Abstract
The way in which ketamine exerts its antidepressant effects has been perplexing. Evidence that a metabolite of the drug is responsible, and acts on a different target from ketamine, might be the key to an answer.
The novelist William Styron, who experienced depression, referred to the disorder as a black and howling tempest in the brain, noting1 that “the wisest books among them underscore the hard truth that serious depressions do not disappear overnight”. Indeed, depression is a painful and often deadly disorder that frequently requires months or more of treatment and that, for around one-third of sufferers, is treatment-resistant2. Ketamine is an attractive therapeutic, because it can act rapidly and effectively against even treatment-resistant depression3–6 — but the drug has side effects and does not always work. An understanding of ketamine’s mechanism of action, which could lead to improved treatments, has been widely sought. In this issue, Zanos et al.7 (page 481) provide several lines of evidence to indicate that it is not ketamine itself, but one of its metabolites, that is responsible for the drug’s antidepressant effects.
Ketamine has a moderately high binding affinity for, and can block the activity of, the NMDA receptor protein (NMDAR)8. This receptor is perhaps best known for its requirement9 in a phenomenon called long-term potentiation (LTP), which occurs widely in the brain, whereby the synaptic connections between neurons are strengthened, enhancing neural signalling10. The enhanced signalling produced by LTP underlies the formation of associative memories11,12.
How can transient blockade of NMDAR, and possibly LTP, have a rapid and long-lasting effect on human depression? Given the role of LTP in memory formation, it might be logical to assume that ketamine causes a brief block in the formation of memories. But even if this were true, how could it alleviate depression? To many physiologists, the idea that blocking NMDAR could treat depression has made no sense.
Zanos and colleagues’ initial experiments placed doubt on an NMDAR-mediated mechanism of action by ketamine (Fig. 1). The authors compared the effects of two different structural forms, or enantiomers, of ketamine, called (S)- and (R)-ketamine, which are normally administered together. (S)-Ketamine is three to four times better at blocking NMDAR than (R)-ketamine13, and so is predicted to be the better antidepressant under the NMDAR-inhibition model. However, the authors found that (R)-ketamine was several times more efficient at reducing depression-like behaviours in mouse models of depression. Furthermore, they confirmed14 that an even more potent NMDAR inhibitor, which binds to the same site as ketamine, fails to produce sustained antidepressant-like effects.
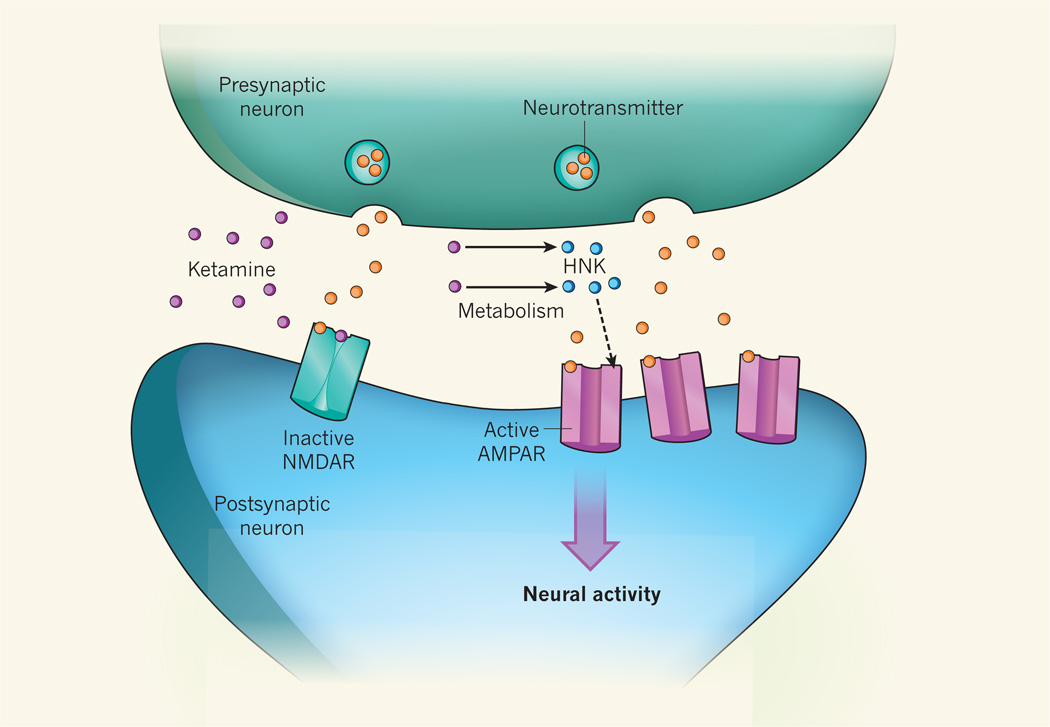
Figure 1. Metabolite mediator of ketamine.
How the drug ketamine exerts its antidepressant effects is unknown, although a common hypothesis states that it acts by binding to the receptor protein NMDAR on postsynaptic neurons, preventing neurotransmitter molecules released by presynaptic neurons from activating NMDAR and so inhibiting signalling processes triggered by the receptor. By contrast, Zanos et al.7 report that it is a metabolite of ketamine called hydroxynorketamine (HNK) that has antidepressant activity. They provide evidence that HNK, through unknown intermediates, increases the levels of another neuronal receptor protein, AMPAR, at synapses (dashed arrow), enhancing neural activity. But how this produces an antidepressant effect remains unclear.
