI with BOLD and dopamine D2/3 receptor binding potential in the dorsal striatum. Synapse 69: 195–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cropley VL, Innis RB, Nathan PJ, Brown AK, Sangare JL, Lerner A et al (2008). Small effect of dopamine release and no effect of dopamine depletion on [18 F]fallypride binding in healthy humans. Synapse 62: 399–408. [DOI] [PubMed] [Google Scholar]
- Culver KE, Szechtman H, Levant B (2008). Altered dopamine D2-like receptor binding in rats with behavioral sensitization to quinpirole: effects of pre-treatment with Ro 41-1049. Eur J Pharmacol 592: 67–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalley JW, Fryer TD, Brichard L, Robinson ES, Theobald DE, Laane K et al (2007). Nucleus accumbens D2/3 receptors predict trait impulsivity and cocaine reinforcement. Science 315: 1267–1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis JF, Tracy AL, Schurdak JD, Tschop MH, Lipton JW, Clegg DJ et al (2008). Exposure to elevated levels of dietary fat attenuates psychostimulant reward and mesolimbic dopamine turnover in the rat. Behav Neurosci 122: 1257–1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodds CM, O'Neill B, Beaver J, Makwana A, Bani M, Merlo-Pich E et al (2012). Effect of the dopamine D3 receptor antagonist GSK598809 on brain responses to rewarding food images in overweight and obese binge eaters. Appetite 59: 27–33. [DOI] [PubMed] [Google Scholar]
- Eisenstein SA, Antenor-Dorsey JA, Gredysa DM, Koller JM, Bihun EC, Ranck SA et al (2013). A comparison of D2 receptor specific binding in obese and normal-weight individuals using PET with (N-[(11)C]methyl)benperidol. Synapse 67: 748–756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erritzoe D, Tziortzi A, Bargiela D, Colasanti A, Searle GE, Gunn RN et al (2014). In vivo imaging of cerebral dopamine D3 receptors in alcoholism. Neuropsychopharmacology 39: 1703–1712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field AE, Coakley EH, Must A, Spadano JL, Laird N, Dietz WH et al (2001). Impact of overweight on the risk of developing common chronic diseases during a 10-year period. Arch Intern Med 161: 1581–1586. [DOI] [PubMed] [Google Scholar]
- Fiorentini C, Savoia P, Bono F, Tallarico P, Missale C (2015). The D3 dopamine receptor: from structural interactions to function. Eur Neuropsychopharmacol 25: 1462–1469. [DOI] [PubMed] [Google Scholar]
- Flegal KM, Carroll MD, Kit BK, Ogden CL (2012). Prevalence of obesity and trends in the distribution of body mass index among US adults, 1999–2010. JAMA 307: 491–497. [DOI] [PubMed] [Google Scholar]
- Gallezot JD, Beaver JD, Gunn RN, Nabulsi N, Weinzimmer D, Singhal T et al (2012). Affinity and selectivity of [(1)(1)C]-(+)-PHNO for the D3 and D2 receptors in the rhesus monkey brain in vivo. Synapse 66: 489–500. [DOI] [PubMed] [Google Scholar]
- Gallezot JD, Kloczynski T, Weinzimmer D, Labaree D, Zheng MQ, Lim K et al (2014. a). Imaging nicotine- and amphetamine-induced dopamine release in rhesus monkeys with [(11)C]PHNO vs [(11)C]raclopride PET. Neuropsychopharmacology 39: 866–874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallezot JD, Zheng MQ, Lim K, Lin SF, Labaree D, Matuskey D et al (2014. b). Parametric imaging and test-retest variability of 11C-(+)-PHNO binding to D2/D3 dopamine receptors in humans on the high-resolution research tomograph PET scanner. J Nucl Med 55: 960–966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geiger BM, Haburcak M, Avena NM, Moyer MC, Hoebel BG, Pothos EN (2009). Deficits of mesolimbic dopamine neurotransmission in rat dietary obesity. Neuroscience 159: 1193–1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginovart N, Galineau L, Willeit M, Mizrahi R, Bloomfield PM, Seeman P et al (2006). Binding characteristics and sensitivity to endogenous dopamine of [11C]-(+)-PHNO, a new agonist radiotracer for imaging the high-affinity state of D2 receptors in vivo using positron emission tomography. J Neurochem 97: 1089–1103. [DOI] [PubMed] [Google Scholar]
