Abstract
Glucagon-like peptide-1 (GLP-1) has shown to influence the oxidative stress status in a number of in vitro, in vivo and clinical studies. Well-known effects of GLP-1 including better glycemic control, decreased food intake, increased insulin release and increased insulin sensitivity may indirectly contribute to this phenomenon, but glucose-independent effects on ROS level, production and antioxidant capacity have been suggested to also play a role. The potential ‘antioxidant’ activity of GLP-1 along with other proposed glucose-independent modes of action related to ameliorating redox imbalance remains a controversial topic but could hold a therapeutic potential against micro- and macrovascular diabetic complications. This review discusses the presently available knowledge from experimental and clinical studies on the effects of GLP-1 on oxidative stress in diabetes and diabetes-related complications.
Keywords: Glucagon-like peptide-1, oxidative stress, diabetes, diabetic complications
1. INTRODUCTION
Diabetes can lead to the development of late complications described as microvascular complications including retinopathy, nephropathy, neuropathy and macrovascular complications comprising cardiovascular diseases (CVD) [1], the latter being the leading cause of death among diabetic patients [2, 3]. The mechanisms underlying the development of complications in diabetes are still not completely understood. However, a hypothesis involving oxidative stress (OS) and inflammation as potent causative initiators of diabetic complications in general has been put forward [4, 5].
Glucagon-like peptide-1 (GLP-1) constitutes a relatively recent therapeutic option for type 2 diabetic patients. GLP-1 receptor agonists, as the approved pharmaceuticals liraglutide, exenatide, lixinatide, albiglutide and dulaglutide are designed to be more resistant to degradation than endogenous GLP-1 that with a half-life of less than two minutes has little therapeutic potential [6]. Pharmacological levels of these GLP-1 analogues are used to mimic incretin effects [7], i.e. glucose lowering, stimulation of glucose-dependent insulin secretion, and decrease of glucagon release [8, 9]. GLP-1 can to some extent preserve β-cell function and inhibit apoptosis [10, 11]. In rodents, GLP-1 analogues have been shown to stimulate proliferation of β-cells, but it is still debated whether this occurs in humans [10, 12]. The clinical effects include decreased appetite and induction of body weight loss [10, 13]. Moreover, GLP-1 and its agonists have the potential to lower postprandial glucose excursions, which may otherwise be associated with endothelial dysfunction, inflammation and OS [14, 15]. Lately, a beneficial role of GLP-1 in inflammation has gained increasing interest as reports have shown that it is released in an interleukin-6 (IL-6) dependent manner [16, 17]. GLP-1 levels has been found to be increased in both atherosclerosis [18] and patients with ventricular systolic dysfunction [19] indicating that GLP-1 could be induced as a compensatory mechanism during disease progression where OS may also play a role. Experimental [20-22] and clinical [23, 24] studies have indicated, that GLP-1 can reduce OS under a variety of conditions making it important to investigate if these effects are dependent or independent of GLP-1’s effects on glucose homeostasis (insulinotropic and glucose-lowering effects). The present review discusses the available literature on the role of GLP-1 as a redox modulator.
2. DIABETES AND OXIDATIVE STRESS
The “unifying theory” proposed by Brownlee [4] in 2001 suggests that hyperglycemia - a hallmark of both type 1 and type 2 diabetes (T1D and T2D) - can lead to increased OS if the antioxidant capacity is exceeded. OS is characterized as an imbalance between produced reactive oxygen species (ROS)/reactive nitrogen species (RNS) and the antioxidant capacity. According to the hypothesis, OS plays a pivotal role in the development of diabetes related late complications in both T1D and T2D [4, 25, 26]. It should be mentioned that OS may also play an important role in the development of both types of diabetes (reviewed in [27]). In short, β-cells are vulnerable to injury from high levels of ROS due to a comparatively low expression of antioxidant enzymes [28] and consequently, elevated OS conditions are likely to cause β-cell loss [28]. Importantly, loss of these cells cannot readily be restored because of their long lifespan and low proliferation rate [29, 30]. The loss of β-cells in turn results in further increasing blood glucose, OS and insulin resistance, thus starting a vicious circle (discussed in 2.1.).
2.1. Sources of Oxidative Stress in Diabetes
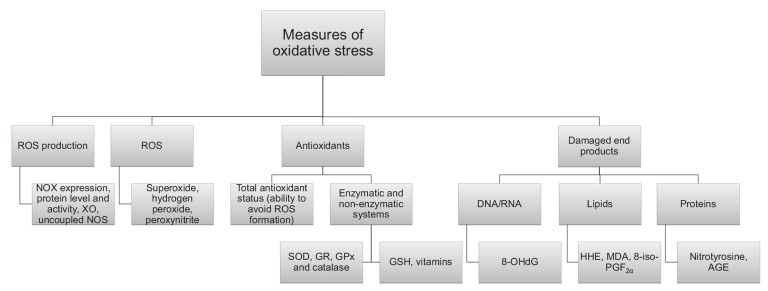
OS as measured both directly as ROS and by a large variety of biomarkers (Fig. 1) has consistently been shown to be elevated in diabetes [4, 14]. Mitochondrial production of the ROS, superoxide and increased activation of the protein kinase C (PKC) pathway in diabetic vascular tissue have been hypothesized to be major contributors to excess ROS production together with enzymatic systems such as nicotinamide adenine dinucleotide phosphate-oxidase (NOX), which is a membrane-bound superoxide producing enzyme [4]. Elevated plasma concentrations of glucose and free fatty acids (FFA), which is observed in T2D and poorly controlled T1D subjects seems to be the main drivers of ROS production [5, 31-34]. This is also the case in other disorders with elevated FFA’s as dyslipidemia and obesity (reviewed in [35]). Proinflammatory cytokines and excessive levels of nutrients can increase expression of phagocytic-like NOX in rat β-cells [36]. Thus, OS is an important factor in glucotoxicity and lipotoxicity as well as the combined glucolipotoxicity [37]. Superoxide produced under these conditions has shown to damage and impair mitochondrial integrity and function in vivo and in vitro [38, 39]. Mitochondrial dysfunction also leads to increased ROS leakage from the electron transport chain, and has been associated with induction of insulin resistance resulting in higher blood glucose levels [40]. Fig. (2) summarizes the putative mechanism leading to OS in diabetes.
Fig. (1).
ROS production/level or antioxidant capacity. OS status can be assessed by different means including the measurement of amounts and activities of ROS producing enzymes, ROS levels per se, antioxidant levels or damaged end products and adducts.
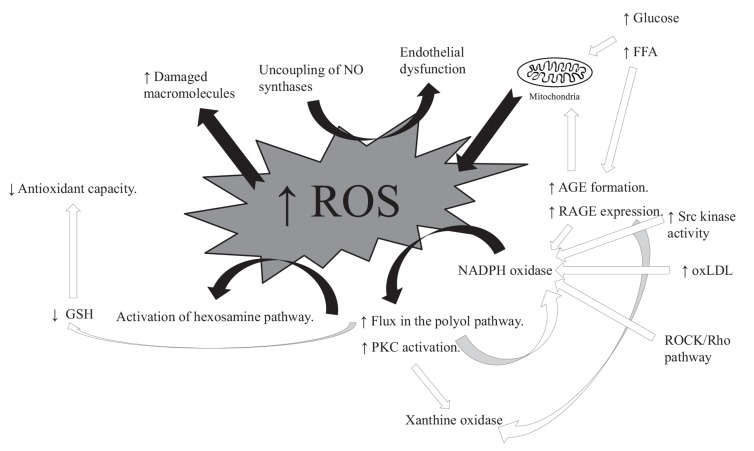
Fig. (2).
Putative mechanisms leading to OS in diabetes. Five pathways are potentially activated, 1) Enlarged flux of monosaccharides through the polyol pathway [5]. 2) Increased formation of advanced glycation end-product (AGE) and 3) expression of advanced glycation end-product receptors (RAGE) [47]. 4) Activation of PKC [5] and 5) increased flux in the hexosamine pathway [5]. These are all downstream pathways from one common event, the production of ROS [5]. Damages through activation of the polyol pathway are conducted as NADPH is degraded, a process leading to a lower level of glutathione (GSH), thus decreasing antioxidant capacity. In addition, the pathway increases the level of the NOX substrate, NADH, thus favoring ROS production [48]. AGEs are formed when proteins or lipids are glycated in contact with for example glucose or other aldose sugars [49], and when these bind to RAGE the result can be production of ROS through activation of NOX [50, 51], xanthine oxidase (XO) and the mitochondrial electron transport chain [51]. The protein kinase C (PKC) pathway is stimulated when diacylglycerol (DAG) levels are elevated during hyperglycemia. The increased activity of PKC has many different effects on various genes, leading to changes in blood flow, increased inflammation and ROS production (by NOX) [52, 53]. A consequence can be an increase in NF-κβ and release of inflammatory cytokines and growth factors in macrophages or mesangial cells [5]. Elevated glucose and FFA concentrations increase flow through the hexosamine pathway as well as induce OS [54]. The activation of these pathways initiates further ROS production. ROS can damage macromolecules including LDL. The resulting oxidized LDL (oxLDL) can lead to further NOX activation and thus further superoxide production [55]. The increase in ROS can uncouple eNOS switching eNOS from NO to superoxide production. This can lead to endothelial dysfunction (further described in section 2.2). The association between hyperglycemia, elevated FFA levels, mitochondrial dysfunction and ROS constitutes a vicious circle, in which the production of ROS during hyperglycemia worsens insulin resistance and thus further induce redox imbalance. These mechanisms are in particularly activated in cell types that are involved in the development of diabetic complications, as they are susceptible to hyperglycemic damage. These cells have a low capacity to reduce intracellular hyperglycemia, and include e.g. endothelial cells, mesangial cells, neurons and Schwann cells in peripheral nerves.
2.2. Oxidative Stress and Diabetic Late Complications
Diabetes induced OS has been suggested to lead to both micro- and macrovascular complications. ROS may induce wide spread damage to cells including e.g. endothelial, retinal, mesangial and neural cells leading to the development of diabetic complications [5].
Damage is collectively caused by both direct effects on cellular components and damaging cells [41, 42] and indirectly through activation of the above mentioned pathways subsequently affecting cellular function (Fig. 2) and through induction of inflammation [4, 43]. Circulating ROS affect endothelial function and initiates inflammatory processes, both of which are thought to play an essential role in early atherogenesis (with atherosclerosis being the very core of diabetic macrovascular complications) as well as in hypertension and heart failures [4, 44-46].
Endothelial dysfunction, characterized by an imbalance between relaxing and contracting factors released by the endothelium, is seen in the early stages of atherosclerosis [56-58]. The vasodilator nitric oxide (NO) is produced by endothelial NO synthase (eNOS) [59]. OS contributes to a decreased NO bioavailability in several ways. OS lowers the production of NO through uncoupling of eNOS, turning production towards superoxide (Fig. 2). Degradation of NO is enhanced, as NO reacts efficiently with superoxide forming the deleterious peroxynitrite and cellular damage is induced [60, 61]. ROS can inactivate eNOS and other anti-atherosclerotic enzymes such as prostacyclin synthase and increase proatherosclerotic events by affecting vascular tone (through activation of PKC, increased flux in the polyol pathway and increased levels of AGE) [44, 62]. Monocytes infiltrate the vascular wall and differentiate into macrophages, which can accumulate oxidized low density lipoproteins (oxLDL) leading to the formation of foam cells [63]. These events in turn stimulate macrophage proliferation, T-lymphocyte attraction, smooth muscle cell proliferation and collagen accumulation, collectively resulting in plaque formation. OxLDL is able to activate NOX and appear to contribute to the development of atherosclerosis through this mechanism as well [55].
Diabetes has been diagnosed in approximately 400 million people worldwide [64] and diabetic late complications is likely to affect a large proportion of these. Trials investigating the relationship between blood glucose status and development of diabetic complications have shown that intensive glucose lowering therapy can decrease the risk of developing in particular microvascular late complications in both T1D and T2D [65, 66], whereas regulation of blood glucose per se does not seem to have as beneficial an effect on the risk of developing macrovascular complications [65, 67]. Even though insulin resistance seems to play a role in the development of macrovascular complications in T2D [68], the mechanism is not fully understood but inflammation and OS could be part of the sequence of events. The effect of FFA’s on OS status should be noted as well. The recognition of these aspects has further prompted the interest in new therapies targeting the redox imbalance associated with diabetes to try to reduce the development of diabetic late complications by lowering the OS.
2.3. GLP-1 and Oxidative Stress
The anti-inflammatory action of GLP-1 has recently gained considerable interest as endogenous GLP-1 has been found to be increased in patients with chronic inflammatory diseases, where OS is implicated, as systolic heart failure (bearing in mind that atherosclerosis can cause heart failure) and to be associated with coronary plaque burden in patients [18, 19, 70]. Moreover, GLP-1 intervention has been shown to decrease inflammation in both preclinical in vivo [21, 71-74] and clinical studies [75-77]. While some studies have incorporated various measures of OS, this area deserves more focused attention. The present review discusses the current literature on GLP-1 with particular focus on the putative effects on OS status beyond anti-hyperglycemia and appetite regulation. The G-protein coupled GLP-1 receptor (GLP-1R) is found in a wide variety of cells. In pancreatic islets, activation of this receptor increases intracellular Ca2+, stimulates adenylate cyclase (increasing production of cAMP) and phospholipase C, and activates a number of pathways including protein kinase A (PKA), PKC, cAMP response element-binding protein (CREB), phospatidylinositol-3 kinase, exchange protein activated by cAMP 2 (Epac2), and mitogen-activated protein kinase pathways ([78] and reviewed in [79]). Both cAMP and PKA are inhibitors of NOX [80-83]. However, some of these pathways vary from cell type to cell type, e.g. isoforms of PKC activates NOX in vascular tissues, where GLP-1 has here been shown to inactivate PKC and thus ROS production by NOX [84]. The role of GLP-1 in different cell types is under investigation and depends on various factors, such as stimuli (e.g. nutrient affecting the cells), species and the expression of the GLP-1R which has to be investigated with sensitive and specific antisera and detected in full length by PCR analysis [85]. Moreover, GLP-1 has been suggested to increase antioxidant capacity. Fig. (3) summarizes potential pathways through which GLP-1 may affect OS status and the following chapters discuss the in vitro, in vivo and clinical literature on GLP-1 and OS.
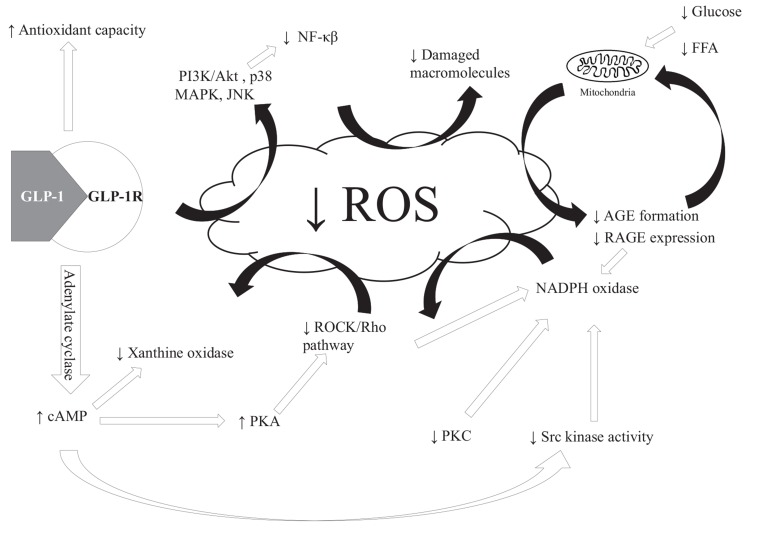
Fig. (3).
Putative mechanisms by which GLP-1 affects OS status by glucose independent mechanisms in cells expressing the GLP-1R and glucose dependent mechanisms in cells ± GLP-1R expression. Black arrows indicate glucose dependent mechanisms, white arrows glucose independent and grey indicates pathways that can be affected by both glucose dependent and glucose independent GLP-1 effects. Black/white arrow indicates that mechanism has not been clarified. GLP-1 lowers OS status through different mechanisms with the glucose dependent effects being the most apparent through reduced food intake, blood glucose and FFA. This reduces the ROS formed by the mitochondria which can decrease the activation of the five pathways outlined in Fig. (2). Glucose independent effects are mediated through GLP-1R stimulation, which can affect pathways involved in ROS formation. Stimulation of GLP-1R leads to increased levels of cAMP in the cell, which results in lower levels of ROS being produced by NOX and XO. The decrease in NOX activity can also be obtained by GLP-1 inactivation of PKC. Increased cAMP level by GLP-1R activation can increase PKA activity and by this route decrease the Rho/ROCK pathway leading to a decreased ROS production. The latter mentioned pathway can be activated by hyperglycemia e.g. though AGE formation (review in [69]) and can thus both be affected by glucose dependent and independent pathways. Decreases in AGE/RAGE interaction can lower XO, NOX and mitochondrial ROS production. Increased cAMP can lower the activity of Src kinase, which is an activator of NOX. This results in decreased NOX activity and ultimately lower superoxide production. Increased antioxidant capacity favors redox homeostasis and has been shown to be induced by GLP-1R activation and/or increased Nrf2 concentration or expression by GLP-1. A decreased ROS production can affect various pathways as PI3K/Akt and p38 MAPK and JNK activities resulting in a decrease in inflammatory (by decreasing NF-κβ activity) and apoptotic pathways as discussed in the text.
3. GLP-1 AND OXIDATIVE STRESS IN VITRO
Numerous in vitro studies have shown that GLP-1 and GLP-1 agonists reduces ROS and protects against apoptosis in several cell types when exposed to different stress factors e.g. high glucose levels or hydrogen peroxide [20, 21, 86-90] (Table 1). However, the observed effects on apoptosis in a number of these studies may not strictly be attributed to effects via OS, as GLP-1 per se activates antiapoptotic genes and thus independently decreases apoptosis [91]. But in general, in vitro studies have reported decreased levels of ROS [20, 21, 76, 84, 88-90, 92-99], NOX subunits or activity [20, 21, 84], malondialdehyde (MDA) [90, 95, 100] and increased levels of antioxidants and/or genes involved in antioxidant activity [21, 86, 87, 90, 92, 95, 100-103] following GLP-1 exposure. Whether these observed effects are GLP-1R dependent or independent can be investigated by using cleavage products of GLP-1 known not to interact with the receptor. The GLP-1 cleavage product, GLP-1(9-36) is not involved in glucose homeostasis [104], but has been shown to have cytoprotective effects on mouse cardiomyocytes exposed to hydrogen peroxide [105] and to decrease super
Table 1.
