Abstract
Background
Cannabis is a widely used illicit drug with various threats of personality syndrome, and Nigella sativa has been widely implicated as having therapeutic efficacy in many neurological diseases. The present study investigates the ameliorative efficacy of Nigella sativa oil (NSO) on cannabis-induced moto-cognitive defects.
Methods
Scopolamine (1 mg/kg i.p.) was given to induce dementia as a standard base line for cannabis (20 mg/kg)-induced cognitive impairment, followed by an oral administration of NSO (1 ml/kg) for 14 consecutive days. The Morris water maze (MWM) paradigm was used to assess the memory index, the elevated plus maze was used for anxiety-like behaviour, and the open field test was used for locomotor activities; thereafter, the rats were sacrificed and their brains were removed for histopathologic studies.
Results
Cannabis-like Scopolamine caused memory impairment, delayed latency in the MWM, and anxiety-like behaviour, coupled with alterations in the cerebello-hippocampal neurons. The post-treatment of rats with NSO mitigated cannabis-induced cognitive dysfunction as with scopolamine and impaired anxiety-like behaviour by increasing open arm entry, line crossing, and histological changes.
Conclusions
The observed ameliorative effects of NSO make it a promising agent against moto-cognitive dysfunction and cerebelo-hippocampal alterations induced by cannabis.
Keywords: cannabis, toxicity, spatial memory, anxiety, brain
Introduction
Cannabis is a hemp plant in the genu flowering plants with three common species, including sativa, indica, and ruderalis (1). Preparations from cannabis, with common names like marijuana, weed, and grass, are the most consumed illicit drugs worldwide.
Cannabis dependency in users outnumbered that of other commonly abused drugs such as cocaine (2), and its exposure has been reported to enhance vulnerability to addiction and psychiatric disorders (3). The vulnerabilities include increased risk of other illicit drug use (4, 5, 6), enhanced intake and sensitivity to opiates (7), and enhanced cocaine-induced motor behaviour (8).
A wide range of neuropsychological studies have linked the consumption of cannabis to learning and memory impairments, as well as impairments to some aspects of executive functioning in humans (9, 10, 11), rodents (12, 13), and non-human primates (14).
It has been reported to impair mood, attention, short-term memory (STM), motor skills (15), and working memory (17), as well as to induce severe transient psychotic symptoms, lasting deficits in attention (16), and depressive disorders.
The major psychoactive constituent of cannabis, Tetrahydrocannabinol (THC), acts on the cannabinoid (CB) receptors that are widely expressed in the cerebral cortex, cerebellum, basal ganglia, Cornu Ammonis (CA)3 region of the hippocampus, and the dentate gyrus (DG) (18), resulting in wide topographical effects and functional alterations to the brain’s integrity.
Herbal and natural plant extracts have gained wide attention for their use in the treatment or management of neurological, psychiatric, and degenerative diseases based on their few or lack of side effects (19). Nigella sativa oil (NSO) is a high-value medicinal solvent used traditionally in the treatment of many diseases. Compelling evidence has shown that NSO exhibits protective activities against many diseases because of its high antioxidant (20) and anti-inflammatory properties (21).
A number of studies have been carried out, and the acclaimed medicinal properties emphasised in terms of the different therapeutic efficacies of NSO include neuro-protective (22), anti-asthmatic (23), anti-inflammatory, immunomodulatory, and anti-tumor properties (21), gastric ulcer healing (24), tumor growth suppression (25), male infertility improvement (26), and milk production stimulation (27). The main active ingredients in NSO are thymoquinone (TQ), alkaloids (nigellidine, nigellimine, and nigellicine), vitamins, such as thiamine, riboflavin, pyridoxine, niacin, and folic acid, minerals, and proteins (28).
This study investigated the effects of cannabis sativa leaf extract on cognitive, locomotor, and anxiety-like behaviours, as well as on differential cortico-hippocampal histology in Wistar rats, and it evaluated the ameliorative efficacies of black seed oil on cannabis-induced moto-cognitive phenotypes and cortico-hippocampal histology.
Materials and Methods
Preparation of cannabis sativa extract
Cannabis plant leaves were obtained from the National Drug and Law Enforcement Agency (NDLEA) in Kwara State, Nigeria, and they were dried, blended to powdery particles, and weighed. The particles were dissolved in distilled water and kept for about 18 hours. The mixture was filtered, the filtrate was oven dried at 45°C, the dried filtrate was weighed, and then it was stored in an airtight container. Experimental approval was obtained from the NDLEA in Kwara State, Nigeria.
Drugs
The Scopolamine hydrobromide used in this study was obtained from SIGMA, USA, and it was dissolved in saline to make a final concentration of 1 mg/kg.bw; it was injected into the animals intraperitoneally (i.p.). The black seed oil (100% pure natural oil) was obtained from Masra warda, Kingdom of Saudi Arabia, and administer at 1 ml/kg.bw.
Animal care
Eighteen adult albino Wistar rats with an average weight of 200±20 g at the time of acquisition and acclimatisation were used in this study. The animals were housed in the animal holding of the Faculty of Basic Medical Sciences, University of Ilorin, Nigeria at six in a cage with free access to water and food and under a standard laboratory condition of 22±2°C temperature and a 12 h:12 h light-dark cycle.
Treatments schedule
The rats were randomly distributed into five groups (n = 6) as follows:
Saline-control: received saline
Experiment 1: received Scopolamine (1 mg/kg/day i.p.)
Experiment 2: received cannabis sativa (20 mg/kg/day orally)
Experiment 3: received black seed oil (1 ml/kg orally)
Experiment 4: received cannabis sativa (20 mg/kg/day orally) + black seed oil (1 ml/kg orally)
All procedures were scheduled and carried out during the light phase between 0900 and 1500 hours. All groups contain six rats each and treatments were given for seven consecutive days.
Morris water maze (MWM) test
The Morris water maze (MWM) is a common paradigm used for testing spatial learning and memory in rodents, and a modification of the procedure was used in the present study. The water maze used in this study consisted of a circular pool with a 1.6 m diameter and 50 cm height and water filled to a 44 cm depth, and it was coloured with a black non-toxic dye and had a controlled temperature of 25°C ± 1°C. Four equally spaced points around the edge of the pool were designated as north (N), east (E), south (S), and west (W). A black-coloured round platform with a 9 cm diameter was placed 1 cm below the water surface. The rats were trained to navigate the submerged platform. The rats were given a maximum time of 120 s (cut–off time) to find the hidden platform and were allowed to stay on it for 30 s. The platform remained in the same position during the training days. Rats that failed to locate the platform within 120 s were placed on the platform only in the first session. The animals were given a daily session of five trials. The escape latency time to reach the platform was recorded in each trial. Three subsequent tests were conducted, 24 h after the last training session on day six of this study. The escape latencies from the first two tests (I and II) were recorded, averaged, and then recorded as the long-term memory (LTM), while the escape latency recorded in the third test was considered the STM, respectively (29).
Open field test (OFT)
To assess the effects of the black seed oil on exploratory activity, experimental animals were evaluated in the open field paradigm. The paradigm is made of Perspex plastic with dimensions 40 × 60 × 50 cm, and the floor was divided into 25 equal squares by lines. Animals were individually placed in the centre of the apparatus and the numbers of squares crossed with all paws (frequent line crossing) were counted in a 5-min session, and all animals were monitored in a balanced design during the procedures (29, 30).
