Abstract
Exosomes are a class of cell-released small vesicles that mediate intercellular communication by delivering functional factors to recipient cells. During hepatitis C virus (HCV) infection, the interaction between liver resident macrophages and hepatocytes is a key component in liver innate immunity. In this study, we explored the role of exosomes in the delivery of innate anti-HCV factors to hepatocytes from macrophages. We showed that supernatant from TLR3-activated macrophage cultures could efficiently inhibit HCV replication in Huh7 cells. This macrophage-mediated anti-HCV activity was through exosomes because inhibiting exosomes could abrogate the action of macrophages. Further analyses demonstrated that TLR3-activated macrophages release exosomes that contain anti-HCV microRNA (miRNA)-29 family members. Inhibiting miRNA29 could restore HCV replication. These findings suggest a novel antiviral mechanism in liver innate immunity against HCV infection and provide insights to support further studies on developing exosome-based delivery system for disease treatment.—Zhou, Y., Wang, X., Sun, L., Zhou, L., Ma, T.-C., Song, L., Wu, J.-G., Li, J.-L., Ho, W.-Z. Toll-like receptor 3-activated macrophages confer anti-HCV activity to hepatocytes through exosomes.
Keywords: exosomes, miRNA, hepatitis C virus
In response to viral infections, the host immune system mounts innate and adaptive immunity to control or eliminate viruses. However, viruses have evolved complex mechanisms to evade or counteract host immune responses (1). Therefore, intercellular communications between immune cells and between immune and nonimmune cells are essential for antiviral immunity. In the setting of hepatitis C virus (HCV) infection, the majority of infected patients develop chronic infection, owing in part to the fact that HCV can counteract host immunity using different mechanisms (2, 3). For example, HCV can utilize the viral protease NS3/4A to block TLR3 signaling in hepatocytes (4, 5). As a major target for HCV infection, hepatocytes alone are less likely to limit or clear HCV (1, 3, 6). However, during HCV infection, viral replication within liver is usually in check, suggesting that live resident immune cells, such as macrophages, are crucial in local control of HCV in liver. Liver-resident macrophages, or Kupffer cells, represent 15–20% of total liver cells and are a key regulator of liver immunity (5, 7). Studies have shown that these liver macrophages can be activated by phagocytizing HCV particles and viral RNAs released from the infected hepatocytes (7–9). Activated macrophages can produce proinflammatory cytokines, which activate liver and systemic immune systems (7, 10). However, it remains to be determined whether macrophages can directly confer immune protection to HCV-infected hepatocytes.
Exosomes are membrane vesicles released from intracellular multivesicular bodies (11–13) of most cell types, including macrophages. Although exosomal content may vary individually based on multiple factors, the common exosome-carried proteins can serve as specific markers, including CD63, Alix, Rab-5, and lysosome-associated membrane glycoprotein 2 (LAMP2) (14–16). Importantly, exosomes are now considered to be a new mode of cell-cell communication (12, 17–19). Free exosomes can be uptaken by recipient cells, where the contents of exosomes can be released and trigger a variety of biologic responses (17–19). A number of studies revealed that exosomes can horizontally transfer functional components to adjacent cells and efficiently mediate intercellular communication (17–21). For instance, exosome-shuttled microRNAs (miRNAs) are implicated in multiple biologic functions (11, 13, 21) and in altering gene expression (20) in recipients. These important findings promote us to examine the role of exosome in mediating intercellular communications between macrophages and hepatocytes (Supplemental Fig. 1). Specifically, we examined whether activate macrophages can transfer the anti-HCV factors to hepatocytes through released exosomes (Supplemental Figs. 1 and 2). We also determined the key factors in exosome-mediated anti-HCV activity in hepatocytes.
MATERIALS AND METHODS
Reagents
The synthetic double-stranded RNAs poly I:C (high molecular mass, rhodamine conjugated–high molecular mass) and Lyovec transfection reagent were purchased from InvivoGen (San Diego, CA, USA). Avian myeloblastosis virus transcriptase and RNasin were from Promega (Madison, WI, USA). Goat anti-human heat shock protein 70 (HSP70) antibody was purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA, USA), and FITC-conjugated donkey anti-goat IgG1 was purchased from Southern Biotechnology Associates (Birmingham, AL, USA). Spiroepoxide was purchased from Sigma-Aldrich (St. Louis, MO, USA). PKH67 Fluorescent cell linker kits and PKH26 Fluorescent kit were purchased from Sigma-Aldrich Co. LLC. Exosome-depleted fetal bovine serum (FBS) was purchased from System Biosciences, Inc. (Mountain View, CA, USA).
Cell culture
Human hepatic cell line Huh7 was kindly provided by Dr. Charles M. Rice (Laboratory of Virology and Infectious Diseases, The Rockefeller University, New York, NY, USA). Cells were maintained in conditioned DMEM supplemented with 10% exosome-free FBS, 10 mM Hepes, 100 units/ml penicillin, 100 μg/ml streptomycin, and 2 mM l-glutamine in 5% CO2. Purified human monocytes obtained from the Human Immunology Core at the University of Pennsylvania were plated in 48-well plates in complete DMEM with 10% exosome-free FBS and differentiated into monocyte-derived macrophages after 5–7 d of culture. For poly I:C treatment, macrophages were seeded in 48-well plates with a density of 2.5 × 105 cells per well. Cells were treated with poly I:C (5 μg/ml) for 12 h, and then the supernatant was removed, followed by the addition of fresh medium containing 10% exosome-free FBS. Untreated cells were included as a negative control. In some experiments, poly I:C was transfected by a cationic lipid-based transfection reagent (LyoVec; InvivoGen) according to the manufacturer's manual. Briefly, poly I:C was mixed gently with 20 μl LyoVec and then incubated at room temperature for 15 min to allow formation of the lipid-RNA complex. The complex was added to cell cultures at a 1:20 volume ratio for further incubation. For antiviral experiments, Huh7 cells were treated with macrophage supernatant or exosome for 6 h before HCV as described below, and culturing was continued for 48 h. Poly I:C-rhodamine-treated macrophages were visualized for rhodamine-positive cells by fluorescence microscopy (Supplemental Fig. 3). The poly I:C-rhodamine-treated macrophage-derived exosomes were isolated and added to Huh7 cells, followed by fluorescence microscope observation. For coculture experiments, monocyte-derived macrophages at a density of 2.5 × 105 cells per milliliter were cultured with Huh7 cells at a density of 8 × 105 cells per milliliter in a Transwell-48 system with a 0.4-μm porous membrane (Corning, Corning, NY, USA) to prevent the transfer of vesicles larger than exosomes and direct cell contact (Supplemental Fig. 2).