In vitro studies of GLP-1 and oxidative stress.
| Species | Cell Type |
Study Design:
Intervention (I), Duration (D) |
Effect of GLP-1
Intervention on Oxidative Stress Markers |
Survival/Apoptosis | Mechanisms | Comments |
|---|---|---|---|---|---|---|
| Mouse | Isolated mouse (C57BL/6 ± DIO) hepatocytes + H4IIE |
I: GLP-1(28-36) amide (10 or 100 nmol/l) + tBHP (0.5 mmol/l). D: 24 hours prior to tBHP for 1 hour. |
GLP-1(28-36)amide: ↓ ROS (CM-H2DCFDA by spectrofluorometer) (hepatocytes: p<0.028 (for both ± DIO), H4IIE: p<0.006 (100 nmol/l) and p<0.002 (10 nmol/l)). |
[98] | ||
| Atrial HL cardiomyocytes |
I: GLP-1 (100 or 200 nmol/l), palmitate (750 µM). D: 30 minutes preincubation ± GLP-1 (7-36) then 6 hours + palmitate. |
GLP-1 (7-36): ↓ XO activity (p<0.05). ↓ ROS and RNS (p<0.05) (dichlorodihydrofluorescin DiOxyQ). ↑ GSH/GSSG ratio (200 nmol/l) (p<0.05). ↓ SOD activity (200 nmol/l) (p<0.05). |
[116] | |||
| Mouse pancreatic islet |
I: GLP-1 (10 nmol/l) + glucose (20.8 mmol/l). D: 90 minutes. |
GLP-1: ↓ Superoxide level (nitroblue tetrazolium assay) (p<0.05). |
ROS may play an antagonistic role in the adenylate cyclase/cAMP/PKA pathway of insulin secretion [97]. | |||
| Hamster | Hamster pancreatic β-cell line (HIT-T15) | I: GLP-1 (10 nmol/l) in GS containing medium. D: 5 days. | GLP-1: ↓ ROS (p<0.01) (DCFH-DA probe). ↑ GR protein expression, thus only significant (p< 0.05) in medium control cells. |
GLP-1: ↑ Nrf2 protein expression (p<0.01). ↑ MafA and PDX-1 protein expression (p<0.05). |
[92] | |
| Hamster pancreatic β-cell line (HIT-T15) | I: GLP-1 (10 nmol/l) in GS containing medium. D: 5 days. | GLP-1: No effect on GSSG levels. ↑ GSH (p<0.001). |
GLP-1 increased proliferation (p<0.01, when compared with control medium, p<0.001 when compared to GS medium) rate and restored LDH release + caspase-3 activity to control cell levels. | GLP-1: ↓ RAGE expression (p<0.05). |
[87] | |
| Species | Cell Type |
Study Design: Intervention (I), Duration (D) |
Effect of GLP-1 Intervention on Oxidative Stress Markers |
Survival/Apoptosis | Mechanisms | Comments |
| Rat | Cardiomyoblasts (H9c2) | I: Exenatide treatment (1 nmol/l) prior to H2O2 (200 µmol/l) exposure. D: 30 minutes | Exenatide: ↓ ROS (DCFH-DA and flow cytometry) and ↓ MDA (p<0.05). ↑ T-SOD (p<0.05). |
Exenatide: ↑ viability (p<0.05) ↓ LDH and CK-MB release (p<0.05). |
[90] | |
| Neonatal rat ventricular myocytes (NRVM) |
I: Exendin-4 (30 mmol/l) ± D-glucose (5 or 33 mmol/l) or H2O2 (250 µmol/l). D: 24-48 hours. |
Exendin-4: No effect on ROS level (DHR123 flourometri). |
Exendin-4: ↓ cell death (p<0.05) (trypan blue). No change in ROS level. |
Exendin-4: ↓ Expression of markers of ER stress (CHOP, GRP78 and PDI). |
Protective effects of exendin-4 on glucose or H2O2 induced cell death might be downstream from OS. PKA dependent effect associated with activation of SERCA2a [109]. | |
| Cardiac microvascular endothelial cells |
I: GLP-1 (10-10, 10-9, 10-8 or 10-7 mol/l) ± glucose (25 mmol/l). D: 24 hours. |
GLP-1 in high-glucose-medium: ↓ superoxide (lucigenin-enhanced chemiluminescence assay and DHE staining) (p<0.05 and 0.01 for 10-8 mol/l). ↓ NOX activity (lucigenin-enhanced chemiluminescence assay) (p<0.05 and 0.01 for 10-8 mol/l). ↓ p47phox, gp91phox, p22phox and p40phox protein level (p<0.05). |
GLP-1 in high-glucose-medium: ↓ apoptosis (p<0.05) (terminal deoxynucleotidyl TUNEL assay). ↓caspase-3 (p<0.05). |
↓ Rho (p<0.05). ↓ ROCK (p<0.05). Fausudil and H89 reduced the effects of GLP-1. |
GLP-1 effects might be through cAMP/PKA/Rho-dependent mechanisms. + in vivo study. [20] |
|
| Neonatal rat ventricular myocytes (NRVM) |
I: Exendin-4 (0.1-10 nmol/l) + high glucose exposure (25 mmol/l). D: 12 hours. |
Exendin-4: ↓ MDA concentration (p<0.05 for 1 and 10 nmol/l). ↑ SOD activity (p<0.05 for 1 and 10 nmol/l). |
Exendin-4: ↓ LDH (p<0.05 for 1 and 10 nmol/l). ↓ CK (p<0.05 for 1 and 10 nmol/l). |
↓ TNF-α, IL-1β and HMGB1 (p<0.05 for 1 and 10 nmol/l). Hypothesis: (Hyperglycemia-induced) OS <-> inflammatory cytokine secretion [100]. | ||
| PC12 |
I: Methylglyoxal (1 mmol/l) ± GLP-1 (3.30 µg/ml). D: 30 min preincubation with GLP-1, then methylglyoxal for 24 hours. |
GLP-1: ↑ GSH (p<0.05). ↑ GSH/GSSG (p<0.001). |
GLP-1: ↓ apoptosis (DAPI staining) (p<0.001). |
Rapamycin (mTOR inhibitor) reduced effects on GSH (p<0.05) and GSH/GSSG (p<0.001). | Pi3K/Akt/mTOR/GCLc pathway. [86] | |
| Species | Cell Type |
Study Design: Intervention (I), Duration (D) |
Effect of GLP-1 Intervention on Oxidative Stress Markers |
Survival/Apoptosis | Mechanisms | Comments |
| Rat insulinoma cells (INS-1) |
I: Exendin-4 (10 or 100 nmol/l) + IFN-γ (1 ng/ml) and IL-1 β (4 ng/ml). D: 5 hour preincubation with exendin-4 then 20 hours of IFN-γ and IL-1 β exposure. |
Exendin-4: ↓ ROS (p<0.01 (10 nmol/l) and p<0.001 (100 nmol/l)) (DCFH-DA probe). |
Exendin-4: ↓ caspase-4 activity (p<0.001). |
Exendin-4: Actions on different proteins in the electron transport chain, e.g. NADH-ubiquinone oxidoreductase 75 kDa SU ↓ (p<0.05). ↑ cAMP dependent PKA (p<0.001). |
[88] | |
| Cardiomyoblasts (H9c2) |
I: Exenatide (50-600 nmol/l) + H/R (hypoxia chamber). D: 30 min prior to H/R. |
Exenatide (200 nmol/l): ↓ ROS (DCFA-DA fluorescence) (p<0.05). ↑ T-SOD (p<0.05). ↓ MDA (p<0.05). |
Exenatide: ↑ Viability (p<0.05 for 100, 200 and 400 nmol/l). ↓ Apoptosis (p<0.05 (200 nmol/l)). ↓ Caspase-3 (cleaved and activity) (p<0.05 (200 nmol/l)). |
Exenatide: ↑ Mitochondrial membrane potential (p<0.05). ↓ mitochondrial Ca2+ (p<0.05). |
Improvement of mitochondrial function [95]. |
|
| Rat insulinoma cells. |
I: Exendin-4 (20 nmol/l) + 0, 5, 20 and 40 mmol/l glucose. D: 4 hours. |
Exendin-4: ↓ TxNIP. |
Exendin-4: ↓ capase-3. |
Forskolin and PDE → similar results as Exendin-4. Inhibitors of: PKA or ePAC → attenuated effect of forskolin. |
cAMP/PKA and/or ePAC and/or PDE pathway leading to ubiquitination and proteasomal degradation of TxNIP [103]. | |
| Goto-Kakizaki islet cells |
I: Exendin-4 (100 µmol/l) + glucose (16.7 mmol/l after preincubation with 2.8 mmol/l for 20 minutes). D: 60 minutes. |
Exendin-4: ↓ ROS (CM-H2DCFDA) (p<0.005). No effect on MnSOD activity. |
Src kinase inhibition by PP2 → ↓ ROS (p<0.05) (exendin-4 and PP2 did not further reduced ROS). Adenylate cyclase activator (forskolin) → ↓ ROS (p<0.01). Exendin-4 or forskolin + PKA inhibition (H-89) → no effect on the decreased ROS. General cAMP analog and ePAC specific cAMP analog → ↓ ROS (p<0.001).PI3K inhibitors (LY294002 and wortmannin) → ↓ ROS (p<0.01). MAPK/ERK kinase inhibitor (PD98059) → no ROS effect. EGFR kinase inhibitor (AG1478) → ↓ ROS (p<0.001). |
The effects of exendin-4 and Src inhibitor (PP2) could not be produced in Wistar islet cells [112]. | ||
| Species | Cell Type |
Study Design: Intervention (I), Duration (D) |
Effect of GLP-1 Intervention on Oxidative Stress Markers |
Survival/Apoptosis | Mechanisms | Comments |
| Rat INS-1E and rat pancreatic islet cells. |
I: Exendin-4 (10 nmol/l) pretreatment ± cytokines (10 ng/ml IL-1β, 50 ng/ml TNF-α, 50 ng/ml IFN-γ). D: Exendin-4 for 18 hours followed by cytokines for 18 hours. |
Exendin-4: INS-1E: ↓ ROS (DCFH-DA probe) (p<0.05) in presence of cytokines. |
Exendin-4: INS-1E: ↑ viability (p<0.01). INS-1E + islet cells: ↓ apoptosis and necrosis. |
Exendin-4: ↑ phosphorylation of PKB (required for the protective effects shown). |
CAT and MnSOD levels are mentioned to have been measured, but no data shown. Exendin-4 did not affect levels of either antioxidant. Conclusion: The reduction in ROS might be through lowering of ROS production rather that elimination. [94] | |
| Human | Human umbilical vein endothelial cells (HUVECs) | GLP-1 (10 nmol/l) or exendin-4 + (30 mol/l). D: GLP-1 for 30 minutes prior to H2O2 for one hour. |
GLP-1: ↑ expression of NQO1 and HO-1 (p<0.05). |
GLP-1 or exendin-4: ↓ senescence (β-galactosidase staining) (p<0.05). |
GLP-1: ↑ CREB (p<0.05). Receptor mediated: exendin(9-39) did not have any effect. Effects blocked by H89 or forskolin. |
cAMP/PKA-dependent pathway involved. [102] |
| HUVECs |
I: GLP-1 (0.03 or 0.3 nmol/l) in glycated bovine serum albumin (100 µg/ml). D: 4 hours. |
GLP-1: ↓ superoxide (DHE staining) (p<0.01). |
GLP-1: ↓ RAGE mRNA level (p<0.01) (in normal bovine serum albumin). This action was inhibited by siRNA’s against GLP-1R and RAGE. 8-Br-cAMP had same effects on RAGE expression as GLP-1. |
0.3 nmol/l GLP-1 decreased VCAM-1 mRNA level and thus might reduce recruitment and adhesion of inflammatory cells. Actions of GLP-1 on OS might be through GLP-1R-cAMP axis, resulting in lower levels of RAGE expression [96]. | ||
| Human aortic endothelial cells (HCAECs) |
I: Liraglutide (30 nmol/l) and/or metformin (10 µM) treatment prior to high glucose exposure (25 mM). D: 48 hours. |
Liraglutide or metformin: ↓ superoxide (DHE staining) (p<0.01). Liraglutide and metformin: ↓ superoxide (DHE staining) when compared to liraglutide or metformin alone (p<0.01). ↓ NOX activity (p<0.05). Prevention of p47 phox translocation. |
No effects on viability (> 94% in all samples). | Liraglutide: ↓ DAG (p<0.01) ↑ AMPK phosphorylation (p<0.05) ↓ PKC (p<0.05) |
Combination of liraglutide and metformin further reduced ROS production. Both by inhibition of the PKC-NOX pathway. [84] |
|
| Species | Cell Type |
Study Design: Intervention (I), Duration (D) |
Effect of GLP-1 Intervention on Oxidative Stress Markers |
Survival/Apoptosis | Mechanisms | Comments |
| HCAECs |
I: Exendin-4 (0.1-10 nmol/l) during palmitate exposure (125 µmol/l). D: 24 hours. |
Exendin-4: ↓ ROS (DCFH-DA probe) (p<0.05). |
Exendin-4: ↓ DNA fragmentation (p<0.05 for 10 nmol/l). |
GLP-1 dependent pathway (effects eliminated by exendin (9-39)) and better effects of GLP-1 than exendin-4 (not significant). No effect of GLP-1 (9-36). | Incubation with BH4 resulted in same reduction in ROS as exendin-4 (p<0.05). The effects on apoptosis might be through lower activation of PI3K/Akt pathway (which can be sensitive to ROS). [89] |
|
| HUVECs |
I: Liraglutide (3, 30 or 300 nmol/l) + TNF-α (5 ng/ml). D: Liraglutide for 30 minutes prior to TNF-α for 90 minutes to 24 hours. |
Liraglutide: ↓ ROS (CM-H2DCFDA by FACS calibur) (p<0.01 (30 nmol/l) and p<0.05 (300 nmol/l)). ↓ gp91phox (protein level and mRNA expression), p22phox (mRNA expression) (p<0.05). ↑ SOD-2 and CAT mRNA expression (p<0.05) (↑ SOD-1 and GPx (NS)). ↑ SOD-2, CAT (p<0.05) and GPx (p<0.01) protein level (↑ SOD-1 (NS)). |
Liraglutide: ↓ Apoptosis (p<0.05 (3nmol/l) and p<0.01 (30 nmol/l)) (Annexin V-FITC apoptosis kit). |
Liraglutide (30 nmol/l): Eliminated PKC-α activation seen by TNF-α exposure. |
Effects on ROS levels not blocked by exendin (9-36). NF-κβ activity decreased by liraglutide. [21] |
|
| Human proximal tubular cells |
I: GLP-1 (0.2 nmol/l) + 100 µg/l AGE. D: 4 hours. |
GLP-1: ↓ Superoxide (DHE staining) (p<0.01). ↓ ADMA levels (p<0.01). |
GLP-1: ↓ RAGE mRNA level (p<0.01). This action was inhibited by siRNA’s against GLP-1R and RAGE. 8-Br-cAMP had same effects on RAGE expression as GLP-1. |
RAGE gene suppression by GLP-1R-cAMP axis [93] | ||
| Human mesangial cells |
I: GLP-1 (0.03 or 0.3 nmol/l) + AGE-BSA (100 µg/ml). D: 4 hours. |
GLP-1: ↓ Superoxide (DHE staining) (p<0.01) (Br-cAMP had same effect). |
GLP-1: ↓ RAGE mRNA level (p<0.01) (in bovine serum albumin alone). This action was inhibited by siRNA’s against GLP-1R and RAGE. 8-Br-cAMP had same effects on RAGE expression as GLP-1. |
RAGE gene suppression by GLP-1R-cAMP axis. GLP-1 decreased MCP-1 (p<0.01) mRNA and protein. [99]. | ||
| Species | Cell Type |
Study Design: Intervention (I), Duration (D) |
Effect of GLP-1 Intervention on Oxidative Stress Markers |
Survival/Apoptosis | Mechanisms | Comments |
| Human (T2D patients) peripheral blood mononuclear cells |
I: Exendin-4 (50 nmol/l). D: 24 hours |
Exendin-4: ↓ Superoxide (chemiluminescence) (p<0.05) |
Activation of cytokine release → NOX activation. [76] | |||
| Rat, mouse and human | INS-1, mouse and human pancreatic islets |
I: Exenatide (100 nmol/l). D: 24 hours. |
Exenatide: ↓ TxNIP mRNA (p<0.001) and protein level. |
Exenatide: INS-1: ↓ H2O2-induced apoptosis (p<0.05). Mouse and humans pancreatic islet cells: ↓ caspase-3 and bax mRNA level (p<0.05). |
[101] |
ADMA = asymmetric dimethyl arginine, AGE = advanced glycation end-products, AGE-BSA = advanced glycation end-product bovine serum albumin, AMPK = AMP-activated kinase, BH4 = tetrahydrobiopterin, CHOP = cytosine-cytosine-adenosine-adenosine-thymidine/enhancer-binding homologous protein, CK = creatinine kinase, CK-MB = creatine kinase-MB, CMECs = cardiac microvascular endothelial cells, CM-H2DCFDA = 2’,7’-dichlorodihydrofluorescein diacetate, CREB = cAMP response element-binding protein, DAG = diacylglycerol, DAPI = 4’,6-diamidino-2-phenylindole, DCFH-DA = 2’,7’-dichloro-fluorescein diacetate, DHE = dihydroethidium, DHR123 = dihydrorhodamine 123, DIO = diet induced obese, EGFR = epidermal growth factor receptor, ePAC = exchange protein activated by cAMP, ER = endoplasmatic reticulum, FACS = fluorescence-activated cell sorting, fausudil = Rho kinase inhibitor, forskolin = activates the enzyme adenylyl cyclase and increases intracellular levels of cAMP, GCLc = catalytic glutamate-l-cysteine ligase, GRP78 = glucose-regulated protein-78, GR = glutathione reductase, GS = glycated serum, GSH = reduced glutathione, GSSG = oxidized glutathione, GPx = glutathione peroxidase, HAECs = Human aortic endothelial cells, HCAECs = Human Coronary Artery Endothelial Cells, HFD = high fat diet, HMGB1 = High-mobility group box 1, HO-1 = heme oxygenase-1, H/R = hypoxia/reperfusion, HUVECs = Human umbilical vein endothelial cells, H4IIe = rat hepatoma cell line, H89 = PKA inhibitor, LDH = lactate dehydrogenase, MafA = v-maf musculoaponeurotic fibrosarcoma oncogene homologue, MAPK/ERK = mitogen-activated protein kinase extracellular signal-regulated, MDA = malondialdehyde, mTOR = mammalian target of rapamycin, NADH = nicotine adenine dinucleotide, NF-κβ = nuclear factor kappa-light-chain-enhancer of activated B cells, NOX = NADPH oxidase, NSC = neuroblastoma spinal cord, Nrf2 = nuclear factor erythroid 2 p45-related factor 2, NRVM = Neonatal rat ventricular myocytes, NQO1= NADPH dehydrogenase quinone 1, PDE = phosphodiesterase, PDI = protein disulfide isomerase, PDX-1 = pancreatic and duodenal homeobox-1, Pi3K = phosphatidylinositol-3 kinase, PKA = protein kinase A, PKC = protein kinase C, RAGE = AGE receptor, ROCK = Rho-associated protein kinase, ROS = reactive oxygen species, SERCA2a = sarco/endoplasmic reticulum Ca2+-ATPase, siRNA = small interfering RNA, SOD = sodium dismutase, SU = subunit, tBHP = tert-butyl hydroperoxide, T-SOD = total superoxide dismutase, TUNEL = terminal deoxynucleotidyl transferase dUTP nick end labeling, TxNIP = thioredoxin interacting protein, XO = xanthine oxidase.
oxide levels in hippocampal slices [106]. This suggests that the effects may not only be mediated through the glucose-lowering effects of GLP-1 or by binding and signaling through the GLP-1R. The following sections examine the available data on GLP-1 mediated effects on antioxidant level, ROS production, quenching of ROS, and protections against ROS induced damage.
3.1. GLP-1 Effect on Antioxidant Level
Intervention with GLP-1 has been shown to upregulate superoxide dismutase (SOD), glutathione peroxidase (GPx) and catalase (CAT) activities and increase glutathione (GSH) concentrations in media containing stressors such as glucose or hydrogen peroxide [21, 86, 87, 90, 95, 100]. PKA activation following GLP-1R stimulation has been shown to protect endothelial cells against ROS through increased mRNA levels of NADPH dehydrogenase quinone 1 (NQO1) and heme-oxygenase 1 (HO-1) [102]. NQO1 and HO-1 both have some antioxidant activity [107, 108] and NQO1 also functions as a superoxide scavenger [107]. While these studies indicate an effect of GLP-1 on antioxidant capacity, increased levels of antioxidants could also result from adaptation to higher ROS levels, or decreased consumption through a lower ROS production and consequently, ROS levels and production should also be assessed [108].