Elevated plus maze (EPM) test
To assess the anxiolytic activity of black seed oil (1 ml/kg orally) in rats, the elevated plus maze (EPM) paradigm was used. The EPM was made of two open arms (OA; 16 × 5 cm) and two closed arms (CA; 16 × 5–12 cm), and it was elevated (60 cm) above the floor. Rats were individually placed at the centre of the EPM with heads facing the OA, i.e., fear-inducing environment, they were allowed a 5-min test session, and the number of entries into the OA was recorded.
Histopathology
The rats were anaesthetised 24 h after the behavioural tests, decapitated, and their brains were excised and stored in a 4% paraformaldehyde preparation. Then, the brains were processed for histo-architectural examination, embedded in paraffin, refrigerated, coronally sectioned into 8-μm sections of the hippocampal formation (from Bregma −2.5 mm to −4.5 mm) and the cerebellar cortices (from Bregma −10 mm to −15 mm) using a rotary microtome (MK 1110), and stained with Hematoxylin and Eosin (H&E) for general cyto-architecture and Cresyl fast violet (CFV) for Nissl granulation acording to routine laboratory procedures (31). An Olympus BX 51 microscope and a DP 12 digital camera were used to produce photomicrographs from all the sections.
Statistical analysis
Data recorded in this study were reported as mean ± standard error of mean. The MWM, EPM, and OFT tests’ data were analysed using one-way analysis of variance (ANOVA) and for post-hoc analyses, we used the Bonfferoni test. A P-value of ≤0.05 was considered statistically significant in all cases. The software package Graph Pad Prism was used to analyse and graphically represent the data.
Results
Cognitive functions
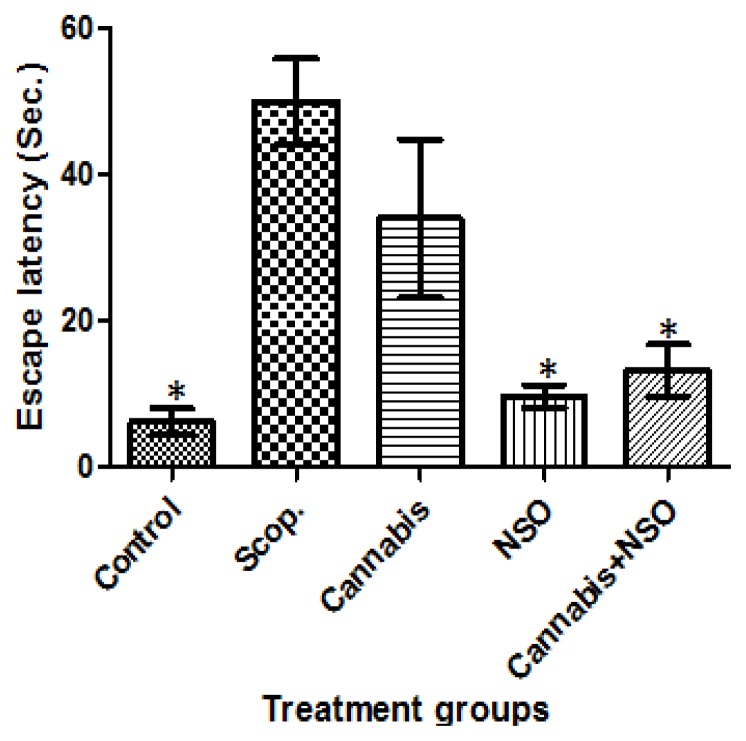
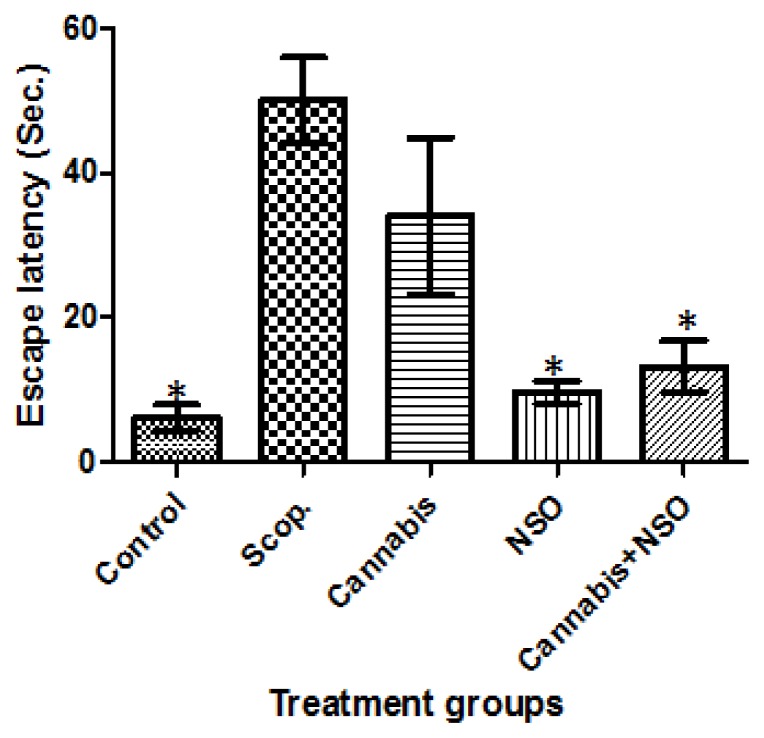
Cannabis significantly (P ≤ 0.05) impaired spatial memory tasks, causing a delayed latency to find the escape platform in the treated rat, an effect comparable to the deficit and delayed latency observed in the Scopolamine-treated rat. These effects are prominent in both LTM (Figure 1) and STM tasks (Figure 2) in the MWM.
Figure 1.
Showing the delayed latency induced by scopolamine and cannabis, the unaffected latency in the control, an improved latency in the NSO and cannabis+NSO treated rats in LTM tasks in the MWM paradigm. Asterisks (*) indicate significant (P ≤ 0.05) lower escape latency of the carriers when compared with the scopolamine and cannabis treated rats.
Figure 2.
Showing the delayed latency induced by scopolamine and cannabis, the unaffected latency in the control, an improved latency in the NSO and cannabis+NSO treated rats in STM tasks in the MWM paradigm. Asterisks (*) indicate significant (P ≤ 0.05) lower escape latency of the carriers when compared with the scopolamine and cannabis treated rats.
The potent efficacy of NSO in cannabis impaired working memory shows that administration of NSO significantly (P ≤ 0.05) reduced the latency to find the platform (Figures 1 and 2). Therefore, rats treated with NSO after cannabis for seven days ameliorated the memory impairments caused by cannabis. Hence, the oral administration of 1 ml/kg/b.wt of NSO can reverse (P ≤ 0.05) the memory impairment induced by cannabis treatment.