- Ginovart N, Willeit M, Rusjan P, Graff A, Bloomfield PM, Houle S et al (2007). Positron emission tomography quantification of [11C]-(+)-PHNO binding in the human brain. J Cereb Blood Flow Metab 27: 857–871. [DOI] [PubMed] [Google Scholar]
- Graff-Guerrero A, Willeit M, Ginovart N, Mamo D, Mizrahi R, Rusjan P et al (2008). Brain region binding of the D2/3 agonist [11C]-(+)-PHNO and the D2/3 antagonist [11C]raclopride in healthy humans. Hum Brain Mapp 29: 400–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo J, Simmons WK, Herscovitch P, Martin A, Hall KD (2014). Striatal dopamine D2-like receptor correlation patterns with human obesity and opportunistic eating behavior. Mol Psychiatry 19: 1078–1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haltia LT, Rinne JO, Merisaari H, Maguire RP, Savontaus E, Helin S et al (2007). Effects of intravenous glucose on dopaminergic function in the human brain in vivo. Synapse 61: 748–756. [DOI] [PubMed] [Google Scholar]
- Hryhorczuk C, Florea M, Rodaros D, Poirier I, Daneault C, Des Rosiers C et al (2016). Dampened mesolimbic dopamine function and signaling by saturated but not monounsaturated dietary lipids. Neuropsychopharmacology 41: 811–821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang XF, Zavitsanou K, Huang X, Yu Y, Wang H, Chen F et al (2006). Dopamine transporter and D2 receptor binding densities in mice prone or resistant to chronic high fat diet-induced obesity. Behav Brain Res 175: 415–419. [DOI] [PubMed] [Google Scholar]
- Innis RB, Cunningham VJ, Delforge J, Fujita M, Gjedde A, Gunn RN et al (2007). Consensus nomenclature for in vivo imaging of reversibly binding radioligands. J Cereb Blood Flow Metab 27: 1533–1539. [DOI] [PubMed] [Google Scholar]
- Ishibashi K, Ishii K, Oda K, Kawasaki K, Mizusawa H, Ishiwata K (2009). Regional analysis of age-related decline in dopamine transporters and dopamine D2-like receptors in human striatum. Synapse 63: 282–290. [DOI] [PubMed] [Google Scholar]
- Kessler RM, Zald DH, Ansari MS, Li R, Cowan RL (2014). Changes in dopamine release and dopamine D2/3 receptor levels with the development of mild obesity. Synapse 68: 317–320. [DOI] [PubMed] [Google Scholar]
- Kim JH, Son YD, Kim HK, Lee SY, Cho SE, Kim YB et al (2011). Effects of age on dopamine D2 receptor availability in striatal subdivisions: a high-resolution positron emission tomography study. Eur Neuropsychopharmacol 21: 885–891. [DOI] [PubMed] [Google Scholar]
- Lakdawalla DN, Bhattacharya J, Goldman DP (2004). Are the young becoming more disabled? Health Aff (Millwood) 23: 168–176. [DOI] [PubMed] [Google Scholar]
- Laruelle M, D'Souza CD, Baldwin RM, Abi-Dargham A, Kanes SJ, Fingado CL et al (1997). Imaging D2 receptor occupancy by endogenous dopamine in humans. Neuropsychopharmacology 17: 162–174. [DOI] [PubMed] [Google Scholar]
