Abstract
Background:
Systemic lupus erythematosus (SLE) is a multifaceted disease, and its diagnosis may be challenging. A blood test for the diagnosis of SLE, the Avise Lupus test, has been recently commercialized and validated in clinical studies.
Objectives:
To evaluate the use of the Avise Lupus test by community rheumatologists.
Methods:
The study is a longitudinal, case-control, retrospective review of medical charts. Cases had a positive test result, and controls had a negative result; all patients were anti-nuclear antibodies (ANA) positive but negative for SLE-specific autoantibodies. Features of SLE, diagnosis, and medications at two time points were recorded.
Results:
Twenty of the 23 cases (87%) and 4 of the 23 controls (17%) were diagnosed with SLE (sensitivity=83%; specificity=86%). More cases than controls (43% vs. 17%) fulfilled 4 American College of Rheumatology (ACR) classification criteria of SLE. Sensitivity of the test was significantly higher than the ACR score (83% vs. 42%, p=0.006). A higher percentage of patients who met the classification criteria had elevated cell-bound complement activation products (CB-CAPs) compared to patients who did not. Anti-rheumatic medications were used in a higher percentage of cases than controls (83% vs. 35% at baseline, p=0.002), suggesting that cases were treated more aggressively early on.
Conclusion:
A positive Avise Lupus test result aids in formulating a SLE diagnosis when diagnosis based on standard-of-care tests and clinical features may be challenging, and impacts patient management. Prospective studies will be performed to better evaluate the clinical utility of the test and of CB-CAPs as biomarkers of SLE.
Keywords: Biomarkers, Case-control study, Diagnostic test, Medical chart review
INTRODUCTION
Systemic lupus erythematosus (SLE) is an autoimmune disease affecting mainly women and characterized by alternating periods of disease activity (flares) and remission. Incidence in the US is approximately 5.5 to 7 per 100,000 person-years and prevalence is 73 to 92 per 100,000 person-years [1, 2]. SLE manifests at an earlier age and is more frequent and aggressive in African-Americans and Hispanics than in Caucasians [2-4]. Symptoms of lupus, especially in the initial stages of the disease, are often non-specific and may occur also in patients with other connective tissue diseases (CTDs) [5], making differential diagnosis challenging [6].
SLE classification criteria have been developed by the American College of Rheumatology (ACR) and the Systemic Lupus International Classification Clinics (SLICC) [7-9] and include clinical and immunological features of SLE. Although non-specific symptoms of SLE, such as fatigue, fever, weight loss, arthralgia, and myalgia [10] are excluded from classification criteria, many features of SLE may be subjective and difficult to assess. In addition, these instruments have not been validated for diagnostic purposes [11, 12].
In clinical practice, the diagnosis of SLE remains particularly challenging because the disease is heterogeneous, and because symptoms are often subjective and mimic those of other diseases [10, 11]. The real-word “gold-standard” for diagnosis of SLE is the physician’s diagnosis [13, 14], which could take time and may be unreliable. SLE is a frequent diagnosis (20-60%) in patients who evolve over time from undifferentiated CTD to a specific CTD [6], however it may take several years for a definitive diagnosis to be made [15, 16]. On the other hand, early diagnosis and targeted and timely therapy may prevent flares and late organ damage [17], improve quality of life [18], and decrease healthcare utilization and costs [19-21].
In addition to the diagnostic challenges outlined above, there are limitations with standard-of-care diagnostic immunology tests [10]; anti-nuclear antibodies (ANA) have high diagnostic sensitivity but high false positivity rate among healthy individuals [22, 23], while antibodies to double-stranded DNA (anti-dsDNA) and/or to Smith antigen (anti-Smith) [24] have low sensitivity and are negative in many SLE patients [25, 26]. Although low levels of complement proteins C3 and C4 may be an indication of ongoing inflammation in SLE and have been incorporated in the SLICC classification criteria [9], measurement of C3 and C4 per se has several drawbacks, including low sensitivity, high inter-subject variability, and increased synthesis during inflammation to compensate for increased consumption [26, 27]. Complement activation results in the formation of complement activation products that bind covalently to blood cells [11, 28, 29]. The performance characteristics of these cell-bound complement activation products (CB-CAPs) have recently been validated in a prospective multicenter clinical study [30] that showed that elevated B lymphocyte complement 4 derived ligand (BC4d) or erythrocyte complement 4 derived ligand (EC4d) have 22% higher sensitivity than low complement for the diagnosis of SLE.
