Abstract
Vascular endothelial growth factor (VEGF) serves an important role in promoting angiogenesis and tissue regeneration. However, the lack of an effective delivery system that can target this growth factor to the injured site reduces its therapeutic efficacy. Therefore, in the current study, collagen-binding VEGF was constructed by fusing a collagen-binding domain (CBD) to the N-terminal of native VEGF. The CBD-VEGF can specifically bind to collagen which is the major component of the extracellular matrix in fibrotic liver. The anti-fibrotic effects of this novel material were investigated by the carbon tetrachloride (CCl4)-induced liver fibrotic mouse model. Mice were injected with CCl4 intraperitoneally to induce liver fibrosis. CBD-VEGF was injected directly into the liver tissue of mice. The liver tissues were stained with hematoxylin and eosin for general observation or with Masson's trichrome staining for detection of collagen deposition. The hepatic stellate cell activation, blood vessel formation and hepatocyte proliferation were measured by immunohistochemical staining for α-smooth muscle actin, CD31 and Ki67 in the liver tissue. The fluorescent TUNEL assay was performed to evaluate the hepatocyte apoptosis. The present study identified that the CBD-VEGF injection could significantly promote vascularization of the liver tissue of fibrotic mice and attenuate liver fibrosis. Furthermore, hepatocyte apoptosis and hepatic stellate cell activation were attenuated by CBD-VEGF treatment. CBD-VEGF treatment could additionally promote hepatocyte regeneration in the liver tissue of fibrotic mice. Thus, it was suggested that CBD-VEGF may be used as a novel therapeutic intervention for liver fibrosis.
Keywords: vascular endothelial growth factor, angiogenesis, collagen, liver fibrosis, collagen-binding domain
Introduction
Liver fibrosis is a wound-healing response to several chronic liver diseases including chronic viral hepatitis, alcoholic liver disease and autoimmune hepatitis (1,2). Liver fibrosis is a serious public health problem which may progress to cirrhosis, liver cancer and death (3).
Certain therapeutic approaches executed in experimental or clinical studies have been demonstrated to prevent the progression of liver fibrosis, however numerous patients do not respond to these strategies (4–7). In addition, numerous pharmacological agents have been investigated in preclinical studies. However, few of them have entered clinical validation (7,8). At present, the only effective treatment for advanced cirrhosis is liver transplantation, however this is limited by numerous factors including donor shortage, risk of rejection and high costs (9). Stem cell therapy is another attractive approach for liver fibrosis that has been researched in recent years. The preliminary results appear promising, however, the experimental design has not been appropriate for the demonstration of its safety and efficacy in liver cirrhosis (10). Thus, novel therapies to reverse fibrosis are urgently required (11).
Vascular endothelial growth factor (VEGF), one of the most widely studied angiogenic growth factors, is critical for migration and proliferation of endothelial cells and formation of new vessels (12). Previous studies have indicated that VEGF gene injection or simultaneous preoperative injection of recombinant adenoviral vectors with VEGF gene effectively stimulates liver regeneration in cirrhotic rats (13,14). However, the half-life of VEGF is short and the metabolic degradation is too fast, thus it cannot be retained at a high enough level at the target site for a sufficient period to execute its function (15). Therefore, increasing the local concentration may improve the efficiency of VEGF.
It has been demonstrated that collagen-binding VEGF (CBD-VEGF) can specifically bind to collagen I and maintain its biological activity in vitro and in vivo (16). Liver fibrosis is characterized by increased deposition of extracellular matrix (ECM), which contains collagens I and III (17). Thus, collagen may be a potential target for VEGF at the fibrotic site.
In the current study, the anti-fibrotic effect of CBD-VEGF was investigated in a carbon tetrachloride (CCl4)-induced liver fibrotic mouse model.
Materials and methods
Preparation of CBD-VEGF
CBD-VEGF was prepared as previously described (16). Briefly, the full-length complementary DNA of human VEGF165 was amplified from the complementary DNA of human breast tumor cell line MCF-7. CBD-VEGF was constructed by linking a sequence that encodes the collagen-binding domain (TKKTLRT) with human VEGF165 complementary DNA. Then, the encoding gene of CBD-VEGF was inserted into pET-28a. The plasmid was transformed into the BL21 strain of Escherichia coli. The protein was expressed and then purified by nickel chelate chromatography and HiTrap heparin HP columns.
