Abstract
β-crystallin B2 (CRYBB2) knockout mice exhibit morphological and functional abnormalities in the ovary. Long non-coding RNAs (lncRNAs) regulate gene transcription and translation, and epigenetic modification of genomic DNA. The present study investigated the role of lncRNAs in mediating the effects of CRYBB2 in the regulation of ovary development in mice. In the current study, ovary tissues from wild-type (WT) and CRYBB2 knockout mice were subjected to lncRNA and mRNA microarray profiling. Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) analyses were performed to group the differentially expressed lncRNAs into regulated gene pathways and functions. The correlation matrix method was used to establish a network of lncRNA and mRNA co-expression. Quantitative reverse transcription-polymerase chain reaction (RT-qPCR) was used to verify expression of a number of these differentially expressed lncRNAs and mRNAs. There were 157 differentially expressed lncRNAs and 1,085 differentially expressed mRNAs between ovary tissues from WT and CRYBB2 knockout mice. The GO and KEGG analyses indicated that these differentially expressed lncRNAs and mRNAs were important in Ca2+ signaling and ligand and receptor interactions. The correlation matrix method established an lncRNA and mRNA co-expression network, consisting of 53 lncRNAs and 45 mRNAs with 98 nodes and 75 connections. RT-qPCR confirmed downregulation of lncRNA A-30-P01019163 expression, which further downregulated its downstream gene purinergic receptor P2X, ligand-gated ion channel, 7 (P2rx7) expression in ovary tissues from CRYBB2 knockout mice. In conclusion, CRYBB2 regulates expression of different lncRNAs to influence ovary development. lncRNA A-30-P01019163 may affect ovarian cell cycle and proliferation by regulating P2rx7 expression in the ovary.
Keywords: β-crystallin B2, long non-coding RNAs, ovary development, signal transduction
Introduction
β-crystallin B2 (CRYBB2) expression was initially reported in the eye lens where it functions to maintain lens transparency and refractive index. CRYBB2 deficiency was demonstrated to result in the generation of age-associated cataracts (1). However, a number of previous studies demonstrated that CRYBB2 is also expressed in the retina, brain, testis, and ovary (2–5). Our previous study observed that CRYBB2 was expressed in human and mouse ovaries, particularly in ovarian granulosa cells (6). CRYBB2 knockout mice exhibited morphological and functional abnormalities in the ovary, including reduced ovarian index (ratio of ovary weight to total body weight) with increased follicle atresia, reduced mature follicles and dysregulated estrous cycle (7). Our data from a previous study also indicated a high level of estrogen in the diestrus and metestrus, but a low level of progesterone in the metestrus compared with wild-type (WT) mice (6). At the genetic level, expression of cell cycle and apoptosis-associated proteins, including B-cell lymphoma 2, cyclin-dependent kinase 4, and cyclin D2, were markedly lower in the CRYBB2 knockout mice compared with WT mice (6). These data suggest that CRYBB2 may be important in ovary development, however, the underlying molecular mechanism by which CRYBB2 regulates ovarian development remains to be elucidated.
To identify and assess the role of CRYBB2 in ovary development, differentially expressed long non-coding RNAs (lncRNAs) were profiled in CRYBB2 knockout mice. lncRNAs are a class of non-coding RNAs with nucleotides >200 bp and transcribed by RNA polymerase II. Functionally, lncRNAs may regulate gene transcription, protein translation, and epigenetic modification of genomic DNA. Altered expression and regulation of lncRNAs has been associated with human diseases and aging (8–12). For example, previous studies have suggested that overexpression of lncRNA HOX transcript antisense RNA associated with the recurrence of hepatocellular carcinoma, poor prognosis in colorectal cancer, and malignant behaviors of gastrointestinal stromal tumors (13–15). lncRNAs modulate cell functions by regulating expression of targeted downstream genes (16), which may in turn affect embryo development (17), inactivate the X chromosome, and regulate genomic imprinting (18). Thus, the current study assessed whether these lncRNAs mediate the functions of CRYBB2 in ovary development and investigated the underlying mechanisms. Microarray profiling between ovarian tissues from CRYBB2 knockout and WT mice was conducted and bioinformatic analysis of differentially expressed lncRNAs and mRNAs was performed. A number of these differentially expressed lncRNAs and mRNAs were verified using quantitative reverse transcription-polymerase chain reaction (RT-qPCR).
Materials and methods
Animals
Male and female C57BL/6 mice were obtained from the Experimental Animal Center of the Second Military Medical University (Shanghai, China). CRYBB2 knockout mice were generated by the Ingenious Targeting Laboratory (Ronkonkoma, NY, USA), as described previously (19). All mice were maintained on a 12 h light/dark cycle with a temperature of 21±1°C and humidity of 50~70% in a pathogen-free facility with access to food and water ad libitum. Bedding material and a plastic house or tube was placed in the cage for environmental enrichment. Daily examinations were performed on all animals throughout the experimental period. Humane euthanasia of mice was performed under isoflurane anesthesia using intracardiac injection of pentobarbitone (150 mg/kg). The present study was conducted in accordance with institutional guidelines and approved by the Animal Care and Use Committee of Changhai Hospital (Shanghai, China).
A total of three 8-9-week-old (weight, 18.0±2.0 g) female CRYBB2 knockout mice and three age-matched WT female mice were obtained. Ovary tissues were collected from mice following a 10-day experimental period.
