Abstract
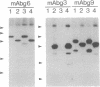
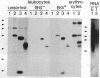
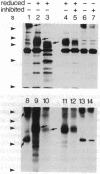
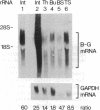
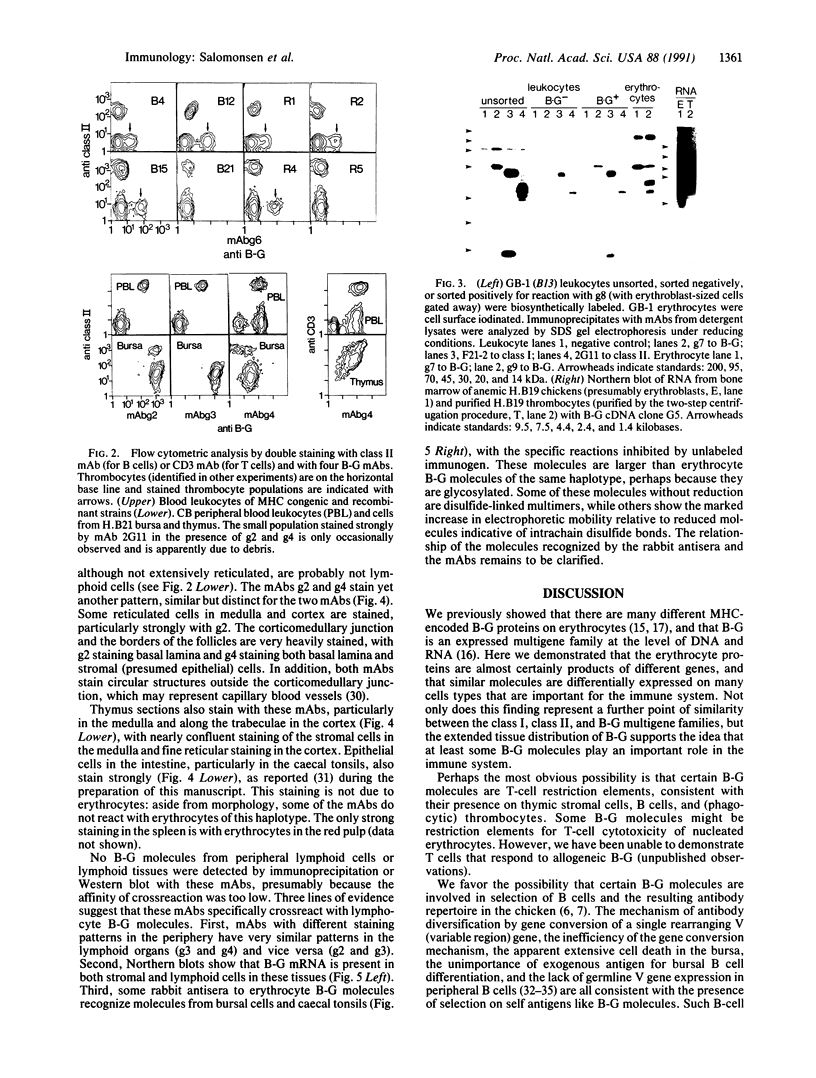
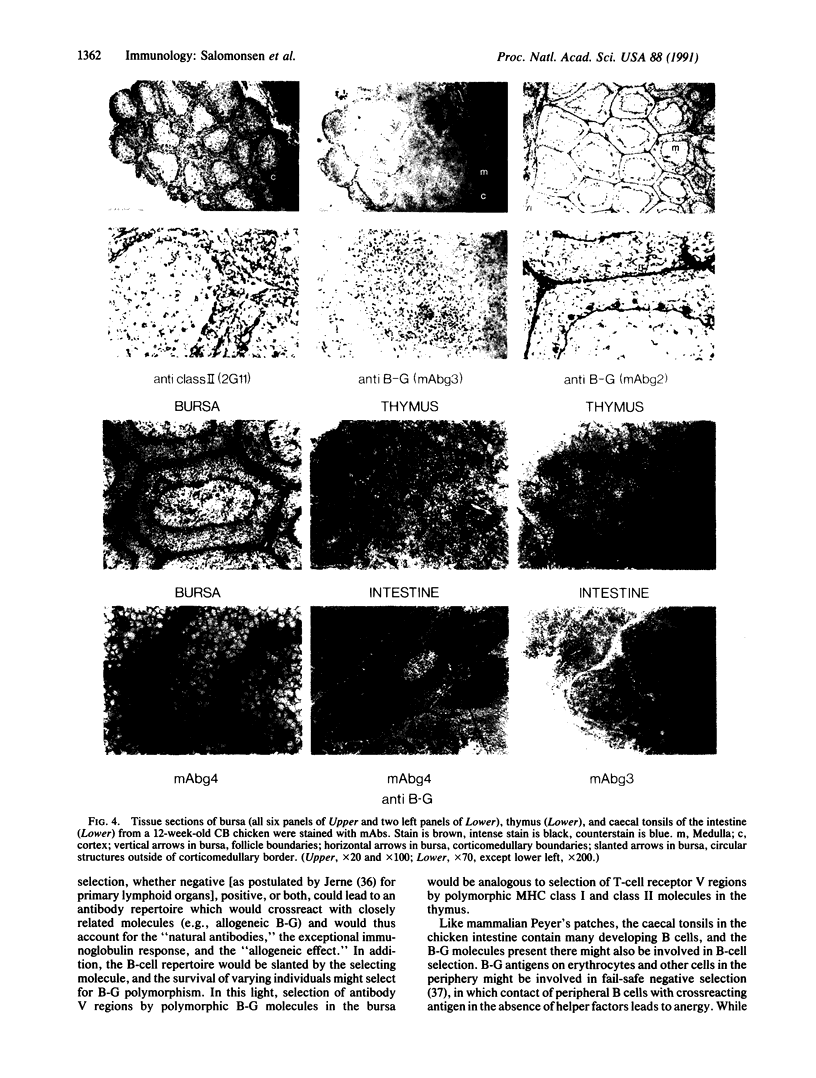
B-G antigens are a polymorphic multigene family of cell surface molecules encoded by the chicken major histocompatibility complex (MHC). They have previously been described only on cells of the erythroid lineage. By using flow cytometry, section staining, and immunoprecipitation with monoclonal antibodies and rabbit antisera to B-G molecules and by using Northern blots with B-G cDNA clones, we demonstrate here that B-G molecules and RNA are present in many other cell types: thrombocytes, peripheral B and T lymphocytes, bursal B cells and thymocytes, and stromal cells in the bursa, thymus, and caecal tonsil of the intestine. The reactions also identify at least one polymorphic B-G determinant encoded by the B-F/B-L region of the chicken MHC. The serology and tissue distribution of B-G molecules are as complex as those of mammalian MHC class I and class II molecules. These facts, taken with certain functional data, lead us to suggest that B-G molecules have an important role in the selection of B cells in the chicken bursa.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Briles W. E., Bumstead N., Ewert D. L., Gilmour D. G., Gogusev J., Hála K., Koch C., Longenecker B. M., Nordskog A. W., Pink J. R. Nomenclature for chicken major histocompatibility (B) complex. Immunogenetics. 1982;15(5):441–447. doi: 10.1007/BF00345903. [DOI] [PubMed] [Google Scholar]
- Chang C. F., Hamilton P. B. The thrombocyte as the primary circulating phagocyte in chickens. J Reticuloendothel Soc. 1979 Jun;25(6):585–590. [PubMed] [Google Scholar]
- Chen C. L., Ager L. L., Gartland G. L., Cooper M. D. Identification of a T3/T cell receptor complex in chickens. J Exp Med. 1986 Jul 1;164(1):375–380. doi: 10.1084/jem.164.1.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunon D., Kaufman J., Salomonsen J., Skjoedt K., Vainio O., Thiery J. P., Imhof B. A. T cell precursor migration towards beta 2-microglobulin is involved in thymus colonization of chicken embryos. EMBO J. 1990 Oct;9(10):3315–3322. doi: 10.1002/j.1460-2075.1990.tb07531.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunon D., Salomonsen J., Skjødt K., Kaufman J., Imhof B. A. Ontogenic appearance of MHC class I (B-F) antigens during chicken embryogenesis. Dev Immunol. 1990;1(2):127–135. doi: 10.1155/1990/75158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodnow C. C., Crosbie J., Jorgensen H., Brink R. A., Basten A. Induction of self-tolerance in mature peripheral B lymphocytes. Nature. 1989 Nov 23;342(6248):385–391. doi: 10.1038/342385a0. [DOI] [PubMed] [Google Scholar]