Activities and anxiety-like behaviours
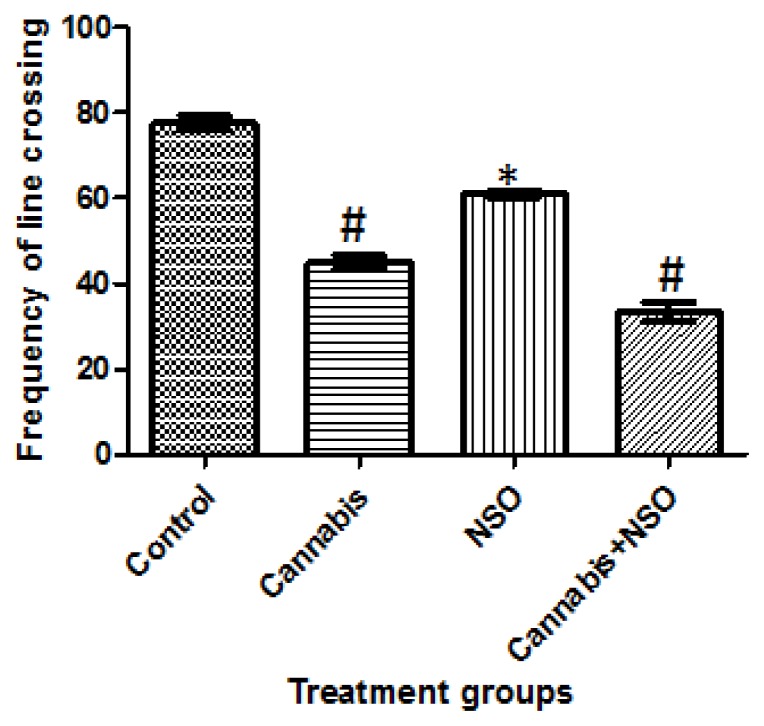
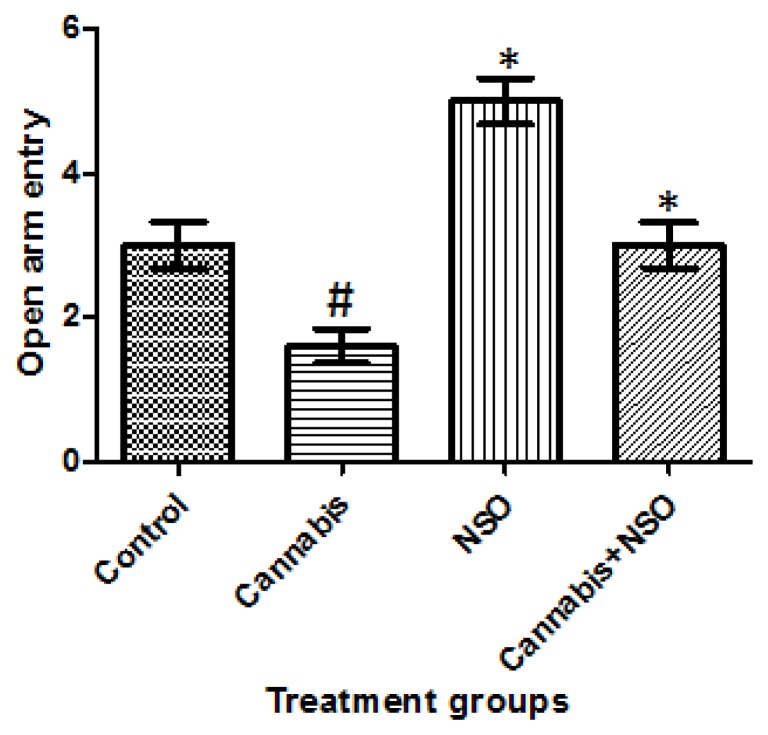
Locomotor activities and anxiety-like behaviours were assessed in the animals following cannabis exposure in the OFT and EPM paradigms, respectively. Cannabis markedly caused a significant (P ≤ 0.05) reduction in frequent line crossing in the OFT paradigm, while NSO only increased line crossing, but NSO after cannabis did not reverse the cannabis-induced deficit (Figure 4). A complementary reduction in the open arm entry (OAE) following cannabis exposure was also recorded in the EPM, while NSO only and NSO after cannabis caused an increase in OAE (Figure 3).
Figure 4.
Showing reduced frequent line crossing following cannabis treatment and cannabis+NSO, while high line crossing frequency were recorded in the NSO and control in the OFT paradigm. Asterisks (*) and hash (#) indicate significant (P ≤ 0.05) higher line crossing in NSO only treated animals when compared with Cannabis and Cannabis + NSO treated animals and a lower line crossing in the Cannabis and Cannabis+NSO treated animals when compared with the Control and NSO treated animals respectively.
Figure 3.
Showing reduced OAE following cannabis treatment, while NSO only and cannabis+NSO improved OAE in the EPM paradigm. Asterisks (*) and hash (#) indicate significant (P ≤ 0.05) lower open arms visit in cannabis treated animals and an improved open arm entry in the NSO only and NSO after Cannabis treated animals respectively.
Histopathological results
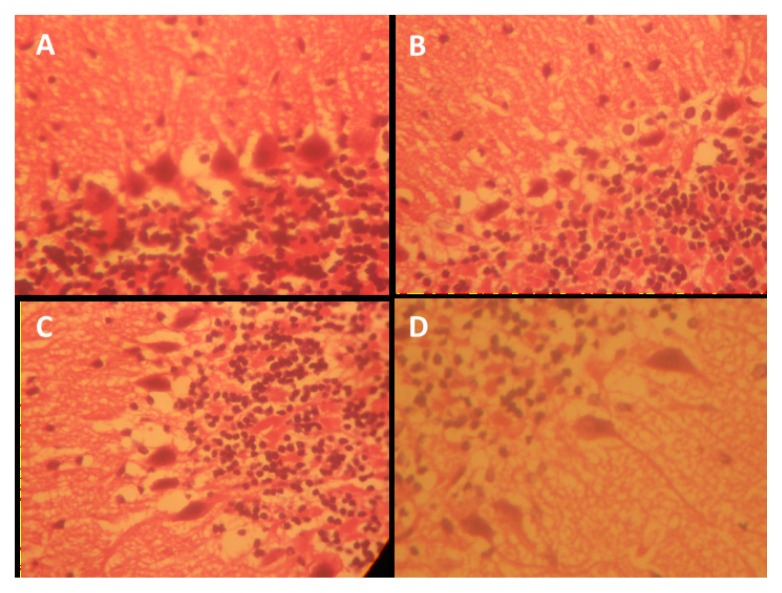
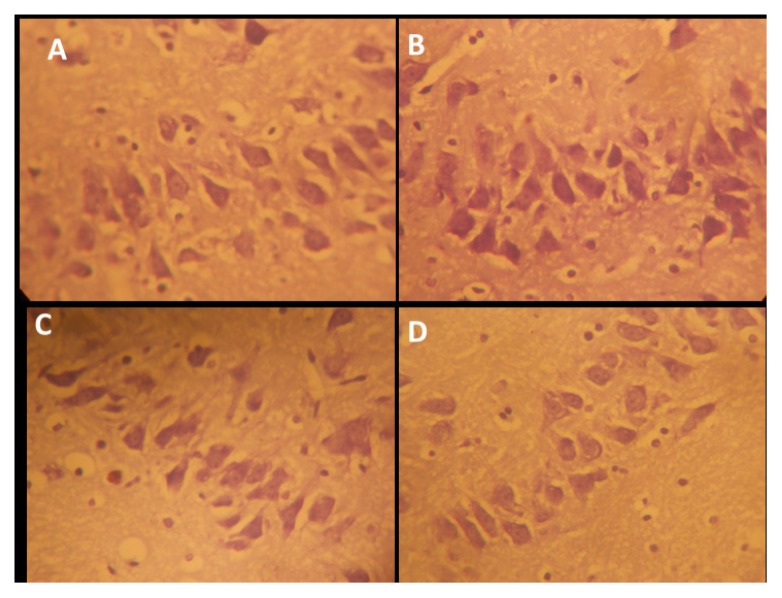
Sections of the cerebella cortices of the animals treated with amine, specifically the pyramidal cells at the internal pyramidal cell layer, show some degree of retraction of processes, vacuolation of the surrounding neuropil of the pyramidal cells, and hyperchromatic and shrunken perikarya (Figure 7). Also observed are corkscrew-shaped apical dendrites and a blending of the membrane into the perikaryal cytoplasm (Figure 7). NSO was observed in this study to enhance axonal-dendritic connections and to reduce scopolamine-induced vacuolation to nearly normal when compared with the control (Figure 7). Scopolamine also caused irregular distributions of Nissl granulation in the perikaryal, increased dark pyknotic nuclei, and selective staining of Nissl granules (tigroid bodies and Nissl bodies), as well as the presence of a dark neuron, glial activation, and a dendritic cytoplasm, suggesting protein denaturing and neural degeneration in the hippocampus and cerebellar cortices (Figures 8, 9, 10). NSO reversed these pathologies to almost normal when compared with the control.
