Abstract
Background:
Pain control after bariatric surgery is a major challenge. Our objective was to study the efficacy and safety of intrathecal (IT) morphine 0.3 mg added to bupivacaine 0.5% for postoperative pain after laparoscopic bariatric surgery.
Methods:
After local ethics committee approval, 100 morbidly obese patients scheduled for laparoscopic bariatric surgery were enrolled in this study. Patients were randomly assigned into two groups: Group I received IT 0.3 mg morphine (0.3 mL) added to 1.2 mL of bupivacaine 0.5%; Group II received IT 0.3 mL saline added to 1.2 mL of bupivacaine 0.5%, immediately before induction of general anaesthesia. For both groups, 60 mg ketorolac and 1000 mg paracetamol were infused 30 minutes before the end of surgery. After wound closure, 20 mL bupivacaine 0.25% was infiltrated at wound edges.
Results:
Visual Analogue Scale (VAS) score was significantly lower in group I immediately, 30 minutes and 1 hour postoperatively. Time to first ambulation, return of intestinal sounds and hospital stay were shorter in group I than group II (p < 0.05); total morphine consumption was significantly lower in group I than group II (p < 0.05). Sedation score was significantly higher in group I immediately postoperatively, while at 30 minutes, 1, 2 and 6 hours postoperatively sedation scores were significantly higher in group II. Itching was significantly higher in group I.
Conclusion:
The addition of IT morphine to a multimodal analgesic regimen after laparoscopic bariatric surgery was an effective and safe method that markedly reduced postoperative pain, systemic opioid consumption and length of hospital stay.
Keywords: Intrathecal morphine, postoperative pain management, multimodal analgesia, laparoscopic bariatric surgery
Introduction
Fear of side effects, such as respiratory depression, over-sedation, nausea and vomiting, from opioid use in patients with morbid obesity often leads to under-treatment of pain. Inadequate analgesia indirectly contributes to multiple postoperative complications in patients with morbid obesity.
In abdominal surgeries, pain prevents deep breathing and further adds to basal lung atelectasis in this patient population. Joris et al.1 demonstrated that postoperative analgesia is directly related to improvement in postoperative pulmonary function tests in patients with morbid obesity undergoing abdominal surgery. Superior pain relief can prevent development of hypoxaemia, atelectasis and pneumonia;2 similarly, adequate analgesia promotes early ambulation. This becomes all the more important in this patient population where deep vein thrombosis rates are significantly higher than in normal population.3–5
Intrathecal (IT) opioid administration is an attractive analgesic technique since the opioid is injected directly into the cerebrospinal fluid, close to the structures of the central nervous system where the opioid acts.6
Morphine has a greater spinal bioavailability and therefore is administered neuraxially; it is a suitable choice for the treatment of acute postoperative pain.7 The neuraxial application of local anaesthetics and opioids combined with general anaesthesia (especially in patients undergoing major abdominal or thoracic procedures) as a multimodal strategy can provide superior pain relief, reduced hormonal and metabolic stress, enhanced normalization of gastrointestinal function and thus a shortened postoperative recovery time, facilitating mobilization and physiotherapy.8
Controlling pain after bariatric surgery is a major challenge; advice on general management includes multimodal analgesic therapy, preference for regional techniques, avoidance of sedatives, non-invasive ventilation with supplemental oxygen and early mobilization and elevation of the head of bed to 30°.9
Our aim was to determine the efficacy and safety of IT morphine (0.3 mg) added to bupivacaine as a part of multimodal analgesia in the management of postoperative pain in morbidly obese patients undergoing laparoscopic bariatric surgery.
Patients and methods
After Hospital Ethics Committee approval and obtaining written informed consent, 100 morbidly obese patients of both sexes, American Society of Anesthesiologists (II and III) scheduled for laparoscopic bariatric surgery (Roux en Y gastric bypass, sleeve gastrectomy and mini bypass) under general anaesthesia, were enrolled in this double-blinded (patient and attending investigator) prospective study. The patients’ inclusion criteria were those scheduled for laparoscopic bariatric surgery with body mass index (BMI) >40 kg/m2 or >35 kg/m2 with at least one co-morbidity.10
Patients with a known allergy to the study drugs; advanced cardiac, respiratory, renal or hepatic diseases; coagulation disorders; infection at or near the site of IT injection; drug or alcohol abuse; and psychiatric illnesses that may interfere with perception and assessment of pain were excluded from the study. Patients were premedicated with 40 mg pantoprazole sodium (Controloc®; Takeda CmbH, Germany) and 8 mg dexamethasone sodium phosphate (Dexamethasone®; Amriya Pharma, Egypt) to guard against IT morphine–induced nausea and vomiting.11
Preoperatively, patients were taught to evaluate their own pain intensity using the Visual Analogue Scale (VAS), scored from 0 to 10 (where 0 = no pain and 10 = worst pain imaginable), and to use patient-controlled analgesia (PCA) device according to their VAS (where they can push the PCA button when VAS score is ⩾3).