Animals
Male Balb/c mice (n=30; age, 6 weeks; weight, 22–25 g) were obtained from Experimental Animal Center of Nanjing Medical University (Nanjing, China) and were kept in a standard laboratory in an air-conditioned room with free access to food and water. All experimental protocols were conducted according to the Guide for the Care and Use of Laboratory Animals (18) and were approved by the Ethics Review Board for Animal Studies of Nanjing Drum Tower Hospital, Nanjing University Medical School (Nanjing, China).
Induction of liver fibrosis
Liver fibrosis was induced as described previously with slight modifications (19). Specifically, 19 mice were treated with CCl4 (diluted 1:5 in olive oil, 5 µl/g) intraperitoneally twice weekly for 12 weeks. Three mice died during the period of CCl4 injection. For the normal control group, 11 mice were injected with the same volume of olive oil. Following 12 weeks of the injection, 6 mice in the normal control group and 6 mice with the CCl4 injection group were sacrificed by cervical dislocation, and blood samples were collected from the heart and the liver samples were also collected.
CBD-VEGF injection
Subsequent to being treated with CCl4 for 12 weeks, a total of 15 mice were randomly assigned to three groups: The i) normal saline (NS) group (n=5); ii) CBD-VEGF group; and Balb/c mice were assigned as the normal control group (n=5). Mice were anesthetized by intraperitoneal injection with 40 mg/kg body weight of 1% pentobarbitol sodium (Merck & Co., Inc., Whitehouse Station, NJ, USA). Subsequent to anesthesia, 50 µl (10 µg) CBD-VEGF was injected directly into 6 sites of the liver tissue of mice. For NS and the normal control group, the same volume of NS was injected into the liver tissue of mice as control. Mice of the CBD-VEGF and NS groups were continued to be injected intraperitoneally with CCl4 with a lower dose (5 µl/g; diluted 1:6 in olive oil) twice weekly for 4 weeks.
Assessment of liver functions
After 4 weeks of CBD-VEGF injection, mice were sacrificed by cervical dislocation and the blood samples were collected from the heart. Serum alanine aminotransferase (ALT) and albumin (ALB) were measured using a 7600-020E full-automatic biochemical analyzer (Hitachi, Ltd., Tokyo, Japan).
Histological analysis and immunohistochemistry
Subsequent to sacrifice, the liver tissue was collected. The liver tissues were then fixed with 4% paraformaldehyde and embedded in paraffin, then sectioned (2 µm) and stained with hematoxylin and eosin (HE) for general observation or with Masson's trichrome staining for detection of collagen deposition. The relative area of liver fibrosis was measured from 6 randomly selected fields in the slide using Image-Pro Plus software (IPP version 6.0; Media Cybernetics, Inc., Silver Spring, MD, USA).
For immunohistochemistry, serial sections were deparaffinized, hydrated and incubated with antibodies from Abcam (Cambridge, MA, USA) against α-smooth muscle actin (α-SMA; 1:800; ab5694), CD31 (1:600; ab28364) and Ki67 (1:300; ab16667), respectively. The sections were incubated at 4 ˚C overnight with the primary antibodies. The sections were washed with phosphate-buffered saline (PBS) three times, and incubated for 20 min at 37 ˚C with IgG antibody conjugated to horseradish peroxidase (HRP; ZhongShan-Golden Bridge Biological Technology Company, Beijing, China; catalog no. PV-8000). The slices were washed with PBS for three times, and incubated in diaminobenzidine (DAB) and counterstained with hematoxylin for 1 min. α-SMA positive areas were quantified from 6 randomly selected fields from each animal sample using Image-Pro Plus software [α-SMA positive area = (percent of α-SMA positive area in the selected region-vascular luminal area)/total α-SMA positive area]. The number of CD31-positive microvessels and Ki67-positive proliferation hepatocytes were counted from 6 randomly selected fields of each sample in the operative region under a magnification of ×400.
TUNEL assay
The fluorescent terminal deoxynucleotidyl transferase dUTP nick-end labeling (TUNEL) assay was performed using an in situ cell death detection kit (Roche Diagnostics GmbH, Mannheim, Germany) to evaluate the hepatocyte apoptosis. Briefly, following routine deparaffinization and treatment with 3% H2O2 for 20 min, the sections were digested with pepsin at 37°C for 30 min and incubated with the TUNEL reaction mixture at 37°C for 60 min. DAB was added and visualized using a Converter-POD. Hematoxylin dye was used for counterstaining. Apoptotic cells were quantified by counting TUNEL-positive nuclei. For each sample, the number of TUNEL-positive cells was counted under a magnification of ×400. Six representative fields were evaluated for each mouse of the experimental groups.