So what could be responsible for the effects of ketamine treatment? The first hint came from comparing the drug’s activity in male and female mice. Zanos et al. confirmed a previous observation15 that a lower dose of ketamine is needed to reduce depression-like behaviours in females than in males. This could not be explained by different levels of ketamine in the brain. However, the authors found that levels of the ketamine metabolite hydroxynorketamine (HNK) were several-fold higher in the brains of females than males after the animals were given the same dose of the drug. Reducing the metabolism of ketamine to HNK reduced the effectiveness of ketamine towards depression-related behaviours in mice. Moreover, treating animals with HNK produced the same rapid and sustained antidepressant-like effects seen after treatment with ketamine. As with ketamine, the (R)-enantiomer of HNK had more-potent antidepressant-like effects than the (S)- form. And, importantly, the researchers showed that HNK neither binds to nor inhibits NMDAR.
The finding that the antidepressant effects of ketamine are not mediated through its actions on the NMDAR is a major advance. Nevertheless, it leads to an obvious, unanswered question — what is the molecular target of HNK responsible for these effects? This question should engender much activity by academic scientists, and possibly by large pharmaceutical companies that have been pouring capital into developing NMDAR inhibitors for treating depression. Candidate targets will probably soon emerge.
Although Zanos and colleagues did not identify such a target, they examined the role of another neural receptor protein, AMPAR, which is concentrated at synapses and mediates most neurotransmission in the brain. They found that a drug called NBQX, which reduces AMPAR activity throughout the brain, prevented and even reversed the antidepressant-like effects of ketamine and HNK in mice. It is surprising that a drug that indiscriminately reduces transmission in almost every brain circuit could alter the very specific effects of HNK and ketamine. The authors also show that transient application of HNK produces a long-lasting increase in AMPAR-mediated synaptic transmission (Fig. 1). How this can alleviate depression is not clear, unless HNK acts specifically to modulate the synapses that exhibit reduced function during depression16. Such a targeted action for HNK remains to be demonstrated.
Finally, Zanos et al. show that HNK does not elicit several of the cognitive and motor side effects that have been linked to ketamine. As such, this study represents important progress. Nonetheless, the molecular target and mechanism of action of HNK remain to be defined. Such advances might further the development of more-specific and effective treatments, allowing people with depression to step out of the darkness of this disorder.
References
- 1.Styron W. Darkness Visible: A Memoir of Madness. Random house: 1990. [Google Scholar]
- 2.Trevino K, McClintock SM, McDonald Fischer N, Vora A, Husain MM. Ann. Clin. Psychiatry. 2014;26:222–232. [PubMed] [Google Scholar]
- 3.Berman RM, et al. Biol. Psychiatry. 2000;47:351–354. doi: 10.1016/s0006-3223(99)00230-9. [DOI] [PubMed] [Google Scholar]
- 4.Zarate CA, Jr, et al. Arch. Gen. Psychiatry. 2006;63:856–864. doi: 10.1001/archpsyc.63.8.856. [DOI] [PubMed] [Google Scholar]
- 5.McGirr A, et al. Psychol. Med. 2015;45:693–704. doi: 10.1017/S0033291714001603. [DOI] [PubMed] [Google Scholar]
- 6.DiazGranados N, et al. J. Clin. Psychiatry. 2010;71:1605–1611. doi: 10.4088/JCP.09m05327blu. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zanos P, et al. Nature. 2016;533:481–486. doi: 10.1038/nature17998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Anis NA, Berry SC, Burton NR, Lodge D. Br. J. Pharmacol. 1983;79:565–575. doi: 10.1111/j.1476-5381.1983.tb11031.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Collingridge GL, Kehl SJ, McLennan H. J. Physiol. (Lond.) 1983;334:33–46. doi: 10.1113/jphysiol.1983.sp014478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bliss TVP, Lømo T. J. Physiol. (Lond.) 1973;232:331–356. doi: 10.1113/jphysiol.1973.sp010273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Morris RG, Anderson E, Lynch GS, Baudry M. Nature. 1986;319:774–776. doi: 10.1038/319774a0. [DOI] [PubMed] [Google Scholar]
- 12.Nabavi S, et al. Nature. 2014;511:348–352. doi: 10.1038/nature13294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ebert B, Mikkelsen S, Thorkildsen C, Borgbjerg FM. Eur. J. Pharmacol. 1997;333:99–104. doi: 10.1016/s0014-2999(97)01116-3. [DOI] [PubMed] [Google Scholar]
- 14.Autry AE, et al. Nature. 2011;475:91–95. doi: 10.1038/nature10130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Carrier N, Kabbaj M. Neuropharmacology. 2013;70:27–34. doi: 10.1016/j.neuropharm.2012.12.009. [DOI] [PubMed] [Google Scholar]
- 16.Li N, et al. Science. 2010;329:959–964. doi: 10.1126/science.1190287. [DOI] [PMC free article] [PubMed] [Google Scholar]