RNA isolation, cDNA synthesis and labeling, and hybridization
Ovarian tissues were homogenized on ice and total cellular RNA was isolated using TRIzol reagent (Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA, USA) according to the manufacturer's protocol and then quantified using NanoDrop ND-1000 (Thermo Fisher Scientific, Inc., Wilmington, DE, USA) and agarose gel electrophoresis. RNA samples were further purified using an RNeasy Mini kit (Qiagen, Inc., Valencia, CA, USA) and reverse transcribed into cDNA with fluorescent labeling for microarray hybridization using using the AffinityScript QPCR cDNA Synthesis kit (Agilent Technologies, Inc., Santa Clara, CA, USA). These labeled cDNA probes were then hybridized to Agilent mouse expression profiling (8×60K) microarray using the Gene Expression Hybridization kit (Agilent Technologies, Inc.) according to the manufacturer's protocol. The arrays were scanned into a file and analyzed using Feature Extraction software, version v10.7.3.1 (Agilent Technologies, Inc.). The arrays were scanned at 5 µm/pixel resolution using an Axon GenePix 4000B scanner (Molecular Devices, LLC, Sunnyvale, CA, USA) piloted by GenePix Pro 6.0 software (Molecular Devices, LLC) and then imported into NimbleScan software (version 2.5; Roche NimbleGen, Inc., Madison, WI, USA) for grid alignment and expression data analysis. Expression data were normalized using quantile normalization and the Robust Multichip Average algorithm included in the NimbleScan software. The probe level files and mRNA level files were generated following normalization. All mRNA level files were imported into Agilent GeneSpringGX software (version 11.0; Agilent Technologies, Inc.) for further analysis. Differentially expressed lncRNAs and mRNAs were identified via fold change filtering.
Functional analysis of microarray data
Gene Ontology (GO; www.geneontology.org) was performed to group differentially expressed lncRNAs and their targeted genes into biological processes, cellular components, and molecular features of the biological functions. Kyoto Encyclopedia of Genes and Genomes (KEGG; www.genome.jp/kegg) analysis was performed to identify roles of the target genes and group them into gene pathways. The correlation matrix method was used to establish a diagram of lncRNA and mRNA co-expression regulatory networks. Prior to functional analyses, the predicted potential lncRNA targets were integrated with the differentially expressed mRNAs using a cut-off value of ≥2.0 fold change or P<0.05.
RT-qPCR
Total RNA was isolated from granulosa cells from the ovaries of WT and CRYBB2 knockout mice using TRIzol reagent and reverse transcribed into cDNA using the PrimeScript RT Reagent Kit (Takara Biotechnology, Co., Ltd., Dalian, China) according to the manufacturer's protocols. These cDNA samples were then subjected to qPCR analysis using a Applied Biosystems 7300 Real-Time PCR system (Applied Biosystems; Thermo Fisher Scientific, Inc.) with the SYBR Green PCR Master Mix kit (Takara Biotechnology, Co., Ltd.). qPCR amplification was conducted at 95°C for 2 min, followed by 40 cycles of 95°C for 15 sec, and 60°C for 30 sec. The relative expression of each target gene compared to β-actin was calculated using the 2−ΔΔCq method (20). Specific primers used were as follows: Forward, 5′-AGCCATGTACGTAGCCATCC-3′ and reverse, 5′-CTCTCAGCTGTGGTGGTGAA-3′ for β-actin; forward, 5′-CGAGTTGGTGCCAGTGTGGA-3′ and reverse, 5′-CCTGCTGTTGGTGGCCTCTT-3′ for P2rx7; and forward, 5′-TCCACTCAGGAAGAGCTGGT-3′ and reverse, 5′-TAGCACCCTCGGGATATCTG-3′ for lncRNAA-30-P01019163.
Statistical analysis
All data were presented as the mean ± standard deviation. The data were Log2-transformed and median centered by genes using Cluster software, version 3.0 (http://bonsai.hgc.jp/~mdehoon/software/cluster/software.htm). Statistical analyses were performed using SPSS 17.0 (SPSS, Inc., Chicago, IL, USA). The data were statistically analyzed using an unpaired Student's t-test or by one-way analysis of variance followed by Tukey's test. P<0.05 was considered to indicate a statistically significant difference.
Results
Microarray profiling of differentially expressed lncRNAs and mRNA in ovary tissues from wild type and CRYBB2 knockout mice
Microarray data on differential lncRNA and mRNA expression levels were analyzed using Cluster software, version 3.0 with the cut-off values set as ≥2-fold difference or P<0.05. The data indicated 157 differentially expressed lncRNAs (42 downregulated vs. 115 upregulated) and 1,085 differentially expressed mRNAs (570 downregulated vs. 515 upregulated; Fig. 1). The genes were selected according to a software forecast associated with the processes of ovarian development, and the lncRNAs associated with these genes were selected (Tables I and II).
Figure 1.