3.2. GLP-1 Effect on ROS Level
GLP-1 is generally effective in lowering ROS levels in vitro [20, 21, 36, 76, 84, 88-90, 92-99]. However in one study, neonatal rat cardiomyocytes exposed to either high glucose concentrations or hydrogen peroxide showed that exendin-4 attenuated apoptosis without lowering ROS levels [109]. This result could suggest that effects of exendin-4 on apoptosis are downstream to that of OS, but differences in the study setup (cell type, glucose concentration and exposure time) may indicate otherwise [20, 84, 100]. A different technique was used for detection of ROS (dihydrorhodamine 123 (DHR123)), which primarily detects peroxynitrite, whereas neither hydrogen peroxide nor superoxide alone oxidizes DHR123 significantly [110]. If exendin-4 is able to reduce other reactive species than peroxynitrite, it would not be detected in this study. Thus, the technique used for measuring ROS levels is of major importance and overall conclusion on the levels of ROS cannot be done by only using techniques specific for only one type of ROS. Another important factor is the cell type. Potential glucose independent effects of GLP-1 is influenced by expression of the GLP-1R (as described in 2.4) and details in the expression of this in different cell types of the heart is still debated (reviewed in [111]. A study in Goto-Kakizaki rat islet cells exposed to high levels of glucose (16.7 mmol/l) showed a ROS concentration increase. Exendin-4 reduced this elevation in ROS without affecting MnSOD activity. Here different signaling pathways of GLP-1 were investigated by adding a Src kinase inhibitor, an adenylate cyclase activator, cAMP and an ePAC-specific cAMP analog, a phosphatidylinositol-3 kinase inhibitor and an epidermal growth factor receptor kinase inhibitor, all of which led to decreased ROS concentrations, while PKA or mitogen-activated protein kinase extracellular signal-regulated (MAPK/ERK) kinase did not affect ROS concentrations. This study indicates that GLP-1 acts through suppression of Src kinase activity by increasing cAMP levels and PKA and MAPK/ERK kinase independently but that ePAC, phosphatidylinositol-3 kinase and epidermal growth factor receptor kinase are involved [112]. Src kinase is an activator of NOX, and the decrease in ROS concentration by Src kinase activity is thus a potential route of mechanism [113].
In human aortic endothelial cells (HAECs) exposed to palmitate, exendin-4 has been shown to lower the level of ROS and at the same time reduce the level of DNA fragmentation used as a measure of apoptosis [89]. ROS can stimulate p38 mitogen-activated protein kinase and Jun N-terminal kinase, which can activate NOX by translocation of p47phox and p67phox subunits leading to the production of superoxide [114, 115]. Adding palmitate to the medium markedly increased the phosphorylation (and subsequently activity) of the kinases, a process that was significantly decreased by co-incubating the cells with exendin-4 [89]. However, addition of trolox (a vitamin E analog) was not effective in decreasing palmitate-induced apoptosis even though it reduced ROS production, suggesting that the antiapoptotic effect of exendin-4 is mediated through lowering p38 MAPK and JNK activities. Another study of palmitate induced lipotoxicity in a cardiomyocyte cell line has shown a decrease in xanthine oxidase (XO) activity and ROS/RNS concentration and an increase in GSH/GSSG ratio. In this study, SOD activity was decreased [116]. The latter could be contributed to a decreased need of SOD as ROS production and concentrations were decreased.
3.3. GLP-1 Effect on ROS Production
GLP-1 and liraglutide has been shown to decrease NOX activity as well as protein and mRNA levels of NOX subunits [20, 21, 84]. By inhibition of Rho through the cAMP/PKA pathway, GLP-1 suppresses ROS production and expression of NOX subunits in cardiac microvascular endothelial cells [20]. High glucose-induced ROS production has been shown to be attenuated by liraglutide in endothelial cells [21, 84]. In a study by Batchuluun, B. et al [84], lower levels of superoxide (detected by DHE) was observed along with a decrease in the PKC phosphorylation, translocation of PKCβ2, translocation of p47phox and decreased activity of NOX [84]. As total diacylglycerol (DAG) was decreased and AMP-activated protein kinase (AMPK) phosphorylation increased by liraglutide stimulation, this study suggests that DAG and AMPK may be involved in the GLP-1 lowering effects on ROS production.
In diabetes, inflammatory conditions in the pancreatic β- cells play a central role, making in vitro studies with differ- ent inflammatory cytokines of interest. Preincubation with exendin-4 was found to decrease ROS levels and caspase-4 activity in rat insulinoma cells exposed to IFN-γ and IL-1β compared to controls [88]. Similar results were obtained when exposing HUVECs to TNF-α after liraglutide preincu- bation. Here, liraglutide resulted in decreased apoptosis, ROS production and expression NOX subunits (gp91phox and p22phox). These observations support findings that NOX activity is reduced by liraglutide and other GLP-1 analogues. A simultaneous increase in mRNA levels of SOD-2 and CAT as well as protein levels of SOD-2, CAT and GPx was found [21]. These data support the hypothesis that GLP-1 analogues improve antioxidant status in pancreatic β-cells and endothelial cells in spite of lower levels of ROS and suggest a mechanism by which OS may potentiate cytokine-induced β-cell apoptosis.
In vitro studies have also shown that GLP-1 can decrease the expression of RAGE [87, 93, 96, 99], which can potentially lead to decreased ROS production from NOX [50, 51], XO and the mitochondrial electron transport chain [51]. The observed effect was accompanied by lower levels of superoxide and higher levels of GSH, indicating an advantageous redox status in the cells.
From these data it can be concluded that GLP-1 shows very positive effects in vitro both by decreasing the production of ROS but also by increasing the antioxidant capacity. These outcomes are seen together with an antiapoptotic effect thus to some extend indicating an effect of these effects on OS status.
4. GLP-1 AND OXIDATIVE STRESS In VIVo
Most studies of GLP-1 in OS have been conducted in various disease models as outlined below. Thus, limited information is available on the effects of GLP-1 on OS status in healthy animals. However, one study examined the effect of exenatide on thioredoxin-interacting protein (TXNIP) [101]. In β-cells, TXNIP is a proapoptotic gene and expression of this gene is increased upon elevated levels of glucose, as seen in diabetes [117]. Expression of the gene inhibits the ROS scavenger, thioredoxin (TRx) and leads to increased ROS levels [118]. Exenatide injections for seven days in nondiabetic mice reduced the expression of TXNIP in pancreatic islets and suggested that this GLP-1 analogue is able to reduce apoptosis of mouse β-cells in vivo [101]. The available in vivo data are summarized in Table 2.
Table 2.
In vivo studies of GLP-1 and oxidative stress.
| Species | Type |
Study Design:
Model (M), Intervention (I) and Treatment Duration (TD) |
Effect of GLP-1 on Oxidative Stress Markers (Compared to Control Unless Otherwise Stated) | Pathological Changes (Compared to Control Unless OTHERWISE Stated) | Comments |
|---|---|---|---|---|---|
| Mice | I/R |
M: C57BL/6 mice. I: MCAO and exendin-4 0.1, 1, 10 or 50 µg/100µL/mouse (iv). TD: Single injection at different time points after I/R. |
Brain: ↓ 8-OHdG and HHE (p<0,001). |
Exendin-4 > 10 µg reduced infarct area and volume (p<0.001), best effect when administrated at time 0 (p<0.001). | ↑ CREB, indicating actions through cAMP/CREB pathways [119]. |
|
M: C57BL/6 mice ♂ (n=12). I: Liraglutide 200 µg/kg (ip twice daily. TD: 7 days prior to MI. |
Heart: ↑ Nrf2 expression and protein concentration (p<0.05). ↑ HO-1 expression and protein concentration (p<0.05). |
Liraglutide: Mice subjected to MI had improved recovery (left ventricular developed pressure) (p<0.01). |
Nrf2 and HO-1 was measured prior to MI in other mice [121]. | ||
| Acute clamp |
M: C57BL/6 + GLP-1R (-/-) mice ♂ (n=62). I: Catheters (lateral cerebral ventricle + left femoral vein) + ultrasonic flow probe. hyperinsulinemic-euglycemic (5.5 mmol/l) or hyperglycemic (20 mmol/l) clamps. Intracerebral infusion of exendin-4 or exendin-9 (0.5 pmol/kg/min). H2O2 (2 or 20 nmol/min) was infused for the last hour. TD: 3 hours. |
Brain (hypothalamus): ↓ ROS (CM-H2DCFDA fluorescence) compared to hyperglycemic and normoglycemic controls (p<0.05). ↓ GSSG/GSH ratio (20 nmol/l) (p<0.05). |
↑ whole body glucose utilization (glucose infusion rate). Glucose dependent exendin-4 induced ROS reduction. Decreased eNOS activity during hyperglycemia. Hypothalamic ROS decrease was associated with reduction of femoral blood flow [141]. |
||
| Cryo-lesion |
M: C57BL/6 mice ♀ (n=20). I: Traumatic brain injury induced applying a liquid nitrogen acclimatized probe to the skull. Liraglutide 200 µg/kg twice daily (sc) ± Ex9. TD: 4 days (from immediately after trauma) |
Brain: ↓ total and average ROS/RNS pr. pixel (p<0.05). |
↓ α-spectrin cleavage pattern (marker of necrosis and apoptosis). | [120] | |
| Prevention |
M: C3H/HeJ mice. I: Exendin-4 24 nmol/kg (ip). TD: 7 days. |
Langerhans islets: ↓ TxNIP expression (p<0.001). |
Langerhans islets: ↓ caspase-3 (p<0.05) and bax (p<0.001) mRNA level. |
[101] | |
|
M: APO E (-/-) mice ♂. I: GLP-1(7-36) amide 2.2 nmol/kg/day (sc. pumps). TD: 30 days. |
Isolated peritoneal macrophages: 45% reduction in oxLDL induced cholesteryl ester accumulation (p<0.0001). |
Oil red O staining and MOMA-2 staining of aorta. Significant reduction in: Surface area of atherosclerotic lesions (p<0.0001), plaque size (p<0.005) and macrophage infiltration (p<0.05). |
oxLDL induced cholesteryl ester accumulation was assessed in cultured macrophages from treated mice. [150] | ||
| Species | Type |
Study Design: Model (M), Intervention (I) and Treatment Duration (TD) |
Effect of GLP-1 on Oxidative Stress Markers (Compared to Control Unless Otherwise Stated) | Pathological Changes (Compared to Control Unless OTHERWISE Stated) | Comments |
|
M: KK/Ta-Akita mice ♂ (n= 5 pr. group). I: Liraglutide 200 µg/kg/day (sc). TD: 4 weeks. |
Kidney: ↓ TBARS level (p<0.05). ↓ NOX activity (p<0.001). ↓ NOX4 expression (p<0.001). ↓ Superoxide level (DHE staining) (p<0.001). ↑ NO fluorescence intensity (p<0.0001). |
Kidney: ↓ albuminuria (p<0.01). ↓ glomerular filtration rate (p<0.01). ↓ Kidney hypertrophy (p<0.05). Kidney histology: ↓ Mesengial expansion score, fibronectin staining intensity, glomerular basement membrane (p<0.001). ↑ Podocyte number (p<0.001). |
+ Adenylate cyclase inhibitor (SQ22536) or PKA inhibitor (H-89) → eliminated effects of liraglutide. No difference in blood glucose or plasma insulin. Experiments GLP-1R knockout mice supports that effects are mediated through the GLP-1R [138] |
||
| Prevention + treatment |
M: Chinese Kun Ming mice (n=24). I: STZ (day 8-10) + rhGLP-1 24 nmol/kg (ip). TD: 30 days from one week prior to STZ. |
Plasma: ↓ MDA (p<0.05). ↑ SOD (p<0.05). ↑ GPx (p<0.05). Pancreas: ↓ MDA (p<0.001). ↑ SOD (p<0.05). |
Pancreas: ↑ Islet number/area (p<0.05). ↓ Histological changes. |
[142]. | |
| Treatment |
M: db/db and m/m ♂ mice. I: Liraglutide 200 µg/kg twice daily (sc). TD: 2 weeks. |
Pancreas: db/db: ↑ GPx (p<0.01) after 2 weeks. |
Pancreas: db/db: ↓ Caspase-1, Caspase-3 and Cad (p<0.05) expression after 2 days and 2 weeks. ↑ Bcl2 (p<0.05) expression after 2 weeks (NS after 2 days). |
↓ ER stress (Xbp1 expression) (p<0.05) after 2 weeks. [135] | |
|
M: KKAy and DIO mice. I: Exendin-4 24 nmol/kg/day (sc pumps). TD: 40 days. |
Heart: ↓ Superoxide (DHE staining) (p<0.05). ↓ Mfn1/Mfn2 (p<0.05). ↓ Mitochondria specific OS (MTR) (p<0.01). PARKIN + PINK-1 (p<0.01 and 0.05). Protein levels of: ↓ Nox-4 (p<0.01). ↑ SOD-1 (DIO) (p<0.05). ↑ GPx (DIO) (p<0.01). Nox-2 and TRx no change. |
Cardiac lipid accumulation ↓ (p<0.01) and fibrosis. | No weight loss observed. [131] | ||
| Species | Type |
Study Design: Model (M), Intervention (I) and Treatment Duration (TD) |
Effect of GLP-1 on Oxidative Stress Markers (Compared to Control Unless Otherwise Stated) | Pathological Changes (Compared to Control Unless OTHERWISE Stated) | Comments |
|
M: db/db (db/m as controls) ♂ (n=27). I: Exendin-4 (0.5 or 1.0 nmol/kg) ip. TD: 8 weeks. |
Urinary (24 hours): ↓ 8-OHdG (p<0.05 for db/db vs. control db/db and p<0.01 for db/m vs. control db/db). Kidney: ↓ 8-OHdG protein concentration in db/db mice (determined with immunostaining). |
Kidney: ↓ glomeruli lipid accumulation (oil red O). ↓ collagen IV (p<0.05 and p<0.01). ↓ TGF-β (p<0.05 and p<0.01). ↓ mesangial matrix expansion. ↓ glomerular hypertrophy. ↓ macrophage infiltration (F4/80 staining) (p<0.05 and p<0.01). ↓ apoptotic cells (caspase-3 positive cells) (p<0.05 in 1.0 nmol/kg). |
Induction of peroxisome proliferator-activator receptor-α and GLP-1R expression. Decrease in GLP-1R positive cells in the glomeruli of db/db mice. Increased by exendin-4 treatment. No effect on food intake and blood glucose but decreased body weight in both db/db dose groups [137]. | ||
|
M: Ob/ob mice. I: Exendin-4 (10 μg/kg for 14 days → 10 μg/kg or 20 μg/kg). TD: 60 days. |
Liver: ↓ TBARS (p<0.05 for 20 µg/kg). |
Significant reduction in liver lipid content (p<0.05 for 10 µg/kg and 0.01 for 20 µg/kg). | [133] | ||
|
M: C57BL/6 ♂ (n=24). I: STZ + exendin-4 (8 µg/kg) sc ± omeprazole. TD: From 14 days after first STZ injection and for 28 days. |
Liver: ↓ MDA (p<0.01). (+ omeprazole p<0.001). ↑ Nrf2 level (p<0.01). |
Liver: ↓ triglycerides (p<0.01). (+ omeprazole p<0.001). |
Nrf2 controls antioxidant genes and is important in protecting the liver against OS [145]. | ||
|
M: BALB/c mice. I: STZ + exendin-4 3 µg/kg (sc). TD: 30 days after STZ injection. |
Pancreas (activity of): ↑ CAT (p<0.05) ↑ GPx (p<0.01) ↑ SOD (p<0.05) |
Pancreas: No observed effects on apoptosis (TUNEL assay and cleaved caspase-3). |
No effect on CAT, GPx and SOD in nondiabetic mice. [143] | ||
| Rats | I/R |
M: SD rat pups. I: Exendin-4 (1.0 nmol/kg) sc. Isolation of heart at 4-6 weeks or 8-9 months and subjected to I/R ex vivo. TD: 6 days from the day of delivery. |
Heart homogenates: No effect on TBARS and MnSOD activity in either group. Nor on nonenzymatic antioxidant capacity (Biovision kit). |
Heart: Improved recovery from I/R (percent recovery of left ventricular end diastolic pressure). |
[129]. |
|
M: Wistar rats ♂. I: MCAO and liraglutide (1 ml, 700 µg/kg (IP) TD: Single dose one hour after reperfusion. |
Peripheral blood: ↓ d-ROMS (0.93) (p<0.05) |
Significant reduction in infarct volume (p< 0.05). | [125] | ||
| Species | Type |
Study Design: Model (M), Intervention (I) and Treatment Duration (TD) |
Effect of GLP-1 on Oxidative Stress Markers (Compared to Control Unless Otherwise Stated) | Pathological Changes (Compared to Control Unless OTHERWISE Stated) | Comments |
|
M: SD rats ♂. I: MCAO and exendin-4 0.5 μg/kg twice daily (ip). TD: 7 days prior to I/R. |
Brain: ↓ MDA (0.75) (p<0.001) ↑ GSH (1.45) (p<0.05) ↑ SOD (1.67) (p<0.01). |
Significant reduction in infarct volume (p<0.001). | [122] | ||
|
M: Sprague Dawley rats ♂ (n=32). I: Exenatide 10 µg/kg/day (ip). Ligation of left coronary artery for 30 min followed by 2 h reperfusion. TD: 2 weeks prior to MI/R. |
Heart homogenates: ↓ MDA (p<0.05). ↑ T-SOD, CAT and GPx (p<0.05). |
[90] | |||
|
M: Wistar rats ♂+♀. I: STZ induced diabetes + surgical induced renal I/R. Exenatide 10 μg twice daily (sc.). TD: 2 weeks prior to I/R. |
Liver: ↓ MDA (p<0.001) ↓ XO (p<0.001) ↑ GSH (p<0.01) ↑ SOD (p<0.001) ↑ GPx (p<0.05) ↑ CAT (p<0.01) |
Significant reduction in ALT, AST and ALP after I/R (p<0.01). | [123] | ||
|
M: Wistar rats ♂+♀. I: STZ + renal I/R (after 10 days of exenatide (10 µg twice daily (sc)) intervention) TD: 2 weeks from 2 weeks after STZ. |
Kidney: ↓ MDA (p<0.001). ↑ GSH (p<0.05). ↑ GPx (p<0.001). ↑ SOD activity (p<0.05). ↑ CAT activity (p<0.001). ↓ XO activity (p<0.001). |
Kidney: ↓ DNA fragmentation. Preservation of normal morphology. |
After STZ but before I/R [124]. | ||
|
M: neonatal SD rats ♂. I: IUGR + exendin-4 1nmol/kg (sc). TD: 6 days from day of life. |
Liver: ↓ MnSOD (p<0.05). ↓ TBARS (p<0.05). ↑ GSH/GSSG (p<0.05). |
↓ BW (p<0.05 in control+exendin-4, not significant in IUGR+exendin-4). No difference between control exendin-4 treated and control vehicle treated [128] | |||
| Treatment |
M: Wistar rats ♂+♀. I: STZ or methionine + exendin-4 1 μg/kg (ip). TD: 7 days. |
Significant reduction in serum TBARS (STZ or methionine +/- exendin-4) (p<0.05). | Significant prevention of diabetes or hyperhomocysteinemia induced VED. | Inhibition of eNOS by L-NAME → no effect of exendin-4 [154] | |
|
M: Wistar ♂ rats. I: STZ + Exendin-4 0.3 or 1.5 µg/kg/h (ip pumps). TD: 2 weeks. |
↓ Urinary 8-OHdG (p<0.01) (1.5 µg/kg/h). ↓ ADMA level (p<0.01) (1.5 µg/kg). |
Kidney: ↓ glomerular area, macrophage infiltration in glomeruli, glomerular and tubulointerstitial fibrosis (p<0.01). |
Inhibition of RAGE gene expression in both dose groups (p<0.01) [93]. | ||
| Species | Type |
Study Design: Model (M), Intervention (I) and Treatment Duration (TD) |
Effect of GLP-1 on Oxidative Stress Markers (Compared to Control Unless Otherwise Stated) | Pathological Changes (Compared to Control Unless OTHERWISE Stated) | Comments |
|
M: SD rats ♂. I: STZ + exenatide (1.0 or 5.0 µg/kg once daily) sc. TD: 1 or 4 weeks from 4 days after STZ. |
Heart: ↓ MDA (p<0.05). ↑ GR (p<0.05). ↑ SOD (p<0.05). ↑ CAT (p<0.05). |
Plasma: ↓ markers of cell damage (LDH, total CK, absolute CK-MB and CK-MB relative index) (p<0.05). |
Low dose treatment does not return blood glucose and serum insulin to normal, but still affects MDA, GR, SOD and CAT [149]. | ||
|
M: Wistar rats ♂+♀. I: STZ + GLP-1 (1 µg/kg twice daily) or exendin-4 (0.1 µg/kg/day) ip. TD: 4 weeks. |
Plasma: ↓ MDA in GLP-1 and exendin-4 treated groups when compared to controls (p<0.001) and diabetic controls (p<0.001). |
Aortic rings: GLP-1 reversed the decreased contractive force in diabetic rats. |
[155]. | ||
|
M: Wistar ♂ rats. I: STZ + liraglutide (0.3 mg/kg twice daily) sc. TD: 4 weeks from one week after STZ. |