- Lee B, London ED, Poldrack RA, Farahi J, Nacca A, Monterosso JR et al (2009). Striatal dopamine d2/d3 receptor availability is reduced in methamphetamine dependence and is linked to impulsivity. J Neurosci 29: 14734–14740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leggio GM, Torrisi SA, Castorina A, Platania CB, Impellizzeri AA, Fidilio A et al (2015). Dopamine D3 receptor-dependent changes in alpha6 GABAA subunit expression in striatum modulate anxiety-like behaviour: responsiveness and tolerance to diazepam. Eur Neuropsychopharmacol 25: 1427–1436. [DOI] [PubMed] [Google Scholar]
- Martinez D, Broft A, Foltin RW, Slifstein M, Hwang DR, Huang Y et al (2004). Cocaine dependence and d2 receptor availability in the functional subdivisions of the striatum: relationship with cocaine-seeking behavior. Neuropsychopharmacology 29: 1190–1202. [DOI] [PubMed] [Google Scholar]
- Martinez D, Greene K, Broft A, Kumar D, Liu F, Narendran R et al (2009). Lower level of endogenous dopamine in patients with cocaine dependence: findings from PET imaging of D(2)/D(3) receptors following acute dopamine depletion. Am J Psychiatry 166: 1170–1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matuskey D, Gaiser EC, Gallezot JD, Angarita GA, Pittman B, Nabulsi N et al (2015). A preliminary study of dopamine D2/3 receptor availability and social status in healthy and cocaine dependent humans imaged with [(11)C](+)PHNO. Drug Alcohol Depend 154: 167–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matuskey D, Gallezot JD, Pittman B, Williams W, Wanyiri J, Gaiser E et al (2014). Dopamine D(3) receptor alterations in cocaine-dependent humans imaged with [(1)(1)C](+)PHNO. Drug Alcohol Depend 139: 100–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matuskey D, Worhunksy P, Correa E, Pittman B, Gallezot JD, Nabulsi N et al (2016). Age-related changes in binding of the D receptor radioligand [C](+)PHNO in healthy volunteers. Neuroimage 130: 241–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mawlawi O, Martinez D, Slifstein M, Broft A, Chatterjee R, Hwang DR et al (2001). Imaging human mesolimbic dopamine transmission with positron emission tomography: I. Accuracy and precision of D(2) receptor parameter measurements in ventral striatum. J Cereb Blood Flow Metab 21: 1034–1057. [DOI] [PubMed] [Google Scholar]
- Mizrahi R, Agid O, Borlido C, Suridjan I, Rusjan P, Houle S et al (2011). Effects of antipsychotics on D3 receptors: a clinical PET study in first episode antipsychotic naive patients with schizophrenia using [11C]-(+)-PHNO. Schizophr Res 131: 63–68. [DOI] [PubMed] [Google Scholar]
- Moerlein SM, Perlmutter JS, Markham J, Welch MJ (1997). In vivo kinetics of [18F](N-methyl)benperidol: a novel PET tracer for assessment of dopaminergic D2-like receptor binding. J Cereb Blood Flow Metab 17: 833–845. [DOI] [PubMed] [Google Scholar]
- Mogg K, Bradley BP, O'Neill B, Bani M, Merlo-Pich E, Koch A et al (2012). Effect of dopamine D(3) receptor antagonism on approach responses to food cues in overweight and obese individuals. Behav Pharmacol 23: 603–608. [DOI] [PubMed] [Google Scholar]
- Mokdad AH, Ford ES, Bowman BA, Dietz WH, Vinicor F, Bales VS et al (2003). Prevalence of obesity, diabetes, and obesity-related health risk factors, 2001. JAMA 289: 76–79. [DOI] [PubMed] [Google Scholar]
- Must A, Spadano J, Coakley EH, Field AE, Colditz G, Dietz WH (1999). The disease burden associated with overweight and obesity. JAMA 282: 1523–1529. [DOI] [PubMed] [Google Scholar]
