Abstract
Background:
Persistent pain in shoulder and arm following post-surgical breast cancer treatment can lead to cognitive and physical deficits. Depression is also common in breast cancer survivors. Virtual reality therapy with integrative cognitive and physical rehabilitation has not been clinically trialed for this population. The novel BrightArm Duo technology improved cognition and upper extremity (UE) function for other diagnoses and has great potential to benefit individuals coping with post-surgical breast cancer pain.
Objectives:
The aim of this study was to explore the feasibility of BrightArm Duo therapy for coping with post-surgical chronic pain and associated disability in breast cancer survivors with depression.
Methods:
BrightArm Duo is a robotic rehabilitation table modulating gravity loading on supported forearms. It tracks arm position and grasping strength while patients play three-dimensional (3D) custom integrative rehabilitation games. Community-dwelling women (N = 6) with post-surgical breast cancer pain in the upper arm trained on the system twice a week for 8 weeks. Training difficulty increased progressively in game complexity, table tilt and session length (20–50 minutes). Standardized assessments were performed before and after therapy for pain, cognition, emotion, UE function and activities of daily living.
Results:
Subjects averaged upwards of 1300 arm repetitions and 850 hand grasps per session. Pain intensity showed a 20% downward trend (p = 0.1) that was corroborated by therapist observations and participant feedback. A total of 10 out of 11 cognitive metrics improved post-training (p = 0.01) with a significant 8.3-point reduction in depression severity (p = 0.04). A total of 17 of 18 range of motion metrics increased (p < 0.01), with five affected-side shoulder improvements above the Minimal Clinically Important Difference (8°). In all, 13 out of 15 strength and function metrics improved (p = 0.02) with lateral deltoid strength increasing 7.4 N on the affected side (p = 0.05).
Conclusion:
This pilot study demonstrated feasibility of using the BrightArm Duo Rehabilitation System to treat cancer survivors coping with upper body chronic pain. Outcomes indicate improvement in cognition, shoulder range, strength, function and depression.
Keywords: Post-surgical pain, breast cancer, integrative virtual rehabilitation, chronic pain, psycho-social impact
Introduction
Breast cancer is the leading cause of cancer in women.1 The prevalence of pain in patients with advanced cancer is 64%, of which 59% is among those on anticancer treatment and 33% after curative treatment.2 Up to 50% of women who have breast cancer also have shoulder pain following mastectomy.3 Post-mastectomy pain syndrome (PMPS) may lead to learned limb non-use, reduced range of shoulder movement, arm weakness, sleep disorders, anti-social behaviour and depression.
Cancer-related pain is a major cause of psychological stress.2,4 Shoulder and arm impairments are a significant complication following many surgical and non-surgical treatments following breast cancer.5 With or without the presence of lymphedema, these can greatly affect the quality of life.6 Anxiety and depression can compound these symptoms and lead to poor long-term outcomes.7 The American Pain Society recognizes opioids as essential for pain management; however, pain medication (especially opioid-based) is highly addictive and can lead to dependency.8 A very recent review of pertinent literature found that 8–12% of American patients with chronic non-cancer pain are opioid-dependent.9
Moreover, studies have found associations between higher doses of opioids and an increased risk of suicide mortality.10 Therefore the American Pain Society also endorses consideration of non-medication treatments (including rehabilitation, hypnosis and behavioural strategies) to address psychological impairments related to pain.
Virtual reality (VR) analgesia, pioneered by Hoffman11 for burn wound care, has subsequently been used for chemotherapy related distress in breast cancer,12 or anxiety for children with cancer.13 VR has great potential to intervene in acute and chronic pain by providing a non-opioid ‘virtual analgesic’ response.14 In a review of 19 studies, Chirico et al.15 found that VR interventions were beneficial to cancer patients by improving their emotional state, and diminishing cancer-related distress, whether due to chemotherapy, painful procedures, or hospitalization. This ‘distraction from pain’ response has also been studied for its ability to provide sustained benefits for pain control in cold pressor tests.16 VR therapy has been tried in breast cancer to alleviate symptoms during chemotherapy.12 However, to the authors’ knowledge, until now no clinical studies focused on VR effectiveness in treating breast cancer survivors with post-surgical chronic shoulder and arm pain and depression. In fact, in a review of pain management, Li et al.17 observed that usage of VR for chronic pain management is still in its infancy.
The BrightArm Duo Rehabilitation System (Bright Cloud International Corp, Highland Park, NJ) is an experimental robotic platform that modulates gravity loading on the upper extremities (UEs), making it appropriate for patients with weak arms and diminished ability to grasp. It uses VR to engage the patient in upper body bimanual exercises while also providing cognitive training and affective relief for the patient. The integration of psychological distraction through VR with controlled increases in motor/cognitive demands has been found to be beneficial in prior BrightArm Duo studies of individuals with chronic stroke.18 These earlier studies have also shown a beneficial effect on cognition and depression reduction, which can benefit breast cancer survivors. Thus, the pilot study described here was conducted to explore the feasibility of BrightArm Duo Rehabilitation System use for coping with chronic pain and associated disability in breast cancer survivors post-surgery, and who may also have depression and cognitive deficits. To the author’s knowledge, this is the first study that utilizes an integrative virtual rehabilitation approach for managing cancer-related upper body chronic pain and its psycho-social correlates.
Methods
The BrightArm Duo Rehabilitation System
The BrightArm Duo Rehabilitation System, shown in Figure 1, consisted of a low-friction robotic rehabilitation table, computerized forearm supports, a display, a laptop computer for the therapist station, a remote clinical server and a library of custom integrative rehabilitation games.
Figure 1.
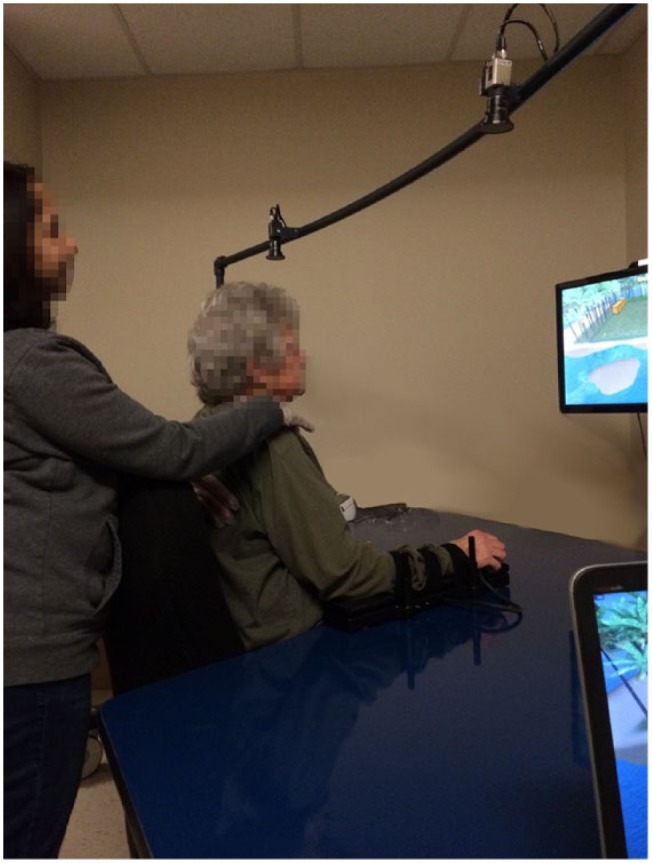
Subject training on BrightArm Duo Rehabilitation System when work surface was tilted upwards. Subject uses left and right arm supports to control virtual paddles in the Breakout 3D game.