Upon arrival to the operating room, routine monitoring probes were introduced including non-invasive blood pressure (NIBP), electrocardiography (ECG), pulse oximetry and capnography. Neuromuscular function was monitored by TOF-watch (train-of-four-watch) (TOF-Watch®; Organon (Ireland) Ltd).
Patients were randomly assigned using a randomization computer program, and sealed envelope method, into two groups who received IT morphine and saline immediately before induction of general anaesthesia:
Group I: IT 0.3 mg morphine (0.3 mL) added to 1.2 mL of bupivacaine 0.5%;
Group II: IT 0.3 mL saline added to 1.2 mL of bupivacaine 0.5%.
The technique was described in detail for the patients. The IT injection was done using the 25-gauge 88-mm spinal needles (Sprotte®; B|BRAUN Melsungen AG, Germany) under complete aseptic precautions at L2-3 interspace. Patients were positioned ideally (sitting with best possible flexion at spine) and the procedure was performed by an experienced anaesthesiologist. Successful block was confirmed before the induction of general anaesthesia to be sure that morphine reached the IT space by testing for motor and sensory block.
General anaesthesia was induced using intravenous (IV) lidocaine 1.5 mg/kg, propofol 1–2 mg/kg, fentanyl 2 µg/kg and rocuronium 0.6 mg/kg to facilitate intubation. Anaesthesia was maintained using isoflurane 1–1.5 MAC (minimum alveolar concentration) in 50% oxygen/air mixture and rocuronium 0.1 mg/kg with mechanical ventilation in parameters to maintain normocapnia; all doses were calculated according to ideal body weight (IBW).
Thirty minutes before the end of surgery, 8 mg ondansetron hydrochloride dihydrate (Zofran®; Glaxosmithkline, UK), as a prophylaxis against IT morphine–induced itching12 and postoperative nausea and vomiting (PONV), 1000 mg of paracetamol infusion (Perfalgan®; Bristol-Myers Squibb, UK) and 60 mg ketorolac tromethamine (Ketolac®; Amriya Pharma, Egypt) were administered.
After wound closure, 20 mL of bupivacaine 0.25% was infiltrated at the sites of insertion of laparoscopic instruments. Before extubation, residual muscle relaxation was reversed using sugammadex 2 mg/kg IBW, and urinary bladder was evacuated. Patients were transferred to post-anaesthesia care unit (PACU) where observation for 24 hours was done. Rescue postoperative analgesia in the form of IV morphine PCA (IV PCA) was used, with an initial bolus of 0.1 mg/kg IBW once the patient expressed pain or if VAS ⩾ 3 followed by 1 mg bolus with a lockout period of 15 minutes with no background infusion allowed.
All patients were maintained postoperatively on 4 mg of ondansetron (Zofran) given every 4 hours to guard against PONV. In uncontrolled cases, IV metoclopramide 10 mg was added.
In PACU, patients were given oxygen through nasal cannula (4 L/min); patients with obstructive sleep apnoea (OSA) were given oxygen at the same flow rate through a nasal airway along with 2% lidocaine gel. All patients were observed and assessed for the following:
The haemodynamics (heart rate and NIBP);
Respiratory monitoring in the form of respiratory rate and oxygen saturation (using pulse oximetry);
The total dose of rescue morphine consumed during the follow-up period;
Pain intensity (while the patients were in bed) by using the VAS (which is a line graded from 0 to 10, where 0 = no pain and 10 = the worst pain imaginable);
Complications of opioids such as nausea and vomiting, itching, respiratory depression, urinary retention and sedation by Ramsay sedation score (1–6; Table 1);13
Time to ambulation and return of intestinal sounds;
Side effects related to the IT (site infection and headache);
Length of hospital stay.
Table 1.