HCV infection and PCR quantification
Production of infectious HCV Japanese fulminant hepatitis virus 1 (JFH1) and infection of Huh7 cells with JFH1 (multiplicity of infection of 0.01) were carried out as previously described. Real-time RT-PCR was used to detect the levels of HCV RNA. Total cellular RNA was extracted from cells using Tri-Reagent (Molecular Research Center, Cincinnati, OH, USA) as described previously. Total RNA was subjected to RT using reagents obtained from Promega (Madison, WI, USA). RT-PCR for the quantification of HCV RNA, as well as messenger RNA for IFN-α, myxovirus resistance A (MxA), 2′-5′-oligoadenylate synthetase 1 (OAS)1, OAS2, TLR3, and glyceraldehyde 3-phosphate dehydrogenase (GAPDH) were performed with the iQ SYBR Green Supermix (Bio-Rad Laboratories, Hercules, CA, USA). The levels of GAPDH mRNA were used as an endogenous reference to normalize the quantities of target mRNA. The special oligonucleotide primers used in this study are as follows: HCV: 5′-RAYCACTCCCCTGTGAGGAAC-3′ (sense) and 5′-TGRTGCACGG TCTACGAGACCTC-3′ (antisense); IFN-α: 5′-TTTCTCCTGCCTGAAGGACAG-3′ (sense) and 5′-GCTCATGATTTCTGCTCTGACA-3′ (antisense); TLR3: 5′-AGCCACCTGAAGTTGACTCAGG-3′ (sense) and 5′-CAGTCAAATTCGTGCAGAAGGC-3′ (antisense); MxA: 5′-GCCGGCTGTGGATATGCTA-3′ (sense) and 5′-TTTATCGAAACATCTGTGAAAGCAA-3′ (antisense); OAS1: 5′-AGAAGGCAGC TCACGAAACC-3′ (sense) and 5′-CCACCACCCAAGTTTCCTGTA-3′ (antisense); OAS2: 5′- CAGTCCTGGTGAGTTTGCAGT-3′ (sense) and 5′-ACAGCGAGGGTAAATCCTTGA-3′(antisense); GAPDH: 5′-GGTGGTCTCCTCTGACTTCAACA-3′ (sense) and 5′-GTTGCTGTAGCCAAATTCGTTGT-3′ (antisense). The oligonucleotide primers were synthesized by Integrated DNA Technologies, Inc. (Coralville, IA, USA).
Real-time PCR quantification of miRNA
Total RNA, including miRNA, was extracted from macrophage culture supernatant or macrophage-derived exosomes using the miRNeasy Mini Kit (Qiagen, Valencia, CA, USA) in accordance with the manufacturer’s instruction. RNA was reverse-transcribed with a miScript Reverse Transcription Kit (Qiagen). RT-PCR for the quantification of a subset of miRNAs was carried out with miScript Primer Assays and with a miScript SYBR Green PCR Kit from Qiagen as previously described (22, 23).
Exosome isolation
Before exosome isolation, macrophages were cultured for 48 h in medium with 10% exosome-free FBS. Exosomes were isolated using an Exosome Isolation Kit (Invitrogen, Grand Island, NY, USA) following the manufacture’s protocol. In brief, cell culture supernatant was subjected to centrifugation (300 g for 10 min and 10,000 g for 20 min) and then filtered through a 0.2-μM Fluoropore hydrophobic membrane filter (EMD Millipore, Billerica, MA, USA). Exosomes were then mixed with total Exosome Isolation Reagent at a ratio of 2:1 (v/v) and incubated at 4°C overnight. The mixture was centrifuged at 10,000 g for 60 min at 4°C. Exosome pellets were then resuspended in 1× PBS.
Electron microscopy of isolated exosomes
Isolated exosomes from macrophages supernatants were resuspended in 10 μl PBS and spotted onto formvar-coated grids (200 mesh). Adsorbed exosomes were then fixed in 2% (vol/vol) paraformaldehyde at room temperature for 5 min. After fixation, the exosomes were negatively stained using uranyl acetate. Grids were observed with an electron microscope (CM100; Philips, Amsterdam, The Netherlands).
Western blotting for cell lysates and exosomes
Macrophage lysates were collected using a Nuclear Extraction Kit (Panomics, Santa Clara, CA, USA) according to the manufacturer’s instructions. Equal amounts of protein lysates from macrophages and exosomes (20 μg) were separated on 4–12% SDS-PAGE precast gels and transferred to an Immunobiolon-P membrane (Millipore, Eschborn, Germany). The blots were incubated with primary antibodies in 2% nonfat milk in PBS with 0.05% Tween 20 overnight at 4°C [Alix, 1:2000; LAMP2, 1:4000; cytochrome c, 1:2000; HSP70, 1:2000; CD63, 1:2000; GAPDH, 1:2000]. Horseradish peroxidase-conjugated anti-rabbit IgG, anti-goat IgG, and anti-mouse IgG were diluted 1:2000 to 1:8000 in 2% nonfat milk PBS with 0.05% Tween 20. Blots were developed with SuperSignal West Pico Chemiluminescent Substrate (Thermo Fisher Scientific, Waltham, MA, USA).
Exosome immunofluorescence
Macrophages were cultured at a density of 2.5 × 105 cells per well in 48-well plates for 48 h. Isolated exosomes from macrophage supernatant were labeled with PKH67 Fluorescent Cell Linker Kits (Sigma-Aldrich) according to the manufacturer’s protocol. PKH67-labeled exosomes were then added to Exosome Spin Columns (MW 3000; Invitrogen) to enable removal of unincorporated dye contamination from exosome labeling reactions. Purified PKH67 exosomes were incubated with Huh7 cells and cultured at 37°C for 2 d in a CO2 incubator. Huh7 cells were then stained with Hoechst 33342 for nuclei and with a PKH26 Fluorescent Cell Linker Kit for membrane and washed 3 times with 1× PBS. The cells were observed under a fluorescence microscope (Olympus IX71; Olympus, Tokyo, Japan).
Transfection of miRNA mimics
The miRNA inhibitors for miRNA-29a, -29b, and -29c were chemically synthesized by Dharmacon (Lafayette, CO, USA). The design of a miRNA inhibitor negative control (antisense miRNA control) was previously described. Huh7 cells in 48-well plates were transfected with either antisense miRNA-29 inhibitor (10 pmol) or antisense miRNA control (10 pmol) using the transfection reagent LyoVec (InvivoGen).
