Despite the important role of cell culture in the study of biology and medicine, evidence has accumulated that cell lines are frequently misidentified or contaminated by other cells or microorganisms. This can be a substantial problem in many fields, such as cancer research, where drugs are initially tested using a cell line derived from the targeted type of tumor (1). If a drug is tested on the wrong cell line, research can lead to unreliable results, and discovery of effective treatments can be delayed. Even in basic research, use of mistaken cell lines can hinder progress because of variations in cell behavior among different cell types. Given these concerns, developing corrective measures for cell line misidentification and contamination warrants renewed attention.
Since the 1960s, more than 400 widely used cell lines worldwide have been shown to have been misidentified (2, 3). Cells originally thought to have been derived from one tissue type have later been found to be from a different tissue. In some cases, even the species of the cells has been misidentified. A 2011 study of 122 different head and neck cancer cell lines revealed that 37 (30%) were misidentified (4). Analyses of a variety of tissue culture collections and cells sent to repositories for curation and storage from labs in the United States, Europe, and Asia suggest that at least 15% of cell lines are misidentified or contaminated (4, 5).
Misidentified cell lines can create problems at many levels of biomedical research. For example, studies using just two misidentified cell lines were included in three grants funded by the U.S. National Institutes of Health (NIH), two clinical trials, 11 patents, and >100 papers (6). Nonetheless, the need for validation and accurate reporting of cell line identity does not appear to be widely recognized by researchers; a 2013 study found that fewer than half of cell lines were unambiguously identified in published studies (7).
A number of factors contribute to the problems of cell line misidentification and contamination. For example, inadvertently using a pipette more than once when working with different cell lines in culture can lead to cross contamination. If the contaminating cell line divides more rapidly than the original cells, it can quickly dominate the population, changing the identity of the culture. This event often goes undetected because cells from different sources can be morphologically similar (see the photo). Cultured cells can also become contaminated with mycoplasma, viruses, or other microbes, which can alter the cells’ behavior. Recent analyses suggest that 5 to 10% of cell culture studies have used cells contaminated with mycoplasma (8, 9). Cell lines in culture can also change over time without any external contamination. As they grow in the lab generation after generation, cells can undergo chromosomal duplications or rearrangements, mutations, and epigenetic changes that alter their phenotypes. Given these factors, in the absence of preventative steps, cell line alteration is inevitable and potentially problematic.
Concerned scientists and research organizations have made efforts to deal with this problem. In 2007, the NIH issued a Guide Notice 10) drawing attention to the misidentification of cell lines and calling on the reviewers of grants and manuscripts to pay special attention to this issue. Despite these efforts, the problem appears to have persisted. The time and cost required for authenticating cell lines likely has been a barrier to widespread adoption of best practices. Shortcomings in training also play a role, as do sociological issues. For example, it is hard for both individual scientists and their fields to accept that their work could be called into question because the wrong cell line was used. For some researchers, the problem may even seem insignificant in the context of their specific research focus; after all, all animal cells have microtubules, motor proteins, and ribosomes, which may lead some scientists to feel that the particular cell type they are studying is irrelevant. These issues may help explain why, even after the misidentification of a cell line has been made public, researchers often continue to publish work attributing the line to its misidentified source [reviewed in (11)].
So what more can we do to address this problem? We believe the biomedical research community—including funding agencies, scientific societies, journals, reagent suppliers, and investigators themselves—needs to give greater focus to the problem of cell line misidentification and must work together to develop new ways to address the complex issues associated with it. A multipronged strategy that combines additional research, alteration of practices, improved training, and investment in the development of new technologies will be necessary.
Some research institutes are urging all their scientists to fingerprint new cell lines as soon as they arrive in the lab and periodically thereafter. Fortunately, the cost of validating cell lines is falling thanks to improvements in testing techniques. One method, using short tandem repeat (STR) analysis to identify DNA sequences unique to a cell line, is now widely available. This approach is inexpensive and rapid, and there are online databases that allow STR fingerprints to be compared to verify cell line identity. For example, ATCC has an STR database of all of its human cell lines. Although it can authenticate commonly used human cell lines, STR cannot distinguish many lines from other species, and it lacks the resolution needed to identify most genetic changes. It is also a technique that is usually done in core facilities rather than routinely by individual researchers, which presents a barrier to frequent use. Thus, we still need technological improvements to address these problems.
Similar cells, different species.