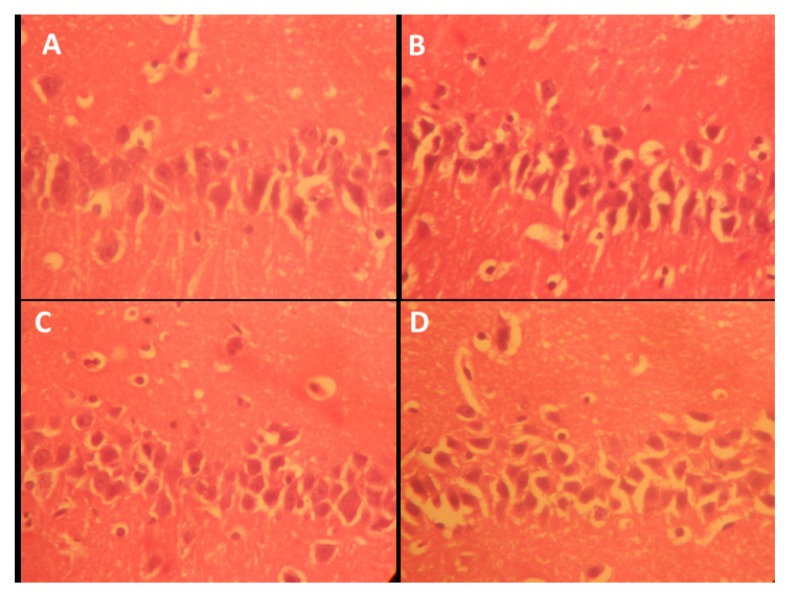
Figure 7.
Representative photo micrograph of the sections of the cerebellar cortices of rats following saline, cannabis, NSO and cannabis+NSO administrations showing: normal and unaffected cytology of the Pukinje cell with projection in A and C; marked affection of the Pukinje cells showing marked retraction of processes with vacuolations, and extreme shrinkening and blending in the perikarya cytoplasm in B, while D shows reduced vacuolation and distinct regenerations in the processes. (H&E ×400)
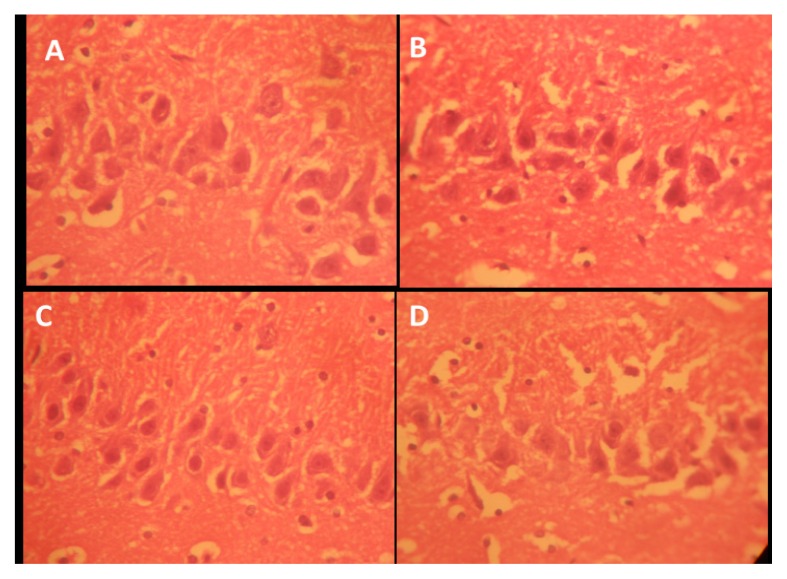
Figure 8.
Representative photo micrograph of the sections of the hippocampi CA1 following saline, cannabis, NSO and cannabis+NSO administrations showing: normal nissl granulation in the mall pyramidal neuron (CA1) in A and C; pyknotic and darkly stained cell body, presence of dark neurons and vacuolation in the surrounding neuropil in B; reduced pyknoticity and disappearance of dark neurons in D. (CFV ×400)
Figure 9.
Representative photo micrograph of the sections of the hippocampi CA3 following saline, cannabis, NSO and cannabis+NSO administrations showing: normal nissl granulation in the large pyramidal neurons (CA3) in A and C; darkly stained cell body, vacuolation in the surrounding neuropil and shrunken perikarya in B; marked regeneration, less shrunken cells and contarasting soma stain in D. (CFV ×400)
Figure 10.
