Abstract Abstract
The ant genus Lenomyrmex was recently discovered and described from mid to high elevation rainforests in southern Central and northwestern South America. Lenomyrmex currently consists of six described species, which are only rarely collected. Here, we add a new species, Lenomyrmex hoelldobleri sp. n., which was discovered in a stomach content sample of the dendrobatid frog, Oophaga sylvatica, from northwestern Ecuador. Lenomyrmex hoelldobleri can be distinguished from other species in the genus by the presence of a well-developed petiolar node, whereas in all other species the node of the petiole is ill-defined. In addition to the shape of the petiolar node, Lenomyrmex hoelldobleri can be distinguished from the morphologically similar Lenomyrmex costatus by (i) the presence of the metanotal suture, (ii) the direction of the striae on dorsum of propodeum (concentrically transverse in Lenomyrmex hoelldobleri, longitudinal in Lenomyrmex costatus), (iii) the finely striate dorsum of postpetiole, (iv) its larger size, and (v) distinctly darker coloration. We also describe the gyne of Lenomyrmex foveolatus. This collection record from northwestern Ecuador extends the geographic distribution of Lenomyrmex foveolatus 400 km south from its previous record in Colombia. A revised taxonomic key to the workers and gynes of all described Lenomyrmex species is provided. We discuss the taxonomic relationship of Lenomyrmex hoelldobleri to other species in the genus and its biology based on the limited information that is currently available. Finally, we briefly discuss the feeding ecology of dendrobatid poison frogs in the context of providing a valuable source of rarely collected and cryptic new ant species.
Keywords: Formicidae, Dendrobatidae, feeding ecology, myrmecophagy, cryptic species
Resumen Abstract
El género de hormigas Lenomyrmex fue recientemente descubierto y descrito de bosques lluviosos tropicales de mediana a gran altitud en el sur de Centro América y del noroeste de Sur América. El género Lenomyrmex está actualmente compuesto de seis especies, las cuales son raramente colectadas. En este artículo, agregamos una especie nueva, Lenomyrmex hoelldobleri sp. n., que fue descubierta en una muestra de contenido estomacal de la rana dendrobátida, Oophaga sylvatica, colectada en el noroeste de Ecuador. Lenomyrmex hoelldobleri se puede distinguir de las otras especies del género por la presencia del nodo del pecíolo bien desarrollado, mientras que en todas las demás especies del género el nodo del pecíolo está mal definido o ausente. Además de la forma del nodo peciolar, Lenomyrmex hoelldobleri se puede distinguir de Lenomyrmex costatus por (i) la presencia de la sutura metanotal, (ii) la dirección de las estrías en el dorso del propodeo (concéntricamente transversal, en Lenomyrmex hoelldobleri, longitudinal en Lenomyrmex costatus), (iii) el dorso del postpecíolo finamente estriado, (iv) su mayor tamaño, y (v) la coloración más oscura. También se describe la reina de la especie Lenomyrmex foveolatus. Esta colección del noroeste de Ecuador amplía la distribución geográfica de Lenomyrmex foveolatus 400 kilómetros al sur de su registro previo en Colombia. Se presenta una clave taxonómica revisada para las obreras y reinas de todas las especies descritas de Lenomyrmex. Se discute la relación taxonómica de Lenomyrmex hoelldobleri con otras especies del género y su biología con base a la información limitada que está disponible actualmente. Finalmente, discutimos brevemente la ecología de la alimentación de las ranas venenosas dendrobátidas en el contexto de ser una valiosa fuente de especies de hormigas crípticas, nuevas y raramente recolectadas.
Introduction
The subfamily Myrmicinae is the most diverse clade of ants with currently more than 6,600 species, which is roughly equivalent to half the number of all described ant species (Bolton 2016). Within the past two decades ten new myrmicine genera and many more species have been discovered and described from the New World, including the extant genera Cryptomyrmex, Cyatta, Diaphoromyrma, Dolopomyrmex, Kalathomyrmex, Kempfidris, Lenomyrmex, Mycetagroicus, Patagonomyrmex, and Tropidomyrmex, testifying to the enormous diversity of this ant subfamily (Sosa-Calvo et al. 2013, and references therein; Fernández et al. 2014, Johnson and Moreau 2016). The myrmicine ants likely originated some 100 Million years ago during the late Cretaceous and the species in this group dispersed to all major ecosystems around the world (Ward et al. 2015). In addition to their hyperdiversity, vast geographic distribution, and old age of the clade, myrmicine ants also occupy diverse ecological niches (Hölldobler and Wilson 1990). Generalist predators and scavengers are common in speciose genera, such as Crematogaster, Monomorium, Myrmica, Pheidole, Solenopsis, and Tetramorium. In addition, highly specialized feeding habits originated in multiple myrmicine clades during the Paleocene and potentially contributed to the species richness and ecological success of these lineages. Especially noteworthy are the intricate behaviors of the seed harvesting ants, the fungus-growing ants, and the highly predaceous dacetine ants, which were expertly reviewed in Hölldobler and Wilson's (1990) landmark monograph “The Ants”.
