Abstract
Background
Single nucleotide polymorphisms (SNPs) in the estrogen receptor 1 (ESR1) and cytochrome P450 19A1 (CYP19A1) genes have been associated with breast cancer risk, endocrine therapy response and side effects, mainly in postmenopausal women with early breast cancer. This analysis aimed to assess the association of selected germline CYP19A1 and ESR1 SNPs with early-onset hot flashes, sweating and musculoskeletal symptoms in premenopausal patients enrolled in the Tamoxifen and Exemestane Trial (TEXT).
Methods
Blood was collected from consenting premenopausal women with hormone-responsive early breast cancer, randomly assigned to 5-years of tamoxifen plus ovarian suppression (OFS) or exemestane plus OFS. DNA was extracted with QIAamp kits and genotyped for two CYP19A1 (rs4646 and rs10046) and three ESR1 (rs2077647, rs2234693 and rs9340799) SNPs by a real-time pyrosequencing technique. Adverse events (AEs) were recorded at baseline and 3-monthly during the first year. Associations of the genotype variants with grade ≥2 early-onset targeted AEs of hot flashes/sweating or musculoskeletal events were assessed using logistic regression models.
Results
There were 2660 premenopausal patients with breast cancer in the intention-to-treat population of TEXT, and 1967 (74 %) are included in this translational study. The CYP19A1 rs10046 variant T/T, represented in 23 % of women, was associated with a reduced incidence of grade ≥2 hot flashes/sweating (univariate odds ratio (OR) = 0.78; 95 % CI 0.63–0.97; P = 0.03), more strongly in patients assigned exemestane + OFS (TT vs CT/CC: OR = 0.65, 95 % CI = 0.48–0.89) than assigned tamoxifen + OFS (OR = 0.94, 95 % CI = 0.69–1.27, interaction P = 0.03). No association with any of the CYP19A1/ESR1 genotypes and musculoskeletal AEs was found.
Conclusion
The CYP19A1 rs10046 variant T/T favors lower incidence of hot flashes/sweating under exemestane + OFS treatment, suggesting endocrine-mediated effects. Based on findings from others, this SNP may potentially enhance treatment adherence and treatment efficacy. We plan to evaluate the clinical impact of this polymorphism during time, pending sufficient median follow up.
Trial registration
ClinicalTrials.gov NCT00066703, registered August 6, 2003.
Keywords: Side effects, Aromatase inhibitors, Tamoxifen, Ovarian suppression, Breast cancer, CYP19A1, ESR1
Background
Adjuvant endocrine therapy significantly prolongs disease-free and overall survival in women with hormone-receptor-positive early breast cancer, but it is associated with several side effects, which may lead to early treatment cessation [1–3]. In the combined analysis of the Tamoxifen and Exemestane Trial (TEXT) and Suppression of Ovarian Function Trial (SOFT) [4], comparing adjuvant exemestane plus ovarian function suppression (OFS) with tamoxifen plus OFS in premenopausal patients with breast cancer, early cessation of OFS and the assigned oral endocrine treatment occurred in 16 % of patients receiving exemestane + OFS and 11 % of those receiving tamoxifen + OFS. Nonetheless, exemestane + OFS significantly improved disease outcome compared to tamoxifen + OFS after 5.7 years median follow up.
The acute onset of menopause induced by gonadotropin-releasing-hormone analogues (GnRHa) is associated with more frequent and severe side effects compared to natural menopause, significantly impacting the quality of life of young patients with breast cancer [5]. The most common side effects associated with early menopause include vasomotor symptoms (hot flashes and sweating), decreased libido, insomnia, and dyspareunia secondary to vaginal dryness. The frequency and severity of hot flashes may depend on the abrupt fall in circulating estrogen levels as observed in several studies among women undergoing a natural menopausal transition [6–8], although other factors also play a role [9, 10]. While chemotherapy, OFS, and aromatase inhibitors (AIs) directly lower circulating estrogen levels, tamoxifen, a selective estrogen receptor modulator, has both agonistic and antagonistic effects on estrogen signaling [11]. In addition to menopausal symptoms, AIs are frequently associated with joint and muscle pain [12], decreased bone density [13] and risk of fracture [3], which appears to increase with better compliance with AIs [14].
Common genetic polymorphisms of the genes involved in estrogen production and estrogen target genes have been linked to breast cancer risk, prognosis, treatment response and side effects. One of these genes, the CYP19A1, encodes for the enzyme aromatase that promotes the bioconversion of androgens to estrogens. Genetic variations at the CYP19A1 locus may result in increased or decreased aromatase activity and influence concentrations of circulating estrogens [15–17]. For example, the rs10046 and rs4646 variants, located in a 3’ untranslated region, were associated with higher estradiol and estrone levels due to increased aromatase activity. Alternatively, these variants could be linked with other gene variants such as the rs749292, which is associated with even higher estrogen levels [17]. A recent review and meta-analysis analyzed the influence of common CYP19A1 polymorphisms on postmenopausal patients with breast cancer treated with AIs [18], indicating a certain heterogeneity between studies.
The estrogen receptor α (ESR1) gene was recently recognized as a low-penetrance breast cancer susceptibility gene. Numerous studies suggest an association between ESR1 gene polymorphisms and breast cancer risk [19]. However, results have been controversial due to heterogeneous data sources, differences in study designs, ethnic background, disease status, and sample size. ESR1 is an important mediator of endocrine pathways involved in breast cancer risk and outcomes, including endocrine treatment response and side effects. Genetic polymorphisms altering the expression of ESR1 have been suggested to affect breast cancer susceptibility [20]. In particular, the restriction enzymes XbaI (rs9340799) and PvuII (rs2234693) have been extensively evaluated. Both are located in the first intron of the ESR1 gene. The association between variant allele T of ESR1 PvuII (C > T) and breast cancer appears to be linked to a higher transcriptional activity of the variant gene [21] and correlated with circulating estrogen levels [22].
A recent meta-analysis [23] found that menopausal status modifies breast cancer risk associated with ESR1 PvuII (C > T), with premenopausal variant carriers being at higher risk, possibly related to differences in circulating estrogen levels [22]. However, an updated meta-analysis restricted the effect to the Asian population [24]. Another meta-analysis of almost 19,000 individuals in eight European centers reported that ESR1 XbaI (A > G) protects against overall fracture risk [25], suggesting an involvement of these polymorphisms in bone metabolism. These ESR1 polymorphisms have also been described to be involved in ovarian hyperstimulation response in assisted reproduction studies [26], further highlighting their role in endocrine-related mechanisms.
Within the phase III TEXT trial in which 2672 premenopausal women were randomized to adjuvant therapy with exemestane + OFS or tamoxifen + OFS, with or without adjuvant chemotherapy, we prospectively designed a translational research project for blood collection to investigate the effect of selected single nucleotide polymorphisms (SNPs) on treatment efficacy and toxicity. The purpose of the current analysis was to investigate the association of common genetic variants of CYP19A1 (rs10046, rs4646) and ESR1 (rs2077647, rs2234693 and rs9340799) with early-onset vasomotor and musculoskeletal symptoms.
Methods
Patients
TEXT is an International Breast Cancer Study Group (IBCSG)-coordinated, randomized, phase III trial that enrolled premenopausal women with histologically proven estrogen receptor (ER) and/or progesterone receptor (PgR)-positive early breast cancer. From November 2003 through April 2011, patients were enrolled within 12 weeks from surgery, prior to the initiation of any systemic adjuvant therapy, and randomized to 5 years of exemestane + OFS or tamoxifen + OFS. OFS was achieved by monthly injection of the GnRHa triptorelin; bilateral oophorectomy or ovarian irradiation was allowed after at least 6 months of triptorelin. Chemotherapy was optional and, if administered, triptorelin and chemotherapy were started concomitantly; oral endocrine treatment was started after the completion of chemotherapy, or if chemotherapy was not administered, it was started 6 to 8 weeks after the initiation of triptorelin, to allow for the suppression of ovarian estrogen production.
Trial procedures
Targeted adverse events (AEs) were systematically collected, using the Common Terminology Criteria for Adverse Events (CTCAE) Version 3.0, at baseline and every 3 months during the first year of treatment: hot flashes was graded 1–3 (1, mild; 2, moderate; 3, interfering with activities of daily living (ADL)); sweating was graded 1–2 (1, mild and occasional; 2, frequent or drenching); and musculoskeletal symptoms, i.e., myalgia, arthralgia (joint pain), stiffness, were graded 1–4 (1, mild pain, not interfering with function; 2, moderate pain, pain or analgesics interfering with function but not interfering with ADL; 3, severe pain, pain or analgesics severely interfering with ADL; 4, disabling). Depending on institutional procedures, patients may have been systematically asked about targeted AEs during the clinical visit, or targeted AEs may have been recorded in the CRFs based on review of the medical reports.
Protocol amendment 2, dated July 2008, increased the sample size and added the collection of a single whole blood sample for DNA isolation for translational research objectives, i.e., to investigate treatment tolerability and disease outcome according to genetic polymorphisms. Samples and consent were prospectively collected for approximately 600 patients enrolled after the amendment, and approximately 2000 patients enrolled prior to the amendment were asked to re-consent and have samples collected at the next scheduled protocol visit. The translational protocol targeted collection was of 2000 total samples.