©Bright Cloud International Corp. Reprinted with permission.
Nine custom games were developed in Unity 3D19 for unimanual and bimanual motor (shoulder, elbow, grasp), emotive and cognitive (executive function, focusing, short-term and delayed memory, working memory and task sequencing) training. Figure 2 shows screen images of these integrative virtual rehabilitation games. Breakout 3D (Figure 2(a)) asked subjects to use paddle avatars to bounce a virtual ball towards an array of crates. It trained shoulder abduction/adduction or flexion/extension depending on crates orientation, as well as focusing and executive function. The games Card Island (Figure 2(b)) and Remember that Card (Figure 2(c)) utilized card matching to train short-term and delayed visual and auditory memory, grasp strength, shoulder abduction/adduction and shoulder flexion/extension.
Figure 2.
Screen images of nine bimanual games: (a) Breakout 3D, (b) Card Island, (c) Remember that Card, (d) Musical Drums, (e) Xylophone, (f) Pick & Place, (g) Arm Slalom, (h) Avalanche and (i) Treasure Hunt.
©Bright Cloud International Corp. Reprinted with permission.
The next three games trained working memory, focusing, short-term visual and auditory memory, shoulder abduction/adduction, shoulder flexion/extension, motor control and grasp strength. Musical Drums asked subjects to strike a series of notes that drifted across (up to four) drums, using drum stick avatars controlled by hand grasps and arm movements. Subjects repeated a sequence of musical notes using mallet avatars in the Xylophone game. In Pick & Place, the subjects grasped and moved virtual balls to a fixed target of matching colour, using either left–right or in–out arm movements.
The last three games shown in Figure 2 trained shoulder/arm strength, range of motion, endurance, task sequencing and dual tasking. Arm Slalom encouraged shoulder rotations in order to guide a skier avatar through a downhill slalom course. Subjects used grasps and arm movements in Avalanche (Figure 2(h)) to control a pick axe and a shovel avatar, so as to break and clear a series of ice walls and free people trapped in a cottage. In the Treasure Hunt game (Figure 2(i)), subjects controlled one or two shovel avatars to clear sand and uncover a series of buried treasures, before periodic sand storms buried the treasures again.
Study design
The pilot study was designed to investigate the feasibility of using the BrightArm Duo System with subjects who were cancer survivors with a history of upper body chronic pain.
The duration of the BrightArm Duo therapy sessions progressed from 20 to 50 minutes of training over a period of 8 weeks, with two sessions every week. Each session consisted of playing a series of custom games (previously described), such that the same game was not played twice in a row (to increase variety and participation).
Session training difficulty was increased from session to session by progressively increasing the table tilt from a minimum of 0° to a maximum table tilt of 20°. Each game had several levels of difficulty, for example, the ball in Breakout 3D was slower (thus easier to bounce) in the first sessions, then progressively faster in following sessions. A dual-task condition was introduced in later sessions, requiring the subject to grasp just before bouncing the ball with the paddle avatar, lest the ball passed through and was lost. All games had progressively higher difficulty levels over the duration of the intervention, which combined with higher gravity loading and longer training sessions to keep the subjects challenged.
Inclusion and exclusion criteria
The study inclusion criteria were as follows: women aged 30 years or older, on regular pain medication in the 3 months prior to enrolment, reported pain levels of at least 4 on the Numeric Pain Rating Scale (NRS),20 depression score of 10 or above (minimal depression or higher) on Patient Health Questionnaire (PHQ-9)21 and an ability to move the UE at least 15° each of active shoulder and elbow range. Potential subjects were excluded if they did not have any ability to move their UE, or had severe vision or hearing problems, or presented with severe cognitive problems, or had a history of violent behaviour, or had metastases to the UE bones (risk of impending fracture) per imaging studies or on clinical examination determined by the physician. Due to difficulty finding subjects in a timely manner, the inclusion criteria were relaxed to include younger subjects (early 20s) and subjects with minimal depression. Thus, the final inclusion criteria were age 22 and up, minimal to severe depression, on regular pain medication and presenting with UE impairments.
The Western Institutional Review Board (an independent board overseeing research involving human subjects) reviewed and approved the study protocol in accordance with Federal Guidelines. The subjects were consented and then underwent 16 training sessions each, in 2014 or 2015. During each session, subjects were assisted by an Occupational Therapist (OT), who warmed up their affected UE, made sure subjects kept good posture, and provided gentle encouragement as needed. The training took place in the Bright Cloud International Corp clinical laboratory at the Roosevelt Care Center (Edison, NJ). Subjects were offered US$25 for each clinical evaluation or rehabilitation session, as well as free transportation to/from the study site.
Experimental group characteristics
A total of 12 female subjects with breast cancer post-surgical chronic pain, who were out-patients at the University Pain Medicine Center (Somerset, NJ), volunteered and signed an informed consent. A total of three subjects were lost to follow-up and nine subjects commenced training on the BrightArm Duo System. Of them, three subjects dropped from the study (after completing 2, 5 and 11 sessions, respectively) due to persistent scheduling conflicts, or prolonged illness causing absences for more than two consecutive weeks.
Data presented here were generated by the remaining N = 6 subjects who completed the experimental therapy (Table 1). The feasibility study group comprised women with average age of 57.8 years (ranging from 22 to 78 years), who were an average of 9.5 years post-cancer surgery and had an average of 4.0 years since the start of upper body pain. The subjects’ depression level varied: minimal (2), mild (2), moderate (1) and severe (1). The pain medications taken included Zoloft®, Medical Marijuana, Norvasc®, Oxycodone® Pain Patch, Flector® Patch, Ambien®, Oxycontin® and Morphine. All subjects ambulated, but some used a cane. One subject wore an arm sling to support her painful UE.
Table 1.
