Abstract
Insulin promotes bone formation via a well-studied canonical signaling pathway. An adapter in this pathway, insulin-receptor substrate (IRS)-1, has been implicated in the diabetic osteopathy provoked by impaired insulin signaling. To further investigate IRS-1’s role in the bone metabolism, we generated Irs-1-deficient Irs-1smla/smla mice. These null mice developed a spontaneous mutation that led to an increase in trabecular thickness (Tb.Th) in 12-mo-old, but not in 2-mo-old mice. Analyses of the bone marrow stromal cells (BMSCs) from these mice revealed their differential expression of osteogenesis-related genes and miRNAs. The expression of miR-342, predicted and then proven to target the gene encoding collagen type Iα2 (COL1A2), was reduced in BMSCs derived from Irs-1-null mice. COL1A2 expression was then shown to be age dependent in osteoblasts and BMSCs derived from Irs-1smla/smla mice. After the induction of osteogenesis in BMSCs, miR-342 expression correlated inversely with that of Col1a2. Further, Col1a2-specific small interfering RNA (siRNA) reduced alkaline phosphatase (ALP) activity and inhibited BMSC differentiation into osteocyte-like cells, both in wild-type (WT) and Irs-1smla/smla mice. Conversely, in Irs-1smla/smla osteocytes overexpressing COL1A2, ALP-positive staining was stronger than in WT osteocytes. In summary, we uncovered a temporal regulation of BMSC differentiation/bone formation, controlled via Irs-1/miR-342 mediated regulation of Col1a2 expression.—Guo, Y., Tang, C.-Y., Man, X.-F., Tang, H.-N., Tang, J., Wang, F., Zhou, C.-L., Tan, S.-W., Feng, Y.-Z., Zhou, H.-D. Insulin receptor substrate-1 time-dependently regulates bone formation by controlling collagen Iα2 expression via miR-342.
Keywords: insulin receptor substrate, bone formation, collagen 1α2, miR-342
Insulin and IGF-1 play important roles in the anabolic regulation of bone metabolism (1), with aberrant insulin signaling correlating with diabetes and diabetic osteopathy (2). In type 1 diabetes mellitus (T1DM) with insulin deficiency, the risk of osteoporosis and fragility fractures are both significantly increased (3). However, in type 2 diabetes mellitus (T2DM) with insulin resistance, results have been contradictory, with higher, lower, or even similar values reported for bone mineral density (BMD) compared to control subjects (4–6). Therefore, it remains unclear as to whether the effects of insulin resistance on bone metabolism are protective or detrimental.
At the molecular level, insulin and IGF-1 stimulate signaling via endogenous tyrosine kinase receptors. The major signaling adapters in these pathways are insulin-receptor substrate (IRS)-1 and -2, which are rapidly phosphorylated on multiple tyrosine residues after ligand stimulation. Phosphorylated IRS-1 and -2 bind to proteins containing Src homology (SH)-2 domains, and these intermediate signaling effectors stimulate a variety of downstream pathways that regulate cell metabolism, growth, and differentiation (7, 8). The number of osteoblasts, and therefore the rate of bone formation, was reduced in mice that specifically lacked insulin receptor (IR) expression in osteoblasts (9). However, the effect of IRSs on bone metabolism remains a controversial issue. Our previous studies showed that Irs-1 promotes bone formation and mineralization in cultured preosteoblasts (10) and that Irs-2 enhances estrogen-induced bone formation (11). Irs-1-knockout mice exhibit low BMD or severe osteopenia, with low bone turnover at 4, 8, 12, and 16 wk of age (12). In contrast, others have shown that bone metabolism is promoted by an Irs-1 deficiency in osteoblasts, with a demonstrable increase in cancellous bone volume and the number of trabeculae (13). These data were based on micro–computed tomography (µCT) of femurs from 450- and 700-d-old female wild-type (WT) and Irs-1−/− mice (13). Such contradictory findings may reflect the use of different mouse strains, distinct gene-knockout methods, and temporal and spatial variations in study design. Nonetheless, the considerable scope for complex, and currently poorly understood, mechanisms for Irs-1’s control of bone metabolism, warrant further study.
It is well known that phosphorylated IRSs act as docking proteins that activate a series of downstream signaling effectors containing SH2 domains. These proteins include PI3K, C-terminal Src kinase, and several growth factor–binding proteins, such as growth factor receptor-bound protein-2 and the transcription factor NF-κB (14, 15). Proteins that contain SH2 domains also participate in bone metabolism by regulating osteogenesis-related gene expression in osteoblasts and osteoclasts. In addition to the above-mentioned classic insulin-signaling pathway, small noncoding RNAs, referred to as micro- (mi)RNAs, are now known to play important roles in bone development and metabolism (16, 17). Currently, how the canonical insulin-signaling pathway and miRNAs intersect regulate bone metabolism is unknown.
In this study, we sought to investigate the role of Irs-1 in regulating bone metabolism in Irs-1smla/smla mice, a mouse strain that carries a spontaneous mutation that abolishes IRS-1 activity. We anticipated that this model could be used to reveal novel mechanism underlying insulin signaling in bone formation.
MATERIALS AND METHODS
Animals
Mice heterozygous for an Irs-1 spontaneous mutation (007240; The Jackson Laboratory, Bar Harbor, ME, USA) were mated to generate homozygotes. The homozygous nonsense mutation (C→A transition) carried by these animals introduces a premature stop codon in place of a serine at position 57 (S57X), which results in a truncated 56-aa N terminus, and a complete loss of active IRS-1 protein (i.e., functionally null). The 3 progeny strains were termed Irs-1smla/smla, Irs-1+/smla, and Irs-1+/+ (WT), with genotypes confirmed by PCR. The primer sequences used were: Irs-1 NM_010570.4 (forward, 5′- CTTCTCAGACGTGCGCAAGG; and reverse, 5′-GTTGATGTTGAAACAGCTCTC). The Animal Ethics Committee of the Central South University provided prior approval of all protocols used for our mouse experiments.
Sample management and μCT
Tibias were fixed in 10% buffered formalin at 4°C overnight and stored in 70% ethanol at 4°C until use. For analyses, tibias were embedded in methylmethacrylate and then scanned with a GE eXplore Locus SP Micro-CT Scanner (GE Healthcare, Pittsburgh, PA, USA) using a 12-μm voxel protocol and the following scan parameters: 80 kV, 80 μA, and 2960 ms exposure time. Bone density was normalized with an acrylic calibration phantom that included densities equivalent to air, water, and bone. Image reconstructions and analyses were performed with Microview and an Advanced Bone Application database (version 2.3; GE Healthcare). Mineralized tissue was segregated from air or soft tissue, based on a threshold of 1758 HU [corresponding to BMD = 300 mg hydroxyapatite acid/cm3]. Trabecular morphometry was characterized by measuring the fraction of bone volume/total volume (BV/TV), the trabecular thickness (Tb.Th), the trabecular number (Tb.N), and the trabecular separation (Tb.Sp).
Cell culture
Bones were harvested from 1-mo-old male mice fed a standard chow diet. Each bone end was severed, and the bone marrow was expelled by flushing with PBS. The liquid containing the bone marrow stromal cells (BMSCs), a subset of which are skeletal stem cells (18), was collected in polypropylene tubes and centrifuged for 10 min. The supernatant was discarded, and the cells were washed twice with PBS, transferred into 60-mm dishes, and cultured at 37°C. Primary osteogenic cells were isolated and cultured as described previously (19). In brief, we excised the craniums from 2-d-old neonatal C57BL/6 mice and digested them with collagenase II at 37°C (digestion repeated 4 times, 15 min each); the last 2 of 4 digestive cells are enriched with cells exhibiting the biochemical characteristics of osteogenic cells. For osteogenic induction, BMSCs or osteogenic cells were cultured in osteogenic medium, which contained 10−8 M dexamethasone, 50 µg/ml l-2-ascorbic acid, and 10 mM β-glycerophosphate. Alkaline phosphatase (ALP) staining was then performed (20). The cells were fixed with 4% paraformaldehyde for 15 min, and ALP staining was performed with an ALP assay kit (Sigma-Aldrich, St. Louis, MO, USA), according to the manufacturer’s instructions.