Representative photo micrograph of the sections of the cerebellar cortices following saline, cannabis, NSO and cannabis+NSO administrations showing: normal nissl distributions in the soma and unaffected proximal dendrites of the large cerebellar Pukinje neurons in A and C; contrasting stains, pyknotic and darkly stained cell body, presence of dark neurons and vacuolation in the surrounding neuropil of Pukinje cells in B; marked regeneration, less shrunken cells. (CFV × 400)
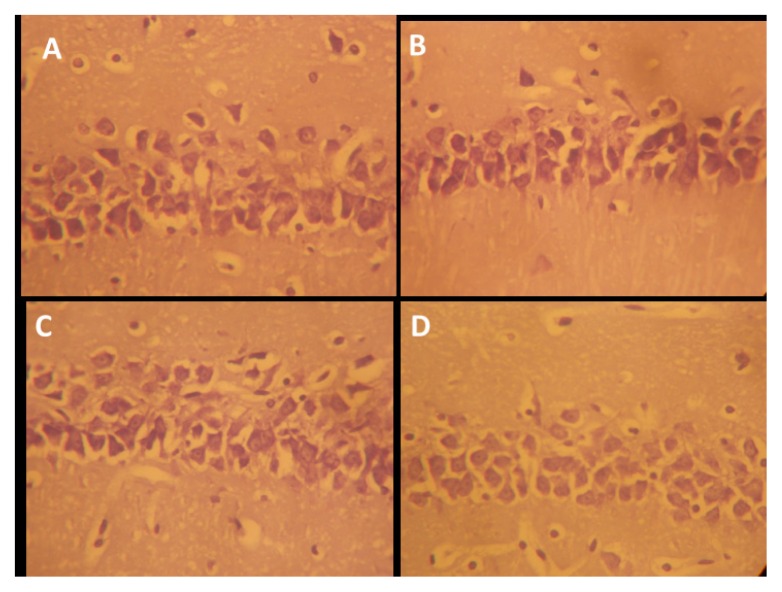
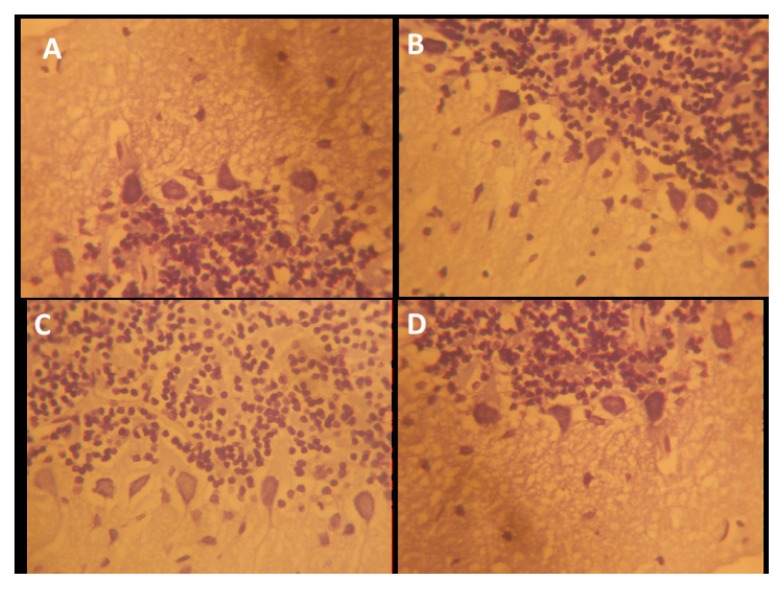
The hippocampus is made up of CA1 and CA2 formed in a zone of small pyramidal cells, as well as CA3 and CA4 formed in a zone of large pyramidal cells. CA4 projects into the DG, made of small granule cells. The neuronal processes (axons and dendrites), glial cells, and scattered nerve cells are found in areas between the compact zones of the cells. NSO was able to repair the damage induced by Scopolamine exposure, including disorganization and a loss of small pyramidal cells (CA1), some of which had pale nuclei, while others were dark (Figure 5). There was also marked shrinkage in the size of the large pyramidal cells (CA3), and the outer layer was more affected, with darkened nuclei and clumped processes (Figure 6). Some darkened, pyknotic nuclei are observed in the granular cells of the DG with contrasting basophilia of some other granular cells’ nuclei; also observed are marked vacuolation and excess glial cells, effects ameliorated by NSO (Figures 5, 6, 7).
Figure 5.
Representative photo micrograph of the sections of the hippocampi CA1 following saline, cannabis, NSO and cannabis + NSO administrations showing: normal and unaffected cytology of the small pyramidal neurons with well outlined soma and unaffected projections in A and C; vacuolation in the surrounding neuropil of the pyramidal cells, shrunken perikarya and blended membrane into the perikaryal cytoplasm in B and marked regeneration, reduced vacuolation and neuropil spaces in D. (H&E ×400)
Figure 6.
Representative photo micrograph of the sections of the hippocampi CA3 following saline, cannabis, NSO and cannabis+NSO administrations showing: normal and unaffected cytology of the large pyramidal neurons (CA3) with outlined soma and unaffected projections in A and C; shrunken perikarya, blended membrane into the perikaryal cytoplasm and glia activation in B and D, but D shows reduced vacuolation and distinct regenerations in the processes. (H&E ×400)
Discussion
Cannabis exposure can potentially function as a cumulative causal factor in some cases of schizophrenia, and its consumption by schizophrenic patients may likely pose a worsening outcome (32), but it remains one of most used illicit substances across boundless ethnicities, languages, classes, and regional diversities (33, 2), and it has been associated with management difficulties. Despite these facts, there is a growing perception that cannabis is safe and possesses medicinal potentials (34, 35).
The present study attempted to elucidate the motor-cognitive responses in cannabis administration in rats, the possible pathology in the hippocampus and cerebellar cortices, and the possible ameliorative efficacy of NSO following these alterations. The delayed escape latency recorded in the measures of LTM and STM in the MWM paradigm induced by cannabis is suggestive evidence of disrupted memory retention in the exposed animals, an effect similarly seen following exposure to Scopolamine, which is a standard amnesic model. Such effects of cannabis on the memory index, especially delayed latency in the MWM, have been previously reported in the literature (36, 37), thereby validating the present results.
NSO, which was hypothesised in this study as having the capacity to restore any memory dysfunction induced by cannabis, quickened latency to find the hidden platform in the treated animals when administered alone. Post-treatment with NSO after cannabis treatment also shows that NSO was able to ameliorate the cognitive dysfunctions induced by cannabis by reducing latency periods in both LTM and STM tasks in the MWM paradigm. These effects of NSO can be supported by various workers who also reported NSO reduced escape latency and increased target quadrant exploration in a MWM paradigm (29, 38), as well as reversed cognitive and novel object recognition in dementia modelled rats (39). Strengthened by various reports of its possible modulating impact on memory, attention, and cognition (40,41), a memory improvement amnesia model (29, 42) and reversed memory impairment in penthylenetetrazole (PTZ)-induced repeated seizures in rats (43) further confirm the memory enhancing and ameliorative capacity of NSO against cannabis-induced memory dysfunction, as observed in the present study.
The hippocampal pathologies observed in this study following cannabis administration in the CA1 and CA3 pyramidal neurons and the Nissl granulation can be supported by studies reporting distinct hippocampal morphological alterations in rats and humans following cannabis toxicity, including the presence of dark neurons, decreased sizes of nuclei, cellular infiltration, reductions in pyramidal cell density (44), reduced hippocampal CA1 and CA3 volumes, reduced DG volume (5, 45, 46), and reduced hippocampal gray matter densities in humans (47).
NSO administration in this study was observed to rescue the impaired hippocampal neural architecture following cannabis toxicity in the small and large pyramidal cells of the CA1 and CA3 regions. These effects could be strengthened by evidences from the literature reporting an improved neuronal cell viability, protection against beta-amyloid protein intoxication (48, 49), protection against cerebral ischemia reperfusion injury in the hippocampus (50), hippocampal neurodegeneration (51), and the prevention of hippocampal neuron cell death in global cerebral ischemia (20). Also supporting the results of this study is evidence of the efficacy of NSO in neurodegenerative diseases, including Parkinson’s and Alzheimer’s, due to its antioxidant potential (51, 52, 53), a property linked to the presence of TQ, which has the potential to prevent neuronal cell death (54).
The effects of cannabis on anxiety-related behaviors (OAEs), an indicator of disrupted emotional behaviours and line crossing frequencies, a marker of activities observed in the EPM and OFT paradigms, show more consistency regarding the anxiogenic properties. As observed in this study, the deleterious effects of cannabis on OAE in the EPM are demonstrated by accumulating evidence showing that cannabis causes a stronger dislike for the open arms of the EPM (55), thereby increasing anxiety-like behaviours (56, 57, 58). Nonetheless, this effect was not reversed by NSO in this study.
The reduced effects of cannabis on activity behaviour in this study are in countenance with earlier studies, where a depression in locomotor activities was reported following cannabis exposure (36, 59). These activity-related deficits and cytological alterations observed in the Pukinje cells of the cerebellar cortices may be linked to the affinity of cannabis to the CB receptors that is highly expressed in the cerebral cortex, cerebellum, and basal ganglia. These effects are repaired to an appreciable amount by NSO, a property that can be associated with its anti-inflammatory efficacy, as it has been reported to ameliorate cerebellar inflammation and demyelination in experimental autoimmune encephalomyelitis-induced rats (60).