Blood collection, DNA extraction and genotyping assays
Venous blood was collected into EDTA-treated tubes provided by IBCSG and either processed and stored locally at −80 °C or shipped immediately to the CALGB Pathology Coordinating Office (USA and Canada), for DNA extraction and temporary storage, until shipping to the IBCSG central biomarker laboratory at the European Institute of Oncology for biobanking, DNA extraction (all countries except USA and Canada) and genotyping. Genomic DNA was extracted with QIAamp DNA Blood Kits (Qiagen, Valencia, CA, USA), according to the manufacturer’s instructions and extraction was performed by the automated platform “QIAcube” (Qiagen, Valencia, CA, USA).
The germline DNA samples were genotyped for SNPs in CYP19A1 (rs4646 and rs10046) and ESR1 (rs207764, rs2234693 and rs9340799). All samples were analyzed using a real-time sequencing method called pyrosequencing (Diatech Pharmacogenetics S.r.l., Jesi, Italy). The DNA was amplified by polymerase chain reaction (PCR) with biotinylated primers on the Real-Time PCR Cycler “Rotor-Gene TM 6000” (Corbett Research, Sydney, Australia), whereas single-stranded DNA templates were prepared using the PyroMark Vacuum Prep Workstation (Biotage, Uppsala, Sweden). The pyrosequencing analysis was performed on the PyroMarkTM Q96 ID instrument (Biotage). Control samples, representing a complete set of genotypes (wt/wt; wt/v; v/v) for all SNPs, were processed in each run. No patient sample failed genotyping.
Statistical analysis
The analysis included 1967 patients from the TEXT intention-to-treat population (ITT) who gave whole blood for genetic profiling (Fig. 1). The endpoint of early-onset hot flushes/sweating was defined as presence or absence of grade 2 or grade 3 hot flashes or grade 2 sweating reported at the 3 month or 6 month visits after randomization. Early-onset musculoskeletal symptoms were defined as presence or absence of grade 2–4 musculoskeletal symptoms reported at the visits at 3, 6, 9 or 12 months after randomization.
Fig. 1.
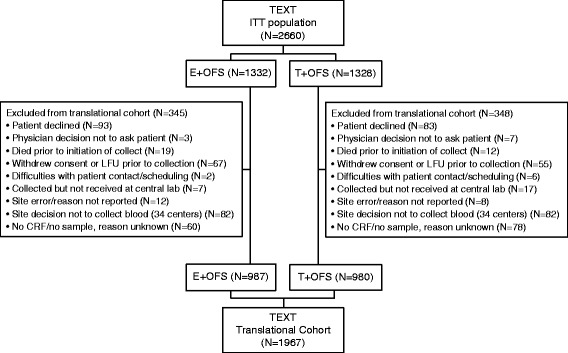
Derivation of the Tamoxifen and Exemestane Trial (TEXT) translational cohort from the intention-to-treat (ITT) population. The translational cohort includes patients whose blood was available for DNA analysis. GNRHa triptorelin was required for the first 6 months, any time after which the patient could choose to undergo bilateral oophorectomy or bilateral ovarian radiotherapy. E exemestane, T tamoxifen, OFS ovarian function suppression, LFU lost to follow up, CRF case report form
Logistic regression modeling assessed the association of the selected genotypes with presence of early-onset AEs. The model also adjusted for patient characteristics at randomization: age (<45 versus ≥45 years); menstruation status (normal versus irregular versus persistent amenorrhea); body mass index (BMI) (normal (<25), overweight (25–29.9) versus obese (≥30) kg/m2)); adjuvant chemotherapy use (yes versus no); treatment assignment (exemestane + OFS versus tamoxifen + OFS); presence of hot flashes/sweating of any grade at baseline; and presence of musculoskeletal symptoms of any grade at baseline. Because concomitant medications may affect the reported AE severity, the impact of relevant concomitant medication use (yes versus no) prior to or continuing at baseline, or introduced during the relevant endpoint time period, was investigated in a sensitivity analysis. Concomitant medications, prescribed for any reason, that might affect the severity of hot flashes/sweating included venlafaxine, SSRIs, clonidine, gabapentin, pregabalin and herbals [27]; medications for musculoskeletal symptoms such as non-steroidal anti-inflammatory drugs (NSAIDs), glucosamine, corticosteroids, gabapentin, and pregabalin. The analyses also assessed whether the association varied by treatment assignment by including genotype variants-by-treatment interaction in the logistic regression models.
We first assessed SNP variant effects in an additive genotype model that compared 0 versus 1 versus 2 minor or variant alleles using a one-degree-of-freedom trend test. The minor or variant homozygote effect was assessed in a recessive model that compared the minor or variant homozygote versus the combined heterozygote and wild-type homozygote (reference group) using the chi-squared test. Hardy-Weinberg equilibrium (HWE) for genotype frequencies was tested using the Monte Carlo simulation method [28] to calculate the P value in order to avoid the reliance on the underlying chi-square approximation.
The study is presented in accordance with the Reporting Recommendations for Tumor Marker Prognostic Studies (REMARK) criteria [29]. All statistical tests were two-sided, without adjustment for multiple comparisons, and a P value <0.05 in the overall cohort or interaction P value ≤0.10 was considered as statistically significant. For a given sample size, assuming 10 % or 20 % homozygous variant and 43 % and 26 % AE rates, the detectable differences in AE rates for homozygous versus combined heterozygous and wild-type would be in the range of 11 % to 7.2 % (Fisher’s exact test, two-sided α = 0.05, power ≥80 %).
Results
Study population
Blood for germline DNA extraction from 1967 consenting women was collected and assessed, representing 74 % of the entire TEXT ITT population of 2660 (Fig. 1). Patients in the analytical cohort were representative of the TEXT trial (Table 1), with the exceptions of race (one country did not participate and some centers with a majority of hispanic ethnicity had low participation rates) and early discontinuation of protocol treatment (retrospective nature of sample collection). Most patients were Caucasian (92 %), median age was 44 years and median body mass index was 24 kg/m2. Adjuvant chemotherapy was given to 58 % of patients.
Table 1.
Characteristics of TEXT intention-to-treat population, overall and according to availability of blood for DNA analysis
| Blood for DNA analysis | TEXT population (n = 2660) |
||
|---|---|---|---|
| No (n = 693) | Yes (n = 1967) | ||
| Characteristics at randomization | |||
| White/Caucasian,% | 73 | 92 | 87 |
| Age (years), median (IQR) | 43 (39, 46) | 44 (40, 47) | 43 (40, 46) |
| Normal menstruation, % | 87 | 88 | 87 |
| BMI (kg/m2), median (IQR) | 24 (22, 29) | 24 (21, 28) | 24 (21, 28) |
| Presence of any grade (1–3) hot flashes, % | 5 | 8 | 7 |
| Presence of any grade (1–2) sweating, % | 4 | 7 | 6 |
| Presence of any grade (1–4) musculoskeletal symptoms, % | 13 | 15 | 15 |
| Concomitant adjuvant therapy | |||
| Adjuvant chemotherapy, % | 64 | 58 | 59 |
| HER2-directed therapy, % | 3 | 7 | 6 |
| Protocol adjuvant therapy | |||
| Treatment assignment | |||
| Exemestane + OFS, % | 50 | 50 | 50 |
| Tamoxifen + OFS, % | 50 | 50 | 50 |
| Oral endocrine therapy (exemestane or tamoxifen) treatment <12 months, % | 19 | 6 | 10 |
| OFS <12 months, % | 16 | 4 | 7 |
| Analysis endpointsa | |||
| Early-onset grade ≥2 hot flashes/sweating, % | 41 | 43 | 43 |
| Early-onset grade ≥2 musculoskeletal symptoms, % | 28 | 26 | 27 |
aAdverse events according to common terminology criteria for adverse events (CTCAE) v3.0 of hot flashes and/or sweating reported at 3 or 6 months after randomization; musculoskeletal symptoms, i.e., myalgia, arthralgia (joint pain), or stiffness, reported at 3, 6, 9 or 12 months after randomization. TEXT Tamoxifen and Exemestane Trial, BMI body mass index, IQR interquartile range, HER2 human epidermal growth factor receptor 2, OFS ovarian function suppression
At baseline, any grade (≥1) of hot flashes and sweating were reported in 8 % and 7 % of patients, respectively, while any grade (≥1) of musculoskeletal symptoms were reported in 15 % of patients (Table 1).
The reference SNP numbers, minor allele frequencies and genotype frequencies for each analyzed SNP are listed in Table 2. No deviations from Hardy-Weinberg equilibrium were observed. Occurrence and grade of hot flashes, sweating and musculoskeletal side effects during the first year of protocol therapy, overall and by treatment assignment, are depicted in Table 3.
Table 2.