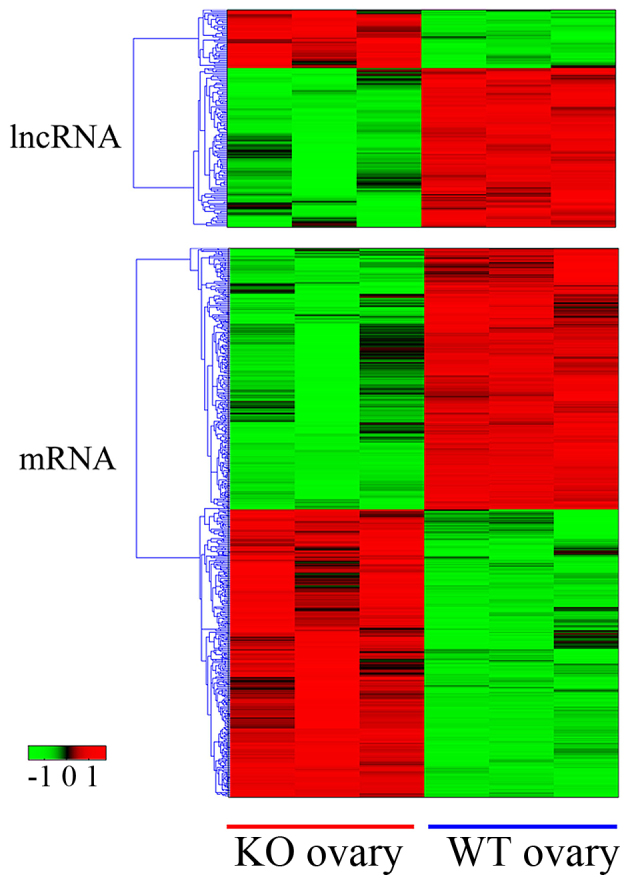
Cluster analysis of differentially expressed lncRNAs and mRNAs in ovary tissues from WT and β-crystallin B2 knockout mice. The horizontal axis represents the sample group and the vertical axis represents the differentially expressed mRNAs or lncRNAs. Upregulated genes are red and downregulated genes are green. Included are 157 lncRNAs (42 downregulated vs. 115 upregulated) and 1,085 mRNAs (570 downregulated vs. 515 upregulated) KO, knockout; WT, wild-type; lncRNA, long non-coding RNA.
Table I.
Differentially expressed long non-coding RNAs in ovary tissues from β-crystallin B2 knockout mice.
| Probe name | P-value | FC (abs) | Regulation |
|---|---|---|---|
| A_30_P01032506 | 0.001900622 | 2.244835 | Down |
| A_30_P01029732 | 0.026376737 | 2.3413253 | Down |
| A_30_P01020038 | 0.020382054 | 2.3550973 | Down |
| A_30_P01019163 | 0.008867065 | 2.3823233 | Down |
| A_30_P01017808 | 0.003141625 | 2.3902857 | Down |
| A_30_P01028465 | 0.026580842 | 2.3909771 | Down |
| A_30_P01024278 | 0.010205811 | 2.4727218 | Down |
| A_30_P01031572 | 0.036648612 | 2.5956807 | Down |
| A_30_P01027590 | 0.034441914 | 2.6343288 | Down |
| A_30_P01022135 | 0.04770927 | 3.120179 | Down |
| A_30_P01031631 | 0.001265911 | 3.135137 | Down |
| A_30_P01032372 | 0.009654216 | 3.4001627 | Down |
| A_30_P01032133 | 0.012271924 | 3.434039 | Down |
| A_30_P01024342 | 2.97×10−4 | 3.7296832 | Down |
| A_30_P01024108 | 0.004697592 | 3.8198798 | Down |
| A_30_P01022445 | 2.66×10−5 | 3.8623033 | Down |
| A_30_P01022656 | 2.08×10−4 | 4.069888 | Down |
| A_30_P01023636 | 0.02164327 | 4.4273615 | Down |
| A_30_P01022538 | 9.77×10−4 | 5.492365 | Down |
| A_30_P01033546 | 0.004913332 | 5.9940124 | Down |
| A_30_P01019023 | 0.024659809 | 3.7894382 | Up |
| A_30_P01024108 | 0.004697592 | 3.8198798 | Up |
| A_30_P01017880 | 0.034943227 | 3.8256395 | Up |
| A_30_P01022445 | 2.66×10−5 | 3.8623033 | Up |
| A_30_P01026472 | 0.022304738 | 3.8728366 | Up |
| A_30_P01030900 | 0.023211088 | 3.9997435 | Up |
| A_30_P01022656 | 2.08×10−4 | 4.069888 | Up |
| A_30_P01018745 | 1.84×10−5 | 4.266393 | Up |
| A_30_P01033353 | 0.03583063 | 4.398616 | Up |
| A_30_P01021636 | 0.01934708 | 4.4207296 | Up |
| A_30_P01023636 | 0.02164327 | 4.4273615 | Up |
| A_30_P01022538 | 9.77×10−4 | 5.492365 | Up |
| A_30_P01019825 | 0.006051722 | 5.5902176 | Up |
| A_30_P01033546 | 0.004913332 | 5.9940124 | Up |
| A_30_P01024788 | 1.11×10−5 | 7.118162 | Up |
| A_30_P01024270 | 6.38×10−5 | 8.226261 | Up |
| A_30_P01027087 | 0.003292078 | 8.981714 | Up |
| A_30_P01031162 | 0.011685452 | 9.579193 | Up |
| A_30_P01021698 | 1.31×10−5 | 10.52238 | Up |
| A_30_P01020936 | 4.52×10−4 | 22.788074 | Up |
FC, fold change.