Statistical analysis
Where appropriate, data are expressed as the means ± sd of triplicate cultures. For comparison of the mean of 2 groups (treated vs. untreated), statistical significance was assessed by a Student’s t test. If there were more than 2 groups, 1-way repeated measures ANOVA was used. Statistical analyses were performed with GraphPad InStat Statistical Software (GraphPad Software, Inc., San Diego, CA, USA). Statistical significance was defined as P < 0.05.
RESULTS
Macrophages confer the anti-HCV activity to hepatocytes without cell-cell contact
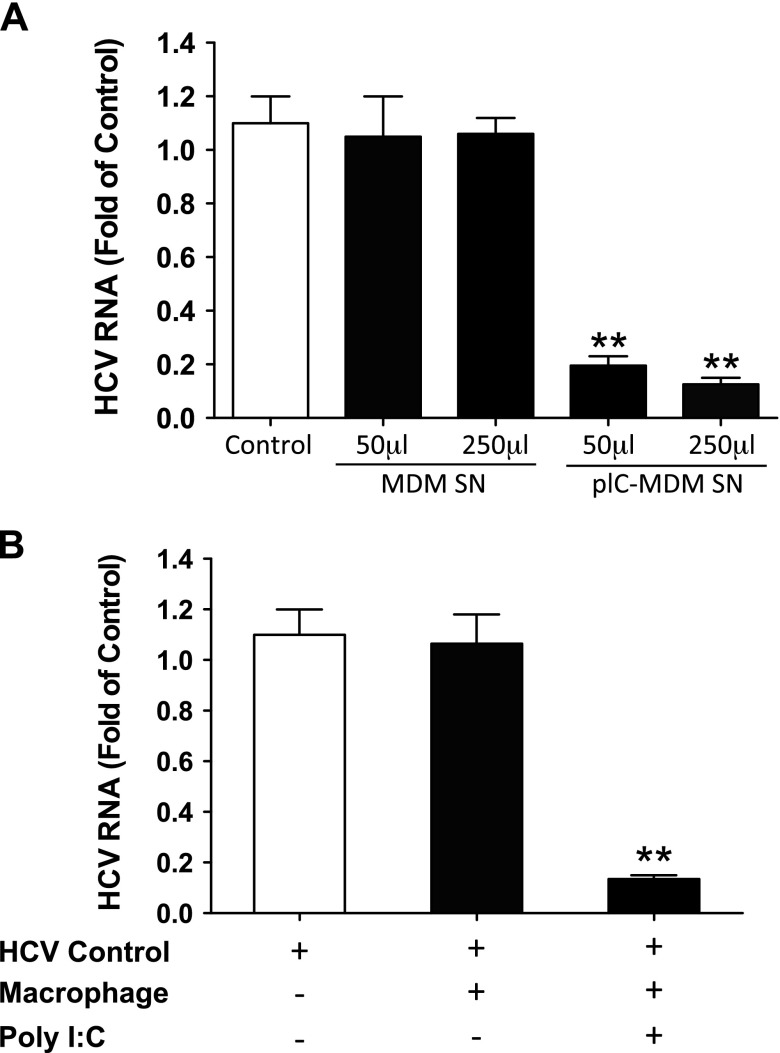
To understand potential mechanisms by which macrophages confer the immune protection to hepatocytes, we first tested the effect of culture supernatant (collected from TLR3-activated macrophages) on HCV infection of hepatocytes. We found that the addition of supernatant from TLR3-activated macrophage cultures to HCV-infected Huh7 cells resulted in viral inhibition, whereas supernatant from unstimulated macrophage cultures had little effect (Fig. 1A). To confirm this observation, we cocultivated macrophages and hepatocytes in a transwell system, where secreted cellular factors can be freely transferred between the 2 cell populations (Supplemental Fig. 2). As shown in Fig. 1B, whereas the coculture with unstimulated macrophages had little effect on HCV replication, the coculture with activated macrophages substantially suppressed HCV replication in Huh7 cells.
Figure 1.
Supernatant from TLR3-activated macrophages inhibits HCV infection of Huh7 cells. A) Macrophages were treated with or without poly I:C (5 μg/ml) for 12 h and then cultured for 48 h after removal of poly I:C. Cell culture supernatant (SN) (50 or 250 μl) was collected and used to treat HCV-infected Huh7 cells in 1 ml (total volume). Total RNA was isolated from Huh7 cells and subjected to real-time PCR for HCV RNA and GAPDH quantification 48 h after treatment. B) Seven-day cultured macrophages were seeded in the low compartment of a 48-well plate, and HCV JFH1-infected Huh7 cells were plated in the upper compartment for coculture. Poly I:C was added to the coculture wells. At 48 h after poly I:C treatment, Huh7 cells were collected for total RNA extraction and then subjected to real-time PCR for HCV and GAPDH RNA quantification. The data are expressed as expression fold change for HCV RNA relative to control (HCV infected Huh7 cells without treatment or coculture, which is defined as 1). The results are the means ± sd of triplicate cultures and are representative of 3 experiments. **P < 0.01. MDM, monocyte-derived macrophages; pIC, poly I:C.
