Abstract
Background:
Clostridium difficile (C. difficile) is a leading cause of infectious diarrhoea in hospitals. Sending faecal samples for testing expedites diagnosis and appropriate treatment. Clinical suspicion of C. difficile based on patient history, signs and symptoms is the basis for sampling. Sending faecal samples from patients with diarrhoea ‘just in case’ the patient has C. difficile may be an indication of poor clinical management.
Aim:
To evaluate the effectiveness of an intervention by an Infection Prevention and Control Team (IPCT) in reducing inappropriate faecal samples sent for C. difficile testing.
Method:
An audit of numbers of faecal samples sent before and after a decision-making algorithm was introduced. The number of samples received in the laboratory was retrospectively counted for 12-week periods before and after an algorithm was introduced.
Findings:
There was a statistically significant reduction in the mean number of faecal samples sent post the algorithm. Results were compared to a similar intervention carried out in 2009 in which the same message was delivered by a memorandum. In 2009 the memorandum had no effect on the overall number of weekly samples being sent.
Conclusion:
An algorithm intervention had an effect on the number of faecal samples being sent for C. difficile testing and thus contributed to the effective use of the laboratory service.
Keywords: Clostridium difficile, healthcare-associated infections, audit
Introduction
Clostridium difficile is a gram-positive, anaerobic spore-forming, toxin-producing bacillus which colonises the intestines of 1–3% of healthy adults and 66% of infants (Public Health England, 2013). In the healthy adult population, C. difficile is kept under control by the normal microflora in the intestines. If this balance is disrupted, then the C. difficile bacteria can proliferate and produce toxins that irritate the lining of the colon, causing diarrhoea with loose, watery and foul-smelling stools. C. difficile infection (CDI) is one of the major causes of healthcare-associated infection (HCAI) and a leading cause of infectious diarrhoea in hospitals in industrialised countries (Crobach et al., 2009). This microorganism has been associated with considerable morbidity and an increased risk of mortality (Mitchell and Gardner, 2012). Severe infection is confirmed when there are more than 10 episodes of diarrhoea a day and other inflammatory markers are evident (Public Health England, 2013). The increase in the number of C. difficile outbreaks and associated deaths across the UK have been the catalyst for the Government to introduce reduction targets (Office for National Statistics, 2012). Significant reductions in cases of CDI have been seen since the introduction of such targets as a result of a number of infection control interventions, including increased compliance with hand hygiene, increased environmental cleaning, antimicrobial stewardship, prompt isolation of symptomatic patients, improved disease management, the introduction of high impact interventions and improved documentation (Hughes et al., 2013).
However, maintaining compliance with these practices over time can be challenging. Ultimately, ownership and responsibility for continual compliance must lie with healthcare practitioners themselves (Scheithauer and Lemmen, 2013), but in a busy working environment healthcare practitioners may require a prompt to assist their compliance.
The primary method of diagnosing CDI is through testing a sample of faeces to identify the toxins that are produced in the colon by these bacteria. However, evidence suggests that many specimens received in the laboratory for C. difficile testing are inappropriate. Crobach et al. (2009) and Goldenberg and French (2011) estimate that laboratories receive on average 4000 faecal samples annually with an approximate 4% C. difficile positivity rate. Khanna (2008) carried out two retrospective audits of faecal sampling in 2007 and 2008 with the aim of assessing compliance with the two national Health Protection Agency (HPA, 2007) standards for C. difficile testing, i.e. no repeat testing within 28 days and no testing of solid stools. Results of the first audit showed that 32.2% of specimens were tested inappropriately by the laboratory; with 28% having already been tested in the previous 28 days and 4.3% of specimens sent for testing were solid stools. Ahmed and Orendi (2012) carried out a retrospective clinical audit from 1 March to 31 May 2010 and a repeat audit during the same time period in 2011. In the 2010 audit, the data suggested that 22% of new episodes of C. difficile had a repeat sample sent within 28 days which was contrary to one of the HPA’s testing standards.