Just prior to the turn of the millennium, Fernández and Palacio (1999) described the myrmicine genus Lenomyrmex from the Neotropical region. Lenomyrmex ants are rarely collected and seven species are currently known from this genus, including Lenomyrmex hoelldobleri sp. n., the species described here. The geographic distribution of Lenomyrmex extends from Costa Rica in the North to southwestern Ecuador in the South, and only Lenomyrmex inusitatus is found on the eastern slope of the Andes (Fernández and Palacio 1999, Fernández 2001, Fernández and Sendoya 2004, Longino 2006, Delsinne and Fernández 2012). So far, all Lenomyrmex species were found in moist tropical rainforests, associated with medium and high elevation between 500 and 1800 meters above sea level (Longino 2006, Delsinne and Fernández 2012). The slender, elongate, and highly conspicuous mandibles with minute peg-like denticles are a synapomorphy of all Lenomyrmex species, suggesting specialized predatory habits (Fernández and Palacio 1999). Unfortunately, the feeding behavior of these rather cryptic ants was never observed and the prey organisms Lenomyrmex feeds on are unknown. Lenomyrmex appears to be a close relative of Daceton trap-jaw ants, which are both part of a monophyletic group of specialized predators (Ward et al. 2015).
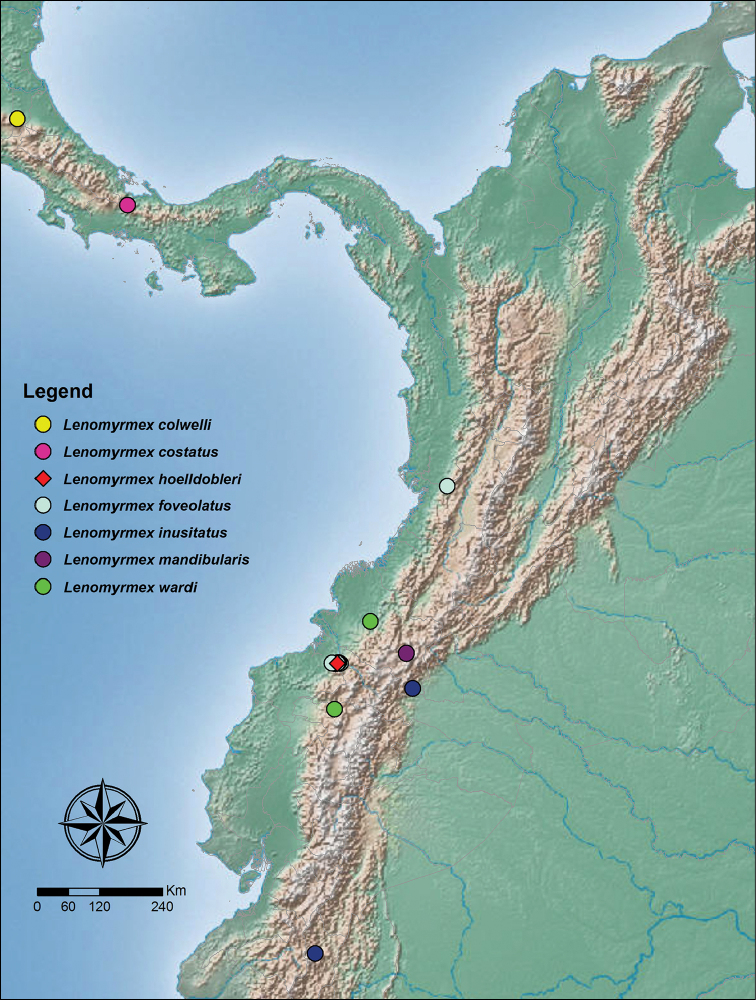
Here, we describe the new species Lenomyrmex hoelldobleri sp. n. from northwest Ecuador (Fig. 1), which was discovered in stomach content samples of the dendrobatid poison frog, Oophaga sylvatica. We also diagnose the gyne of Lenomyrmex foveolatus (Fig. 3), which also fell prey to Oophaga sylvatica. This new record of Lenomyrmex foveolatus from northwest Ecuador expands the known geographic distribution range of this species from Colombia to Ecuador (Fig. 4). Many amphibians, including species of the aposematic poison frogs in the family Dendrobatidae, and non-avian reptiles are known to be specialized predators of ants (Weber 1938, Darst et al. 2005, Esteves et al. 2008, Sosa-Calvo 2015), and therefore they provide interesting sources of rarely collected and new arthropod species. Dendrobatid poison frogs sequester alkaloids that are found in their skin toxins from their diet (Daly et al. 2000, Saporito et al. 2004, 2007, Darst et al. 2005, McGugan et al. 2016), and therefore we briefly discuss the ecology of the specialized ant feeding behavior, or myrmecophagy, of dendrobatid frogs.
Figure 1.
Worker of Lenomyrmex hoelldobleri in full-face (A), dorsal (B), and lateral (C) views. The depicted worker is the holotype with the unique specimen identifier USNMENT01124322. Scale bars: 0.5 mm (A), 1 mm (B, C).
Figure 3.
Dealate gyne of Lenomyrmex foveolatus in full-face (A), dorsal (B), and lateral (C) views. The depicted gyne has the unique specimen identifier USNMENT01127956. Scale bars: 0.5 mm (A), 1 mm (B, C).
Figure 4.
Geographic distribution of the genus Lenomyrmex in Central and South America.
Materials and methods
Material examined. The examined ant and frog specimens have been deposited at the following institutions.
CJ
CRC
DZUP
ICN
MCZC
QCAZ
USNM
Morphological analysis. Specimens were examined and measured using a Leica M165 C stereomicroscope fitted with a stage micrometer. Measurements were recorded to the nearest 0.01 mm at 40x magnification. To generate composite images of the specimens we utilized a Leica DFC450 digital camera mounted on a Leica M205 C stereomicroscope. Composite images were assembled using the Leica Application Suite (Version 4.5) and the Helicon Focus (Version 6.2.2) software packages. Conventions for morphological terminology, measurements, and indices follow those utilized in recent taxonomic studies of Neotropical ants and frogs (Fernández and Palacio 1999, Brown et al. 2011, Delsinne and Fernández 2012, Rabeling et al. 2015). Measurements are given in millimeters. Measurements and indices are defined as follows:
EL
GL
HL
HW
ML
PL
PPL
PPW
PW
SL
SVL
TL
WL
CI
MI
OI
SI
Results
Taxonomy
Lenomyrmex hoelldobleri sp. n.
http://zoobank.org/AD681140-8B64-4835-A2B7-E9730BD2CA70
Holotype worker.