Macrophage-derived exosomes can be taken up by the cocultivated hepatocytes
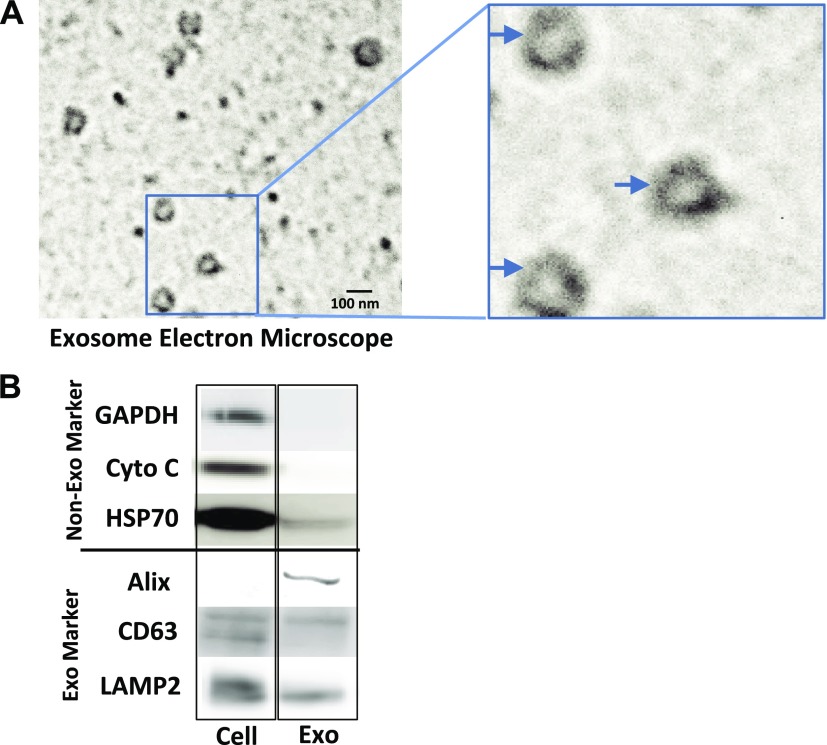
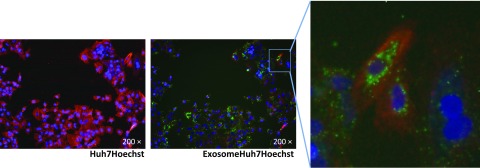
Exosomes released from donor cells can carry an array of cellular components to recipient cells, representing a key mode of intercellular communications (9, 11, 12, 24). To determine the role of exosomes in intercellular communications between macrophages and hepatocytes in the coculture system, we first determined whether TLR3 signaling of macrophages can produce and release exosomes. As shown in Supplemental Fig. 4, exosomes could be isolated from macrophage culture supernatant. Electronic microscopy showed the sizes (50–100 nm) and cup-like shape of the isolated exosomes (Fig. 2A). Characterization of the isolated exosomes showed that exosomes contained the common exosome-carried proteins (Alix, CD63, and LAMP2) (Fig. 2). We next determined whether exosomes isolated from macrophage culture supernatant could be efficiently delivered into Huh7 cells. We incubated Huh7 cells with exosomes labeled with fluorescent dye PKH67 (green). As shown in Fig. 3 and in Supplemental Fig. 5, PKH67-labeled exosomes were observed within Huh7 cells after incubation.
Figure 2.
Characterization of exosomes. A) Electronic microscope observation of macrophage-derived exosomes. Exosomes were isolated from supernatant of macrophage culture. Isolated exosomes were then fixed in 2% (v/v) paraformaldehyde. The fixed exosomes were negatively stained with uranyl acetate and then observed by a Philips CM100 electron microscope. B) Western blot assay of exosome markers and nonexosome markers. Macrophages were cultured in exosome-free culture medium for 48 h in a 48-well culture plate. Total proteins from macrophages and the isolated exosomes were extracted for Western blot analysis. Protein levels of specific exosomal markers (Alix, CD63, and LAMP2) and nonexosomal markers (GAPDH, cytochrome c and HSP70) were detected.
Figure 3.
Delivery of macrophage exosomes to Huh7 cells. Macrophages were cultured in 48-well plates for 48 h, and cell culture supernatant was collected for exosome isolation. Isolated exosomes were labeled with PKH67 fluorescent cell linker (green) and then added to exosome spin columns. Purified PKH67 exosomes were incubated with Huh7 cells and cultured for 48 h. Huh7 cells were stained with Hoechst 33342 (blue) for nuclei and PKH26 fluorescent cell linker (red) and then observed under fluorescence microscope. Original magnification, ×200.
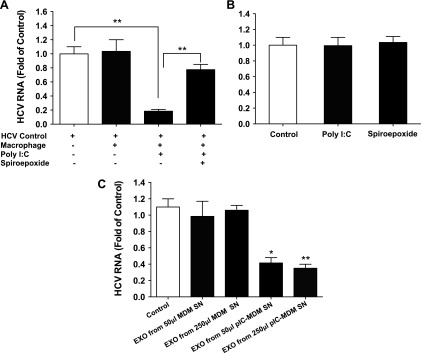
Macrophage-derived exosomes contribute to HCV inhibition in hepatocytes
To evaluate the role of exosomes in macrophage-mediated anti-HCV activity in hepatocytes, we added an exosome release enzyme inhibitor (spiroepoxide) to the cocultures. We found that inhibition of exosome release by spiroepoxide partially but significantly compromised the macrophage-mediated anti-HCV effect in Huh7 cells (Fig. 4A). As controls, direct treatment of HCV-infected Huh7 cells with spiroepoxide or poly I:C alone in the absence of macrophages had little effect on HCV replication (Fig. 4B). To directly examine the role of macrophage-derived exosomes in inhibiting HCV in Huh7 cells, exosomes from macrophage cultures with or without TLR3 activation were added to the HCV-infected Huh7 cells. Whereas exosomes released from unstimulated macrophages had a negligible effect, exosomes isolated from the poly I:C-stimulated macrophages suppressed HCV replication in Huh7 cells in a dose-dependent manner (Fig. 4C).
Figure 4.
Effect of macrophage exosomes (EXO) on HCV replication in Huh7 cells. A) Seven-day-cultured macrophages were seeded in the low compartment of a 48-well plate, and HCV JFH1-infected Huh7 cells were plated in the upper compartment for coculture. Poly I:C (5 μg/ml) and/or 5 μM spiroepoxide were added into the coculture wells. At 48 h after poly I:C or spiroepoxide treatment, Huh7 cells were collected for total RNA extraction and then subjected to real-time PCR for HCV and GAPDH RNA quantification. B) Huh7 cells were infected with HCV JFH1 for 72 h and then treated with or without poly I:C (5 μg/ml) or spiroepoxide (5 μM) for 48 h. Cells were then collected for total RNA extraction and subjected to real-time PCR for HCV and GAPDH RNA quantification. C) Macrophages were treated with or without poly I:C (5 μg/ml) for 12 h and then cultured for 48 h after removal of poly I:C. Cell culture supernatant was collected for exosome isolation. Macrophage SN (50 or 250 μl) was used to treat HCV-infected Huh7 cells in 1 ml total volume. In a parallel experiment, exosomes (EXO) isolated from the same amount of SN (50 or 250 μl) were used to treat HCV-infected Huh7 cells. After 48 h of treatment, total RNA from Huh7 cells was isolated and subjected to real-time PCR for HCV and GAPDH RNA quantification. The data are expressed as fold of HCV RNA levels relative to control (HCV infected Huh7 cells without treatment, which is defined as 1). The results are the means ± sd of triplicate cultures and are representative of 3 experiments.