Conclusion
In this study, cannabis impaired delayed escape latency in the MWM, thereby impairing memory and reducing OAE and frequent line crossing in the EPM and OFT, inducing anxiety-like behaviour and impaired activities. Post-treatment with NSO ameliorated the delayed latency and anxiogenic effects of cannabis. It also improved cortico-hippocampal granulation and the histo-architecture. Hence, NSO could be a potential supplement in the management of memory dysfunction and emotional deregulation seen in cannabis psychosis and toxicity.
Acknowledgements
We appreciate the Faculty of Basic Medical Sciences, University of Ilorin for the faculty research support and provision of routine histological consumables.
Footnotes
Ethical Approval
All experimental procedures were performed in accordance with institutional guidelines for animal care and use, and ethical approvals were obtained from the University of Ilorin Ethics Committee and the NDLEA in Kwara State, Nigeria.
Conflict of Interests
None
Funds
The authors received no financial support from any government or not for profit organizations to conduct this work.
Authors’ Contributions
Conception and design: IA, AMS, AMI, BWG
Analysis and interpretation of the data: IA, AA, AWI, IA, OOJ, AOA
Drafting of the article: IA, AA
Critical revision of the article for important intellectual content: IA, AMS, AA
Final approval of the article: IA, AMS, AA
Provision of study materials or patients: AMS, IA, AA, AA
Statistical expertise: AWI, IA
Administrative, technical, or logistic support: IA, AMS
Collection and assembly of data: AA
References
- 1.Elsohly MA, editor. Marijuana and the cannabinoids. Totowa, NJ: Humana Press; 2007. [Google Scholar]
- 2.SAMHSA. Office of Applied Studies, NSDUH Series H-41, HHS Publication. Rockville, MD: 2011. Results from the 2010 National Survey on Drug Use and Health: National Findings. No. (SMA) 11e4658. [Google Scholar]
- 3.Hurd YL, Michaelides M, Miller ML, Jutras-Aswad D. Trajectory of adolescent cannabis use on addiction vulnerability. Neuropharmacology. 2014;76:416–424. doi: 10.1016/j.neuropharm.2013.07.028. http://dx.doi.org/10.1016/j.neuropharm.2013.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Agrawal A, Neale MC, Prescott CA, Kendler KS. Cannabis and other illicit drugs: comorbid use and abuse/dependence in males and females. Behav Genet. 2004;34(3):217–228. doi: 10.1023/B:BEGE.0000017868.07829.45. [DOI] [PubMed] [Google Scholar]
- 5.Hall WD, Lynskey M. Is cannabis a gateway drug? Testing hypotheses about the relationship between cannabis use and the use of other illicit drugs. Drug Alcohol Rev. 2005;24(1):39–48. doi: 10.1080/09595230500126698. [DOI] [PubMed] [Google Scholar]
- 6.Fergusson DM, Boden JM. Cannabis use and later life outcomes. Addiction. 2008;103(6):969–976. doi: 10.1111/j.1360-0443.2008.02221.x. doi: http://dx.doi.org/10.1111/j.1360-0443.2008.02221.x. [DOI] [PubMed] [Google Scholar]
- 7.Biscaia M, Fernandez B, Higuera-Matas A, Miguens M, Viveros MP, Garcia-Lecumberri C, et al. Sex-dependent effects of periadolescent exposure to the cannabinoid agonist CP-55,940 on morphine self administration behaviour and the endogenous opioid system. Neuropharmacology. 2008;54(5):863–873. doi: 10.1016/j.neuropharm.2008.01.006. http://dx.doi.org/10.1016/j.neuropharm.2008.01.00. [DOI] [PubMed] [Google Scholar]
- 8.Dow-Edwards D, Izenwasser S. Pretreatment with Delta9-tetrahydrocannabinol (THC) increases cocaine-stimulated activity in adolescent but not adult male rats. Pharmacol Biochem Behav. 2012;100(3):587–591. doi: 10.1016/j.pbb.2011.09.003. http://dx.doi.org/10.1016/j.pbb.2011.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bolla KI, Brown K, Eldreth D, Tate K, Cadet JL. Dose-related neurocognitive effects of marijuana use. Neurology. 2002;59(9):1337–1343. doi: 10.1212/01.wnl.0000031422.66442.49. http://dx.doi.org/10.1212/01.WNL.0000031422.66442.49. [DOI] [PubMed] [Google Scholar]
- 10.Pope HG., Jr Cannabis, cognition, and residual confounding. JAMA. 2002;287(9):1172–1174. doi: 10.1001/jama.287.9.1172. http://dx.doi.org/10.1001/jama.287.9.1172. [DOI] [PubMed] [Google Scholar]
- 11.Grant I, Gonzalez R, Carey CL, Natarajan L, Wolfson T. Nonacute (residual) neurocognitive effects of cannabis use: a meta-analytic study. J Int Neuropsychol Soc. 2003;9(5):679–689. doi: 10.1017/S1355617703950016. http://dx.doi.org/10.1017/S1355617703950016. [DOI] [PubMed] [Google Scholar]
- 12.Terranova JP, Storme JJ, Lafon N, Perio A, Rinaldi-Carmona M, Le Fur G, et al. Improvement of memory in rodents by the selective CB1 cannabinoid receptor antagonist, SR 141716. Psychopharmacology (Berl) 1996;126(2):165–172. doi: 10.1007/BF02246352. http://dx.doi.org/10.1007/BF02246352. [DOI] [PubMed] [Google Scholar]
- 13.Nava F, Carta G, Colombo G, Gessa GL. Effects of chronic Delta(9)-tetrahydrocannabinol treatment on hippocampal extracellular acetylcholine concentration and alternation performance in the T-maze. Neuropharmacology. 2001;41(3):392–399. doi: 10.1016/s0028-3908(01)00075-2. http://dx.doi.org/10.1016/S0028-3908(01)00075-2. [DOI] [PubMed] [Google Scholar]
- 14.Evans EB, Wenger GR. Effects of drugs of abuse on acquisition of behavioral chains in squirrel monkeys. Psychopharmacology (Berl) 1992;107(1):55–60. doi: 10.1007/BF02244965. http://dx.doi.org/10.1007/BF02244965. [DOI] [PubMed] [Google Scholar]
- 15.Kano M, Ohno-Shosaku T, Hashimotodani Y, Uchigashima M, Watanabe M. Endocannabinoid-mediated control of synaptic transmission. Physiology Rev. 2009;89(1):309–380. doi: 10.1152/physrev.00019.2008. http://dx.doi.org/10.1152/physrev.00019.2008. [DOI] [PubMed] [Google Scholar]
- 16.Ehrenreich H, Rinn T, Kunert HJ, Moeller MR, Poser W, Schilling L, et al. Specific attentional dysfunction in adults following early start of cannabis use. Psychopharmacology. 1999;142:295–301. doi: 10.1007/s002130050892. http://dx.doi.org/10.1007/s002130050892. [DOI] [PubMed] [Google Scholar]
- 17.Schwartz RH, Gruenewald PJ, Klitzner M, Fedio P. Short-term memory impairment in cannabis-dependent adolescents. Am J Dis Child. 1989;143:1214–1219. doi: 10.1001/archpedi.1989.02150220110030. http://dx.doi.org/10.1001/archpedi.1989.02150220110030. [DOI] [PubMed] [Google Scholar]