Participant characteristics for the group (N = 6) of cancer survivors coping with chronic pain.
| Variable | Group statistics (N = 6) |
|---|---|
| Age | 57.8 (20.4) years |
| Race | 4 White, 2 African American |
| Formal education | 16.7 (2.7) years |
| Years since surgery | 9.5 (5.1) years |
| Years since pain start | 4.0 (3.3) years |
| Affected side | 2 left, 4 right |
| Depression level | 2 minimal, 2 mild, 1 moderate and 1 severe |
| Medications | Zoloft®, Medical Marijuana, Norvasc®, Oxycodone® Pain Patch, Flector® Patch, Ambien®, Oxycontin® and Morphine |
©Bright Cloud International Corp. Reprinted with permission.
Data collection instruments
The study used an ABAA protocol, with data being collected pre-training (A), during training (B), post-training (A) and at 8-week follow-up (A). Therapy session data (B) consisted of supported arm reach baseline on the BrightArm Duo table (as measured by overhead digital cameras), power grasp strength baseline (as measured by a forearm support grasp sensor), heart rate and blood pressure, number of active movements and grasp repetitions for each arm during a session collected during play. Pain was assessed using the NRS administered verbally by the attending OT. The NRS has established validity in measuring cancer-related pain intensity.20 At the end of weeks 4 and 8 of VR training, the subjects rated their experience on a custom paper-based subjective evaluation questionnaire. The 10 questions were rated using a 5-point Likert scale, from 1 meaning ‘strongly disagree’ (least desirable outcome) to 5 meaning ‘strongly agree’ (most desirable one). Subjects could add free form comments on the evaluation form.
Occupational therapy evaluations were done pre-training, post-training and at 8-week follow-up by a blinded Senior OT consultant who was not training the subjects. This OT evaluation involved assessment of UE function using the Fulg–Meyer Assessment (FMA) Upper Extremity Section,22 the Chedokee Arm and Hand Activity Inventory-9 (CAHAI-9)23 for bimanual tasks and the Jebsen Hand Function Test (JHFT)24 for hand function. Arm and hand range of motion were measured using mechanical goniometers, shoulder strength was assessed using wrist weights, and grasp strength was measured with a Jamar mechanical dynamometer and a Jamar pinchmeter. In addition, subjects were assessed for their degree of independence in activities of daily living (ADL) involving the UE, using the Upper Extremity Functional Index 20 (UEFI-20).25
Neuropsychological evaluations were done by a blinded research assistant under the supervision of a licenced clinical neuropsychologist pre-training, post-training and at follow-up. These were measures of depression, attention/concentration, processing speed, learning, memory, and executive function. The standardized measures used were the Beck Depression Inventory, Second Edition (BDI-II),26 the Neuropsychological Assessment Battery (NAB) Attention Module (Orientation, Digit Span and Dots) and Executive Functioning Module (Generation subtest),27 the Hopkins Verbal Learning Test, Revised (HVLT-R),28 the Brief Visuospatial Memory Test, Revised (BVMT-R)29 and the Trail Making Test (TMT) A and B.30 Alternate test forms were used whenever possible to minimize test-taking practice effects. Raw scores were utilized in all data analysis. Both evaluating clinicians were blinded to the therapy methodology and scope.
Continuous variables were compared pre-to-post using paired t-tests. p values <0.05 were deemed statistically significant, without multiple-testing adjustment. Outcome changes were compared against the Minimum Clinically Important Difference (MCID) in the corresponding category, whenever possible. Additionally, metrics were grouped in functional categories and a non-parametric sign test was employed to determine if the number of tests that showed improvement yielded a statistically significant result. Although low power makes a negative statement less reliable, positive statistically significant findings imply the findings are robust and not obscured by the small sample size.
Results
Training results
The BrightArm Duo Rehabilitation System captured baselines of supported arm reach at the start of each training session. The average areas for the affected and unaffected arms were 238 cm2 (standard deviation (SD) = 160 cm2) and 441 cm2 (SD = 467 cm2) at the start of training. These increased to 577 cm2 (SD = 504 cm2) and 818 cm2 (SD = 791 cm2), respectively, by the final training session. These measures were done with the table kept flat so as to remove the influence of gravity loading for sessions when the table was otherwise tilted. This corresponds to increases in supported reach of 143% and 85%, respectively, for the affected and unaffected arms.
The BrightArm Duo software also tracked the number, length and intensity of the games played each session by the subjects. They started with 20-minute sessions and were able to sustain up to 50 minutes of training by the end of the therapy, against a 20° upwards table tilt.
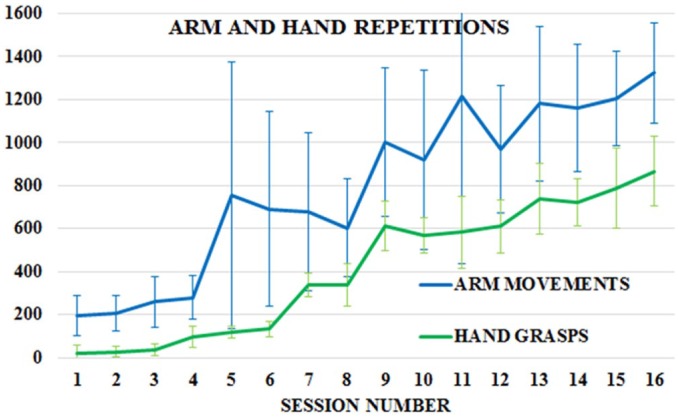
Figure 3 shows that active arm movement and hand grasp repetitions steadily increased from 195 and 22, respectively, in Session 1, to over 1300 movement repetitions and 850 grasps, respectively, by the end of the therapy. The intensity of training increased with each session as well. When session length was normalized to the final duration of 50 minutes, the regression fit of arm movement and hand grasp repetitions revealed an increase of 829 (p = 0.0002) and 913 (p < 0.0001) repetitions, respectively, between Sessions 1 and 16. Arm repetitions more than doubled in Session 5 corresponding to the start of bimanual exercises; increases at Sessions 9 and 13 corresponded to longer exercise time and the introduction of new games (Avalanche, Drums, Xylophone). Hand grasp increases at Sessions 7 and 9, respectively, corresponded to the introduction of hand grasp requirement for the Breakout 3D game and the introduction of Avalanche, a grasp-intensive game.
Figure 3.
Mean (and standard deviations) of active arm movement and hand grasps repetitions per session for the group (N = 6) of cancer survivors experiencing chronic upper body pain.
©Bright Cloud International Corp. Reprinted with permission.