Minor allele frequency and genotype of the five genotyped SNPs in CYP19A1 and ESR1
| Genotype, n (%) | |||||||
|---|---|---|---|---|---|---|---|
| Gene | SNP | Number assessed | Minor allele frequency | Wild-type | Heterozygous | Homozygous | HWE P value |
| CYP19A1 | rs4646 (G > T) | 1967 | 0.29 | 989 (50) | 822 (42) | 156 (8) | 0.44 |
| CYP19A1 | rs10046 (C > T) | 1967 | 0.48 | 532 (27) | 989 (50) | 446 (23) | 0.75 |
| ESR1 | rs2077647 (A > G) | 1967 | 0.47 | 550 (28) | 999 (51) | 418 (21) | 0.39 |
| ESR1 | rs2234693 (Pvull) (T > C) | 1967 | 0.45 | 594 (30) | 993 (50) | 380 (19) | 0.36 |
| ESR1 | rs9340799 (Xbal) (A > G) | 1967 | 0.36 | 806 (41) | 923 (47) | 238 (12) | 0.30 |
CYP19A1 Cytochrome P450 19A1, ESR1 Estrogen receptor 1, HWE Hardy-Weinberg equilibrium, SNP single nucleotide polymorphism
Table 3.
Analysis endpoints and side effects during first year of protocol therapy according to treatment assignment
| Treatment | ||||
|---|---|---|---|---|
| Overall | Exemestane + OFS | Tamoxifen + OFS | ||
| (n = 1967a) | (n = 987) | (n = 980) | ||
| Analysis endpoint | ||||
| Early-onset hot flashes/sweating, grade ≥2 | 848 (43) | 411 (42) | 437 (45) | |
| Early-onset musculoskeletal symptoms, grade ≥2 | 516 (26) | 331 (34) | 185 (19) | |
| Side effect and time point | Grade | |||
| Hot flashes | ||||
| Baseline | Unk | 2 (0) | 2 (0) | 0 (0) |
| Gr0 | 1812 (92) | 924 (94) | 888 (91) | |
| Gr1 | 134 (7) | 51 (5) | 83 (8) | |
| Gr2 | 19 (1) | 10 (1) | 9 (1) | |
| 3 months | Unk | 8 (0) | 3 (0) | 5 (1) |
| Gr0 | 637 (32) | 325 (33) | 312 (32) | |
| Gr1 | 766 (39) | 386 (39) | 380 (39) | |
| Gr2 | 500 (25) | 248 (25) | 252 (26) | |
| Gr3 | 56 (3) | 25 (3) | 31 (3) | |
| 6 months | Unk | 20 (1) | 9 (1) | 11 (1) |
| Gr0 | 525 (27) | 294 (30) | 231 (24) | |
| Gr1 | 797 (41) | 401 (41) | 396 (40) | |
| Gr2 | 573 (29) | 260 (26) | 313 (32) | |
| Gr3 | 52 (3) | 23 (2) | 29 (3) | |
| Sweating | ||||
| Baseline | Unk | 3 (0) | 3 (0) | 0 (0) |
| Gr0 | 1832 (93) | 920 (93) | 912 (93) | |
| Gr1 | 119 (6) | 56 (6) | 63 (6) | |
| Gr2 | 13 (1) | 8 (1) | 5 (1) | |
| 3 months | Unk | 9 (0) | 4 (0) | 5 (1) |
| Gr0 | 1332 (68) | 675 (68) | 657 (67) | |
| Gr1 | 444 (23) | 219 (22) | 225 (23) | |
| Gr2 | 182 (9) | 89 (9) | 93 (9) | |
| 6 months | Unk | 22 (1) | 9 (1) | 13 (1) |
| Gr0 | 1285 (65) | 688 (70) | 597 (61) | |
| Gr1 | 447 (23) | 206 (21) | 241 (25) | |
| Gr2 | 213 (11) | 84 (9) | 129 (13) | |
| Musculoskeletal symptoms | ||||
| Baseline | Unk | 3 (0) | 3 (0) | 0 (0) |
| Gr0 | 1669 (85) | 837 (85) | 832 (85) | |
| Gr1 | 248 (13) | 125 (13) | 123 (13) | |
| Gr2 | 47 (2) | 22 (2) | 25 (3) | |
| Gr3 | 0 (0) | 0 (0) | 0 (0) | |
| 3 months | Unk | 9 (0) | 5 (1) | 4 (0) |
| Gr0 | 1360 (69) | 670 (68) | 690 (70) | |
| Gr1 | 467 (24) | 231 (23) | 236 (24) | |
| Gr2 | 118 (6) | 74 (7) | 44 (4) | |
| Gr3 | 13 (1) | 7 (1) | 6 (1) | |
| 6 months | Unk | 21 (1) | 9 (1) | 12 (1) |
| Gr0 | 1070 (54) | 463 (47) | 607 (62) | |
| Gr1 | 648 (33) | 366 (37) | 282 (29) | |
| Gr2 | 198 (10) | 128 (13) | 70 (7) | |
| Gr3 | 30 (2) | 21 (2) | 9 (1) | |
| 9 months | Unk | 41 (2) | 24 (2) | 17 (2) |
| Gr0 | 995 (51) | 386 (39) | 609 (62) | |
| Gr1 | 670 (34) | 392 (40) | 278 (28) | |
| Gr2 | 228 (12) | 159 (16) | 69 (7) | |
| Gr3 | 33 (2) | 26 (3) | 7 (1) | |
| 12 months | Unk | 37 (2) | 23 (2) | 14 (1) |
| Gr0 | 965 (49) | 388 (39) | 577 (59) | |
| Gr1 | 708 (36) | 400 (41) | 308 (31) | |
| Gr2 | 235 (12) | 158 (16) | 77 (8) | |
| Gr3 | 22 (1) | 18 (2) | 4 (0) | |
Reports of hot flashes and sweating side effects and of musculoskeletal symptoms according to common terminology criteria for adverse events (CTCAE) v3.0 at time points during the first year of protocol therapy. All data are summarized as number (%) of patients. aPatients without any adverse event data (two patients without hot flashes/sweating and one without musculoskeletal symptoms) were excluded from summary. Unk unknown, Gr grade OFS ovarian function suppression
Association of CYP19A1 and ESR1 with early-onset hot flashes/sweating
A total of 43 % of patients reported early-onset grade 2–3 hot flashes/sweating during the first 6 months of protocol treatment (42 % of women receiving exemestane + OFS (411/987) and 45 % of women allocated to tamoxifen + OFS (437/980)). Most side effects were reported by the month-3 visit (Table 3). Overall, patients with CYP19A1 rs10046 (C > T) minor variant (T/T) had a 22 % reduced odds of reporting early-onset grade 2–3 hot flashes/sweating (odds ratio (OR) = 0.78, 95 % CI 0.63–0.97; P = 0.03) when compared to patients with the C/T or C/C variants. The effect was consistent, showing a multivariable OR of 0.83 (95 % CI 0.66–1.04; P = 0.10) after adjusting for patient and treatment characteristics and concomitant medications (Table 4). A differential effect according to treatment assignment (treatment-by-genotype interaction, P = 0.03) was observed for the association between CYP19A1 rs10046 (C > T) genotype variants and early-onset hot flashes/sweating. Patients treated with exemestane + OFS and having the T/T variant had a 35 % reduced odds of early-onset hot flashes/sweating (Table 5; univariate OR = 0.65, 95 % CI 0.48–0.89; multivariable OR = 0.67, 95 % CI 0.49–0.93), which was not apparent for patients treated with tamoxifen + OFS (univariable OR = 0.94, 95 % CI 0.69–1.27; multivariable OR = 1.04, 95 % CI 0.75–1.43). There was no statistically significant association between the other four SNPs of CYP19A1 or ESR1 and early-onset hot flashes/sweating side effects.
Table 4.
