Abstract
Alterations in sphingolipid metabolism, especially ceramide and sphingosine 1-phosphate, have been linked to colon cancer, suggesting that enzymes of sphingolipid metabolism may emerge as novel regulators and targets in colon cancer. Neutral ceramidase (nCDase), a key enzyme in sphingolipid metabolism that hydrolyzes ceramide into sphingosine, is highly expressed in the intestine; however, its role in colon cancer has not been defined. Here we show that molecular and pharmacological inhibition of nCDase in colon cancer cells increases ceramide, and this is accompanied by decreased cell survival and increased apoptosis and autophagy, with minimal effects on noncancerous cells. Inhibition of nCDase resulted in loss of β-catenin and inhibition of ERK, components of pathways relevant for colon cancer development. Furthermore, inhibition of nCDase in a xenograft model delayed tumor growth and increased ceramide while decreasing proliferation. It is noteworthy that mice lacking nCDase treated with azoxymethane were protected from tumor formation. Taken together, these studies show that nCDase is pivotal for regulating initiation and development of colon cancer, and these data suggest that this enzyme is a suitable and novel target for colon cancer therapy.—García-Barros, M., Coant, N., Kawamori, T., Wada, M., Snider, A. J., Truman, J.-P., Wu, B. X., Furuya, H., Clarke, C. J., Bialkowska, A. B., Ghaleb, A., Yang, V. W., Obeid, L. M., Hannun, Y. A. Role of neutral ceramidase in colon cancer.
Keywords: sphingolipids ceramide, aberrant crypt foci, azoxymethane, apoptosis
Colorectal cancer (CRC) remains one of the most commonly diagnosed cancers in the western world, contributing to almost 50,000 premature deaths annually in the United States (http://www.cancer.gov). Most colon carcinomas are nonhereditary and sporadic, and it is believed that they develop through an accumulation of molecular and genetic alterations (1), including activation of specific oncogenes, inactivation of tumor suppressors, and additional epigenetic alterations (2).
Evidence has been forthcoming about the roles of bioactive sphingolipids in cancer pathogenesis and therapeutics, including CRC. Bioactive sphingolipids include sphingosine (SPH), ceramide, and sphingosine-1-phosphate (S1P) that have been implicated in the regulation of cell growth and death, autophagy, angiogenesis, cell adhesion, differentiation, migration, senescence, stress, and inflammatory responses (3–7).
Initial studies indicate that human colon cancer specimens had 50% less ceramide compared with adjacent normal colon mucosa (8), and expression of sphingosine kinase (SK)1 (an enzyme that phosphorylates SPH to generate S1P) was elevated in human colon adenocarcinomas (9, 10). Additional studies indicate that CRC exhibits down-regulation of enzymes that decrease the overall S1P levels by dephosphorylation by S1P-phosphatase or irreversible degradation by S1P lyase (SPL) (11). Furthermore, the activity of alkaline-sphingomyelinase (alk-SMase), the enzyme responsible for hydrolysis of sphingomyelin (SM) into ceramide, was decreased significantly in patients with colon cancer (12). Altogether, these studies indicate that alterations in the metabolism of sphingolipids may play an important role in colon cancer development. Thus, colon cancer seems to be associated with a decrease of ceramide and an increase in S1P. It is noteworthy that dietary SM, as well as ceramide analogs, have been shown to attenuate colon tumorigenesis in vivo (13–15; reviewed in 16).
Neutral ceramidase (encoded by the ASAH2 gene) is a key enzyme of sphingolipid metabolism that hydrolyzes ceramide into SPH, potentially regulating the balance between cell death-associated and proliferation-associated lipids (ceramide and S1P, respectively). Neutral ceramidase (nCDase) is a type II integral membrane protein that can be cleaved to generate a soluble secreted enzyme (17). In addition, a truncated neutral ceramidase has been localized to the mitochondria (18). Several studies have implicated nCDase as having essential roles in proliferative and antiapoptotic biological processes. In transformed murine endothelial cells, a role for nCDase was demonstrated in mediating the effects of the anticancer agent gemcitabine on growth inhibition (19), and studies of neuroblastoma indicated a suppressive effect of all-trans retinoic acid on nCDase (20). In turn, induction of nCDase protected keratinocytes from UV-induced death (21) and hepatocytes from TNF-induced death in vitro and in vivo (22).