In the authors’ NHS Trust a root cause analysis (RCA) carried out in relation to diarrhoeal samples from 2010 to 2013 found that in one-quarter of cases, clinical signs of CDI were not present and no further episodes of diarrhoea had been recorded after the sample had been sent. In some instances, patients had been given a laxative in the previous 24 h or a second repeat sample had been sent. Patients’ sampled while prescribed laxatives, in which a positive result is found, may be carriers of this microorganism and not necessarily infected (Awad-el-Kariem et al., 2012). Thus, treatment would not be required in such instances unless other clinical signs and symptoms suggestive of CDI were present.
The reason for this inappropriate sampling may be that healthcare practitioners do not have the necessary knowledge or skills to risk assess every patient presenting with diarrhoea, therefore they send a sample to the laboratory ‘just in case’ an infection is present. Ahmed and Orendi (2012) surmised that this may be due to over-cautiousness in investigating patients’ conditions. Fryer and Smellie (2013) postulate that healthcare professionals inappropriately request tests for a wide range of reasons including fear of litigation, lack of experience, lack of awareness of guidance or an inability to access previous results.
The Royal College of Nursing (2013, p. 14) caution that ‘it is essential that a thorough risk assessment takes place as to the potential cause of a new onset of acute diarrhoea… Faecal specimens should not be taken “just in case” or on a repeat basis.’ Clinical signs and symptoms must inform the clinical suspicion of C. difficile and should be the main driver for faecal sampling. Crobach et al. (2009, p. 1064) recommend that the diagnosis of C. difficile should be based on ‘clinical signs and symptoms in combination with laboratory tests’ and that the interpretation of the laboratory results ‘should be done in the clinical context, taking into account the background prevalence of C. difficile in the institution’.
Treatment prescribed on the basis of laboratory result alone is inappropriate and detrimental to good patient management. A review of laboratory services by the Department of Health (England) reported that 25% of tests were unnecessary and that the reduction of inappropriate faecal samples could see significant cost savings without any detriment to patient care. (Department of Health, 2008).
Algorithms have been used extensively and effectively to guide healthcare staff in the appropriate management of patients or outbreaks of infectious diseases (Health Protection Scotland, 2013). They are a useful tool in infection prevention and control where often complex messages need to be conveyed (Freeman et al., 2013). Khanna (2008) showed the effectiveness of introducing written easily accessible guidance to correct inappropriate sampling and testing for C. difficile. This resulted in a reduction in the number of inappropriate sampling from 32.3% to 15.6%.
In July 2013, a service intervention based around a decision-making algorithm was introduced within a NHS Trust aiming to reduce the number of inappropriate faecal samples sent for C. difficile testing (see Figure 1). The algorithm used in this intervention was designed to act as a permanent visual aid to the assessment of the patient presenting with diarrhoea. Using this visual reminder as a checklist, the healthcare practitioner would be guided to make an informed decision on the need for C. difficile testing and thus only send appropriate faecal samples. The content of the algorithm outlined the same parameters for testing as in a previous Memorandum sent in 2009 (box 1). However, the algorithm intervention was designed to be eye-catching and presented in the form of bullet points including the ‘Do’ and ‘Don’t’ parameters for appropriate sampling and the red, amber, green ‘traffic light’ system used extensively in the healthcare setting.
Figure 1.
Box 1.
Memorandum.