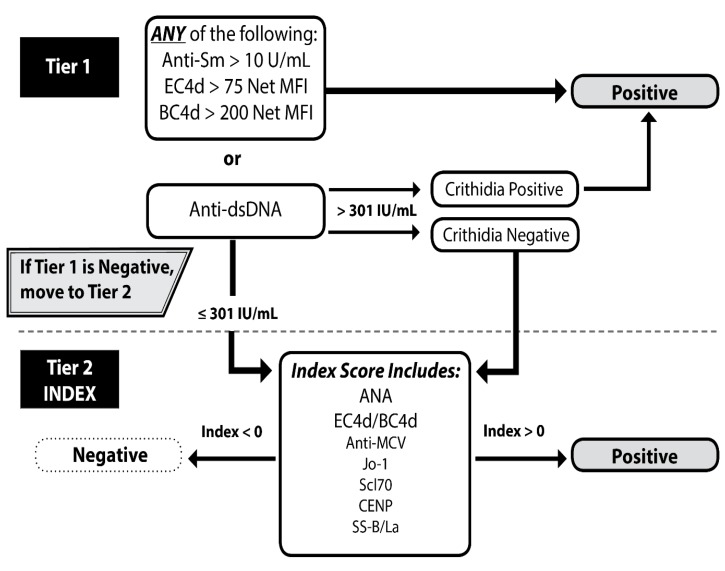
The Avise Lupus test incorporates the levels of autoantibodies and of Exagen’s proprietary biomarkers, EC4d and BC4d, into a 2-tiered method (Fig. 1) [30]. Patients are considered tier 1 positive if titer of specific autoantibodies is above cut-off (anti-dsDNA > 301 units and positivity is confirmed by the Crithidia luciliae immunofluorescence test, or anti-Smith > 10 units), or if CB-CAPs are strongly positive (EC4d > 75 units or BC4d > 200 units). If tier 1 is negative, an algorithm that takes into account the levels of ANA, EC4d, BC4d, and the specificity component is calculated [30]. The specificity component is represented by autoantibodies associated with diseases other than SLE, i.e., antibodies to MCV, SS-B/La, CENP, Jo-1, and Scl-70. The specificity component is assigned a value of 0 in the algorithm if all these antibodies are negative (≤ 10 units) and a value of 1 if any of these antibodies is positive (> 10 units). The 2-tiered method has high sensitivity and specificity in differentiating patients diagnosed with SLE based on the ACR classification criteria, including childhood onset SLE [31], from patients diagnosed with other CTDs [30] or fibromyalgia [32].
Fig. (1).
The 2-tiered model of the Avise Lupus test.
The present study is a retrospective chart review to evaluate whether the test, in combination with clinical features, aids the differential and early diagnosis of SLE - and, consequently, timely therapeutic intervention and improved prognosis - in patients treated by community rheumatologists.
METHODS
This study is a retrospective case-control study that required review of patients’ medical records by the treating rheumatologists. The study was approved by a central Institutional Review Board; because no patient visits were necessary, and risk to patients was minimal, a waiver of informed consent was granted.
Selection of Patients
Patients were selected by Exagen on the basis of the results of the Avise Lupus test conducted as described [30] between February and December 2014. As SLE affects mainly females, females 18 years and older at the time the test was performed were included; males were not included in this study to avoid a possible gender imbalance between cases and controls. Patient selection was carried out with the intent of identifying patients for whom a differential diagnosis, based on their immunological profile, may be challenging. In fact, all patients selected for the study were ANA positive by enzyme-linked immunosorbent assay, but negative for biomarkers specific for SLE: all patients had anti-dsDNA ≤ 301 units and anti-Smith ≤ 10 units. In addition, all patients were negative for the specificity component of the test (anti-MCV, anti-SS-B/La, anti-CENP, anti-Jo-1, anti-Scl-70 all negative: ≤ 10 units). Cases are defined as having a positive result for the Avise Lupus test (tier 1 positive or tier 2 index between 0.5 and 5), while controls had a negative test result (tier 1 negative and tier 2 index between -0.5 and -5) [30]. Patients with an index score between -0.5 and 0.5 were not selected for the study as this interval is borderline negative or positive, and provides lower confidence in the clinical interpretation of the test result.
Less than 5% of the patients for whom the Avise Lupus test was conducted between February and December 2014 met the rigorous selection criteria described above for cases. To avoid selection bias, each case was matched with a control from the same clinic and with the same ANA level (≥ 20-60 or > 60 units). The selection of controls based on these requirements further decreased the number of patients who could be included in the study; 41 pairs of cases and controls - for a total of 82 patients - were identified for the study, representing approximately 0.3% of the initial pool of patients.
Study Design
The rheumatologists who participated in the study were asked to review medical charts of patients they suspected had SLE, but for whom they had not made a definite diagnosis prior to ordering the test, and to exclude patients who they had treated with immunosuppressants (methotrexate, anti-TNFs, belimumab, mycophenolate mofetil, azathioprine, cyclophosphamide) or high doses of corticosteroids (e.g. prednisone > 7.5 mg per day) prior to ordering the test.
Investigators, who were not blinded to the results of the test, reviewed all the medical charts during the observation period, which started with the baseline visit (Time 0 [T0]), defined as the visit when the results of the Avise Lupus test became available. Two Case Report Forms per patient were completed: at T0, and at Time 1 (T1), defined as the time when the chart review was performed. The interval between T0 and T1 was approximately 1 year, and at least 1 visit in the rheumatologist office occurred during this time.
Investigators recorded features of SLE according to the ACR [8] or SLICC criteria [9] at T0 and at T1. They also indicated at both time points what diagnosis they made, based on their clinical expertise, and medications; in addition, they indicated at T1 whether the patient experienced flares between T0 and T1.
Statistical Analysis
For all 2x2 contingency tables, the statistical significance of differences was assessed using the Fisher’s exact test. For all paired two sample comparisons, differences in biomarker values for the two paired samples were assessed using a Wilcoxon signed rank test.