Kidney: ↓ Superoxide level (DHE in situ). ↓ Nox4 mRNA (p<0.05) and protein (p<0.05). ↓ gp91phox mRNA (p<0.05) and protein (p<0.05). ↓ p22phox mRNA (p<0.05). ↓ p47phox mRNA (p<0.01) and protein (p<0.05). Urine: ↓ 8-OHdG (p<0.01). ↓ MDA (p<0.01). |
Urine: ↓ albumin secretion (p<0.05). |
Treatment [148]. | ||
|
M: Sprague Dawley rats ♂ (n=23) I: STZ + exendin-4 10 µg/kg/day (ip). TD: 8 weeks from one week after STZ. |
Diabetic animals: ↓ Urinary 8-OHdG (p<0.01). ↓ Glomeruli 8-OHdG content (p<0.001). ↓ Cortex Nox-4 expression (p<0.05). ↓ NOX-4 content in kidney endothelial cells (p<0.05). |
Kidney morphology: ↓ glomerular hypertrophy and mesangial matrix expansion. Kidney microinflammation: ↓ expression of Cd14 (p<0.001), ICAM1 (p<0.05) and TGF-β1 (p<0.05) in cortex. ↓ level of ICAM1 (p<0.001), type IV collagen (p<0.001) and macrophage infiltration in glomeruli. |
NF-κβ p65 binding activity decreased by exendin-4 in diabetic group [71] | ||
|
M: Wistar rats ♂. I: STZ + exenatide 1 µg/kg (ip). TD: 10 weeks from day five after STZ. |
Pancreas: ↑ CAT containing cells (p<0.05) ↑ GR containing cells (p<0.05) ↑ GPx gene expression (p<0.05) |
Serum: ↓ alanine and aspartic aminotransferases, cholesterol, triglyceride, uric acids, blood urea nitrogen and creatinine. |
Focus on insulin release. [146] |
||
|
M: Wistar rats ♂ (n=40). I: STZ + GLP-1 50 ng/kg (ip). TD: 10 weeks from day five after STZ. |
Pancreas: ↑ GR containing cells (p<0.05) ↑ GR gene expression in both normal and diabetic animals (p<0.01). |
Pancreas: ↑ heat shock protein-70 in both normal and diabetic animals (p<0.05). ↑ pancreatic duodenal homeobox-1 in both normal and diabetic animals (p<0.05). |
[147] | ||
| Species | Type |
Study Design: Model (M), Intervention (I) and Treatment Duration (TD) |
Effect of GLP-1 on Oxidative Stress Markers (Compared to Control Unless Otherwise Stated) | Pathological Changes (Compared to Control Unless OTHERWISE Stated) | Comments |
| Pigs | I/R |
M: Pigs ♀+♂ (n=18). I: Cardiac arrest. Human rGLP-1 10pmol/kg/min (iv infusion pump). TD: 4 hours from one minute after resuscitation. |
Plasma: ↓ 8-iso-PGF2α (0.78) in coronary sinus (p<0.05). No effect on SOD activity. |
Preservation of coronary microvascular function (compared to baseline). No decline in coronary flow reserve (p<0.05) (compared to controls). |
No difference in blood glucose or plasma insulin [130]. |
|
M: Pigs I: Cardiac I/R. Exenatide 10 µg and 10 µg (iv. and sc). TD: 5 min. before reperfusion and two days after (twice daily). |
Heart (72 hours after reperfusion): ↓ 8-OHdg (p<0.005 (positive cells/mm2)). ↑ SOD activity (p<0.05). ↑ CAT activity (p<0.05). |
↓ (40%) in infarct size (p<0.05). ↑ improved cardiac function. ↓ caspase-3 protein (p<0.05) (four hours after reperfusion). |
[22] |
AGE = advanced glycation end-product, ADMA = asymmetric dimethyl arginine, ALT = alanine aminotransferase, ALP = alkaline phosphatase, AST = aspartate aminotransferase, APO E = Apolipoprotein E, Bax = BCL2-associated X protein, CAD = Caspase-activated DNase, CAT = catalase, CK = creatinine kinase, CK-MB = creatinine kinase-MB, CM-H2DCFDA = 2',7'-dichlorodihydrofluorescein diacetate, CREB = cAMP response element-binding protein, DIO = diet induced obese, d-ROMs = derivatives of reactive oxygen metabolites, GSH = reduced glutathione, GSSG = oxidized glutathione, GPx = glutathione peroxidase, GR = Glutathione reductase, HHE = 4-hydroxy 2-hexenal, ICAM-1 = intercellular Adhesion Molecule 1, I/R = ischemia reperfusion, IUGR = intrauterine growth retardation, LDH = lactate dehydrogenase, MCAO = middle cerebral artery occlusion, MDA = malondialdehyde, Mfn = mitofusin, MOMA-2 = Monocyte/Macrophage Marker Antibody-2, MI = myocardial infarction, MI/R = myocardial ischemia/ reperfusion, MTR = mitotracker red, NOS = nitric oxide synthase, Nrf2 = nuclear factor erythroid 2 p45-related factor 2, PGF2α = prostaglandin 2α, PKA = protein kinase A, RAGE = advanced glycation end-product receptor, rhGLP-1 = recombinant human GLP-1, RNS = reactive ROS = reactive oxygen species, SOD = superoxide dismutase, STZ = streptozotocin, TBARS = thiobarbituric acid reactive substances, TGF-β = transforming growth factor β, TxNIP = thioredoxin interacting protein, TRx = Thioredoxin, TRxR = TRx reductase, T-SOD = total superoxide dismutase, TUNEL = terminal deoxynucleotidyl transferase dUTP nick end labeling VED = vascular endothelial dysfunction, XO = xanthine oxidase, 8-OHdg = 8-hydrodeoxyguanosine.
4.1. Ischemia/reperfusion (I/R) Models
In mice subjected to acute left middle cerebral artery occlusion (MCAO), single injections of exendin-4 at different time points after reperfusion reduced 8-hydroxy-2'-deoxyguanosine (8-OHdG) and anti-4-hydroxy 2-hexenal (HHE) positive cells in the brain at both 24 hours, 72 hours and seven days after I/R [119]. This effect of GLP-1 on OS has been supported by a study showing that four days of liraglutide treatment, in a model of traumatic brain injury induced by cryolesion (probe cooled in liquid nitrogen), can decrease in vivo visualization of ROS/RNS in female C57BL/6 mice. The effect was accompanied by a reduction in α-spectrin, a marker of necrosis and apoptosis, indicating a reduction in these cellular events [120]. In CL57BL/6 mice, seven days of liraglutide treatment resulted in higher expression and protein concentration of Nrf2 and HO-1 in the heart suggesting that liraglutide can increase antioxidant capacity [121].
In rats, the preventive effect of exendin-4 has also been examined in a MCAO model. Administration seven days prior to I/R resulted in significantly lower levels of MDA and higher levels of SOD and GSH [122]. This suggests that exendin-4 exerted its protective effect through increased antioxidant status capable of protecting against macromolecular damage induced by ROS. Similar results using exenatide were obtained in a model were the left coronary artery was ligated for 30 minutes followed by two hours of reperfusion. Here, exenatide administration for two weeks prior to I/R resulted in a significant decrease in MDA and increases in total SOD, CAT, and GPx [90]. Similarly, in a renal model of I/R, exenatide treatment for two weeks prior to I/R showed significant reductions in XO, MDA, combined with an increase in antioxidant capacity (GSH, GPx, SOD and CAT) [123]. In this study, a reduction in ROS producing enzymes was also observed, indicating that GLP-1 can prevent the production of ROS in vivo. Comparable effects of exenatide has been found after induction of diabetes with streptozotocin (STZ); thus, in kidney I/R induced after 10 days of exenatide, decreased kidney content of MDA and XO activity was observed and accompanied by increases in GSH concentration, GPx protein concentration, and SOD and CAT activities [124]. Collectively, these results suggest that prophylactic administration of GLP-1 analogues in I/R models may partially protect against OS both by induction of antioxidant capacity and by reduction of ROS producing enzymes. Therapeutic administration of GLP-1 in rats following I/R has been shown effective as well. A single administration of liraglutide acutely after reperfusion has been shown to reduce infarct volumes in a transient MCAO model [125]. Derivatives of reactive oxygen metabolites (d-ROMs) - a crude measure of peroxides [126] were significantly reduced in plasma in the liraglutide treated rats. Likewise, intrauterine growth retardation (IUGR) followed by six days of exendin-4 administration resulted in decreased thiobarbituric acid reactive substances (TBARS; a crude measure of lipid hydroperoxides with questionable specificity and quite inconsistent findings [127]) in the livers of the offspring, thus indicating a reduction in lipid peroxidation in newborn rats treated with exendin-4 [128]. Furthermore, antioxidant status was improved as shown by increased liver MnSOD and GSH/GSSG ratio.
The potential long term effect of a 6-day exendin-4 administration to newborn rats on cardiac OS status was examined by evaluating the cardiac response to ex vivo I/R after 4-6 weeks and 8-9 months [129]. Surprisingly, the study showed improved recovery in hearts from the exendin-4 treated groups, which was attributed to reductions in oxidative phosphorylation possibly through epigenetic alterations, as no differences between groups in TBARS and MnSOD activity were observed [129].
Two studies have been performed using porcine models of I/R. Exenatide intervention (started just prior to reperfusion and continued for two days after) in a cardiac I/R model resulted in higher activity of SOD and CAT as well as decreased 8-OHdG in the heart tissue [22]. In the same study, the exenatide group showed a 40% reduction in infarction size. In the second experiment, treatment with human recombinant GLP-1 for four hours from one minute after reperfusion in a model of cardiac arrest resulted in lower levels of 8-iso-Prostagladin F2α (8-iso-PGF2α) in plasma [130]. In contrast to the first study, the GLP-1 analogue did not have any effect on the SOD activity. The authors concluded that the decrease in the level of 8-iso-PGF2α might be through non-enzymatic pathways, i.e. through a reduction in ROS [130]. Firstly, the decrease in 8-iso-PGF2α could be due to changes in many other pathways as discussed through this review. Secondly, the decrease in 8-iso-PGF2α could still be explained by SOD activity as the duration of these two experiments are different, maybe SOD is utilized to decrease superoxide levels and the amount of 8-iso-PGF2α, and the difference in the time allowed to increase SOD in these experiments are different. Catalase and SOD was only elevated at measurements after 72 hours. Unfortunately, the studies did not measure other antioxidants such as GSH or CAT. If the overall antioxidant capacity was indeed unchanged, the decrease in 8-iso-PGF2α may be attributed to a decreased production of ROS by GLP-1, as GLP-1 has shown glucose independent effects on ROS production by down regulating NOX in both heart and kidney in in vivo studies [71, 131]. Importantly, the observation in Dokken et al. [130] shows that GLP-1 protects against I/R in isolated hearts, suggesting that the effects are independent of circulating insulin and blood glucose levels [130].
Collectively, these results support the hypothesis that GLP-1 can reduce tissue damage in I/R models (decrease infarct volume size, improve recovery, decrease DNA fragmentation etc.) [22, 119, 122, 124, 125, 129, 130] by lowering ROS production, oxidative damaged macromolecules, and/or increase antioxidant status. This indication of lower nuclear DNA damage and lipid peroxidation accompanied by increased intracellular cAMP supports the hypothesis that induction of cAMP signaling takes part in the protective effects of GLP-1. Further mechanistic details are needed to determine if the effects of GLP-1 are mediated through lowering the production of ROS or scavenging of ROS. It has already been summarized by Birnbaum et al (2012) that the protective effects observed by GLP-1 on injury after I/R are primarily due to GLP-1R activation, cAMP concentration increase intracellular and PKA activation, which leads to activation of several pathways as mentioned previously [132]. E.g. CREB has shown to be involved in reducing OS status both in vitro and in vivo potentially by activation of OS defense mechanisms [102, 119].
4.2. Obese and/or Spontaneously Diabetic Animal Models
As mentioned in the introduction, hyperglycemia may affect OS status in several ways. This issue becomes particularly important when studying effects of GLP-1 on OS in obese and diabetic animal models, as GLP-1’s effects on body weight and blood glucose are easily mistaken for glucose independent effects, i.e. direct effects of GLP-1 on OS. Thus, body weight and blood glucose must be closely monitored, properly controlled for and taken into account when concluding on data from such experiments.
A 60-day exendin-4 treatment of ob/ob mice showed a reduction in TBARS in liver contents compared to untreated animals [133]. The treated animals lost 14% of their body weight compared to vehicle controls, and as the experiment did not include a weight-matched control group, it cannot be ruled out that the effect of exendin-4 on TBARS in the liver may to some extent result from the reduction in body weight. Additionally normalization to liver lipids could help exclude the possibility that the observed effects on TBARS were due to the reduction in the liver lipid content. Measurement of antioxidant levels and ROS producing enzymes could be helpful on evaluation on potential glucose independent effects as well. Forty days of exendin-4 infusion has been shown to reduce the degree of cardiac remodeling in two different obese pre diabetic animal models, the hyperphagic, insulin resistance and dyslipidemic, KKay mouse and the diet induced obese (DIO) mouse [131]. Exendin-4 decreased the levels of cardiac superoxide and NOX4 in both models. In the DIO model - but not the KKay mouse - cardiac SOD-1 and GPx increased concurrently, showing that the choice of model often affects the readouts obtained in the evaluation of OS. Here, factors such as difference in obesity status and GLP-1R expression could play a role. In the same study, exendin-4 decreased both total cholesterol and triglycerides, systemically and in the heart but the potential indirect effect on ROS status caused by the improvement of dyslipidemia was not independently controlled for. Moreover, as NOX is down regulated by exendin-4, the GLP-1R activator might have a glucose independent effect on the ROS producing enzyme. Exendin-4 did not affect food intake (given as an individual average of the entire experimental course), body weight or blood glucose, but only the blood glucose during a challenge (oral glucose tolerance test) [131]. Even though the KKay model is generally considered insulin resistant [134], the effect of exendin-4 was significant in the oral glucose tolerance test, indicating that the treatment ameliorates glucose intolerance in the model, which was also the case in the DIO model. Similar difficulties in determining the independent effects of weight loss, food intake and blood glucose lowering were apparent in a study in db/db mice, in which two weeks of liraglutide intervention, in a relevant dosing regime in rodent models (200 µg/kg twice daily), resulted in increased GPx activity and a no significant increase in CAT in pancreatic tissue, while no effect on antioxidant gene expression was observed [135].
The first study on the effect of GLP-1 in the kidney was performed in db/db mice. Here, exendin-4 treatment for 8 weeks decreased urinary 8-OHdG and decreased immunohistochemical expression of 8-OHdG in the kidney. Exendin-4 did not decrease blood glucose, which could be due to a low number of β-cells in db/db mice [136], but body weight was decreased by exendin-4 treatment making it difficult to rule out an effect of body weight on the OS status. However, the observed effects could be induced by increased GLP-1R and peroxisome proliferator-activator receptor-α expression [137]. In a mouse model of diabetic nephropathy, the KK/Ta-Akita mouse, activation of the GLP-1R by liraglutide for four weeks has been shown to decrease NOX4 expression and its activity, a decrease in glomerular superoxide and an increase in glomerular NO, via an elevation of cAMP and PKA levels [138]. This resulted in lower levels of TBARS and a reduction in kidney damage as measured by decreased albuminuria, glomerular filtration rate, kidney hypertrophy, mesengial expansion score, fibronectin staining intensity, glomerular basement membrane and podocyte number. SOD-1 down-regulation in this mouse model plays an important role in the development of nephropathy [139] and thus the reduced superoxide production in the kidneys in the presence of liraglutide is likely to cause the attenuated kidney damage observed in this study. Importantly, no changes were observed in blood glucose or plasma insulin in the diabetic animals, as this mouse model has dysfunctional β-cells [140]. The body weight was not affected either, suggesting that the observed protective mechanisms did not occur through the known metabolic effects of liraglutide.
4.3. Animal Models of Induced Diabetes
The acute effect of exendin-4 under hyperglycemic conditions (blood glucose = 20 mmol/l) has been investigated in one study using C57BL/7 mice undergoing hyperglycemic clamp studies, where blood glucose was fixed at a predetermined concentration. Intracerebral infusion of exendin-4 lowered ROS level in the hypothalamus and decreased the GSSG/GSH ratio. This study showed increased whole body glucose utilization in hyperglycemic mice [141]. The study indicates an improved OS status but glucose dependent effects cannot be ruled out as the intracerebral injection of exendin-4 could improve glucose homeostasis locally even though the systemic blood glucose is kept constant.
Prophylactic intervention with human recombinant GLP-1 seven days prior to STZ administration and 23 days after diabetes induction has been shown to decrease plasma and pancreatic levels of MDA, increased SOD and GPx (ns in pancreas) in Chinese Kun Ming mice [142]. Treatment with exendin-4 for 30 days to BALB/c mice in a post STZ administration regimen, resulted in a protection of β-cells from apoptosis probably through increased antioxidant capacity [143]. Exendin-4 increased pancreatic CAT, GPx and SOD in diabetic but not in non-diabetic exendin-4 treated animals. In addition, exendin-4 reduced blood glucose and increased body weight, an effect that may be attributed to increased β-cell mass and improved blood glucose regulation. Food intake was not recorded but effects of altered food intake would be expected to be most pronounced in the beginning of the study. Therefore, decreased blood glucose may at least in part explain the higher levels of antioxidants observed. Increased expression of Nrf2, a nuclear factor involved in expression of antioxidant genes and genes involved in the antioxidant defense [144], has been shown in a STZ induced T1D mouse model treated with exendin-4 after STZ injections [145]. MDA levels were decreased in the liver indicating a protective effect of exendin-4 through regulation of antioxidant genes.