| TO: | All Ward Managers |
| FROM: | Infection Prevention & Control Team |
| DATE: | 7th July 2009 |
| SUBJECT | Clarification on Testing for C. difficile |
|
C. difficile infection is defined as one episode of diarrhoea (Bristol Stool Chart types 6 & 7) that is not attributable to any other cause including medications and that occurs at the same time as a positive toxin result. When to send samples Stool samples should be sent for C. difficile testing if:- • Samples should be sent to the laboratory for C. difficile testing if a patient presents with diarrhoea that is not attributable to any other cause. • Speed of diagnosis is important to minimise the risk of transmission and to ensure efficient use of isolation facilities. In line with the SIGHT protocol, clinical staff should ensure that stool samples are sent for toxin testing as soon as infective diarrhoea is suspected and that the patient is isolated. When Not to Send Samples Stool samples should not be sent for C. difficile testing if:- • The stool specimen is type 1–5 on the Bristol Stool Chart. • Diarrhoea is part of the patient’s normal bowel pattern. • There is another cause for the patient’s diarrhoea, e.g. ulcerative colitis. • The patient is on laxatives. • The patient has constipation with overflow. NB. Remember that diarrhoea is a common reaction to many medications. Therefore, before sampling consideration should be given to when new medications were commenced and the onset of diarrhoeal symptoms. | |
This algorithm included parameters for testing based on the national standards outlined by Public Health England (2013). Clinical signs and symptoms were also outlined in order to guide healthcare practitioners on the case definition for the presence of CDI. It reminded staff to review the patient’s clinical condition in order to assess the likelihood of CDI before sending a specimen. By following the guidance in this algorithm, it was anticipated that healthcare practitioners would have increased confidence in their decision to sample, thus correcting the tendency to send a sample ‘just in case’.
Methodology
A retrospective audit of faecal samples sent before and after the decision-making algorithm was introduced by the Infection Prevention and Control Team in order to evaluate the effectiveness in reducing the number of inappropriate faecal samples sent for C. difficile testing.
Objectives
To ascertain if there was a statistically significant reduction in inappropriate faecal samples sent for C. difficile testing, following the algorithm intervention.
To ascertain if there was a statistically significant reduction in inappropriate faecal samples sent for C. difficile testing following the 2009 memorandum intervention.
To compare data on C. difficile sampling following the algorithm in 2013 with data following a memorandum intervention in 2009.
Data collection
The data for this audit were obtained retrospectively from the laboratory records. Data were collected before and after the introduction of the algorithm and the memorandum interventions.
The total weekly number of faecal samples received in the laboratory from all inpatient departments was recorded along with the number of positive C. difficile over a 3-month period before and after the interventions. Samples from patients aged under 16 years and community specimens were not included.
Results
Table 1 shows the number of weekly samples received in the laboratory for both the 12 weeks before the intervention and 12 weeks after. The figures given in brackets refer to the 2009 data.
Table 1.
C. difficile samples by week and % positive 2013 [2009].
| Toxin results 2013 [2009] |
|||||
|---|---|---|---|---|---|
| Week | Negative | Positive | Samples sent | % Positive | |
| Pre intervention April to June |
Week 1 | 100 [144] | 1 [5] | 101 [149] | 0.99 [3.36] |
| Week 2 | 98 [117] | 0 [9] | 98 [126] | 0 [7.14] | |
| Week 3 | 95 [130] | 1 [6] | 96 [136] | 1.04 [4.41] | |
| Week 4 | 52 [154] | 1 [5] | 53 [159] | 1.89 [3.14] | |
| Week 5 | 70 [157] | 4 [2] | 74 [159] | 5.41 [1.26] | |
| Week 6 | 102 [117] | 4 [6] | 106 [123] | 3.77 [4.88] | |
| Week 7 | 94 [144] | 2 [3] | 96 [147] | 2.08 [2.04] | |
| Week 8 | 76 [111] | 1 [5] | 77 [116] | 1.3 [4.31] | |