- Grossi C. E., Lydyard P. M., Cooper M. D. B-cell ontogeny in the chicken. Ann Immunol (Paris) 1976 Nov-Dec;127(6):931–941. [PubMed] [Google Scholar]
- Guillemot F., Billault A., Pourquié O., Béhar G., Chaussé A. M., Zoorob R., Kreibich G., Auffray C. A molecular map of the chicken major histocompatibility complex: the class II beta genes are closely linked to the class I genes and the nucleolar organizer. EMBO J. 1988 Sep;7(9):2775–2785. doi: 10.1002/j.1460-2075.1988.tb03132.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillemot F., Kaufman J. F., Skjoedt K., Auffray C. The major histocompatibility complex in the chicken. Trends Genet. 1989 Sep;5(9):300–304. doi: 10.1016/0168-9525(89)90112-1. [DOI] [PubMed] [Google Scholar]
- Hála K., Plachý J., Schulmannová J. Role of the B-G-region antigen in the humoral immune response to the B-F-region antigen of chicken MHC. Immunogenetics. 1981;14(5):393–401. doi: 10.1007/BF00373319. [DOI] [PubMed] [Google Scholar]
- Jerne N. K. The somatic generation of immune recognition. Eur J Immunol. 1971 Jan;1(1):1–9. doi: 10.1002/eji.1830010102. [DOI] [PubMed] [Google Scholar]
- Kaufman J., Ferrone S., Flajnik M., Kilb M., Völk H., Parisot R. MHC-like molecules in some nonmammalian vertebrates can be detected by some cross-reactive monoclonal antibodies. J Immunol. 1990 Mar 15;144(6):2273–2280. [PubMed] [Google Scholar]
- Kaufman J., Ferrone S., Flajnik M., Kilb M., Völk H., Parisot R. MHC-like molecules in some nonmammalian vertebrates can be detected by some cross-reactive monoclonal antibodies. J Immunol. 1990 Mar 15;144(6):2273–2280. [PubMed] [Google Scholar]
- Kaufman J., Salomonsen J., Skjødt K. B-G cDNA clones have multiple small repeats and hybridize to both chicken MHC regions. Immunogenetics. 1989;30(6):440–451. doi: 10.1007/BF02421176. [DOI] [PubMed] [Google Scholar]
- Kaufman J., Salomonsen J., Skjødt K., Thorpe D. Size polymorphism of chicken major histocompatibility complex-encoded B-G molecules is due to length variation in the cytoplasmic heptad repeat region. Proc Natl Acad Sci U S A. 1990 Nov;87(21):8277–8281. doi: 10.1073/pnas.87.21.8277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman J., Skjoedt K., Salomonsen J. The MHC molecules of nonmammalian vertebrates. Immunol Rev. 1990 Feb;113:83–117. doi: 10.1111/j.1600-065x.1990.tb00038.x. [DOI] [PubMed] [Google Scholar]