Microarray analyses and bioinformatics prediction
A sixth generation miRCURY LNA Array (v.16.0; Exiqon, Vedbaek, Denmark) was used to analyze differentially expressed miRNAs isolated from 2-mo-old Irs-1smla/smla mice. In all samples, miRNAs with intensities ≥50 were chosen to calculate the median intensity, with expression data normalized to that median intensity. Differentially expressed osteogenesis-related genes were analyzed with a mouse osteogenesis PCR Array (PAMM-026; SA Biosciences, Frederick, MD, USA), which comprises 84-key osteogenesis-related genes. The array was hybridized with 3 independent mouse RNA samples for each genotype. The raw data were analyzed to determine relative expression levels, calculated with the ΔΔCt method, with relative expression reported as 2−ΔΔCt according to the manufacturer’s instructions. After normalization, differentially expressed miRNAs and genes were identified with fold-change filtering. Three prediction algorithms (TargetScanS, miRanda, and PicTar) were used to identify the target genes of differentially expressed miRNAs. When the target gene of a differentially expressed miRNA was also a differentially expressed gene in the osteogenesis PCR array analyses, the gene and miRNA were selected for further study.
Luciferase reporter gene assay
The PmirGLO Dual-Luciferase miRNA Target Expression Vector (Promega, Madison, WI, USA) was used for quantitative evaluations of miRNA activity in regulating luciferase gene expression. We inserted the 3′-UTR of the Col1a2 DNA sequence (Col1a2 3′-UTR) immediately downstream of the firefly luciferase gene (pmirGLO/Col1a2UTR). In addition, we constructed the pmirGLO/mUTR, which contained a Col1a2 3′UTR with a deleted miR-342 core binding sequence downstream of the luciferase gene. HEK293T cells were cotransfected with the pmirGLO/Col1a2UTR or pmirGLO/mUTR construct and with either an miR-342 mimic or an miR-342 inhibitor. After 48 h of incubation, HEK293T cells were analyzed for luciferase activity with the Dual-Glo Luciferase Assay System (Promega) and a MicroLumatPlus LB96V luminometer (Berthold Technologies, Oak Ridge, TN, USA). The normalized firefly luciferase activity for each construct was compared to that of the pmirGLO Vector control (no insert). For each transfection, luciferase activity was averaged from 6 replicates.
Cell transfection
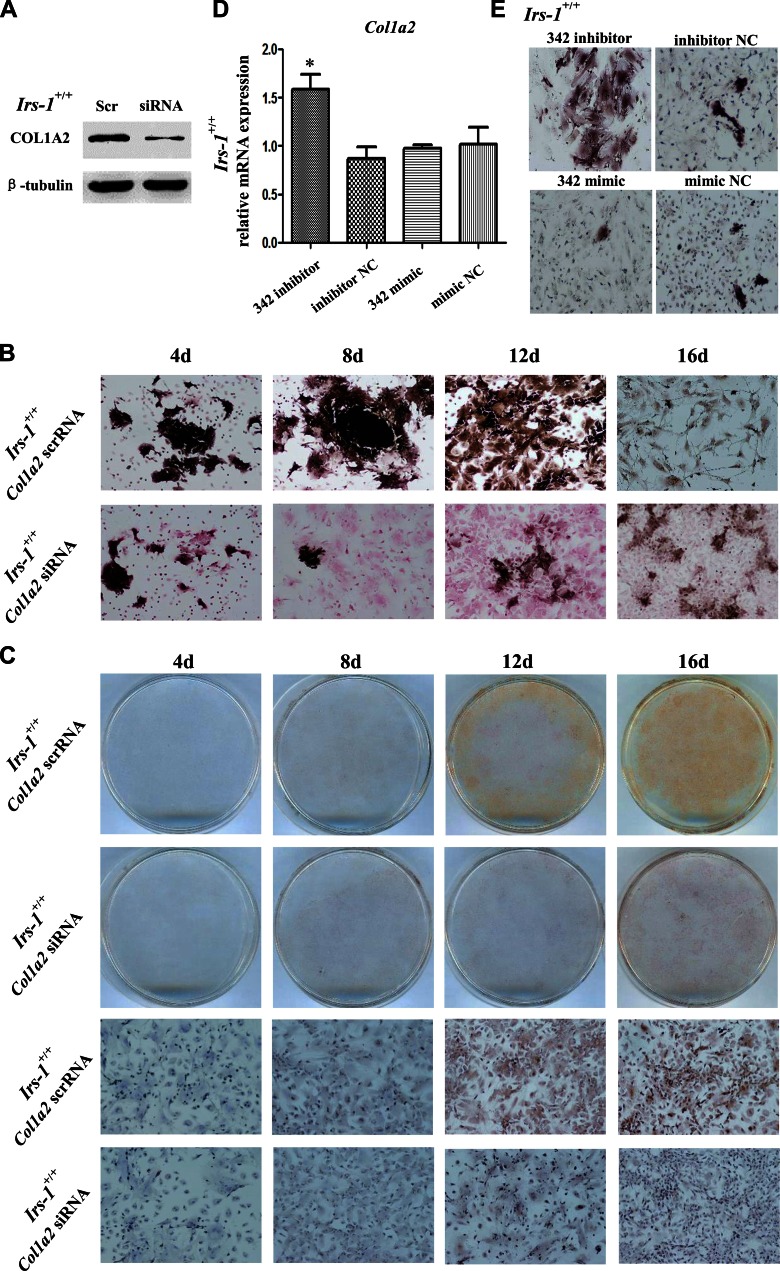
We purchased 4 miRNAs from Shanghai GenePharma Co., Ltd (Shanghai, China): an miR-342-3p mimic and its negative control (NC), and an miR-342-3p inhibitor (anti-miR-342-3p) and its NC (anti-miR-342-3p NC). A predesigned small interfering (si)RNA was also used to target the murine Col1a2 gene (GenBank accession number, NM_12843), with an appropriate NC scrambled small interfering RNA (siRNA) (scrRNA). Transfections of primary mouse calvarial osteogenic cells or BMSCs were performed with Lipofectamine 2000, according to the manufacturer’s suggested protocol (Thermo Fisher Scientific, Waltham, MA, USA). Primary mouse calvarial osteogenic cells were seeded in 6-well plates (5 × 105 cells/well) and were then transfected with miR-342-3p mimics, miR-342-3p inhibitors, and their controls. For Col1a2-specific siRNA transfections, BMSCs of WT mice were transfected for 6 h and then switched to an osteogenic differentiation medium for 4, 8, 12, or 16 d. Western blots and PCR analyses were performed to evaluate Col1a2 expression.
ALP staining analyses
Left femurs were fixed in 10% neutral-buffered formalin for 24–48 h and then decalcified in 10% EDTA (pH 7.4) for 21 d. Decalcified 5-μm-thick left femur sections were deparaffinized with xylene and hydrated with graded alcohol solutions. ALP staining was performed with an ALP assay kit (Sigma-Aldrich), according to the manufacturer’s instructions.
RNA isolation and quantitative real-time PCR
RNA was extracted from BMSCs and primary osteogenic cells with TRIzol reagent, according to the manufacturer’s instructions (Thermo Fisher Scientific). Reverse transcription was performed to generate cDNA from 1 μg total RNA with the PrimeScript RT Reagent Kit, the gDNA Eraser (TaKaRa, Otsu, Japan), and random primers. Quantitative PCR was performed using primers synthesized by Shanghai Generay Biotech Co., Ltd. (Shanghai, China). The primer sequences were: Col1a2 NM_007743.3 (forward, 5′-CAGAACATCACCTACCACTGCAA-3′, reverse, 5′-TTCAACATCGTTGGAACCCTG-3′), and β-actin NM_007393.5 (forward, 5′-CAACGAGCGGTTCCGATG-3′, reverse, 5′-GCCACAGGATTCCATACCCA-3′). For miRNAs, the reverse transcription reaction was performed with 0.5 µg total RNA and a PrimeScript miRNA quantitative PCR (qPCR) Starter Kit (TaKaRa). For real-time PCR (RT-PCR) assays, we used primers specific for mouse miR-342-3p and primers specific for U6, as the internal control (GeneCopoeia, Inc., Rockville, MD, USA; FulenGen, Guangzhou, China). Amplification and detection were performed with the SYBR Premix Ex Taq II kit (TaKaRa) and the LightCycler Real-time PCR system (Roche). The cycling conditions were: 30 s polymerase activation at 95°C, followed by 40 cycles at 95°C for 5 s and 60°C for 20 s. In addition, melting curves were examined to ensure primer specificity.