RESULTS
The study sample consisted of a total of 46 patients whose charts were reviewed between May and July 2015 by 10 rheumatologists. Although a total of 41 pairs of patients were identified for this period - as T0 was approximately 1 year earlier - 23 pairs were actually completed (56%). The remaining 18 pairs could not be included in the study; the most common reason for exclusion was that 1 of the patients in the pair did not have any follow up visits, or had less than 9 months of follow up. All patients selected for the study were females and had similar demographic and clinical characteristics (Table 1). Most patients had arthritis (tender and/or swollen joints); other common features of SLE (photosensitivity, malar rash, and ulcers) were more prevalent amongst cases than controls (Table 1).
Table 1. Characteristics of the patients included in the study.
| Cases (n=23) | Controls (n=23) | ||
|---|---|---|---|
| Age (years) at T0 | Median (range) |
53 (21-77) |
55 (21-82) |
| Race/ethnicity N, (%) |
Whites | 14 (61%) |
14 (61%) |
| African-Americans | 6 (26%) |
6 (26%) |
|
| Hispanics | 3 (13%) |
2 (9%) |
|
| Asians | 0 (0%) |
1 (4%) |
|
| Common clinical features of SLE at T0 N, (%) |
Tender joints | 15 (65%) |
15 (65%) |
| Swollen joints | 7 (30%) |
7 (30%) |
|
| Photosensitivity | 11 (48%) |
4 (17%) |
|
| Malar rash | 5 (22%) |
3 (13%) |
|
| Ulcers | 3 (13%) |
2 (9%) |
Values are the number (percentage) of cases and controls within a category. T0: visit when the results of the Avise Lupus test became available.
As indicated under Methods, all cases were Avise Lupus test positive; of these, 4 were tier 1 positive [30] because either EC4d or BC4d were strongly positive. Twenty of the 23 cases (87%) were diagnosed by the investigator with SLE at T0 (n=17) or between T0 and T1 (n=3). Of the 3 cases diagnosed with SLE after T0, 1 was tier 1 positive; of the 3 cases not diagnosed with SLE, 1 of them was tier 1 positive. One of the 3 cases not diagnosed with SLE presented with photosensitivity and ulcers; the other 2 did not present with clinical features of SLE. All cases with a diagnosis of SLE at T0 had the same diagnosis at T1.
The majority (n=19) of controls had a diagnosis different from SLE, or no diagnosis; the negative Avise Lupus test result was overruled for only 4 controls, who were diagnosed with SLE during the study period (3 at T0 and 1 after T0). The controls diagnosed with SLE at T0 had the same diagnosis at T1. Interestingly, only 1 case (4%) vs. 7 controls (30%) did not get any definite diagnosis during the study period.
The Avise Lupus test had excellent performance characteristics, as compared to the physician’s diagnosis, with sensitivity of 83.3%, specificity of 86.4%, positive predictive value of 87.0%, and negative predictive value of 82.6% (positive likelihood ratio: 6.125 and negative likelihood ratio: 0.19).
Fulfillment of 4 ACR classification criteria of SLE is considered the gold-standard in SLE research. A higher percentage of cases met the ACR classification criteria at T0 than controls (10 cases (43%) vs. 4 controls (17%)). On average, the ACR score at T0 was higher in cases than controls (2.9 vs. 2.3, respectively, p=0.044). The 10 cases who met the ACR classification criteria of SLE were all diagnosed with SLE: 9 at T0 and 1 after T0, when the patient developed a fifth feature. Only 4 controls (17%) met the same classification criteria, however none of them was diagnosed with SLE. The difference in sensitivity between the ACR score and the Avise Lupus test was highly significant (p=0.006) (Table 2).
Table 2. Sensitivity and specificity of the ACR score and of the Avise Lupus test.
| Physician diagnosis of SLE (n=24) | ||||
|---|---|---|---|---|
| ACR score ≥ 4 | ACR score 1 to 3 | Cases | Controls | |
| Number of patients | 10 | 14 | 20 | 4 |
| Sensitivity | ACR score: 41.7% | Avise Lupus test: 83.3% | ||
| Physician diagnosis different from SLE, or no diagnosis (n=22) | ||||
| ACR score ≥ 4 | ACR score 1 to 3 | Cases | Controls | |
| Number of patients | 4 | 18 | 3 | 19 |
| Specificity | ACR score: 81.8% | Avise Lupus test: 86.4% | ||
Number of patients indicates the number of patients within a category. Sensitivity and specificity values are also indicated. Physician diagnosis was based on the clinical expertise of the rheumatologist investigator, and is considered the gold-standard for the calculation of sensitivity and specificity values reported above. Difference in sensitivity between ACR score (41.7%) and Avise Lupus test (83.3%): p=0.006. ACR: American College of Rheumatology; SLE: Systemic Lupus Erythematosus.
Some immunological features that are part of the SLICC classification criteria (in particular, complement and Coomb’s test) were often indicated as “not evaluated” by the rheumatologists, precluding the calculation of the SLICC score.
A higher percentage of patients who met the ACR criteria had EC4d above the cutoff value of 14 mean fluorescence intensity units, compared to patients who did not meet the ACR criteria (43% vs. 22%). A similar trend was observed for BC4d, although difference did not reach statistical significance. This data is in agreement with a previous study where diagnosis of SLE was based on fulfillment of the ACR criteria [30].