Table II.
Differentially expressed mRNAs in ovary tissues from β-crystallin B2 knockout mice.
| Gene | P-value | FC (abs) | Regulation |
|---|---|---|---|
| Prkar2b | 0.040216 | 2.1857524 | Down |
| Lrp11 | 0.006975 | 2.9040618 | Down |
| P2rx7 | 4.11×10−4 | 4.6213336 | Down |
| Calml3 | 0.011701 | 5.126569 | Down |
| Dclre1c | 3.39×10−4 | 5.1453366 | Down |
| Lpcat2 | 0.002695 | 5.828531 | Down |
| Stk32a | 0.001242 | 5.833555 | Down |
| Cyp19a1 | 0.034041 | 6.1799 | Down |
| Megf10 | 0.045621 | 6.1827664 | Down |
| Plcxd1 | 1.04×10−4 | 6.82413 | Down |
| Gm5103 | 0.005622 | 8.389297 | Down |
| Fermt1 | 0.001363 | 8.656997 | Down |
| Mlxip | 3.10×10−4 | 8.746344 | Down |
| Slc6a2 | 0.046581 | 8.750824 | Down |
| Gm7969 | 0.012795 | 9.970471 | Down |
| Ces2a | 0.028129 | 10.472376 | Down |
| Dclre1c | 1.23×10−4 | 10.645219 | Down |
| Ostn | 9.07×10−6 | 11.019576 | Down |
| Jph4 | 0.001674 | 12.722166 | Down |
| Onecut2 | 0.048542 | 13.894124 | Down |
| Ces2a | 0.028128909 | 10.472376 | Up |
| Dclre1c | 1.23×10−4 | 10.645219 | Up |
| Jph4 | 0.001673621 | 12.722166 | Up |
| Onecut2 | 0.04854157 | 13.894124 | Up |
| Itln1 | 5.97×10−5 | 14.339393 | Up |
| Arsk | 7.13×10−5 | 16.06716 | Up |
| Sfrp4 | 0.023781205 | 17.065975 | Up |
| Plekhg4 | 0.047409292 | 17.067673 | Up |
| Nuf2 | 6.05×10−5 | 17.872303 | Up |
| Adh7 | 0.0064019 | 18.950857 | Up |
| Itln1 | 4.63×10−5 | 19.05549 | Up |
| Ptgfr | 0.032550577 | 20.059986 | Up |
| Pou6f1 | 1.71×10−4 | 23.766891 | Up |
| Saa2 | 0.023944996 | 25.063555 | Up |
| Wnt10b | 0.02046059 | 27.648937 | Up |
| Ifi202b | 0.001051159 | 28.72067 | Up |
| Dcpp1 | 0.014649089 | 40.37458 | Up |
| Cd5l | 4.12×10−4 | 42.74245 | Up |
| Dcpp2 | 0.012848383 | 45.13117 | Up |
FC, fold change.
Bioinformatic analysis of differentially expressed lncRNAs and mRNA in ovary tissues from WT and CRYBB2 knockout mice
GO analysis was performed for functional annotation of differentially expressed lncRNAs and mRNAs and it was observed that they were predominantly involved in cell cycle regulation, cell proliferation, metabolism, and signal transduction (Table III). One particular gene, P2rx7, localized in the cytoplasm, functions to regulate cell cycle progression and proliferation, and the signal transduction process.
Table III.
Gene ontology analysis of differentially expressed genes.
| Term | Number of genes (%) | P-value |
|---|---|---|
| Biological process | ||
| Cell cycle and proliferation | 3 (4) | 0.479979 |
| Stress response | 4 (6) | 0.205102 |
| Transport | 5 (7) | 0.563981 |
| Developmental processes | 6 (9) | 0.459856 |
| RNA metabolism | 7 (10) | 0.315857 |
| Other metabolic processes | 12 (17) | 0.007676 |
| Cell organization and biogenesis | 6 (9) | 0.193590 |
| Cell-cell signaling | 1 (1) | 0.502761 |
| Signal transduction | 11 (16) | 0.120754 |
| Protein metabolism | 6 (9) | 0.502818 |
| Death | 1 (1) | 0.827556 |
| Other biological processes | 8 (11) | 0.990425 |
| Cellular component | ||
| Cytosol | 1 (2) | 0.505969 |
| Mitochondrion | 1 (2) | 0.862146 |
| Endoplasmic reticulum/golgi | 3 (5) | 0.460760 |
| Other cytoplasmic organelle | 1 (2) | 0.604395 |
| Nucleus | 10 (18) | 0.306385 |
| Plasma membrane | 6 (1) | 0.438812 |
| Other membranes | 14 (26) | 0.661329 |
| Translational apparatus | 1 (2) | 0.330956 |
| Non-structural extracellular | 2 (4) | 0.830334 |
| Other cellular component | 15 (28) | 0.354726 |
| Molecular function | ||
| Enzyme regulator activity | 3 (5) | 0.141417 |
| Transcription regulatory activity | 4 (7) | 0.174629 |
| Transporter activity | 2 (4) | 0.623057 |
| Signal transduction activity | 11 (20) | 0.082302 |
| Nucleic acid binding activity | 7 (13) | 0.252320 |
| Kinase activity | 2 (4) | 0.503159 |
| Other molecular function | 26 (47) | 0.535279 |
Subsequently, KEGG analysis was conducted to group differentially expressed lncRNAs and mRNA into gene pathways. The data from the present study indicated that the genes predominantly formed signaling pathways associated with the regulation of Ca2+ signaling, ligand and receptor interactions, and other cell signaling pathways (Table IV). For example, P2rx7 was identified to be predominantly involved in Ca2 + signaling.