Associations of CYP19A1 and ESR1 genotypes with analysis endpoints
| Univariate model | Multivariableb model | Multivariablec model | ||||||
|---|---|---|---|---|---|---|---|---|
| Gene: SNP | Comparisons | Numbera (events) | Odds ratio (95 % CI) | P value | Odds ratio (95 % CI) | P value | Odds ratio (95 % CI) | P value |
| Hot flashes/sweating | ||||||||
| CYP19A1: rs4646 | Dose effectd | 1965 (848) | 1.05 (0.91,1.21) | 0.50 | 1.04 (0.90,1.20) | 0.63 | 1.08 (0.93,1.25) | 0.30 |
| CYP19A1: rs10046 | T/T vs. C/T,C/C (ref) | 446 (172) vs. 1519 (676) | 0.78 (0.63,0.97) | 0.03 | 0.82 (0.66,1.02) | 0.08 | 0.83 (0.66,1.04) | 0.10 |
| ESR1:rs2077647 | Dose effect | 1965 (848) | 0.95 (0.84,1.08) | 0.47 | 0.96 (0.84,1.09) | 0.51 | 0.97 (0.85,1.11) | 0.69 |
| ESR1:rs2234693 (PvuII) | Dose effect | 1965 (848) | 0.92 (0.80,1.04) | 0.18 | 0.92 (0.80,1.04) | 0.19 | 0.94 (0.82,1.07) | 0.36 |
| ESR1:rs9340799 (XbaI) | Dose effect | 1965 (848) | 0.94 (0.82,1.08) | 0.38 | 0.94 (0.82,1.07) | 0.34 | 0.98 (0.85,1.12) | 0.73 |
| Musculoskeletal symptoms | ||||||||
| CYP19A1: rs4646 | Dose effectd | 1966 (516) | 1.01 (0.86,1.18) | 0.90 | 1.05 (0.89,1.24) | 0.55 | 1.11 (0.93,1.31) | 0.25 |
| CYP19A1: rs10046 | T/T vs. C/T,C/C (ref) | 446 (110) vs. 1520 (406) | 0.90 (0.70,1.15) | 0.39 | 0.86 (0.66,1.10) | 0.23 | 0.84 (0.65,1.09) | 0.18 |
| ESR1:rs2077647 | Dose effect | 1966 (516) | 1.08 (0.94,1.25) | 0.28 | 1.12 (0.96,1.30) | 0.15 | 1.11 (0.95,1.29) | 0.20 |
| ESR1:rs2234693 (PvuII) | Dose effect | 1966 (516) | 1.03 (0.90,1.19) | 0.65 | 1.07 (0.92,1.25) | 0.37 | 1.06 (0.91,1.24) | 0.47 |
| ESR1:rs9340799 (XbaI) | Dose effect | 1966 (516) | 1.06 (0.91,1.23) | 0.44 | 1.10 (0.94,1.29) | 0.22 | 1.11 (0.94,1.30) | 0.22 |
Analysis endpoints were early-onset (within 6 months) grade ≥2 hot flashes/sweating or (within 12 months) grade ≥2 musculoskeletal symptoms. aPatients without any adverse event data, excluded from analyses (2 without hot flashes/sweating and one without musculoskeletal symptoms). bMultivariable logistic regression model adjusted for characteristics: age, menstruation status, BMI, adjuvant chemotherapy use, treatment assignment, and presence of hot flashes/sweating at baseline or of musculoskeletal symptoms at baseline (according to endpoint). cMultivariable model also adjusted for relevant concomitant medications prior to or continuing at baseline, and use during relevant time period for the endpoint. dDose effect: comparisons of variant (Var) allele groups: 0 (Var) vs. 1 (Var) vs. 2 (Var). SNP single nucleotide polymorphism
Table 5.
Associations of endpoints with SNP CYP19A1 rs10046 variants, overall and according to treatment assignments
| CYP19A1: rs10046 | |||||
|---|---|---|---|---|---|
| Endpoint | Cohort | T/T vs T/C, C/C patients (events) | Univariate model OR (95 % CI)a | Univariate model Interaction P valueb | Multivariable model OR (95 % CI)c |
| Hot flashes/sweating | All patients | 446 (172) vs 1519 (676) | 0.78 (0.63, 0.97) | 0.03 | 0.83 (0.66, 1.03) |
| Exemestane + OFS | 227 (77) vs 759 (334) | 0.65 (0.48, 0.89) | 0.67 (0.49, 0.93) | ||
| Tamoxifen + OFS | 219 (95) vs 760 (342) | 0.94 (0.69, 1.27) | 1.04 (0.75, 1.43) | ||
| Musculoskeletal events | All patients | 446 (110) vs 1520 (406) | 0.90 (0.70, 1.15) | 0.39 | 0.85 (0.66, 1.1) |
| Exemestane + OFS | 227 (75) vs 759 (256) | 0.97 (0.71, 1.33) | 0.96 (0.69, 1.32) | ||
| Tamoxifen + OFS | 219 (35) vs 761 (150) | 0.77 (0.52, 1.16) | 0.77 (0.51, 1.17) | ||
aEstimates from univariate logistic regression model. b P value from test of rs10046 variants ((T/T) vs. T/C, C/C) by treatment interaction in logistic regression model (univariable) assessing association between the SNP variants and early-onset adverse events in the overall cohort. cAdjusted for baseline characteristics: age, menstrual status, body mass index, adjuvant chemotherapy use, treatment assignment (for “all patients” cohort), baseline hot flashes/sweating or baseline musculoskeletal symptoms (according to endpoint) and prior to or baseline concomitant medications use and use during relevant time period for the endpoint (yes or no). SNP single nucleotide polymorphism, OFS ovarian function suppression, OR odds ratio, CI confidence interval
Association of CYP19A1 and ESR1 with early-onset musculoskeletal symptoms
Within the first year of treatment, 26 % of patients reported early-onset grade 2–4 musculoskeletal symptoms (34 % of patients (331/987) assigned to exemestane + OFS and 19 % of patients (185/980) assigned to tamoxifen + OFS. There was no statistically significant association between any of the five SNPs of CYP19A1 and ESR1 and early-onset musculoskeletal side effects (Table 4), nor of treatment-by-genotype interaction. The presence of CYP19A1 rs10046 (C > T) minor variant (T/T) was not associated with early-onset grade 2–4 musculoskeletal symptoms (univariate OR = 0.90, 95 % CI 0.70–1.15; P = 0.39; OR = 0.84, 95 % CI 0.65–1.09; P = 0.18 after adjusting for patient and treatment characteristics and concomitant medications (Table 4). There was no evidence of a differential effect according to treatment assignment (exemestane + OFS, OR = 0.97, 95 % CI 0.71–1.33 versus tamoxifen + OFS, OR = 0.77, 95 % CI 0.52–1.16, treatment-by-genotype interaction; P = 0.39 from the univariate model). The results were consistent after adjusting for patient and treatment characteristics and concomitant medications (exemestane + OFS, OR = 0.96, 95 % CI 0.69–1.32 versus tamoxifen + OFS, OR = 0.77, 95 % CI 0.51 − 1.17) (Table 5).
Discussion
This study provides evidence that CYP19A1 rs10046 variant carriers may face milder vasomotor symptoms under combined endocrine treatment. Notably, the effect was restricted to patients under OFS combined with exemestane (treatment-by-genotype interaction, P = 0.03) and not tamoxifen, after adjusting for patient characteristics and concomitant medications, including the selective serotonin-reuptake inhibitors known to reduce hot flashes/sweating.
This finding is in line with evidence from others, linking this SNP to enhanced aromatase activity and higher circulating estrogens [15, 17] and underscores a possible relationship between the effect of this variant polymorphism (T/T), hot flashes/sweating and exemestane activity. This result may in fact be related to less effective estrogen suppression by exemestane + OFS in these women as a consequence of higher circulating estrogens compared to patients with wild-type SNPs, although the exact mechanism by which this SNP may affect exemestane efficacy in suppressing the aromatase activity is not known. One study recently reported similar associations in postmenopausal patients with breast cancer [30] enrolled in the TEAM trial: CYP19A1 variants linked with lower estrogen levels were associated with increased risk of early vasomotor and musculoskeletal symptoms under exemestane. The TEAM substudy, however, only included 27 % of the patients enrolled, less than two-thirds of patients, which represents a smaller proportion than is recommended by Simon et al. for evaluating predictive biomarkers [31].
The ELPh trial was designed to address genetic associations with toxicity-related discontinuation of AI therapy for breast cancer [32], including the SNP rs10046. The authors did not specifically report on vasomotor symptoms, but did not find any relationship between rs10046 and toxicity-related treatment discontinuation. In another study, in which the impact of CYP19A1 SNPs with estrogen suppression during letrozole treatment was assessed, the degree of suppression was independent of the SNPs [33].
To our knowledge this is the first study to evaluate the associations between common germline polymorphisms of the CYP19A1 and ESR1 genes and early-onset side effects under combined endocrine treatment in premenopausal patients with hormone receptor-positive early breast cancer. The strength of this translational research is its considerable sample size of 1967 patients, which represents 74 % of women enrolled in TEXT. Furthermore, blood samples were collected specifically for this research, i.e., to investigate treatment tolerability and disease outcome. Women enrolled prior to the amendment were asked to re-consent, but 693 TEXT participants were not assessed due to the retrospective nature of blood collection. As a result, we may have missed some patients who discontinued treatment early, possibly due to treatment-related side effects.
The combined analysis of TEXT and SOFT [4] showed that adjuvant treatment with exemestane + OFS as compared with tamoxifen + OFS, significantly reduces the risk of recurrence. Although the overall incidence of adverse events and the quality of life were similar in the two treatment groups, between-group differences were observed with respect to specific symptoms. While vasomotor AEs (hot flashes and sweating) were quite frequent and evenly distributed amongst treatment groups, musculoskeletal AEs were more frequently reported in patients assigned to exemestane + OFS.
We did not observe any direct association between the CYP19A1 SNPs and musculoskeletal symptoms, nor any interaction by endocrine treatment. This is in contrast with findings from the TEAM trial [30], but as mentioned they studied a very small proportion of patients. Furthermore, genotyping in that study was performed on DNA extracted from tumor samples. A cross-sectional study of patients receiving AIs [34] found that women carrying at least one 8-repeat allele of the tetranucleotide repeat polymorphism of CYP19A1, associated with higher estrogen concentrations, had lower odds of AI-associated arthralgia. Conversely, they also did not find any association between the rs10046 SNP and arthralgia.