- Longenecker B. M., Mosmann T. R. "Natural" antibodies to chicken MHC antigens are present in mice, rats, humans, alligators and allogeneic chickens. Immunogenetics. 1980;11(3):293–302. doi: 10.1007/BF01567795. [DOI] [PubMed] [Google Scholar]
- Longenecker B. M., Mosmann T. R. Restricted expression of an MHC alloantigen in cells of the erythroid series: a specific marker for erythroid differentiation. J Supramol Struct. 1980;13(3):395–400. doi: 10.1002/jss.400130310. [DOI] [PubMed] [Google Scholar]
- Miller M. M., Goto R., Clark S. D. Structural characterization of developmentally expressed antigenic markers on chicken erythrocytes using monoclonal antibodies. Dev Biol. 1982 Dec;94(2):400–414. doi: 10.1016/0012-1606(82)90357-8. [DOI] [PubMed] [Google Scholar]
- Miller M. M., Goto R., Young S., Liu J., Hardy J. Antigens similar to major histocompatibility complex B-G are expressed in the intestinal epithelium in the chicken. Immunogenetics. 1990;32(1):45–50. doi: 10.1007/BF01787328. [DOI] [PubMed] [Google Scholar]
- Neu N., Hála K., Wick G. "Natural" chicken antibodies to red blood cells are mainly directed against the B-G antigen, and their occurrence is independent of spontaneous autoimmune thyroiditis. Immunogenetics. 1984;19(4):269–277. doi: 10.1007/BF00345400. [DOI] [PubMed] [Google Scholar]
- Salomonsen J., Skjødt K., Crone M., Simonsen M. The chicken erythrocyte-specific MHC antigen. Characterization and purification of the B-G antigen by monoclonal antibodies. Immunogenetics. 1987;25(6):373–382. doi: 10.1007/BF00396103. [DOI] [PubMed] [Google Scholar]
- Traill K. N., Böck G., Boyd R., Wick G. Chicken thrombocytes. Isolation, serological and functional characterisation using the fluorescence activated cell sorter. Dev Comp Immunol. 1983 Winter;7(1):111–125. doi: 10.1016/0145-305x(83)90060-5. [DOI] [PubMed] [Google Scholar]
- Vainio O., Ratcliffe M. J. Proliferation of chicken peripheral blood leukocytes in response to pokeweed mitogen is macrophage dependent. Cell Immunol. 1984 Apr 15;85(1):235–243. doi: 10.1016/0008-8749(84)90293-4. [DOI] [PubMed] [Google Scholar]
- Weill J. C., Reynaud C. A. The chicken B cell compartment. Science. 1987 Nov 20;238(4830):1094–1098. doi: 10.1126/science.3317827. [DOI] [PubMed] [Google Scholar]