Abstract
The role of resolvins in abdominal aortic aneurysm (AAA) has not been established. We hypothesized that treatment with D-series resolvins (RvD2 or RvD1) would attenuate murine AAA formation through alterations in macrophage polarization and cytokine expression. Male C57/B6 mice (n = 9 per group) 8 to 12 wk old received RvD2 (100 ng/kg/treatment), RvD1 (100 ng/kg/treatment), or vehicle only every third day beginning 3 d before abdominal aortic perfusion with elastase as prevention. Aortas were collected 14 d after elastase perfusion. Cytokine analysis (n = 5 per group) or confocal microscopy (n = 4 per group) was performed. In a separate experiment, RvD2 was provided to mice with small AAAs 3 d after elastase treatment (n = 8 per group). Additionally, apolipoprotein E knockout mice treated with angiotensin II (1000 ng/kg) were treated with RvD2 or vehicle alone (n = 10 per group) in a nonsurgical model of AAA. To determine the effect of RvD2 on macrophage polarization, confocal staining for macrophages, M1 and M2 macrophage subtypes, α-actin, and DAPI was performed. Mean aortic dilation was 96 ± 13% for vehicle-treated mice, 57 ± 9.7% for RvD2-treated mice, and 61 ± 11% for RvD1-treated mice (P < 0.0001). Proinflammatory cytokines macrophage chemotactic protein 1, C-X-C motif ligand 1, and IL-1β were significantly elevated in control animals compared to RvD2- and RvD1-treated animals (P < 0.05), resulting in a reduction of matrix metalloproteinase 2 and 9 activity in resolvin-treated mice in both elastase and angiotensin II models. Treatment of existing small AAAs with RvD2 demonstrated a 25% reduction in aneurysm size at d 14 compared to vehicle alone (P = 0.018). Confocal histology demonstrated a prevalence of M2 macrophages within the aortic medium in mice treated with RvD2. Resolvin D2 exhibits a potent protective effect against experimental AAA formation. Treatment with RvD2 significantly influences macrophage polarization and decreases several important proinflammatory cytokines. Resolvins and the alteration of macrophage polarization represent potential future targets for prevention of AAA.—Pope, N. H., Salmon, M., Davis, J. P., Chatterjee, A., Su, G., Conte, M. S., Ailawadi, G., Upchurch, G. R., Jr. D-series resolvins inhibit murine abdominal aortic aneurysm formation and increase M2 macrophage polarization.
Keywords: inflammation, proresolving mediators, vascular biology
Resolvins have been shown to play a protective role in neointimal hyperplasia; however, their role in abdominal aortic aneurysm (AAA) has not been established (1). Resolvins are a family of endogenous proresolving mediators derived from polyunsaturated fatty acid precursors. Resolvins derived from docosahexaenoic acid (D-series resolvins) occur naturally as part of the resolution phase of inflammation. These lipid-derived mediators have been shown to have a beneficial, quiescent effect in a diverse group of inflammatory disease models, including neointimal hyperplasia, obesity-induced adipose inflammation, peritonitis, colitis, and atherosclerosis, at nanomolar doses (2–6).
AAAs are responsible for over 15,000 deaths annually and are present in as many as 7% of older men (7–10). While the exact order of events leading to AAA formation is not known, inflammation of the aortic wall is known to play a key role in the creation of aneurysms (11). Several proinflammatory cytokines, including IL-1β and macrophage chemotactic protein 1 (MCP-1), have been shown to be important in experimental AAA formation. Macrophages are known to play a key role in regulating the inflammatory process in AAA, although their exact mechanism of action remains less clear (12, 13). Macrophages exist along a spectrum from the classic proinflammatory (M1) phenotype to an alternatively anti-inflammatory (M2) subtype. Modulation between M1 and M2 phenotypes depends largely on local conditions, including chemokine expression (14–17).
D-series resolvins have previously been shown to both decrease M1 and paradoxically increase M2 expression without influencing the absolute number of infiltrating macrophages, indicating a role in the polarization of activated macrophages (6). We hypothesized that treatment with D-series resolvins would inhibit AAA progression in murine AAAs by altering macrophage polarization, with the ultimate goal of developing novel pharmacological therapies for treatment and prevention of AAA.
MATERIALS AND METHODS
Creation of murine AAAs
Eight- to 10-wk-old C57/B6 mice were anesthetized with intraperitoneal delivery of 0.1 ml ketamine/xylazine solution. Male mice were chosen because of their propensity to generate larger aortic aneurysms, thus allowing differences in treatment effect to be more readily demonstrable and to eliminate well-established sex differences in experimental AAA expression. Animals were weighed and a midline laparotomy performed. Viscera were retraced cephalad and the infrarenal abdominal aorta exposed. The aorta was then isolated to the level of the aortic bifurcation by ligating side branches with cautery or 10-0 nylon suture. Proximal and distal aortic control was obtained, and a small aortotomy was made near the aortic bifurcation. The aorta was then cannulated with a 0.033-inch polyethylene catheter (Braintree Scientific, Braintree, MA, USA) and perfused with 0.45 U/ml solution of porcine pancreatic elastase (Sigma-Aldrich, St. Louis, MO, USA) in 0.9% sodium chloride for 5 min. The catheter was then removed and the remaining elastase solution evacuated from the aorta. The aortotomy was then repaired with 10-0 nylon suture. The abdominal contents were returned and the abdominal wall closed in 2 layers. Mice received intraperitoneal buprenorphine for pain relief.
At collection, mice were again anesthetized with ketamine/xylazine as above. Midline laparotomy was made through the previous incision. Abdominal contents were freed of retroperitoneal adhesions and reflected superiorly. The aorta was then dissected from surrounding tissues from the level of the left renal vein to the aortic bifurcation. Measurements of aortic diameter in perfused, and unperfused segments were made by video photomicrometry using Leica Application Suite 4.3 software (Leica Microsystems, Buffalo Grove, IL, USA). Aortas were either snap frozen in liquid nitrogen or fixed in 4% paraformaldehyde at the time of collection.
Murine elastase model
Eight- to 12-wk-old wild-type (WT) male mice (C57BL/6J, stock number 000664; The Jackson Laboratory, Bar Harbor, ME, USA) were injected with intraperitoneal ketamine solution, elastase perfused, and collected as previously described and carried out by Johnston and colleagues (18–20). Aortas were collected 14 d after elastase perfusion. Aortic diameters were determined by video micrometry using Leica Application Suite 4.3 software (Leica Microsystems). The aortas (or aneurysms, when present) were collected and either snap frozen in liquid nitrogen for analysis by real-time PCR or protein extraction, or incubated overnight for histology or immunohistochemistry. Animal care and use were in accordance with the Guide for the Care and Use of Laboratory Animals. The animal protocol was approved by the University of Virginia Institutional Animal Care and Use Committee (#3634) in compliance with the Office of Laboratory Animal Welfare.