nCDase is highly expressed in the small intestine along the brush border and, together with alk-SMase, has relevant roles in the metabolism of bioactive and dietary sphingolipids. nCDase−/− mice are viable, indicating that the enzyme is not essential for mammalian development (23). However, these animals have modified basal intestinal bioactive sphingolipids and greater C16:0 ceramide accompanied by less SPH.
Although nCDase is highly expressed in the intestinal tract and has an active role in balancing the metabolism of dietary and bioactive sphingolipids, its role in colon cancer has not been examined. Therefore, we undertook this study to define a role for nCDase in colon cancer pathogenesis using cellular and in vivo models, and the data suggest that nCDase permits the development of colon cancer and, as such, may be a novel chemotherapeutic target.
MATERIALS AND METHODS
Reagents
Cell culture medium and fetal bovine serum were obtained from Invitrogen (Carlsbad, CA, USA). The following antibodies were obtained: β-catenin from Santa Cruz Biotechnology (Santa Cruz, CA, USA); Ki67 (#AB9260) from Millipore (Billerica, MA, USA); LC-3 antibody (NB100-2220) from Novus Biologicals (Littleton, CO, USA); and phospho-β-catenin (Ser33-37) rabbit mAb (#2009), GAPDH rabbit mAb (#2118), caspase-3 rabbit mAb (#9665), p-ERK (Thr202/Tyr204) rabbit antibody (#9101), c-myc rabbit antibody (#5605), cyclin D1 rabbit antibody (#2978), and ERK rabbit antibody (#9102) from Cell Signaling (Beverly, MA, USA). nCDase antibody (Ab174) was a kind gift from Dr. Rick Proia (Genetics of Development and Disease, National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, Bethesda, MD, USA) (23).
The enhanced chemiluminescence kit was from Thermo Fisher Scientific (Rockford, IL, USA). Bafilomycin A1 (Baf-1) and chloroquine diphosphate salt (CQ) were purchased from Sigma-Aldrich (#1793 and #C6628, respectively; St. Louis, MO, USA). C6 urea-ceramide was provided by the Lipidomics Core facility at the Medical University of South Carolina (Charleston, SC, USA).
Cell culture
Colon cancer cells (HT29, HCT116, T84, SW837, SW480, SW620, DLD-1, and HCA-7) were obtained from ATCC (Manassas, VA, USA) and maintained as recommended. RIE-1 cells were obtained from K. D. Brown (Babraham Institute, Cambridge, United Kingdom) and maintained in DMEM supplemented with 10% fetal bovine serum. All cell lines were grown in a 5% CO2 incubator at 37°C. Mycoplasma tests were performed on a monthly basis for each cell line.
Small interfering RNA transfection
Cells were seeded in 35-mm dishes (10,000 cells). After 24 h, cells were transfected with 20 nM ASAH2 small interfering RNA (siRNA) (Hs_ASAH2_7; Qiagen, Hilden, Germany) or negative control siRNA (AllStars; Qiagen) using Lipofectamine RMAiMax according to the manufacturer’s instructions (Invitrogen, ). The next day, cells were trypsinized and seeded at a 1/5 dilution, and a day later, cells were transfected again with 20 nM nCDase siRNA or control siRNA as described previously. Cells were then collected and analyzed as indicated.
Lipid analysis
Lipid analysis was performed as described previously (24). In brief, cells were harvested at indicated times, and sphingolipid species were identified on a triple quadruple mass spectroscope (TSQ700; Thermo Finnigan, San Jose, CA, USA) at the Lipidomis Core at Stony Brook University. Sphingolipids from cellular extracts were normalized to total lipid phosphates present in cells after a Blight and Dyer extraction. For lipid analysis of mouse tissues, tumors were harvested after treatment at the indicated times, snap-frozen in liquid nitrogen, and stored at −80°C.