Contrary to findings from case–control studies conducted in different treatment settings, i.e., postmenopausal or premenopausal women with breast cancer treated with tamoxifen alone, we found no association between the three ESR1 polymorphisms and endocrine-mediated side effects (hot flashes/sweating and musculoskeletal symptoms). Postmenopausal Chinese patients with breast cancer carrying an ESR1 rs2234693 CC genotype or rs9340799 AA genotype had an increased risk of AI-related musculoskeletal AEs [35]. In fact, several studies suggest that the effect of the ESR1 polymorphisms on breast cancer risk is hormone-related and dependent on the woman’s hormonal context, showing statistically significant associations mainly in premenopausal women [23]. Likewise, an association with increased mammographic density [36] was shown only in women taking hormone replacement therapy. Possibly, the concurrent OFS by the GnRH analogue triptorelin masked the effect of these polymorphisms due to its complete estrogen deprivation effect. Thus, in the context of adjuvant combined endocrine treatment, these ESR1 polymorphisms may be unlikely to exert their effect.
Musculoskeletal events are a common toxicity, leading to premature discontinuation of AI therapy [37]. In the TEXT-SOFT combined analysis, early cessation of protocol treatment was more frequent among patients receiving exemestane + OFS than among those receiving tamoxifen + OFS. Several studies have investigated the relationship between endocrine treatment efficacy and associated side effects in different settings. Recent findings support an inverse association between the reporting of early side effects under adjuvant endocrine treatment and breast cancer recurrence [38–40]. Vasomotor symptoms were associated with improved disease-free and overall survival in the TEAM trial [38] and reduced breast cancer recurrence in the ATAC trial [40], but not in the BIG 1–98 [41] and MA.27 trials [42]. Thus, we cannot exclude that the CYP19A1 rs10046 (T/T) genotype might be associated with reduced exemestane + OFS efficacy: women with this polymorphism possibly lack complete estrogen suppression, despite receiving concomitant OFS. On the other hand, because the rs10046 polymorphism is located in a 3’ untranslated region, upstream of the coding sequence, it may interfere with aromatase transcription in a tissue-specific manner, depending on the transcriptional modulators present, thus influencing the degradation rate of the aromatase differently according to tissue and independently from circulating estrogen [9].
Conclusions
This translational study within the TEXT trial for premenopausal patients with hormone-receptor-positive early breast cancer provides evidence that the CYP19A1 rs10046 polymorphism may influence endocrine treatment side effects under combined endocrine therapy. The CYP19A1 rs10046 variant favors lower incidence of hot flashes/sweating under exemestane plus ovarian function suppression treatment, suggesting endocrine-mediated effects that might enhance treatment adherence and potentially impact long-term treatment efficacy. No effect of any other tested SNPs was evident on hot flashes/sweating and no interaction on musculoskeletal symptoms emerged overall. Monitoring of musculoskeletal and bone events, known to occur later during treatment are warranted. Although our results must be considered hypothesis-generating, longer follow up will allow us to assess the clinical relevance of this finding, in particular its potential impact on disease outcome, and will be the subject of a future report after the TEXT results are further updated.
Acknowledgements
The authors thank the patients who participated and the staff who conducted the study at the participating centers, and the CALGB Pathology Coordinating Office. Investigators and the International Breast Cancer Study Group participants include Steering Committee: P.A. Francis (Chair, SOFT Co-Chair), G.F. Fleming (SOFT Co-Chair), O. Pagani (TEXT Co-Chair), B. A. Walley (TEXT Co-Chair), M.M. Regan (Trial Statistician), L. Blacher, H. Bonnefoi, E. Ciruelos, A.S. Coates, M. Colleoni, N. Dif, R.D. Gelber, A. Goldhirsch, A. Hiltbrunner, R. Kammler, R. Maibach, O. Ortmann, K.N. Price, M. Rabaglio, B. Ruepp, H. Shaw, G. Viale, G. von Minckwitz, V. Katkade (Pfizer), E. Chetaille (Ipsen). IBCSG Scientific Executive Committee: M. Colleoni, F. Boyle, A. DiLeo, G. Jerusalem, K.N. Price, M.M. Regan, G. Viale. IBCSG Foundation Council: R. Stahl (President), S. Aebi, A.S. Coates, M. Colleoni, R.D. Gelber, A. Goldhirsch, P. Karlsson, I. Kössler. IBCSG Coordinating Center, Bern, Switzerland: A. Hiltbrunner (Director), R. Kammler, R. Maibach, M. Rabaglio, S. Roux, B. Ruepp, P. Sicher. IBCSG Statistical Center, Dana-Farber Cancer Institute, Boston, MA, USA: R.D. Gelber (Director), M.M. Regan (Group Statistician), M. Bonetti, Y. Feng, A. Giobbie-Hurder, K.P. Gray, H. Huang, W. Luo, K.N. Price, L. Zickl. IBCSG Data Management Center, Frontier Science & Technology Research Foundation, Amherst, NY, USA: L. Blacher (Director), K. Scott (DM Section Head), M. Blackwell, A. Cesario, A. Dickinson, K. Donahue, M. Greco, P. Gonzalez, T. Heckman-Scolese, R. Hecker, R. Hinkle, M. Kalera, K. Lupejkis, A. Mora de Karausch, V. Palermo, H. Shaw, R. Starkweather, J. Swick-Jemison. IBCSG Central Biomarker Laboratory, European Institute of Oncology, Division of Cancer Prevention and Genetics, Milan, Italy: B. Bonanni, H. Johansson, D. Macis. IBCSG Central Pathology Office, European Institute of Oncology, Division of Pathology, Milan, Italy: G. Viale, D. Lepanto, O. Pala. IBCSG Quality of Life Office, Bern, Switzerland: J. Bernhard, K. Ribi. U.S. National Cancer Institute: J. Abrams, J.A. Zujewski. U.S. NCI Clinical Trials Support Unit (CTSU)/Westat: M. Hering, M. Greene, A. Nelson, M. Balois-Ouellette, S. Riordan, O. Santos. ALMAC: W. Mahon, E. Whitney, J. Bryant. CTSU Regulatory Office: R. Catalano, D. Marinucci, B. Niewood, R. Lambersky. Alliance (CALGB) Pathology Coordinating Office, Ohio State University, Columbus, OH, USA: W. Frankel, S. Jewell. Dana-Farber Cancer Institute, Boston, MA, USA (US FDA IND): E.P. Winer, J. Savoie. Pfizer Study Support: B. Campanelli, S. Duong, J.A. Graham, C. Grant, B. Klingele, J. Passmore. Ipsen Study Support: E. Chetaille, J. Amauri Soares, C. Descot, S. Hemont-Dacosta, F. Bismuth, P. Chevreau, H. Bibas. TEXT Participating Centers and Principal Investigators include Centers with accrual of more than one patient: Breast International Group (BIG); International Breast Cancer Study Group (IBCSG). Australia and New Zealand Breast Cancer Trials Group (ANZBCTG): Austin Health, Heidelberg, Victoria: J. Stewart; Box Hill Hospital, Box Hill, Victoria: J. Chirgwin; Calvary Mater Newcastle, Waratah, New South Wales: A. van der Westhuizen; Coffs Harbour Health Campus, Coffs Harbour, New South Wales: K. Briscoe; Flinders Medical Centre, Bedford Park, South Australia: B. Koczwara; Launceston General Hospital, Launceston, Tasmania: S. Gauden; Liverpool Hospital, Liverpool, New South Wales: E. Moylan; Maroondah Hospital, Ringwood East, Victoria: J. Chirgwin; Peter MacCallum Cancer Centre, East Melbourne, Victoria: P. A. Francis; Royal Brisbane and Women’s Hospital, Herston, Queensland: M. Nottage; Royal Hobart Hospital, Hobart, Tasmania: D. Boadle; Royal Perth Hospital, Perth, Western Australia: E. Bayliss; St. Vincent’s Hospital Melbourne, Fitzroy, Victoria: R. Snyder; Tamworth Rural Referral Hospital, Tamworth, New South Wales: F. Sardelic; Tweed Hospital, The, Tweed Heads, New South Wales: E. Abdi; Victorian Breast and Oncology Care, East Melbourne, Victoria: M. Chipman. Belgium: Institute Jules Bordet, Brussels: A. Gombos; Centre Hospitalier Peltzer-La Tourelle, Verviers: A. Barbeaux; Centre Hospitalier Universitarie Sart Tilman, Liège: G. Jerusalem; U.Z. Gasthuisberg, Leuven: P. Neven. Hungary: National Institute of Oncology, Budapest; I. Láng. Italy: Dipartimento di Oncologia, Azienda