Macrophage-derived exosomes induce innate antiviral status in hepatocytes
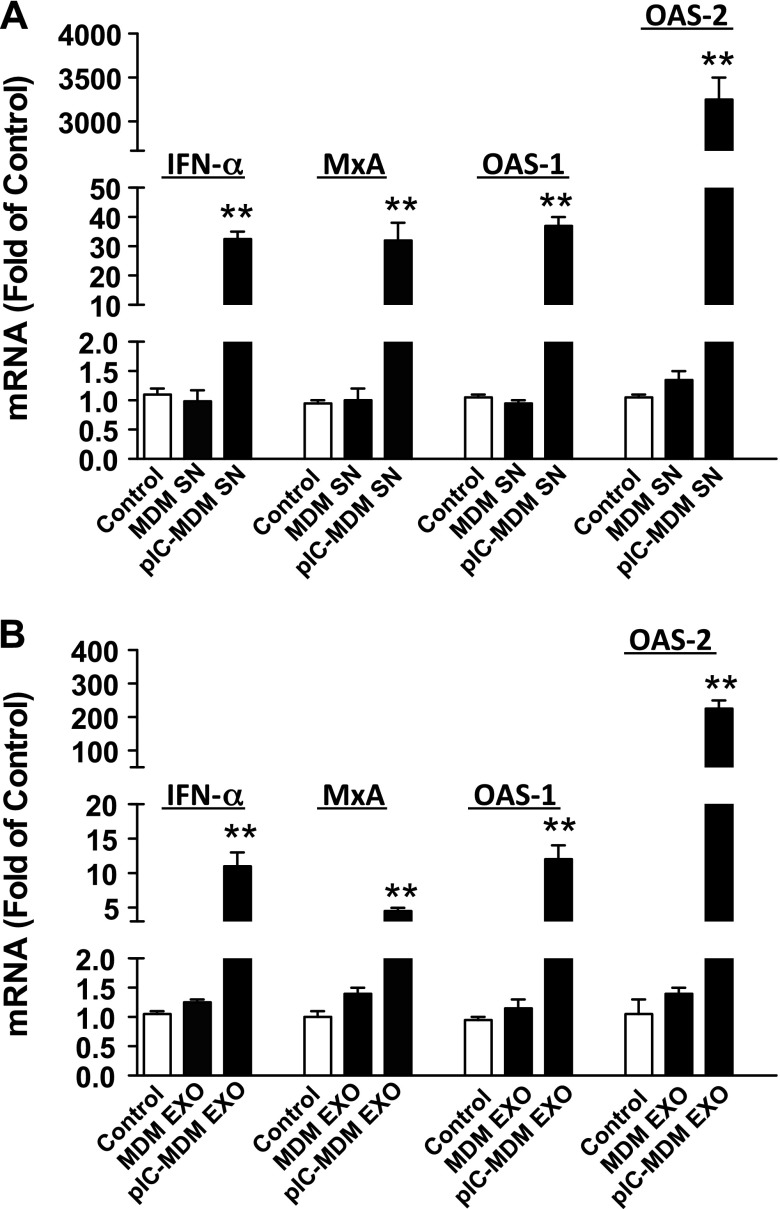
We next examined the downstream immune responses in hepatocytes after exposure to macrophage-derived exosomes. We found that culture supernatant or purified exosomes from poly I:C-stimulated macrophages could induce the expression of IFN-α- and IFN-stimulated genes (ISGs, MxA, OAS-1, and OAS-2) in Huh7 cells (Fig. 5). Induction of these antiviral genes in Huh7 cells by exosomes was less potent than macrophage culture supernatant (Fig. 5), which was correlated with the levels of HCV inhibition (Figs. 1A and 4C).
Figure 5.
Macrophage culture SN and exosomes induce innate antiviral factors in Huh7 cells. Macrophages were treated with or without poly I:C (5 μg/ml) for 12 h and then cultured for 48 h after removal of poly I:C. Cell culture SN was collected for exosome isolation. Macrophage SN (A) or exosomes (B) isolated from SN were used to treat HCV-infected Huh7 cells. Total RNA was isolated from Huh7 cells and subjected to the real-time PCR for IFN-α, MxA, OAS-1, OAS-2, and GAPDH RNA quantification 48 h after treatment. The data are expressed as fold of mRNA levels relative to control (HCV-infected Huh7 cells without treatment, which is defined as 1). The results are the means ± sd of triplicate cultures and are representative of 3 experiments. **P < 0.01.
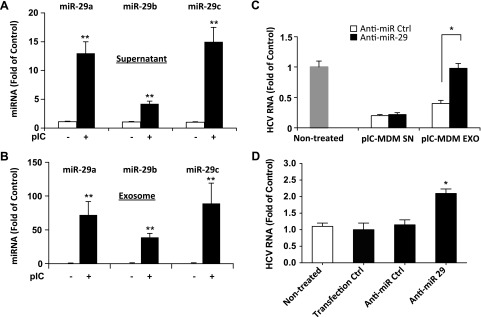
Exosome-derived microRNAs inhibit HCV infection
It is known that miRNAs can be compartmentalized in cell-released exosomes and exert biologic functions on recipient cells. We found that the levels of miRNA-29 family members were substantially increased in both culture supernatant (Fig. 6A) and purified exosomes (Fig. 6B) from poly I:C-stimulated macrophages as compared with the unstimulated macrophages. To determine the role of miRNA-29 in macrophage exosome-mediated HCV inhibition, HCV-infected Huh7 cells were transfected with anti-miRNA-29 or antisense miRNA control, followed by exposure to culture supernatant or purified exosomes from poly I:C-stimulated macrophages. We observed that the addition of culture supernatant or purified exosomes to Huh7 cells resulted in a significant inhibition of HCV. However, transfection of Huh7 cells with specific anti-miRNA-29 abrogated exosome-mediated HCV inhibition (Fig. 6C). In contrast, transfection of antisense miRNA control had little effect (Fig. 6C). The transfection reagent alone or transfection with antisense miRNA control had little effect on HCV replication in Huh7 cells (Fig. 6D).
Figure 6.