Table IV.
KEGG pathways analysis of differentially expressed genes.
| Term | Count | P-value | Genes | Up | Down |
|---|---|---|---|---|---|
| Glutathione metabolism | 1 | 0.106032 | Gsta2 | 0 | 1 |
| Metabolism of xenobiotics by cytochrome P450 | 1 | 0.143567 | Gsta2 | 0 | 1 |
| Drug metabolism-cytochrome P450 | 1 | 0.147434 | Gsta2 | 0 | 1 |
| Ribosome | 1 | 0.172182 | Rps3a | 0 | 1 |
| PPAR signaling pathway | 1 | 0.143567 | Adipoq | 0 | 1 |
| Calcium signaling pathway | 3 | 0.00614 | P2rx7, Ptger1, Ptafr | 3 | 0 |
| Cytokine-cytokine receptor interaction | 1 | 0.45143 | Cxcl1 | 0 | 1 |
| Chemokine signaling pathway | 1 | 0.353888 | Cxcl1 | 0 | 1 |
| Neuroactive ligand-receptor interaction | 4 | 0.001745 | P2rx7, Ptger1, Ptafr, Vipr1 | 4 | 0 |
| Cell cycle | 1 | 0.226819 | Hdac1 | 0 | 1 |
| Apoptosis | 1 | 0.179664 | Prkar2b | 1 | 0 |
| Notch signaling pathway | 1 | 0.099971 | Hdac1 | 0 | 1 |
| Insulin signaling pathway | 1 | 0.268146 | Prkar2b | 1 | 0 |
| Adipocytokine signaling pathway | 1 | 0.139685 | Adipoq | 0 | 1 |
| Type II diabetes mellitus | 1 | 0.099971 | Adipoq | 0 | 1 |
| Huntington's disease | 1 | 0.334126 | Hdac1 | 0 | 1 |
| Pathways in cancer | 1 | 0.530933 | Hdac1 | 0 | 1 |
| Chronic myeloid leukemia | 1 | 0.155119 | Hdac1 | 0 | 1 |
Building the lncRNA-mRNA regulatory network
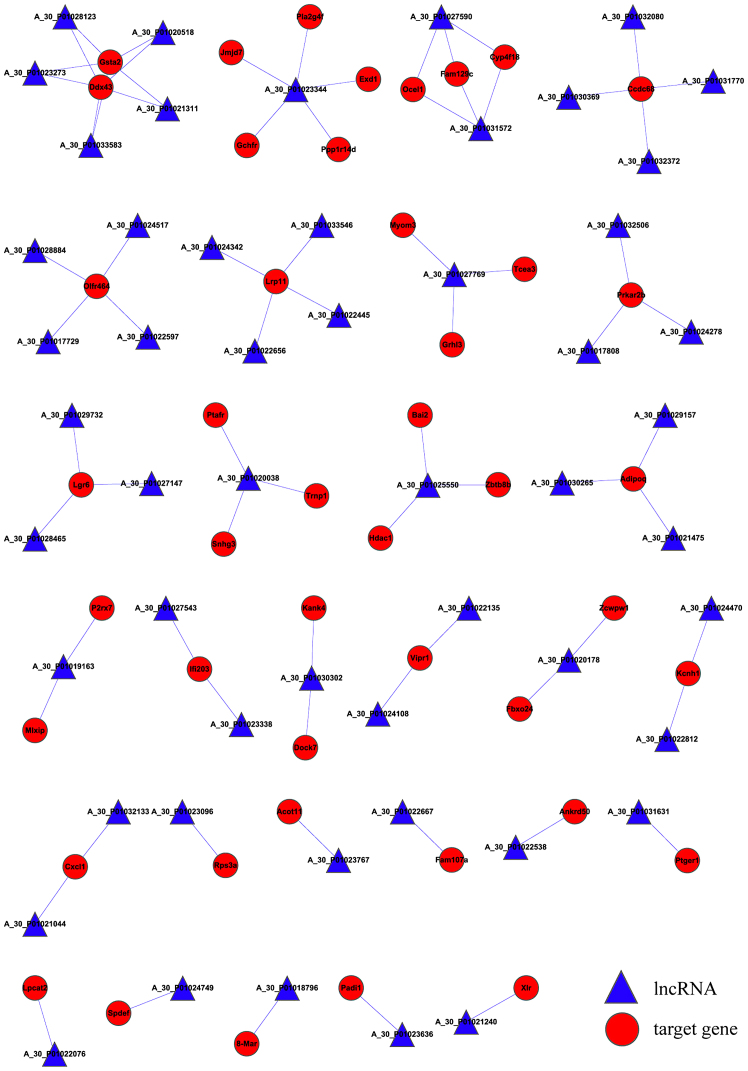
The lncRNA target predictions were superimposed into the lncRNA-mRNA correlation network. As presented in Fig. 2, the network has a total of 98 nodes, 75 connections, 53 lncRNAs and 45 mRNAs. Expression of glutathione S-transferase α 2 (Gsta2) and DEAD (Asp-Glu-Ala-Asp) box polypeptide 43 (Ddx43) was regulated by a total of five lncRNAs, while expression of coiled-coil domain containing 68 (Ccdc68), olfactory receptor 464 (Olfr464) and low density lipoprotein receptor-related protein 11 (Lrp11) were regulated by four lncRNAs. In addition, expression levels of protein kinase, cAMP dependent regulatory, type II β (Prkar2b), leucine-rich repeat-containing G protein-coupled receptor 6 (Lgr6) and adiponectin, C1Q and collagen domain containing (Adipoq) were regulated by a total of three lncRNAs.