Measurement of aortic cytokines
Abdominal aortas (n = 5 per group) were snap frozen and analyzed using a murine antibody cytokine array (R&D Systems, Minneapolis, MN, USA). Tissue was processed according to the manufacturer’s protocol. Samples were run in duplicate. Densitometric volume was determined by spectrophotometry using Thermo Scientific software (Thermo Fisher Scientific, Waltham, MA, USA).
Prevention and treatment strategy with 2 D-series resolvins in an elastase model of aneurysm formation
For prevention studies, 8- to 12-wk-old WT male (n = 9 per group; C57BL/6J, stock number 000664; The Jackson Laboratory) received either resolvin D1 (RvD1, 100 ng/kg, Cayman Chemicals, Ann Arbor, MI, USA), resolvin D2 (RvD2, 100 ng/kg, Cayman Chemicals), or vehicle (% DMSO, Sigma-Aldrich) by intraperitoneal injection beginning 3 d before elastase perfusion and continuing every third day (d 0, 3, 6, 9, 12) until collection. Aortas were then collected for antibody cytokine arrays (n = 5 per group), Western blot analysis (n = 5 per group), or immunocytochemistry (n = 4 per group).
To assess the effects of treatment with D-series resolvins on AAA formation, WT mice (n = 8 per group; C57BL/6J, stock number 000664; The Jackson Laboratory) were treated with RvD2 beginning 3 d after elastase perfusion and continuing every third day until collection at d 14 (d 3, 6, 9, 12). Aortas were analyzed for cytokine antibody arrays and immunohistochemistry as above.
Angiotensin II infusion
Osmotic pumps (Alzet 2004; Durect, Cupertino, CA, USA) containing angiotensin II (Ang II; 1000 ng/kg/min; Sigma-Aldrich) were introduced into 10-wk-old WT male mice (n = 10 per group) as described previously (20–22). Mice were then treated every third day with either RvD2 (100 ng/kg; Cayman Chemicals) or vehicle as control (1% DMSO; Sigma-Aldrich). Mice were housed and maintained at 70°F and 50% humidity in 12-h light–dark cycles according to institutional animal protocols. All mice were fed water ad libitum and placed on a high-fat diet (TD 88137; Harlan Teklad, Indianapolis, IN, USA) with no restrictions on movement as previously described (20, 23). Aneurysmal segments of the aortas (proximal to the renal arteries) were collected after 28 d and processed for histology.
Histology
Murine aortas were collected at the time of humane killing for histological analysis after undergoing left ventricular puncture and 4% paraformaldehyde antegrade perfusion at physiologic pressure and rinsed with 70% ethanol. Further fixation was achieved by overnight incubation in 4% paraformaldehyde at 4°C, followed by embedding in paraffin and sectioning at 5 µm. After microwave antigen retrieval, antibodies were bound and detected using the VectaStain Elite Kit (Vector Laboratories, Burlingame, CA, USA). Antibodies for immunohistochemical staining were anti-rat Mac-2 for macrophages (1:10,000; Cedarlane Laboratories, Burlington, ON, Canada), CD3 for T cells (1:500; Santa Cruz Biotechnology, Santa Cruz, CA, USA), and anti-mouse α smooth muscle actin (α-SMA) for α-SMA (1:1000; Santa Cruz Biotechnology). Visualization color development was completed using diaminobenzidine (Dako, Glostrup, Denmark) for α-SMA, Mac-2, anti-neutrophil, and CD3ε.
Mouse confocal immunofluorescent staining was performed to measure the effect of D-series resolvins on macrophage polarization. Confocal analysis was performed for arginine type 1 (Arg-1; 1:200; Abcam, Cambridge, MA, USA) for M2 macrophages, MCP-1 for M1 macrophages (1:200, Santa Cruz Biotechnology), Mac-2 for macrophages (1:1000, Cedarline Biotechnology) followed by α-SMA for smooth muscle cells (1:1000; Abcam), and nuclei using DAPI (1:10,000; Thermo Fisher Scientific). Quantification of Mac-2-, MCP-1-, and Arg-1-positive cells within the aortic medium was scored independently using 2 blinded observers with Zen software according to the following grading system: 1 = none to few, 2 = mild staining, 3 = moderate staining, and 4 = extensive staining (Carl Zeiss GmbH, Jena, Germany).
Images were acquired using AxioCam 4.6 software with ×10, ×40, and ×100 objectives and an AxioCam MRc camera (Carl Zeiss GmbH). Threshold gated positive signal was detected within the area of interest and quantified using Image-Pro Plus 7.0 (Media Cybernetics, Bethesda, MD, USA). Elastin degradation was quantified by counting the number of breaks per vessel, then was averaged and graphed. Images were quantified and counted using 2 independent observers and are graphed as means ± sd.
Cytokine array
For the purpose of determining the effects of resolvins on proinflammatory cytokines in the aortic wall, mouse cytokine arrays (R&D Systems) were performed using isolated protein from mouse aortas at d 14 according to the manufacturer’s instructions (n = 5 mice per group). Protein samples from each group were pooled for analysis, and all samples were run in duplicate (18–20, 24).
Western blot analysis of MCP-1
To further quantify the decreased levels of MCP-1 seen in the cytokine antibody array, WT mice (C57BL/6J male mice) pretreated with D-series resolvins were collected and then subjected to protein Western blot analysis from both the elastase and, separately, from the Ang II–infused mouse models of aneurysm formation. In detail, 10 µg of whole-cell protein extracts were loaded onto a 10–20% Bis-Tris polyacrylamide gel at 120 V for 1 h, followed by transfer of the protein to a nitrocellulose membrane at 25 V for 30 min. The membranes were then blocked for 1 h in Tris-buffered saline and Tween 20 with 5% nonfat milk for 1 h. After blocking, membranes were incubated with MCP-1 (1:1000; Santa Cruz Biotechnology) for 1 h, followed by 3 washes. Membranes were then incubated in horseradish peroxidase–conjugated secondary antibody, followed by another series of washes. Protein was visualized using X-ray film and then analyzed by Image Analysis software (Bio-Rad, Hercules, CA, USA).