| Week 9 | 75 [138] | 0 [8] | 75 [146] | 0 [5.48] | |
| Week 10 | 69 [152] | 4 [4] | 73 [156] | 5.48 [2.56] | |
| Week 11 | 60 [118] | 1 [5] | 61 [123] | 1.64 [4.07] | |
| Week 12 | 77 [128] | 1 [12] | 78 [140] | 1.28 [8.57] | |
| Total | 973 [1610] | 20 [70] | 993 [1680] | 2 [4] | |
| Mean = 80.66 [134.1] | Mean = 1.66 [5.83] | Mean = 82.33 [140] | |||
|
| |||||
| Intervention period – Algorithm [Memorandum] | |||||
| Post intervention July to October |
Week 13 | 62 [139] | 4 [2] | 66 [141] | 6.06 [1.42] |
| Week 14 | 61 [110] | 4 [1] | 65 [111] | 6.15 [0.90] | |
| Week 15 | 71 [129] | 5 [3] | 76 [132] | 6.58 [2.27] | |
| Week 16 | 47 [149] | 1 [0] | 48 [149] | 2.08 [0.00] | |
| Week 17 | 73 [95] | 0 [3] | 73 [98] | 0 [3.06] | |
| Week 18 | 65 [138] | 5 [4] | 70 [142] | 7.14 [2.82] | |
| Week 19 | 61 [128] | 2 [2] | 63 [130] | 3.17 [1.54] | |
| Week 20 | 85 [126] | 1 [5] | 86 [131] | 1.16 [3.82] | |
| Week 21 | 76 [124] | 1 [2] | 77 [126] | 1.3 [1.59] | |
| Week 22 | 48 [143] | 1 [1] | 49 [144] | 2.04 [0.69] | |
| Week 23 | 96 [142] | 1 [9] | 97 [151] | 1.03 [5.96] | |
| Week 24 | 56 [149] | 0 [3] | 56 [152] | 0 [1.97] | |
| Total | 801 [1572) | 25 [35] | 826 [1607] | 3 [2] | |
| Mean = 64 [131] | Mean = 2.08 [2.91] | Mean = 68.83 [133.9] | |||
In 2013, the weekly number of samples received in the pre-intervention 12 weeks was 993. Of this number, 20 (2%) of the samples were positive for the C. difficile toxin. The total number of samples received each week in both the pre-intervention and post-intervention periods was in the range of 53–106 and the percentage of C. difficile toxin-positive samples was in the range of 0–5.48%. The mean was 82.33, Standard deviation (SD) 16.789, (Q1) 73.25.
In the 2013 post intervention, there were 826 samples received, of which 25 (3%) were positive for the C. difficile toxin. The number of samples received each week was in the range of 48–97 and the percentage of C. difficile toxin-positive samples was in the range of 0–7%. The mean was 68.83, SD 14.377, Q1 57.75. In 2013, the total number of samples received was 1819, of which (2.5%) were positive for the C. difficile toxin.
In 2009 in the pre-intervention 12 weeks, there were 1680 faecal samples received, of which 70 (4%) were positive for the C. difficile toxin. The number of samples received each week was in the range of 116–159 and the percentage of C. difficile toxin-positive samples was in the range of 1.26–8.57%. The mean was 140, SD 15.106, Q1 123.75. In 2009, in the post-intervention 12 weeks there were 1607 samples received, of which 35 (2%) were positive for the C. difficile toxin. The number of samples received each week was in the range of 98–152 and the percentage of C. difficile toxin-positive samples was in the range of 0–5.96%. The mean was 133.92, SD 16.429, Q1 127. Over the 24 weeks the total number of samples received was 3287, of which 90 (2.7%) were positive. The number of CDI episodes in inpatients recorded for the year 2008–2009 was 233 and for the year 2013–2014 was 110.
Analysis
Analysis of the data was carried out using Statistical Package for the Social Sciences (SPSS) version 22. Parametric statistical methods deal with the estimation of population parameters and are described using the range, mean and standard deviation. The standard ‘Bell Curves’ for 2013 for the periods before and after the algorithm intervention indicated a normal distribution of the data. There was also normal distribution of the data for 2009. The paired t-test is used for normally distributed continuous parameters in two paired groups. Analysis of the data for 2013 and 2009 were therefore undertaken using parametric t-tests. The t-test allows for mean comparisons. An independent-samples t-test was conducted to compare the mean number of samples received before and after interventions in 2013 and 2009. A t-test comparing the mean number of samples sent for the 12 week periods before and after the memorandum in 2009 indicated that there was a change downwards in the mean between these two periods but this was not statistically significant (p = 0.36). The change downwards in the mean number of samples sent between the pre-intervention period and post-intervention period in 2013 was 13.5 and was statistically significant (p = 0.046).