Statistical analysis
All the data were expressed as mean ± standard deviation and were analyzed with SPSS software for Windows (version 16.0; SPSS, Inc., Chicago, IL, USA). An independent sample t-test was used to determine the statistical differences between two groups. Multiple group comparisons were tested using one-way analysis of variance. P<0.05 was considered to be indicate a statistically significant difference.
Results
CCl4-induced liver fibrosis
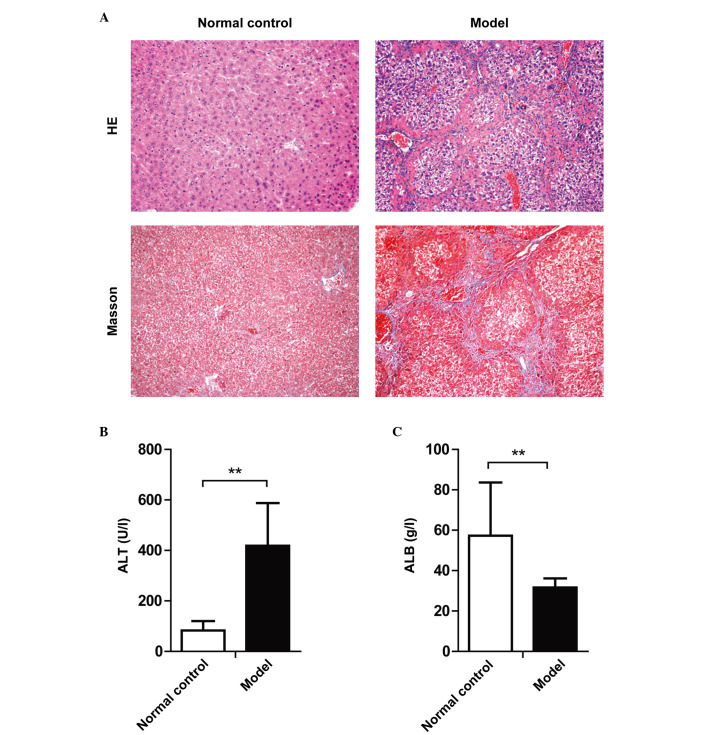
As indicated by the HE and Masson's trichrome staining, significant liver cell degeneration, necrosis, inflammatory cell infiltration and collagen deposition were identified in the liver tissue of mice after 12 weeks of CCl4 injection (Fig. 1A). In addition, significant elevation of serum ALT and reduction of serum ALB levels was observed (Fig. 1B and C). Serological and histological analysis demonstrated that the liver fibrosis mouse model was constructed successfully.
Figure 1.
Serological and histological alterations of mice following 12 weeks of CCl4 injection. Mice were intraperitoneally injected with CCl4 repeatedly for 12 weeks to induce liver fibrosis. (A) Histological changes of the liver tissues of CCl4-treated mice. Upper panels, HE staining; lower panels, Masson's trichrome staining. Magnification, ×100. (B) Serum ALT levels and (C) ALB levels. Data are presented as the mean ± standard deviation; **P<0.01. HE, hematoxylin and eosin; ALT, alanine aminotransferase; ALB, albumin.
CBD-VEGF alleviated CCl4-induced liver inflammation and fibrosis
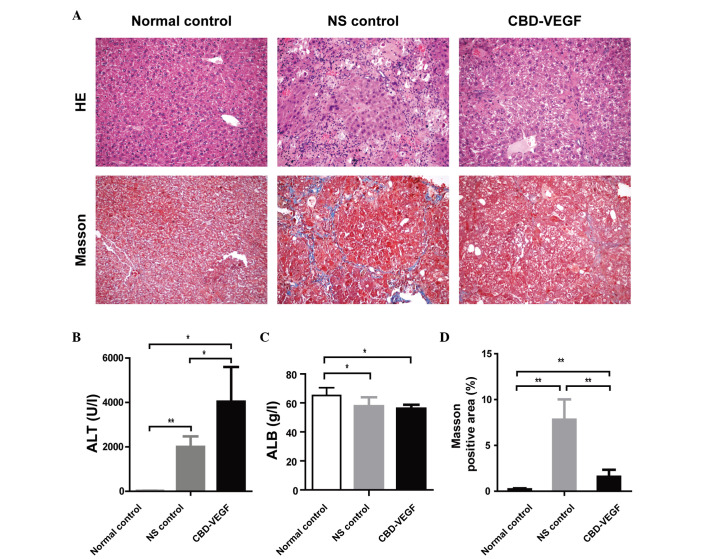
As demonstrated in tissue sections stained with HE, compared with sections from livers in the normal controls, CCl4 injection resulted in prominent degeneration, necrosis and inflammatory cell infiltration. However, the necrosis and inflammation of the liver in the CBD-VEGF group were slighter than NS group (Fig. 2A).