Corticosteroids (prednisone and methylprednisolone), methotrexate, and hydroxychloroquine were more utilized in cases than in controls at T0 (p=0.002) (Table 3) and throughout the study; in fact, difference in the use of these medications was observed also when combining data at T0 and T1 (data not shown).
Table 3. Use of medications (corticosteroids, methotrexate, and hydroxychloroquine) in cases and controls at T0.
| One or more medication (n=27) |
None of the medications (n=19) |
|
|---|---|---|
| Cases (n=23) | 19 (83%) |
4 (17%) |
| Controls (n=23) | 8 (35%) |
15 (65%) |
Values are the number (percentage) of patients within a category; p=0.002. T0: visit when the results of the Avise Lupus test became available.
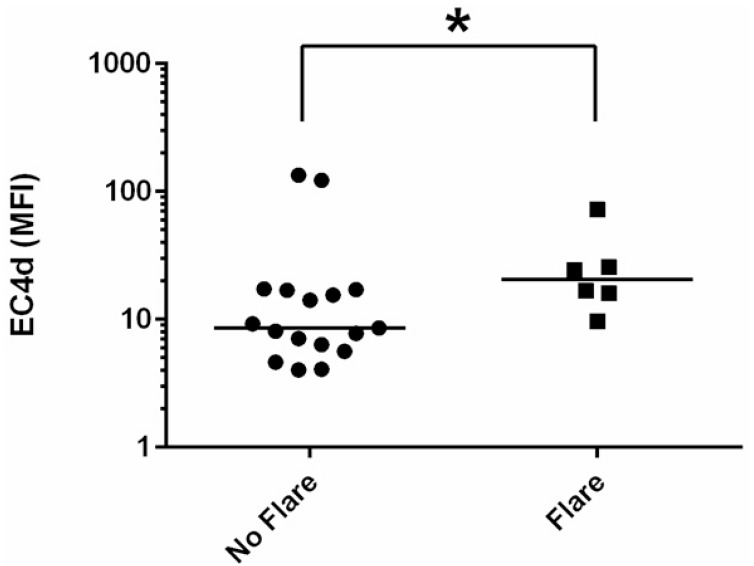
SLE-related flares were reported for 6 cases (6/23, 26%) between T0 and T1, and were of mild or moderate severity. Cases who experienced flares had higher EC4d than cases who did not (n=17) (p=0.0499) (Fig. 2). Although African-Americans and Hispanics were the minority of cases (9/23, 39%), they were the majority of cases who experienced flares (4/6, 67%).
Fig. (2).
EC4d values in patients with flares. EC4d values (in mean fluorescence intensity units, MFI) and median (horizontal bars) in cases who experienced SLE-related flares (n=6) vs. cases who did not (n=17). *: p = 0.0499. EC4d: erythrocyte complement 4 derived ligand.
DISCUSSION
Diagnostic tests for SLE that measure autoantibodies (ANA, anti-dsDNA, and anti-Smith) and complement proteins have low sensitivity or specificity [22-25]. The Avise Lupus test was developed to address these limitations: by incorporating objective measurement of CB-CAPs and autoantibodies associated with CTDs, validation studies demonstrated that the test has high sensitivity and specificity [30-32].
We decided to investigate in this study whether the test aids the diagnosis of SLE in community rheumatology settings. To this end, we selected patients for whom a diagnosis of SLE would be particularly challenging based on standard-of-care tests that measure circulating autoantibodies. In fact, all patients selected for the study were positive for ANA - which has high sensitivity but low specificity [22, 23] - but negative for autoantibodies specific for SLE [24-26]. Cases were defined as patients with a positive Avise Lupus test result, while controls had a negative test result. To avoid selection bias, each case was matched with a control with the same ANA status (positive or strong positive), and chart reviews were done by the treating rheumatologist only if both patients in a pair could be included in the study. This assured that each investigator reviewed charts of matched cases and controls who had approximately 1 year of history. This interval of time was chosen to evaluate whether new features of SLE manifested, if features that were present at T0 resolved, or if diagnosis changed over time. It should be noted, however, that a period of time of 1 year may be insufficient to evaluate whether new features of SLE presented, as progression from undifferentiated CTD to SLE takes, on average, a longer period of time [15, 16]. In fact, minimal changes in clinical features occurred in our patient population between T0 and T1. Because of the rigorous selection criteria based on immunological characteristics, and because of the steps taken to avoid selection bias, only a small percentage of the entire pool of patients could be selected for this study. In addition, approximately 40% of the selected pairs could not be included in the study, mainly because 1 of the 2 patients did not have sufficient follow up. This suggests that the remaining patients might have been more involved in their healthcare, or were otherwise more motivated to continue to see their rheumatologist. It is unlikely that the patients included in the study were sicker, because the average physician’s global assessment was medium/low (1.36±0.77 on a scale 0-3) for the 18 patients (15 cases and 3 controls) for whom the value was provided at T0 (data not shown). Although the observation that our patient population had features of SLE commonly observed in patients with early or established disease [1, 3, 15] indicates that the patients in our study are a good representation of SLE patients encountered in clinical practice, it is unclear whether the results of this study can be extrapolated to the general population of patients in community rheumatology clinics.