Role of miRNA-29 in TLR3-induced macrophage exosome-mediated HCV inhibition. A, B) Macrophages cultured in exosome-free medium were treated with or without poly I:C (5 μg/ml) for 12 h. Cells were then cultured for 48 h after removal of poly I:C. SN was collected for exosome isolation. Macrophage SN (A) and isolated exosomes (B) were subjected for RNA extraction. Total RNA was then subjected to real-time RT-PCR to quantify levels of miRNA-29a, -29b, and -29c in SN (A) and exosomes (B). Synthetic Caenorhabditis elegans miRNA-39 (cel-miR-39) was used as a spiked-in miRNA for normalization. Levels of miRNAs were expressed as fold of control (without poly I:C treatment). C) Macrophages were treated with or without poly I:C (5 μg/ml) for 12 h. Cells were cultured for an additional 48 h after removal of poly I:C. SN was collected for exosome isolation. SN and isolated exosomes were used to treat HCV JFH1-infected Huh7 cells for 48 h. Huh7 cells were transfected with antisense miRNA-29 inhibitors (10 pmol) or miRNA control (10 pmol) 6 h before treatment. Huh7 cells were then collected for total RNA extraction and subjected to real-time PCR for HCV and GAPDH RNA quantification. D) Huh7 cells were infected with HCV JFH1 for 72 h and transfected with antisense miRNA-29 inhibitors (10 pmol) or miRNA control (10 pmol) using the transfection reagent LyoVec. Cells were then collected for total RNA extraction and subjected to real-time PCR for HCV and GAPDH RNA quantification. The data are expressed as fold of HCV RNA level relative to control (without transfection, which is defined as 1). The results are the means ± sd of triplicate cultures and are representative of 3 experiments. The data are shown as scatter plots. Mean values are indicated by horizontal bars.
DISCUSSION
As an important element of liver antiviral immunity, macrophages can protect hepatocytes from HCV infection (8, 9). Macrophages are an important source of IFN and possess a functional TLR3-IFN signaling pathway, which plays a key role in innate immunity against viral infections (10, 25). Although human hepatocytes also express functional TLR3 and produce IFNs (26, 27), HCV is capable of evading TLR3-mediated innate immune responses in hepatocytes (1, 28, 29). Studies (30, 31) have shown the HCV serine protease (NS3/4A) was able to cleave cellular adaptor protein TRIF, an essential element in TLR3 signaling pathway. Therefore, resident macrophages in liver are critical in local control of HCV infection.
However, the mechanisms by which macrophages confer immune protection to hepatocytes are largely unknown. In this study, we found that macrophages produced and released a significant amount of exosomes (Fig. 6). Exosomes are known to have the ability to shuttle biologically active molecules, including proteins, mRNAs, and miRNAs, from donor cells to recipient cells (17–19, 21). The transporting functions of exosomes playing a vital role in a variety of biologic processes, such as cell proliferation, apoptosis, and immune responses (8, 24, 32). For examples, exosomes are important mediators in the immunologic synapse between B cells and T cells for intercellular communication during an immune response. B cells can secrete exosomes, which carry major histocompatibility complex class II molecules and thereby stimulate antigen-specific major histocompatibility complex class II-restricted T-cell responses when taken up by T cells (33). Previous studies (32, 34–37) demonstrated that during viral infections, such as HIV, human T-cell lymphotropic virus and dengue virus exosomes secreted by infected cells harbor and deliver many viral elements (viral RNA and proteins) and host cellular factors to neighboring cells, promoting or inhibiting infections. We found that macrophage-derived exosomes could be taken up by Huh7 cells, where they exerted the biologic function of inhibiting HCV replication.
One of the important discoveries in exosome research is that cellular RNA components, in particular miRNAs, can be packaged into the exosomes (20, 38–41). More importantly, miRNAs shuttled by exosomes could exert biologic functions (20, 41). It was reported that exosomes derived from human (HMC-1) and mouse (MC/9) mast cell lines were able to deliver miRNAs to neighboring cells, influencing the biologic process of the recipient cells (11, 42). The miRNAs carried by exosomes at the immune synapse could regulate gene expression of the recipient antigen-presenting cells (20). We found that the cellular miRNAs were readily detectable in macrophage-derived exosomes. Among the cellular miRNAs identified, miRNA-29 family members (miR-29a, -29b, and -29c) are involved in host-HCV interactions. It was reported that the levels of miR-29 were significantly decreased in both in vitro HCV-infected hepatic cells and the liver of patients with chronic HCV infection as compared with uninfected individuals (43). In addition, it was shown that miR-29 overexpression suppressed HCV replication (43), and the levels of miR-29 family (miR-29a, -29b, and -29c) were found to be higher in livers of chronic HCV-infected patients with sustained virologic response when compared with HCV-infected patients without sustained virologic response (44). These findings suggest the importance of miR-29 in the control of HCV infection. Mechanisms for HCV infection-mediated down-regulation of miR-29 in hepatocytes are currently unclear. It was suggested that the HCV infection-mediated miR-29 down-regulation is due to the activation of TGF-β (45). We showed that miR-29 family members are presented in exosomes released by poly I:C-activated macrophages. More importantly, these miRNAs contributed to HCV inhibition in the recipient hepatocytes.
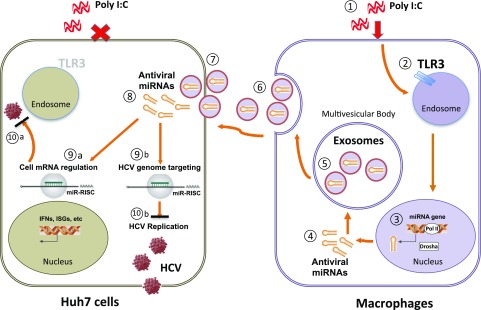
In summary, we have presented a significant in vitro model to study the intercellular communications between human macrophages and hepatocytes. We showed that macrophages could transmit the innate anti-HCV factors to HCV-infected hepatocytes through exosomes (Fig. 7). This observation is highly significant given the fact that HCV infection/replication impairs the intracellular immunity in hepatocytes. It has been reported that macrophages could be activated by HCV infection or HCV RNA and release the antiviral factors, including the type I IFNs and the HCV restriction miRNAs (46). We demonstrate here that some of these important cellular factors can be packaged into cell-free exosomes, which could be taken up by the infected hepatocytes, resulting in HCV inhibition. These findings demonstrate a previously unidentified mechanism by which macrophages confer the immune protection for HCV-infected hepatocytes, which provides a novel strategy using exosomes as an efficient tool to deliver the therapeutic agents to target cells.
Figure 7.
Hypothetical model of macrophage-to-Huh7 cell transmission of TLR3-induced anti-HCV activity by exosomes. In the presence of poly I:C (1), the TLR3 pathway is activated in macrophages (2), leading to the induction of multiple cellular factors, including antiviral miRNAs (3). After exporting out of the nucleus (4), miRNAs were selectively incorporated into exosomes, which are contained in the multivesicular body (5). The multivesicular body then fuses with the plasma membrane, leading to secretion of exosomes into the extracellular space (6). Exosomes subsequently bind to plasma membrane and enter into a Huh7 cell (7). Recruited exosomes release antiviral miRNAs into the cytosol of Huh7 cell (8), where they either activate cellular anti-HCV response or directly target HCV gene expression (9, 10). ISG, IFN-stimulated genes; miR-RISC, miR-RNA induced silencing complex.