- 18.Tsou K, Brown S, Sanudo-Pena MC, Mackie K, Walker JM. Immunohistochemical distribution of cannabinoid CB1 receptors in the rat central nervous system. Neuroscience. 1998;83(2):393–411. doi: 10.1016/s0306-4522(97)00436-3. http://dx.doi.org/10.1016/S0306-4522(97)00436-3. [DOI] [PubMed] [Google Scholar]
- 19.Kumar H, More SV, Han SD, Choi JY, Choi DK. Promising therapeutics with natural bioactive compounds for improving learning and memory review of randomized trials. Molecules. 2012;17(9):10503–10539. doi: 10.3390/molecules170910503. http://dx.doi.org/10.3390/molecules170910503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kanter M, Coskun O, Kalayci M, Cagavi F. Neuroprotective effects of Nigella sativa on experimental spinal cord injury in rats. Hum Exp Toxicol. 2008;25(3):127–133. doi: 10.1191/0960327106ht608oa. http://dx.doi.org/10.1191/0960327106ht608oa. [DOI] [PubMed] [Google Scholar]
- 21.Majdalawieh AF, Hmaidan R, Carr RI. Nigella sativa modulates splenocyte proliferation, Th1/Th2 cytokine profile, macrophage function and NK anti-tumor activity. J Ethnopharmacol. 2010;131:268–275. doi: 10.1016/j.jep.2010.06.030. http://dx.doi.org/10.1016/j.jep.2010.06.030. [DOI] [PubMed] [Google Scholar]
- 22.Yaman I, Balikci E. Protective effects of Nigella sativa against gentamicin-induced nephrotoxicity in rats. Exp Toxicol Pathol. 2010;62:183–190. doi: 10.1016/j.etp.2009.03.006. http://dx.doi.org/10.1016/j.etp.2009.03.006. [DOI] [PubMed] [Google Scholar]
- 23.Boskabady MH, Mohsenpoor N, Takaloo L. Anti-asthmatic effect of Nigella sativa in airways of asthmatic patients. Phytomedicine. 2010;17:707–713. doi: 10.1016/j.phymed.2010.01.002. http://dx.doi.org/10.1016/j.phymed.2010.01.002. [DOI] [PubMed] [Google Scholar]
- 24.Bukhari MH, Khalil J, Qamar S, Qamar Z, Zahid M, Ansari N, et al. Comparative gastroprotective effects of natural honey, Nigella sativa and cimetidine against acetylsalicylic acid induced gastric ulcer in albino rats. J Coll Physicians Surg Pak. 2011;21:151–156. [PubMed] [Google Scholar]
- 25.Salim EI. Cancer chemopreventive potential of volatile oil from black cumin seeds, Nigella sativa L., in a rat multi-organ carcinogenesis bioassay. Oncol Lett. 2010;1:913–924. doi: 10.3892/ol_00000162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kolahdooz M, Nasri S, Modarres SZ, Kianbakht S, Huseini HF. Effects of Nigella sativa L. seed oil on abnormal semen quality in infertile men: A randomized, doubleblind, placebo controlled clinical trial. Phytomedicine. 2014;21:901–905. doi: 10.1016/j.phymed.2014.02.006. http://dx.doi.org/10.1016/j.phymed.2014.02.006. [DOI] [PubMed] [Google Scholar]
- 27.Hosseinzadeh H, Tafaghodi M, Mosavi MJ, Taghiabadi E. Effect of aqueous and ethanolic extracts of Nigella sativa seeds on milk production in rats. J Acupunct Meridian Stud. 2013;6:18–23. doi: 10.1016/j.jams.2012.07.019. http://dx.doi.org/10.1016/j.jams.2012.07.019. [DOI] [PubMed] [Google Scholar]
- 28.Salem ML. Immunomodulatory and therapeutic properties of the Nigella sativa L. seed. Int Immunopharmacol. 2005;5:1749–1770. doi: 10.1016/j.intimp.2005.06.008. [DOI] [PubMed] [Google Scholar]
- 29.Imam A, Ajao MS, Ajibola MI, Amin A, Abdulmajeed AI, Lawal AZ, et al. Black seed oil reversed scopolamine-induced Alzheimer and cortico-hippocampal neural alterations in male Wistar rats. Bulletin of Faculty of Pharmacy, Cairo University. 2016;54(1):49–57. http://dx.doi.org/10.1016/j.bfopcu.2015.12.005. [Google Scholar]
- 30.Wahab IA, Habeeb BS, Maymunah OZ, Aminu I, Abdulbasit A, Sikiru AB, Lukuman AO, Bamidele VO. Honey prevents neurobehavioural deficit and oxidative stress induced by lead acetate exposure in male wistar rats- a preliminary study. Metabolic Brain Diseases. 2016;31:37–44. doi: 10.1007/s11011-015-9733-6. http://dx.doi.org/10.1007/s11011-015-9733-6. [DOI] [PubMed] [Google Scholar]
- 31.Bancroft JD, Gamble M. Theory and practice of histological techniques. Elsevier Health Sciences; 2008. [Google Scholar]
- 32.Castle DJ. Cannabis and psychosis: what causes what? F1000 Med Reports. 2013;5:1. doi: 10.3410/M5-1. http://dx.doi.org/10.3410/M5-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Johnston LD, O’Malley PM, Bachman JG, Schulenberg JE. Monitoring the future national results on adolescent drug use: overview of key findings 2011. Ann Arbor, MI: Institute for Social Research, University of Michigan; 2012. [Google Scholar]
- 34.Hermanson DJ, Marnett LJ. Cannabinoids, endocannabinoids, and cancer. Cancer Metastasis Rev. 2011;30:599–612. doi: 10.1007/s10555-011-9318-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hill AJ, Williams CM, Whalley BJ, Stephens GJ. Phytocannabinoids as novel therapeutic agents in CNS disorders. Pharmacol Ther. 2012;133:79–97. doi: 10.1016/j.pharmthera.2011.09.002. [DOI] [PubMed] [Google Scholar]
- 36.Omar MEA, Neveen AS, Marwa ES, Noha AA, Jihan SH, Zakaria AE. Cannabis-induced impairment of learning and memory: effect of different nootropic drugs. Excli J. 2013;12:193–214. [PMC free article] [PubMed] [Google Scholar]
- 37.Keeley RJ, Trow J, Bye C, McDonaldet RJ. Part II: Strain- and sex-specific effects of adolescent exposure to THC on adult brain and behaviour: variants of learning, anxiety and volumetric estimates. Behav Brain Res. 2015;288:132–152. doi: 10.1016/j.bbr.2015.01.001. http://dx.doi.org/10.1016/j.bbr.2015.01.001. [DOI] [PubMed] [Google Scholar]
- 38.Beheshti F, Hosseini M, Vafaee F, Shafei MN, Soukhtanloo M. Feeding of Nigella sativa during neonatal and juvenile growth improves learning and memory of rats. J Tradit Complement Med. 2015;6(2):146–152. doi: 10.1016/j.jtcme.2014.11.039. http://dx.doi.org/10.1016/j.jtcme.2014.11.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Salma AE, Siham ME, Aiman SE, Osama AE, Sanaa AK. Effect of Nigella sativa and wheat germ oils on scopolamine-induced memory impairment in rats. Bulletin of Faculty of Pharmacy, Cairo University. 2012;50:81–88. http://dx.doi.org/10.1016/j.bfopcu.2012.05.001. [Google Scholar]