The majority of patients (20/23, 87%; 17 at T0, and 3 after T0) with a positive test result (cases) had a diagnosis of SLE, while the majority of controls (19/23, 83%) did not get this diagnosis. Thus, the test has excellent performance characteristics: sensitivity of 83.3%, specificity of 86.4%, positive predictive value of 87.0%, negative predictive value of 82.6%, positive likelihood ratio of 6.125, and negative likelihood ratio of 0.19. As none of the SLE diagnosis given at T0 was changed to a non-SLE diagnosis afterwards, our data suggest that a positive test result helped the rheumatologists assign a definite diagnosis of SLE early on, and that confidence in the initial diagnosis was high. In addition, the test was able to predict a SLE diagnosis in the 3 cases who were diagnosed after T0.
A higher percentage of cases than controls was treated with corticosteroids or anti-rheumatic medications (methotrexate and hydroxychloroquine), consistent with the hypothesis that a diagnosis of SLE led to a more aggressive and targeted treatment, possibly improving patient outcome over time [17-19].
A higher percentage of cases met the ACR classification criteria than controls and the average ACR score was higher in cases than controls demonstrating, as expected, that the Avise Lupus test parallels the ACR score. Interestingly, all cases who met the ACR classification criteria of SLE (10/23, 43%) were diagnosed with SLE. On the contrary, only 4 controls (17%) met the same classification criteria, and none of them was diagnosed with SLE. The ACR classification criteria had low sensitivity in our study, strongly suggesting that classification criteria are not widely used in clinical practice to make a diagnosis of SLE in early lupus [12]. In addition, it is well accepted that some features of SLE are often subjective or overlapping, and that more objective tools are required to make an accurate and timely diagnosis. Our study suggests that the Avise Lupus test could represent such tool as it has higher sensitivity and specificity than the ACR score; the difference in sensitivity (83.3% vs. 41.7%) was highly significant (p=0.006). The ACR score has been reported to have higher sensitivity than what we observed [9]; different patient populations and investigators, and different use of the classification criteria may explain this difference. Although the SLICC criteria could represent an advantage for the diagnosis of SLE [13, 14], SLICC criteria are not diagnostic criteria, either. In addition, we could not calculate a SLICC score for many patients because of missing data, as some features necessary for the calculation of the SLICC score were not evaluated by the rheumatologists. Taken together, these findings confirm the limited utility of classification criteria in clinical practice.
Although it is unclear, from our study, what led to a diagnosis of SLE in the absence of fulfillment of classification criteria, it is well accepted that rheumatologists take into account symptoms that are somewhat non-specific, e.g. fatigue, fever, weight loss, arthralgia and myalgia, or Raynaud’s phenomenon [10]: it is possible that some of these symptoms were present in our patient population and were taken into account for the physician diagnosis, although were not recorded for the purpose of this study.
We observed that a higher percentage of patients who met the ACR criteria had elevated CB-CAPs compared to the percentage of patients who did not. This finding indicates that previous data [30, 32] that showed elevated CB-CAPs in patients who fulfilled the ACR criteria for SLE hold true in community rheumatology settings where diagnosis is not necessarily based on classification criteria.
Interestingly, 6 cases, all diagnosed with SLE at T0, experienced a mild to moderate flare based on the investigators’ assessment. Cases with a flare had higher EC4d at T0 than cases who did not have a flare. Several studies have attempted to identify predictors of flares in patients with SLE [26, 33, 34]. A recent study found that low baseline C3 levels predict severe flares in a large patient population [33], and another demonstrated that EC3d and EC4d parallel SLE disease activity [27], supporting our findings. Although African-Americans were a minority in the overall case population, they were the majority of cases with flares, consistent with the fact that SLE is more aggressive in African-Americans than whites [1-4].
Our study has limitations, including its retrospective nature, the small number of patients, and the fact that it is uncertain whether only patients not previously diagnosed with a particular condition by the investigators were included. It cannot be excluded that a selection bias existed on the part of the physician based on his or her concern for lupus, and the ability of the patient or health insurance to pay for the test. In addition, investigators were not blinded to the results of the test and a diagnostic review bias cannot be excluded. Nonetheless, this bias was limited, as apparent by the following observations: i) In a small but significant number of patients, the investigators overruled the Avise Lupus test results, for both cases (3/23, 13%) and controls (4/23, 17%), even when test results were strongly positive (tier 1 positive); ii) Three cases, 1 of whom was strongly positive, did not get any diagnosis at T0 and were diagnosed with SLE later. This indicates that clinical evaluation over time was incorporated in the final diagnosis; iii) In the period of approximately 1 year between T0 and T1, none of the SLE diagnosis was changed, suggesting high confidence in the initial diagnosis; iv) The investigators who completed chart reviews of multiple pairs of patients (5 and 4 pairs) not always made a diagnosis consistent with the test result, indicating limited bias.
The results of this study indicate that the Avise Lupus test, as an objective laboratory test that combines 2 components of complement activation and autoantibodies associated with rheumatic diseases, aids the diagnosis of SLE in patients for whom a diagnosis, based on standard-of-care immunological tests and clinical features, could be difficult. Timely diagnosis impacts patient management, and may prevent late organ damage. Additional studies will be performed to evaluate prospectively the value of the test and of CB-CAPs in establishing a diagnosis of SLE in difficult to diagnose patients, and to evaluate whether the test predicts disease progression over time.