- Nakajima S, Caravaggio F, Boileau I, Chung JK, Plitman E, Gerretsen P et al (2015). Lack of age-dependent decrease in dopamine D3 receptor availability: a [(11)C]-(+)-PHNO and [(11)C]-raclopride positron emission tomography study. J Cereb Blood Flow Metab 35: 1812–1818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narendran R, Slifstein M, Guillin O, Hwang Y, Hwang DR, Scher E et al (2006). Dopamine (D2/3) receptor agonist positron emission tomography radiotracer [11C]-(+)-PHNO is a D3 receptor preferring agonist in vivo. Synapse 60: 485–495. [DOI] [PubMed] [Google Scholar]
- Payer DE, Behzadi A, Kish SJ, Houle S, Wilson AA, Rusjan PM et al (2014). Heightened D3 dopamine receptor levels in cocaine dependence and contributions to the addiction behavioral phenotype: a positron emission tomography study with [11C]-+-PHNO. Neuropsychopharmacology 39: 311–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rada P, Bocarsly ME, Barson JR, Hoebel BG, Leibowitz SF (2010). Reduced accumbens dopamine in Sprague-Dawley rats prone to overeating a fat-rich diet. Physiol Behav 101: 394–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson CL, Ishibashi K, Chudzynski J, Mooney LJ, Rawson RA, Dolezal BA et al (2015). Effect of exercise training on striatal dopamine D2/D3 receptors in methamphetamine users during behavioral treatment. Neuropsychopharmacology 41: 1629–1636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savage SW, Zald DH, Cowan RL, Volkow ND, Marks-Shulman PA, Kessler RM et al (2014). Regulation of novelty seeking by midbrain dopamine D2/D3 signaling and ghrelin is altered in obesity. Obesity (Silver Spring) 22: 1452–1457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Searle G, Beaver JD, Comley RA, Bani M, Tziortzi A, Slifstein M et al (2010). Imaging dopamine D3 receptors in the human brain with positron emission tomography, [11C]PHNO, and a selective D3 receptor antagonist. Biol Psychiatry 68: 392–399. [DOI] [PubMed] [Google Scholar]
- Seeman P (2012). Dopamine agonist radioligand binds to both D2High and D2Low receptors, explaining why alterations in D2High are not detected in human brain scans. Synapse 66: 88–93. [DOI] [PubMed] [Google Scholar]
- Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E et al (1998). The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry 59(Suppl 20): 22–33; quiz 34–57. [PubMed] [Google Scholar]
- Shotbolt P, Tziortzi AC, Searle GE, Colasanti A, van der Aart J, Abanades S et al (2012). Within-subject comparison of [(11)C]-(+)-PHNO and [(11)C]raclopride sensitivity to acute amphetamine challenge in healthy humans. J Cereb Blood Flow Metab 32: 127–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skinbjerg M, Sibley DR, Javitch JA, Abi-Dargham A (2012). Imaging the high-affinity state of the dopamine D2 receptor in vivo: fact or fiction? Biochem Pharmacol 83: 193–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokoloff P, Leriche L, Diaz J, Louvel J, Pumain R (2013). Direct and indirect interactions of the dopamine D(3) receptor with glutamate pathways: implications for the treatment of schizophrenia. Naunyn Schmiedebergs Arch Pharmacol 386: 107–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sturm R, Ringel JS, Andreyeva T (2004). Increasing obesity rates and disability trends. Health Aff (Millwood) 23: 199–205. [DOI] [PubMed] [Google Scholar]