In diabetic Wistar rats, ten weeks of exenatide treatment initiated five days after STZ exposure led to decreased blood glucose levels after four and eight weeks and improved glucose tolerance as measured by intraperitoneal glucose tolerance test (IPGTT) at the end of the ten weeks [146]. Immunofluorescence staining of the pancreas for CAT and glutathione reductase (GR) revealed an increase in treated animals (co-localized with insulin in the islets of Langerhans) together with an elevation in GPx gene expression. The results may suggest that exenatide increases antioxidant level and gene expression, but an indirect effect through blood glucose lowering cannot be excluded. A similar study with GLP-1 yielded comparable results as ten weeks of GLP-1 treatment increased the percentage of GR containing cells in the pancreas and increased GR gene expression in STZ induced diabetic Wistar rats. The GLP-1 treated diabetic rats had a higher body weight and a lower blood glucose (both fasting and during an IPGTT), while GLP-1 did not affect body weight in nondiabetic rats. Moreover, the latter only showed a reduction in blood glucose at two time points during the IPGTT (no effect on fasting blood glucose) but still displayed increased GR gene expression [147]. This could indicate a glucose independent effect of GLP-1 on GR gene expression. Exendin-4 treatment of Sprague Dawley rats for eight weeks starting one week after STZ administration decreased urinary 8-OHdG, glomerular 8-OHdG content, expression of Nox-4 in cortex and Nox-4 content in kidney endothelial cells (detected by immunofluorescence staining) without any effect on blood glucose level, blood pressure, food intake or body weight [71]. These results indicate a glucose independent effect of exendin-4 on ROS induced DNA damage, a decrease in ROS production and a decrease in NF-κβ, implicating a favorable effect on inflammation [71]. However, these measurements were made at the end of the experiment (week 8) and the initial glucose dependent effects were not accounted for. Similar results were obtained in a four week liraglutide treatment study in Wistar rats, resulting in decreased mRNA expression of Nox-4, gp91phox, p22phox and p47phox and protein levels of NOX4, gp91phox and p47phox. Kidney superoxide levels were lower in the liraglutide treated group compared to controls and both urinary 8-OHdG and MDA were decreased. The observed effects were not accompanied by a change in food intake, body weight, blood glucose or blood pressure at day 28 [148]. In another study in Sprague Dawley rats, antioxidant enzyme activity (GR, SOD and CAT) was increased by four weeks of exenatide treatment together with a decrease in MDA, a result shown in a low dose and high dose regime (1.0 µg/kg and 5.0 µg/kg once daily) without returning blood glucose and insulin levels to normal in the low dose regime [149]. However, a significant decrease in blood glucose and an increase in serum insulin were observed. Even though their levels did not return to normal, the data do not seem to support the authors’ conclusions of a glucose independent effect of exenatide on lipid peroxidation resulting from an improved oxidative status seemingly by increased antioxidant activity. In the same study a short term intervention with exenatide had the same effects on OS measurements, but as neither food intake or body weight was measured in these experiments, it is likely that the effect of exenatide on food intake (especially in the beginning of the treatment period) and on body weight could cause these effects. Shorter treatment regimens (two weeks) has also proven effective as e.g. a two-week exendin-4 administration by intraperitoneal infusion pump led to a reduction in urinary 8-OHdG content and increased body weight in male STZ treated diabetic Wistar rats [93]. In this study, RAGE expression was decreased and as depicted in Fig. (3), this could provide a mechanism explaining the effects of exendin-4 on ROS production and damage. Again, food intake was unfortunately not measured, which could have been interesting in this short treatment regimen.
Effects of GLP-1 treatment on body weight in STZ-induced diabetic animals are interesting as the animals both are hyperphagic and diabetic and when treatment increases body weight in some of these studies, it could indicate a remaining β-cell mass with the potential for GLP-1 to exert insulinotropic effects. C-peptide measures could be used to reveal the degree of β-cell function in such studies.
4.4. Animal Models of Cardiovascular Function and Disease
GLP-1 has shown protective effects on cardiovascular function in both apolipoprotein E (APO E) knockout mice and humans making the underlying mechanisms of these effects relevant to investigate [72, 150-152]. In APO E knockout mice, prophylactic intervention with human GLP-1 for four weeks (initiated simultaneously with a change to atherogenic diet) suppressed the formation of atherosclerotic lesions (surface area of atherosclerotic lesions, plaque size and macrophage infiltration) [150]. A reduction in oxLDL accumulation in isolated macrophages was also observed but without concurrent effects on plasma glucose or insulin when compared to control groups. However, as atherosclerotic development and OS status is highly dependent on food intake in this model [153], the duration of study becomes important as effects of lowered food intake (which are most pronounced in the beginning of GLP-1 intervention) may be misinterpreted as potential glucose independent effects of GLP-1 on atherosclerosis and OS. Administration of GLP-1 prior to change in diet can circumvent the most pronounced effect of GLP-1 on food intake and thereby on the intake of an atherogenic diet. Longer studies are needed both in prevention and treatment regimens to evaluate further on the latter effects of GLP-1.
In Wistar rats, seven days of exendin-4 injections prevented the induction of vascular endothelial dysfunction following streptozotocin treatment as evaluated by Ach-induced relaxation and integrity of endothelial lining [154]. The data suggest that the effect result from an increased production of eNOS by activation of Akt, as the effects of exendin-4 were attenuated by the eNOS inhibitor L-NAME. A significantly reduction in TBARS was observed in the exendin-4 treated when compared with control animals [154] implying effects on OS status. However, as a report showed that contractile force in aortic rings can be preserved by GLP-1 in STZ induced diabetic rats but not with exendin-4 while both treatments lead to reduced plasma MDA [155], the two effects may not be related. On the other hand, the lack of contractile effects of exendin-4 may be due to the low doses used, although they were clearly high enough to reduce lipid oxidation.
Collectively, the above results suggest that GLP-1 can lower the OS status in various animal models, both preventive and treatment studies, by lowering ROS producing enzymes and increasing antioxidant capacity, thus lowering the damage induced by high levels of ROS in the animals. Many different organ systems are involved and thus more detailed studies are needed to elucidate the effects of GLP-1 on OS
and mechanisms involved. Particularly, by assessing the balance between ROS, ROS production and antioxidant concentration and activity in all studies and trying to eliminate the confounding of GLP-1 on blood glucose, body weight and food intake. Moreover, it is important to investigate if and to what extent the effects observed in animal models may translate to humans bearing in mind the comparatively high doses used in animals compared to clinically relevant dosing regimens.
5. Effect of GLP-1 on OXIDATIVE STRESS in clinical studies
The relationship between fasting GLP-1 concentration and OS has been investigated in a retrospective study where 72 T2D patients (65.3±8.6 yrs.) and 14 (63.4±8.7 yrs.) non-diabetic subjects were included. It revealed an inverse correlation between nitrotyrosine concentrations and GLP-1 independently of age, gender, BMI, HbA1c, antidiabetic treatment, and antihypertensive treatment. In this study, 8-OHdG was increased in the diabetic patients as well [116]. The effect of GLP-1 on OS status in humans has been investigated in both acute studies and longer term clinical studies. The available clinical data are summarized in Table 3.
Table 3.
Clinical studies including measurements of oxidative stress level.
| Duration of treatment |
Study Design:
Subjects (S), Intervention (I) and Treatment Duration (TD) |
End point: Effect of GLP-1 Intervention on Oxidative Stress Markers (Compared to control Unless Otherwise Stated) | Comments |
|---|---|---|---|
| Hours |
S: T1D (n=30). I: Hypo- (2.9 ± 0.1 mmol/l) or hyperglycemic (15 mmol/l) clamp ± GLP-1 infusion (0.4 pmol/kg/min). TD: 2 hours. |
Plasma: ↓ 8-iso-PGF2α + nitrotyrosine (hypo- and hyperglycemia) (p<0.01) but significantly higher than baseline (p<0.01). |
[77] |
|
S: T2D (n=16) + controls (n=12). I: Hyperglycemic (15 mmol/l 0-1 hours and 12.8 mmol/l at 1-2 hours) clamp. Repeated after two months of better glycemic control (insulin therapy). TD: 2 hours. |
Plasma: ↓ 8-iso-PGF2α + nitrotyrosine (p<0.05) (after one and two hours in T2D, after one in controls). More pronounced effect after two months of better glycemic control. |
[156] | |
|
S: T1D (n=20). I: Hypoglycemic (2.9 ± 0.1 mmol/l) clamp. Vitamin C (30 mg/min) and/or GLP-1 (0.4 pmol/kg/min). TD: 2 hours. |
Plasma: ↓ 8-iso-PGF2α + nitrotyrosine (GLP-1 or vitamin C (more pronounced (p<0.05)). GLP-1 + vitamin C: 8-iso-PGF2α and nitrotyrosine at one and two hours not significantly different from baseline measurements. |
[158] | |
|
S: T1D (n=15). I: Hypoglycemia (2.9 ± 0.1 mmol/l) superseded with either normoglycemia (4.5 mmol/l) or hyperglycemia (15 mmol/l) Vitamin C (30 mg/min) and/or GLP-1 (0.4 pmol/kg/min). TD: 4 hours (2 hours of hypoglycemia and 2 hours of hyper- or normoglycemia). |
Plasma: ↓ 8-iso-PGF2α + nitrotyrosine (p<0.01) (GLP-1 or vitamin C). Not significantly different from baseline when administrated together. |
[157] | |
|
S: T2D (n=24) receiving dietary or metformin intervention. I: Gr. 1: Normoglycaemic-normoinsulinaemic (blood glucose = 5 mmol/l) or normoglycaemic-hyperinsulinaemic (insulin infusion 0.1 mU/kg/min, blood glucose = 5 mmol/l) ± GLP-1 (0.4 pmol/kg/min). Gr. 2: Hyperglycaemic-normoinsulinaemic (blood glucose = 15 mmol/l) or hyperglycaemic-hyperinsulinaemic (insulin infusion 0.1 mU/kg/min, blood glucose = 15 mmol/l) ± GLP-1 (0.4 pmol/kg/min). TD: 2 hours. |
Plasma: ↓ 8-iso-PGF2α + nitrotyrosine (p<0.05), further decreased by hyperinsulinaemia. |
[159] | |
| < 6 months |
S: T2D (n=20 ♂+♀) on metformin. I: Liraglutide (0.6 mg daily for two weeks then 1.2 mg daily). TD: 2 months. |
Blood; ↓ LOOH (p<0.05). ↑ GSH (p<0.01). ↓HO-1 (p<0.05). |
Decreased HbA1c (p<0.0001) and increased ghrelin concentrations (p<0.01) [166]. |
|
S: T2D (n=12) on metformin. I: Exenatide (10 μg twice daily). Measuring superoxide release from isolated peripheral blood mononuclear cells. TD: 3 months. |
Isolated peripheral blood MNCs: ↓ superoxide release (chemiluminescence) (p<0.05). |
And acute study was performed as well testing superoxide generation in MNCs after a single dose of exenatide (5 µg) → superoxide was reduced after 6 hours compared to baseline and placebo (p<0.05) [24]. | |
|
S: Prediabetic patients (n=50) I: Exenatide (5 μg for 4 weeks then 10 μg twice daily) or metformin (500 mg for 4 weeks then 1000 mg twice daily). TD: 3 months. |
Plasma: ↓ oxLDL in metformin group (p<0.05) but no significant difference between the metformin and exenatide group. |
[171] | |
|
S: Obese but nondiabetic children and adolescents (age 9–16 years old) (n=12). I: Exenatide (5 μg for 4 weeks then 10 μg twice daily). TD: 3 months. |
Plasma: No effect on oxLDL. |
Significant BMI decrease [174] | |
| Duration of treatment |
Study Design: Subjects (S), Intervention (I) and Treatment Duration (TD) |
End point: Effect of GLP-1 Intervention on Oxidative Stress Markers (Compared to control Unless Otherwise Stated) | Comments |
|
S: T2D (n=23) on metformin and/or sulfonylurea. I: Exenatide (5 μg for 4 weeks then 10 μg twice daily). TD: 5 months. |
Plasma: ↓ 8-iso-PGF2α (p<0.05). |
Covariance analysis indicated glucose independent effect on 8-iso-PGF2α levels that was independent of HbA1c, mean SD, body weight, and BMI. [75] | |
| > 6 months |
S: T2D (n=65) (58.7±10.2 years) no more than 15 years of disease duration. n=32 on sulfonylurea. I: Liraglutide (started at 0.3 mg/day then 0.6-0.9 mg/day (mean dose 0.74 mg/day). TD: 8 months. |
Blood: ↓ d-ROMs (p<0.05) (compared to baseline). No change in MDA. |
Decreased HbA1c and blood glucose when compared to baseline (p<0.01). No effect on body weight [176]. |
|
S: T2D ♀+♂ (n=60) on metformin. I: Exenatide (5 μg for 4 weeks then 10 μg twice daily) or insulin glargine (starting at 10 U once daily). TD: 1 year. |
Plasma: ↓ MDA (compared to baseline and insulin glargine group (p<0.001)). ↓ oxLDL/LDL in exenatide group (compared to baseline) (p<0.05). |
Decrease in blood glucose (p<0.001) and triglycerides (p<0.05) both compared to baseline and insulin glargine group. No correlation between change in body weight and effects on blood glucose and triglycerides (body weight change not given) [23]. |
BMI = body mass index, d-ROMs = derivatives of reactive oxygen metabolites, HbA1c = hemoglobin A1c, HO-1 = heme oxygenease-1, GSH = glutathione, LDL = low density lipoprotein, LOOH = lipid hydroperoxide, MDA = malondialdehyde, MNCs = mononuclear cells, oxLDL = oxidized low density lipoprotein, SD = standard deviation, T1D = type 1 diabetes, T2D = type 2 diabetes, 8-iso-PGF2α = 8-iso-prostaglandin F2α.
5.1. Acute Effects in Diabetic Patients
Ceriello and coworkers have performed several acute cross over experiments with hypo- or hyperglycemia in both T1D and T2D diabetic patients with focus on the effects of GLP-1 on OS [77, 156-159]. In one study, 30 male and female T1D patients, mean aged about 24 years and diagnosed for 7-8 years, were exposed to hypo- or hyperglycemic conditions [77]. Hypoglycemia has been shown to induce ROS production in an in vivo rat model in the CNS comparable to that of hyperglycemia [43, 160]. In the above study, two hours of hypo- or hyperglycemia and an simultaneous GLP-1 infusion to T1D patients decreased plasma 8-iso-PGF2α and nitrotyrosine levels, although they remained significantly elevated compared to baseline. In a similar study of hyperglycemia in T2D patients, GLP-1 also reduced plasma 8-iso-PGF2α and nitrotyrosine levels significantly [156]. The study was repeated after two months of optimized glycemic control of the diabetic patients. In these clamp experiments, which do not correspond to any physiological situation, two months of improved glycemic control per se decreased basal levels of 8-iso-PGF2α and nitrotyrosine while the following GLP-1 treatment no longer improved the measures as it had done at baseline. The study suggests that the effect of GLP-1 on OS status is more pronounced when subjects are exposed to hyperglycemic conditions for longer periods of time, which may indicate that GLP-1 exerts a protective effect only under hyperglycemic conditions. However, these patients might have lost weight during the two months, and to evaluate on the effect of better glycemic control, a group of body weight and/or HbA1c matched patients should have been included, depending on whether the glycemic control aimed at lowering the HbA1c or fluctuations in blood glucose.
Effects of the antioxidant vitamin C and/or GLP-1 on OS status were examined in 20 T1D patients during acute hypo- glycemia [158]. GLP-1 alone reduced both 8-iso-PGF2α and nitrotyrosine significantly and vitamin C alone reduced the levels of these two biomarkers even more. Interestingly, si- multaneous administration of both GLP-1 and vitamin C resulted in levels of 8-iso-PGF2α and nitrotyrosine not being different from baseline measurements. This can be contributed to different actions of vitamin C and GLP-1, vitamin C being a scavenger of ROS and GLP-1 acting through induction of intracellular antioxidant defenses, respectively. Similar results were obtained in T1D patients in a study of effects of GLP-1 and/or vitamin C on the glycemic changes during I/R like conditions, where hypoglycemic conditions were followed by either normo- or hyperglycemia [157]. During hypoglycemia, levels of both 8-iso-PGF2α and nitrotyrosine increased, and these levels increased even more when hyperglycemia replaced hypoglycemia as compared to normoglycemia. GLP-1 lowered 8-iso-PGF2α and nitrotyrosine but again, vitamin C had a more pronounced effect. In this case, simultaneous infusions of GLP-1 and vitamin C lowered 8-iso-PGF2α and nytrosine to baseline level. The finding that GLP-1 is less effective than vitamin C could be caused by a reduction of sensitivity to GLP-1 in hypoglycemia as hyperglycemic conditions can increase the effects of GLP-1 on OS status as discussed above.
In the above experiments, the clamp technique was used and insulin was administrated. As insulin has effects on OS level and vasodilatory effects as well, the administration of insulin should be considered when evaluating on the effects of GLP-1 on OS [161-165]. It has been shown that combined administration of insulin and GLP-1 in such clamp studies can lower 8-iso-PGF2α and nitrotyrosine levels more than GLP-1 by itself [159].
5.2. Short-Term Intervention Studies (< 6 Months)
A pilot study in 20 female and male T2D patients on metformin (57 ± 13 years) liraglutide treatment for two months lowered HbA1c significantly together with a decrease in blood concentrations of lipid peroxides (LOOH) and HO-1 and an increase in GSH and ghrelin. Body weight, fasting blood glucose and waist circumference were not affected in this study, and there was no correlation between LOOH and GSH concentrations, which implies a glucose independent effect of liraglutide on OS status [166]. Ghrelin has previously been associated with decrease in OS status in obese subjects [167]. Liraglutide induced ghrelin regulation could have an effect on OS status. The data on HO-1 in this study are not in line with the in vitro data of Oeseburg et al (2010), where HO-1 were increased by GLP-1, as it has antioxidant properties. T2D patients have increased levels of HO-1 [168] and the effect of liraglutide in this study could lower the need of HO-1 protection against OS and thus the concentration of HO-1. ROS was measured using DHE staining in this study, but no significant effect was found, which could support the hypothesis that antioxidant protection is present in diabetes. No metabolic studies were performed and only fasting blood glucose was measured, which together with the lowering of HbA1c makes it difficult to rule out that the effects on OS could be attributed in part to improved glycemic control.
In isolated mononuclear cells from obese T2D patients undergoing diabetes therapy, a 22 ± 9% reduction in superoxide production was observed when the patients were treated with exenatide for 6 or 12 weeks prior to sampling. This was significantly different from baseline and from placebo, an effect seemingly independent of weight loss as no change in body weight was observed over the course of the study. The lack of weight loss could be due to the short duration of the study and that the patients were on insulin therapy throughout the study. An acute study was also performed; here a single injection of exenatide (5 µg) reduced superoxide release from isolated mononuclear cells as well [24]. FFA was reduced in the single injection experiment but glucose and insulin were not measured. In the 12 week study fasting blood glucose, HbA1c and FFA were significantly lowered and insulin increased by exenatide treatment. Thus, regulatory effects on glucose, lowered FFA and increased insulin cannot be excluded to have an effect in this study. NF-κβ binding and mRNA levels of inflammatory markers (TNF-α, JNK-1, IL-1β, TLR-2, and TLR-4) were all decreased as well. The link between inflammation and OS has been suggested to be initiated by ROS activating NF-κβ subsequently inducing proinflammatory cytokines. However, increased NF-κβ may be induced by elevated blood glucose [169].
Reductions in inflammatory markers and 8-iso-PGF2α have been shown in T2D patients following five months treatment with exenatide. Consistent with the expected effects of GLP-1 in T2D patients, these biochemical changes were accompanied by reduced body weights and BMIs, improved glucose profiles, reduced HbA1c, mean glucose levels and limited blood glucose excursions. To investigate the potential glucose independent effects of exenatide on 8-iso-PGF2α, an analysis of covariance analysis was performed, showing that the effect of exenatide was independent of glycemic regulation and body weight [75]. Thus, this study supports that glucose excursions per se increase OS [170], since the glucose excursions measured in this study correlated with 8-iso-PGF2α. However, as 8-iso-PGF2α is decreased by exenatide, a glucose dependent effects of GLP-1 on OS status cannot be ruled out.