Ramsay13 sedation score.
| Score | Description |
|---|---|
| 1 | Anxious and agitated or restless or both |
| 2 | Co-operative, oriented and calm |
| 3 | Responsive to commands only |
| 4 | Exhibiting brisk response to light glabellar tap or loud auditory stimulus |
| 5 | Exhibiting a sluggish response to light glabellar tap or loud auditory stimulus |
| 6 | Unresponsive |
All those observations (except for length of hospital stay) were recorded at these time points: immediately postoperatively (after extubation and before transportation to PACU), 30 minutes, 1, 2, 6, 12, 18 and 24 hours postoperatively.
For ambulation and discharge of our patients, we used a clinical protocol that was frequently applied to allow patient ambulation; the patient has to meet the following criteria: stable and average vital signs; oriented; pain, nausea or vomiting adequately controlled; all drains, catheters, tubes and dressings secured; no new symptoms that may threaten a safe recovery; minimal wound oozing; adequate peripheral circulation; and minimal dizziness or shortness of breath after sitting for 10 minutes.
For discharge from the PACU, patients had to meet these criteria: the acute effects of anaesthesia and surgery are resolved, continuous monitoring is no longer required and the risk of abrupt airway obstruction or profound hypotension is minimal.
The same criteria are applied to discharge from hospital with the addition of ability of the patient to void, giving discharge medications to the patient and giving patient discharge instructions.
Our primary outcome measure was the efficacy of 0.3 mg of IT morphine added to bupivacaine 0.5% in reducing postoperative total analgesic consumption in this special group of patients. Secondary outcome measures included postoperative pain scores, time to first request of rescue analgesia, the length of hospital stay and the tolerability of the used doses represented by the side effects during the follow-up period of 24 hours.
Statistical analysis
Calculation of the sample size was based on the difference in the total dose of morphine consumption, with an expected background standard deviation of 2.0, an alpha error not exceeding 0.05 and power of 90%; we estimated that 25 patients in each group would be required. To compensate for dropouts, we recruited 50 patients in each group to account for random errors and additional comparisons.
Analysis was performed using SPSS® version 17 (Chicago, USA). Data were presented as mean ± standard deviation (SD), numbers and percentages. The Mann–Whitney test was used to compare the numeric data of two groups, while the Chi-square test was used for comparison between percentages and frequencies. A value of p < 0.05 was considered significant.
Results
In this study, there were no significant differences between the studied groups in the demographic data (age, sex, weight, height and BMI) and associated co-morbidities (diabetes, asthma, hypertension and OSA) (p > 0.05; Table 2).
Table 2.
Demographic data and co-morbidities in both study groups.
| Item | Group I (n = 50) | Group II (n = 50) | p-value |
|---|---|---|---|
| 1. Age (years), mean ± SD | 35.40 ± 10.62 | 36.58 ± 11.05 | p = 0.588 |
| 2. Sex | |||
| Male | 16 (32.0%) | 18 (36.0%) | p = 0.47 |
| Female | 34 (68.0%) | 32 (64.0%) | |
| 3. Weight (kg), mean ± SD | 141.00 ± 25.85 | 134.80 ± 22.06 | p = 0.200 |
| 4. Height (cm), mean ± SD | 168.89 ± 4.83 | 170.40 ± 5.19 | p = 0.119 |
| 5. BMI (kg/m2), mean ± SD | 49.35 ± 8.00 | 47.29 ± 6.27 | p = 0.261 |
| 6. Diabetes | 21 (42.0%) | 20 (40.0%) | p = 0.50 |
| 7. Asthma | 3 (6.0%) | 5 (10.0%) | p = 0.357 |
| 8. Hypertension | 24 (48.0%) | 26 (52.0%) | p = 0.421 |
| 9. OSA | 4 (8.0%) | 5 (10.0%) | p = 0.500 |
SD: standard deviation; OSA: obstructive sleep apnoea; BMI: body mass index; PCA: patient-controlled analgesia.
Group I: intrathecal morphine group; Group II: PCA group.
There were no significant differences between both groups as regards the type of operation, the time of the block, operative time and time in the operative room (Table 3). Postoperative haemodynamic variables (systolic and diastolic blood pressure and heart rate) remained within normal range, without statistically significant differences between the two groups (p > 0.05).
Table 3.