Pain results
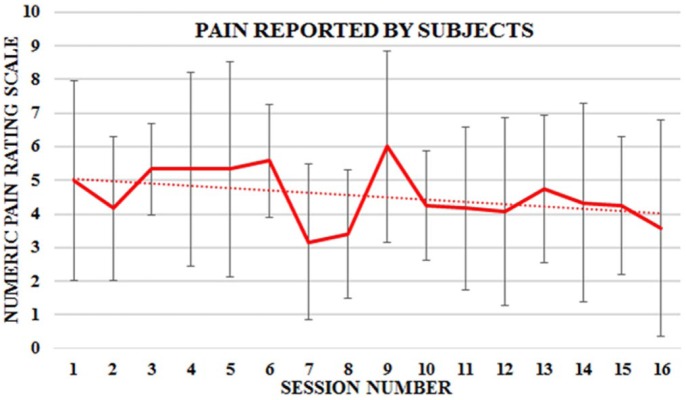
The subjects’ reported pain level was captured at the start and end of each session using the NRS scale. The type of pain described by subjects ranged from stabbing and throbbing, to burning and electrical jolt–like sensation. Figure 4 shows a plot of the worst reported pain in each session averaged over the N = 6 study participants. The SD values were about half the magnitude of the mean values. The regression fit of the plot yielded a trend line with downward slope of 1.1 over the 8-week protocol (p = 0.1). This translated to a 20% drop in reported pain severity given the initial mean pain value of 5.0. Individually, four of six subjects showed improvement on the NRS scale, but only the results of Subject No. 5 (aged 22 years) with a downward slope of 4.3 was statistically significant (p = 0.01). She reported additional lower back pain due to an injury experienced at home, but the associated pain was excluded from the graph as its cause was unrelated to the study. Furthermore, lower back pain was not the aim of this study.
Figure 4.
Mean and standard deviation of reported pain for a group (N = 6) of cancer survivors over the 16 BrightArm Duo therapy sessions.
©Bright Cloud International Corp. Reprinted with permission.
The therapist notes indicated change in medications (Tramadol, Percocet or Ibuprofen) needed by the youngest subject (No. 5, aged 22 years). The subject indicated that no pain medications were taken towards the end of the 8-week protocol. Therapist notes also indicated that subject No. 6 (aged 68 years) had a 3- to 5-point decrease in pain intensity after most sessions, although the subject consistently reported high pain intensity in the arm on arrival at the therapy location. The same subject remarked an improvement in her ability to perform heavy housework such as vacuuming following the training. Therapist notes also indicate a reduction in reported pain intensity in subject No. 6’s right knee during the VR sessions, even though her lower extremity was not trained. Similar reduction in pain intensity was reported by subject No. 4 for pain in both her knees during the VR sessions. She also reported an improved ability to engage in gardening, cooking and cleaning activities.
UE range of motion
In Table 2, the averages of 10 different range of motion metrics were captured for the affected and unaffected arms of the subject group. A total of 18 of 19 non-zero changes in metrics were improvements (+) between pre-training (T0) and post-training (T1), which is statistically significant (p < 0.0001). A total of 17 of 18 non-zero changes were improvements (+) at follow-up (T2) as compared to pre-training (T0), also a statistically significant result (p = 0.0001). Notably, all 10 range of motion metrics for the affected arm improved (p = 0.002). Post-training, the greatest group average increases were in the affected shoulder: flexion 20.2° (p = 0.23), abduction 16.5° (p = 0.50), adduction 10.0° (p = 0.33), external rotation 10.8° (p = 0.27) and internal rotation 9.8° (p = 0.05). These increases were maintained at follow-up: flexion 20.2° (p = 0.21), abduction 21.2° (p = 0.29), adduction 14.7° (p = 0.05), external rotation 14.3° (p = 0.05) and internal rotation 9.5° (p = 0.01). These mean improvements in shoulder range of motion exceeded the MCID of 8°.31 For each of the six affected shoulder range of motion metrics, the individual improvement of about half of the subjects at follow-up (T2) exceeded the MCID.
Table 2.
Range of motion (degrees) statistical analysis for the experimental group (N = 6) of cancer survivors coping with chronic pain.
| Variable | T0 | T1 | T2 | T1 – T0 | p | T2 – T0 | p |
|---|---|---|---|---|---|---|---|
| Affected shoulder | |||||||
| Flexion | 111.3 (29.2) | 131.5 (11.9) | 131.5 (11.9) | +20.2 | 0.23 | +20.2 | 0.21 |
| Extension | 47.7 (19.9) | 54.8 (16.0) | 54.5 (19.2) | +7.2 | 0.47 | +6.8 | 0.35 |
| Abduction | 113.0 (44.4) | 129.5 (19.0) | 134.2 (12.9) | +16.5 | 0.50 | +21.2 | 0.29 |
| Adduction | 21.7 (11.1) | 31.7 (12.1) | 36.3 (8.2) | +10.0 | 0.33 | +14.7 | 0.05 |
| Internal rot. | 40.0 (12.0) | 49.8 (15.2) | 49.5 (9.0) | +9.8 | 0.05 | +9.5 | 0.01 |
| External rot. | 63.5 (10.3) | 74.3 (10.4) | 77.8 (12.4) | +10.8 | 0.27 | +14.3 | 0.04 |
| Affected elbow | |||||||
| Flexion | 140.8 (7.4) | 143.7 (5.7) | 143.8 (5.8) | +2.8 | 0.40 | +3.0 | 0.38 |
| Extension | 6.3 (10.8) | 3.5 (5.4) | 5.5 (8.6) | +2.8a | 0.30 | +0.8a | 0.61 |
| Pronation | 85.2 (4.5) | 86.3 (6.6) | 91.8 (2.9) | +1.2 | 0.73 | +6.7 | 0.03 |
| Supination | 81.3 (6.8) | 81.8 (8.8) | 86.5 (5.2) | +0.5 | 0.86 | +5.2 | 0.12 |
| Unaffected shoulder | |||||||
| Flexion | 132.3 (17.5) | 139.3 (14.7) | 137.8 (8.7) | +7.0 | 0.53 | +5.5 | 0.31 |
| Extension | 62.7 (9.5) | 65.0 (11.9) | 65.3 (13.1) | +2.3 | 0.73 | +2.7 | 0.63 |
| Abduction | 130.8 (11.6) | 143.2 (10.9) | 142.2 (5.0) | +3.3 | 0.19 | +2.3 | 0.68 |
| Adduction | 32.2 (6.6) | 39.8 (11.1) | 39.5 (8.6) | +7.7 | 0.13 | +7.3 | 0.04 |
| Internal rot. | 60.7 (7.9) | 61.0 (15.2) | 61.0 (8.3) | +0.3 | 0.93 | +0.3 | 0.95 |
| External rot. | 74.5 (9.0) | 73.3 (4.0) | 69.2 (7.0) | −1.2 | 0.61 | −5.3 | 0.15 |
| Unaffected elbow | |||||||
| Flexion | 148.2 (4.4) | 148.8 (1.7) | 148.2 (4.6) | +0.7 | 0.71 | 0.0 | – |
| Extension | 0.0 (0.0) | 0.0 (0.0) | 0.0 (0.0) | 0.0 | – | 0.0 | – |
| Pronation | 86.8 (5.6) | 87.8 (5.9) | 92.8 (2.5) | +1.0 | 0.45 | +6.0 | 0.10 |
| Supination | 83.5 (6.1) | 83.8 (7.1) | 85.0 (4.2) | +0.3 | 0.88 | +1.5 | 0.65 |
©Bright Cloud International Corp. Reprinted with permission.