Gelatin zymography for matrix metalloproteinase levels
To evaluate the levels of matrix metalloproteinase 2 (MMP2) and MMP9 in WT male mice after D-series resolvins, protein samples from mice pretreated with RvD2 in the elastase perfusion and Ang II infusion mouse studies were loaded onto a 10% zymogram gel (Thermo Fisher Scientific) with 3 µg protein per lane (n = 4 per group per time point). Protein samples were loaded individually, separated electrophoretically, and renatured with renaturing buffer (Thermo Fisher Scientific) for 30 min, and then placed in developing buffer (Thermo Fisher Scientific) overnight. The gels were then stained with SimplyBlue SafeStain (Thermo Fisher Scientific), and the MMP bands were observed and quantitated by Bio-Rad Image Lab 4.0 software (Bio-Rad).
Statistical methods
Statistical analysis was performed with GraphPad Prism 5 software (GraphPad Software, La Jolla, CA, USA). Maximal aortic dilation (%) was calculated as follows: [(maximal aortic diameter − internal control diameter)/(internal control diameter × 100%)]. The internal control was a small segment of normal abdominal aorta just distal to the renal arteries that was above the proximal ligation. This section was not perfused with elastase, but it was susceptible to blood pressure changes from volume loss during the collection as well as expected animal growth over time. Values are reported as mean ± sem. Aortic dilation between groups was compared by Fisher’s exact test. Post hoc Tukey correction was applied to determine the significance of individual comparisons with α = 0.05 (20). When groups were compared, the Fisher’s exact test was used for significance. Pearson correlation coefficients (R) were used to determine strength of linear dependence and are reported as R value and 95% confidence interval (20). Ang II experiment differences were measured by a log-rank (Mantel-Cox) test (20). All assays were performed in triplicate unless stated otherwise. Comparisons between multiple groups were accounted for in analysis of cytokine data. Blinding to experimental groups was maintained as feasible. Data are presented as means ± sd.
RESULTS
Prevention of AAA formation with RvD2
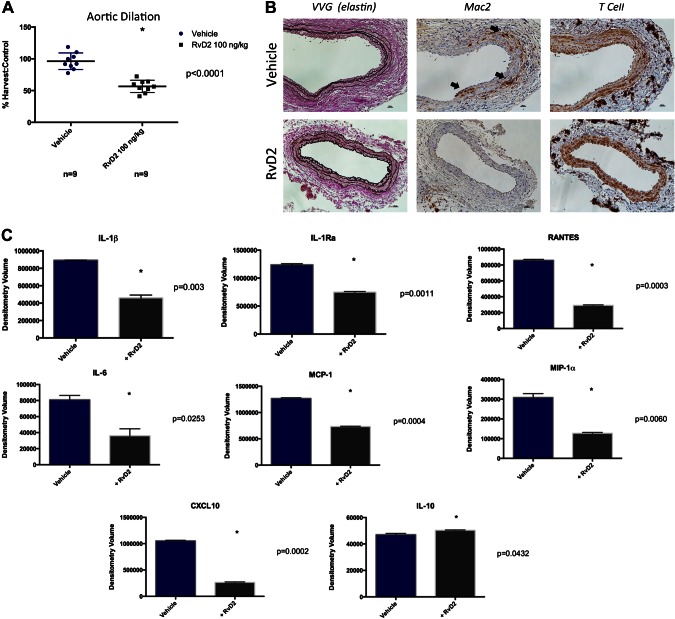
Animals treated with RvD2 showed significantly attenuated mean aortic dilation compared to those receiving vehicle alone (RvD2 56.7 ± 9.7% vs. vehicle only 96 ± 13%, P < 0.0001, Fig. 1A). RvD2 animals demonstrated significant protection from elastin degradation and decreased macrophage infiltration compared to untreated animals. No significant difference was observed in T-cell or neutrophil infiltration at d 14 (Fig. 1B; neutrophil not shown). Cytokine arrays of murine aortas from RvD2-treated animals demonstrated a significant decrease in inflammatory cytokines. MCP-1, IL-1β, IL-Ra, RANTES, IL-6, macrophage inflammatory protein 1α, and C-X-C motif ligand 10 were significantly reduced in RvD2-treated animals (P < 0.05 for all comparisons, Fig. 1C). It is noteworthy that treated animals had significantly higher levels of IL-10 than vehicle-treated animals. There was no significant difference between groups in IL-17, IL-23, TNF-α, or complement 5a (Supplemental Fig. 1).
Figure 1.
Aortic dilation 14 d after elastase perfusion in animals treated with vehicle only or RvD2 100 ng/kg per dose every 72 h. A) Animals in treatment group showed mean decrease of 40% compared to animals treated only with vehicle. B) Histology demonstrated increased elastin preservation and decrease in macrophage staining within aortic media (arrows). No significant difference in T-cell staining was observed. Aortic cytokine array of RvD2 treated and untreated animals. There is significant reduction in several inflammatory cytokine with RvD2 treatment. C) It is noteworthy that RvD2-treated animals exhibited higher levels of anti-inflammatory IL-10.
Western blot analysis for MCP-1 in vehicle- and RvD2-treated aortas demonstrated significantly higher expression of MCP-1 in vehicle-treated aortas (1.005 ± 0.057 vs. 0.257 ± 0.233, respectively; P = 0.0003, Supplemental Fig. 2). Gelatin zymography for pro-MMP2, MMP2, and MMP9 demonstrated significantly higher activity of these important metalloproteases in vehicle compared to RvD2-treated animals (pro-MMP2: vehicle 103,224 ± 16,428 vs. RvD2 12,169 ± 4667; MMP2: vehicle 104,016 ± 1307 vs. RvD2 11,756 ± 2929; MMP9: vehicle 154,242 ± 10,454 vs. RvD2 12,782 ± 15,832, P < 0.0001, Supplemental Fig. 2D–F).
RvD2 alters macrophage polarization toward an M2 phenotype
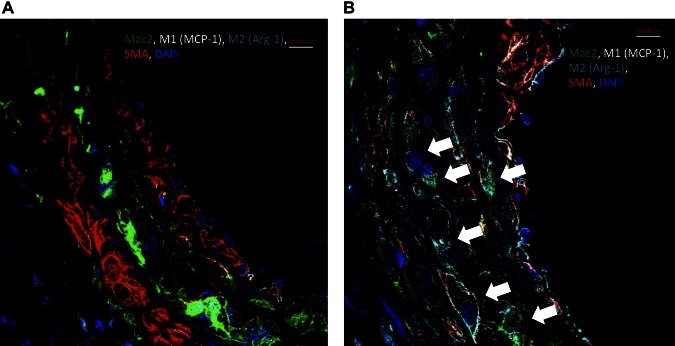
Confocal microscopy demonstrated a significant increase in M2 macrophage marker Arg-1 and decreased M1 macrophage marker MCP-1 in animals given RvD2 compared with vehicle only (Fig. 2). M2 polarization was significantly increased in RvD2-treated animals by the previously stated scoring system (Table 1, vehicle 1.3 ± 0.48 vs. RvD2 3.3 ± 0.48, P < 0.0001). Additionally, RvD2-treated animals had significantly lower M1 staining scores compared to nontreated animals (vehicle 2.1 ± 0.74 vs. RvD2 1.4 ± 0.52, P = 0.03). Channel images for confocal microscopy are shown in Supplemental Fig. 3.