In 2009, the mean of C. difficile toxin-positive samples was 4.27% before and 2.17% after intervention. This was statistically significant at P = 0.011. The estimated change downwards was 2.10%.
In 2013, the C. difficile positive rate in the 12-week period before the algorithm was introduced was 2.07%. This rose to 3.06% in the 12-week period after the algorithm was introduced, but the change was not statistically significant, 0.99%.
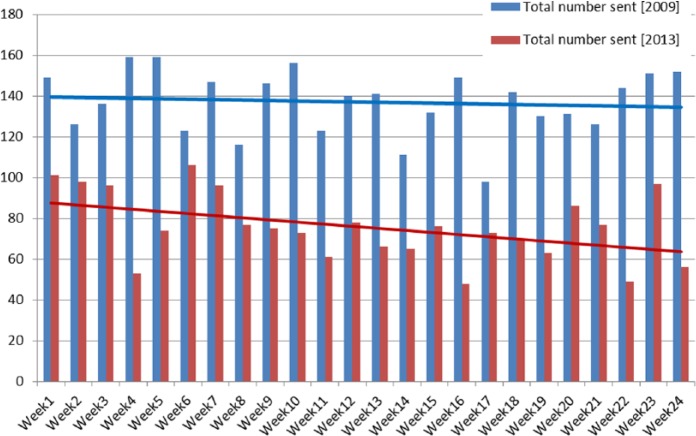
Figure 2 compares the number of specimens sent in 2009 and 2013. The linear line for the number of specimens sent in 2009 showed minimal change.
Figure 2.
Total number of specimens sent in 2009 and 2013.
The number of specimens sent in 2013 shows an overall weekly downward trend suggesting a general reduction in the number of samples received with the number of toxin-positive samples remaining relatively unchanged. There was no significant difference in the number of samples received after the 2009 memorandum intervention. From these results we can infer that the memorandum had no value as an intervention tool to correct sampling behaviour.
There was a significant difference in the number of samples received after the memorandum intervention in 2009 (M = 134, SD = 16) and before the algorithm intervention in 2013 (M = 69, SD = 14) P = 0.0001. This may suggest that the ongoing education and the introduction of other infection prevention and control initiatives were having a continuing effect over this period of time. The Introduction of further guidance from the Department of Health in 2012 may have been one reason for this change but no definitive conclusion can be made here.
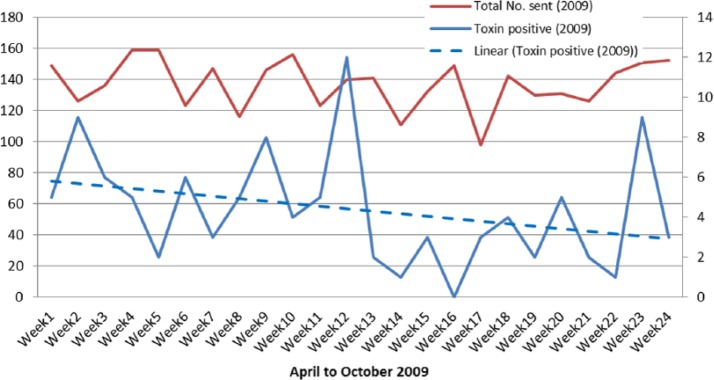
The numbers of specimens sent and the number of toxin-positive samples by week in 2009 are shown in Figure 3.
Figure 3.
Specimens sent and toxin positive results 2009.