Protein isolation and Western blot analyses
Total protein extracts were prepared in RIPA buffer [10 mM Tris-HCl, 1% Nonidet P-40, 0.1% SDS, 150 mM NaCl, and 1 mM EDTA (pH7.5)] containing 1 mM PMSF, 5 μM leupeptin, and 10 μM aprotinin. The concentrations of purified proteins were determined with the bicinchoninic acid protein assay (Thermo Fisher Scientific) using bovine serum albumin (BSA) to standardize protein loading. Total cell lysates were resolved on 8% wt/vol or 10% wt/vol SDS-polyacrylamide gels and proteins transferred to Immobilon PVDF membranes (EMD–Millipore, Billerica, MA, USA). The membranes were blocked with 5% nonfat dry milk in Tris-buffered saline-Tween [TBST; 50 mM Tris (pH 7.6), 150 mM NaCl, 0.1% Tween 20] and incubated overnight at 4°C in 3% nonfat dry milk in TBST supplemented with primary antibodies against COL1A2 (OAAF02899, 1:2000, Aviva Systems Biology, San Diego, CA, USA) and β-actin (bs-0061R, 1:1000, Bioss, Shanghai, China). Immunoreactive bands were detected with an antirabbit peroxidase-conjugated secondary antibody (1:5000; Bioss) and visualized with enhanced chemiluminescence (GE Healthcare, Little Chalfont, United Kingdom).
Histomorphology and immunohistochemistry
For immunohistochemical analyses, decalcified femur sections were deparaffinized and heat treated to retrieve antigens. Endogenous peroxidase activity was blocked with 0.3% H2O2 in PBS. After treating with 0.1% trypsin for 30 min, sections were incubated at 4°C overnight with a COL1A2 polyclonal antibody (OAAF01757; 1:100, Aviva Systems Biology) followed by incubation with secondary antibodies. Antibodies were detected by staining with a horseradish peroxidase (HRP)-conjugated rabbit anti-mouse IgG and diaminobenzidine (GTVision III Detection System/Mo&Rb Kit; Gene Tech, Shanghai, China). Specimens were counterstained with hematoxylin. For immunocytochemistry, BMSCs were seeded in culture medium, with 10 mM β-sodium glycerophosphate, 50 mg/ml ascorbic acid, and 10−8 M dexamethasone added on d 0, 4, 8, 12, or 16. Cells were then fixed in 4% paraformaldehyde, permeabilized with 0.05% Triton X-100 for 15 min, and incubated with 1% BSA in PBS for 10 min at room temperature. Osteocyte-like cells were detected with a goat anti-dentin matrix acidic phosphoprotein (DMP)-1 polyclonal antibody (sc-54181, 1:100; Santa Cruz Biotechnology, Santa Cruz, CA, USA), and the UltraSensitive S-P Kit (Maxin Biotechnology Ltd., Fuzhou, China), used according to the manufacturer’s suggested protocol.
Statistical analyses
Data are expressed as means ± sd. All experiments were performed at least 3 times, with similar results obtained. Differences between groups were analyzed with a 1-way ANOVA or Student’s t test. P < 0.05 indicated significant results.
RESULTS
IRS-1 deficiency led to an age-related increase in BMD and trabecular thickness
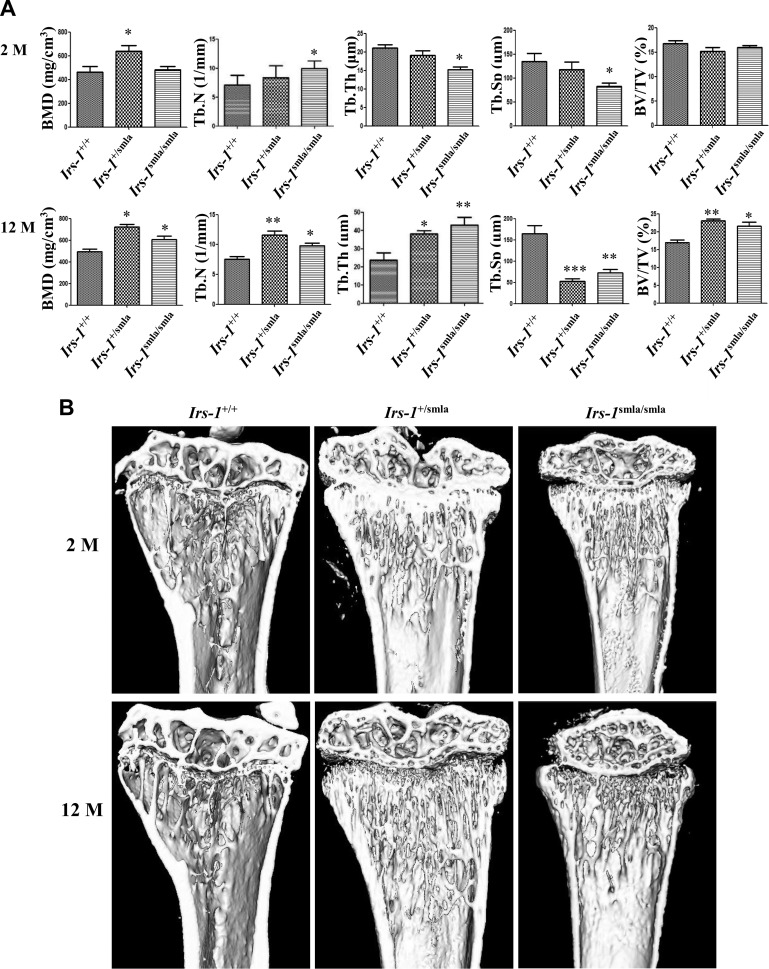
After μCT analyses, we found elevated Tb.N, but reduced Tb.Th and Tb.Sp in 2-mo-old Irs-1smla/smla mice, compared with age-matched Irs-1+/+ mice. No significant differences in BMD or BV/TV were identified (Fig. 1A). However, by 12 mo of age, μCT showed that the Irs-1-null mice demonstrated more regenerated mineralized tissue than their Irs-1+/+ counterparts (Fig. 1B). Moreover, they expressed a significant elevation in BMD, Tb.N, Tb.Th, and BV/TV, together with a significant reduction in Tb.Sp, compared to the Irs-1+/+ mice (Fig. 1A). Tb.Th was reduced at 2 mo of age, but elevated at 12 mo in Irs-1smla/smla mice relative to controls. In 2- and 12-mo-old Irs-1 heterozygotes, values for Tb.Th lay between those of the Irs-1smla/smla and Irs-1+/+ mice. Likewise, 2-mo-old heterozygote values for Tb.N, and Tb.Sp were intermediate between those of Irs-1smla/smla and Irs-1+/+ control mice. In contrast, the greatest values for BMD were recorded for the 2- and 12-mo-old heterozygotes, whereas at 12 mo of age, heterozygotes expressed the highest Tb.N and BV/TV ratios, but the lowest Tb.Sp values.
Figure 1.
Bone histomorphometric analyses and images of Irs-1smla/smla mice by μCT. A) Results of histomorphometric analyses in 2- and 12-mo-old mice. Compared with age-matched WT Irs-1+/+ mice, 2-mo-old Irs-1smla/smla mice demonstrated comparable BMD and BV/TV, increased Tb.N, and decreased Tb.Th and Tb.Sp. In 12-mo-old Irs-1smla/smla mice, BMD, Tb.N, Tb.Th, and BV/TV were all increased, but Tb.Sp was decreased compared with age-matched WT mice. B) Representative 3-D rendered μCT images of proximal tibiae from different groups. Compared with the WT group, dramatic bone gain was identified in 12-mo-old Irs-1smla/smla trabeculae. *P < 0.05; **P < 0.01, compared to WT mice. Data are means ± sd.