Figure 2.
Confocal microscopy of elastase-perfused aortas in animals treated with vehicle only (A) and in RvD2-treated animals (B) at d 14. Evident was marked increase in M2 macrophage marker (Arg-1, arrows) in RvD2-treated animals. Confocal images were scored by 2 independent observers for M1 (MCP-1) and M2 (Arg-1) staining density as follows: 1 = none, 2 = mild staining, 3 = moderate staining, 4 = extensive staining. RvD2-treated animals had significantly higher M2 scores (P < 0.0001) and lower M1 scores (P = 0.03) compared to nontreated animals.
TABLE 1.
Scoring of M1 and M2 polarization in vehicle- and RvD2-treated groups
| Phenotype | Vehicle only | RvD2, 100 ng/kg per dose |
|---|---|---|
| M1 | 2.1 ± 0.74 | 1.4 ± 0.52 |
| M2 | 1.3 ± 0.48 | 3.3 ± 0.48 |
Values are reported as averages ± sd. Confocal images were scored by 2 independent observers for M1 (MCP-1) and M2 (Arg-1) staining density as follows: 1 = none, 2 = mild staining, 3 = moderate staining, 4 = extensive staining. RvD2-treated animals had higher average M2 scores compared with nontreated animals.
Alternative D-series resolvin pretreatment inhibits AAA formation
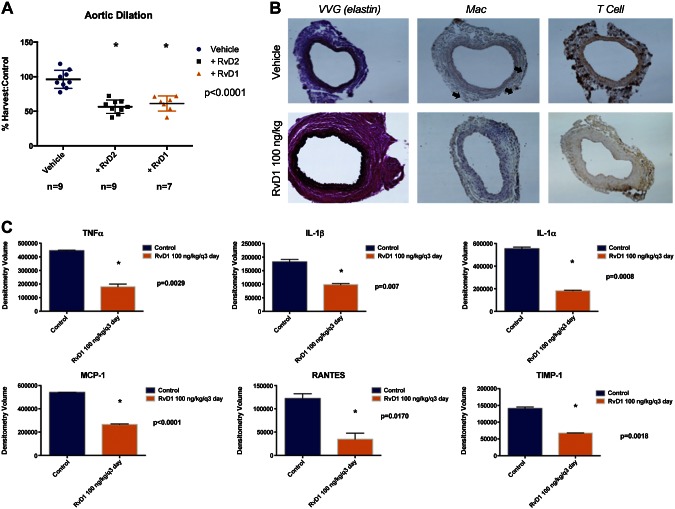
Likewise, protection from AAA formation was observed with RvD1 pretreatment (RvD1 61 ± 11%, vehicle only 96 ± 13, P < 0.0001, Fig. 3). Furthermore, similar preservation of elastin and protection from macrophage infiltration was observed with RvD1 as with RvD2. Cytokine analysis of RvD1-treated animals demonstrated reduction in proinflammatory cytokines including TNF-α (RvD1 179,734 ± 19,695 vs. 445,095 ± 3924, P = 0.003), IL1-β (RvD1 98,392 ± 3881 vs. 182,631 ± 8871, P = 0.007), IL1-α (RvD1 181,538 ± 4851 vs. 553,683 ± 14498, P = 0.0008), MCP-1 (RvD1 265,167 ± 4391 vs. 541,463 ± 496, P < 0.0001), and RANTES (RvD1 34,650 ± 12,881 vs. 122,330 ± 10,112, P = 0.017, Fig. 3A). Furthermore, histology demonstrated preservation of elastin and decreased macrophage infiltration in RvD1-treated animals (Fig. 3B). There was no significant difference in T-cell infiltration.
Figure 3.
A) RvD1 AAA prevention experiments. RvD1 (100 ng/kg per dose) significantly (P < 0.001) inhibited aneurysm formation in proportion to inhibition seen with RvD2 experiments. B) Histology demonstrates reduction in elastin degradation and macrophage infiltration (arrows) and T-cell infiltration. C) Proinflammatory cytokines TNF-α, IL-1β, MCP-1, and RANTES were attenuated in animals treated with RvD1.
RvD2 is effective in attenuation of aneurysm formation in Ang II model of AAA
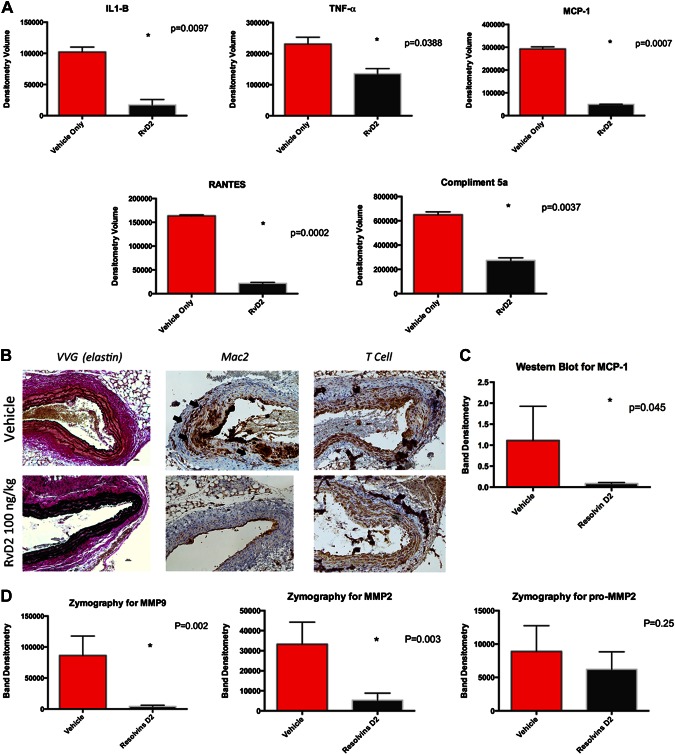
While there was no significant survival difference between treatment and control groups (Supplemental Fig. 4, P = 0.96), inflammatory cytokines including IL-1β, TNF-α, RANTES, and complement 5a were significantly lower in animals treated with RvD2 compared to control animals (IL-1β RvD2 1704 ± 8977 vs. 102,156 ± 7891, P = 0.0097; TNF-α RvD2 135,144 ± 16,699 vs. 230,951 ± 21,864, P = 0.039; MCP-1 RvD2 49,236 ± 1069 vs. 292,704 ± 8994, P = 0.0007; RANTES RvD2 21,624 ± 2070 vs. 163,620 ± 2019, P = 0.002, Fig. 4A). By immunohistochemistry, RvD2-treated animals demonstrated preservation of elastin and decreased macrophage infiltration compared with controls (Fig. 4B). Western blot analysis for MCP-1 in vehicle- and RvD2-treated aortas again demonstrated significantly higher expression of MCP-1 in vehicle-treated aortas (1.112 ± 0.814 vs. 0.0835 ± 0.026 respectively, P = 0.045, Fig. 4C).