Figure 2.
CBD-VEGF alleviated CCl4-induced liver inflammation and fibrosis. CBD-VEGF was injected to the liver of CCl4-induced mice. After 4 weeks, the mice were sacrificed. (A) Conventional HE staining (upper panels) and Masson's trichrome staining (lower panels) were performed in liver paraffin sections. Magnification, ×100. (B) Serum ALT and (C) ALB levels were detected. (D) Comparisons of Masson-positive areas in each group. n=5 for each group. Data are presented as the mean ± standard deviation; **P<0.01, *P<0.05. CBD-VEGF, collagen-binding domain-vascular endothelial growth factor; HE, hematoxylin and eosin; ALT, alanine aminotransferase; ALB, albumin; NS, normal saline.
After 4 weeks of CBD-VEGF injection, the serum ALT levels were significantly increased in the CBD-VEGF and NS groups as compared with the normal control group (Fig. 2B). The serum ALT levels of the CBD-VEGF group were significantly higher than that of the NS group (P<0.05). The ALB levels were significantly reduced in the CBD-VEGF group and NS group as compared with the normal control group. However, no significant difference was identified between the CBD-VEGF and NS groups (Fig. 2C).
To assess the impact of CBD-VEGF on hepatic fibrogenesis resulting by CCl4, liver sections were stained with Masson's trichrome for detecting the deposition of collagens (Fig. 2A). Compared with sections from normal controls, liver sections from NS group indicated prominent blue staining in the fibrotic septa between nodules, suggesting a high level of collagen deposition. However, the CBD-VEGF injection could significantly reduce the size stained with Masson's trichrome in the livers when compared with the NS group (P<0.01; Fig. 2D).
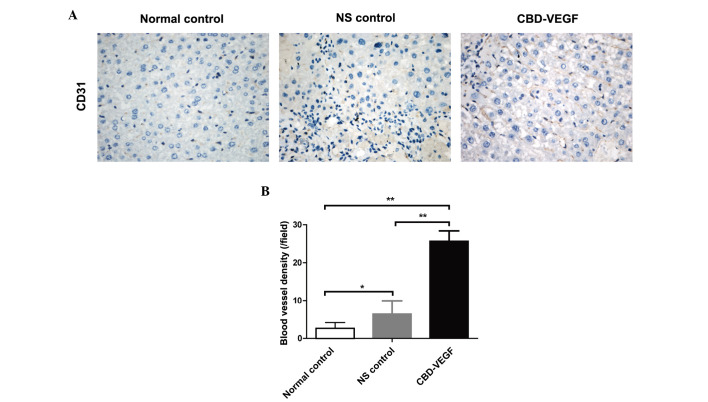
CBD-VEGF increased blood vessel formation
To detect the angiogenesis effect of CBD-VEGF, microvessels in the liver tissue where measured by immunohistochemical staining for CD31 (Fig. 3A). Following 4 weeks of CBD-VEGF injection, the blood vessel density in the CBD-VEGF group was significantly higher than that of NS group (Fig. 3B; P<0.01).
Figure 3.
CBD-VEGF improved blood vessel formation. (A) Immunohistochemical staining of CD31 for blood vessel density in the liver tissue. (B) Comparison of blood vessel density of each group. n=5 for each group. Data are presented as the mean ± standard deviation; **P<0.01, *P<0.05. CBD-VEGF, collagen-binding domain-vascular endothelial growth factor; NS, normal saline.