Ospedaliero-Universitaria di Udine, Udine: F. Plugisi; Centro di Riferimento Oncologico, Aviano: D. Crivellari; Fondazione Salvatore Maugeri, Pavia: L. Pavesi; Istituto Europeo di Oncologia, Milano: M. Colleoni; Ospedale degli Infermi, Rimini: L. Gianni; Ospedale di Circolo e Fondazione Macchi, Varese: G. Pinotti; Ospedali Riuniti di Bergamo, Bergamo: C. Tondini; Sandro Pitigliani Medical Oncology Unit, Hospital of Prato, Prato: A. Di Leo; Azienda Sanitaria di Bolzano, Bolzano: C. Graiff. Peru: Instituto de Enfermedades Neoplásicas, Lima: H. Gomez. Slovenia: Institute of Oncology, Ljubljana: E. Skof. South Africa: Sandton Oncology Centre, Johannesburg; D. Vorobiof. Sweden: Sahlgrenska University Hospital, Gothenburg; P. Karlsson. Switzerland: Swiss Association for Clinical Cancer Research (SAKK), Centre Hospitalier Universitaire Vaudois, Lausanne: K. Zamin; Inselspital, Bern: M. Rabaglio; Oncocare Engeried, Bern: K. Buser; Institute of Oncology of Southern Switzerland (Ospedale San Giovanni, Bellinzona; Ospedale Regionale di Lugano, (Civico & Italiano), Lugano; Ospedale Regionale Beata Vergine, Mendrisio; Ospedale Regionale La Carità, Locarno; Istituto Cantonale di Patologia, Locarno): O. Pagani; Kantonsspital St. Gallen, St. Gallen: T. Ruhstaller; Rätisches Kantonos-/Regionalspital, Chur: R. von Moos; Kantonsspital Basel, Basel: C. Rochlitz; Onkologiezentrum Thun-Berner Oberland, Thun: D. Rauch; Zürich Frauenklinik, Zürich: N. Gabriel. Germany: German Breast Group (GBG), Caritas-Krankenhaus St. Josef, Regensburg: S. Buchholz; Dr. Horst Schmidt Kliniken, Wiesbaden: F. Lorenz-Salehi. North American Breast Cancer Group: American College of Surgeons Oncology Group (ACOSOG, now part of Alliance for Clinical Trials in Oncology); Cancer and Leukemia Group B (CALGB, now part of Alliance for Clinical Trials in Oncology); Eastern Cooperative Oncology Group (ECOG, now part of ECOG-ACRIN Cancer Research Group); NCIC Clinical Trials Group (NCIC CTG); National Surgical Adjuvant Breast and Bowel Project (NSABP, now part of NRG Oncology); North Central Cancer Treatment Group (NCCTG, now part of Alliance for Clinical Trials in Oncology); Radiation Therapy Oncology Group (RTOG, now part of NRG Oncology); South West Oncology Group (SWOG); North American Participating Centers. Canada: Cross Cancer Institute, Edmonton, Alberta: K.S. Tonkin; Tom Baker Cancer Center, Calgary, Alberta: B.A. Walley (Chair), M. Webster (PI); London Regional Cancer Center, London, Ontario: K.R. Potvin; Juravinski Cancer Centre at Hamilton Health Sciences, Hamilton, Ontario: R.G. Tozer; Trillium Health Centre - W Toronto, Toronto, Ontario: J.A. Gapski; Hôpital Charles LeMoyne, Greenfield Park, Quebec: C. Prady; Allan Blair Cancer Center, Regina, Saskatchewan; M. Salim; Saskatoon Cancer Center, Saskatoon, Saskatchewan: A. Sami; The Vitalite Health Network - Dr. Leon Richard Oncology Centre, Moncton, New Brunswick: P. Whitlock. USA: Presbyterian Hospital, Whittier, CA: J.H. Freimann; University of California at San Diego, San Diego, CA: J.E. Mortimer; St. Joseph Medical Center, Burbank, CA: R.R. Mena; San Francisco General, San Francisco, CA: H.S. Rugo; University of California at San Francisco, San Francisco, CA: C.J. Ryan; University of California San Diego Cancer Center, San Diego, CA: B.A. Parker; University of Colorado, Aurora, CO: A.D. Elias; The Shaw Regional Cancer Center, Aurora, CO: A.D. Elias; University of Connecticut, Farmington, CT: S. Tannenbaum; Walter Reed Army Medical Center, Washington, DC: D.C. Van Echo; Northeast Georgia Medical Center, Gainesville, GA: R.J. LoCicero; Siouxland Hematology - Oncology Associates, Sioux City, IA: D.B. Wender; Saint Luke's Mountain States Tumor Institute, Boise, ID: T.A. Walters; Evanston Northwestern Healthcare, Evanston, IL: D.E. Merkel; Resurrection Medical Center, Chicago, IL: C. G. Rose; University of Chicago, Chicago, IL: H.L. Kindler; Saint Joseph's Medical Center, South Bend, IN: R.H. Ansari; Memorial Hospital of South Bend, South Bend, IN: R.H. Ansari; Northern Indiana Cancer Research Co, South Bend, IN: R.H. Ansari; Mount Carmel Regional Cancer Center, Pittsburg, KS; Stormont-Vail Regional Health Center, Topeka, KS: S.J. Vogel; Cancer Center of Kansas Wichita, Wichita, KS: S.R. Dakhil; Via Christi Regional Medical Center, Wichita, KS: S.R. Dakhil; Addison Gilbert, Gloucester, MA: A.P. McIntyre; Tufts Medical Center, Boston, MA: J.K. Erban; Massachusetts General Hospital, Boston, MA: H.J. Burstein; Dana-Farber Cancer Institute, Boston, MA: H.J. Burstein; Beth Israel Deaconess Medical Center, Boston, MA: H.J. Burstein; Faulkner Hospital, Boston, MA: H.J. Burstein; North Shore Cancer Center, Salem, MA: K.J. Krag; Emerson Hospital, Boston, MA: H.J. Burstein; Suburban Hospital, Bethesda, MD: C.B. Hendricks; University of Maryland Greenebaum Cancer Center, Baltimore, MD: K.H. Rak Tkaczuk; Mercy Medical Center, Baltimore, MD: D.A. Riseberg; Frederick Memorial Hospital, Frederick, MD: E.D. Eskander; William Beaumont Hospital, Royal Oak, MI: D. Zakalik; United Hospital, St. Paul, MN: P.J. Flynn; Abbott-Northwestern Hospital, St. Louis Park, MN: P.J. Flynn; Mercy Hospital, Coon Rapids, MN: P.J. Flynn; Mayo Clinic, Rochester, MN: J.N. Ingle; Saint John's Hospital - Healtheast, Minneapolis, MN: D.J. Schneider; Metro-Minnesota CCOP, Minneapolis, MN: P.J. Flynn; Washington School of Medicine, St Louis, MO: M.J. Naughton; Kansas City CCOP, Kansas City, MO: W.T. Stephenson; Moses H. Cone Memorial, Greensboro, NC: J.E. Feldmann; Mission Hospitals Inc, Asheville, NC: M.J. Messino; Hope, A Women's Cancer Center, Asheville, NC: D.J. Hetzel; Medcenter One Health Systems, Bismarck, ND: E.J. Wos; Dakota Clinic, Fargo, ND: K. Sen; University of Nebraska Medical Center, Omaha, NE: E.C. Reed; Portsmouth Regional Hospital, Portsmouth, NH: E.M. Bonnem; South Jersey Healthcare, Vineland, NJ: D.H. Blom; New York University Medical Center, New York, NY: A.D. Tiersten; Albert Einstein College/Medicine, Bronx, NY: C.M. Pellegrino; Roswell Park Cancer Institute, Buffalo, NY: E.G. Levine; Geisinger Medical Center, Danville, PA: G.D.A. Padula; Greenville CCOP, Greenville, SC: J.K. Giguere; Sioux Valley Clinic - Oncology, Sioux Falls, SD: M.A. Mazurczak; University of Vermont, Burlington, VT: S. Burdette-Radoux; Mountainview Medical, Berlin, VT: S. Burdette-Radoux; Swedish Hospital Medical Center, Seattle, WA: S.E. Rivkin; University of Washington Medical Center, Seattle, WA: S.E. Rivkin; Aspirus Wausau Hospital Center, Wausau, WI: U. Gautam; Oncology Alliance-Glendale, Glendale, WI: R.D. Hart; West Virginia University, Morgantown, WV: J. Abraham.
Funding
The translational project presented here is supported by Susan G. Komen for the Cure Promise Grant (KG080081 to GV, OP, MMR). The translational project in Australia and New Zealand was supported by an Australia and New Zealand Breast Cancer Trials Group (ANZBCTG) Discretionary Funding Research Grant (PF, AC). TEXT receives financial support for trial conduct from Pfizer, the International Breast Cancer Study Group and the US National Cancer Institute. Pfizer and Ipsen provided the drug supply, and the IBCSG received funding from Ipsen for additional data analyses. Support for the coordinating group, IBCSG: Frontier Science and Technology Research Foundation, Swiss Group for Clinical Cancer Research (SAKK), US National Cancer Institute (NCI) (CA75362), Cancer Research Switzerland/Oncosuisse, and the Foundation for Clinical Cancer Research of Eastern Switzerland (OSKK). Neither the pharmaceutical companies nor Susan G. Koment for the Cure have a role in the reporting or interpretation of the results, other than a minority representation on the Steering Committee. Grant support of cooperative groups: ANZBCTG (NHMRC 351161, 510788, 1105058); SWOG (US NIH CA32102); Alliance/CALGB (US NIH U10CA180821); ECOG-ACRIN (US NIH CA21115 and CA16116); NSABP/NRG (US NIH U10-CA-12027, U10-CA-69651, U10-CA-37377, U10-CA-69974); NCIC (US NIH CA077202 and CCSRI 015469 and 021039).