Mean (and standard deviation) of variables are shown pre-training (T0), post-training (T1) and at 8-week follow-up (T2). Bold p values indicate statistical significance.
Sign reversed so all positive differences in table indicate improvement.
UE strength and functional assessments
Table 3 lists the strength metrics captured for the affected and unaffected arms including anterior and lateral deltoid shoulder, hand grip, 2 and 3 finger pinch grip, as well as the functional assessments FMA, CAHAI, UEFI-20 and JHFT (both arms). A total of 13 out of the 15 strength and functional assessment measures improved between pre-training (T0) and either post-training (T1) or follow-up (T2). The result of the binomial sign test was statistically significant (p = 0.02).
Table 3.
Hand and arm strength and functional assessments statistical analysis for the experimental group (N = 6) of cancer survivors coping with chronic pain.
| Variable | T0 | T1 | T2 | T1 – T0 | p | T2 – T0 | p |
|---|---|---|---|---|---|---|---|
| Affected arm strength (N) | |||||||
| Grip | 159.4 (54.5) | 199.1 (59.3) | 199.9 (64.0) | +39.7 | 0.27 | +40.6 | 0.34 |
| Pinch | 23.9 (17.3) | 33.9 (14.3) | 32.4 (12.3) | +10.0 | 0.12 | +8.5 | 0.18 |
| 3 Jaw | 31.9 (18.2) | 40.0 (11.4) | 39.9 (14.1) | +8.2 | 0.31 | +8.0 | 0.35 |
| Anterior deltoid | 15.6 (8.8) | 23.0 (11.0) | 25.2 (10.8) | +7.4 | 0.05 | +9.6 | 0.06 |
| Lateral deltoid | 15.6 (8.8) | 23.0 (11.0) | 25.2 (10.8) | +7.4 | 0.05 | +9.6 | 0.06 |
| Unaffected arm strength (N) | |||||||
| Grip | 181.6 (71.9) | 202.7 (32.5) | 213.1 (42.5) | +21.1 | 0.52 | +31.5 | 0.33 |
| Pinch | 33.8 (15.6) | 32.5 (10.6) | 31.3 (10.5) | −1.3 | 0.85 | −2.4 | 0.77 |
| 3 Jaw | 34.9 (11.3) | 42.6 (8.3) | 43.1 (12.1) | +7.7 | 0.08 | +8.2 | 0.38 |
| Anterior deltoid | 28.9 (13.6) | 31.9 (11.0) | 34.8 (12.1) | +3.0 | 0.17 | +5.9 | 0.08 |
| Lateral deltoid | 26.7 (12.3) | 31.9 (11.0) | 34.9 (12.1) | +5.2 | 0.08 | +8.2 | 0.03 |
| Functional assessments | |||||||
| FMA | 59.7 (6.3) | 61.8 (2.3) | 61.8 (3.0) | +2.2 | 0.28 | +1.3 | 0.44 |
| CAHAI-9 | 59.8 (5.0) | 62.5 (1.2) | 62.5 (1.2) | +2.7 | 0.26 | +2.7 | 0.26 |
| UEFI-20 | 41.8 (15.9) | 50.3 (12.9) | 55.7 (11.8) | +8.5 | 0.32 | +13.8 | 0.004 |
| JHFT affected | 57.0 (14.9) | 49.2 (11.5) | 47.7 (12.8) | +7.8a | 0.34 | +6.3a | 0.40 |
| JHFT unaffected | 58.2 (10.2) | 60.5 (12.5) | 59.5 (10.4) | −2.3a | 0.37 | −1.3a | 0.64 |
©Bright Cloud International Corp. Reprinted with permission.
FMA: Fulg–Meyer Assessment; CAHAI-9: Chedokee Arm and Hand Activity Inventory-9; UEFI-20: Upper Extremity Functional Index 20; JHFT: Jebsen Hand Function Test.
Mean (and standard deviation) of variables are shown pre-training (T0), post-training (T1) and at 8-week follow-up after training ended (T2). Bold p values indicate statistical significance.
Sign reversed so all positive differences in table indicate improvement.
The group average anterior deltoid shoulder strength improved by 7.4 N at T1 (p = 0.05) and 9.6 N at T2 (p = 0.06) for the affected shoulder. The group average Lateral Deltoid Strength improved by 5.2 N at T1 (p = 0.08) and 8.2 N at T2 (p = 0.03) for the unaffected shoulder. The average grasp strength for the affected and unaffected hands increased by 39.7 and 21.1 N, respectively, post-training, and increased by 40.6 and 31.5 N, respectively, at follow-up compared to pre-training. The improvement in grip strength for both arms of Subject No. 5 (aged 22 years) at both post-training and follow-up exceeded the MCID of 49 N for hand grip.
CAHAI improved 2.7 points from 59.8 (SD = 5.0) pre-training (T0) to 62.5 (SD = 1.2) post-training (T1) and follow-up (p = 0.26). Only the improvement for Subject No. 5 exceeded the MCID of 6.3 for CAHAI,32 as all assessments for the other five subjects were within 3 points of the maximum CAHAI scale (63). FMA score on average improved by 2.2 points (p = 0.28) from 59.7 (SD = 6.3) at pre-training (T0) to 61.8 (SD = 2.3) at post-training (T1). At follow-up (T2), the improvement was 1.3 points (p = 0.44) with an average FMA of 61.0 (SD = 3.0). Again, only the improvement for Subject No. 5 exceeded the MCID of 9 points for FMA,33 as the other five subjects consistently scored within 6 points of the maximum UE score of 66 points on the FMA UE subset test.
The ability to perform ADL improved on average 8.5 points (p = 0.32) from 41.8 (SD = 15.9) at T0 to 50.3 (SD = 12.9) at T1. At follow-up (T2), the improvement increased 13.8 points to 55.7 (SD = 11.8), a statistically significant result (p = 0.0004). Both mean changes in ADL exceeded the MCID of 8 points for the UEFI-20 questionnaire.25 The individual improvement in ADL exceeded MCID for two of six subjects at post-training and for five of six subjects at follow-up. The youngest subject (No. 5, aged 22 years) acquired the ability to pick up and carry a large heavy shoulder tote bag using the affected UE. Her better mobility resulted in improved socialization with both family and friends. Furthermore, she returned to working full-time as certified nursing assistant after therapy, which she maintained at follow-up.