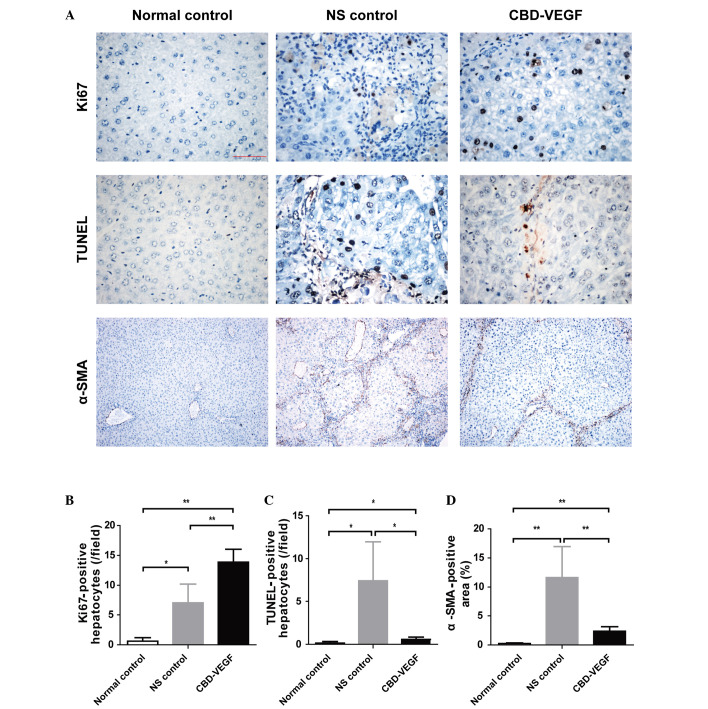
CBD-VEGF promoted hepatocyte proliferation, suppressed hepatocyte apoptosis and activated hepatic stellate cell (HSC) activation
Ki67, a nuclear antigen for cell proliferation, was used to determine whether CBD-VEGF could promote hepatocyte proliferation (Fig. 4A). Following 4 weeks of CBD-VEGF injection, the numbers of proliferative hepatocytes in the CBD-VEGF group were significantly increased compared with that of the NS group (P<0.01; Fig. 4B).
Figure 4.
CBD-VEGF promoted hepatocyte proliferation and suppressed hepatocyte apoptosis and HSC activation. Immunohistochemical staining of Ki67 and α-SMA in addition to a TUNEL assay were performed in the liver sections of each group. (A) Upper panels, Ki67 immunohistochemical staining (magnification, ×400); middle panels, TUNEL assay for evaluation of hepatocyte apoptosis (magnification, ×400); lower panels, α-SMA immunohistochemical staining (magnification, ×100). (B) Statistical analysis of proliferative hepatocytes, (C) TUNEL-positive hepatocytes and (D) α-SMA positive area of each group. n=5 for each group. Data are presented as the mean ± standard deviation; **P<0.01, *P<0.05. CBD-VEGF, collagen-binding domain-vascular endothelial growth factor; HSC, hepatic stellate cells; α-SMA, α-smooth muscle actin; NS, normal saline.
TUNEL assay was performed to evaluate the hepatocyte apoptosis (Fig. 4C). The NS group indicated a significant increase of hepatocyte apoptosis as compared with the normal control group (P<0.01). However, the CBD-VEGF group indicated a significantly smaller number of TUNEL-positive hepatocytes than that of NS group (P<0.05).
α-SMA is the unique marker for HSC. To evaluate the effect of CBD-VEGF on HSC activation, α-SMA immunohistochemistry was examined in liver tissue (Fig. 4A). Compared with the normal control group, the α-SMA positive areas were significantly increased in the NS group (Fig. 4D; P<0.01). However, the SMA-positive areas were significantly reduced following CBD-VEGF injection (P<0.01).
Discussion
Liver fibrosis is a characteristic of numerous types of chronic liver diseases resulting from chronic injury to the liver (20). Following chronic damage, HSC are activated and transdifferentiate into myofibroblast-like cells, secreting large amounts of ECM. Accumulation of the ECM would distort the hepatic vasculature and lead to shunting of the portal and arterial blood, impairing the material exchange between hepatocyte and hepatic sinusoidal blood (3). Hepatocytes cannot obtain the necessary oxygen and nutrients and thus this results in cell apoptosis (21). Therefore, promoting angiogenesis can improve hepatic microcirculation, and in turn reduce fibrosis (22).
VEGF is an endothelial cell-specific mitogen that improves the proliferation and migration of endothelial cells in vitro and induces new blood vessel growth in vivo (12). VEGF-induced angiogenesis has been used in tissue repair and regeneration (23). Administrating exogenous VEGF in injury site is an effective therapeutic method for promoting tissue repair and regeneration.