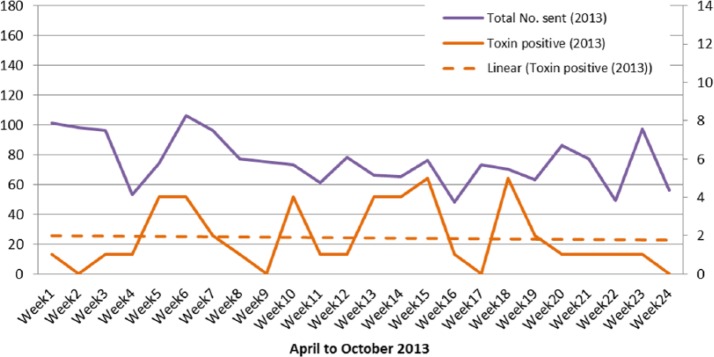
The figures for the number of toxin-positive samples show a clear downward trend in 2009. The mean positivity rate fell from 4% pre intervention to 2% post intervention, which is statistically highly significant; before memorandum (M = 6, SD = 3) and after (M = 3, SD = 2) P = 0.010. The numbers of specimens sent and the number of toxin-positive samples by week in 2013 are shown in Figure 4.
Figure 4.
Specimens sent and toxin positive results 2013.
The positivity rate post intervention in 2009 (2%) was comparable to the pre-intervention period in 2013 (2.5%) despite a lower number of samples being sent. In 2009, the total number of C. difficile samples recorded during the 24 weeks of this service intervention was 3287 and 105 (3%) were positive. In comparison, in 2013 in the 24 weeks, the total number of samples received was 1819, of which 45 (2.5%) were positive for the C. difficile toxin.
Discussion
The aim of this audit was to examine whether an algorithm intervention was effective in reducing the number of samples sent for C. difficile testing. Ultimately, the purpose was to reduce the number of inappropriate or ‘just in case’ samples being sent to the laboratory. It was believed that the benefits of changing this pattern of sampling would lead to a more appropriate clinical response and ensure better laboratory utilisation. This intervention did not impact on the Trust policy to isolate all patients in a single en suite room immediately on presenting with diarrhoea. All infection prevention and control interventions were maintained at all times in all cases of diarrhoea.
This issue of oversampling by Trust clinical staff had previously been identified in 2009 and a memorandum was circulated to healthcare staff. The objectives were to ascertain if there was a statistically significant reduction in C. difficile specimens post an algorithm intervention 2013, post a memorandum intervention 2009 and then compare the two.
The two methods of communication reported in this service intervention, i.e. the memorandum sent out in 2009 and the algorithm in 2013, conveyed the same information based on the national standards on when to send a sample for C. difficile (HPA, 2007) along with the clinical signs and symptoms of a CDI (Department of Health, 2012). However, the difference in the impact of the message between these two interventions is evident. The evidence in this service intervention suggests that the algorithm intervention was a much more effective method than the memorandum. Memorandums are considered to be more of a diktat and delivered through a downward hierarchical flow usually seeking to mandate staff to carry out an action. This type of authoritarian approach may explain why there was little clinician’s engagement or behavioural change. This intervention did not appear to have any major impact on the number of samples being sent.
Spillan et al. (2002) believe that in complex healthcare organisations communication must flow horizontally/laterally. The purpose of horizontal/lateral communication is that this takes place among peers and co-workers, often during staff meetings and informational presentations. The communication on this level generally exists to provide updates on existing polices or procedures or information on new practices. In this intervention the algorithm was sent out by the IPCT directly to their medical and nursing peers. Clinicians are more likely to deem this as valuable advice between peers rather than as a directive from management. The results of this service intervention show that they are a very useful tool in infection prevention and control where complex messages need to be conveyed in a simplified format.