Figure 2.
lncRNA-mRNA regulatory network. Circles represent genes and triangles represent lncRNAs; lines represent the association between the two types of regulation (solid line represents a cis-regulation, the dashed line represents the trans-regulation). lncRNA, long non-coding RNA.
Validation of differentially expressed lncRNAs and mRNAs in ovary tissues of WT and CRYBB2 knockout mice
RT-qPCR analysis of different lncRNAs and mRNAs was performed and it was demonstrated that expression levels of lncRNA A-30-P01019163 and P2rx7 were significantly different between10 cases of ovary tissues from WT and CRYBB2 knockout mice (P<0.05; Fig. 3), whereas other lncRNAs and mRNAs did not indicate any difference between WT and CRYBB2 knockout mouse ovary tissues (data not shown).
Figure 3.
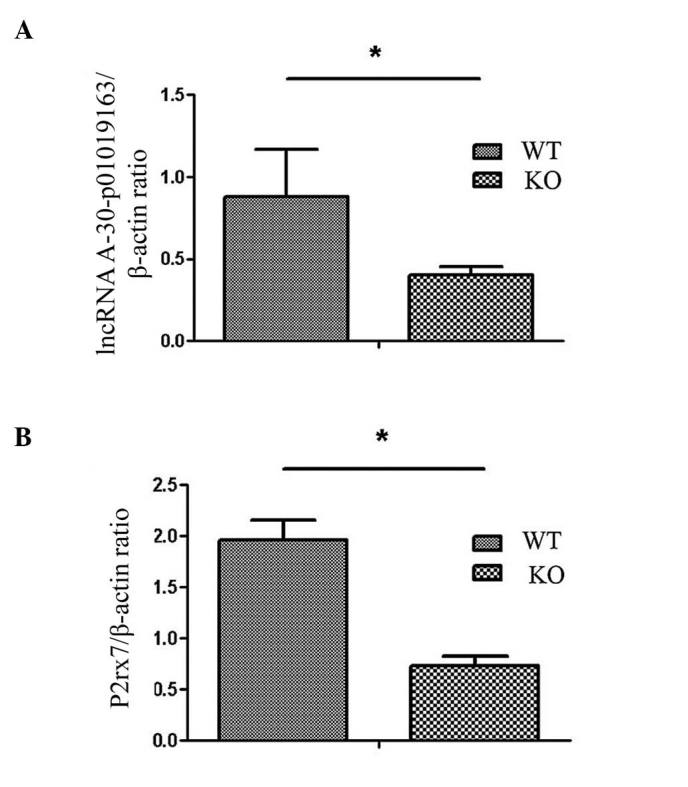
Validation of differentially expressed lncRNAs and mRNAs in ovary tissues from WT and CRYBB2 knockout mice. (A) RT-qPCR indicated lncRNA A-30-P01019163 was downregulated in ovary tissues from CRYBB2 KO mice; (B) RT-qPCR indicated P2rx7 was downregulated in ovary tissues from the CRYBB2 KO mice. *P<0.05. RT-qPCR, reverse transcription-quantitative polymerase chain reaction; KO, knock out; WT, wild-type.
Discussion
CRYBB2 knockout mice exhibit abnormal morphological and functional mouse ovaries. The current study investigated the underlying molecular mechanisms by which CRYBB2 affects expression of lncRNAs to alter mouse ovary structure and function. Certain aberrant lncRNA expression in the regulation of other genes was assessed. A total of 157 differentially expressed lncRNAs and 1,085 mRNAs were observed. The GO database analysis indicated that these altered gene expressions and were predominantly distributed in the biological process of metabolism, immune system, and signal transduction. The KEGG database pathway analysis suggested that the predominant signaling pathways were associated with Ca2+ signaling and ligand-receptor interaction.
Subsequent to establishing a correlation matrix lncRNA and mRNA co-expression network map, a total of 98 nodes, 75 connections, 53 lncRNAs, and 45 mRNAs were identified. Gsta2 and Ddx43 were regulated by five lncRNAs, Ccdc68, Olfr464 and Lrp11 by four lncRNAs, Prkar2b, Lgr6, and Adipoq by three lncRNAs. Gsta2 participates in cytochrome P450 metabolism and cytochrome P450 is important in the conversion of androgens to estrogens. P2rx7, Olfr464, Lrp11, Prkar2b, Lgr6, and Adipoq are involved in cellular signal transduction and P2rx7, Prkar2b, and Hdac1 are involved in regulation of the cell cycle and cell proliferation, while Prkar2b, Hdac1, and Dock7 are important in cell development. lncRNA A-30-P01019163 and P2rx7 were differentially expressed in ovarian tissues of CRYBB2 knockout mice.