ECUADOR: Esmeraldas; 4 Km SW of Alto Tambo, next to Reserve Otokiki; elevation 676 meters above sea level; GPS coordinates: 0.912306, -78.583528; 09.vii.2013; from the stomach content of a male specimen (frog voucher number: CJ1689; SVL = 36.7 mm) of the Little Devil poison frog, Oophaga sylvatica; leg. L. A. O'Connell, E. E. Tapia, L. A. Coloma; unique ant specimen identifier: USNMENT01124322; deposited in USNM.
Measurements of holotype.
HL: 1.02; HW: 0.78; ML: 0.45; SL: 0.81; EL: 0.18; WL: 1.58; PL: 0.73; PW: 0.23; PPL: 0.46; PPW: 0.35; GL: 1.00; TL: 4.77; CI: 76; OI: 23; SI 79.
Description, holotype worker.
Mandibles elongate, triangular with masticatory margin crenulated, 3 times longer than basal margin, sclerotized blunt peg-like denticles barely visible at 80x magnification (Fig. 1A). Clypeus without carinae, apical margin mostly convex and with a median angle; posterior margin convex, barely projects backward between frontal carinae. Frontal lobes inconspicuous, little expanded laterally, only partially covering antennal condyles. Antennal fossae large, deep, 1.5x longer than broad. Antennal scrobes absent. In full-face view, head with a broadly convex posterior cephalic margin; in full-face view, maximum width, just behind eyes, slightly narrowing posterad. Compound eyes large, protruding, with 15 facets along maximum diameter. Mesosomal profile with pronotum, mesonotum, and propodeum differentiated. Metanotal impression clearly marked (Fig. 1C). Propodeum armed with 2 long, acute spines, clearly longer than distance between their bases (Figs 1B, C). In lateral view, inferior lobes of propodeum triangular. Femora claviform. Meso- and metatibiae lacking spurs. Tarsal claws simple, elongated. In lateral view, petiole long, fusiform, pedunculate; petiolar node well-defined; antero-ventral subpetiolar process directed forward, compressed in anterior-posterior direction, giving appearance of a spine in lateral view; anterolateral edges of process continue dorsally toward sides of petiolar peduncle. In lateral view, postpetiole dome-like, lacking a ventral process.
Mandibles smooth, slightly shining (Fig. 1A). Head, mesosoma, dorsum of petiolar node and postpetiole costate. The costae longitudinal in the head frons, concentric around eyes, predominantly transverse on pronotal dorsum, transverse on mesonotum, concentrically transverse on dorsum of propodeum (Fig. 1B), longitudinal on disc of petiole and postpetiole (Figs 1B, C). Lateral margins of mesosoma with longitudinal costae, coxae with transverse costae, discrete in meso- and meta-coxae. Petiolar peduncle with granulations. Postpetiole mostly shining, and dorsolaterally with fine longitudinal striae and granulations ventrolaterally. Gaster smooth, shining except for dense punctures on pygidium and hypopygium.
Clypeal apical margin with several short, erect hairs. Head frons, leading edge of antennal scape, pronotum, node of petiole, disc of postpetiole, and gaster with scattered erect hairs, most of them longer than maximum diameter of eye (Fig. 1A & B). Erect hairs on dorsum of petiole and legs as long as, or shorter than, maximum eye diameter. Hairs on antennal scape longer than maximum diameter of antennal scape. Funicular antennal segment with numerous short decumbent hairs. Otherwise body devoid of hairs. Body black; legs and coxae lighter; antennal club, mandibles, and gastric apex yellowish-brown.
Distribution and ecology.
The single known specimen of Lenomyrmex hoelldobleri was recovered from a stomach content sample of the dendrobatid poison frog, Oophaga sylvatica. The habitat where the poison frog Oophaga sylvatica was collected was a secondary habitat with forest fragments and pastureland. The region encompasses remnant Evergreen Foothill Forests of the Western Cordillera (Ministerio del Ambiente del Ecuador 2012). This area is located in the Chocó Ecoregion, one of the most biologically diverse areas in the world with exceptionally high levels of endemism. The Chocó is considered one of the biodiversity hotspots for conservation purposes (Mittermeier et al. 1998, Myers et al. 2000) and one of the most threatened areas in the world (Brooks et al. 2002). The coastal northwest region of Ecuador, where the Alto Tambo area is found, is part of the wettest ecosystem known in Ecuador, with rainfalls ranging from 2000 up to 4000 mm annually (Ministerio del Ambiente del Ecuador 2012). Temperatures range from an annual average of 20 to 25° C (Ministerio del Ambiente del Ecuador 2012). The Foothill Forests are characterized by the dominance of tree species that can exceed 30 m in height. Trees are covered by orchids, bromeliads, ferns, and aroids. These forests have a dense herbaceous undergrowth layer dominated by Marantaceae, Araceae, and Polypodiopsida (Cerón et al. 1999). Two species of Lenomyrmex (Lenomyrmex foveolatus, Lenomyrmex hoelldobleri) occur in sympatry in the Alto Tambo area (Fig. 4).
Queen and male.
Unknown.