A comparison of the first line drug for prediabetic patients, metformin, and exenatide has been performed in male and female non-diabetic but obese and prediabetic patients with a mean age of 58.5±10.0 yrs. having either impaired fasting glucose, elevated HbA1c, or impaired glucose tolerance [171]. Three months of treatment resulted in no significant differences between exenatide or metformin groups on either oxLDL levels or endothelial function. The metformin group had a significantly lower oxLDL level after treatment than when compared to baseline. Unfortunately, the study had a small sample size and did not include a placebo group as baseline was used as control and albeit not significantly different, there was a difference in baseline data between the groups. Thus, the metformin treated group had a higher baseline concentration of oxLDL perhaps leaving a higher window of opportunity for effect of treatment. The effects of metformin could be due to altered antioxidant activity and antioxidant homeostasis [172, 173]. In another study, three months of exenatide treatment to obese children and adolescents did not reduce oxLDL levels even though BMI were significantly decreased [174]. These studies indicate that the effect of GLP-1 on OS may be restricted to or at least most pronounced when individuals are challenged with diabetes and e.g. postprandial glucose excursions. This is suggested
by Tesauro et al. (2013) as well, as healthy obese subjects do not respond to GLP-1 therapy when measuring endothelial vasodilation [175]. But aspects such as duration of the studies, obesity level of the patients, and its effect on blood glucose should be evaluated more thoroughly in future studies, which could also include more biomarkers of OS as a single marker does not give a complete picture.
5.3. Longer Term Intervention studies (> 6 Months)
Very limited data are available on long-term effects of GLP-1 administration on OS in humans as only two studies have been performed. Here, one year of exenatide treatment resulted in improved postprandial glucose homeostasis and dyslipidemia in 60 metformin treated T2D patients subjected to a standardized meal after an overnight fasting [23]. Exenatide treatment lowered plasma MDA significantly both compared to baseline and to a parallel insulin glargine treatment group. The postprandial oxLDL/LDL ratio was significantly reduced from baseline measures in the exenatide group, but no significant difference was observed between the two treatment groups. The glucose lowering effects of both treatments may explain these findings. The treatment period of one year was followed by a period of five weeks off drug, after which the subjects had the standardized mixed meal and the above measurements were repeated, displaying a return of all parameters to their baseline values [23]. The study shows that exenatide lowers excursions in glucose and lipids after a meal and improved biochemical markers of oxidative damage to lipids but does not provide a lasting effect following discontinuation of treatment. Again measuring other biomarkers of ROS level, antioxidant capacity and damaged end products could help elucidate the mechanisms behind the observed effects. In another long-term study, eight months of liraglutide intervention (mean dose 0.74 mg/day) in T2D patients did not result in a reduction of blood MDA even though it lowered d-ROMs when compared to baseline [176]. Blood glucose and plasma insulin was significantly lower, which may explain the effect on d-ROMs. The absence of effect on MDA could be due to the low doses used in this study as the normal human dose is 1.2 mg/day in T2D. In the former mentioned exenatide study, the normal dosing regimen of exenatide was used.
Part of the evaluation of the effects of GLP-1 on diabetic late complications and in particular CVD will be done in the LEADER trial, which was initiated in 2010 and will follow patients for up to five years. This trial evaluates the effect of liraglutide on CVD [177]. Such a trial makes studying the mechanisms behind a potential effect even more warranted, as the clinical studies available so far have not been suitable for separating glucose dependent effects from glucose independent effects.
Conclusion
Besides the well-established metabolic effects of GLP-1 that in several ways may positively affect redox homeostasis, glucose independent effect on OS may also play a role and can be investigated by choosing other compounds exerting distinct parts of the GLP-1 activity complex. Better glycemic control is known to lower the risk of developing diabetic complications such as CVD [2]. Decreased food intake caused by diet restriction has been shown repeatedly to affect OS levels in rodents, primarily lowering oxidative damage and increasing GSH, but the effect on antioxidant status varies from tissue to tissue [178]. Moreover, caloric restriction is an effective way to extend lifespan in rodents supporting the role of OS in late onset disease development [179]. However, from the clinical studies available so far, it seems that lowering blood glucose may be the main driver and not body weight reduction in humans.
This review summarizes the effects of GLP-1 on OS status in different treatment or intervention regimes, both in in vitro, in vivo and in the clinical setting. The less pronounced effects of GLP-1 in clinical studies may possibly be explained by something as simple as the lower doses used, as in vitro and in vivo studies in general have used doses well above those approved clinically while the differences between “mouse and man” are not to be neglected. There is no clear evidence supporting a single mechanism driving the effects of GLP-1 on redox homeostasis, but it seems that both ROS production and antioxidant capacity can be affected in a positive direction, favoring lower OS status and lower levels of damaged end products. The binding of GLP-1 to the GLP-1R increases cAMP, activates PKA and downstream pathways from this, which can explain the glucose independent pathways of GLP-1 induced improvement of OS status.
Collectively, the available data demonstrates that GLP-1 can decrease OS but also that this effect is dependent on dose, diabetic status, obesity etc. This effect of GLP-1 may have therapeutic potential if OS is in fact causally related to the development of late-onset diabetic complications.
ACKNOWLEDGEMENTS
KEP and JL are supported by the Lifepharm Centre for in vivo pharmacology at University of Copenhagen. The present review was conceived by all authors. KEP wrote the draft manuscript, which was subsequently edited by all authors.
CONFLICT OF INTEREST
KR and GR are employees at Novo Nordisk A/S. The authors declare no conflicts of interest regarding the present work.
REFERENCES
- 1.Nathan D.M. Long-term complications of diabetes mellitus. N. Engl. J. Med. 1993;328(23):1676–1685. doi: 10.1056/NEJM199306103282306. [DOI] [PubMed] [Google Scholar]
- 2.Mazzone T. Intensive glucose lowering and cardiovascular disease prevention in diabetes: Reconciling the recent clinical trial data. Circulation. 2010;122(21):2201–2211. doi: 10.1161/CIRCULATIONAHA.109.913350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rydén L., Standl E., Malgorzata B., et al. Guidelines on diabetes, pre-diabetes, and cardiovascular diseases: Executive summary. The task force on diabetes and cardiovascular diseases of the European Society of Cardiology (ESC) and of the European Association for the Study of Diabetes (EASD). Eur. Heart J. 2007;28(1):88–136. doi: 10.1093/eurheartj/ehl260. [DOI] [PubMed] [Google Scholar]
- 4.Brownlee M. Biochemistry and molecular cell biology of diabetic complications. Nature. 2001;414(6865):813–820. doi: 10.1038/414813a. [DOI] [PubMed] [Google Scholar]
- 5.Brownlee M. The pathobiology of diabetic complications: A unifying mechanism. Diabetes. 2005;54(6):1615–1625. doi: 10.2337/diabetes.54.6.1615. [DOI] [PubMed] [Google Scholar]
- 6.Deacon C.F., Johnsen A.H., Holst J.J. Degradation of glucagon-like peptide-1 by human plasma in vitro yields an N-terminally truncated peptide that is a major endogenous metabolite in vivo. J. Clin. Endocrinol. Metab. 1995;80(3):952–957. doi: 10.1210/jcem.80.3.7883856. [DOI] [PubMed] [Google Scholar]
- 7.Holst J.J. Treatment of type 2 diabetes mellitus with agonists of the GLP-1 receptor or DPP-IV inhibitors. Expert Opin. Emerg. Drugs. 2004;9(1):155–166. doi: 10.1517/eoed.9.1.155.32952. [DOI] [PubMed] [Google Scholar]
- 8.Schmidt W.E., Siegel E.G., Creutzfeldt W. Glucagon-like peptide-1 but not glucagon-like peptide-2 stimulates insulin release from isolated rat pancreatic islets. Diabetologia. 1985;28(9):704–707. doi: 10.1007/BF00291980. [DOI] [PubMed] [Google Scholar]
- 9.Kreymann B., Williams G., Ghatei M.A., et al. Glucagon-like peptide-1 7-36: A physiological incretin in man. Lancet. 1987;2(8571):1300–1304. doi: 10.1016/s0140-6736(87)91194-9. [DOI] [PubMed] [Google Scholar]
- 10.Zander M., Madsbad S., Madsen J.L., et al. Effect of 6-week course of glucagon-like peptide 1 on glycaemic control, insulin sensitivity, and beta-cell function in type 2 diabetes: a parallel-group study. Lancet. 2002;359(9309):824–830. doi: 10.1016/S0140-6736(02)07952-7. [DOI] [PubMed] [Google Scholar]
- 11.Urusova I.A., Farilla L., Hui H., et al. GLP-1 inhibition of pancreatic islet cell apoptosis. Trends Endocrinol. Metab. 2008;19(1):27–33. doi: 10.1016/j.tem.2003.11.006. [DOI] [PubMed] [Google Scholar]
- 12.Tourrel C., Bailbé D., Meile M.J., et al. Glucagon-Like Peptide-1 and Exendin-4 Stimulate beta-Cell Neogenesis in Streptozotocin-Treated Newborn Rats Resulting in Persistently Improved Glucose Homeostasis at Adult Age. Diabetes. 2001;50(7):1562–1570. doi: 10.2337/diabetes.50.7.1562. [DOI] [PubMed] [Google Scholar]
- 13.Gutzwiller JP, Drewe J, Göke B, et al. Glucagon-like peptide-1 promotes satiety and reduces food intake in patients with diabetes mellitus type 2. Am J Physiol-Reg I. 1999;276(5):45–5. doi: 10.1152/ajpregu.1999.276.5.R1541. 1541-4. [DOI] [PubMed] [Google Scholar]
- 14.Ceriello A. Acute hyperglycaemia and oxidative stress generation. Diabet. Med. 1997;14(Suppl. 3):45–49. doi: 10.1002/(sici)1096-9136(199708)14:3+<s45::aid-dia444>3.3.co;2-i. [DOI] [PubMed] [Google Scholar]
- 15.Monnier L., Mas E., Ginet C., et al. Activation of oxidative stress by acute glucose fluctuations compared with sustained chronic hyperglycemia in patients with type 2 diabetes. JAMA. 2006;295(14):1681–1687. doi: 10.1001/jama.295.14.1681. [DOI] [PubMed] [Google Scholar]
- 16.Ellingsgaard H., Hauselmann I., Schuler B., et al. Interleukin-6 enhances insulin secretion by increasing glucagon-like peptide-1 secretion from L cells and alpha cells. Nat. Med. 2011;17(11):1481–1489. doi: 10.1038/nm.2513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kahles F., Meyer C., Möllmann J., et al. GLP-1 Secretion Is Increased by Inflammatory Stimuli in an IL-6-Dependent Manner, Leading to Hyperinsulinemia and Blood Glucose Lowering. Diabetes. 2014;63(11):3221–3229. doi: 10.2337/db14-0100. [DOI] [PubMed] [Google Scholar]
- 18.Piotrowski K., Becker M., Zugwurst J., et al. Circulating concentrations of GLP-1 are associated with coronary atherosclerosis in humans. Cardiovasc. Diabetol. 2013;12(1) doi: 10.1186/1475-2840-12-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hattori A., Kawamura I., Yamada Y., et al. Elevated plasma GLP-1 levels and enhanced expression of cardiac GLP-1 receptors as markers of left ventricular systolic dysfunction: A cross-sectional study. BMJ Open. 2013;3(9) doi: 10.1136/bmjopen-2013-003201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang D., Luo P., Wang Y., et al. Glucagon-like peptide-1 protects against cardiac microvascular injury in diabetes via a cAMP/PKA/Rho-dependent mechanism. Diabetes. 2013;62(5):1697–1708. doi: 10.2337/db12-1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shiraki A., Oyama J.I., Komoda H., et al. The glucagon-like peptide 1 analog liraglutide reduces TNF-alfa-induced oxidative stress and inflammation in endothelial cells. Atherosclerosis. 2012;221(2):375–382. doi: 10.1016/j.atherosclerosis.2011.12.039. [DOI] [PubMed] [Google Scholar]
- 22.Timmers L., Henriques J.P., de Kleijn D.P., et al. Exenatide Reduces Infarct Size and Improves Cardiac Function in a Porcine Model of Ischemia and Reperfusion Injury. J. Am. Coll. Cardiol. 2009;53(6):501–510. doi: 10.1016/j.jacc.2008.10.033. [DOI] [PubMed] [Google Scholar]
- 23.Bunck M.C., Cornér A., Eliasson B., et al. One-year treatment with exenatide vs. Insulin Glargine: Effects on postprandial glycemia, lipid profiles, and oxidative stress. Atherosclerosis. 2010;212(1):223–229. doi: 10.1016/j.atherosclerosis.2010.04.024. [DOI] [PubMed] [Google Scholar]
- 24.Chaudhuri A., Ghanim H., Vora M., et al. Exenatide exerts a potent antiinflammatory effect. J. Clin. Endocrinol. Metab. 2012;97(1):198–207. doi: 10.1210/jc.2011-1508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ceriello A., Motz E. Is oxidative stress the pathogenic mechanism underlying insulin resistance, diabetes, and cardiovascular disease? The common soil hypothesis revisited. Arterioscler. Thromb. Vasc. Biol. 2004;24(5):816–823. doi: 10.1161/01.ATV.0000122852.22604.78. [DOI] [PubMed] [Google Scholar]
- 26.Maiese K., Chong Z.Z., Shang Y.C. Mechanistic insights into diabetes mellitus and oxidative stress. Curr. Med. Chem. 2007;14(16):1729–1738. doi: 10.2174/092986707781058968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Maritim A.C., Sanders R.A., Watkins J.B., III Diabetes, oxidative stress, and antioxidants: A review. J. Biochem. Mol. Toxicol. 2003;17(1):24–38. doi: 10.1002/jbt.10058. [DOI] [PubMed] [Google Scholar]
- 28.Lenzen S., Drinkgern J., Tiedge M. Low antioxidant enzyme gene expression in pancreatic islets compared with various other mouse tissues. Free Radic. Biol. Med. 1996;20(3):463–466. doi: 10.1016/0891-5849(96)02051-5. [DOI] [PubMed] [Google Scholar]
- 29.Boden G., Shulman G.I. Free fatty acids in obesity and type 2 diabetes: Defining their role in the development of insulin resistance and beta-cell dysfunction. Eur. J. Clin. Invest. 2002;32(Suppl. 3):14–23. doi: 10.1046/j.1365-2362.32.s3.3.x. [DOI] [PubMed] [Google Scholar]
- 30.Cnop M., Hughes S.J., Igoillo-Esteve M., et al. The long lifespan and low turnover of human islet beta cells estimated by mathematical modeling of lipofuscin accumulation. Diabetologia. 2010;53(2):321–330. doi: 10.1007/s00125-009-1562-x. [DOI] [PubMed] [Google Scholar]
- 31.Reaven G.M., Hollenbeck C., Jeng C.Y., et al. Measurement of plasma glucose, free fatty acid, lactate, and insulin for 24 h in patients with NIDDM. Diabetes. 1988;37(8):1020–1024. doi: 10.2337/diab.37.8.1020. [DOI] [PubMed] [Google Scholar]
- 32.Zuniga-Guajardo S., Zinman B. The metabolic response to the euglycemic insulin clamp in type I diabetes and normal humans. Metabolis. 1985;34(10):926–930. doi: 10.1016/0026-0495(85)90140-4. [DOI] [PubMed] [Google Scholar]
- 33.Fraze E, Donner CC. Ambient plasma free fatty acid concentrations in noninsulin-dependent diabetes mellitus: Evidence for insulin resistance. J. Clin. Endocrinol. Metab. 1985;61(5):807–811. doi: 10.1210/jcem-61-5-807. [DOI] [PubMed] [Google Scholar]
- 34.McGarry J.D. What if Minkowski had been ageusic? An alternative angle on diabetes. Science. 1992;258(5083):766–770. doi: 10.1126/science.1439783. [DOI] [PubMed] [Google Scholar]
- 35.Matsuda M., Shimomura I. Increased oxidative stress in obesity: Implications for metabolic syndrome, diabetes, hypertension, dyslipidemia, atherosclerosis, and cancer. Obes. Res. Clin. Pract. 2013;7(5):330–341. doi: 10.1016/j.orcp.2013.05.004. [DOI] [PubMed] [Google Scholar]
- 36.Morgan D., Oliveira-Emilio H.R., Keane D., et al. Glucose, palmitate and pro-inflammatory cytokines modulate production and activity of a phagocyte-like NADPH oxidase in rat pancreatic islets and a clonal beta cell line. Diabetologia. 2007;50(2):359–369. doi: 10.1007/s00125-006-0462-6. [DOI] [PubMed] [Google Scholar]
- 37.Poitout V., Robertson R.P. Glucolipotoxicity: Fuel excess and beta-cell dysfunction. Endocr. Rev. 2008;29(3):351–366. doi: 10.1210/er.2007-0023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bonnard C., Durand A., Peyrol S., et al. Mitochondrial dysfunction results from oxidative stress in the skeletal muscle of diet-induced insulin-resistant mice. J. Clin. Invest. 2008;118(2):789–800. doi: 10.1172/JCI32601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Choksi K.B., Boylston W.H., Rabek J.P., et al. Oxidatively damaged proteins of heart mitochondrial electron transport complexes. Biochim Biophys Acta Mol Basis Dis. 2004;1688(2):95–101. doi: 10.1016/j.bbadis.2003.11.007. [DOI] [PubMed] [Google Scholar]
- 40.Kim J.A., Wei Y., Sowers J.R. Role of mitochondrial dysfunction in insulin resistance. Circ. Res. 2008;102(4):401–414. doi: 10.1161/CIRCRESAHA.107.165472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Trachootham D., Lu W., Ogasawara M.A., et al. Redox regulation of cell survival. Antioxid. Redox Signal. 2008;10(8):1343–1374. doi: 10.1089/ars.2007.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Poulsen H.E., Specht E., Broedbaek K., et al. RNA modifications by oxidation: A novel disease mechanism? Free Radic. Biol. Med. 2012;52(8):1353–1361. doi: 10.1016/j.freeradbiomed.2012.01.009. [DOI] [PubMed] [Google Scholar]
- 43.Giacco F., Brownlee M. Oxidative stress and diabetic complications. Circ. Res. 2010;107(9):1058–1070. doi: 10.1161/CIRCRESAHA.110.223545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schaffer S.W., Jong C.J., Mozaffari M. Role of oxidative stress in diabetes-mediated vascular dysfunction: Unifying hypothesis of diabetes revisited. Vascul. Pharmacol. 2012;57(5-6):139–149. doi: 10.1016/j.vph.2012.03.005. [DOI] [PubMed] [Google Scholar]