The reduction in C. difficile toxin-positive numbers can be clearly seen in 2009 and shows a downward trend which was statistically significant (P <0.01). This reduction is in line with comparative data in the rest of the UK at that time. This may be indicative of the reduction in the number of people succumbing to this infection and thus fewer patients showing signs and symptoms of the disease. It would seem, therefore, that many of the specimens being sent were not appropriate or in line with current standards for C. difficile testing. The positivity rate is similar to the rate of positives found in the studies by Goldenberg and French (2011) and Crobach et al. (2009). Both these studies reported a 4% positivity rate in all populations in and around 2009. It can be assumed therefore that the 2009 data collected and reported in this service intervention, which showed a positivity rate of 4% before the memorandum intervention and 2% after, are within the expected rate for that time. The reduction in the number of positive cases reflects the impact of the implementation of infection prevention and control interventions introduced during 2009 and this has been borne out by subsequent PHE surveillance reports. It is also apparent from the data post-memorandum intervention in 2009 (2%) and the pre-algorithm intervention (2%) in 2013, that this reduction has been sustained over time.
The percentage of positive specimens was 3% in both years during these interventions despite the difference in the number of overall number of samples sent. Many more samples are still being sent for testing than ultimately turn out to be C. difficile toxin-positive. Some authors have argued that it is safer to send specimens ‘just in case’ so cases are not missed (Spencer et al., 2001). To approve of ‘just in case’ sampling is to proclaim that healthcare practitioners cannot do their job. Unlike a pathology test on a tissue biopsy, which makes the diagnosis in its own right, a microbiology test in itself cannot and must be used in conjunction with the clinical signs and symptoms and the patients’ medical history. It is more important to ensure the correct criteria for testing is understood to ensure the most effective management of the patient is being carried out.
There were some factors that may have led to bias in this evaluation such as seasonal variations. The data in this audit were recorded over the spring and summer periods in 2009 and 2013 during which there were no documented outbreaks of C. difficile or Norovirus. Polgreen et al. (2010) suggest that there is an increase of C. difficile in the winter months, which is associated with an increase in influenza and the use of antibiotics. Wilcox and Fawley (2007) report an increase in C. difficile numbers during viral gastroenteritis outbreaks. The number of specimens sent in 2009 and 2013 showed an overall downward trend suggesting a general reduction in the number of samples being sent. It is possible that some of this trend may have been as a result of Department of Health guidance on the diagnosis and reporting of C. difficile (Department of Health, 2012). However, despite this there is evidence of a significant change following the introduction of the algorithm.
Conclusion and recommendations
The intervention of an algorithm was effective in reducing the number of samples being sent to the laboratory. In comparison, a memorandum had little impact on the number of samples being sent. The layout of the information and the method of implementation of these two interventions had a very different impact on sampling. The contention is that memoranda have little value in changing behaviour at a clinical level and algorithms can play a vital role in conveying and supporting good clinical management. Due to the likelihood of an increase in morbidity and mortality in patients with CDI, there has been a tendency to err on the side of caution and test more specimens than would be appropriate for any other disease. The influencing factors are thought to be: public disquiet due to the high profile outbreaks reported in the media; and political impetus which culminated in the setting of reduction targets leading to NHS Trusts being possibly accused of wanting to control the number of specimens sent to keep within the target range.
Currently there is a paucity of literature on the appropriateness of sampling for C. difficile outside of the two criteria set out by the HPA. Audits carried out focus on the re-sampling of faeces within 28 days from a previously positive sample and the sending of formed stools. The added benefit of auditing the impact of a clinical intervention to ensure an appropriate sample is sent can only be beneficial for staff as well as patients. This type of audit is extremely useful from the clinical viewpoint as it can highlight immediately the issue of concern, provide the information required to help change practice and thus improve patient care.
Footnotes
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Peer review statement: Not commissioned; blind peer-reviewed.