ACKNOWLEDGMENTS
This work was supported by Grants DA022177 and DA041302 (to W.-Z.H.) and Grant DA040329 (to J.-L.L.) from the U.S. National Institutes of Health, National Institute on Drug Abuse, Grants 81271334 and 81571962 from National Natural Science Foundation of China, Grant 2012CB518901 from the Development Program (973) from Ministry of Science and Technology of China, and Grant 2014ZX10001003-005 from the National Science and Technology Major Project of China. The authors declare no conflicts of interest.
Glossary
- FBS
fetal bovine serum
- GAPDH
glyceraldehyde 3-phosphate dehydrogenase
- HCV
hepatitis C virus
- HSP70
heat shock protein 70
- JFH1
Japanese fulminant hepatitis virus 1
- LAMP2
lysosome-associated membrane glycoprotein 2
- miRNA
microRNA
- MxA
myxovirus resistance A
- OAS
2′-5′-oligoadenylate synthetase
Footnotes
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
AUTHOR CONTRIBUTIONS
Y. Zhou and W.-Z. Ho conceived of and designed the experiments; Y. Zhou performed the experiments; Y. Zhou and X. Wang analyzed the data; X. Wang, L. Sun, J.-L. Li, L. Zhou, J.-G. Wu, T.-C. Ma, and L. Song contributed reagents, materials, analysis tools, and discussions; and Y. Zhou, J.-L. Li, and W.-Z. Ho wrote the paper.
REFERENCES
- 1.Park S. H., Rehermann B. (2014) Immune responses to HCV and other hepatitis viruses. Immunity 40, 13–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Battaglia A. M., Hagmeyer K. O. (2000) Combination therapy with interferon and ribavirin in the treatment of chronic hepatitis C infection. Ann. Pharmacother. 34, 487–494 [DOI] [PubMed] [Google Scholar]
- 3.Gale M. Jr., Foy E. M. (2005) Evasion of intracellular host defence by hepatitis C virus. Nature 436, 939–945 [DOI] [PubMed] [Google Scholar]
- 4.Li K., Foy E., Ferreon J. C., Nakamura M., Ferreon A. C., Ikeda M., Ray S. C., Gale M. Jr., Lemon S. M. (2005) Immune evasion by hepatitis C virus NS3/4A protease-mediated cleavage of the Toll-like receptor 3 adaptor protein TRIF. Proc. Natl. Acad. Sci. USA 102, 2992–2997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Foy E., Li K., Sumpter R. Jr., Loo Y. M., Johnson C. L., Wang C., Fish P. M., Yoneyama M., Fujita T., Lemon S. M., Gale M. Jr (2005) Control of antiviral defenses through hepatitis C virus disruption of retinoic acid-inducible gene-I signaling. Proc. Natl. Acad. Sci. USA 102, 2986–2991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Koziel M. J. (2005) Cellular immune responses against hepatitis C virus. Clin. Infect. Dis. 41(Suppl 1), S25–S31 [DOI] [PubMed] [Google Scholar]
- 7.Heydtmann M. (2009) Macrophages in hepatitis B and hepatitis C virus infections. J. Virol. 83, 2796–2802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Negash A. A., Ramos H. J., Crochet N., Lau D. T., Doehle B., Papic N., Delker D. A., Jo J., Bertoletti A., Hagedorn C. H., Gale M. Jr (2013) IL-1β production through the NLRP3 inflammasome by hepatic macrophages links hepatitis C virus infection with liver inflammation and disease. PLoS Pathog. 9, e1003330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Revie D., Salahuddin S. Z. (2014) Role of macrophages and monocytes in hepatitis C virus infections. World J. Gastroenterol. 20, 2777–2784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yang L., Seki E. (2012) Toll-like receptors in liver fibrosis: cellular crosstalk and mechanisms. Front. Physiol. 3, 138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Valadi H., Ekström K., Bossios A., Sjöstrand M., Lee J. J., Lötvall J. O. (2007) Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat. Cell Biol. 9, 654–659 [DOI] [PubMed] [Google Scholar]
- 12.Théry C., Zitvogel L., Amigorena S. (2002) Exosomes: composition, biogenesis and function. Nat. Rev. Immunol. 2, 569–579 [DOI] [PubMed] [Google Scholar]
- 13.Joyce D. P., Kerin M. J., Dwyer R. M. (2016) Exosome-encapsulated microRNAs as circulating biomarkers for breast cancer. Int. J. Cancer 139,1443– 1448 [DOI] [PubMed] [Google Scholar]
- 14.Van Niel G., Wubbolts R., Ten Broeke T., Buschow S. I., Ossendorp F. A., Melief C. J., Raposo G., van Balkom B. W., Stoorvogel W. (2006) Dendritic cells regulate exposure of MHC class II at their plasma membrane by oligoubiquitination. Immunity 25, 885–894 [DOI] [PubMed] [Google Scholar]
- 15.Federici C., Petrucci F., Caimi S., Cesolini A., Logozzi M., Borghi M., D’Ilio S., Lugini L., Violante N., Azzarito T., Majorani C., Brambilla D., Fais S. (2014) Exosome release and low pH belong to a framework of resistance of human melanoma cells to cisplatin. PLoS One 9, e88193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Parolini I., Federici C., Raggi C., Lugini L., Palleschi S., De Milito A., Coscia C., Iessi E., Logozzi M., Molinari A., Colone M., Tatti M., Sargiacomo M., Fais S. (2009) Microenvironmental pH is a key factor for exosome traffic in tumor cells. J. Biol. Chem. 284, 34211–34222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.De Toro J., Herschlik L., Waldner C., Mongini C. (2015) Emerging roles of exosomes in normal and pathological conditions: new insights for diagnosis and therapeutic applications. Front. Immunol. 6, 203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Meckes D. G. Jr., Raab-Traub N. (2011) Microvesicles and viral infection. J. Virol. 85, 12844–12854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Raposo G., Stoorvogel W. (2013) Extracellular vesicles: exosomes, microvesicles, and friends. J. Cell Biol. 200, 373–383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mittelbrunn M., Gutiérrez-Vázquez C., Villarroya-Beltri C., González S., Sánchez-Cabo F., González M. A., Bernad A., Sánchez-Madrid F. (2011) Unidirectional transfer of microRNA-loaded exosomes from T cells to antigen-presenting cells. Nat. Commun. 2, 282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rabinowits G., Gerçel-Taylor C., Day J. M., Taylor D. D., Kloecker G. H. (2009) Exosomal microRNA: a diagnostic marker for lung cancer. Clin. Lung Cancer 10, 42–46 [DOI] [PubMed] [Google Scholar]