Altered expression of genes and miRNAs related to bone metabolism in Irs-1smla/smla mice
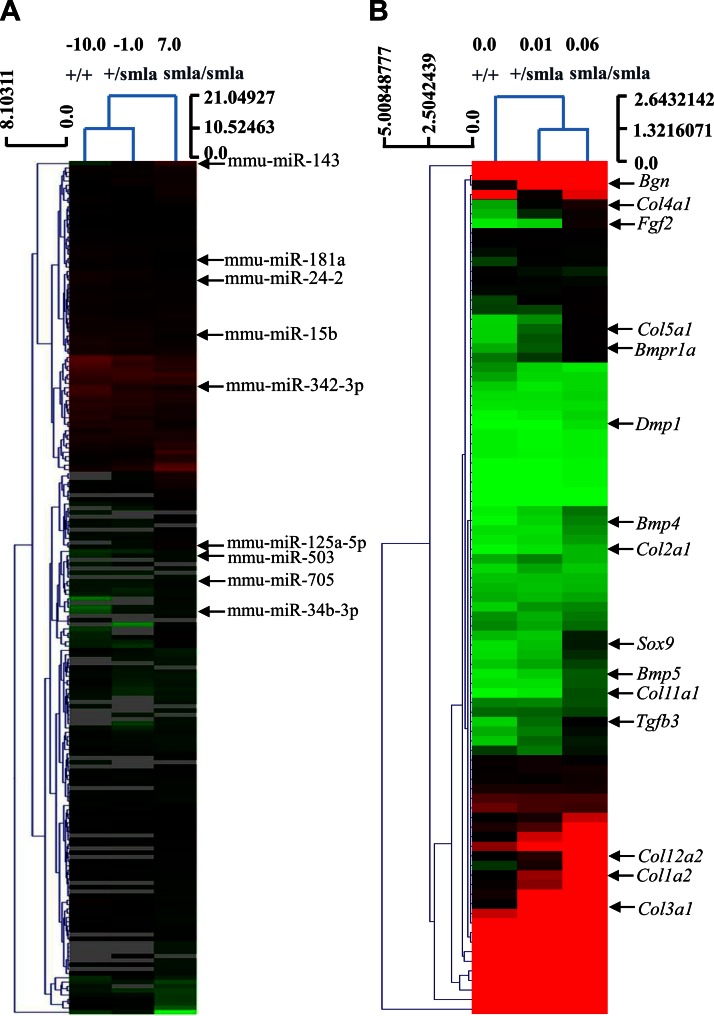
To discover the molecular mechanisms underlying the age-related differences in bone microarchitecture in Irs-1smla/smla mice, we identified differentially expressed genes and miRNAs related to osteogenesis using high-throughput PCR-arrays and miRNA-array analyses, respectively. The PCR-array results showed that multiple genes related to bone metabolism were affected by Irs-1 deficiency (Fig. 2B). In all, 36 genes were up-regulated (>2.0-fold change) in BMSCs derived from Irs-1smla/smla mice. These comprised genes related to bone mineral metabolism and skeletal development, cell growth and differentiation, extracellular matrix (ECM) proteins, and catabolic enzymes. We also identified 5 genes that were down-regulated, including matrix metalloproteinase-9 and CD36 antigen (Supplemental Tables 1 and 2). The miRNA array results showed that 34 miRNAs were up-regulated (>2.0-fold change) and 46 down-regulated (>2.0-fold change) in BMSCs from Irs-1smla/smla mice (Fig. 2A; Supplemental Tables 3 and 4). Among the down-regulated miRNAs, miR-705 and -125b were found to inhibit BMSC differentiation, together with miR-34b and -143, which inhibit osteoblast differentiation. Of the up-regulated miRNAs, miR-24-2 was found to inhibit osteoblast apoptosis, and miR-181a, -15b, and -29a all promote osteoblast differentiation (16, 17). The data discussed in this publication have been deposited in NCBI’s Gene Expression Omnibus (GEO) and are accessible through the GEO Series accession number GSE84310 (https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE84310).
Figure 2.
Differential expression of osteogenesis-related genes and miRNAs in BMSCs isolated from Irs-1smla/smla mice. A) Heat map shows differentially expressed miRNAs in BMSCs from Irs-1+/+, Irs-1+/smla, and Irs-1smla/smla mice. B) Heat map shows differentially expressed osteogenesis-related genes in BMSCs from Irs-1+/+, Irs-1+/smla, and Irs-1smla/smla mice.
Col1a2 is directly regulated by miR-342
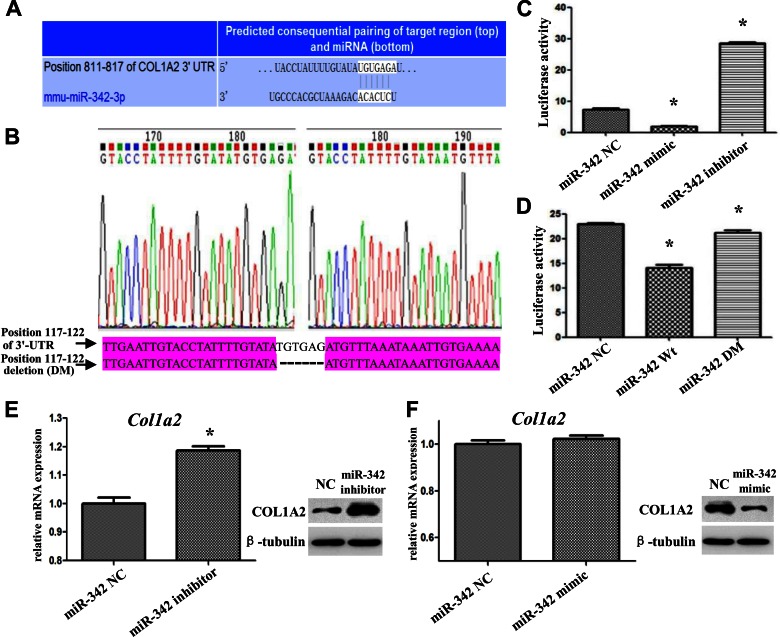
The role of miRNAs is to regulate gene expression by binding target sequences that can be identified using the miRNA database. Accordingly, we identified all the target genes of the 80 miRNAs that were differentially expressed in the Irs-1smla/smla mice. Next, we compared these target genes with the differentially expressed osteogenesis-related genes identified by our PCR array analyses. We found that the expression of Col1a2 was elevated in BMSCs derived from Irs-1smla/smla mice, whereas the expression of miR-342, which targets this gene, was reduced. We then performed a luciferase reporter assay to confirm that miR-342 could bind to the 3′-UTR of the Col1a2 gene and regulate promoter activity. As shown in Fig. 3, we predicted the DNA-binding sequence of murine miR-342 (mmu-miR-342-3p) and its complementary sequence in the 3′-UTR of the Col1a2 gene (Fig. 3A). We then constructed 2 luciferase reporter pmirGLO vectors, one driven by miRNA binding to the WT Col1a2 3′-UTR (pmirGLO/Col1a2UTR), with a second containing a Col1a2 3′-UTR-deletion mutant (DM; pmirGLO/Col1a2UTR, sequence shown in Fig. 3A; labeled DM in Fig. 3B). After transfection of HEK293T cells with these constructs, we found that an miR-342 mimic inhibited and an miR-342 inhibitor enhanced luciferase activity regulated by the WT Col1a2 3′-UTR (Fig. 3C). However, the miR-342 mimic could not inhibit luciferase activity in cells transfected with the DM Col1a2 3′-UTR, which lacks its requisite target sequence (Fig. 3D). Similarly, transfection of primary mouse calvarial osteogenic cells showed that the miR-342 inhibitor increased, and the miR-342 mimic reduced, Col1a2 RNA and protein expression (Fig. 3E, F).
Figure 3.
Validation that murine miR-342 targets and controls the expression of Col1a2. A) Bioinformatics prediction of the binding region of mouse miR-342 (mmu-miR-342-3p) within the Col1a2 3′-UTR. B) Sequence comparison of the Col1a2 3′-UTR (WT) and the core sequence DM. These fragments were amplified, isolated, and inserted into the luciferase expression vector, pmirGLO, to examine luciferase reporter activity. C) In HEK293T cells transfected with WT Col1a2, an miR-342 mimic inhibited luciferase activity, and an miR-342 inhibitor enhanced luciferase activity. D) In HEK293 cells transfected with the DM, the miR-342 mimic lost the ability to inhibit luciferase activity. E, F) In osteogenic cells, the miR-342 inhibitor increased Col1a2 RNA (E, left) and protein (E, right) expression, but an miR-342 mimic reduced Col1a2 protein expression (F, right). *P < 0.05, compared to NC. Data are means ± sd.