Operative data in both study groups.
| Item | Group I (n = 50) | Group II (n = 50) | p-value |
|---|---|---|---|
| 1. Type of operation | |||
| Roux en Y gastric bypass | 23 (46.0%) | 14 (28.0%) | p = 0.088 |
| Sleeve gastrectomy | 13 (26.0%) | 12 (24.0%) | |
| Mini bypass | 14 (28.0%) | 24 (48.0%) | |
| 2. Time of block (minutes), mean ± SD | 6.74 ± 2.76 | 6.94 ± 2.56 | p = 0.482 |
| 3. Operative time (minutes), mean ± SD | 66.00 ± 8.63 | 65.80 ± 8.99 | p = 0.910 |
| 4. Time in OR (minutes), mean ± SD | 100.80 ± 10.06 | 101.10 ± 10.16 | p = 0.832 |
SD: standard deviation; OR: operative room; PCA: patient-controlled analgesia.
Group I: intrathecal morphine group; Group II: PCA group.
Also, postoperative peripheral oxygen saturation and respiratory rate (Figures 1 and 2, respectively) showed no significant difference among groups during the study period (p > 0.05).
Figure 1.
SPO2 (oxygen saturation percentage) of both study groups.
GI: group I (intrathecal morphine group); GII: group II (PCA (patient-controlled analgesia) group).
Figure 2.
Respiratory rate (RR) of both study groups.
GI: group I (intrathecal morphine group); GII: group II (PCA (patient-controlled analgesia) group).
The mean times to ambulation (3.27 ± 1.03; 3.97 ± 1.01), return of intestinal sounds (12.22 ± 5.83; 16.72 ± 5.19) and length of hospital stay (30.12 ± 5.34; 35.86 ± 5.6) were observed in groups I and II, respectively; all of those parameters were significantly shorter in group I than in group II (p < 0.001, 0.01 and 0.03, respectively), and the total morphine consumption (2.06 ± 1.50; 39.10 ± 8.49) was significantly lower in group I than in group II (p < 0.000).
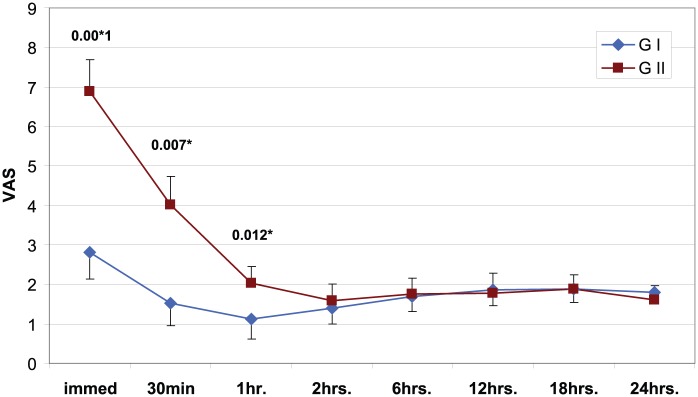
Moreover, postoperative VAS score showed significant decrease in group I compared to group II during the immediate (p = 0.000), 30 minutes (p = 0.001) and 1 hour (p = 0.016) postoperatively (Figure 3).
Figure 3.
Visual Analogue Scale (VAS) of both study groups.
GI: group I (intrathecal morphine group); GII: group II (PCA (patient-controlled analgesia) group).
*Significant p-value.
The sedation score in the immediate postoperative period was significantly higher in group I compared with group II, but at 30 minutes, 1, 2 and 6 hours postoperatively, it was significantly higher in group II compared with group I. During the rest of the study period, all patients of both groups were co-operative, oriented and calm (Figure 4).
Figure 4.
Sedation score (SS) of both study groups.
GI: group I (intrathecal morphine group); GII: group II (PCA (patient-controlled analgesia) group).
*Significant p-value.
No significant side effects were observed except for itching which occurred in 12 (24%) patients in group I compared to group II (p = 0.000) and it was tolerated, so no additional treatment was required. Three (6%) patients in group I and four (8%) in group II had nausea (p = 0.695); two (4%) patients in group I and three (6%) in group II had vomiting (p = 0.646). Otherwise, no side effects (respiratory depression, headache, hypotension, bradycardia and arrhythmia) were observed.
Discussion
Effective pain control can contribute to several clinically valuable outcomes, including earlier patient mobilization and quicker recovery, which can result in a shortened hospital stay and reduced costs.14
Optimal pain control can be better achieved by a multimodal technique; the use of IT opiates as an effective form of postoperative pain relief has been established for many years. Morphine was the first opioid used by IT route. In clinical practice, morphine is regarded as the gold standard used to relieve intense pain.15
In the light of the well-documented clinical advantages associated with neuraxial blockade in the non-obese patient undergoing major abdominal surgery,16 we assumed that grossly obese patients undergoing bariatric surgery would benefit particularly from regional anaesthesia techniques.