Figure 4.
Ang II model of aortic aneurysm in apolipoprotein E–knockout animals. Mice were treated with vehicle only or RvD2 every 72 h. A) Aortic cytokine levels at d 28 showed decrease in proinflammatory cytokines (IL-1B, TNF-α, RANTES, MCP-1) and decreased complement activation in RvD2-treated animals. B) Histology at d 28 demonstrated significant elastin preservation and decreased macrophage infiltration compared to control animals (arrows). C) Western blot analysis for MCP-1 showed significant reduction in RvD2-treated animals compared with controls (P = 0.045). D) Gelatin zymography showed decreased MMP2 and MMP9 in RvD2-treated animals compared with controls.
Gelatin zymography for pro-MMP2, MMP2, and MMP9 demonstrated significantly higher MMP2 and MMP9 in vehicle compared to RvD2-treated animals in the Ang II model (MMP2: vehicle 33,221 ± 11,070 vs. RvD2 5313 ± 3483, P = 0.003; MMP9: vehicle 86,460 ± 31,237 vs. RvD2 4059 ± 1988, P = 0.002, Fig. 4D). There was a trend toward lower pro-MMP2 in RvD2-treated animals, which did not reach statistical significance (vehicle 8894 ± 3847 vs. RvD2 6221 ± 262, P = 0.294).
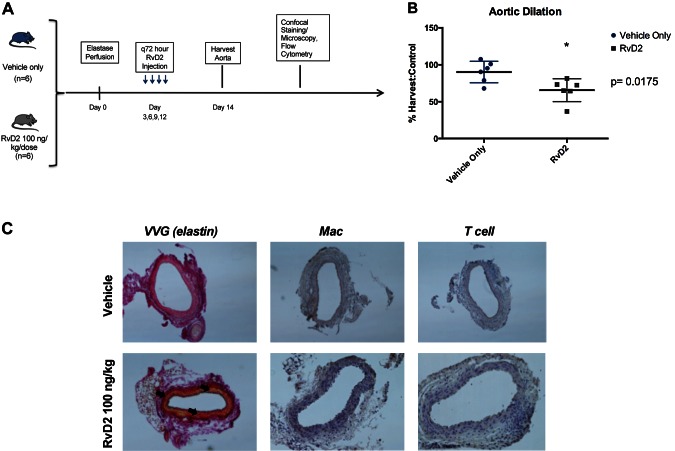
RvD2 treatment prevents small aneurysm propagation in existing murine AAA
The above findings demonstrated a preventative effect on aneurysm formation when begun before elastase perfusion. To test if RvD2 treatment could decrease aneurysm formation in developing aneurysms, RvD2 treatment was begun 3 d after elastase perfusion. RvD2 either halted progression or encouraged resolution of developing murine AAAs (Fig. 5A). Treatment with 100 ng/kg per dose every third day after development of small aneurysm demonstrated an average decrease in aortic diameter of 25% compared to vehicle alone (RvD2 66 ± 15% vs. vehicle only 90.1 ± 15%, P = 0.0175, Fig. 5B). Immunohistochemistry showed preservation of elastin and decreased macrophage infiltration in RvD2-treated animals (Fig. 5C).
Figure 5.
Study design to examine role of RvD2 in treating growing aortic aneurysms. A) Animals were treated with vehicle only or RvD2 100 ng/kg per dose every third day beginning 3 d after elastase perfusion. B) Aortic dilation data demonstrated 25% mean reduction in aortic diameter at d 14 (P = 0.0175). C) Histology demonstrated elastin preservation and decreased macrophage infiltration in treated animals (arrows).
DISCUSSION
Although the majority of research into the prevention and treatment of AAA has focused on decreasing the proinflammatory phase of inflammation, we present evidence of AAA prevention and attenuation through administration of naturally occurring proresolving mediators. Metabolic pathways have recently been implicated in the formation and growth of AAAs. The balance between pro- and anti-inflammatory mediators dictates both the onset and resolution of acute and chronic inflammation. Resolvins are an important class of mediators of inflammation that have been shown to be active the quiescent phase of inflammation (4, 25–27).
Both RvD1 and RvD2 demonstrated significant inhibition of AAA formation when administered before elastase treatment. Previous studies have implicated several inflammatory cytokines as important in aneurysm formation (11, 28, 29). In addition to phenotypic changes in aneurysm size, both RvD1 and RvD2 treatment resulted in significant attenuation of several important inflammatory cytokines known to be involved in AAA formation. In particular, IL-1β, and MCP-1 have both been previously shown to be important in AAA development and were decreased by treatment with either RvD1 or RvD2, and in both the elastase perfusion and the Ang II models. It is noteworthy that RvD2 pretreatment resulted in increased IL-10 expression. IL-10 is generally considered to be an anti-inflammatory cytokine and has been shown to be increased with docosahexaenoic acid and resolvin administration (2, 6). Additionally, Western blot analysis of aortic tissue demonstrated significantly reduced expression of MCP-1, which has been shown to be important in AAA formation (30). Finally, pretreatment with D-series resolvins led to a decrease in MMPs, known to be necessary for AAA formation (31–34). Together, these data suggest an overall reduction in the inflammatory process integral to the formation of AAA. While this change in IL-10 is small but significant, the overall reduction in proinflammatory cytokines combined with an increase in IL-10 suggests both a proresolving and an anti-inflammatory role for RvD2.