Etymology.
This species is named in honor of our colleague and friend Bert Hölldobler on the occasion of his 80th birthday. Because of Bert's passion for ants, his pioneering and high-caliber contributions to entomology and behavioral ecology, as well as his dedication to mentoring the next generation of myrmecologists, myrmecology has become its own discipline in entomology, and continues to attract enthusiastic students who share Bert's love for ants.
Comments.
Lenomyrmex hoelldobleri can be distinguished from all other Lenomyrmex species by the following combination of character states: (i) petiolar node conspicuous, well-defined; (ii) a well-defined metanotal suture; (iii) conspicuous costae on its body; (iv) long erect hairs on the scape, and (v) size, being larger than all known species. Lenomyrmex costatus is morphologically most similar to Lenomyrmex hoelldobleri and both share the integumental sculpturing and the presence of long setae on the antennal scapes. However, Lenomyrmex hoelldobleri can be clearly distinguished from Lenomyrmex costatus by its well-defined petiolar node, the presence of the metanotal suture, its larger size, by having concentrically transverse striae on dorsum of propodeum (longitudinal in Lenomyrmex costatus), and the distinctly darker coloration (compare Figs 1, 2). To differentially diagnose Lenomyrmex hoelldobleri and Lenomyrmex costatus, we examined the holotype of Lenomyrmex costatus (Fig. 2). The specimen is deposited at Museum of Comparative Zoology at Harvard University. The specimen information is as follows: Panama; Bocas del Toro; Fortuna to Chiriqui Grande rd.; elevation 1050 meters above sea level; GPS coordinates: 8°47'N, 82°12'W; 14.vii.1987; leg. D. M. Olson (DMO523); unique ant species identifier: MCZ-ENT00036069.
Figure 2.
Worker of Lenomyrmex costatus in full-face (A), dorsal (B), and lateral (C) views. The depicted worker is the holotype with the unique specimen identifier MCZ-ENT00036069. Scale bars: 0.5 mm (A), 1 mm (B, C).
Lenomyrmex foveolatus
Fernández & Palacio
Gyne.
ECUADOR: Esmeraldas; Reserve Otokiki-Alto Tambo; elevation 723 meters above sea level; GPS coordinates: 0.918533, -78.566800; 08.vii.2013; from the stomach content of a female specimen (frog voucher number: CJ1658, SVL = 36.7 mm) of the Little Devil frog, Oophaga sylvatica; leg. L. A. O'Connell, E. E. Tapia, L. A. Coloma; unique ant specimen identifier: USNMENT01127956; deposited in USNM.
Gyne measurements.
HL: 0.91; HW: 0.83; ML: 0.49; SL: 0.75; EL: 0.23; WL: 1.47; PL: 0.78; PW: 0.25; PPL: 0.35; PPW: 0.29; GL: 1.41; TL: 5.40; CI: 91; MI: 55; OI: 0.29; SI 90 (n=1).
Description, dealate gyne.
As in the worker description (Fernández and Palacio 1999: 13–14) but mesosoma with caste-specific morphology related to wing-bearing and with the following differences: in full-face view, mid portion of anterior margin of clypeus weakly concave, forming a pair of lateral angles; compound eyes larger than in worker, with 12 ommatidia in maximum diameter; three small but conspicuous ocelli present. Dorsum of pronotum, mesoscutum, axillae, and scutellum lustrous and weakly coriaceous; dorsolateral portion of pronotum with small and sparse foveae; in dorsal view, posterior lateral portions of pronotum concave. In dorsal view, mesoscutum somewhat triangular anteriorly; parapsidal lines short, conspicuous; scuto-scutellar sulcus well-developed; posterior margin of scutellum subquadrate, lacking tubercles. Dorsum and declivity of propodeum lustrous; posterior margin of propodeum angulate, lacking tubercles or spines (as in worker). Mesopleuron clearly divided to anepisternum and katepisternum by oblique mesopleural sulcus. Pilosity of body consisting of small, simple, appressed hairs.
Additional material examined.
ECUADOR: Esmeraldas; Alto Tambo; elevation 788 meters above sea level; GPS coordinates: 0.907450, -78.540583; 05.vii.2013; from the stomach content of a male specimen (frog voucher number: CJ1770) of the Little Devil frog, Oophaga sylvatica; leg. L. A. O'Connell, E. E. Tapia, L. A. Coloma; [1w, CRC, USNMENT01127960]. Same as previous entry but, 200–300 m SW El Placer; elevation 551 meters above sea level; GPS coordinates: 0.901050, -78.618233; 07.vii.2013; from the stomach content of a male specimen (frog voucher number: CJ1632; SVL = 35.6 mm) of the Little Devil frog, Oophaga sylvatica; leg. L. A. O'Connell, E. E. Tapia, L. A. Coloma; [1w, QCAZ, USNMENT01127955]. Same as previous entry but, next to Reserva Otokiki (farm next to railway); elevation 676 meters above sea level; GPS coordinates: 0.912306, -78.583528; 09.vii.2013; from the stomach content of a male specimen (frog voucher number: CJ1690; SVL = 38.2 mm) of the Little Devil frog, Oophaga sylvatica; leg. L. A. O'Connell, E. E. Tapia, L. A. Coloma; [3w, DZUP, ICN, USNM; USNMENT01127957, USNMENT01127935, USNMENT01127958]. Same as previous entry but, from the stomach content of a female specimen (frog voucher number: CJ1691; SVL = 34.7 mm) of the Little Devil frog, Oophaga sylvatica [1w, QCAZ; USNMENT01127954]. Same as previous entry but, Lita; around bamboo forest; elevation 326 meters above sea level; GPS coordinates: 0.911944, -78.680833; 10.vii.2013; from the stomach content of a female specimen (frog voucher number: CJ1695; SVL = 32 mm) of the Little Devil frog, Oophaga sylvatica; leg. L. A. O'Connell, E. E. Tapia, L. A. Coloma; [1w, CRC, USNMENT01127936].