Time-dependent Col1a2 and miR-342 expression in vivo and in vitro
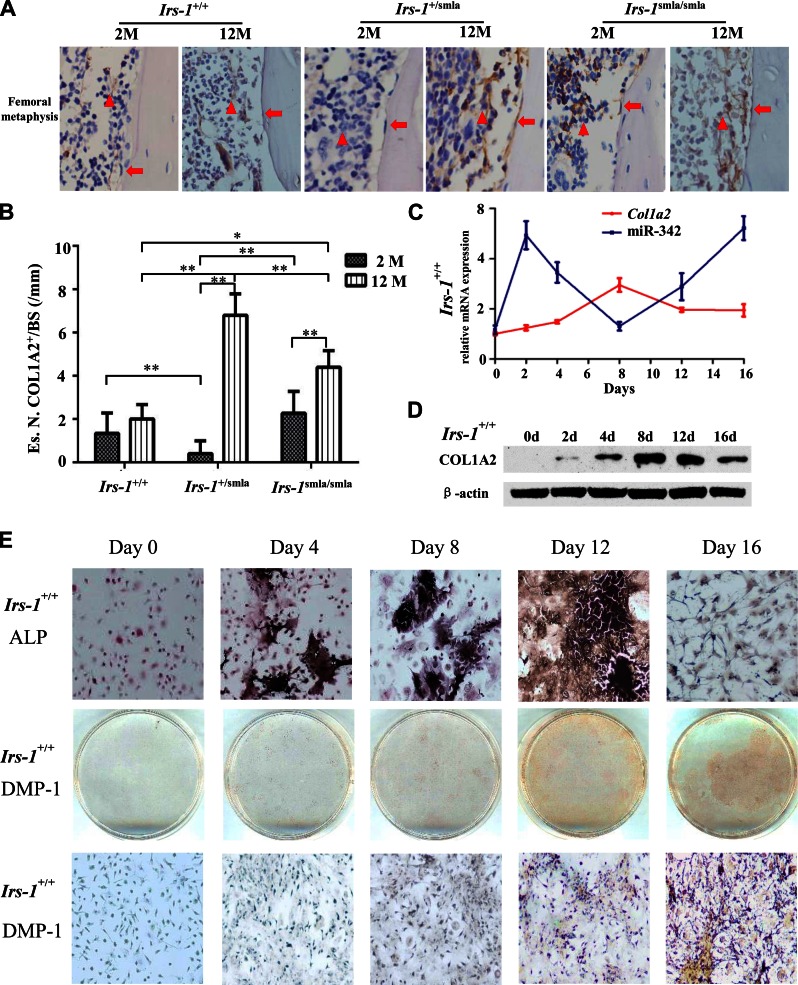
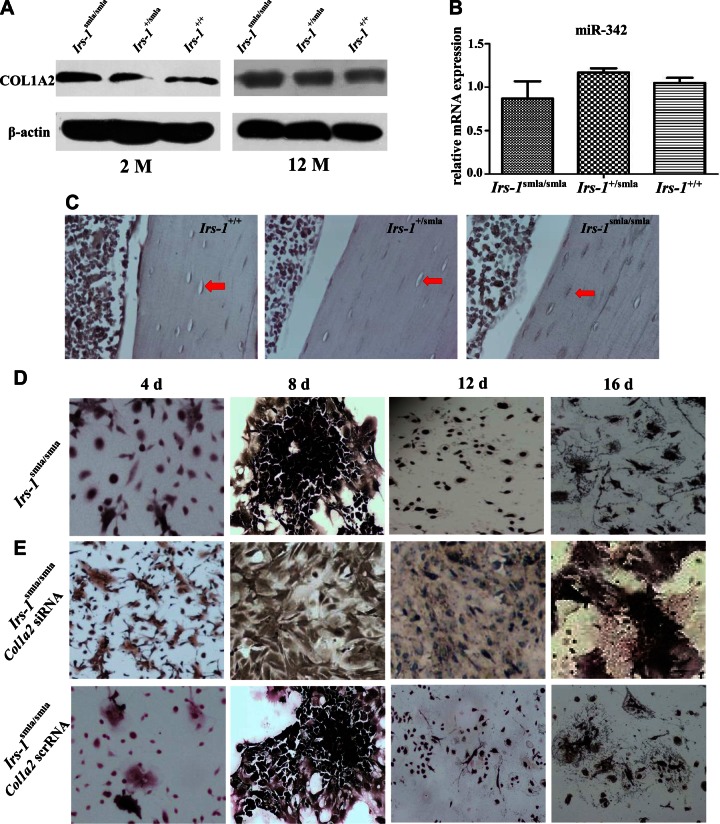
To identify the dynamic expression patterns of COL1A2 and miR-342 in bone formation, we analyzed their expression over time in vivo. Femurs isolated from 2- and 12-mo-old mice, respectively, showed an age-dependent expression of COL1A2 (Fig. 4A). At 2 mo of age, COL1A2 expression was higher in the BMSCs derived from Irs-1smla/smla mice than in BMSCs from Irs-1+/+ mice, but no clear differences in COL1A2 expression or number of COL1A2+ osteoblasts at the endosteal bone surfaces were identified (Fig. 4B). By 12 mo of age, COL1A2 expression levels for Irs-1smla/smla and Irs-1+/+ mice were broadly comparable in BMSCs, but were higher in osteoblasts derived from Irs-1smla/smla mice than in those from Irs-1+/+ mice (Fig. 4B). In the heterozygotes (Irs-1+/smla mice), osteoblasts and BMSCs showed the highest COL1A2 expression at 12 mo of age, but the lowest at 2 mo of age.
Figure 4.
Time-dependent expression of Col1a2 and miR-342 in Irs-1smla/smla mice and in bone marrow cells induced to osteogenic differentiation in vitro. A) Immunohistochemical analyses of bone tissue shows COL1A2 expression (brown stain) in BMSCs (triangles), and osteoblasts (arrows) from Irs-1+/+, Irs-1+/smla, and Irs-1smla/smla mice at 2 mo (left) and 12 mo (right) of age. At 2 mo of age, Irs-1smla/smla mice showed a higher COL1A2 expression in BMSCs than Irs-1+/+ mice. However, by 12 mo of age, COL1A2 expression levels were higher in osteoblasts from Irs-1smla/smla mice than in osteoblasts from Irs-1+/+ mice. B) Representative images of COL1A2 immunohistochemical stains with quantification of the osteoblast number on endosteal bone surface of the distal femora of 2-mo-old (2 M) and 12-mo-old (12 M) C57BL/6 mice. Es.N. COL1A2+/BS, number of COL1A2+ cells per endosteal bone surface (n = 5 per group). *P < 0.05, **P < 0.01, by 1-way ANOVA. C) Time course of Col1a2 mRNA and miR-342 expression during osteogenic induction in BMSCs isolated from WT mice. Col1a2 expression increased gradually, peaked after d 8, then decreased. Conversely, miR-342 expression increased up to d 2, reached a nadir after d 8, then increased. D) COL1A2 protein expression also increased gradually during osteogenic induction of BMSCs from WT mice, then, after d 8, protein levels decreased to d 16. E) During BMSCs osteogenic induction, ALP expression (black stain) increased, peaked on d 12, and then decreased. Expression of the osteocyte marker, DMP-1, was detected on d 12 and 16.
During osteogenic induction in BMSCs, Col1a2 mRNA and miR-342 levels changed over time. Col1a2 mRNA levels gradually increased from 0 to 8 d of osteogenic induction, peaking after d 8, then gradually falling. miR-342 levels were clearly negatively correlated to Col1a2 levels. Initially, miR-342 levels increased, but after d 2 they declined, reaching a nadir after d 8 before increasing again (Fig. 4C). Accordingly, COL1A2 protein expression gradually increased in early stages, peaking on d 8–12, and then falling (Fig. 4D).
We used ALP staining to determine the differentiation status of the induced BMSCs. ALP staining was strongest on d 12 of osteogenic induction, then declined. At 16 d after osteogenic induction, cells became small and asteroid, with low ALP staining (Fig. 4E). When these cells were stained with the preosteocyte (21) or osteocyte marker (22), DMP-1, they showed positive DMP-1 staining at 16 d of induction. This result suggested that these BMSCs had successfully differentiated into osteocyte-like cells.
Col1a2 silencing and an miR-342 mimic inhibits BMSC differentiation
Next, we investigated the roles of Col1a2 and miR-342 in the time-dependent regulation of BMSC differentiation. When a Col1a2-specific siRNA was transfected into BMSCs, COL1A2 expression was successfully silenced (Fig. 5A). Compared with controls (transfected with scrRNA), Col1a2-silenced cells showed reduced ALP content after 4, 8, 12, and 16 d of osteogenic induction (Fig. 5B). After 16 d of induction, no small, asteroid osteocyte-like cells were observed (Fig. 5B). Immunocytochemistry also showed that Col1a2 siRNA silenced DMP-1 expression in BMSCs after 12 and 16 d of osteogenic induction (Fig. 5C). In osteogenic-induced BMSCs, transfection of miR-342 inhibitors increased Col1a2 expression (Fig. 5D) and ALP levels (Fig. 5E). Conversely, transfection of an miR-342 mimic reduced ALP levels (Fig. 5E). These results indicated that miR-342 inhibited Col1a2 expression and BMSC differentiation.
Figure 5.
A Col1a2-specific siRNA and miR-342 mimic inhibited BMSC differentiation into osteoblasts and osteocyte-like cells. BMSCs from WT mice were transfected with either a Col1a2 siRNA/scrRNA or miR-342 mimic/inhibitor, then subjected to osteogenic differentiation. A) The Col1a2 siRNA, but not control (scrRNA), transfections inhibited the expression of COL1A2. B) ALP staining showed that control-transfected BMSCs (scrRNA) differentiated into small, asteroid osteocyte-like cells, but the transfection of a Col1a2 siRNA inhibited differentiation, even after 16 d of induction. C) DMP-1 staining (brown) was observed in control-transfected cells but not in Col1a2 siRNA-transfected cells. D, E) Transfections with an miR-342 inhibitor increased Col1a2 mRNA expression (D) and ALP content (E) in BMSCs.