CONCLUSION
This case-control study used rigorous criteria to identify patients for whom a diagnosis of SLE, based on standard-of-care immunological tests such as ANA, anti-dsDNA, and anti-Smith, may be difficult. The Avise Lupus test, which measures CB-CAPs and autoantibodies commonly observed in patients with CTDs, showed excellent performance characteristics, as compared to the rheumatologist diagnosis, and had higher sensitivity than the ACR score. These results support earlier findings that CB-CAPs are valuable biomarkers of SLE. Use of anti-rheumatic medications was more prevalent in test positive patients than in patients who had a negative test result, indicating that the Avise Lupus test impacts patient management and may improve outcome. In conclusion, the Avise Lupus test aids in the diagnosis of SLE when immunological and clinical feature of disease, which are often subjective, are insufficient.
ACKNOWLEDGEMENTS
We wish to thank all the investigators and their staff who participated in the study. We gratefully acknowledge Dr. Thierry Dervieux of Exagen Diagnostics and Dr. Wade Aubry, Hoang-Lan Tran, and Jacob Graham of Quorum Consulting for critically reviewing the manuscript. The technical assistance of John Conklin for data management is gratefully acknowledged.
SOURCE OF FUNDING
The study was funded by Exagen Diagnostics, Inc.
CONFLICT OF INTEREST
Drs. Alexander and Barken are employees of Exagen Diagnostics, Inc.
REFERENCES
- 1.Lim S.S., Bayakly A.R., Helmick C.G., Gordon C., Easley K.A., Drenkard C. The incidence and prevalence of systemic lupus erythematosus, 2002-2004. Arthritis Rheum. 2014;66:357–368. doi: 10.1002/art.38239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Somers E.C., Marder W., Cagnoli P., Lewis E.E., DeGuire P., Gordon C., Helmick C.G., Wang L., Wing J.J., Dhar J.P., Leisen J., Shaltis D., McCune W.J. Population-based incidence and prevalence of systemic lupus erythematosus: the Michigan Lupus Epidemiology and Surveillance program. Arthritis Rheum. 2014;66(2):369–378. doi: 10.1002/art.38238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alarcón G.S., McGwin G., Jr, Roseman J.M., Uribe A., Fessler B.J., Bastian H.M., Friedman A.W., Baethge B., Vilá L.M., Reveille J.D., Lumina Study Group. Natural History of the Accrual of the American College of Rheumatology Criteria Prior to the Occurrence of Criteria Diagnosis Systemic lupus erythematosus in three ethnic groups. XIX. Natural history of the accrual of the American College of Rheumatology criteria prior to the occurrence of criteria diagnosis. Arthritis Rheum. 2004;51(4):609–615. doi: 10.1002/art.20548. [DOI] [PubMed] [Google Scholar]
- 4.Urowitz M.B., Gladman D.D., Ibañez D., Fortin P.R., Bae S.C., Gordon C., Clarke A., Bernatsky S., Hanly J.G., Isenberg D., Rahman A., Sanchez-Guerrero J., Wallace D.J., Ginzler E., Alarcón G.S., Merrill J.T., Bruce I.N., Sturfelt G., Nived O., Steinsson K., Khamashta M., Petri M., Manzi S., Ramsey-Goldman R., Dooley M.A., van Vollenhoven R.F., Ramos M., Stoll T., Zoma A., Kalunian K., Aranow C. Evolution of disease burden over five years in a multicenter inception systemic lupus erythematosus cohort. Arthritis Care Res. (Hoboken) 2012;64(1):132–137. doi: 10.1002/acr.20648. [DOI] [PubMed] [Google Scholar]
- 5.Doria A., Mosca M., Gambari P.F., Bombardieri S. Defining unclassifiable connective tissue diseases: incomplete, undifferentiated, or both? J. Rheumatol. 2005;32(2):213–215. [PubMed] [Google Scholar]
- 6.Mosca M., Tani C., Vagnani S., Carli L., Bombardieri S. The diagnosis and classification of undifferentiated connective tissue diseases. J. Autoimmun. 2014;48-49:50–52. doi: 10.1016/j.jaut.2014.01.019. [DOI] [PubMed] [Google Scholar]
- 7.Tan E.M., Cohen A.S., Fries J.F., Masi A.T., McShane D.J., Rothfield N.F., Schaller J.G., Talal N., Winchester R.J. The 1982 revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1982;25(11):1271–1277. doi: 10.1002/art.1780251101. [DOI] [PubMed] [Google Scholar]
- 8.Hochberg M.C. Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1997;40(9):1725. doi: 10.1002/art.1780400928. [DOI] [PubMed] [Google Scholar]