Cognitive and emotive outcomes
The neuropsychology consultant evaluated subjects for the 12 cognitive and emotive metrics shown in Table 4. A total of 10 out of the 11 cognitive metrics with non-zero changes improved between pre-training (T0) and post-training (T1). The result of the binomial sign test was statistically significant (p = 0.01). The most notable improvements included a drop in group average depression severity of 8.3 points from 17.7 (SD = 12.8) at T0 to 9.3 (6.7) at T1 (p = 0.04), and a drop of 5.7 points at follow-up from 17.7 (SD = 12.8) at T0 to 12.0 (SD = 11.5) at T2 (p = 0.07). Both changes were above the MCID of 5 points for BDI-II.34 The individual improvement in depression exceeded MCID for five of six subjects at post-training and for three of six subjects at follow-up. The BVMT-R assessment of visuospatial memory showed a group average improvement from 13.0 (SD = 2.7) at T0 to 21.0 (SD = 5.7) at T1, an 8.0-point difference (p = 0.0007).
Table 4.
Emotive and cognitive outcomes for the group (N = 6) of cancer survivors coping with chronic pain.
| Variable | T0 | T1 | T2 | T1 – T0 | p | T2 – T0 | p |
|---|---|---|---|---|---|---|---|
| BDI-II | 17.7 (12.8) | 9.3 (6.7) | 12.0 (11.5) | +8.3a | 0.04 | +5.7a | 0.07 |
| TMT-A | 32.0 (8.7) | 38.3 (19.3) | 31.6 (12.8) | −6.3a | 0.37 | −0.4a | 0.78 |
| TMT-B | 103.3 (36.8) | 83.5 (20.7) | 74.2 (20.6) | +19.8a | 0.32 | +29.1a | 0.37 |
| NAB Person | 14.0 (0.0) | 14.0 (0.0) | 13.8 (0.4) | 0.0 | – | −0.2 | 0.36 |
| NAB Time | 9.7 (0.8) | 9.8 (0.4) | 9.8 (0.4) | +0.2 | 0.70 | +0.1 | 0.37 |
| NAB Place | 3.8 (0.4) | 4.0 (0.0) | 3.8 (0.4) | +0.2 | 0.36 | 0.0 | – |
| Digits forward | 7.7 (2.5) | 8.0 (1.7) | 8.2 (2.2) | +0.3 | 0.68 | +0.5 | 0.27 |
| Digits back | 3.3 (1.2) | 3.7 (1.9) | 3.8 (0.8) | +0.3 | 0.71 | +0.5 | 0.18 |
| NAB dots | 5.3 (2.7) | 5.8 (2.3) | 5.6 (1.7) | +0.5 | 0.46 | +0.3 | 0.87 |
| NAB word gen | 8.8 (5.1) | 11.0 (4.9) | 10.6 (4.0) | +2.2 | 0.07 | +2.0 | 0.08 |
| HVLT-R | 22.3 (3.4) | 22.7 (5.3) | 21.6 (6.6) | +0.3 | 0.86 | −0.7 | 0.96 |
| BVMT-R | 13.0 (2.7) | 21.0 (5.7) | 17.6 (8.9) | +8.0 | 0.007 | +6.2 | 0.09 |
©Bright Cloud International Corp. Reprinted with permission.
BDI-II: Beck Depression Inventory, Second Edition; TMT-A: Trail Making Test A; TMT-B: Trail Making Test B; NAB: Neuropsychological Assessment Battery; HVLT-R: Hopkins Verbal Learning Test, Revised; BVMT-R: Brief Visuospatial Memory Test, Revised.
Mean (and standard deviation) of variables are shown pre-training (T0), after 8 weeks of training (T1), and at 8-week follow-up after training (T2). Bold p values indicate statistical significance.
Sign reversed so all positive differences in table indicate improvement.
Attendance to protocol and technology acceptance
The six participants completed the 16 sessions of the protocol on average over a period of 8.5 weeks. Missed sessions due to medical issues on any given day were typically made up in the same week. There were three additional subjects who dropped from the study (after completing 2, 5 and 11 sessions, respectively) due to prolonged illness causing absences for more than two consecutive weeks and inability to complete the post-assessment. One participant reported prior issues with lymphedema at grade 1 (mild) with no current complaints due to the same. No motion sickness due to VR therapy was reported by any participant.
The six participants provided their subjective evaluation of the system by answering the 10 questions of the custom subjective evaluation form. The average of all responses was 3.9/5 at week 4, which improved to 4.3/5 at week 8. The lowest post-training ratings were for the questions ‘I had no pain or discomfort in my upper body?’ (3.3), ‘Playing games with my affected arm(s) was easy?’ (3.7), ‘Playing games with both arms was easy?’ (3.7), and ‘The length of the exercising in a day was appropriate?’ (3.83). The responses averaged 4.3 or better for the questions, ‘There were few technical problems?’ (4.3), ‘The system was easy to use’ (4.5), ‘I would encourage others to used it?’ (4.7), ‘I was not bored while exercising?’ (4.7) and ‘Instructions given to me were useful?’ (4.8). The highest score was 4.83/5 for the question ‘I liked the system overall?’ which shows good technology acceptance.
Discussion
Cancer pain management is challenging.35 The nature and complexity of chronic pain does not easily lend itself to changes in perception of pain intensity.36 Many survivors of breast cancer continue to report pain years post-mastectomy.37 In this study, the pain intensity measured using the NRS showed a 20% downward trend (p = 0.1). The subjects reported their pain as burning, electrical sensation, and stabbing. This characteristic neuropathic nature of the cancer-related upper body pain has been reported in the literature38 and is complex to treat. However, improvements in arm strength and range can lead to improved participation in daily activities for people with chronic cancer pain. In this study, BrightArm Duo training mediated 1300 active arm repetitions per session at the end of therapy, increased shoulder range of motion, and improved lateral deltoid strength. These gains need to be studied in a larger sample to further examine their benefit.
The most remarkable finding was a significant improvement in depression without an increase in depression medication dosage. This finding relates to a positive effect of VR on emotional well-being as discussed in a recent systematic review of VR in cancer treatments.15 The ability to interact with virtual media has been shown to be beneficial in mental health as opposed to passively watching television.15 Immersion in VR two times a week for an 8-week protocol reduced depression an average of 8.3 points in this study (p = 0.04) as measured by the BDI-II. This improvement in depression, which is larger than the MCID of 5 points,34 can be attributed to the positive motivational aspect of VR therapy as reported in the literature.39 Moreover, a maintenance effect was seen at follow-up after 8 weeks where the BDI-II scores, although higher than post-training (T2 = 12.0), did not reach the pre-training levels (T0 = 17.7). This is an encouraging finding. Furthermore, one of the subjects had worsened her depression at follow-up versus post-training since she had increased lower extremity pain due to her other medical care, as well as family issues. For a small N such as in this study, the worsening in depression for causes unrelated to the study had a negative effect for the group average result at follow-up. Maintenance effect of VR has been reported for treatment of obesity40 and surgical technique training studies;41 however, no such effect has been previously studied in post-surgical breast cancer survivors.