Resolvins are derived from polyunsaturated fatty acids. Interestingly, recent studies have demonstrated attenuation of experimental AAAs with administration of diets rich in resolvin precursors (35–37). Aspirin has been previously shown to both inhibit proinflammatory prostaglandins and thromboxanes, and to initiate the production of anti-inflammatory lipoxins and resolvins (1, 37, 39). Taken together, these findings provide a potential mechanism by which aspirin therapy has been shown to retard the progression of AAAs, as previous studies have established an association between low-dose aspirin treatment and AAA growth and need for surgical intervention (40)
The most clinically useful application of a drug to treat AAAs is in halting the growth of small existing aortic aneurysms. On the basis of data from the United Kingdom small aneurysm trial, a period of close observation of aneurysms less than 4.5 cm in diameter is often warranted, as the risk of rupture is low (41). Furthermore, this study demonstrated that this observation period frequently lasted for longer than 5 yr, which would allow ample time for pharmacologic treatment to attenuate aneurysmal growth. To this end, we investigated the ability of RvD2 in the treatment of existing experimental AAA by delaying administration until 3 d after elastase treatment. The ability of RvD2 to decrease both final aneurysm size and inflammatory cytokine expression indicates that its protective effect is preserved when delivered after the inflammatory process has begun.
Although macrophage expression was reduced in both the prevention and treatment arms of this study, both groups demonstrated mild macrophage staining in their treatment groups. D-series resolvins have previously been shown to alter macrophage expression toward an anti-inflammatory M2 phenotype (6). Confocal microscopy of treated animals demonstrated significant increase in M2 macrophage expression within the aortic media, providing a potential mechanism by which D-series resolvins are able to alter aortic cytokine expression, MMP expression, and ultimately aneurysm size. Although this change in macrophage polarization is evident at d 14 by confocal microscopy, earlier reduction in MCP-1 in RvD2-treated animals, combined with not only a prevention but also a treatment effect, suggest that this change in macrophage polarization is important in aneurysm reduction earlier than d 14.
It is noteworthy that recent studies examining patients undergoing surgical repair of AAA identified 2 distinct subgroups: those with a longer, more pronounced proinflammatory phenotype, and those with increased and earlier production of proresolving mediators including D- and E-series resolvins (1). Although this study did not examine the relationship between metabolic profiles and outcomes, it does suggest that inherent differences in proresolving mediators may provide a logical target for pharmacologic intervention.
Translation to human subjects remains a challenge in AAA research. Large animal models of AAA are lacking, and many human subject trials have chosen to trial pharmacotherapy for AAA on human subjects on the basis of murine data alone. Many of these trial therapies involve treatment with drugs already approved by the U.S. Food and Drug Administration for other indications. A human subject trial of resolvins in preventing AAA progression would first require phase 1 safety trials and would likely require industry support to accomplish.
A common criticism of the elastase perfusion model is the acute nature of aortic injury. To this end, we used the Ang II model to test our results in a more chronic model of inflammation. While measurement of aortic size is difficult, the result of both the tortuous nature of these aneurysms and their peridiaphragmatic location, we were able to demonstrate significant reduction in inflammatory cytokines in animals treated with RvD2, suggesting that our results are not limited to an acute, surgical model of AAA.
There are several limitations to our study. Large animal translational models of AAA are limited, and thus the translatability of our results remains in question. In addition, while we were able to demonstrate the anti-inflammatory nature of RvD2 in a 30 d medical model of AAA, the inflammatory process that leads to aneurysm formation in humans is likely far more chronic. Unfortunately, more chronic models of AAA are lacking and are time and resource intensive, and they have not supplanted the models used in this study. Finally, resolvin receptors remain poorly understood. Whereas two D-series receptors have been identified, their downstream signaling cascade remains poorly understood. Resolvin levels have been notoriously difficult to measure in tissue, and although their half-life may be short, the downstream effects of receptor binding may be much longer lasting (2, 42–45). Although the proinflammatory cytokines IL-1β, RANTES, and MCP-1 were all reduced with both RvD1 and RvD2 treatment, differences existed between the RvD1 and RvD2 groups in other important pro- and anti-inflammatory cytokines, including TNF-α. Although TNF-α is typically associated with M1 macrophage polarization, RvD2 treatment did not demonstrate a significant reduction in TNF-α despite a convincing reduction in M1 macrophage expression. Additionally, differences were noted in T-cell infiltration between RvD1- and RvD2-treated animals. Whereas T-cell infiltration was significantly attenuated in RvD1-treated animals, there was no difference in T-cell infiltration in RvD2-treated animals and controls. These differences may suggest differing mechanisms of action or differences in downstream signaling after RvD1 or RvD2 treatment.
In conclusion, both D-series resolvins significantly attenuated AAA formation in prevention and treatment models. In addition, RvD1 and RvD2 significantly attenuated inflammatory cytokine expression in surgical and nonsurgical models of the disease. RvD2 administration led to a significant increase in M2 macrophage polarization, which may provide a mechanism for its potent anti-inflammatory action. D-series resolvins are a novel and exciting candidate for nonsurgical treatment of human AAA, and further investigation into their effects in human subjects is needed.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by the U.S. National Institutes of Health (NIH) National Heart, Lung, and Blood Institute (NHLBI) Grants K08-HL098560 (to G.A.) and R01-HL081629 (to G.R.U.). This project was supported by NIH NHLBI Award T32-HL007849 (to N.H.P., principal investigator: I. L. Kron). The content is solely the responsibility of the authors and does not necessarily represent the views of the NHLBI. The authors thank A. Herring, C. Dodson, and M. Bevard for their technical expertise.
Glossary
- AAA
abdominal aortic aneurysm
- α-SMA
α smooth muscle actin
- Ang II
angiotensin II
- Arg-1
arginine type 1
- MCP-1
macrophage chemotactic protein 1
- MMP
matrix metalloproteinase
- RvD1
resolvin D1
- RvD2
resolvin D2
- WT
wild-type
Footnotes
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
AUTHOR CONTRIBUTIONS
N. H. Pope, M. Salmon, M. Conte, A. Chatterjee, G. Ailawadi, and G. R. Upchurch, Jr., designed research; N. H. Pope, M. Salmon, G. Su, and J. P. Davis performed the research; N. H. Pope, M. Salmon, and G. Su analyzed data; and N. H. Pope and G. R. Upchurch, Jr. wrote the paper.