- 45.Montezano A.C., Touyz R.M. Oxidative stress, Noxs, and hypertension: Experimental evidence and clinical controversies. Ann. Med. 2012;44(Suppl. 1):2–16. doi: 10.3109/07853890.2011.653393. [DOI] [PubMed] [Google Scholar]
- 46.Tsutsui H., Kinugawa S., Matsushima S. Oxidative stress and heart failure. Am. J. Physiol. Heart Circ. Physiol. 2011;301(6):2181–2190. doi: 10.1152/ajpheart.00554.2011. [DOI] [PubMed] [Google Scholar]
- 47.Yao D., Brownlee M. Hyperglycemia-induced reactive oxygen species increase expression of the receptor for advanced glycation end products (RAGE) and RAGE ligands. Diabetes. 2010;59(1):249–255. doi: 10.2337/db09-0801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chung S.S., Ho E.C., Lam K.S., et al. Contribution of polyol pathway to diabetes-induced oxidative stress. J. Am. Soc. Nephrol. 2003;14(Suppl. 3):233–236. doi: 10.1097/01.asn.0000077408.15865.06. [DOI] [PubMed] [Google Scholar]
- 49.Singh R., Barden A., Mori T., et al. Advanced glycation end-products: A review. Diabetologia. 2001;44(2):129–146. doi: 10.1007/s001250051591. [DOI] [PubMed] [Google Scholar]
- 50.Wautier MP, Chappey O, Corda S, et al. Activation of NADPH oxidase by AGE links oxidant stress to altered gene expression via RAGE. Am J Physiol Endocrinol Metab. 2001;280(5):43–5. doi: 10.1152/ajpendo.2001.280.5.E685. 685- 94. [DOI] [PubMed] [Google Scholar]
- 51.Basta G., Lazzerini G., Del Turco S., et al. At least 2 distinct pathways generating reactive oxygen species mediate vascular cell adhesion molecule-1 induction by advanced glycation end products. Arterioscler. Thromb. Vasc. Biol. 2005;25(7):1401–1407. doi: 10.1161/01.ATV.0000167522.48370.5e. [DOI] [PubMed] [Google Scholar]
- 52.Geraldes P., King G.L. Activation of protein kinase C isoforms and its impact on diabetic complications. Circ. Res. 2010;106(8):1319–1331. doi: 10.1161/CIRCRESAHA.110.217117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Inoguchi T., Sonta T., Tsubouchi H., et al. Protein kinase C-dependent increase in reactive oxygen species (ROS) production in vascular tissues of diabetes: Role of vascular NAD(P)H oxidase. J. Am. Soc. Nephrol. 2003;14(Suppl. 3):227–232. doi: 10.1097/01.asn.0000077407.90309.65. [DOI] [PubMed] [Google Scholar]
- 54.Kaneto H, Xu G, Song KH, et al. Activation of the Hexosamine Pathway Leads to Deterioration of Pancreatic Cell Function through the Induction of Oxidative Stress. J Biol Chem. 2001. pp. 31099–104. [DOI] [PubMed]
- 55.O'Donnell R.W., Johnson D.K., Ziegler L.M., et al. Endothelial NADLPH oxidase: Mechanism of activation by low-density lipoprotein. Endothelium J Endothelial Cell Res. 2003;10(6):291–297. doi: 10.1080/10623320390272280. [DOI] [PubMed] [Google Scholar]
- 56.Lusis A.J. Atherosclerosis. Nature. 2000;407(6801):233–241. doi: 10.1038/35025203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lüscher T.F., Barton M. Biology of the endothelium. Clin. Cardiol. 1997;20(Suppl. 12):3–10. [PubMed] [Google Scholar]
- 58.Miller J.D., Chu Y., Castaneda L.E., et al. Vascular function during prolonged progression and regression of atherosclerosis in mice. Arterioscl Throm Vas. 2013;33(3):459–465. doi: 10.1161/ATVBAHA.112.252700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mayer B., Hemmens B. Biosynthesis and action of nitric oxide in mammalian cells. Trends Biochem. Sci. 1997;22(12):477–481. doi: 10.1016/s0968-0004(97)01147-x. [DOI] [PubMed] [Google Scholar]
- 60.Pitocco D., Tesauro M., Alessandro R., et al. Oxidative stress in diabetes: Implications for vascular and other complications. Int. J. Mol. Sci. 2013;14(11):21525–21550. doi: 10.3390/ijms141121525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mortensen A., Lykkesfeldt J. Does vitamin C enhance nitric oxide bioavailability in a tetrahydrobiopterin-dependent manner? in vitro, in vivo and clinical studies. Nitric Oxide Biol Chem. 2014;36:51–57. doi: 10.1016/j.niox.2013.12.001. [DOI] [PubMed] [Google Scholar]
- 62.Folli F., Corradi D., Fanti P., et al. The role of oxidative stress in the pathogenesis of type 2 diabetes mellitus micro-and macrovascular complications: Avenues for a mechanistic-based therapeutic approach. Curr. Diabetes Rev. 2011;7(5):313–324. doi: 10.2174/157339911797415585. [DOI] [PubMed] [Google Scholar]
- 63.D'Souza A., Hussain M., Howarth F.C., et al. Pathogenesis and pathophysiology of accelerated atherosclerosis in the diabetic heart. Mol. Cell. Biochem. 2009;331(1-2):89–116. doi: 10.1007/s11010-009-0148-8. [DOI] [PubMed] [Google Scholar]
- 64.Federation I.D. IDF Diabetes Atlas. Int Diabetes Fed.Available from:http://www.idf.org/diabetesatlas. 2013;6 [Google Scholar]
- 65.Turner R. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet. 1998;352(9131):837–853. [PubMed] [Google Scholar]
- 66.The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N. Engl. J. Med. 1993;329(14):977–986. doi: 10.1056/NEJM199309303291401. [DOI] [PubMed] [Google Scholar]
- 67.Boussageon R, Bejan-Angoulvant T, Saadatian-Elahi M, et al. Effect of intensive glucose lowering treatment on all cause mortality, cardiovascular death, and microvascular events in type 2 diabetes: Meta-analysis of randomised controlled trials. Br Med J(Online). 2011:343. doi: 10.1136/bmj.d4169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hanley A.J., Williams K., Stern M.P., et al. Homeostasis model assessment of insulin resistance in relation to the incidence of cardiovascular disease: The San Antonio heart study. Diabetes Care. 2002;25(7):1177–1184. doi: 10.2337/diacare.25.7.1177. [DOI] [PubMed] [Google Scholar]
- 69.Nunes K.P., Rigsby C.S., Webb R.C. RhoA/Rho-kinase and vascular diseases: What is the link? Cell. Mol. Life Sci. 2010;67(22):3823–3836. doi: 10.1007/s00018-010-0460-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Heymes C., Bendall J.K., Ratajczak P., et al. Increased myocardial NADPH oxidase activity in human heart failure. J. Am. Coll. Cardiol. 2003;41(12):2164–2171. doi: 10.1016/s0735-1097(03)00471-6. [DOI] [PubMed] [Google Scholar]
- 71.Kodera R., Shikata K., Kataoka H.U., et al. Glucagon-like peptide-1 receptor agonist ameliorates renal injury through its anti-inflammatory action without lowering blood glucose level in a rat model of type 1 diabetes. Diabetologia. 2011;54(4):965–978. doi: 10.1007/s00125-010-2028-x. [DOI] [PubMed] [Google Scholar]
- 72.Arakawa M., Mita T., Azuma K., et al. Inhibition of Monocyte Adhesion to Endothelial Cells and Attenuation of Atherosclerotic Lesion by a Glucagon-like Peptide-1 Receptor Agonist, Exendin-4. Diabetes. 2010;59(4):1030–1037. doi: 10.2337/db09-1694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lee Y.S., Park M.S., Choung J.S., et al. Glucagon-like peptide-1 inhibits adipose tissue macrophage infiltration and inflammation in an obese mouse model of diabetes. Diabetologia. 2012;55(9):2456–2468. doi: 10.1007/s00125-012-2592-3. [DOI] [PubMed] [Google Scholar]
- 74.Wang Y., Parlevliet E.T., Geerling J.J., et al. Exendin-4 decreases liver inflammation and atherosclerosis development simultaneously by reducing macrophage infiltration. Br. J. Pharmacol. 2014;171(3):723–734. doi: 10.1111/bph.12490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wu J.D., Xu X.H., Zhu J., et al. Effect of exenatide on inflammatory and oxidative stress markers in patients with type 2 diabetes mellitus. Diabetes Technol. Ther. 2011;13(2):143–148. doi: 10.1089/dia.2010.0048. [DOI] [PubMed] [Google Scholar]
- 76.He L., Wong C.K., Cheung K.K., et al. Anti-inflammatory effects of exendin-4, a glucagon-like peptide-1 analog, on human peripheral lymphocytes in patients with type 2 diabetes. J. Diabetes Investig. 2013;4(4):382–392. doi: 10.1111/jdi.12063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ceriello A., Novials A., Ortega E., et al. Glucagon-like peptide 1 reduces endothelial dysfunction, inflammation, and oxidative stress induced by both hyperglycemia and hypoglycemia in type 1 diabetes. Diabetes Care. 2013;36(8):2346–2350. doi: 10.2337/dc12-2469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Drucker D.J., Philippe J., Mojsov S., et al. Glucagon-like peptide I stimulates insulin gene expression and increases cyclic AMP levels in a rat islet cell line. Proc. Natl. Acad. Sci. USA. 1987;84(10):3434–3438. doi: 10.1073/pnas.84.10.3434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Baggio L.L., Drucker D.J. Biology of Incretins: GLP-1 and GIP. Gastroenterology. 2007;132(6):2131–2157. doi: 10.1053/j.gastro.2007.03.054. [DOI] [PubMed] [Google Scholar]
- 80.Savitha G., Salimath B.P. Cross-talk between protein kinase C and protein kinase A down-regulates the respiratory burst in polymorphonuclear leukocytes. Cell. Signal. 1993;5(2):107–117. doi: 10.1016/0898-6568(93)90063-r. [DOI] [PubMed] [Google Scholar]
- 81.Bengis-Garber C., Gruener N. Protein kinase A downregulates the phosphorylation of p47 phox in human neutrophils: A possible pathway for inhibition of the respiratory burst. Cell. Signal. 1996;8(4):291–296. doi: 10.1016/0898-6568(96)00052-6. [DOI] [PubMed] [Google Scholar]
- 82.Saha S., Li Y., Anand-Srivastava M.B. Reduced levels of cyclic AMP contribute to the enhanced oxidative stress in vascular smooth muscle cells from spontaneously hypertensive rats. Can. J. Physiol. Pharmacol. 2008;86(4):190–198. doi: 10.1139/Y08-012. [DOI] [PubMed] [Google Scholar]
- 83.Kim J.S., Diebold B.A., Babior B.M., et al. Regulation of Nox1 activity via protein kinase A-mediated phosphorylation of NoxA1 and 14-3-3 binding. J. Biol. Chem. 2007;282(48):34787–34800. doi: 10.1074/jbc.M704754200. [DOI] [PubMed] [Google Scholar]
- 84.Batchuluun B., Inoguchi T., Sonoda N., et al. Metformin and liraglutide ameliorate high glucose-induced oxidative stress via inhibition of PKC-NAD(P)H oxidase pathway in human aortic endothelial cells. Atherosclerosis. 2014;232(1):156–164. doi: 10.1016/j.atherosclerosis.2013.10.025. [DOI] [PubMed] [Google Scholar]
- 85.Campbell J.E., Drucker D.J. Pharmacology, physiology, and mechanisms of incretin hormone action. Cell Metab. 2013;17(6):819–837. doi: 10.1016/j.cmet.2013.04.008. [DOI] [PubMed] [Google Scholar]
- 86.Kimura R., Okouchi M., Fujioka H., et al. Glucagon-like peptide-1 (GLP-1) protects against methylglyoxal-induced PC12 cell apoptosis through the PI3K/Akt/mTOR/GCLc/redox signaling pathway. Neuroscience. 2009;162(4):1212–1219. doi: 10.1016/j.neuroscience.2009.05.025. [DOI] [PubMed] [Google Scholar]
- 87.Puddu A., Storace D., Durante A., et al. Glucagon-like peptide-1 counteracts the detrimental effects of Advanced Glycation End-Products in the pancreatic beta cell line HIT-T 15. Biochem. Biophys. Res. Commun. 2010;398(3):462–466. doi: 10.1016/j.bbrc.2010.06.100. [DOI] [PubMed] [Google Scholar]
- 88.Tews D., Lehr S., Hartwig S., et al. Anti-apoptotic action of exendin-4 in INS-1 beta cells: comparative protein pattern analysis of isolated mitochondria. Horm. Metab. Res. 2009;41(4):294–301. doi: 10.1055/s-0028-1105911. [DOI] [PubMed] [Google Scholar]
- 89.Erdogdu O., Eriksson L., Xu H., et al. Exendin-4 protects endothelial cells from lipoapoptosis by PKA, PI3K, eNOS, P38 MAPK, and JNK pathways. J. Mol. Endocrinol. 2013;50(2):229–241. doi: 10.1530/JME-12-0166. [DOI] [PubMed] [Google Scholar]
- 90.Chang G., Zhang D., Yu H., et al. Cardioprotective effects of exenatide against oxidative stress-induced injury. Int. J. Mol. Med. 2013;32(5):1011–1020. doi: 10.3892/ijmm.2013.1475. [DOI] [PubMed] [Google Scholar]
- 91.Buteau J., El-Assaad W., Rhodes C.J., et al. Glucagon-like peptide-1 prevents beta cell glucolipotoxicity. Diabetologia. 2004;47(5):806–815. doi: 10.1007/s00125-004-1379-6. [DOI] [PubMed] [Google Scholar]
- 92.Puddu A, Sanguineti R, Durante A, et al. Glucagon-like peptide-1 triggers protective pathways in pancreatic beta-cells exposed to glycated serum. Mediat Inflamm. 2013. pp. 1–10. [DOI] [PMC free article] [PubMed]
- 93.Ojima A., Ishibashi Y., Matsui T., et al. Glucagon-like peptide-1 receptor agonist inhibits asymmetric dimethylarginine generation in the kidney of streptozotocin-induced diabetic rats by blocking advanced glycation end product-induced protein arginine methyltranferase-1 expression. Am. J. Pathol. 2013;182(1):132–141. doi: 10.1016/j.ajpath.2012.09.016. [DOI] [PubMed] [Google Scholar]
- 94.Li L., El-Kholy W., Rhodes C.J., et al. Glucagon-like peptide-1 protects beta cells from cytokine-induced apoptosis and necrosis: Role of protein kinase B. Diabetologia. 2005;48(7):1339–1349. doi: 10.1007/s00125-005-1787-2. [DOI] [PubMed] [Google Scholar]
- 95.Chang G., Zhang D., Liu J., et al. Exenatide protects against hypoxia/reoxygenation-induced apoptosis by improving mitochondrial function in H9c2 cells. Exp. Biol. Med. 2014;239(4):414–422. doi: 10.1177/1535370214522177. [DOI] [PubMed] [Google Scholar]
- 96.Ishibashi Y., Matsui T., Takeuchi M., et al. Glucagon-like peptide-1 (GLP-1) inhibits advanced glycation end product (AGE)-induced up-regulation of VCAM-1 mRNA levels in endothelial cells by suppressing AGE receptor (RAGE) expression. Biochem. Biophys. Res. Commun. 2010;391(3):1405–1408. doi: 10.1016/j.bbrc.2009.12.075. [DOI] [PubMed] [Google Scholar]
- 97.Li N., Li B., Brun T., et al. NADPH oxidase NOX2 defines a new antagonistic role for reactive oxygen species and cAMP/PKA in the regulation of insulin secretion. Diabetes. 2012;61(11):2842–2850. doi: 10.2337/db12-0009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Tomas E., Stanojevic V., Habener J.F. GLP-1-derived nonapeptide GLP-1(28-36)amide targets to mitochondria and suppresses glucose production and oxidative stress in isolated mouse hepatocytes. Regul. Pept. 2011;167(2-3):177–184. doi: 10.1016/j.regpep.2011.01.003. [DOI] [PubMed] [Google Scholar]
- 99.Ishibashi Y., Nishino Y., Matsui T., et al. Glucagon-like peptide-1 suppresses advanced glycation end product-induced monocyte chemoattractant protein-1 expression in mesangial cells by reducing advanced glycation end product receptor level. Metabolism. 2011;60(9):1271–1277. doi: 10.1016/j.metabol.2011.01.010. [DOI] [PubMed] [Google Scholar]
- 100.Cai Y., Hu X., Yi B., et al. Glucagon-like peptide-1 receptor agonist protects against hyperglycemia-induced cardiocytes injury by inhibiting high mobility group box 1 expression. Mol. Biol. Rep. 2012;39(12):10705–10711. doi: 10.1007/s11033-012-1961-9. [DOI] [PubMed] [Google Scholar]
- 101.Chen J., Couto F.M., Minn A.H., et al. Exenatide inhibits beta-cell apoptosis by decreasing thioredoxin-interacting protein. Biochem. Biophys. Res. Commun. 2006;346(3):1067–1074. doi: 10.1016/j.bbrc.2006.06.027. [DOI] [PubMed] [Google Scholar]
- 102.Oeseburg H., De Boer R.A., Buikema H., et al. Glucagon-like peptide 1 prevents reactive oxygen species-induced endothelial cell senescence through the activation of protein kinase A. Arterioscl Thromb Vasc. 2010;30(7):1407–1414. doi: 10.1161/ATVBAHA.110.206425. [DOI] [PubMed] [Google Scholar]
- 103.Shao W., Yu Z., Fantus I.G., et al. Cyclic AMP signaling stimulates proteasome degradation of thioredoxin interacting protein (TxNIP) in pancreatic beta-cells. Cell. Signal. 2010;22(8):1240–1246. doi: 10.1016/j.cellsig.2010.04.001. [DOI] [PubMed] [Google Scholar]
- 104.Rolin B., Deacon C.F., Carr R.D., et al. The major glucagon-like peptide-1 metabolite, GLP-1-(9-36)-amide, does not affect glucose or insulin levels in mice. Eur. J. Pharmacol. 2004;494(2-3):283–288. doi: 10.1016/j.ejphar.2004.05.013. [DOI] [PubMed] [Google Scholar]
- 105.Ban K., Kim K.H., Cho C.K., et al. Glucagon-Like Peptide (GLP)-1(9-36)amide-mediated cytoprotection is blocked by exendin(9-39) yet does not require the known GLP-1 receptor. Endocrinology. 2010;151(4):1520–1531. doi: 10.1210/en.2009-1197. [DOI] [PubMed] [Google Scholar]
- 106.Ma T., Du X., Pick J.E., et al. Glucagon-like peptide-1 cleavage product GLP-1(9-36) amide rescues synaptic plasticity and memory deficits in Alzheimer's disease model mice. J. Neurosci. 2012;32(40):13701–13708. doi: 10.1523/JNEUROSCI.2107-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Siegel D., Gustafson D.L., Dehn D.L., et al. NAD(P)H:quinone oxidoreductase 1: Role as a superoxide scavenger. Mol. Pharmacol. 2004;65(5):1238–1247. doi: 10.1124/mol.65.5.1238. [DOI] [PubMed] [Google Scholar]
- 108.Turkseven S, Kruger A, Mingone CJ, et al. Antioxidant mechanism of heme oxygenase-1 involves an increase in superoxide dismutase and catalase in experimental diabetes. 2005. [DOI] [PubMed]
- 109.Younce C.W., Burmeister M.A., Ayala J.E. Exendin-4 attenuates high glucose-induced cardiomyocyte apoptosis via inhibition of endoplasmic reticulum stress and activation of SERCA2a. Am. J. Physiol. Cell Physiol. 2013;304(6):508–518. doi: 10.1152/ajpcell.00248.2012. [DOI] [PubMed] [Google Scholar]
- 110.Crow J.P. Dichlorodihydrofluorescein and dihydrorhodamine 123 are sensitive indicators of peroxynitrite in vitro: Implications for intracellular measurement of reactive nitrogen and oxygen species. Nitric Oxide Biol Chem. 1997;1(2):145–157. doi: 10.1006/niox.1996.0113. [DOI] [PubMed] [Google Scholar]
- 111.Ussher J.R., Drucker D.J. Cardiovascular actions of incretin-based therapies. Circ. Res. 2014;114(11):1788–1803. doi: 10.1161/CIRCRESAHA.114.301958. [DOI] [PubMed] [Google Scholar]
- 112.Mukai E., Fujimoto S., Sato H., et al. Exendin-4 suppresses Src activation and reactive oxygen species production in diabetic Goto-Kakizaki rat islets in an Epac-dependent manner. Diabetes. 2011;60(1):218–226. doi: 10.2337/db10-0021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Chowdhury A.K., Watkins T., Parinandi N.L., et al. Src-mediated tyrosine phosphorylation of p47phox in hyperoxia-induced activation of NADPH oxidase and generation of reactive oxygen species in lung endothelial cells. J. Biol. Chem. 2005;280(21):20700–20711. doi: 10.1074/jbc.M411722200. [DOI] [PubMed] [Google Scholar]