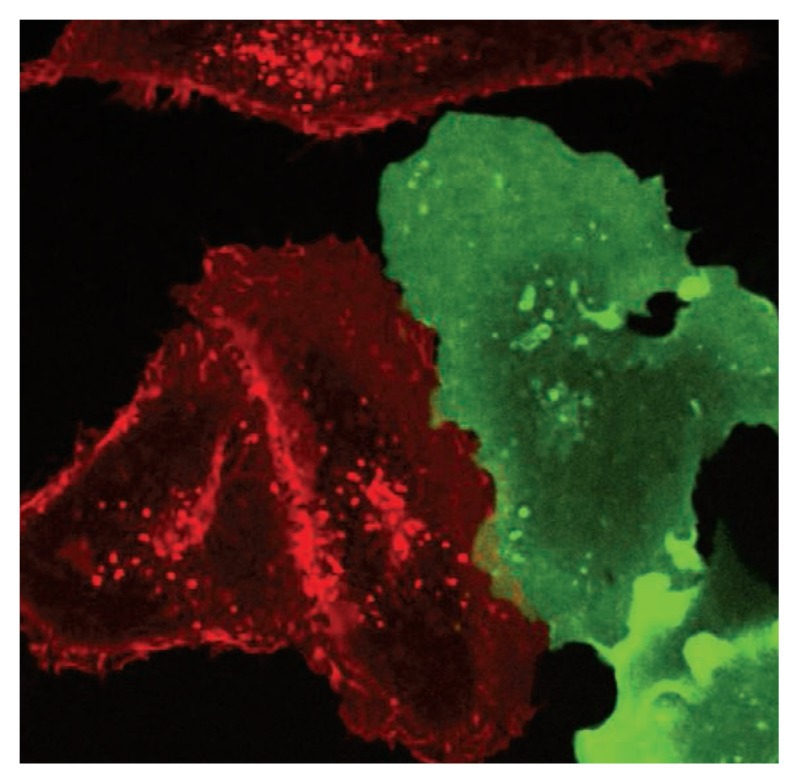
Monkey cells (red) and human cells (green) growing together in culture.
For its part, the NIH is considering several approaches to help catalyze improvements in identifying cell lines and maintaining their integrity. First, grant applicants may be required to provide information on how they intend to address concerns about the identity of their cell lines, the composition of their key reagents, and contamination of their cells, similar to the model organism—sharing plans that are already included in NIH grant applications. Standards and suggested best practices will be developed with the help of academic and industrial researchers, the professional societies, and other organizations and government agencies, such as the American Society for Cell Biology, the Global Biological Standards Institute, and the U.S. National Institute of Standards and Technology.
The NIH is also considering investing in development of improved technologies for cell culture studies, including faster, cheaper, and easier methods for the validation of cell lines and inexpensive, defined, and controllable media for cell growth. These are areas where reagent suppliers and equipment manufacturers will also need to play a role. In addition, the NIH is exploring funding studies to determine the extent to which variables such as cell type and genetic drift affect the reproducibility and generalizability of biomedical research results. Recently, several components of the NIH launched an initiative to help universities and other organizations enhance training in good laboratory practices (12), an effort in which the professional societies could also be instrumental. Given the global nature of the cell line authentication problem, the NIH will engage additional funding agencies in the United States and around the world to develop concerted approaches to address these and other problems related to reproducibility in cell culture studies and overall rigor of experimental design in life sciences research (13).
The journals and their reviewers also have an important role to play; they can ensure that authors include in published manuscripts data on cell line quality and identity, as well as details about key reagents used in their studies. Some journals have already adopted guidelines and checklists to help make sure these goals are achieved (14), and we urge widespread adoption of these standards. Of course, the authors themselves are ultimately responsible for authenticating their cell lines as rigorously and carefully as possible and for training the next generation of scientists to do the same.
If all of these groups work together, we are confident that the reproducibility and rigor of cell culture studies will improve.
REFERENCES
- 1.Weinstein JN. Nature. 2012;483:544. doi: 10.1038/483544a. [DOI] [PubMed] [Google Scholar]
- 2.Capes-Davis A, et al. Int J Cancer. 2010;127:1. doi: 10.1002/ijc.25242. [DOI] [PubMed] [Google Scholar]
- 3.ATCC; The role of ICLAC. International Cell Line Authentication Committee; http://standards.atcc.org/kwspub/home/the_international_cell_line_authentication_committee-iclac_/ [Google Scholar]
- 4.Zhao M, et al. Clin Cancer Res. 2011;17:7248. doi: 10.1158/1078-0432.CCR-11-0690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.MacLeod RAF, Drexler HG. Nature. 2006;439:912. doi: 10.1038/439912b. [DOI] [PubMed] [Google Scholar]
- 6.Boonstra JJ, et al. J Natl Cancer Inst. 2010;102:271. doi: 10.1093/jnci/djp499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vasilevsky NA, et al. Peer J. 2013;1:e148. doi: 10.7717/peerj.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Armstrong SE, Mariano JA, Lundin DJ. Biologicals. 2010;38:211. doi: 10.1016/j.biologicals.2010.03.002. [DOI] [PubMed] [Google Scholar]
- 9.Olarerin-George AO, Hogenesch JB. bioRXiv. 2014. http://biorxiv.org/content/early/2014/07/11/007054.
- 10.NIH Guide Notice, NOT-OD-08-017. Nov 28, 2007. http://grants.nih.gov/grants/guide/notice-files/NOT-OD-08-017.html.
- 11.American Type Culture Collection Standards Development Organization Workgroup ASN-0002. Nat Rev Cancer. 2010;10:441. doi: 10.1038/nrc2852. [DOI] [PubMed] [Google Scholar]
- 12.NIH Guide Notice, RFA-GM-15-006. Aug 28, 2014. http://grants.nih.gov/grants/guide/rfa-files/RFA-GM-15-006.html.
- 13.Collins FS, Tabak LA. Nature. 2014;505:612. doi: 10.1038/505612a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McNutt M. Science. 2014;346:679. doi: 10.1126/science.aaa1724. [DOI] [PubMed] [Google Scholar]