In patients with liver cirrhosis, the VEGF levels have been observed to be significantly reduced and therefore, administration of exogenous VEGF may be beneficial (21,24,25). Administering an adenoviral vector encoding murine VEGF into bile duct ligation fibrotic mice can promote tissue repair, and blockade of VEGF can significantly delay tissue repair (26). Injection of a plasmid encoding VEGF through the portal vein has been reported to induce the formation of fenestrae and reduce portal vein pressure in CCl4-induced cirrhotic rats (21).
However, it is difficult for VEGF to retain an effective local concentration due to its short half-time and rapid diffusion to extracellular fluids (15). Multiple injections may be required to maintain VEGF concentration. This would thus increase both the risk and cost of this potential therapy. Furthermore, excessive VEGF at injection sites and the diffusion of VEGF may cause possible adverse effects. Thus, a highly efficient and safe delivery system is required to enhance its local therapeutic efficiency and reduce its possible adverse effects.
Research groups are investigating how to localize and sustain VEGF proteins at the sites of injury. A previous study has demonstrated that CBD-VEGF had the characteristics of both specific binding to collagen and a stimulating effect on endothelial cell proliferation in vitro (16). It has been demonstrated that CBD-VEGF can be retained and concentrated at the border zone of infarction to improve cardiac function in the rat myocardial infarction model (16). CBD-VEGF can also be retained and concentrated at the local wound and promote diabetic wound healing in a rat diabetic wound model (27). In the full-thickness rat uterus injury model, CBD-VEGF injection was observed to result in a high local concentration and prolonged biological effect of the growth factor, and thus promote remodeling of the scarred uterus and improve uterine function (28).
Liver fibrosis is associated with major alterations in the composition of the ECM. In advanced cirrhosis, the liver contains approximately 6 times more ECM than normal, including collagens I and III (29). The native collagen could serve as a potential target for CBD-VEGF. In the present study, CBD-VEGF was injected directly into the liver tissue of the CCl4 induced fibrotic mice. The results indicated that CBD-VEGF injection could significantly attenuate the CCl4-induced chronic liver injury and fibrosis.
It remains unclear how CBD-VEGF promotes liver regeneration. The current study demonstrated that angiogenesis is significantly increased in the CBD-VEGF group compared with the NS group. It is suggested that the liver fibrotic attenuation by CBD-VEGF may be due to the increased vascularity which leads to the increased access to nutrients and oxygen for the hepatocytes. Ki67 was used to determine whether CBD-VEGF may promote hepatocyte proliferation. The results indicated that CBD-VEGF may promote hepatocyte regeneration in the mouse fibrotic liver model. To evaluate whether CBD-VEGF injection may protect hepatocytes from apoptosis, the TUNEL assay was performed. The results indicated that hepatocyte apoptosis was significantly reduced following injection of CBD-VEGF. Thus, it is hypothesized that exogenous CBD-VEGF may facilitate the transportation of nutrients and growth factors to hepatocytes through the microcirculation by upregulating angiogenesis and thus promoting hepatocyte regeneration and preventing hepatocyte apoptosis and HSC activation.
Taken together, the current study demonstrated that injection of CBD-VEGF could significantly promote angiogenesis and attenuate liver fibrosis in the liver of CCl4-induced fibrotic mice. The anti-fibrotic mechanisms of CBD-VEGF may be associated with the promotion of hepatocyte regeneration and the reduction of hepatocyte apoptosis and HSC activation. CBD-VEGF may potentially provide a novel treatment option for liver fibrosis.
Acknowledgements
The current study was supported by the Strategic Priority Research Program of the Chinese Academy of Sciences (grant no. XDA01030000), the National Natural Science Foundation of China (grant nos. 81470093 and 81672025), the Jiangsu Province's Outstanding Medical Academic Leader Program (grant no. LJ201154), the Jiangsu Province Clinical Medicine and Technology Special Program (grant no. BL2012034) and the Natural Science Foundation of Jiangsu Province for Young Scholars (grant no. BK20160121).