- 9.Petri M., Orbai A.M., Alarcón G.S., Gordon C., Merrill J.T., Fortin P.R., Bruce I.N., Isenberg D., Wallace D.J., Nived O., Sturfelt G., Ramsey-Goldman R., Bae S.C., Hanly J.G., Sánchez-Guerrero J., Clarke A., Aranow C., Manzi S., Urowitz M., Gladman D., Kalunian K., Costner M., Werth V.P., Zoma A., Bernatsky S., Ruiz-Irastorza G., Khamashta M.A., Jacobsen S., Buyon J.P., Maddison P., Dooley M.A., van Vollenhoven R.F., Ginzler E., Stoll T., Peschken C., Jorizzo J.L., Callen J.P., Lim S.S., Fessler B.J., Inanc M., Kamen D.L., Rahman A., Steinsson K., Franks A.G., Jr, Sigler L., Hameed S., Fang H., Pham N., Brey R., Weisman M.H., McGwin G., Jr, Magder L.S. Derivation and validation of the Systemic Lupus International Collaborating Clinics classification criteria for systemic lupus erythematosus. Arthritis Rheum. 2012;64(8):2677–2686. doi: 10.1002/art.34473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bertsias G.K., Pamfil C., Fanouriakis A., Boumpas D.T. Diagnostic criteria for systemic lupus erythematosus: has the time come? Nat. Rev. Rheumatol. 2013;9(11):687–694. doi: 10.1038/nrrheum.2013.103. [DOI] [PubMed] [Google Scholar]
- 11.Manzi S., Navratil J.S., Ruffing M.J., Liu C.C., Danchenko N., Nilson S.E., Krishnaswami S., King D.E., Kao A.H., Ahearn J.M. Measurement of erythrocyte C4d and complement receptor 1 in systemic lupus erythematosus. Arthritis Rheum. 2004;50(11):3596–3604. doi: 10.1002/art.20561. [DOI] [PubMed] [Google Scholar]
- 12.Liu C.C., Manzi S., Danchenko N., Ahearn J.M. New advances in measurement of complement activation: lessons of systemic lupus erythematosus. Curr. Rheumatol. Rep. 2004;6(5):375–381. doi: 10.1007/s11926-004-0012-5. [DOI] [PubMed] [Google Scholar]
- 13.Amezcua-Guerra L.M., Higuera-Ortiz V., Arteaga-García U., Gallegos-Nava S., Hübbe-Tena C. Performance of the 2012 Systemic Lupus International Collaborating Clinics and the 1997 American College of Rheumatology classification criteria for systemic lupus erythematosus in a real-life scenario. Arthritis Care Res. (Hoboken) 2015;67(3):437–441. doi: 10.1002/acr.22422. [DOI] [PubMed] [Google Scholar]
- 14.Pons-Estel G.J., Wojdyla D., McGwin G., Jr, Magder L.S., Petri M.A., Pons-Estel B.A., Alarcón G.S., Grupo Latino Americano De Estudio del Lupus (GLADEL) LUMINA cohort The American College of Rheumatology and the Systemic Lupus International Collaborating Clinics classification criteria for systemic lupus erythematosus in two multiethnic cohorts: a commentary. Lupus. 2014;23(1):3–9. doi: 10.1177/0961203313512883. [DOI] [PubMed] [Google Scholar]
- 15.Bodolay E., Csiki Z., Szekanecz Z., Ben T., Kiss E., Zeher M., Szücs G., Dankó K., Szegedi G. Five-year follow-up of 665 Hungarian patients with undifferentiated connective tissue disease (UCTD). Clin. Exp. Rheumatol. 2003;21(3):313–320. [PubMed] [Google Scholar]
- 16.Guerrero L.F., Rueda J.C., Arciniegas R., Rueda J.M. Undifferentiated connective tissue disease in a rheumatology center in Cali, Colombia: clinical features of 94 patients followed for a year. Rheumatol. Int. 2013;33(4):1085–1088. doi: 10.1007/s00296-011-2234-y. [DOI] [PubMed] [Google Scholar]
- 17.Doria A., Gatto M., Zen M., Iaccarino L., Punzi L. Optimizing outcome in SLE: treating-to-target and definition of treatment goals. Autoimmun. Rev. 2014;13(7):770–777. doi: 10.1016/j.autrev.2014.01.055. [DOI] [PubMed] [Google Scholar]
- 18.Urowitz M., Gladman D.D., Ibañez D., Sanchez-Guerrero J., Bae S.C., Gordon C., Fortin P.R., Clarke A., Bernatsky S., Hanly J.G., Wallace D.J., Isenberg D., Rahman A., Merrill J., Ginzler E., Alarcón G.S., Fessler B., Khamashta M., Steinsson K., Petri M., Dooley M., Bruce I.N., Manzi S., Sturfelt G., Nived O., Ramsey-Goldman R., Zoma A., Maddison P., Kalunian K., van Vollenhoven R., Aranow C., Romero Diaz J., Stoll T. Changes in quality of life in the first 5 years of disease in a multicenter cohort of patients with systemic lupus erythematosus. Arthritis Care Res. (Hoboken) 2014;66(9):1374–1379. doi: 10.1002/acr.22299. [DOI] [PubMed] [Google Scholar]
- 19.Oglesby A., Korves C., Laliberté F., Dennis G., Rao S., Suthoff E.D., Wei R., Duh M.S. Impact of early versus late systemic lupus erythematosus diagnosis on clinical and economic outcomes. Appl. Health Econ. Health Policy. 2014;12(2):179–190. doi: 10.1007/s40258-014-0085-x. [DOI] [PubMed] [Google Scholar]
- 20.Meacock R., Dale N., Harrison M.J. The humanistic and economic burden of systemic lupus erythematosus : a systematic review. Pharmacoeconomics. 2013;31(1):49–61. doi: 10.1007/s40273-012-0007-4. [DOI] [PubMed] [Google Scholar]