Col1a2 overexpression in Irs-1smla/smla mice promotes BMSC differentiation
Given that collagen I comprises ∼90% of the organic matrix of bone, Col1a2 expression is clearly expected to be important in BMSC differentiation. Therefore, we assessed the relationships between Irs-1, Col1a2, and BMSC differentiation in Irs-1-deficient mice. Compared to BMSCs from Irs-1+/smla and Irs-1+/+ mice, BMSCs from Irs-1smla/smla mice showed elevated COL1A2 expression at 2 mo but not at 12 mo of age (Fig. 6A). The expression levels of miR-342 showed no statistically significant differences when comparing Irs-1smla/smla, Irs-1+/smla, and Irs-1+/+ mice at 12 mo of age (Fig. 6B). Osteocyte ALP staining was increased in Irs-1smla/smla mice compared to Irs-1+/smla and Irs-1+/+ mice (Fig. 6C). However, ALP staining in BMSCs from Irs-1smla/smla mice was low after 4 d of osteogenic induction, but differentiation into osteocyte-like cells was observed after 12 d of induction (Fig. 6D), which was faster than the differentiation pattern observed in BMSCs from WT mice (16 d of induction). In addition, the osteocyte-like cells derived from the differentiation of Irs-1smla/smla BMSCs showed more intense ALP staining than BMSCs from WT mice after 16 d of induction (Fig. 4E). When BMSCs from Irs-1smla/smla mice were transfected with a Col1a2 siRNA, they lost their ability to differentiate into osteocyte-like cells, even after 16 d of induction (Fig. 6E).
Figure 6.
The relationship of COL1A2 expression in BMSCs, ALP staining in osteocytes, and in the capacity for in vitro osteogenesis in BMSCs derived from Irs-1smla/smla, Irs-1+/smla, and Irs-1+/+ mice. A) Western blots showing that compared to BMSCs from Irs-1+/smla and Irs-1+/+ mice, COL1A2 expression was clearly elevated in BMSCs from Irs-1smla/smla mice at 2 mo but not at 12 mo of age. B) Real-time PCR assays shows that the expression levels of miR-342 showed no significant statistical differences among Irs-1smla/smla, Irs-1+/smla, and Irs-1+/+ mice. C) ALP staining was also increased in osteocytes from Irs-1smla/smla mice compared to osteocytes from Irs-1+/smla and Irs-1+/+ mice. D) BMSCs from the Irs-1smla/smla mice showed low ALP staining after 4 d of osteogenesis induction and then differentiated into osteocyte-like cells after 12 d of osteogenesis induction. These osteocyte-like cells showed a more intense ALP staining than those from WT mice (see Fig. 4E). E) When BMSCs from the Irs-1smla/smla mice were transfected with a Col1a2 siRNA, they lost the ability to differentiate into osteocyte-like cells, even after 16 d of induction.
DISCUSSION
Previous in vitro studies have shown that Irs-1 plays significant roles in bone formation (23, 24). However, conflicting data have been reported for the influence of Irs-1 on BMD for patients with T2DM that were both deficient in IRS-1 expression and expressed insulin resistance. Several groups reported elevated BMD at the lumbar spine and femoral neck among postmenopausal women or men with metabolic syndrome and insulin resistance (25, 26). In contrast, many other studies have shown the opposite, significant reductions in lumbar spine BMD among women with metabolic syndrome and diabetic osteopathy in patients with T2DM and IRS-1 deficiency (27, 28). The conflicting data suggest that insulin and IRS-1 play complex roles in bone metabolism. To investigate these mechanisms, we first analyzed bone morphometric changes in Irs-1-deficient mice. Although no significant difference was found in BMD when comparing Irs-1smla/smla and Irs-1+/+ mice at 2 mo of age, by 12 mo of age, the Irs-1-null mice exhibited a significantly higher BMD (than the Irs-1+/+ mice), suggested an age-dependent transition (Fig. 1). More significantly, the Tb.Th was increased in 12-mo-old mice, but reduced in 2-mo-old Irs-1smla/smla mice compared with WT controls (Fig. 1). That finding indicated that osteoblasts and osteocytes in embryonic or newborn Irs-1smla/smla mice that were initially deficient in bone formation, later developed an increased capacity to make bone during the growth stages. Because osteoblasts and osteocytes are derived from BMSCs (29), we then investigated changes in the gene and miRNA expression profiles in Irs-1smla/smla BMSCs to reveal additional mechanistic data.
Using PCR array analyses, we compared 84 key osteogenesis-related genes in the 3 genotypes of Irs-1 mice. We found that 41 osteogenesis-related genes were affected by Irs-1 deficiency (Fig. 2; Supplemental Table 1). These genes were involved in bone and skeletal development and cell growth and differentiation and in extracellular matrix synthesis and degradation. However, their net effects were difficult to unravel, given that bone-promoting genes were both up- and down-regulated. For example, bone morphogenetic protein (BMP)-4 and fibroblast growth factor (FGF)-2 promote bone formation (30, 31), but BMP-4 was up-regulated, whereas FGF-2 was down-regulated in Irs-1smla/smla BMSCs. These findings indicated that Irs-1-mediated regulation of bone metabolism is of substantial complexity, which is also reflected by the number (80 miRNAs) of differentially expressed miRNAs that were found. Irs-1 deficiency provoked a down-regulation of several miRNAs (miR-705 and -143) that inhibit osteoblastic differentiation, together with miRNAs (miR-125a) that inhibit osteoclastic differentiation (32, 33) (Supplemental Table 3). To determine the connection between differentially expressed genes and miRNAs, we then performed bioinformatics analyses to identify the target genes of the 80 differentially expressed miRNAs. In particular, Col1a2 was identified as the target gene of miR-342. In BMSCs from Irs-1smla/smla mice, miR-342 expression was reduced, and Col1a2 expression increased. In dual luciferase reporter assays, we confirmed that Col1a2 was the target gene of miR-342, and we identified a 6-nt core binding sequence in the 3′-UTR of the Col1a2 gene (Fig. 3).
As an important component of bone tissue, Col1a2 plays crucial roles in organizing mineral deposition and bone growth (34). A previous study found that a Col1a2 mutation led to ineffective osteogenesis because of increased bone resorption (35, 36). In Irs-1smla/smla mice, we investigated the relationships between COL1A2 expression and age-dependent changes in BMD and trabecular bone. First, we detected dynamic COL1A2 expression changes in the bone marrow of mice at different ages. We then examined COL1A2 expression during the osteogenic induction of BMSCs and found that COL1A2 expression in Irs-1smla/smla mice was age-dependent (Fig. 4A). During in vitro osteogenic induction of BMSCs, COL1A2 expression was also time dependent (Fig. 4C, D). ALP staining and morphologic observations showed that ALP secretion peaked on d 8. By d 12, induced BMSCs had become mineralized osteoblasts with diminished ALP staining, indicating that mineralized osteoblasts express little COL1A2 or ALP (Fig. 4E), as reported by Lian and Stein (37). After 16 d of osteogenic induction, mineralized osteoblasts became small, asteroid osteocyte-like cells with weak ALP staining (Fig. 4E). Indeed, positive staining with the preosteocyte- or osteocyte-specific marker (21, 22), DMP-1 indicated that these BMSCs had differentiated into osteocyte-like cells. These results demonstrated that COL1A2 and ALP expression was increased before mineralization and osteoblast differentiation, then decreased afterward. Moreover, normal osteocytes expressed relatively low levels of ALP. The regulator of Col1a2 was found to be miR-342. During in vitro osteogenic induction, miR-342 expression correlated inversely with Col1a2 expression, consistent with the notion that miR-342 regulates the time-dependent expression of Col1a2.
COL1A2 belongs to the family of ECM proteins. The ECM provides structural support, mediates cell–matrix adhesion; binds soluble growth factors, regulates growth factor distribution, activation, and presentation to cells; and integrates complex, multivalent signals for cells (38, 39). It remains unclear as to whether ECM proteins can regulate osteoblast differentiation. However, collagen was identified as a ligand for the osteoclast-associated receptor (OSCAR) in osteoclasts and dendritic cells (40). The binding of collagen peptides to OSCAR on blood-derived monocytes has been shown to promote receptor activator for NF-κB ligand-induced differentiation to osteoclasts (41). Accordingly, we analyzed the effects of Col1a2 on the differentiation of BMSCs and osteoblasts. We found that BMSCs transfected with either a Col1a2 siRNA, or miR-342, expressed reduced ALP after 4, 8, 12, and 16 d of osteogenic induction. Ultimately, these cells could not be induced to differentiate into DMP-1 expressing osteocyte-like cells (Fig. 5), demonstrating that COL1A2, an ECM protein, could directly regulate BMSC differentiation.