Glossary
Abbreviations
- ALB
albumin
- ALT
alanine aminotransferase
- CBD
collagen-binding domain
- CBD-VEGF
collagen-binding vascular endothelial growth factor
- CCl4
carbon tetrachloride
- ECM
extracellular matrix
- HE
hematoxylin and eosin
- HSC
hepatic stellate cells
- NS
normal saline
- SMA
smooth muscle actin
- TUNEL
terminal deoxynucleotidyl transferase dUTP nick-end labeling
- VEGF
vascular endothelial growth factor
References
- 1.Bataller R, Brenner DA. Liver fibrosis. J Clin Invest. 2005;115:209–218. doi: 10.1172/JCI200524282C1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tsukada S, Parsons CJ, Rippe RA. Mechanisms of liver fibrosis. Clin Chim Acta. 2006;364:33–60. doi: 10.1016/j.cca.2005.06.014. [DOI] [PubMed] [Google Scholar]
- 3.Schuppan D, Afdhal NH. Liver cirrhosis. Lancet. 2008;371:838–851. doi: 10.1016/S0140-6736(08)60383-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Inagaki Y, Nemoto T, Kushida M, Sheng Y, Higashi K, Ikeda K, Kawada N, Shirasaki F, Takehara K, Sugiyama K, et al. Interferon alfa down-regulates collagen gene transcription and suppresses experimental hepatic fibrosis in mice. Hepatology. 2003;38:890–899. doi: 10.1002/hep.1840380415. [DOI] [PubMed] [Google Scholar]
- 5.Veldt BJ, Heathcote EJ, Wedemeyer H, Reichen J, Hofmann WP, Zeuzem S, Manns MP, Hansen BE, Schalm SW, Janssen HL. Sustained virologic response and clinical outcomes in patients with chronic hepatitis C and advanced fibrosis. Ann Intern Med. 2007;147:677–684. doi: 10.7326/0003-4819-147-10-200711200-00003. [DOI] [PubMed] [Google Scholar]
- 6.Bruno S, Stroffolini T, Colombo M, Bollani S, Benvegnù L, Mazzella G, Ascione A, Santantonio T, Piccinino F, Andreone P, et al. Sustained virological response to interferon-alpha is associated with improved outcome in HCV-related cirrhosis: A retrospective study. Hepatology. 2007;45:579–587. doi: 10.1002/hep.21492. [DOI] [PubMed] [Google Scholar]
- 7.Schuppan D, Pinzani M. Anti-fibrotic therapy: Lost in translation? J Hepatol. 2012;56:S66–S74. doi: 10.1016/S0168-8278(12)60008-7. (Suppl 1) [DOI] [PubMed] [Google Scholar]
- 8.Pinzani M, Rombouts K, Colagrande S. Fibrosis in chronic liver diseases: Diagnosis and management. J Hepatol. 2005;42:S22–S36. doi: 10.1016/j.jhep.2004.12.008. (Suppl) [DOI] [PubMed] [Google Scholar]
- 9.Alqahtani SA. Update in liver transplantation. Curr Opin Gastroenterol. 2012;28:230–238. doi: 10.1097/MOG.0b013e3283527f16. [DOI] [PubMed] [Google Scholar]
- 10.Lorenzini S, Andreone P. Stem cell therapy for human liver cirrhosis: A cautious analysis of the results. Stem Cells. 2007;25:2383–2384. doi: 10.1634/stemcells.2007-0056. [DOI] [PubMed] [Google Scholar]
- 11.Popov Y, Schuppan D. Targeting liver fibrosis: Strategies for development and validation of antifibrotic therapies. Hepatology. 2009;50:1294–1306. doi: 10.1002/hep.23123. [DOI] [PubMed] [Google Scholar]
- 12.Yancopoulos GD, Davis S, Gale NW, Rudge JS, Wiegand SJ, Holash J. Vascular-specific growth factors and blood vessel formation. Nature. 2000;407:242–248. doi: 10.1038/35025215. [DOI] [PubMed] [Google Scholar]
- 13.Oe H, Kaido T, Mori A, Onodera H, Imamura M. Hepatocyte growth factor as well as vascular endothelial growth factor gene induction effectively promotes liver regeneration after hepatectomy in Solt-Farber rats. Hepatogastroenterology. 2005;52:1393–1397. [PubMed] [Google Scholar]
- 14.Oe H, Kaido T, Furuyama H, Mori A, Imamura M. Simultaneous transfer of vascular endothelial growth factor and hepatocyte growth factor genes effectively promotes liver regeneration after hepatectomy in cirrhotic rats. Hepatogastroenterology. 2004;51:1641–1647. [PubMed] [Google Scholar]