Cognition improved for the subjects in 10 out of 11 evaluation metrics used in this study (p = 0.01) and aligns with the benefits described in cognition after VR therapy in other studies related to brain injury.42 Specifically, visual memory improved significantly (p = 0.0007) as measured by BVMT-R, indicating that the custom games designed in this study greatly increased the visual memory ability of the subject group. The reason for cognitive impairments seen in this group of individuals could be age related, the consequence of cancer per se43 or an effect of chemotherapy and has a prevalence of 25.9% in women with breast cancer.44 Cognitive impairments and VR therapy need to be further studied in this population. As observed in other studies of women with breast cancer,12 VR therapy was very well accepted. Although the questionnaire is limited by the custom design tailored to this VR system, the BrightArm Duo technology was well liked by participants in this first feasibility study of the system with a chronic pain population. As with immersive games, no ‘cyber sickness’ (symptoms such as vomiting or dizziness)45 was reported by participants in this study. We do not consider the 33% dropout rate as reflecting negatively on the system acceptance. The three subjects who dropped out from study did so due to persistent scheduling conflicts, or prolonged illness causing absences for more than two consecutive weeks.
A limitation of this study was small sample size. Although the study aimed to recruit more subjects, only six were able to successfully complete the 8-week protocol. The challenges reported by subjects were unrelated to VR therapy and were inability to drive to appointments, changes in medical status with prolonged illness, scheduling issues in-between other treatments, religious observances and family obligations.
Another limitation was a lack of additional measures. The measurement of pain was done only in terms of pain intensity using the NRS. Questionnaires targeting fear of pain and pain catastrophizing are needed for capturing better information about the nature of pain. Also, there is a difference in arm and breast pain in women with cancer and the results of this study can only be generalized to women with arm pain post breast cancer surgery46 since breast pain and arm pain were not assessed separately in this study. Furthermore, lymphedema, a common problem post-mastectomy, was not measured in this study and could complicate PMPS. However, other than one participant with prior reported lymphedema, none of the participants had any current problems.
In conclusion, this pilot study demonstrated the feasibility of using the BrightArm Duo Rehabilitation System. The findings indicate improvement in cognition, shoulder range, strength, function, and in reducing depression for people coping with chronic pain post cancer surgery. Future research studies need to take into consideration the need for randomized controls, overcoming the obstacles to scheduling and transportation issues faced by subjects, and including additional pain and behaviour measures.
Acknowledgments
The authors wish to thank Abby Eisner OT (Occupational Therapist) and Daniel Saldana for performing clinical evaluations, Pooja Joshi OT for training some of the subjects, as well as Ms Noreen Rana, the clinical coordinator of this study.
Footnotes
Declaration of conflicting interests: The authors declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: G.H. is CTO of Bright Cloud International Corp (BCI). G.B. is inventor on a patent related to technology described above and majority shareholder of BCI. N.G. is a part-time employee and K.P. is a full-time employee of BCI. D.R. is a part-time employee and shareholder of BCI. F.D. is an Administrator of Roosevelt Care Center where the project study took place. J.H. has been a contractor for BCI. D.D. is the founder of the University Pain Medicine Center that recruited the subjects for this study.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Research reported here was supported by grant 1R43DA032224-01 from the National Institutes for Health/National Institute on Drug Abuse (NIDA).
References
- 1. CDC. Invasive cancer incidence – United States. MMWR Weekly, 2010, http://www.cdc.gov/mmwr/preview/mmwrhtml/mm6312a1.htm (accessed 5 January 2016).
- 2. van den Beuken-van Everdingen MHJ, de Rijke JM, Kessels AG, et al. Prevalence of pain in patients with cancer: a systematic review of the past 40 years. Ann Oncol 2007; 18(9): 1437–1449. [DOI] [PubMed] [Google Scholar]
- 3. Rietman JS, Dijkstra PU, Hoekstra HJ, et al. Late morbidity after treatment of breast cancer in relation to daily activities and quality of life: a systematic review. Eur J Surg Oncol 2003; 29(3): 229–238. [DOI] [PubMed] [Google Scholar]
- 4. Riva G. Virtual reality in neuro-psycho-physiology: cognitive, clinical and methodological issues in assessment and rehabilitation. Amsterdam: IOS Press, 1997, p. 226. [PubMed] [Google Scholar]
- 5. Ebaugh D, Spinelli B, Schmitz KH. Shoulder impairments and their association with symptomatic rotator cuff disease in breast cancer survivors. Med Hypotheses 2011; 77(4): 481–487. [DOI] [PubMed] [Google Scholar]
- 6. Nesvold I-L, Fosså SD, Holm I, et al. Arm/shoulder problems in breast cancer survivors are associated with reduced health and poorer physical quality of life. Acta Oncol 2010; 49(3): 347–353. [DOI] [PubMed] [Google Scholar]
- 7. Schreiber KL, Kehlet H, Belfer I, et al. Predicting, preventing and managing persistent pain after breast cancer surgery: the importance of psychosocial factors. Pain Manag 2014; 4(6): 445–459. [DOI] [PubMed] [Google Scholar]
- 8. Metrebian N. Diagnosis of dependence to prescribed pain medication. Lancet Psychiatry 2015; 2(4): 283–284. [DOI] [PubMed] [Google Scholar]
- 9. Just J, Mücke M, Bleckwenn M. Dependence on prescription opioids. Dtsch Ärztebl Int 2016; 113(13): 213–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ilgen MA, Bohnert ASB, Ganoczy D, et al. Opioid dose and risk of suicide. Pain 2016; 157(5): 1079–1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hoffman HG, Doctor JN, Patterson DR, et al. Virtual reality as an adjunctive pain control during burn wound care in adolescent patients. Pain 2000; 85(1–2): 305–309. [DOI] [PubMed] [Google Scholar]
- 12. Schneider SM, Ellis M, Coombs WT, et al. Virtual reality intervention for older women with breast cancer. Cyberpsychol Behav 2003; 6(3): 301–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Gershon J, Zimand E, Pickering M, et al. A pilot and feasibility study of virtual reality as a distraction for children with cancer. J Am Acad Child Adolesc Psychiatry 2004; 43(10): 1243–1249. [DOI] [PubMed] [Google Scholar]
- 14. Triberti S, Repetto C, Riva G. Psychological factors influencing the effectiveness of virtual reality-based analgesia: a systematic review. Cyberpsychol Behav Soc Netw 2014; 17(6): 335–345. [DOI] [PubMed] [Google Scholar]
- 15. Chirico A, Lucidi F, De Laurentiis M, et al. Virtual reality in health system: beyond entertainment. A mini-review on the efficacy of VR during cancer treatment. J Cell Physiol 2016; 231(2): 275–287. [DOI] [PubMed] [Google Scholar]
- 16. Rutter CE, Dahlquist LM, Weiss KE. Sustained efficacy of virtual reality distraction. J Pain 2009; 10(4): 391–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Li A, Montaño Z, Chen VJ, et al. Virtual reality and pain management: current trends and future directions. Pain Manag 2011; 1(2): 147–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. House G, Burdea G, Polistico K, et al. Integrative rehabilitation of residents chronic post-stroke in skilled nursing facilities: the design and evaluation of the BrightArm Duo. Disabil Rehabil Assist Technol 2015; 1–12. [DOI] [PubMed] [Google Scholar]
- 19. Unity. Unity Manual (Internet), http://docs.unity3d.com/Manual/index.html (accessed 18 January 2016).