Worker measurements.
HL: 0.81–0.90; HW: 0.73–0.83; ML: 0.42–0.47; SL: 0.61–0.73; EL: 0.17–0.20; WL: 1.06–1.42; PL: 0.65–0.73; PW: 0.21–0.23; PPL: 0.29–0.35; PPW: 0.25–0.28; GL: 0.98–1.34; TL: 4.31–5.19; CI: 90–94; MI: 51–57; OI: 0.25–0.28; SI 82–95 (n=7).
Comments.
Specimens from the Colombian type series could not been examined, but based on the Fernández and Palacio's (1999) description, the worker specimens collected from Ecuador closely resemble the specimens from Colombia. The main differences between the specimens belonging to these two populations are: (i) the fovea on dorsum of head are scattered in the Colombian specimens and more densely clustered in the Ecuadorian individuals; (ii) the specimens from Ecuador have rounded propodeal lobes differing from the acute propodeal lobes observed in the type series from Colombia; (iii) in the specimens from Ecuador the metapleural gland bulla is striate, and striae seem absent from bulla of the Colombian specimens.
Distribution and ecology.
Previously only known from the type locality in western Colombia, Departamento del Valle, Darién, middle Río Calima basin. The current record near Alto Tambo extends the species geographic range 400 km south of the type locality (Fig. 4). General habitat data is the same as in the Lenomyrmex hoelldobleri account, except that the frog was collected in a banana plantation.
Key to the workers of Lenomyrmex (modified from Delsinne and Fernández 2012)
| 1 | Mesosoma predominantly smooth and shiny, without erect hairs | 2 |
| – | Mesosoma with conspicuous sculpture and at least one pair of erect hairs | 3 |
| 2(1) | Propodeum without spines; head only foveolate (SW Colombia) | Lenomyrmex foveolatus |
| – | Propodeum with a pair of acute and well-defined spines; head foveolate, with median longitudinal striae (Cordillera Oriental of the Andes in S Colombia and S Ecuador) | Lenomyrmex inusitatus |
| 3(1) | Dorsum of head and petiole with longitudinal conspicuous costae; erect hairs of antennal scape as long as or longer than maximum diameter of scape | 4 |
| – | Dorsum of head densely rugo-reticulate; sculpture of the petiole variable, rugulate to rugo-reticulate or longitudinally striate but never costate; erect hairs of antennal scape not longer than maximum diameter of the scape | 5 |
| 4(3) | Node of petiole inconspicuous and ill-defined; dorsum of propodeum with longitudinal striae; in dorsal view, disc of postpetiole weakly sculptured; body ferruginous yellow (W Panama) | Lenomyrmex costatus |
| – | Node of petiole conspicuous, well-defined; dorsum of propodeum with transverse striae; in dorsal view, disc of postpetiole finely striate; body black (W Ecuador) | Lenomyrmex hoelldobleri |
| 5(3) | Length of propodeal spines approximately equal to distance between their bases; mesopleuron with some irregular longitudinal striae, but mostly smooth and shiny; metapleuron with irregular longitudinal striae; HL > 0.80 mm; mesosoma with only two suberect hairs on the pronotum (SW Colombia) | Lenomyrmex mandibularis |
| – | Length of propodeal spines variable, either shorter or longer than distance between their bases; metapleuron and subsequent portion of mesopleuron with fine transverse rugulae or rugo-reticulate, without smooth areas; HL < 0.80 mm; mesosoma with numerous erect to suberect hairs | 6 |
| 6(5) | Propodeal spines shorter than distance between their bases; eyes with six or seven facets in maximum diameter; petiolar node protruding over the peduncle and well defined; postpetiolar dorsum with longitudinal striae (NW Ecuador, SW Colombia) | Lenomyrmex wardi |
| – | Propodeal spines longer than distance between their bases; eyes with about nine facets in maximum diameter; petiolar node undifferentiated from the peduncle; postpetiolar dorsum smooth and polished (Costa Rica) | Lenomyrmex colwelli |
Key to the known queens of Lenomyrmex (modified from Delsinne and Fernández 2012)
| 1 | Head foveolate; median longitudinal striae may be present. Body lacking erect hairs | 2 |
| – | Head densely rugo-reticulate. Body with erect hairs | 3 |
| 2(1) | Propodeal spines present. Mesosoma shiny with sparse punctures on pronotum, mesopleuron, metapleuron, and propodeum. Scutellum and axillae foveolate, mesoscutum foveolate-striate | Lenomyrmex inusitatus |
| – | Propodeal spines absent. Mesosoma predominantly smooth and shiny, lacking punctures in mesopleuron, metapleuron, and propodeum. Pronotum with a few foveae on lateral portions. Scutellum and axillae smooth. Mesoscutum smooth and shining | Lenomyrmex foveolatus |
| 3(1) | Propodeal spines approximately equal in length to distance between their bases; integument predominantly shiny; HL > 0.80 | Lenomyrmex mandibularis |
| – | Propodeal spines notably shorter than distance between their bases; integument predominantly opaque; HL <0.80 | Lenomyrmex wardi |
Discussion