Protein extraction and immunoblot analysis
Protein extracts from cells and animal tissues were obtained after harvesting or homogenization with RIPA buffer (with PMSF, orthovanadate, and protease inhibitor cocktail) (Santa Cruz Biotechnology). Extracts were sonicated, and protein was measured using a Pierce BCA Protein Assay Kit (#23225, Thermo Fisher Scientific). Proteins were separated by SDS-PAGE using the Criterion system (Bio-Rad, Hercules, CA, USA) and immunoblotted as described previously (25).
3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide assay
3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide(MTT) was purchased from Sigma-Aldrich (#M5655). Cells were treated with MTT (1 mg/ml) for 1 h, and supernatants were discarded. DMSO was added, and absorbance was measured at 560 nm using a SpectraMax microplate reader (Molecular Devices, Sunnyvale, CA, USA). Each experimental condition tested was performed in triplicate.
Cell cycle analysis
Cells treated with C6 urea-ceramide (at the indicated doses and times) were permeabilized with 0.1% Triton X-100, and the nuclei were stained with propidium iodide. The nuclear DNA content was measured using BD FACSCalibur system (BD Biosciences, San Jose, CA, USA). Each experiment was repeated at least 3 times.
Apoptosis
Apoptosis was measured using the following 3 techniques: 1) morphologic changes in cell nuclear chromatin undergoing apoptosis after treatment with C6 urea-ceramide were quantified by staining with DNA-binding fluorochrome bis-benzimide trihydrochloride (Hoechst dye 33258 #B1155; Sigma-Aldrich) as described previously (26); 2) caspase-3 activity was measured in HT29 and RIE-1 cells after treatment with C6 urea-ceramide at doses and time specified using a caspase-3 fluormetric assay kit (#K105-100; BioVision, Milpitas, CA, USA) as indicated by the manufacturer; and 3) to evaluate apoptosis in tissue specimens, tumor tissues were obtained at the indicated time after treatment, fixed in 4% formaldehyde, and embedded in paraffin, and 5-μm sections were stained for measurement of apoptosis using TUNEL with a TACS 2TdT DAB In Situ apoptosis detect kit (#4810; Trevigen, Gaithersburg, MD, USA). Apoptotic cells were brown TUNEL-positive nuclear signals.
Senescence
To measure cell and tissue senescence, β-galactosidase activity at pH 6 was evaluated with a senescence β-galactosidase staining kit (#9860; Cell Signaling). Cells that stained blue were positively senescent.
Immunofluorescence and confocal microscopy
HCT116 colon cancer cells were treated with nCDase inhibitor at the indicated doses, and immunofluorescent analysis was performed as described in the literature (27). Samples were evaluated using a LSM510 META confocal microscope (Carl Zeiss, Inc., Oberkochen, Germany) and a Leica SP8 confocal microscopy system (Leica Microsystems, Wetzlar, Germany), and photos were obtained and analyzed using LSM Browser software and Leica software, respectively.
Mice
C57BL/6 and athymic male nude mice (Nu/J) (6–8 wk old) were purchased from The Jackson Laboratory (Bar Harbor, ME, USA), and nCDase-knockout (KO) mice (23) were backcrossed at least 6 generations into the C57BL/6 background. Mice were maintained in accordance with the regulations and standard of the U.S. Department of Agriculture and the U.S. Department of Health and Human Services. Animal protocols were approved by the Internal Review Board of Stony Brook University. Mice were fed regular chow (PicoLab Rodent Diet; LabDiet, St. Louis, MO) and had free access to food and water.
Xenograft implantation and C6 urea-ceramide treatment
HT29 colon cancer cells were maintained as indicated, and 5 × 106 cells were transplanted subcutaneously into the right limbs of Null/J mice. Mice were treated with C6 urea-ceramide at the indicated doses intraperitoneally for 5 consecutive days when tumors reached 100–150 mm3. Tumor volume was evaluated daily using calipers and calculated according to the formula of Kim et al. (28).