References
- Ahmed BLR, Orendi J. (2012) An audit on inappropriate repeat testing of Clostridium difficile toxin (CDT). Bulletin of the Royal College of Pathologists 157: 42–44. [Google Scholar]
- Awad-el-Kariem F, Brown N, Malone C, Gough H, Yates C, O’Connor H. (2012) Clostridium difficile detection: identification of colonization, subclinical and overt disease. Journal of Hospital Infection 82: 138–139. [DOI] [PubMed] [Google Scholar]
- Crobach MTJ, Dekkers OM, Wilcox MH, Kiuper EJ. (2009) European Society of Clinical Microbiology and Infectious Diseases (ESCMID): Data review and recommendations for diagnosing Clostridium difficile-infection. Clinical Microbiology Infection 15: 1053–1066. [DOI] [PubMed] [Google Scholar]
- Department of Health. (2008) Report of the Review of NHS Pathology Services in England Chaired by Lord Carter of Coles, Part 2. London: Department of Health. [Google Scholar]
- Department of Health. (2012) Updated guidance on the diagnosis and reporting of clostridium difficile (March 6) DH/HCAI/ infectious disease. London: Department of Health. [Google Scholar]
- Freeman R, Moore LSP, Garcıa Alvarez L, Charlett A, Holmes A. (2013) Advances in electronic surveillance for healthcare- associated infections in the 21st Century: a systematic review. Journal of Hospital Infection 84: 106–119. [DOI] [PubMed] [Google Scholar]
- Fryer A A, Smellie WSA. (2013) Managing demand for laboratory tests: a laboratory toolkit. Journal of Clinical Pathology 66: 62–72. [DOI] [PubMed] [Google Scholar]
- Goldenberg SD, French GL. (2011) Diagnostic testing for Clostridium difficile: a comprehensive survey of laboratories in England. Journal of Hospital Infection 79: 4–7. [DOI] [PubMed] [Google Scholar]
- Health Protection Scotland. (2013) Healthcare Outbreak Algorithm for Patient, Healthcare Worker and Visitor (PHV) Safety. Glasgow: Health Protection Scotland; Available at: http://www.documents.hps.scot.nhs.uk/hai/infection-control/toolkits/hospitaloutbreak-management-2013–05.pdf (accessed 28 May 2015). [Google Scholar]
- HPA. (2007) Processing of Faeces for C. difficile. Health Protection Agency National Standard Method (BSOP 10i1). London: HPA Standards Unit. [Google Scholar]
- Hughes GJ, Nickerson E, Enoch DA, Ahluwalia J, Wilkinson C, Ayers R, Brown NM. (2013) Impact of cleaning and other interventions on the reduction of hospital-acquired Clostridium difficile infections in two hospitals in England assessed using a breakpoint model. Journal of Hospital Infection 84: 227–234. [DOI] [PubMed] [Google Scholar]
- Khanna N. (2008) Audit of Clostridium difficile toxin (CDT) testing. Bulletin of the Royal College of Pathologists 144: 288–290. [Google Scholar]
- Mitchell BG, Gardner A. (2012) Mortality and Clostridium difficile infection: a review. Antimicrobial Resistance and Infection Control 1: 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polgreen PM, Yang M, Bohnett LC, Cavanaugh JE. (2010) A time series analysis of Clostridium difficile and its seasonal association with influenza. Infection Control and Hospital Epidemiology 31: 382–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Public Health England. (2013) Updated guidance on the management and treatment of Clostridium difficile infection. London: Public Health England. [Google Scholar]
- Royal College of Nursing. (2013) The Management of diarrhoea in adults. RCN guidance for nursing staff. London: Royal College of Nursing. [Google Scholar]
- Scheithauer S, Lemmen SW. (2013) How can compliance with hand hygiene be improved in specialized areas of a university hospital? Journal of Hospital Infection 83 (Suppl. 1): S17–S22. [DOI] [PubMed] [Google Scholar]
- Spencer HL, Donnelly MT, Teahon K. (2001) Culturing of stool samples from hospital inpatients. Lancet 358: 151–152. [DOI] [PubMed] [Google Scholar]
- Spillan JE, Mino M, Rowles MS. (2002). Sharing organizational messages through effective lateral communication. Communication Quarterly 50: Research Library Core, Q96. [Google Scholar]
- Wilcox M, Fawley W. (2007) Viral gastroenteritis increases the reports of Clostridium difficile infection. Journal of Hospital Infection 66: 395–396. [DOI] [PubMed] [Google Scholar]