In Irs-1smla/smla mice, COL1A2 expression was increased in the bone marrow, which may enhance BMSC differentiation and promote bone formation at the surface of trabecular bone. These activities may partially explain why Tb.Th and BMD were elevated in 12-mo-old Irs-1smla/smla mice. Overexpression of COL1A2 in Irs-1smla/smla mice promoted BMSC differentiation into osteocyte-like cells. Conversely, when Col1a2 was silenced in BMSCs from Irs-1smla/smla mice (with siRNAs), those BMSCs could no longer differentiate into osteocyte-like cells (Fig. 6). Collectively, these results suggest that Col1a2 might play a key role in the age-dependent changes in BMD and Tb.Th that manifest in Irs-1smla/smla mice. In vivo ALP staining of osteocytes in bone tissues also showed a high ALP content in the osteocytes of Irs-1smla/smla mice, but not in osteocytes of WT mice. Therefore, the time-dependent expression of COL1A2 appeared to lead to age-related changes in BMD and bone trabeculae in Irs-1smla/smla mice. However, the imbalance between bone formation and absorption in Irs-1smla/smla mice likely involves many other genes and miRNAs. It remains to be determined which genes regulate bone formation in the embryonic phase and which are involved in the regulation of bone formation from birth to 2 mo of age. Actually, the BMD and Tb.Th in newborn Irs-1smla/smla mice were found to be reduced relative to WT and heterozygotes. Further studies will be needed to reveal the mechanisms that underlie these phenotypes. In addition, an Irs-1 conditional knockout, targeted to osteocytes, would enable robust validation of these data.
In summary, our results indicate that Irs-1 regulates bone formation in a time-dependent fashion by changes to Col1a2 expression that necessitate miR-342 activity. In addition, we demonstrated that Col1a2 could regulate osteoblast and osteocyte differentiation, which represents a new mechanism underlying insulin signaling in bone formation.
AUTHOR CONTRIBUTIONS
H.-D. Zhou designed and conducted of the study; Y. Guo, C.-Y. Tang, X.-F. Man, and J. Tang provided the data and performed the research; Y. Guo, F. Wang, C.-L. Zhou, and S.-W. Tan analyzed and interpreted the data; H.-N. Tang and Y.-Z. Feng developed software necessary to perform and record experiments; and Y. Guo wrote the paper.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by National Natural Scientific Foundation of China Grants 81370975 and 81070278, and Science and Technology Department of Hunan Province Grants 2015JC3012 and 100JJ1007. The authors declare no conflicts of interest.
Glossary
- μCT
micro–computed tomography
- ALP
alkaline phosphatase
- anti-miR-342-3p NC
miR-342-3p inhibitor negative control
- anti-miR-342-3p
miR-342-3p inhibitor
- BMD
bone mineral density
- BMP
bone morphogenetic protein
- BMSC
bone marrow stromal cell
- BSA
bovine serum albumin
- BV/TV
bone volume/total volume
- COL1A2
collagen type Iα2
- DM
deletion mutant
- DMP
dentin matrix acidic phosphoprotein
- ECM
extracellular matrix
- FGF
fibroblast growth factor
- GEO
Gene Expression Omnibus
- HRP
horseradish peroxidase
- IR
insulin receptor
- IRS
IR substrate
- miRNA
microRNA
- NC
negative control
- OSCAR
osteoclast-associated receptor
- scrRNA
scrambled small interfering RNA
- SH2
Src homology-2
- siRNA
small interfering RNA
- T1DM
type 1 diabetes mellitus
- T2DM
type 2 diabetes mellitus
- Tb.N
trabecular number
- Tb.Sp
trabecular separation
- TBST
Tris-buffered saline-Tween
- Tb.Th
trabecular thickness
- WT
wild-type
Footnotes
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
REFERENCES
- 1.Wang T., Wang Y., Menendez A., Fong C., Babey M., Tahimic C. G., Cheng Z., Li A., Chang W., Bikle D. D. (2015) Osteoblast-specific loss of IGF1R Signaling results in impaired endochondral bone formation during fracture healing. J. Bone Miner. Res. 30, 1572–1584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fowlkes J. L., Bunn R C., Thrailkill K. M. (2011) Contributions of the insulin/insulin-like growth factor-1 axis to diabetic osteopathy. J. Diabetes Metab. 1, S1–003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Thrailkill K., Bunn R. C., Lumpkin C. Jr., Wahl E., Cockrell G., Morris L., Kahn C. R., Fowlkes J., Nyman J. S. (2014) Loss of insulin receptor in osteoprogenitor cells impairs structural strength of bone. J. Diabetes Res. 2014, 703589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dhaliwal R., Cibula D., Ghosh C., Weinstock R. S., Moses A. M. (2014) Bone quality assessment in type 2 diabetes mellitus. Osteoporos. Int. 25, 1969–1973 [DOI] [PubMed] [Google Scholar]
- 5.Mathen P. G., Thabah M. M., Zachariah B., Das A. K. (2015) Decreased bone mineral density at the femoral neck and lumbar spine in South Indian patients with type 2 diabetes. J. Clin. Diagn. Res. 9, OC08–OC12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shu A., Yin M. T., Stein E., Cremers S., Dworakowski E., Ives R., Rubin M. R. (2012) Bone structure and turnover in type 2 diabetes mellitus. Osteoporos. Int. 23, 635–641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yamaguchi M., Ogata N., Shinoda Y., Akune T., Kamekura S., Terauchi Y., Kadowaki T., Hoshi K., Chung U. I., Nakamura K., Kawaguchi H. (2005) Insulin receptor substrate-1 is required for bone anabolic function of parathyroid hormone in mice. Endocrinology 146, 2620–2628 [DOI] [PubMed] [Google Scholar]
- 8.Akune T., Ogata N., Hoshi K., Kubota N., Terauchi Y., Tobe K., Takagi H., Azuma Y., Kadowaki T., Nakamura K., Kawaguchi H. (2002) Insulin receptor substrate-2 maintains predominance of anabolic function over catabolic function of osteoblasts. J. Cell Biol. 159, 147–156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fulzele K., Riddle R. C., DiGirolamo D. J., Cao X., Wan C., Chen D., Faugere M. C., Aja S., Hussain M. A., Brüning J. C., Clemens T. L. (2010) Insulin receptor signaling in osteoblasts regulates postnatal bone acquisition and body composition. Cell 142, 309–319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bu Y. H., He Y. L., Zhou H. D., Liu W., Peng D., Tang A. G., Tang L. L., Xie H., Huang Q. X., Luo X. H., Liao E. Y. (2010) Insulin receptor substrate 1 regulates the cellular differentiation and the matrix metallopeptidase expression of preosteoblastic cells. J. Endocrinol. 206, 271–277 [DOI] [PubMed] [Google Scholar]
- 11.Bu Y. H., Peng D., Zhou H. D., Huang Q. X., Liu W., Luo X. B., Tang L. L., Tang A. G. (2009) Insulin receptor substrate 2 plays important roles in 17beta-estradiol-induced bone formation. J. Endocrinol. Invest. 32, 682–689 [DOI] [PubMed] [Google Scholar]
- 12.Ogata N., Chikazu D., Kubota N., Terauchi Y., Tobe K., Azuma Y., Ohta T., Kadowaki T., Nakamura K., Kawaguchi H. (2000) Insulin receptor substrate-1 in osteoblast is indispensable for maintaining bone turnover. J. Clin. Invest. 105, 935–943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Selman C., Lingard S., Choudhury A. I., Batterham R. L., Claret M., Clements M., Ramadani F., Okkenhaug K., Schuster E., Blanc E., Piper M. D., Al-Qassab H., Speakman J. R., Carmignac D., Robinson I. C., Thornton J. M., Gems D., Partridge L., Withers D. J. (2008) Evidence for lifespan extension and delayed age-related biomarkers in insulin receptor substrate 1 null mice. FASEB J. 22, 807–818 [DOI] [PubMed] [Google Scholar]