Availability of data and materials
The authors are not sharing the data in this article because the primary results of the main trial, TEXT, have not been shared in a public venue. The International Breast Cancer Study Group has data-sharing policies; please contact the corresponding author for details.
Authors’ contributions
HJ, OP, MMR, GV, AG, RDG, ASC, BB, and BAW contributed to conception and design of the TEXT translational study. KNP, RM, SR, and RK gave administrative support of study procedures. OP, MR, and BAW contributed to collection of clinical data. HJ coordinated biobanking, oversaw the laboratory analysis and performed quality control. HJ, VA, DM, and AP extracted DNA and performed genotyping analysis. KPG and MMR performed the statistical analysis, coordinated the conduct and oversaw the study for data quality control. HJ, KPG, OP, MMR, BB, and BAW contributed to analysis and interpretation of data and drafted the manuscript. All authors participated in writing and revising the manuscript, and approved the final version.
Competing interests
The authors declare that they have no competing interests.
Consent for publication
Not applicable.
Ethics approval and consent to participate
The International Breast Cancer Study Group Ethical Committee approved the TEXT trial and Amendment 2. As a requirement for study participation, each of the 137 participating centers was required to submit proof of ethics committee approval of the protocol and Amendment 2. A list of participating centers is included in “Acknowledgements”. Written informed consent was obtained for all patients enrolled in the TEXT trial. Consent to use collected blood samples was obtained prior to sample collection.
Abbreviations
- ADL
activities of daily living
- AE
adverse event
- AI
aromatase inhibitor
- BMI
body mass index
- CALGB
Cancer and Leukemia Group B
- CTCAE
common terminology criteria for adverse events
- CYP19A1
Cytochrome P450 19A1 gene
- E + OFS
exemestane plus ovarian function suppression
- EDTA
ethylenediaminetetetraacetic acid
- ER
estrogen receptor
- ESR1
estrogen receptor α gene
- GnRHa
gonadotropin-releasing-hormone analogues
- HWE
Hardy-Weinberg equilibrium
- IBCSG
International Breast Cancer Study Group
- IQR
interquartile range
- ITT
intention to treat
- NSAID
nonsteroidal anti-inflammatory drugs
- OFS
ovarian function suppression
- PCR
polymerase chain reaction
- PgR
progesterone receptor
- REMARK
Reporting recommendations for tumor marker prognostic studies
- SNP
single nucleotide polymorphism
- SOFT
Suppression of Ovarian Function Trial
- T + OFS
tamoxifen plus ovarian function suppression
- TEXT
Tamoxifen and Exemestane Trial
- Unk
unknown
Contributor Information
Harriet Johansson, Phone: +390294372654, Email: harriet.johansson@ieo.it.
Kathryn P. Gray, Email: pkruan@jimmy.harvard.edu
Olivia Pagani, Email: olivia.pagani@ibcsg.org.
Meredith M. Regan, Email: mregan@jimmy.harvard.edu
Giuseppe Viale, Email: giuseppe.viale@ieo.it.
Valentina Aristarco, Email: valentina.aristarco@ieo.it.
Debora Macis, Email: debora.macis@ieo.it.
Antonella Puccio, Email: antonellapuccio@tiscali.it.
Susanne Roux, Email: Susanne.Roux@ibcsg.org.
Rudolf Maibach, Email: rudolf.maibach@ibcsg.org.
Marco Colleoni, Email: marco.colleoni@ieo.it.
Manuela Rabaglio, Email: manuela.rabaglio@ibcsg.org.
Karen N. Price, Phone: +1-617-632-2459, Email: price@jimmy.harvard.edu
Alan S. Coates, Email: alan.coates@ibcsg.org
Richard D. Gelber, Email: gelber@jimmy.harvard.edu
Aron Goldhirsch, Email: aron.goldhirsch@ibcsg.org.
Roswitha Kammler, Email: Rosita.Kammler@ibcsg.org.
Bernardo Bonanni, Email: bernardo.bonanni@ieo.it.
Barbara A. Walley, Email: bwalley@ucalgary.ca
References
- 1.Burstein HJ, Lacchetti C, Anderson H, Buchholz TA, Davidson NE, Gelmon KE, et al. Adjuvant endocrine therapy for women with hormone receptor-positive breast cancer: American Society of Clinical Oncology clinical practice guideline update on ovarian suppression. J Clin Oncol. 2016;34:1689–701. doi: 10.1200/JCO.2015.65.9573. [DOI] [PubMed] [Google Scholar]
- 2.Burstein HJ, Temin S, Anderson H, Buchholz TA, Davidson NE, Gelmon KE, et al. Adjuvant endocrine therapy for women with hormone receptor-positive breast cancer: American Society of Clinical Oncology clinical practice guideline focused update. J Clin Oncol. 2014;32:2255–69. doi: 10.1200/JCO.2013.54.2258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dowsett M, Forbes JF, Bradley R, Ingle J, Aihara T, Bliss J, et al. Aromatase inhibitors versus tamoxifen in early breast cancer: patient-level meta-analysis of the randomised trials. Lancet. 2015;386:1341–52. doi: 10.1016/S0140-6736(15)61074-1. [DOI] [PubMed] [Google Scholar]
- 4.Pagani O, Regan MM, Walley BA, Fleming GF, Colleoni M, Lang I, et al. Adjuvant exemestane with ovarian suppression in premenopausal breast cancer. N Engl J Med. 2014;371:107–18. doi: 10.1056/NEJMoa1404037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schover LR. Premature ovarian failure and its consequences: vasomotor symptoms, sexuality, and fertility. J Clin Oncol. 2008;26:753–8. doi: 10.1200/JCO.2007.14.1655. [DOI] [PubMed] [Google Scholar]
- 6.Gallicchio L, Miller SR, Kiefer J, Greene T, Zacur HA, Flaws JA. Risk factors for hot flashes among women undergoing the menopausal transition: baseline results from the Midlife Women's Health Study. Menopause. 2015;22:1098–107. doi: 10.1097/GME.0000000000000434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dennerstein L, Lehert P, Burger HG, Guthrie JR. New findings from non-linear longitudinal modelling of menopausal hormone changes. Hum Reprod Update. 2007;13:551–7. doi: 10.1093/humupd/dmm022. [DOI] [PubMed] [Google Scholar]
- 8.Dugan SA, Powell LH, Kravitz HM, Everson Rose SA, Karavolos K, Luborsky J. Musculoskeletal pain and menopausal status. Clin J Pain. 2006;22:325–31. doi: 10.1097/01.ajp.0000208249.07949.d5. [DOI] [PubMed] [Google Scholar]
- 9.Freedman RR. Pathophysiology and treatment of menopausal hot flashes. Semin Reprod Med. 2005;23:117–25. doi: 10.1055/s-2005-869479. [DOI] [PubMed] [Google Scholar]
- 10.Freeman EW, Sammel MD, Sanders RJ. Risk of long-term hot flashes after natural menopause: evidence from the Penn Ovarian Aging Study cohort. Menopause. 2014;21:924–32. doi: 10.1097/GME.0000000000000196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stearns V, Ullmer L, Lopez JF, Smith Y, Isaacs C, Hayes D. Hot flushes. Lancet. 2002;360:1851–61. doi: 10.1016/S0140-6736(02)11774-0. [DOI] [PubMed] [Google Scholar]
- 12.Niravath P. Aromatase inhibitor-induced arthralgia: a review. Ann Oncol. 2013;24:1443–9. doi: 10.1093/annonc/mdt037. [DOI] [PubMed] [Google Scholar]
- 13.Villa P, Lassandro AP, Amar ID, Vacca L, Moruzzi MC, Ferrandina G, et al. Impact of aromatase inhibitor treatment on vertebral morphology and bone mineral density in postmenopausal women with breast cancer. Menopause. 2016;23:33–9. doi: 10.1097/GME.0000000000000515. [DOI] [PubMed] [Google Scholar]
- 14.Schimdt N, Jacob L, Coleman R, Kostev K, Hadji P. The impact of treatment compliance on fracture risk in women with breast cancer treated with aromatase inhibitors in the United Kingdom. Breast Cancer Res Treat. 2016;155:151–7. doi: 10.1007/s10549-015-3661-3. [DOI] [PubMed] [Google Scholar]
- 15.Dunning AM, Dowsett M, Healey CS, Tee L, Luben RN, Folkerd E, et al. Polymorphisms associated with circulating sex hormone levels in postmenopausal women. J Natl Cancer Inst. 2004;96:936–45. doi: 10.1093/jnci/djh167. [DOI] [PubMed] [Google Scholar]