Crybb2 not only functions to maintain lens transparency and refractive index, but also affects ovary development in mice. At the gene level, Crybb2 may mediate Ca2+ signal transduction (21,22). The current study demonstrated that Crybb2 also regulates expression of lncRNAs in ovarian tissues, which may be a novel area of research on Crybb2-regulation of gene expression. Previous studies using large scale cDNA library sequencing and next generation sequencing demonstrated abundant lncRNAs in mammals, however, not all lncRNAs are functional and only a relatively small proportion have been demonstrated to be biologically relevant (11,23). For example, as of mid-2014, 197 lncRNAs have been functionally annotated in lncRNA databases (24). However, other lncRNAs may be translated into proteins (25). In addition, lncRNAs, unlike miRNAs, can down- or upregulate gene expression by targeting transcriptional activators or repressors (10), in addition to post-transcriptional regulation of protein expression.
In the current study differentially expressed lncRNAs in ovary tissues of CRYBB2 knockout mice were profiled and a total of 157 differentially expressed lncRNAs were observed. Furthermore, differentially expressed mRNAs were profiled and a total of 1,085 differentially expressed mRNAs in ovary tissues of CRYBB2 knockout mice were observed. GO and KEGG pathway analyses were performed to determine associations between these lncRNAs and mRNAs, and a number of these were subsequently verified using RT-qPCR. It was observed that lncRNA A-30-P01019163 and P2rx7 were significantly differentially expressed between10 cases of ovary tissues from WT and CRYBB2 knockout mice. Thus, CRYBB2 knockout could downregulate expression of lncRNA A-30-P01019163 and, subsequently, suppress expression of the downstream gene P2rx7 and affect ovarian cell signal transduction, cell cycle, and ultimately ovarian development.
The current study is a proof-of-principle study and there are certain limitations. For example, it was not confirmed how lncRNA A-30-P01019163 regulates P2rx7 expression and the mechanistic investigation into how lncRNA A-30-P01019163-regulated P2rx7 expression mediates the effects of CRYBB2 on ovary development could be expanded on.
In conclusion, CRYBB2 regulates expression of different lncRNAs to influence ovary development. lncRNA A-30-P01019163 may affect ovarian cell cycle and proliferation by regulating P2rx7 expression in the ovary.
Acknowledgements
The authors would like to thank Shanghai Sensichip Infotech Co., Ltd. (Shanghai, China) for assistance with bioinformatic analysis and Medjaden Bioscience Ltd. for assisting in the preparation of the manuscript. The present study was supported in part by grants from the National Natural Science Foundation of China (grant nos. 81170834 and 81300748).
References
- 1.Zhang J, Li J, Huang C, Xue L, Peng Y, Fu Q, Gao L, Zhang J, Li W. Targeted knockout of the mouse betaB2-crystallin gene (Crybb2) induces age-related cataract. Invest Ophthalmol Vis Sci. 2008;49:5476–5483. doi: 10.1167/iovs.08-2179. [DOI] [PubMed] [Google Scholar]
- 2.Ganguly K, Favor J, Neuhüuser-Klaus A, Sandulache R, Puk O, Beckers J, Horsch M, Schädler S, Weisenhorn D Vogt, Wurst W, Graw J. Novel allele of crybb2 in the mouse and its expression in the brain. Invest Ophthalmol Vis Sci. 2008;49:1533–1541. doi: 10.1167/iovs.07-0788. [DOI] [PubMed] [Google Scholar]
- 3.Liedtke T, Schwamborn JC, Schröer U, Thanos S. Elongation of axons during regeneration involves retinal crystallin beta b2 (crybb2) Mol Cell Proteomics. 2007;6:895–907. doi: 10.1074/mcp.M600245-MCP200. [DOI] [PubMed] [Google Scholar]
- 4.Magabo KS, Horwitz J, Piatigorsky J, Kantorow M. Expression of betaB(2)-crystallin mRNA and protein in retina, brain, and testis. Invest Ophthalmol Vis Sci. 2000;41:3056–3060. [PMC free article] [PubMed] [Google Scholar]
- 5.Duprey KM, Robinson KM, Wang Y, Taube JR, Duncan MK. Subfertility in mice harboring a mutation in betaB2-crystallin. Mol Vis. 2007;13:366–373. [PMC free article] [PubMed] [Google Scholar]
- 6.Gao Q, Sun LL, Xiang FF, Gao L, Jia Y, Zhang JR, Tao HB, Zhang JJ, Li WJ. Crybb2 deficiency impairs fertility in female mice. Biochem Biophys Res Commun. 2014;453:37–42. doi: 10.1016/j.bbrc.2014.09.049. [DOI] [PubMed] [Google Scholar]
- 7.Gao Q, Yang XY, Gu ML, Zhu RR, Zhang JJ, Li WJ. Effect of beta-B2 crystallin on ovary development and estrous cycle of mouse. Reprod Contracept. 2013;33:217–223. [Google Scholar]