- 14.Pramojanee S. N., Phimphilai M., Chattipakorn N., Chattipakorn S. C. (2014) Possible roles of insulin signaling in osteoblasts. Endocr. Res. 39, 144–151 [DOI] [PubMed] [Google Scholar]
- 15.Ma H., Ma J. X., Xue P., Gao Y., Li Y. K. (2015) Osteoblast proliferation is enhanced upon the insulin receptor substrate 1 overexpression via PI3K signaling leading to down-regulation of NFκB and BAX pathway. Exp. Clin. Endocrinol. Diabetes 123, 126–131 [DOI] [PubMed] [Google Scholar]
- 16.Papaioannou G., Mirzamohammadi F., Kobayashi T. (2014) MicroRNAs involved in bone formation. Cell. Mol. Life Sci. 71, 4747–4761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ell B., Kang Y. (2014) MicroRNAs as regulators of bone homeostasis and bone metastasis. Bonekey Rep. 3, 549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sacchetti B., Funari A., Michienzi S., Di Cesare S., Piersanti S., Saggio I., Tagliafico E., Ferrari S., Robey P. G., Riminucci M., Bianco P. (2007) Self-renewing osteoprogenitors in bone marrow sinusoids can organize a hematopoietic microenvironment. Cell 131, 324–336 [DOI] [PubMed] [Google Scholar]
- 19.Bakker A. D., Klein-Nulend J. (2012) Osteoblast isolation from murine calvaria and long bones. Methods Mol. Biol. 816, 19–29 [DOI] [PubMed] [Google Scholar]
- 20.Wang S., Mu J., Fan Z., Yu Y., Yan M., Lei G., Tang C., Wang Z., Zheng Y., Yu J., Zhang G. (2012) Insulin-like growth factor 1 can promote the osteogenic differentiation and osteogenesis of stem cells from apical papilla. Stem Cell Res. (Amst.) 8, 346–356 [DOI] [PubMed] [Google Scholar]
- 21.Sun Q., Gu Y., Zhang W., Dziopa L., Zilberberg J., Lee W. (2015) Ex vivo 3D osteocyte network construction with primary murine bone cells. Bone Res. 3, 15026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rios H. F., Ye L., Dusevich V., Eick D., Bonewald L. F., Feng J. Q. (2005) DMP1 is essential for osteocyte formation and function. J. Musculoskelet. Neuronal Interact. 5, 325–327 [PubMed] [Google Scholar]
- 23.Shimoaka T., Kamekura S., Chikuda H., Hoshi K., Chung U. I., Akune T., Maruyama Z., Komori T., Matsumoto M., Ogawa W., Terauchi Y., Kadowaki T., Nakamura K., Kawaguchi H. (2004) Impairment of bone healing by insulin receptor substrate-1 deficiency. J. Biol. Chem. 279, 15314–15322 [DOI] [PubMed] [Google Scholar]
- 24.Hoshi K., Ogata N., Shimoaka T., Terauchi Y., Kadowaki T., Kenmotsu S., Chung U. I., Ozawa H., Nakamura K., Kawaguchi H. (2004) Deficiency of insulin receptor substrate-1 impairs skeletal growth through early closure of epiphyseal cartilage. J. Bone Miner. Res. 19, 214–223 [DOI] [PubMed] [Google Scholar]
- 25.Szulc P., Varennes A., Delmas P. D., Goudable J., Chapurlat R. (2010) Men with metabolic syndrome have lower bone mineral density but lower fracture risk: the MINOS study. J. Bone Miner. Res. 25, 1446–1454 [DOI] [PubMed] [Google Scholar]
- 26.de Paula F. J., de Araújo I. M., Carvalho A. L., Elias J. Jr., Salmon C. E., Nogueira-Barbosa M. H. (2015) The relationship of fat distribution and insulin resistance with lumbar spine bone mass in women. PLoS One 10, e0129764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ornstrup M. J., Kjær T. N., Harsløf T., Stødkilde-Jørgensen H., Hougaard D. M., Cohen A., Pedersen S. B., Langdahl B. L. (2015) Adipose tissue, estradiol levels, and bone health in obese men with metabolic syndrome. Eur. J. Endocrinol. 172, 205–216 [DOI] [PubMed] [Google Scholar]
- 28.Hernández J. L., Olmos J. M., González-Macías J. (2011) Metabolic syndrome, fractures and gender. Maturitas 68, 217–223 [DOI] [PubMed] [Google Scholar]
- 29.Titorencu I., Pruna V., Jinga V. V., Simionescu M. (2014) Osteoblast ontogeny and implications for bone pathology: an overview. Cell Tissue Res. 355, 23–33 [DOI] [PubMed] [Google Scholar]
- 30.Zappitelli T., Chen F., Aubin J. E. (2015) Up-regulation of BMP2/4 signaling increases both osteoblast-specific marker expression and bone marrow adipogenesis in Gja1Jrt/+ stromal cell cultures. Mol. Biol. Cell 26, 832–842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kitamura M., Akamatsu M., Kawanami M., Furuichi Y., Fujii T., Mori M., Kunimatsu K., Shimauchi H., Ogata Y., Yamamoto M., Nakagawa T., Sato S., Ito K., Ogasawara T., Izumi Y., Gomi K., Yamazaki K., Yoshie H., Fukuda M., Noguchi T., Takashiba S., Kurihara H., Nagata T., Hamachi T., Maeda K., Yokota M., Sakagami R., Hara Y., Noguchi K., Furuuchi T., Sasano T., Imai E., Ohmae M., Koizumi H., Watanuki M., Murakami S. (2016) Randomized placebo-controlled and controlled non-inferiority phase III trials comparing trafermin, a recombinant human fibroblast growth factor 2, and enamel matrix derivative in periodontal regeneration in intrabony defects. J. Bone Miner. Res. 31, 806–814 [DOI] [PubMed] [Google Scholar]
- 32.Liao L., Su X., Yang X., Hu C., Li B., Lv Y., Shuai Y., Jing H., Deng Z., Jin Y. (2016) TNF-alpha inhibits FoxO1 by up-regulating MiR-705 to aggravate oxidative damage in bone marrow-derived mesenchymal stem cells during osteoporosis. Stem Cells 34, 1054–1067 [DOI] [PubMed] [Google Scholar]
- 33.Li E., Zhang J., Yuan T., Ma B. (2014) MiR-143 suppresses osteogenic differentiation by targeting Osterix. Mol. Cell. Biochem. 390, 69–74 [DOI] [PubMed] [Google Scholar]
- 34.Komori T. (2010) Regulation of bone development and extracellular matrix protein genes by RUNX2. Cell Tissue Res. 339, 189–195 [DOI] [PubMed] [Google Scholar]
- 35.Kalajzic I., Terzic J., Rumboldt Z., Mack K., Naprta A., Ledgard F., Gronowicz G., Clark S. H., Rowe D. W. (2002) Osteoblastic response to the defective matrix in the osteogenesis imperfecta murine (oim) mouse. Endocrinology 143, 1594–1601 [DOI] [PubMed] [Google Scholar]
- 36.Zhang H., Doty S. B., Hughes C., Dempster D., Camacho N. P. (2007) Increased resorptive activity and accompanying morphological alterations in osteoclasts derived from the oim/oim mouse model of osteogenesis imperfecta. J. Cell. Biochem. 102, 1011–1020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lian J. B., Stein G. S. (1995) Development of the osteoblast phenotype: molecular mechanisms mediating osteoblast growth and differentiation. Iowa Orthop. J. 15, 118–140 [PMC free article] [PubMed] [Google Scholar]
- 38.Egusa H., Iida K., Kobayashi M., Lin T. Y., Zhu M., Zuk P. A., Wang C. J., Thakor D. K., Hedrick M. H., Nishimura I. (2007) Downregulation of extracellular matrix-related gene clusters during osteogenic differentiation of human bone marrow- and adipose tissue-derived stromal cells. Tissue Eng. 13, 2589–2600 [DOI] [PubMed] [Google Scholar]
- 39.Hynes R. O. (2009) The extracellular matrix: not just pretty fibrils. Science 326, 1216–1219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Barrow A. D., Raynal N., Andersen T. L., Slatter D. A., Bihan D., Pugh N., Cella M., Kim T., Rho J., Negishi-Koga T., Delaisse J. M., Takayanagi H., Lorenzo J., Colonna M., Farndale R. W., Choi Y., Trowsdale J. (2011) OSCAR is a collagen receptor that costimulates osteoclastogenesis in DAP12-deficient humans and mice. J. Clin. Invest. 121, 3505–3516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schultz H. S., Nitze L. M., Zeuthen L. H., Keller P., Gruhler A., Pass J., Chen J., Guo L., Fleetwood A. J., Hamilton J. A., Berchtold M. W., Panina S. (2015) Collagen induces maturation of human monocyte-derived dendritic cells by signaling through osteoclast-associated receptor. J. Immunol. 194, 3169–3179 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.