- 16.Wang L, Ellsworth KA, Moon I, Pelleymounter LL, Eckloff BW, Martin YN, et al. Functional genetic polymorphisms in the aromatase gene CYP19 vary the response of breast cancer patients to neoadjuvant therapy with aromatase inhibitors. Cancer Res. 2010;70:319–28. doi: 10.1158/0008-5472.CAN-09-3224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Haiman CA, Dossus L, Setiawan VW, Stram DO, Dunning AM, Thomas G, et al. Genetic variation at the CYP19A1 locus predicts circulating estrogen levels but not breast cancer risk in postmenopausal women. Cancer Res. 2007;67:1893–7. doi: 10.1158/0008-5472.CAN-06-4123. [DOI] [PubMed] [Google Scholar]
- 18.Artigalas O, Vanni T, Hutz MH, Shton-Prolla P, Schwartz IV. Influence of CYP19A1 polymorphisms on the treatment of breast cancer with aromatase inhibitors: a systematic review and meta-analysis. BMC Med. 2015;13:139. doi: 10.1186/s12916-015-0373-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ding SL, Yu JC, Chen ST, Hsu GC, Hsu HM, Ho JY, et al. Diverse associations between ESR1 polymorphism and breast cancer development and progression. Clin Cancer Res. 2010;16:3473–84. doi: 10.1158/1078-0432.CCR-09-3092. [DOI] [PubMed] [Google Scholar]
- 20.Zheng W, Long J, Gao YT, Li C, Zheng Y, Xiang YB, et al. Genome-wide association study identifies a new breast cancer susceptibility locus at 6q25.1. Nat Genet. 2009;41:324–8. doi: 10.1038/ng.318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Herrington DM, Howard TD, Brosnihan KB, McDonnell DP, Li X, Hawkins GA, et al. Common estrogen receptor polymorphism augments effects of hormone replacement therapy on E-selectin but not C-reactive protein. Circulation. 2002;105:1879–82. doi: 10.1161/01.CIR.0000016173.98826.88. [DOI] [PubMed] [Google Scholar]
- 22.Onland-Moret NC, van Gils CH, Roest M, Grobbee DE, Peeters PH. The estrogen receptor alpha gene and breast cancer risk (The Netherlands) Cancer Causes Control. 2005;16:1195–202. doi: 10.1007/s10552-005-0307-5. [DOI] [PubMed] [Google Scholar]
- 23.Li LW, Xu L. Menopausal status modifies breast cancer risk associated with ESR1 PvuII and XbaI polymorphisms in Asian women: a HuGE review and meta-analysis. Asian Pac J Cancer Prev. 2012;13:5105–11. doi: 10.7314/APJCP.2012.13.10.5105. [DOI] [PubMed] [Google Scholar]
- 24.Zhang Y, Zhang M, Yuan X, Zhang Z, Zhang P, Chao H, et al. Association between ESR1 PvuII, XbaI, and P325P polymorphisms and breast cancer susceptibility: a meta-analysis. Med Sci Monit. 2015;21:2986–96. doi: 10.12659/MSM.894010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ioannidis JP, Ralston SH, Bennett ST, Brandi ML, Grinberg D, Karassa FB, et al. Differential genetic effects of ESR1 gene polymorphisms on osteoporosis outcomes. JAMA. 2004;292:2105–14. doi: 10.1001/jama.292.17.2105. [DOI] [PubMed] [Google Scholar]
- 26.de Mattos CS, Trevisan CM, Peluso C, Adami F, Cordts EB, Christofolini DM, et al. ESR1 and ESR2 gene polymorphisms are associated with human reproduction outcomes in Brazilian women. J Ovarian Res. 2014;7:114. doi: 10.1186/s13048-014-0114-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.L'Esperance S, Frenette S, Dionne A, Dionne JY. Pharmacological and non-hormonal treatment of hot flashes in breast cancer survivors: CEPO review and recommendations. Support Care Cancer. 2013;21:1461–74. doi: 10.1007/s00520-013-1732-8. [DOI] [PubMed] [Google Scholar]
- 28.Hope ACBA. Simplified Monte Carlo significance test procedure. J Roy Stat Soc B. 1968;30:582–98. [Google Scholar]
- 29.McShane LM, Altman DG, Sauerbrei W, Taube SE, Gion M, Clark GM. Reporting recommendations for tumor marker prognostic studies. J Clin Oncol. 2005;23:9067–72. doi: 10.1200/JCO.2004.01.0454. [DOI] [PubMed] [Google Scholar]
- 30.Fontein DB, Houtsma D, Nortier JW, Baak-Pablo RF, Kranenbarg EM, van der Straaten TR, et al. Germline variants in the CYP19A1 gene are related to specific adverse events in aromatase inhibitor users: a substudy of Dutch patients in the TEAM trial. Breast Cancer Res Treat. 2014;144:599–606. doi: 10.1007/s10549-014-2873-2. [DOI] [PubMed] [Google Scholar]
- 31.Simon RM, Paik S, Hayes DF. Use of archived specimens in evaluation of prognostic and predictive biomarkers. J Natl Cancer Inst. 2009;101:1446–52. doi: 10.1093/jnci/djp335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Henry NL, Skaar TC, Dantzer J, Li L, Kidwell K, Gersch C, et al. Genetic associations with toxicity-related discontinuation of aromatase inhibitor therapy for breast cancer. Breast Cancer Res Treat. 2013;138:807–16. doi: 10.1007/s10549-013-2504-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lunardi G, Piccioli P, Bruzzi P, Notaro R, Lastraioli S, Serra M, et al. Plasma estrone sulfate concentrations and genetic variation at the CYP19A1 locus in postmenopausal women with early breast cancer treated with letrozole. Breast Cancer Res Treat. 2013;137:167–74. doi: 10.1007/s10549-012-2306-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mao JJ, Su HI, Feng R, Donelson ML, Aplenc R, Rebbeck TR, et al. Association of functional polymorphisms in CYP19A1 with aromatase inhibitor associated arthralgia in breast cancer survivors. Breast Cancer Res. 2011;13:R8. doi: 10.1186/bcr2813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang J, Lu K, Song Y, Xie L, Zhao S, Wang Y, et al. Indications of clinical and genetic predictors for aromatase inhibitors related musculoskeletal adverse events in Chinese Han women with breast cancer. PLoS ONE. 2013;8:e68798. doi: 10.1371/journal.pone.0068798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.van Duijnhoven FJ, Peeters PH, Warren RM, Bingham SA, Uitterlinden AG, Van Noord PA, et al. Influence of estrogen receptor alpha and progesterone receptor polymorphisms on the effects of hormone therapy on mammographic density. Cancer Epidemiol Biomarkers Prev. 2006;15:462–7. doi: 10.1158/1055-9965.EPI-05-0754. [DOI] [PubMed] [Google Scholar]
- 37.Henry NL, Azzouz F, Desta Z, Li L, Nguyen AT, Lemler S, et al. Predictors of aromatase inhibitor discontinuation as a result of treatment-emergent symptoms in early-stage breast cancer. J Clin Oncol. 2012;30:936–42. doi: 10.1200/JCO.2011.38.0261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fontein DB, Seynaeve C, Hadji P, Hille ET, van de Water W, Putter H, et al. Specific adverse events predict survival benefit in patients treated with tamoxifen or aromatase inhibitors: an international tamoxifen exemestane adjuvant multinational trial analysis. J Clin Oncol. 2013;31:2257–64. doi: 10.1200/JCO.2012.45.3068. [DOI] [PubMed] [Google Scholar]
- 39.Mortimer JE, Flatt SW, Parker BA, Gold EB, Wasserman L, Natarajan L, et al. Tamoxifen, hot flashes and recurrence in breast cancer. Breast Cancer Res Treat. 2008;108:421–6. doi: 10.1007/s10549-007-9612-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cuzick J, Sestak I, Cella D, Fallowfield L. Treatment-emergent endocrine symptoms and the risk of breast cancer recurrence: a retrospective analysis of the ATAC trial. Lancet Oncol. 2008;9:1143–8. doi: 10.1016/S1470-2045(08)70259-6. [DOI] [PubMed] [Google Scholar]
- 41.Huober J, Cole BF, Rabaglio M, Giobbie-Hurder A, Wu J, Ejlertsen B, et al. Symptoms of endocrine treatment and outcome in the BIG 1–98 study. Breast Cancer Res Treat. 2014;143:159–69. doi: 10.1007/s10549-013-2792-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Stearns V, Chapman JA, Ma CX, Ellis MJ, Ingle JN, Pritchard KI, et al. Treatment-associated musculoskeletal and vasomotor symptoms and relapse-free survival in the NCIC CTG MA.27 adjuvant breast cancer aromatase inhibitor trial. J Clin Oncol. 2015;33:265–71. doi: 10.1200/JCO.2014.57.6926. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The authors are not sharing the data in this article because the primary results of the main trial, TEXT, have not been shared in a public venue. The International Breast Cancer Study Group has data-sharing policies; please contact the corresponding author for details.