All seven species of the myrmicine ant genus Lenomyrmex are characterized by their elongate, highly modified mandibles, which are indicative of specialized predatory habits (Fernández and Palacio 1999, Fernández 2001, Longino 2006, Delsinne and Fernández 2012). Interestingly, Lenomyrmex ants combine morphological characters typical of highly specialized predators with plesiotypic characters, such as the flexible suture between pronotom and mesonotum, which is atypical for myrmicine ants, but characteristic of early ant lineages with a predatory lifestyle in low-light environments (Bolton 1990, Rabeling et al. 2008, Yamane et al. 2008). This combination of plesiomorphic and derived morphological characters made it difficult to place the genus Lenomyrmex within the myrmicine phylogeny and its phylogenetic relationship to other members of the subfamily remained uncertain at first (Fernández and Palacio 1999). A recent molecular phylogenetic reconstruction of the subfamily Myrmicinae inferred Lenomyrmex as a close relative of the genus Daceton (Ward et al. 2015), which are predatory, arboreal ants (Wilson 1962, Azorsa and Sosa-Calvo 2008). Interestingly, the Daceton-species group is the sister group of the fungus-growing ants. Unfortunately, the sister-group relationship of the predatory trap-jaw ants and fungus-growing ants does not provide new insights into the much-debated evolutionary origins of the unique and highly derived fungus-growing behavior (Hölldobler and Wilson 1990, 2011, Mueller et al. 2001, Rabeling et al. 2006, Mehdiabadi and Schultz 2010). The current phylogenetic hypothesis suggests that either ant fungiculture evolved from a predatory ancestral state or, alternatively, the fungicultural and the predatory behaviors evolved along independent evolutionary trajectories from a common ancestor with generalist feeding habits. The discovery of a “missing link” would mark a real advance in our understanding about the evolutionary trajectories towards highly derived behaviors.
Lenomyrmex ants are rare in museum collections and the majority of the specimens have been collected sporadically in leaf-litter samples (Fernández and Palacio 1999, Fernández 2001, Longino 2006, Delsinne and Fernández 2012). So far only colonies of Lenomyrmex mandibularis have been collected manually because this species constructs nests in stems of a Palicourea species in the plant family Rubiaceae and in rotten logs (Fernández and Palacio 1999). In addition to systematic leaf litter sampling and hand collecting, the examination of stomach contents of leaf-litter foraging amphibians is a valuable source of cryptic and rarely collected ant species (Weber 1938, Delsinne and Fernández 2012, Sosa-Calvo 2015). Many species of amphibians and non-avian reptiles specialize on ant feeding and some species are predominantly myrmecophagous (Solé et al. 2002, Darst et al. 2005, Esteves et al. 2008). In the Neotropical poison frog family Dendrobatidae, myrmecophagy evolved at least twice, possibly three times independently (Santos et al. 2003, Darst et al. 2005), and the frogs sequester the skin alkaloids mostly from their ant and mite diet (McGugan et al. 2016). In addition to ants and mites, other arthropods, such as beetles and millipedes, are considered alkaloid sources for poison frogs (Dumbacher et al. 2004, Saporito et al. 2003, 2004, 2007).
To study the feeding ecology of the Little Devil poison frog, Oophaga sylvatica, the stomach contents of more than 300 individuals from different populations in Ecuador have been examined recently (McGugan et al. 2016, O'Connell, Sosa-Calvo et al., unpublished data). The majority of the frogs' diet consisted of ants, constituting between 40 and 86 % of diet volume in different frog populations. Of the more than 3000 examined prey items, 44 different ant genera could be identified, representing nine different subfamilies (Sosa-Calvo, O'Connell et al., unpublished data). The majority of the eaten ant genera belong to the subfamily Myrmicinae, including the rarely collected genus Lenomyrmex, with a total of nine specimens belonging to two species, Lenomyrmex hoelldobleri (the holotype worker) and Lenomyrmex foveolatus (seven workers and one gyne). Other cryptic and rarely collected ant genera include Leptanilloides, Stigmatomma, and Cerapachys, among others. To sample stomach contents of amphibians and other vertebrates solely for nutritional studies, it is not necessary to kill the animals. Stomach flushing methods have been developed and successfully applied in numerous studies, which avoids killing individuals of the study species (Solé et al. 2005). To conclude, the study of vertebrate stomach contents is not only a way of studying the trophic ecology of vertebrates themselves, but also an interesting source of cryptic and new arthropod species, including ants.
Supplementary Material
Acknowledgements
We gratefully acknowledge the Ministerio de Ambiente de Ecuador for permission to collect frog specimens in Ecuador (001-13 IC-FAU-DNB/MA) and export them to the United States (CITES 32V/S). We thank Stefan Cover (MCZ) for providing access to the holotype of Lenomyrmex costatus. Rodrigo Feitosa and one anonymous reviewer improved the manuscript with helpful comments. Lina Pedraza assisted in taking morphometric measurements of the ants. CR and JSC are supported by the National Science Foundation (DEB-1456964). LAO is supported by a Bauer Fellowship from Harvard University, a For Women in Science Fellowship from the L'Oreal Foundation, and is grateful to Lola Guarderas and Elicio E. Tapia for providing assistance during field research in Ecuador. LAC acknowledges support from Wikiri and the Saint Louis Zoo.