REFERENCES
- 1.Pillai P. S., Leeson S., Porter T. F., Owens C. D., Kim J. M., Conte M. S., Serhan C. N., Gelman S. (2012) Chemical mediators of inflammation and resolution in post-operative abdominal aortic aneurysm patients. Inflammation 35, 98–113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Krishnamoorthy S., Recchiuti A., Chiang N., Fredman G., Serhan C. N. (2012) Resolvin D1 receptor stereoselectivity and regulation of inflammation and proresolving microRNAs. Am. J. Pathol. 180, 2018–2027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Merched A. J., Ko K., Gotlinger K. H., Serhan C. N., Chan L. (2008) Atherosclerosis: evidence for impairment of resolution of vascular inflammation governed by specific lipid mediators. FASEB J. 22, 3595–3606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Miyahara T., Runge S., Chatterjee A., Chen M., Mottola G., Fitzgerald J. M., Serhan C. N., Conte M. S. (2013) D-series resolvin attenuates vascular smooth muscle cell activation and neointimal hyperplasia following vascular injury. FASEB J. 27, 2220–2232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Serhan C. N. (2014) Pro-resolving lipid mediators are leads for resolution physiology. Nature 510, 92–101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Titos E., Rius B., González-Périz A., López-Vicario C., Morán-Salvador E., Martínez-Clemente M., Arroyo V., Clària J. (2011) Resolvin D1 and its precursor docosahexaenoic acid promote resolution of adipose tissue inflammation by eliciting macrophage polarization toward an M2-like phenotype. J. Immunol. 187, 5408–5418 [DOI] [PubMed] [Google Scholar]
- 7.Lederle F. A., Johnson G. R., Wilson S. E., Chute E. P., Hye R. J., Makaroun M. S., Barone G. W., Bandyk D., Moneta G. L., Makhoul R. G.; Aneurysm Detection and Management Veterans Affairs Cooperative Study Investigators (2000) The aneurysm detection and management study screening program: validation cohort and final results. Arch. Intern. Med. 160, 1425–1430 [DOI] [PubMed] [Google Scholar]
- 8.Lederle F. A., Larson J. C., Margolis K. L., Allison M. A., Freiberg M. S., Cochrane B. B., Graettinger W. F., Curb J. D.; Women’s Health Initiative Cohort Study (2008) Abdominal aortic aneurysm events in the Women’s Health Initiative: cohort study. BMJ 337, a1724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Norman P. E., Jamrozik K., Lawrence-Brown M. M., Le M. T., Spencer C. A., Tuohy R. J., Parsons R. W., Dickinson J. A. (2004) Population based randomised controlled trial on impact of screening on mortality from abdominal aortic aneurysm. BMJ 329, 1259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Scott R. A., Bridgewater S. G., Ashton H. A. (2002) Randomized clinical trial of screening for abdominal aortic aneurysm in women. Br. J. Surg. 89, 283–285 [DOI] [PubMed] [Google Scholar]
- 11.Shimizu K., Mitchell R. N., Libby P. (2006) Inflammation and cellular immune responses in abdominal aortic aneurysms. Arterioscler. Thromb. Vasc. Biol. 26, 987–994 [DOI] [PubMed] [Google Scholar]
- 12.Blomkalns A. L., Gavrila D., Thomas M., Neltner B. S., Blanco V. M., Benjamin S. B., McCormick M. L., Stoll L. L., Denning G. M., Collins S. P., Qin Z., Daugherty A., Cassis L. A., Thompson R. W., Weiss R. M., Lindower P. D., Pinney S. M., Chatterjee T., Weintraub N. L. (2013) CD14 directs adventitial macrophage precursor recruitment: role in early abdominal aortic aneurysm formation. J. Am. Heart Assoc. 2, e000065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tieu B. C., Lee C., Sun H., Lejeune W., Recinos A. III, Ju X., Spratt H., Guo D. C., Milewicz D., Tilton R. G., Brasier A. R. (2009) An adventitial IL-6/MCP1 amplification loop accelerates macrophage-mediated vascular inflammation leading to aortic dissection in mice. J. Clin. Invest. 119, 3637–3651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ariel A., Serhan C. N. (2012) New lives given by cell death: macrophage differentiation following their encounter with apoptotic leukocytes during the resolution of inflammation. Front. Immunol. 3, 4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mantovani A., Sica A., Locati M. (2005) Macrophage polarization comes of age. Immunity 23, 344–346 [DOI] [PubMed] [Google Scholar]
- 16.Mantovani A., Sica A., Sozzani S., Allavena P., Vecchi A., Locati M. (2004) The chemokine system in diverse forms of macrophage activation and polarization. Trends Immunol. 25, 677–686 [DOI] [PubMed] [Google Scholar]
- 17.Martinez F. O., Sica A., Mantovani A., Locati M. (2008) Macrophage activation and polarization. Front. Biosci. 13, 453–461 [DOI] [PubMed] [Google Scholar]
- 18.Johnston W. F., Salmon M., Su G., Lu G., Stone M. L., Zhao Y., Owens G. K., Upchurch G. R. Jr., Ailawadi G. (2013) Genetic and pharmacologic disruption of interleukin-1β signaling inhibits experimental aortic aneurysm formation. Arterioscler. Thromb. Vasc. Biol. 33, 294–304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Johnston W. F., Salmon M., Su G., Lu G., Ailawadi G., Upchurch J. (2014) Aromatase is required for female abdominal aortic aneurysm protection. J. Vasc. Surg. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Salmon M., Johnston W. F., Woo A., Pope N. H., Su G., Upchurch G. R. Jr., Owens G. K., Ailawadi G. (2013) KLF4 regulates abdominal aortic aneurysm morphology and deletion attenuates aneurysm formation. Circulation 128(11, Suppl 1)S163–S174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Daugherty A., Manning M. W., Cassis L. A. (2000) Angiotensin II promotes atherosclerotic lesions and aneurysms in apolipoprotein E–deficient mice. J. Clin. Invest. 105, 1605–1612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Daugherty A., Cassis L. (1999) Chronic angiotensin II infusion promotes atherogenesis in low density lipoprotein receptor −/− mice. Ann. N. Y. Acad. Sci. 892, 108–118 [DOI] [PubMed] [Google Scholar]
- 23.Daugherty A., Cassis L. A. (2004) Mouse models of abdominal aortic aneurysms. Arterioscler. Thromb. Vasc. Biol. 24, 429–434 [DOI] [PubMed] [Google Scholar]