- Thanos PK, Michaelides M, Ho CW, Wang GJ, Newman AH, Heidbreder CA et al (2008). The effects of two highly selective dopamine D3 receptor antagonists (SB-277011 A and NGB-2904) on food self-administration in a rodent model of obesity. Pharmacol Biochem Behav 89: 499–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tziortzi AC, Searle GE, Tzimopoulou S, Salinas C, Beaver JD, Jenkinson M et al (2011). Imaging dopamine receptors in humans with [11C]-(+)-PHNO: dissection of D3 signal and anatomy. Neuroimage 54: 264–277. [DOI] [PubMed] [Google Scholar]
- Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N et al (2002). Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage 15: 273–289. [DOI] [PubMed] [Google Scholar]
- Ujike H, Onoue T, Akiyama K, Hamamura T, Otsuki S (1989). Effects of selective D-1 and D-2 dopamine antagonists on development of methamphetamine-induced behavioral sensitization. Psychopharmacology (Berl) 98: 89–92. [DOI] [PubMed] [Google Scholar]
- Val-Laillet D, Aarts E, Weber B, Ferrari M, Quaresima V, Stoeckel LE et al (2015). Neuroimaging and neuromodulation approaches to study eating behavior and prevent and treat eating disorders and obesity. Neuroimage Clin 8: 1–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valenza M, Steardo L, Cottone P, Sabino V (2015). Diet-induced obesity and diet-resistant rats: differences in the rewarding and anorectic effects of D-amphetamine. Psychopharmacology (Berl) 232: 3215–3226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Chang L, Wang GJ, Fowler JS, Ding YS, Sedler M et al (2001). Low level of brain dopamine D2 receptors in methamphetamine abusers: association with metabolism in the orbitofrontal cortex. Am J Psychiatry 158: 2015–2021. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS, Wolf AP, Schlyer D, Shiue CY, Alpert R et al (1990). Effects of chronic cocaine abuse on postsynaptic dopamine receptors. Am J Psychiatry 147: 719–724. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Logan J, Fowler JS, Wang GJ, Gur RC, Wong C et al (2000). Association between age-related decline in brain dopamine activity and impairment in frontal and cingulate metabolism. Am J Psychiatry 157: 75–80. [DOI] [PubMed] [Google Scholar]
- Wang GJ, Volkow ND, Logan J, Pappas NR, Wong CT, Zhu W et al (2001). Brain dopamine and obesity. Lancet 357: 354–357. [DOI] [PubMed] [Google Scholar]
- Willeit M, Ginovart N, Graff A, Rusjan P, Vitcu I, Houle S et al (2008). First human evidence of d-amphetamine induced displacement of a D2/3 agonist radioligand: A [11C]-(+)-PHNO positron emission tomography study. Neuropsychopharmacology 33: 279–289. [DOI] [PubMed] [Google Scholar]
- Willeit M, Ginovart N, Kapur S, Houle S, Hussey D, Seeman P et al (2006). High-affinity states of human brain dopamine D2/3 receptors imaged by the agonist [11C]-(+)-PHNO. Biol Psychiatry 59: 389–394. [DOI] [PubMed] [Google Scholar]
- Worhunsky P, Matuskey D, Gallezot J, Gaiser E, Nabulsi N, Angarita G et al (2016). Concurrently upregulated and downregulated D2/D3 receptor systems in cocaine use disorder using [11C]PHNO. J Nucl Med Suppl 57: 16. [Google Scholar]
- Wu Y, Carson RE (2002). Noise reduction in the simplified reference tissue model for neuroreceptor functional imaging. J Cereb Blood Flow Metab 22: 1440–1452. [DOI] [PubMed] [Google Scholar]
- Yasuno F, Suhara T, Sudo Y, Yamamoto M, Inoue M, Okubo Y et al (2001). Relation among dopamine D(2) receptor binding, obesity and personality in normal human subjects. Neurosci Lett 300: 59–61. [DOI] [PubMed] [Google Scholar]
- Yi SJ, Johnson KM (1990). Chronic cocaine treatment impairs the regulation of synaptosomal 3H-DA release by D2 autoreceptors. Pharmacol Biochem Behav 36: 457–461. [DOI] [PubMed] [Google Scholar]