Citation
Rabeling C, Sosa-Calvo J, O'Connell LA, Coloma LA, Fernández F (2016) Lenomyrmex hoelldobleri: a new ant species discovered in the stomach of the dendrobatid poison frog, Oophaga sylvatica (Funkhouser). ZooKeys 618: 79–95. doi: 10.3897/zookeys.618.9692
References
- Azorsa F, Sosa-Calvo J. (2008) Description of a remarkable new species of ant in the genus Daceton Perty (Formicidae: Dacetini) from South America. Zootaxa 1749: 27–38. [Google Scholar]
- Bolton B. (1990) The higher classification of the ant subfamily Leptanillinae (Hymenoptera: Formicidae). Systematic Entomology 15: 267–282. doi: 10.1111/j.1365-3113.1990.tb00063.x [Google Scholar]
- Bolton B. (2016) An online catalog of the ants of the world. Available from http://antcat.org [accessed 20 June, 2016]
- Brooks TM, Mittermeier RA, Mittermeier CG, da Fonseca GA, Rylands AB, Konstant WR, Flick P, Pilgrim J, Oldfield S, Magin G, Hilton‐Taylor C. (2002) Habitat loss and extinction in the hotspots of biodiversity. Conservation Biology 16: 909–923. doi: 10.1046/j.1523-1739.2002.00530.x [Google Scholar]
- Brown JL, Twomey E, Amezquita A, Souza MB, Caldwell JP, Lötters S, May R, Melo-Sampaio PR, Mejía-Vargas D, Perez-Peña P, Pepper M, Poelman EH, Sanchez-Rodriguez M, Summers K. (2011) A taxonomic revision of the Neotropical poison frog genus Ranitomeya (Amphibia: Dendrobatidae). Zootaxa 3083: 1–20. [Google Scholar]
- Cerón C, Palacios W, Valencia R, Sierra R. (1999) Las formaciones naturales de la Costa del Ecuador. In: Sierra R. (Ed.) Propuesta preliminar de un sistema de clasificación de vegetación para el Ecuador continental. Proyecto INEFAN/GERF-BIRF y Ecociencia, Quito. [Google Scholar]
- Daly JW, Garraffo HM, Poonam J, Spande TF, Snelling RR, Jaramillo C, Rand AS. (2000) Arthropod-frog connection: decahydroquinoline and pyrrolizidine alkaloids common to microsympatric myrmicine ants and dendrobatid frogs. Journal of Chemical Ecology 26: 73–85. doi: 10.1023/A:1005437427326 [Google Scholar]
- Darst CR, Menéndez-Guerrero PA, Coloma LA, Cannatella DC. (2005) Evolution of dietary specialization and chemical defense in poison frogs (Dendrobatidae): a comparative analysis. The American Naturalist 165: 56–69. doi: 10.1086/426599 [DOI] [PubMed] [Google Scholar]
- Delsinne T, Fernández F. (2012) First record of Lenomyrmex inusitatus (Formicidae: Myrmicinae) in Ecuador and description of the queen. Psyche 2012: . doi: 10.1155/2012/145743 [Google Scholar]
- Dumbacher JP, Wako A, Derrickson SR, Samuelson A, Spande TF, Daly JW. (2004) Melyrid beetles (Choresine): a putative source for the batrachotoxin alkaloids found in poison-dart frogs and toxic passerine birds. Proceedings of the National Academy of Science USA 101: 15857–15860. doi: 10.1073/pnas.0407197101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esteves EA, Brandão CRF, Viegas K. (2008) Subterranean ants (Hymenoptera,Formicidae) as prey of fossorial reptiles (Reptilia, Squamata: Amphisbaenidae) in Central Brazil. Papéis Avulsos de Zoologia, Museu de Zoologia da Universidade de São Paulo 48: 329–334. doi: 10.1590/S0031-10492008002800001 [Google Scholar]
- Fernández F, Palacio EE. (1999) Lenomyrmex, an enigmatic new ant genus from the Neotropical Region (Hymenoptera: Formicidae: Myrmicinae). Systematic Entomology 24: 7–16. doi: 10.1046/j.1365-3113.1999.00063.x [Google Scholar]
- Fernández F. (2001) Hormigas de Colombia. IX: Nueva especie de Lenomyrmex (Formicidae: Myrmicinae). Revista Colombiana de Entomología 27: 201–204 [Google Scholar]
- Fernández F, Sendoya S. (2004) Synonymic list of Neotropical ants (Hymenoptera: Formicidae). Biota Colombiana 5: 3–105 [Google Scholar]
- Fernández F, Feitosa RM, Lattke J. (2014) Kempfidris, a new genus of myrmicine ants from the Neotropical region (Hymenoptera: Formicidae). European Journal of Taxonomy 85: 1–10. doi: 10.5852/ejt.2014.85 [Google Scholar]
- Hölldobler B, Wilson EO. (1990) The Ants. Harvard University Press, Harvard, 732 pp. [Google Scholar]
- Hölldobler B, Wilson EO. (2011) The leafcutter ants—civilization by instinct. W. W. Norton & Company, New York, 157 pp. [Google Scholar]
- Johnson RA, Moreau CS. (2016) A new ant genus from southern Argentina and southern Chile, Patagonomyrmex (Hymenoptera: Formicidae). Zootaxa 4139: 1–31. doi: 10.11646/zootaxa.4139.1.1 [DOI] [PubMed] [Google Scholar]
- Longino JT. (2006) New species and nomenclatural changes for the Costa Rican ant fauna (Hymenoptera: Formicidae). Myrmecologische Nachrichten 8: 131–143 [Google Scholar]
- McGugan JR, Byrd GD, Roland AB, Caty SN, Kabir N, Tapia EE, Trauger SA, Coloma LA, O'Connell LA. (2016) Ant and mite diversity drives toxin variation in the Little Devil Poison frog. Journal of Chemical Ecology 42: 537–551. doi: 10.1007/s10886-016-0715-x [DOI] [PubMed] [Google Scholar]