- 21.Narayanan S., Wilson K., Ogelsby A., Juneau P., Durden E. Economic burden of systemic lupus erythematosus flares and comorbidities in a commercially insured population in the United States. J. Occup. Environ. Med. 2013;55(11):1262–1270. doi: 10.1097/JOM.0000000000000008. [DOI] [PubMed] [Google Scholar]
- 22.Satoh M., Chan E.K., Ho L.A., Rose K.M., Parks C.G., Cohn R.D., Jusko T.A., Walker N.J., Germolec D.R., Whitt I.Z., Crockett P.W., Pauley B.A., Chan J.Y., Ross S.J., Birnbaum L.S., Zeldin D.C., Miller F.W. Prevalence and sociodemographic correlates of antinuclear antibodies in the United States. Arthritis Rheum. 2012;64(7):2319–2327. doi: 10.1002/art.34380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Meroni P.L., Schur P.H. ANA screening: an old test with new recommendations. Ann. Rheum. Dis. 2010;69(8):1420–1422. doi: 10.1136/ard.2009.127100. [DOI] [PubMed] [Google Scholar]
- 24.Suresh E. Systemic lupus erythematosus: diagnosis for the non-specialist. Br. J. Hosp. Med. (Lond.) 2007;68(10):538–541. doi: 10.12968/hmed.2007.68.10.27324. [DOI] [PubMed] [Google Scholar]
- 25.Hanly J.G., Thompson K., McCurdy G., Fougere L., Theriault C., Wilton K. Measurement of autoantibodies using multiplex methodology in patients with systemic lupus erythematosus. J. Immunol. Methods. 2010;352(1-2):147–152. doi: 10.1016/j.jim.2009.10.003. [DOI] [PubMed] [Google Scholar]
- 26.Liu C.C., Manzi S., Kao A.H., Navratil J.S., Ahearn J.M. Cell-bound complement biomarkers for systemic lupus erythematosus: from benchtop to bedside. Rheum. Dis. Clin. North Am. 2010;36(1):161–172. doi: 10.1016/j.rdc.2009.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kao A.H., Navratil J.S., Ruffing M.J., Liu C.C., Hawkins D., McKinnon K.M., Danchenko N., Ahearn J.M., Manzi S. Erythrocyte C3d and C4d for monitoring disease activity in systemic lupus erythematosus. Arthritis Rheum. 2010;62(3):837–844. doi: 10.1002/art.27267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kalunian K.C., Chatham W.W., Massarotti E.M., Reyes-Thomas J., Harris C., Furie R.A., Chitkara P., Putterman C., Gross R.L., Somers E.C., Kirou K.A., Ramsey-Goldman R., Hsieh C., Buyon J.P., Dervieux T., Weinstein A. Measurement of cell-bound complement activation products enhances diagnostic performance in systemic lupus erythematosus. Arthritis Rheum. 2012;64(12):4040–4047. doi: 10.1002/art.34669. [DOI] [PubMed] [Google Scholar]
- 29.Yang D.H., Chang D.M., Lai J.H., Lin F.H., Chen C.H. Usefulness of erythrocyte-bound C4d as a biomarker to predict disease activity in patients with systemic lupus erythematosus. Rheumatology (Oxford) 2009;48(9):1083–1087. doi: 10.1093/rheumatology/kep161. [DOI] [PubMed] [Google Scholar]
- 30.Putterman C., Furie R., Ramsey-Goldman R., Askanase A., Buyon J., Kalunian K., Chatham W.W., Massarotti E., Kirou K., Jordan N., Blanco I., Weinstein A., Chitkara P., Manzi S., Ahearn J., OMalley T., Conklin J., Ibarra C., Barken D., Dervieux T. Cell-bound complement activation products in systemic lupus erythematosus: comparison with anti-double-stranded DNA and standard complement measurements. Lupus Sci. Med. 2014;1(1):e000056. doi: 10.1136/lupus-2014-000056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Askanase A, Hui-Yuen J, Conklin J, et al. Cell-Bound Complement Activation Products have high sensitivity and specificity in childhood-onset systemic lupus erythematosus and juvenile idiopathic arthritis [abstract]. Arthritis Rheumatol. 2015;67(Suppl 10) [Google Scholar]
- 32.Wallace D.J., Silverman S.L., Conklin J., Barken D., Dervieux T. Systemic lupus erythematosus and primary fibromyalgia can be distinguished by testing for cell-bound complement activation products. Lupus Sci. Med. 2016;3(1):e000127. doi: 10.1136/lupus-2015-000127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Petri M.A., van Vollenhoven R.F., Buyon J., Levy R.A., Navarra S.V., Cervera R., Zhong Z.J., Freimuth W.W., BLISS-52 and BLISS-76 Study Groups Baseline predictors of systemic lupus erythematosus flares: data from the combined placebo groups in the phase III belimumab trials. Arthritis Rheum. 2013;65(8):2143–2153. doi: 10.1002/art.37995. [DOI] [PubMed] [Google Scholar]
- 34.Floris A., Piga M., Cauli A., Mathieu A. Predictors of flares in Systemic Lupus Erythematosus: Preventive therapeutic intervention based on serial anti-dsDNA antibodies assessment. Analysis of a monocentric cohort and literature review. Autoimmun. Rev. 2016;15(7):656–663. doi: 10.1016/j.autrev.2016.02.019. [DOI] [PubMed] [Google Scholar]