To our knowledge, this is the first study to evaluate the efficacy and safety of IT morphine for the management of postoperative pain after laparoscopic bariatric surgery. According to our results, the addition of 0.3 mg of IT morphine with local anaesthetic to a multimodal analgesic regimen was safe and effective and was associated with decreased VAS scores, reduced total morphine consumption in the first 24 hours postoperatively, earlier time to ambulation and return of intestinal sounds, all of which decreased the length of hospital stay without serious side effects.
Rathmell et al.17 reported that the optimal dose of IT morphine depends on the specific surgical procedure, with doses of <0.1 mg often sufficing for pain control after caesarean delivery, whereas doses in the area of 0.5 mg may be required for more extensive surgery. As morbidly obese patients are opioid-sensitive and as opioids increase sensitivity to therapeutic doses producing unpredictable results,18 safety concerns necessitate that analgesic techniques in patients with morbid obesity must be tailored to achieve optimal analgesia with minimal opioids;18 this is why we have chosen a dose of 0.3 mg IT morphine in order to suite the intensity of the associated pain.
In our study, although the difference in pain scores between the two groups was significant only during the first hour of postoperative period, the 24-hour intravenous morphine consumption was significantly decreased in the IT morphine group. Meanwhile, IT morphine was associated with VAS scores of <3 all over the study period. This is in agreement with the meta-analysis by Meylan et al.6 who included five trials in which patients underwent abdominal surgery and the median dose of IT morphine was 0.3 mg; in these trials, the postoperative morphine-sparing was pronounced and pain intensity, at rest, was significantly decreased at 2, 4, 12 and 24 hours.
The analgesic efficacy of IT morphine in our study, including pain score and total morphine consumption, is similar to the meta-analyses by Engelman and Marsala,19 Meylan et al.6 and Popping et al.20
The accumulated clinical experience suggests that morphine administered IT has about 100-fold greater analgesic potency than morphine given IV, allowing for decreases in the total systemic opioid consumption by substituting much lower IT doses in place of higher IV doses.
Also in our study, the duration of hospital stay was decreased in the IT morphine group which confirmed the results of the meta-analysis by Meylan et al.,6 who reported that IT morphine decreased the duration of hospital stay by 0.5 days.
In our study, the only complication was itching in the IT morphine group. There were no cases of respiratory depression or urinary retention; there were a limited number of cases of nausea (group I: 3, group II: 4) and vomiting (group I: 2, group II: 3) in both study groups. This contradicts what was reported by Bujedo,7 who reported that using IT morphine at doses ⩾0.3 mg is associated with higher incidence of pruritus, respiratory and depression, although there was not a higher rate of nausea or vomiting.
From our work, it seems that administration of low dose of IT morphine (0.3 mg) added much to the postoperative analgesia offered by a multimodal analgesic regimen in this special group of patients. Remarkably, this was not associated with serious side effects or complications. This encourages us to recommend its further use in a similar fashion in similar cases, where achievement of optimal postoperative analgesia is expected to be difficult.
This work is relatively limited by its sample size; a larger sample size would have enforced our results – the relatively few time points of measurements during follow-up period – as more frequent measurements and extending the follow-up period to 48 hours might have been more informative.
In conclusion, the addition of IT morphine to a multimodal analgesic regimen after laparoscopic bariatric surgery was an effective and safe method that markedly reduced postoperative pain, systemic opioid consumption and length of hospital stay.
Acknowledgments
This study is registered at www.clinicaltrial.gov under number NCT02731430.
Footnotes
Declaration of conflicting interest: The authors declared no potential conflicts of interest with respect to the research, authorship and/or publication of this article.
Funding: The authors received no financial support for the research, authorship and/or publication of this article.