- 22.Wang X., Ye L., Hou W., Zhou Y., Wang Y. J., Metzger D. S., Ho W. Z. (2009) Cellular microRNA expression correlates with susceptibility of monocytes/macrophages to HIV-1 infection. Blood 113, 671–674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhou Y., Wang X., Liu M., Hu Q., Song L., Ye L., Zhou D., Ho W. (2010) A critical function of toll-like receptor-3 in the induction of anti-human immunodeficiency virus activities in macrophages. Immunology 131, 40–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Abusamra A. J., Zhong Z., Zheng X., Li M., Ichim T. E., Chin J. L., Min W. P. (2005) Tumor exosomes expressing Fas ligand mediate CD8+ T-cell apoptosis. Blood Cells Mol. Dis. 35, 169–173 [DOI] [PubMed] [Google Scholar]
- 25.Wang Y., Li J., Wang X., Ye L., Zhou Y., Ho W. (2013) Induction of interferon-λ contributes to Toll-like receptor-3-activated hepatic stellate cell-mediated hepatitis C virus inhibition in hepatocytes. J. Viral Hepat. 20, 385–394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kanda T., Steele R., Ray R., Ray R. B. (2007) Hepatitis C virus infection induces the beta interferon signaling pathway in immortalized human hepatocytes. J. Virol. 81, 12375–12381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ye L., Wang X., Wang S., Wang Y., Song L., Hou W., Zhou L., Li H., Ho W. (2009) CD56+ T cells inhibit hepatitis C virus replication in human hepatocytes. Hepatology 49, 753–762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang Y., Li J., Wang X., Ye L., Zhou Y., Thomas R. M., Ho W. (2014) Hepatitis C virus impairs TLR3 signaling and inhibits IFN-λ 1 expression in human hepatoma cell line. Innate Immun. 20, 3–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lemon S. M. (2010) Induction and evasion of innate antiviral responses by hepatitis C virus. J. Biol. Chem. 285, 22741–22747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kaukinen P., Sillanpää M., Kotenko S., Lin R., Hiscott J., Melén K., Julkunen I. (2006) Hepatitis C virus NS2 and NS3/4A proteins are potent inhibitors of host cell cytokine/chemokine gene expression. Virol. J. 3, 66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Horner S. M., Park H. S., Gale M. Jr (2012) Control of innate immune signaling and membrane targeting by the Hepatitis C virus NS3/4A protease are governed by the NS3 helix α0. J. Virol. 86, 3112–3120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sampey G. C., Meyering S. S., Asad Zadeh M., Saifuddin M., Hakami R. M., Kashanchi F. (2014) Exosomes and their role in CNS viral infections. J. Neurovirol. 20, 199–208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Buschow S. I., van Balkom B. W., Aalberts M., Heck A. J., Wauben M., Stoorvogel W. (2010) MHC class II-associated proteins in B-cell exosomes and potential functional implications for exosome biogenesis. Immunol. Cell Biol. 88, 851–856 [DOI] [PubMed] [Google Scholar]
- 34.Fang Y., Wu N., Gan X., Yan W., Morrell J. C., Gould S. J. (2007) Higher-order oligomerization targets plasma membrane proteins and HIV gag to exosomes. PLoS Biol. 5, e158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jaworski E., Narayanan A., Van Duyne R., Shabbeer-Meyering S., Iordanskiy S., Saifuddin M., Das R., Afonso P. V., Sampey G. C., Chung M., Popratiloff A., Shrestha B., Sehgal M., Jain P., Vertes A., Mahieux R., Kashanchi F. (2014) Human T-lymphotropic virus type 1-infected cells secrete exosomes that contain Tax protein. J. Biol. Chem. 289, 22284–22305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jaworski E., Saifuddin M., Sampey G., Shafagati N., Van Duyne R., Iordanskiy S., Kehn-Hall K., Liotta L., Petricoin E. III, Young M., Lepene B., Kashanchi F. (2014) The use of Nanotrap particles technology in capturing HIV-1 virions and viral proteins from infected cells. PLoS One 9, e96778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhu X., He Z., Yuan J., Wen W., Huang X., Hu Y., Lin C., Pan J., Li R., Deng H., Liao S., Zhou R., Wu J., Li J., Li M. (2015) IFITM3-containing exosome as a novel mediator for anti-viral response in dengue virus infection. Cell. Microbiol. 17, 105–118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stone L. (2016) Kidney cancer: exosome transmission of sunitinib resistance. Nat. Rev. Urol. 13, 297 [DOI] [PubMed] [Google Scholar]
- 39.Stone L. (2016) Prostate cancer: exosome RNA expression predicts high-grade disease. Nat. Rev. Urol. 13, 298–299 [DOI] [PubMed] [Google Scholar]
- 40.Gibbings D. J., Ciaudo C., Erhardt M., Voinnet O. (2009) Multivesicular bodies associate with components of miRNA effector complexes and modulate miRNA activity. Nat. Cell Biol. 11, 1143–1149 [DOI] [PubMed] [Google Scholar]
- 41.Hu G., Drescher K. M., Chen X. M. (2012) Exosomal miRNAs: biological properties and therapeutic potential. Front. Genet. 3, 56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schorey J. S., Bhatnagar S. (2008) Exosome function: from tumor immunology to pathogen biology. Traffic 9, 871–881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bandyopadhyay S., Friedman R. C., Marquez R. T., Keck K., Kong B., Icardi M. S., Brown K. E., Burge C. B., Schmidt W. N., Wang Y., McCaffrey A. P. (2011) Hepatitis C virus infection and hepatic stellate cell activation downregulate miR-29: miR-29 overexpression reduces hepatitis C viral abundance in culture. J. Infect. Dis. 203, 1753–1762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sulkowski M. S., Moore R. D., Mehta S. H., Chaisson R. E., Thomas D. L. (2002) Hepatitis C and progression of HIV disease. JAMA 288, 199–206 [DOI] [PubMed] [Google Scholar]
- 45.Hoffmann T. W., Duverlie G., Bengrine A. (2012) MicroRNAs and hepatitis C virus: toward the end of miR-122 supremacy. Virol. J. 9, 109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang Y., Li J., Wang X., Zhou Y., Zhang T., Ho W. (2015) HCV dsRNA-activated macrophages inhibit HCV replication in hepatocytes. Hepat. Mon. 15, e29282 [DOI] [PMC free article] [PubMed] [Google Scholar]