- 114.Klaunig J.E., Kamendulis L.M., Hocevar B.A. Oxidative stress and oxidative damage in carcinogenesis. Toxicol. Pathol. 2010;38(1):96–109. doi: 10.1177/0192623309356453. [DOI] [PubMed] [Google Scholar]
- 115.Kim J.Y., Yu S.J., Oh H.J., et al. Panaxydol induces apoptosis through an increased intracellular calcium level, activation of JNK and p38 MAPK and NADPH oxidase-dependent generation of reactive oxygen species. Apoptosis. 2011;16(4):347–358. doi: 10.1007/s10495-010-0567-8. [DOI] [PubMed] [Google Scholar]
- 116.Ravassa S., Beaumont J., Huerta A., et al. Association of low GLP-1 with oxidative stress is related to cardiac disease and outcome in patients with type 2 diabetes mellitus: A pilot study. Free Radic. Biol. Med. 2015;81:1–12. doi: 10.1016/j.freeradbiomed.2015.01.002. [DOI] [PubMed] [Google Scholar]
- 117.Minn A.H., Hafele C., Shalev A. Thioredoxin-interacting protein is stimulated by glucose through a carbohydrate response element and induces B-cell apoptosis. Endocrinology. 2005;146(5):2397–2405. doi: 10.1210/en.2004-1378. [DOI] [PubMed] [Google Scholar]
- 118.Schulze P.C., Yoshioka J., Takahashi T., et al. Hyperglycemia promotes oxidative stress through inhibition of thioredoxin function by thioredoxin-interacting protein. J. Biol. Chem. 2004;279(29):30369–30374. doi: 10.1074/jbc.M400549200. [DOI] [PubMed] [Google Scholar]
- 119.Teramoto S., Miyamoto N., Yatomi K., et al. Exendin-4, a glucagon-like peptide-1 receptor agonist, provides neuroprotection in mice transient focal cerebral ischemia. J. Cereb. Blood Flow Metab. 2011;31(8):1696–1705. doi: 10.1038/jcbfm.2011.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.DellaValle B., Hempel C., Johansen F.F., et al. GLP-1 improves neuropathology after murine cold lesion brain trauma. Ann. Clin. Transl. Neurol. 2014;1(9):721–732. doi: 10.1002/acn3.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Noyan-Ashraf M.H., Abdul Momen M., Ban K., et al. GLP-1R agonist liraglutide activates cytoprotective pathways and improves outcomes after experimental myocardial infarction in mice. Diabetes. 2009;58(4):975–983. doi: 10.2337/db08-1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Briyal S., Gulati K., Gulati A. Repeated administration of exendin-4 reduces focal cerebral ischemia-induced infarction in rats. Brain Res. 2012;1427:23–34. doi: 10.1016/j.brainres.2011.10.026. [DOI] [PubMed] [Google Scholar]
- 123.Vaghasiya J.D., Sheth N.R., Bhalodia Y.S., et al. Exaggerated liver injury induced by renal ischemia reperfusion in diabetes: Effect of exenatide. Saudi J. Gastroenterol. 2010;16(3):174–180. doi: 10.4103/1319-3767.65187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Vaghasiya J., Sheth N., Bhalodia Y., et al. Exenatide protects renal ischemia reperfusion injury in type 2 diabetes mellitus. Int. J. Diabetes Dev. Ctries. 2010;30(4):217–225. [Google Scholar]
- 125.Sato K., Kameda M., Yasuhara T., et al. Neuroprotective effects of liraglutide for stroke model of rats. Int. J. Mol. Sci. 2013;14(11):21513–21524. doi: 10.3390/ijms141121513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Cesarone M.R., Belcaro G., Carratelli M., et al. A simple test to monitor oxidative stress. 18(2). Int. Angiol. 1999:127–130. [PubMed] [Google Scholar]
- 127.Esterbauer H., Gebicki J., Puhl H., et al. The role of lipid peroxidation and antioxidants in oxidative modification of LDL. Free Radic. Biol. Med. 1992;13(4):341–390. doi: 10.1016/0891-5849(92)90181-f. [DOI] [PubMed] [Google Scholar]
- 128.Raab E.L., Vuguin P.M., Stoffers D.A., et al. Neonatal exendin-4 treatment reduces oxidative stress and prevents hepatic insulin resistance in intrauterine growth-retarded rats. Am J Physiol-Reg I. 2009;297(6):1785–1794. doi: 10.1152/ajpregu.00519.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Brown S.B., Libonati J.R., Selak M.A., et al. Neonatal Exendin-4 leads to protection from reperfusion injury and reduced rates of oxidative phosphorylation in the adult rat heart. Cardiovasc. Drugs Ther. 2010;24(3):197–205. doi: 10.1007/s10557-010-6242-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Dokken B.B., Piermarini C.V., Teachey M.K., et al. Glucagon-like peptide-1 preserves coronary microvascular endothelial function after cardiac arrest and resuscitation: Potential antioxidant effects. Am J Physiol-Heart C. 2013;304(4):538–546. doi: 10.1152/ajpheart.00282.2012. [DOI] [PubMed] [Google Scholar]
- 131.Monji A., Mitsui T., Bando Y.K., et al. Glucagon-like peptide-1 receptor activation reverses cardiac remodeling via normalizing cardiac steatosis and oxidative stress in type 2 diabetes. Am J Physiol-Heart C. 2013;305(3):295–304. doi: 10.1152/ajpheart.00990.2012. [DOI] [PubMed] [Google Scholar]
- 132.Birnbaum Y., Ye Y., Bajaj M. Myocardial protection against Ischemia-Reperfusion Injury by GLP-1: Molecular mechanisms. Metab. Syndr. Relat. Disord. 2012;10(6):387–390. doi: 10.1089/met.2012.0095. [DOI] [PubMed] [Google Scholar]
- 133.Ding X., Saxena N.K., Lin S., et al. Exendin-4, a glucagon-like protein-1 (GLP-1) receptor agonist, reverses hepatic steatosis in ob/ob mice. Hepatology. 2006;43(1):173–181. doi: 10.1002/hep.21006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Alberts P., Rönquist-Nii Y., Larsson C., et al. Effect of high-fat diet on KKAy and ob/ob mouse liver and adipose tissue corticosterone and 11-dehydrocorticosterone concentrations. Horm. Metab. Res. 2005;37(7):402–407. doi: 10.1055/s-2005-870228. [DOI] [PubMed] [Google Scholar]
- 135.Shimoda M., Kanda Y., Hamamoto S., et al. The human glucagon-like peptide-1 analogue liraglutide preserves pancreatic beta cells via regulation of cell kinetics and suppression of oxidative and endoplasmic reticulum stress in a mouse model of diabetes. Diabetologia. 2011;54(5):1098–1108. doi: 10.1007/s00125-011-2069-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Dalbøge L.S., Almholt D.L., Neerup T.S., et al. Characterisation of age-dependent beta cell dynamics in the male db/db mice. PLoS One. 2013;8(12) doi: 10.1371/journal.pone.0082813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Park C.W., Kim H.W., Ko S.H., et al. Long-term treatment of glucagon-like peptide-1 analog exendin-4 ameliorates diabetic nephropathy through improving metabolic anomalies in db/db mice. J. Am. Soc. Nephrol. 2007;18(4):1227–1238. doi: 10.1681/ASN.2006070778. [DOI] [PubMed] [Google Scholar]
- 138.Fujita H., Morii T., Fujishima H., et al. The protective roles of GLP-1R signaling in diabetic nephropathy: Possible mechanism and therapeutic potential. Kidney Int. 2014;85(3):579–589. doi: 10.1038/ki.2013.427. [DOI] [PubMed] [Google Scholar]
- 139.Fujita H., Fujishima H., Takahashi K., et al. SOD1, but not SOD3, deficiency accelerates diabetic renal injury in C57BL/6-Ins2Akita diabetic mice. Metabolism. 2012;61(12):1714–1724. doi: 10.1016/j.metabol.2012.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Wang J., Takeuchi T., Tanaka S., et al. A mutation in the insulin 2 gene induces diabetes with severe pancreatic beta-cell dysfunction in the Mody mouse. J. Clin. Invest. 1999;103(1):27–37. doi: 10.1172/JCI4431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Cabou C., Campistron G., Marsollier N., et al. Brain glucagon-like peptide-1 regulates arterial blood flow, heart rate, and insulin sensitivity. Diabetes. 2008;57(10):2577–2587. doi: 10.2337/db08-0121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Wu Y.L., Huang J., Liu J., et al. Protective effect of recombinant human glucagon-like peptide-1 (rhGLP-1) pretreatment in STZ-induced diabetic mice. J. Pept. Sci. 2011;17(7):499–504. doi: 10.1002/psc.1352. [DOI] [PubMed] [Google Scholar]
- 143.Gezginci-Oktayoglu S., Bolkent S. Exendin-4 exerts its effects through the NGF/p75NTR system in diabetic mouse pancreas. Biochem. Cell Biol. 2009;87(4):641–651. doi: 10.1139/o09-046. [DOI] [PubMed] [Google Scholar]
- 144.Kobayashi A., Kang M.I., Watai Y., et al. Oxidative and electrophilic stresses activate Nrf2 through inhibition of ubiquitination activity of Keap1. Mol. Cell. Biol. 2006;26(1):221–229. doi: 10.1128/MCB.26.1.221-229.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Patel V., Joharapurkar A., Dhanesha N., et al. Combination of omeprazole with GLP-1 agonist Therapy improves insulin sensitivity and antioxidant activity in liver in type 1 diabetic mice. Pharmacol. Rep. 2013;65(4):927–936. doi: 10.1016/s1734-1140(13)71074-0. [DOI] [PubMed] [Google Scholar]
- 146.Lotfy M., Singh J., Rashed H., et al. Mechanism of the beneficial and protective effects of exenatide in diabetic rats. J. Endocrinol. 2014;220(3):291–304. doi: 10.1530/JOE-13-0426. [DOI] [PubMed] [Google Scholar]
- 147.Lotfy M., Singh J., Rashed H., et al. The effect of glucagon-like peptide-1 in the management of diabetes mellitus: cellular and molecular mechanisms. Cell Tissue Res. 2014;358(2):343–358. doi: 10.1007/s00441-014-1959-9. [DOI] [PubMed] [Google Scholar]
- 148.Hendarto H., Inoguchi T., Maeda Y., et al. GLP-1 analog liraglutide protects against oxidative stress and albuminuria in streptozotocin-induced diabetic rats via protein kinase A-mediated inhibition of renal NAD(P)H oxidases. Metabolism. 2012;61(10):1422–1434. doi: 10.1016/j.metabol.2012.03.002. [DOI] [PubMed] [Google Scholar]
- 149.Elbassuoni E.A. Incretin attenuates diabetes-induced damage in rat cardiac tissue. J. Physiol. Sci. 2014;64(5):357–364. doi: 10.1007/s12576-014-0327-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Nagashima M., Watanabe T., Terasaki M., et al. Native incretins prevent the development of atherosclerotic lesions in apolipoprotein e knockout mice. Diabetologia. 2011;54(10):2649–2659. doi: 10.1007/s00125-011-2241-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Gaspari T., Welungoda I., Widdop R.E., et al. The GLP-1 receptor agonist liraglutide inhibits progression of vascular disease via effects on atherogenesis, plaque stability and endothelial function in an ApoE-/- mouse model. Diab. Vasc. Dis. Res. 2013;10(4):353–360. doi: 10.1177/1479164113481817. [DOI] [PubMed] [Google Scholar]
- 152.Koska J., Schwartz E.A., Mullin M.P., et al. Improvement of postprandial endothelial function after a single dose of exenatide in individuals with impaired glucose tolerance and recent-onset type 2 diabetes. Diabetes Care. 2010;33(5):1028–1030. doi: 10.2337/dc09-1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Guo Z., Mitchell-Raymundo F., Yang H., et al. Dietary restriction reduces atherosclerosis and oxidative stress in the aorta of apolipoprotein E-deficient mice. Mech. Ageing Dev. 2002;123(8):1121–1131. doi: 10.1016/s0047-6374(02)00008-8. [DOI] [PubMed] [Google Scholar]
- 154.Goyal S., Kumar S., Bijjem K.V., et al. Role of glucagon-like peptide-1 in vascular endothelial dysfunction. Indian J. Exp. Biol. 2010;48(1):61–69. [PubMed] [Google Scholar]
- 155.Özyazgan S., Kutluata N., Afsar S., et al. Effect of glucagon-like peptide-1(7-36) and exendin-4 on the vascular reactivity in streptozotocin/nicotinamide-induced diabetic rats. Pharmacology. 2005;74(3):119–126. doi: 10.1159/000084277. [DOI] [PubMed] [Google Scholar]
- 156.Ceriello A., Esposito K., Testa R., et al. The possible protective role of glucagon-like peptide1 on endothelium during the meal and evidence for an “endothelial resistance” to glucagon-like peptide 1 in diabetes. Diabetes Care. 2011;34(3):697–702. doi: 10.2337/dc10-1949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Ceriello A., Novials A., Ortega E., et al. Vitamin C further improves the protective effect of GLP-1 on the ischemia-reperfusion-like effect induced by hyperglycemia post-hypoglycemia in type 1 diabetes. Cardiovasc. Diabetol. 2013;12(1) doi: 10.1186/1475-2840-12-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Ceriello A., Novials A., Ortega E., et al. Vitamin C further improves the protective effect of glucagon-like peptide-1 on acute hypoglycemia-induced oxidative stress, inflammation, and endothelial dysfunction in type 1 diabetes. Diabetes Care. 2013;36(12):4104–4108. doi: 10.2337/dc13-0750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Ceriello A., Novials A., Canivell S., et al. Simultaneous GLP-1 and insulin administration acutely enhances their vasodilatory, antiinflammatory, and antioxidant action in type 2 diabetes. Diabetes Care. 2014;37(7):1938–1943. doi: 10.2337/dc13-2618. [DOI] [PubMed] [Google Scholar]
- 160.Singh P., Jain A., Kaur G. Impact of hypoglycemia and diabetes on CNS: Correlation of mitochondrial oxidative stress with DNA damage. Mol. Cell. Biochem. 2004;260(1):153–159. doi: 10.1023/b:mcbi.0000026067.08356.13. [DOI] [PubMed] [Google Scholar]
- 161.Dandona P., Chaudhuri A., Ghanim H., et al. Insulin as an Anti-Inflammatory and Antiatherogenic Modulator. J. Am. Coll. Cardiol. 2009;53(Suppl. 5):14–20. doi: 10.1016/j.jacc.2008.10.038. [DOI] [PubMed] [Google Scholar]
- 162.Monnier L., Colette C., Mas E., et al. Regulation of oxidative stress by glycaemic control: Evidence for an independent inhibitory effect of insulin therapy. Diabetologia. 2010;53(3):562–571. doi: 10.1007/s00125-009-1574-6. [DOI] [PubMed] [Google Scholar]
- 163.Dandona P., Aljada A., Mohanty P., et al. Insulin inhibits intranuclear nuclear factor kB and stimulates kB in mononuclear cells in obese subjects: Evidence for an anti-inflammatory effect? J. Clin. Endocrinol. Metab. 2001;86(7):3257–3265. doi: 10.1210/jcem.86.7.7623. [DOI] [PubMed] [Google Scholar]
- 164.Taddei S., Virdis A., Mattei P., et al. Effect of insulin on acetylcholine-induced vasodilation in normotensive subjects and patients with essential hypertension. Circulation. 1995;92(10):2911–2918. doi: 10.1161/01.cir.92.10.2911. [DOI] [PubMed] [Google Scholar]
- 165.Rask-Madsen C., Domínguez H., Ihlemann N., et al. Tumor Necrosis Factor-alfa Inhibits Insulin's Stimulating Effect on Glucose Uptake and Endothelium-Dependent Vasodilation in Humans. Circulation. 2003;108(15):1815–1821. doi: 10.1161/01.CIR.0000091406.72832.11. [DOI] [PubMed] [Google Scholar]
- 166.Rizzo M., Abate N., Chandalia M., et al. Liraglutide reduces oxidative stress and restores heme oxygenase-1 and ghrelin levels in patients with type 2 diabetes: A prospective pilot study. J. Clin. Endocrinol. Metab. 2015;100(2):603–606. doi: 10.1210/jc.2014-2291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Suematsu M., Katsuki A., Sumida Y., et al. Decreased circulating levels of active ghrelin are associated with increased oxidative stress in obese subjects. Eur. J. Endocrinol. 2005;153(3):403–407. doi: 10.1530/eje.1.01977. [DOI] [PubMed] [Google Scholar]
- 168.Bao W., Song F., Li X., et al. Plasma heme oxygenase-1 concentration is elevated in individuals with type 2 diabetes mellitus. PLoS One. 2010;5(8) doi: 10.1371/journal.pone.0012371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Dhindsa S., Tripathy D., Mohanty P., et al. Differential effects of glucose and alcohol on reactive oxygen species generation and intranuclear nuclear factor-kB in mononuclear cells. Metabolism. 2004;53(3):330–334. doi: 10.1016/j.metabol.2003.10.013. [DOI] [PubMed] [Google Scholar]
- 170.Rakipovski G., Raun K., Lykkesfeldt J. Fluctuating hyperglycaemia increases oxidative stress response in lean rats compared to sustained hyperglycaemia despite lower glycaemic exposure. Diab. Vasc. Dis. Res. 2011;8(4):295–298. doi: 10.1177/1479164111421033. [DOI] [PubMed] [Google Scholar]
- 171.Kelly A.S., Bergenstal R.M., Gonzalez-Campoy J.M., et al. Effects of Exenatide vs. Metformin on endothelial function in obese patients with pre-diabetes: a randomized trial. Cardiovasc. Diabetol. 2012:11. doi: 10.1186/1475-2840-11-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Rojas L.B., Gomes M.B. Metformin: An old but still the best treatment for type 2 diabetes. Diabetol. Metab. Syndr. 2013;5(1) doi: 10.1186/1758-5996-5-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Araki E., Nishikawa T. Oxidative stress: A cause and therapeutic target of diabetic complications. J. Diabetes Investig. 2010;1(3):90–96. doi: 10.1111/j.2040-1124.2010.00013.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Kelly A.S., Metzig A.M., Rudser K.D., et al. Exenatide as a weight-loss therapy in extreme pediatric obesity: A randomized, controlled pilot study. Obesity (Silver Spring) 2012;20(2):364–370. doi: 10.1038/oby.2011.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Tesauro M., Schinzari F., Adamo A., et al. Effects of GLP-1 on forearm vasodilator function and glucose disposal during hyperinsulinemia in the metabolic syndrome. Diabetes Care. 2013;36(3):683–689. doi: 10.2337/dc12-0763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Okada K., Kotani K., Yagyu H., et al. Effects of treatment with liraglutide on oxidative stress and cardiac natriuretic peptide levels in patients with type 2 diabetes mellitus. Endocrine. 2014;47(3):962–964. doi: 10.1007/s12020-014-0246-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Marso S.P., Poulter N.R., Nissen S.E., et al. Design of the liraglutide effect and action in diabetes: Evaluation of cardiovascular outcome results (LEADER) trial. Am. Heart J. 2013;166(5):823–830. doi: 10.1016/j.ahj.2013.07.012. [DOI] [PubMed] [Google Scholar]
- 178.Walsh M.E., Shi Y., Van Remmen H. The effects of dietary restriction on oxidative stress in rodents. Free Radic. Biol. Med. 2014;66:88–99. doi: 10.1016/j.freeradbiomed.2013.05.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Swindell W.R. Dietary restriction in rats and mice: A meta-analysis and review of the evidence for genotype-dependent effects on lifespan. Ageing Res. Rev. 2012;11(2):254–270. doi: 10.1016/j.arr.2011.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]