- Mehdiabadi NJ, Schultz TR. (2010) Natural history and phylogeny of the fungus-farming ants (Hymenoptera: Formicidae: Myrmicinae: Attini). Myrmecological News 13: 37–55 [Google Scholar]
- Ministerio del Ambiente del Ecuador (2012) Sistema de clasificación de los ecosistemas del Ecuador continental. Subsecretaría de Patrimonio Natural, Quito. [Google Scholar]
- Mittermeier RA, Myers N, Thomsen J, da Fonseca GA, Olivieri S. (1998) Biodiversity hotspots and major tropical wilderness areas: Approaches to setting conservation priorities. Conservation Biology 12: 516–520. doi: 10.1046/j.1523-1739.1998.012003516.x [Google Scholar]
- Mueller UG, Schultz TR, Currie C, Adams R, Malloch D. (2001) The origin of the attine ant-fungus mutualism. Quarterly Review of Biology 76: 169–197. doi: 10.1086/393867 [DOI] [PubMed] [Google Scholar]
- Myers N, Mittermeier RA, Mittermeier CG, da Fonseca GA, Kent J. (2000) Biodiversity hotspots for conservation priorities. Nature 403: 853–858. doi: 10.1038/35002501 [DOI] [PubMed] [Google Scholar]
- Rabeling C, Verhaagh M, Mueller UG. (2006) Behavioral ecology and natural history of Blepharidatta brasiliensis (Formicidae, Blepharidattini). Insectes Sociaux 53: 300–306. doi: 10.1007/s00040-006-0872-y [Google Scholar]
- Rabeling C, Brown JM, Verhaagh M. (2008) Newly discovered sister lineage sheds light on early ant evolution. Proceedings of the National Academy of Sciences USA 105: 14913–14917. doi: 10.1073/pnas.0806187105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabeling C, Schultz TR, Bacci Jr M, Bollazzi M. (2015) Acromyrmex charruanus: a new inquiline social parasite species of leaf-cutting ants. Insectes Sociaux 62: 335–349. doi: 10.1007/s00040-015-0406-6 [Google Scholar]
- Solé M, Ketterl J, Di-Bernardo M, Kwet A. (2002) Ants and termites are the diet of the microhylid frog Elachistocleis ovalis (Schneider, 1799) at an Auracaria forest in Rio Grande do Sul. Herpetological Bulletin 79: 14–17. [Google Scholar]
- Solé M, Beckmann O, Pelz B, Kwet A, Engels W. (2005) Stomach-flushing for diet analysis in anurans: an improved protocol evaluated in a case study in Araucaria forests, southern Brazil. Studies on Neotropical Fauna and Environment 40: 23–28. doi: 10.1080/01650520400025704 [Google Scholar]
- Santos JC, Coloma LA, Cannatella DC. (2003) Multiple, recurring origins of aposematism and diet specialization in poison frogs. Proceedings of the National Academy of Science USA 100: 12792–12797. doi: 10.1073/pnas.2133521100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saporito RA, Donnelly MA, Hoffman RL, Garraffo HM, Daly JW. (2003) A siphonotid millipede (Rhinotus) as the source of spiropyrrolizidine oximes of dendrobatid frogs. Journal of Chemical Ecology 29: 2781–2786. doi: 10.1023/B:JOEC.0000008065.28364.a0 [DOI] [PubMed] [Google Scholar]
- Saporito RA, Garraffo HM, Donnelly MA, Edwards AL, Longino JT, Daly JW. (2004) Formicine ants: an arthropod source for the pumiliotoxin alkaloids of dendrobatid poison frogs. Proceedings of the National Academy of Science USA 101: 8045–8050. doi: 10.1073/pnas.0402365101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saporito RA, Donnelly MA, Norton RA, Garraffo HM, Spande TF, Daly JW. (2007) Oribatid mites as a major dietary source for alkaloids in poison frogs. Proceedings of the National Academy of Sciences USA 104: 8885–8890. doi: 10.1073/pnas.0702851104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sosa-Calvo J, Schultz TR, Brandão CR, Klingenberg C, Feitosa RM, Rabeling C, Bacci M Jr., Lopes CT, Vasconcelos HL. (2013) Cyatta abscondita: taxonomy, evolution, and natural history of a new fungus-farming ant genus from Brazil. PLoS ONE 8(11): . doi: 10.1371/journal.pone.0080498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sosa-Calvo J. (2015) Systematics of the cryptic fungus-farming ant genus Myrmicocrypta Fr. Smith, with the description of a new genus and species of fungus-farming ants (Hymenoptera: Myrmicinae). Ph.D. Dissertation, University of Maryland. [Google Scholar]
- Ward PS, Brady SG, Fisher BL, Schultz TR. (2015) The evolution of myrmicine ants: phylogeny and biogeography of a hyperdiverse ant clade (Hymenoptera: Formicidae). Systematic Entomology 40: 61–81. doi: 10.1111/syen.12090 [Google Scholar]
- Weber NA. (1938) The food of the giant toad, Bufo marinus (L.), in Trinidad and British Guiana with special reference to the ants. Annals of the Entomological Society of America 31: 499–503. doi: 10.1093/aesa/31.4.499 [Google Scholar]
- Wilson EO. (1962) Behavior of Daceton armigerum (Latreille), with a classification of self-grooming movements in ants. Bulletin of the Museum of Comparative Zoology 127: 401–422. [Google Scholar]
- Yamane S, Bui TV, Eguchi K. (2008) Opamyrma hungvuong, a new genus and species of ant related to Apomyrma (Hymenoptera: Formicidae: Amblyoponinae). Zootaxa 1767: 55–63. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.