- 20. Paice JA, Cohen FL. Validity of a verbally administered numeric rating scale to measure cancer pain intensity. Cancer Nurs 1997; 20(2): 88–93. [DOI] [PubMed] [Google Scholar]
- 21. Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med 2001; 16(9): 606–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Duncan PW, Propst M, Nelson SG. Reliability of the Fugl-Meyer assessment of sensorimotor recovery following cerebrovascular accident. Phys Ther 1983; 63(10): 1606–1610. [DOI] [PubMed] [Google Scholar]
- 23. Barreca S, Gowland C, Stratford P, et al. Development of the Chedoke Arm and Hand Activity Inventory: theoretical constructs, item generation, and selection. Top Stroke Rehabil 2004; 11(4): 31–42. [DOI] [PubMed] [Google Scholar]
- 24. Jebsen RH, Taylor N, Trieschmann RB, et al. An objective and standardized test of hand function. Arch Phys Med Rehabil 1969; 50(6): 311–319. [PubMed] [Google Scholar]
- 25. Chesworth BM, Hamilton CB, Walton DM, et al. Reliability and validity of two versions of the upper extremity functional index. Physiother Can 2014; 66(3): 243–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Beck A, Steer R, Brown G. Manual for beck depression inventory, 2nd edn. Antonio, TX: Psychology Corp, 1996. [Google Scholar]
- 27. White T, Stern R. Neuropsychological assessment battery. Lutz, FL: Psychological Assessment Resources, 2003. [Google Scholar]
- 28. Brandt J, Benedict R. Hopkins verbal learning test: revised. Lutz, FL: Psychological Assessment Resources, 2001. [Google Scholar]
- 29. Benedict R. Brief visuospatial memory test: revised. Odessa, FL: Psychological Assessment Resources, 1997. [Google Scholar]
- 30. Reitan R. Validity of the trail making test as an indicator of organic brain damage. Percept Mot Skills 1958; 8: 271–276. [Google Scholar]
- 31. Roy J-S, MacDermid JC, Woodhouse LJ. Measuring shoulder function: a systematic review of four questionnaires. Arthritis Rheum 2009; 61(5): 623–632. [DOI] [PubMed] [Google Scholar]
- 32. Barreca SR. Chedoke arm and hand activity inventory (CAHAI), 2015, http://www.cahai.ca/manual.html (accessed 26 October 2015). [PubMed]
- 33. Arya KN, Verma R, Garg RK. Estimating the minimal clinically important difference of an upper extremity recovery measure in subacute stroke patients. Top Stroke Rehabil 2011; 18 (Suppl. 1): 599–610. [DOI] [PubMed] [Google Scholar]
- 34. Hiroe T, Kojima M, Yamamoto I, et al. Gradations of clinical severity and sensitivity to change assessed with the Beck Depression Inventory-II in Japanese patients with depression. Psychiatry Res 2005; 135(3): 229–235. [DOI] [PubMed] [Google Scholar]
- 35. Thronæs M, Raj SX, Brunelli C, et al. Is it possible to detect an improvement in cancer pain management? A comparison of two Norwegian cross-sectional studies conducted 5 years apart. Support Care Cancer 2016; 24(6): 2565–2574. [DOI] [PubMed] [Google Scholar]
- 36. Marty M, Rozenberg S, Legout V, et al. Influence of time, activities, and memory on the assessment of chronic low back pain intensity. Spine 2009; 34(15): 1604–1609. [DOI] [PubMed] [Google Scholar]
- 37. Macdonald L, Bruce J, Scott NW, et al. Long-term follow-up of breast cancer survivors with post-mastectomy pain syndrome. Br J Cancer 2005; 92(2): 225–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Smith WC, Bourne D, Squair J, et al. A retrospective cohort study of post mastectomy pain syndrome. Pain 1999; 83(1): 91–95. [DOI] [PubMed] [Google Scholar]
- 39. Molina KI, Ricci NA, de Moraes SA, et al. Virtual reality using games for improving physical functioning in older adults: a systematic review. J Neuroeng Rehabil 2014; 11: 156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Cesa GL, Manzoni GM, Bacchetta M, et al. Virtual reality for enhancing the cognitive behavioral treatment of obesity with binge eating disorder: randomized controlled study with one-year follow-up. J Med Internet Res 2013; 15(6):e113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Khan MW, Lin D, Marlow N, et al. Laparoscopic skills maintenance: a randomized trial of virtual reality and box trainer simulators. J Surg Educ 2014; 71(1): 79–84. [DOI] [PubMed] [Google Scholar]
- 42. Shin H, Kim K. Virtual reality for cognitive rehabilitation after brain injury: a systematic review. J Phys Ther Sci 2015; 27(9): 2999–3002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Lange M, Giffard B, Noal S, et al. Baseline cognitive functions among elderly patients with localised breast cancer. Eur J Cancer 2014; 50(13): 2181–2189. [DOI] [PubMed] [Google Scholar]
- 44. Cheung YT, Ong YY, Ng T, et al. Assessment of mental health literacy in patients with breast cancer. J Oncol Pharm Pract 2015; 22: 437–447. [DOI] [PubMed] [Google Scholar]
- 45. Holden MK. Virtual environments for motor rehabilitation: review. Cyberpsychology Behav 2005; 8(3): 187–211. [DOI] [PubMed] [Google Scholar]
- 46. Langford DJ, Paul SM, West C, et al. Persistent arm pain is distinct from persistent breast pain following breast cancer surgery. J Pain 2014; 15(12): 1238–1247. [DOI] [PMC free article] [PubMed] [Google Scholar]