- 40.Bin Sayeed MS, Asaduzzaman M, Morshed H, Hossain MM, Kadir MF, Rahman MR. The effect of Nigella sativa Linn. seed on memory, attention and cognition in healthy human volunteers. J Ethnopharmacol. 2013;148:780–786. doi: 10.1016/j.jep.2013.05.004. [DOI] [PubMed] [Google Scholar]
- 41.Bin Sayeed MS, Shams T, Fahim HS, Rahman MR, Mostofa A, Fahim Kadir M, et al. Nigella sativa L. seeds modulate mood, anxiety and cognition in healthy adolescent males. J Ethnopharmacol. 2014;152:156–162. doi: 10.1016/j.jep.2013.12.050. http://dx.doi.org/10.1016/j.jep.2013.12.050. [DOI] [PubMed] [Google Scholar]
- 42.Hosseini M, Mohammad T, Karami R, Rajaei Z, Sadeghnia HR, Soukhtanloo M. Effects of the hydro-alcoholic extract of Nigella sativa on scopolamine-induced spatial memory impairment in rats and its possible mechanism. Chin J Integr Med. 2014:1–7. doi: 10.1007/s11655-014-1742-5. [DOI] [PubMed] [Google Scholar]
- 43.Farzaneh V, Mahmoud H, Zahra H, Mohammad AE, Hamid RS, Masoumeh S, et al. the effects of Nigella Sativa hydro alcoholic extract on memory and brain tissues oxidative damage after repeated seizures in rats. Iran J of Pharm Res. 2015;14(2):547–557. [PMC free article] [PubMed] [Google Scholar]
- 44.Lawston J, Borella A, Robinson JK, Whitaker-Azmitia PM. Changes in hippocampal morphology following chronic treatment with the synthetic cannabinoid WIN 55,212-2. Brain Res. 2000;877(2):407–410. doi: 10.1016/s0006-8993(00)02739-6. [DOI] [PubMed] [Google Scholar]
- 45.Ashtari M, Avants B, Cyckowski L, Cervellione KL, Roofeh D, Cook P, et al. Medial temporal structures and memory functions in adolescents with heavy cannabis use. J Psychiatr Res. 2011;45(8):1055–1066. doi: 10.1016/j.jpsychires.2011.01.004. http://dx.doi.org/10.1016/j.jpsychires.2011.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schacht JP, Hutchison KE, Filbey FM. Associations between cannabinoid receptor-1 (CNR1) variation and hippocampus and amygdala volumes in heavy cannabis users. Neuropsychopharm. 2012;37(11):2368–2376. doi: 10.1038/npp.2012.92. doi: http://dx.doi.org/10.1038/npp.2012.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cousijn J, Wiers RW, Ridderinkhof KR, van den Brink W, Veltman DJ, Goudriaan AE. Grey matter alterations associated with cannabis use: results of a VBM study in heavy cannabis users and healthy controls. NeuroImage. 2012;59:3845–3851. doi: 10.1016/j.neuroimage.2011.09.046. http://dx.doi.org/10.1016/j.neuroimage.2011.09.046. [DOI] [PubMed] [Google Scholar]
- 48.El-Naggar T, Gomez-Serranillos MP, Palomino OM, Arce C, Carretero ME. Nigella sativa L. seed extract modulates the neurotransmitter amino acids release in cultured neurons in vitro. J Biomed Biotechnol. 2010;2010:398312. doi: 10.1155/2010/398312. http://dx.doi.org/10.1155/2010/398312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ismail N, Ismail M, Mazlan M, Latiff LA, Imam MU, et al. Thymoquinone prevents beta-amyloid neurotoxicity in primary cultured cerebellar granule neurons. Cell Mol Neurobiol. 2013;33(8):1159–1169. doi: 10.1007/s10571-013-9982-z. http://dx.doi.org/10.1007/s10571-013-9982-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hossein zadeh H, Parvardeh S, Asl MN, Sadeghnia HR, Ziaee T. Effect of thymoquinone and Nigella sativa seeds oil on lipid peroxidation level during global cerebral ischemia reperfusion injury in rat hippocampus. Phytomedicine. 2007;14:621–627. doi: 10.1016/j.phymed.2006.12.005. [DOI] [PubMed] [Google Scholar]
- 51.Hobbenaghi R, Javanbakht J, Sadeghzadeh Sh, Kheradmand D, Abdi FS, Jaberi MH, et al. Neuroprotective effects of Nigella sativa extract on cell death in hippocampal neurons following experimental global cerebral ischemia reperfusion injury in rats. J Neuro Sci. 2014;337:74–79. doi: 10.1016/j.jns.2013.11.019. http://dx.doi.org/10.1016/j.jns.2013.11.019. [DOI] [PubMed] [Google Scholar]
- 52.Hajra N. Nigella sativa: the miraculous herb. Pak J Biochem Mol Biol. 2011;44(1):44–48. [Google Scholar]
- 53.Amir K, Thapliyal RP, Chauhan SK. Antioxidant impacts of Boerhaavia Diffusa & black caraway oil on conjugated diene, lipid hydroperoxidation & MDA content in DMBA induced hypercholesterolemia in rats. Int J Pharm Pharm Sci. 2012;4(3):600–607. [Google Scholar]
- 54.Kim SJ, Lee JH, Chung HS, Song JH, Ha J, Bae H. Neuroprotective effects of AMP-activated protein kinase on scopolamine induced memory impairment. Korean J Physiol Pharmacol. 2013;17:331–338. doi: 10.4196/kjpp.2013.17.4.331. http://dx.doi.org/10.4196/kjpp.2013.17.4.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Moreira F, Lutz B. The endocannabinoid system: emotion, learning and addiction. Addict Biol. 2008;13(2):196–212. doi: 10.1111/j.1369-1600.2008.00104.x. http://dx.doi.org/10.1111/j.1369-1600.2008.00104.x. [DOI] [PubMed] [Google Scholar]
- 56.D’Souza DC, Perry E, MacDougall L, Ammerman Y, Cooper T, Wu YT, et al. The psychotomimetic effects of intravenous delta-9-tetrahydrocannabinol in healthy individuals: implications for psychosis. Neuropsychopharm. 2004;29(8):1558–1572. doi: 10.1038/sj.npp.1300496. http://dx.doi.org/10.1038/sj.npp.1300496. [DOI] [PubMed] [Google Scholar]
- 57.Genn RF, Tucci S, Marco EM, Viveros MP, File SE. Unconditioned and conditioned anxiogenic effects of the cannabinoid receptor agonist CP 55,940 in the social interaction test. Pharm Biochem Behav. 2004;77(3):567–573. doi: 10.1016/j.pbb.2003.12.019. http://dx.doi.org/10.1016/j.pbb.2003.12.019. [DOI] [PubMed] [Google Scholar]
- 58.Viveros MP, Marco EM, File SE. Endocannabinoid system, stress and anxiety responses. Pharm Biochem Behav. 2005;81:331–342. doi: 10.1016/j.pbb.2005.01.029. http://dx.doi.org/10.1016/j.pbb.2005.01.029. [DOI] [PubMed] [Google Scholar]
- 59.Okon VE, Obembe AO, Nna VU, Osim EE. Long-term administration of cannabis sativa on locomotor and exploratory behavior in mice. Res Neur Sci. 2014;3(1):7–21. [Google Scholar]
- 60.Noor NA, Fahmy HM, Mohammed FF, Elsayed AA, Radwan NM. Nigella sativa amliorates inflammation and demyelination in the experimental autoimmune encephalomyelitis-induced Wistar rats. Int J Clin Exp Pathol. 2015;8(6):6269–6286. [PMC free article] [PubMed] [Google Scholar]