- 15.Silva EA, Mooney DJ. Spatiotemporal control of vascular endothelial growth factor delivery from injectable hydrogels enhances angiogenesis. J Thromb Haemost. 2007;5:590–598. doi: 10.1111/j.1538-7836.2007.02386.x. [DOI] [PubMed] [Google Scholar]
- 16.Zhang J, Ding L, Zhao Y, Sun W, Chen B, Lin H, Wang X, Zhang L, Xu B, Dai J. Collagen-targeting vascular endothelial growth factor improves cardiac performance after myocardial infarction. Circulation. 2009;119:1776–1784. doi: 10.1161/CIRCULATIONAHA.108.800565. [DOI] [PubMed] [Google Scholar]
- 17.Friedman SL. Molecular regulation of hepatic fibrosis, an integrated cellular response to tissue injury. J Biol Chem. 2000;275:2247–2250. doi: 10.1074/jbc.275.4.2247. [DOI] [PubMed] [Google Scholar]
- 18.National Research Council, corp-author. Guide for the Care and Use of Laboratory Animals. NIH Publications (USA); Bethesda: 1985. pp. 85–23. [Google Scholar]
- 19.Zhang D, Jiang M, Miao D. Transplanted human amniotic membrane-derived mesenchymal stem cells ameliorate carbon tetrachloride-induced liver cirrhosis in mouse. PLoS One. 2011;6:e16789. doi: 10.1371/journal.pone.0016789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Friedman SL. Liver fibrosis-from bench to bedside. J Hepatol. 2003;38:S38–S53. doi: 10.1016/S0168-8278(02)00429-4. (Suppl 1) [DOI] [PubMed] [Google Scholar]
- 21.Xu H, Shi BM, Lu XF, Liang F, Jin X, Wu TH, Xu J. Vascular endothelial growth factor attenuates hepatic sinusoidal capillarization in thioacetamide-induced cirrhotic rats. World J Gastroenterol. 2008;14:2349–2357. doi: 10.3748/wjg.14.2349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kantari-Mimoun C, Castells M, Klose R, Meinecke AK, Lemberger UJ, Rautou PE, Pinot-Roussel H, Badoual C, Schrödter K, Österreicher CH, et al. Resolution of liver fibrosis requires myeloid cell-driven sinusoidal angiogenesis. Hepatology. 2015;61:2042–2055. doi: 10.1002/hep.27635. [DOI] [PubMed] [Google Scholar]
- 23.Ferrara N. Vascular endothelial growth factor: Basic science and clinical progress. Endocr Rev. 2004;25:581–611. doi: 10.1210/er.2003-0027. [DOI] [PubMed] [Google Scholar]
- 24.Assy N, Paizi M, Gaitini D, Baruch Y, Spira G. Clinical implication of VEGF serum levels in cirrhotic patients with or without portal hypertension. World J Gastroenterol. 1999;5:296–300. doi: 10.3748/wjg.v5.i4.296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Akiyoshi F, Sata M, Suzuki H, Uchimura Y, Mitsuyama K, Matsuo K, Tanikawa K. Serum vascular endothelial growth factor levels in various liver diseases. Dig Dis Sci. 1998;43:41–45. doi: 10.1023/A:1018863718430. [DOI] [PubMed] [Google Scholar]
- 26.Yang L, Kwon J, Popov Y, Gajdos GB, Ordog T, Brekken RA, Mukhopadhyay D, Schuppan D, Bi Y, Simonetto D, Shah VH. Vascular endothelial growth factor promotes fibrosis resolution and repair in mice. Gastroenterology. 2014;146:1339–1350.e1. doi: 10.1053/j.gastro.2014.01.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yan X, Chen B, Lin Y, Li Y, Xiao Z, Hou X, Tan Q, Dai J. Acceleration of diabetic wound healing by collagen-binding vascular endothelial growth factor in diabetic rat model. Diabetes Res Clin Pract. 2010;90:66–72. doi: 10.1016/j.diabres.2010.07.001. [DOI] [PubMed] [Google Scholar]
- 28.Lin N, Li X, Song T, Wang J, Meng K, Yang J, Hou X, Dai J, Hu Y. The effect of collagen-binding vascular endothelial growth factor on the remodeling of scarred rat uterus following full-thickness injury. Biomaterials. 2012;33:1801–1807. doi: 10.1016/j.biomaterials.2011.11.038. [DOI] [PubMed] [Google Scholar]
- 29.Benyon RC, Iredale JP. Is liver fibrosis reversible? Gut. 2000;46:443–446. doi: 10.1136/gut.46.4.443. [DOI] [PMC free article] [PubMed] [Google Scholar]