References
- 1. Joris JL, Hinque VL, Laurent PE, et al. Pulmonary function and pain after gastroplasty performed via laparotomy or laparoscopy in morbidly obese patients. Br J Anaesth 1998; 80(3): 283–288. [DOI] [PubMed] [Google Scholar]
- 2. Ballantyne JC, Carr DB, deFerranti S, et al. The comparative effects of postoperative analgesic therapies on pulmonary outcome: cumulative meta-analyses of randomized, controlled trials. Anesth Analg 1998; 86(3): 598–612. [DOI] [PubMed] [Google Scholar]
- 3. Escalante-Tattersfield T, Tucker O, Fajnwaks P, et al. Incidence of deep vein thrombosis in morbidly obese patients undergoing laparoscopic Roux-en-Y gastric bypass. Surg Obes Relat Dis 2008; 4(2): 126–130. [DOI] [PubMed] [Google Scholar]
- 4. Kehlet H, Holte K. Effect of postoperative analgesia on surgical outcome. Br J Anaesth 2001; 87(1): 62–72. [DOI] [PubMed] [Google Scholar]
- 5. Flancbaum L, Choban PS. Surgical implications of obesity. Annu Rev Med 1998; 49: 215–234. [DOI] [PubMed] [Google Scholar]
- 6. Meylan N, Elia N, Lysakowski C, et al. Benefit and risk of intrathecal morphine without local anaesthetic in patients undergoing major surgery: meta-analysis of randomized trials. Br J Anaesth 2009; 102: 156–167. [DOI] [PubMed] [Google Scholar]
- 7. Bujedo BM. Spinal opioid bioavailability in postoperative pain. Pain Pract 2014; 14(4): 350–364. [DOI] [PubMed] [Google Scholar]
- 8. Schug S, Chong C. Pain management after ambulatory surgery. Curr Opin Anaesthesiol 2009; 22(6): 738–743. [DOI] [PubMed] [Google Scholar]
- 9. Schug SA, Raymann A. Postoperative pain management of the obese patient. Best Pract Res Clin Anaesthesiol 2011; 25(1): 73–81. [DOI] [PubMed] [Google Scholar]
- 10. Gastrointestinal surgery for severe obesity: National Institutes of Health Consensus Development Conference Statement. Am J Clin Nutr 1992; 55: 615S–619S. [DOI] [PubMed] [Google Scholar]
- 11. Allen TK, Jones CA, Habib AS. Dexamethasone for the prophylaxis of postoperative nausea and vomiting associated with neuraxial morphine administration: a systematic review and meta-analysis. Anesth Analg 2012; 114: 813–822. [DOI] [PubMed] [Google Scholar]
- 12. Bonnet MP, Marret E, Josserand J, et al. Effect of prophylactic 5-HT3 receptor antagonists on pruritus induced by neuraxial opioids: a quantitative systematic review. Br J Anaesth 2008; 101: 311–319. [DOI] [PubMed] [Google Scholar]
- 13. Ramsay MA, Kuterman DL. Dexmedetomidine as a total intravenous anesthetic agent. Anesthesiology 2004; 101: 787–790. [DOI] [PubMed] [Google Scholar]
- 14. Apfelbaum JL, Chen C, Mehta SS, et al. Postoperative pain experience: results from a national survey suggest postoperative pain continues to be undermanaged. Anesth Analg 2003; 97(2): 534–540. [DOI] [PubMed] [Google Scholar]
- 15. DeSousa KA, Chandran R. Intrathecal morphine for postoperative analgesia: current trends. World J Anesthesiol 2014; 3(3): 191–202. [Google Scholar]
- 16. Cindea J, Balcan A, Gherghina V, et al. The impact of epidural analgesia on postoperative outcome after major abdominal surgery. In: Sotonye Fyneface-Ogan (ed.) Epidural analgesia: current views and approaches, 2012. Available at: http://www.intechopen.com/books/epidural-analgesia-current-views-andapproaches/the-impact-of-epidural-analgesia-on-postoperative-outcome-after-major-abdominal-surgery
- 17. Rathmell JP, Lair TR, Nauman B. The role of intrathecal drugs in the treatment of acute pain. Anesth Analg 2005; 101: S30–S43. [DOI] [PubMed] [Google Scholar]
- 18. Moss IR, Brown KA, Laferrière A. Recurrent hypoxia in rats during development increases subsequent respiratory sensitivity to fentanyl. Anesthesiology 2006; 105(4): 715–718. [DOI] [PubMed] [Google Scholar]
- 19. Engelman E, Marsala C. Efficacy of adding clonidine to intrathecal morphine in acute postoperative pain: meta-analysis. Br J Anaesth 2013; 110(1): 21–27. [DOI] [PubMed] [Google Scholar]
- 20. Popping DM, Elia N, Marret E, et al. Opioids added to local anesthetics for single-shot intrathecal anesthesia in patients undergoing minor surgery: a meta-analysis of randomized trials. Pain 2012; 153: 784–793. [DOI] [PubMed] [Google Scholar]