- 24.Johnston W. F., Salmon M., Pope N. H., Meher A., Su G., Stone M. L., Lu G., Owens G. K., Upchurch G. R. Jr., Ailawadi G. (2014) Inhibition of interleukin-1β decreases aneurysm formation and progression in a novel model of thoracic aortic aneurysms. Circulation 130(11, Suppl 1)S51–S59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Akagi D., Chen M., Toy R., Chatterjee A., Conte M. S. (2015) Systemic delivery of proresolving lipid mediators resolvin D2 and maresin 1 attenuates intimal hyperplasia in mice. FASEB J. 29, 2504–2513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Duffield J. S., Hong S., Vaidya V. S., Lu Y., Fredman G., Serhan C. N., Bonventre J. V. (2006) Resolvin D series and protectin D1 mitigate acute kidney injury. J. Immunol. 177, 5902–5911 [DOI] [PubMed] [Google Scholar]
- 27.Hasturk H., Kantarci A., Ohira T., Arita M., Ebrahimi N., Chiang N., Petasis N. A., Levy B. D., Serhan C. N., Van Dyke T. E. (2006) RvE1 protects from local inflammation and osteoclast-mediated bone destruction in periodontitis. FASEB J. 20, 401–403 [DOI] [PubMed] [Google Scholar]
- 28.Freestone T., Turner R. J., Coady A., Higman D. J., Greenhalgh R. M., Powell J. T. (1995) Inflammation and matrix metalloproteinases in the enlarging abdominal aortic aneurysm. Arterioscler. Thromb. Vasc. Biol. 15, 1145–1151 [DOI] [PubMed] [Google Scholar]
- 29.Newman K. M., Jean-Claude J., Li H., Ramey W. G., Tilson M. D. (1994) Cytokines that activate proteolysis are increased in abdominal aortic aneurysms. Circulation 90, II224–II227 [PubMed] [Google Scholar]
- 30.Moehle C. W., Bhamidipati C. M., Alexander M. R., Mehta G. S., Irvine J. N., Salmon M., Upchurch G. R. Jr., Kron I. L., Owens G. K., Ailawadi G. (2011) Bone marrow–derived MCP1 required for experimental aortic aneurysm formation and smooth muscle phenotypic modulation. J. Thorac. Cardiovasc. Surg. 142, 1567–1574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ailawadi G., Eliason J. L., Upchurch G. R. Jr (2003) Current concepts in the pathogenesis of abdominal aortic aneurysm. J. Vasc. Surg. 38, 584–588 [DOI] [PubMed] [Google Scholar]
- 32.Ailawadi G., Knipp B. S., Lu G., Roelofs K. J., Ford J. W., Hannawa K. K., Bishop K., Thanaporn P., Henke P. K., Stanley J. C., Upchurch G. R. Jr (2003) A nonintrinsic regional basis for increased infrarenal aortic MMP-9 expression and activity. J. Vasc. Surg. 37, 1059–1066 [DOI] [PubMed] [Google Scholar]
- 33.Woodrum D. T., Ford J. W., Ailawadi G., Pearce C. G., Sinha I., Eagleton M. J., Henke P. K., Stanley J. C., Upchurch G. R. Jr (2005) Gender differences in rat aortic smooth muscle cell matrix metalloproteinase-9. J. Am. Coll. Surg. 201, 398–404 [DOI] [PubMed] [Google Scholar]
- 34.Newman K. M., Malon A. M., Shin R. D., Scholes J. V., Ramey W. G., Tilson M. D. (1994) Matrix metalloproteinases in abdominal aortic aneurysm: characterization, purification, and their possible sources. Connect. Tissue Res. 30, 265–276 [DOI] [PubMed] [Google Scholar]
- 35.Kavazos K., Nataatmadja M., Wales K. M., Hartland E., Williams C., Russell F. D. (2015) Dietary supplementation with omega-3 polyunsaturated fatty acids modulate matrix metalloproteinase immunoreactivity in a mouse model of pre-abdominal aortic aneurysm. Heart Lung Circ. 24, 377–385 [DOI] [PubMed] [Google Scholar]
- 36.Wang J. H., Eguchi K., Matsumoto S., Fujiu K., Komuro I., Nagai R., Manabe I. (2014) The ω-3 polyunsaturated fatty acid, eicosapentaenoic acid, attenuates abdominal aortic aneurysm development via suppression of tissue remodeling. PLoS One 9, e96286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yoshihara T., Shimada K., Fukao K., Sai E., Sato-Okabayashi Y., Matsumori R., Shiozawa T., Alshahi H., Miyazaki T., Tada N., Daida H. (2015) Omega 3 polyunsaturated fatty acids suppress the development of aortic aneurysms through the inhibition of macrophage-mediated inflammation. Circ. J. 79, 1470–1478 [DOI] [PubMed] [Google Scholar]
- 38.Serhan C. N., Clish C. B., Brannon J., Colgan S. P., Chiang N., Gronert K. (2000) Novel functional sets of lipid-derived mediators with anti-inflammatory actions generated from omega-3 fatty acids via cyclooxygenase 2–nonsteroidal anti-inflammatory drugs and transcellular processing. J. Exp. Med. 192, 1197–1204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Serhan C. N., Hong S., Gronert K., Colgan S. P., Devchand P. R., Mirick G., Moussignac R. L. (2002) Resolvins: a family of bioactive products of omega-3 fatty acid transformation circuits initiated by aspirin treatment that counter proinflammation signals. J. Exp. Med. 196, 1025–1037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lindholt J. S., Sorensen H. T., Michel J. B., Thomsen H. F., Henneberg E. W. (2008) Low-dose aspirin may prevent growth and later surgical repair of medium-sized abdominal aortic aneurysms. Vasc. Endovascular Surg. 42, 329–334 [DOI] [PubMed] [Google Scholar]
- 41.Brady A. R., Thompson S. G., Fowkes F. G., Greenhalgh R. M., Powell J. T.; UK Small Aneurysm Trial Participants (2004) Abdominal aortic aneurysm expansion: risk factors and time intervals for surveillance. Circulation 110, 16–21 [DOI] [PubMed] [Google Scholar]
- 42.Arita M., Bianchini F., Aliberti J., Sher A., Chiang N., Hong S., Yang R., Petasis N. A., Serhan C. N. (2005) Stereochemical assignment, anti-inflammatory properties, and receptor for the omega-3 lipid mediator resolvin E1. J. Exp. Med. 201, 713–722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Arita M., Yoshida M., Hong S., Tjonahen E., Glickman J. N., Petasis N. A., Blumberg R. S., Serhan C. N. (2005) Resolvin E1, an endogenous lipid mediator derived from omega-3 eicosapentaenoic acid, protects against 2,4,6-trinitrobenzene sulfonic acid–induced colitis. Proc. Natl. Acad. Sci. USA 102, 7671–7676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Serhan C. N. (2007) Resolution phase of inflammation: novel endogenous anti-inflammatory and proresolving lipid mediators and pathways. Annu. Rev. Immunol. 25, 101–137 [DOI] [PubMed] [Google Scholar]
- 45.Serhan C. N., Chiang N., Van Dyke T. E. (2008) Resolving inflammation: dual anti-inflammatory and pro-resolution lipid mediators. Nat. Rev. Immunol. 8, 349–361 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.