- 8.Hangauer MJ, Vaughn IW, McManus MT. Pervasive transcription of the human genome produces thousands of previously unidentified long intergenic noncoding RNAs. PLoS Genet. 2013;9:e1003569. doi: 10.1371/journal.pgen.1003569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Faghihi MA, Modarresi F, Khalil AM, Wood DE, Sahagan BG, Morgan TE, Finch CE, St Laurent G, III, Kenny PJ, Wahlestedt C. Expression of a noncoding RNA is elevated in Alzheimer's disease and drives rapid feed-forward regulation of beta-secretase. Nat Med. 2008;14:723–730. doi: 10.1038/nm1784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Goodrich JA, Kugel JF. Non-coding-RNA regulators of RNA polymerase II transcription. Nat Rev Mol Cell Biol. 2006;7:612–616. doi: 10.1038/nrm1946. [DOI] [PubMed] [Google Scholar]
- 11.Mercer TR, Dinger ME, Mattick JS. Long non-coding RNAs: Insights into functions. Nat Rev Genet. 2009;10:155–159. doi: 10.1038/nrg2521. [DOI] [PubMed] [Google Scholar]
- 12.Reinius B, Shi C, Hengshuo L, Sandhu KS, Radomska KJ, Rosen GD, Lu L, Kullander K, Williams RW, Jazin E. Female-biased expression of long non-coding RNAs in domains that escape X-inactivation in mouse. BMC Genomics. 2010;11:614. doi: 10.1186/1471-2164-11-614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yang Z, Zhou L, Wu LM, Lai MC, Xie HY, Zhang F, Zheng SS. Overexpression of long non-coding RNA HOTAIR predicts tumor recurrence in hepatocellular carcinoma patients following liver transplantation. Ann Surg Oncol. 2011;18:1243–1250. doi: 10.1245/s10434-011-1581-y. [DOI] [PubMed] [Google Scholar]
- 14.Kogo R, Shimamura T, Mimori K, Kawahara K, Imoto S, Sudo T, Tanaka F, Shibata K, Suzuki A, Komune S, et al. Long noncoding RNA HOTAIR regulates polycomb-dependent chromatin modification and is associated with poor prognosis in colorectal cancers. Cancer Res. 2011;71:6320–6326. doi: 10.1158/0008-5472.CAN-11-1021. [DOI] [PubMed] [Google Scholar]
- 15.Niinuma T, Suzuki H, Nojima M, Nosho K, Yamamoto H, Takamaru H, Yamamoto E, Maruyama R, Nobuoka T, Miyazaki Y, et al. Upregulation of miR-196a and HOTAIR drive malignant character in gastrointestinal stromal tumors. Cancer Res. 2012;72:1126–1136. doi: 10.1158/0008-5472.CAN-11-1803. [DOI] [PubMed] [Google Scholar]
- 16.Huang MD, Chen WM, Qi FZ, Xia R, Sun M, Xu TP, Yin L, Zhang EB, De W, Shu YQ. Long non-coding RNA ANRIL is upregulated in hepatocellular carcinoma and regulates cell apoptosis by epigenetic silencing of KLF2. J Hematol Oncol. 2015;8:50. doi: 10.1186/s13045-015-0146-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kiefer JC. Epigenetics in development. Dev Dyn. 2007;236:1144–1156. doi: 10.1002/dvdy.21094. [DOI] [PubMed] [Google Scholar]
- 18.Lee JT. Gracefully ageing at 50, X-chromosome inactivation becomes a paradigm for RNA and chromatin control. Nat Rev Mol Cell Biol. 2011;12:815–826. doi: 10.1038/nrm3231. [DOI] [PubMed] [Google Scholar]
- 19.Zhang J, Huang CG, Li WJ, Weng W, Wang JQ. Establishment of a βB2crystallin gene knockout mice model. Acad J Sec Mil Med Univ. 2006;27:1246–1248. [Google Scholar]
- 20.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 21.Jobby MK, Sharma Y. Calcium-binding to lens betaB2- and betaA3-crystallins suggests that all beta-crystallins are calcium-binding proteins. FEBS J. 2007;274:4135–4147. doi: 10.1111/j.1742-4658.2007.05941.x. [DOI] [PubMed] [Google Scholar]
- 22.Kang D, Hur CG, Park JY, Han J, Hong SG. Acetylcholine increases Ca2+ influx by activation of CaMKII in mouse oocytes. Biochem Biophys Res Commun. 2007;360:476–482. doi: 10.1016/j.bbrc.2007.06.083. [DOI] [PubMed] [Google Scholar]
- 23.Dinger ME, Amaral PP, Mercer TR, Mattick JS. Pervasive transcription of the eukaryotic genome: Functional indices and conceptual implications. Brief Funct Genomic Proteomic. 2009;8:407–423. doi: 10.1093/bfgp/elp038. [DOI] [PubMed] [Google Scholar]
- 24.Amaral PP, Clark MB, Gascoigne DK, Dinger ME, Mattick JS. lncRNAdb: A reference database for long noncoding RNAs. Nucleic Acids Res. 2011;39:D146–D151. doi: 10.1093/nar/gkq1138. (Database Issue) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Smith JE, Alvarez-Dominguez JR, Kline N, Huynh NJ, Geisler S, Hu W, Coller J, Baker KE. Translation of small open reading frames within unannotated RNA transcripts in Saccharomyces cerevisiae. Cell Rep. 2014;7:1858–1866. doi: 10.1016/j.celrep.2014.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]