Aberrant crypt focus formation assay
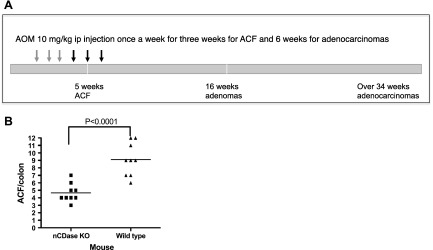
Starting at 6 wk of age, wild-type (WT) and Asah2−/− mice were given azoxymethane (AOM) (10 mg/kg, i.p.) (NCI Chemical Carcinogen Reference Standard Repository, Bethesda, MD, USA) once weekly for 3 consecutive weeks. Animals were euthanized 2 wk after the last AOM treatment (see Fig. 5A). Colons were fixed and stained with 0.1% methylene blue (Sigma-Aldrich) in saline, and aberrant crypt foci (ACFs) per colon were counted under a light microscope as described previously (29).
Figure 5.
Effect of nCDase deficiency on AOM-induced ACF formation. A) Scheme of treatment with AOM and time points when mice were euthanized. Colons were collected for ACF, adenoma, and adenocarcinoma evaluation. B) ACF formation in nCDase-KO mice vs. WT mice. Bars represent group median; symbols represent ACF numbers per animal. A 2-tailed Student’s t test was used to analyze the data in B (9 mice per group).
AOM-induced colon cancer
WT and Asah2−/− mice (6 wk of age) were treated with AOM (10 mg/kg, i.p.) for 6 consecutive weeks. Animals were euthanized at 50 wk of age. After laparotomy, colons were examined, and tumor numbers and sizes were recorded. Diagnosis of intestinal tumors using H&E-stained sections was performed according to the classification of Krutovskikh and Turusov (30).
5-Bromo-2-deoxyuridine staining
Mice were injected with 5-bromo-2-deoxyuridine (BrdU) (100 µg/g, i.p.) (Sigma-Aldrich) 2 h before being euthanized. Immunohistochemistry was performed using an anti-BrdU antibody (#B8434; Sigma-Aldrich) on hydrated sections. Secondary biotinylated anti-mouse antibody and streptavidin-horseradish peroxidase (Sigma-Aldrich) were quantified with a Vectastain ABC kit (Vector Laboratories Inc., Burlingame, CA, USA). Nuclei were visualized with hematoxylin. Data means were compiled from at least 20 random high-resolution fields (original magnification, ×10) from each mouse. At least 5 mice were evaluated per group.
Complete blood counts
Whole blood was collected from euthanized mice into EDTA-coated tubes. Complete blood counts were performed with a Hemavet 950FS (Drew Scientific Inc., Waterbury, CT, USA).
Statistical analysis
Statistical analysis was performed using 1-way ANOVA or a Student’s t test for strain and treatment effects.
Study approval
All animal studies were reviewed and approved by the Institutional Animal Care and Use Committee of Stony Brook University.
RESULTS
Effects of suppression of ASAH2 expression on cell survival in colon cancer cells
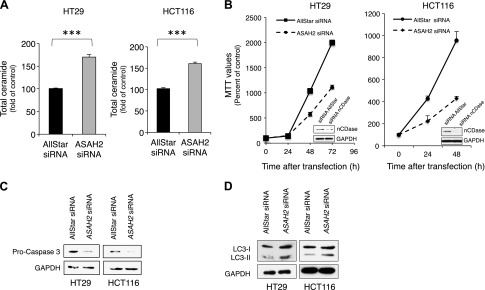
To determine the effects of ASAH2 suppression on lipids and the consequent implications in cell survival for colon cancer, HT29 and HCT116 colon cancer cells were transfected with a siRNA for the ASAH2 gene (gene encoding for nCDase). Total ceramide in HT29 and HCT116 colon cells after ASAH2 knockdown increased to 170 ± 5 and 160 ± 3% of control, respectively (Fig. 1A). Suppression of ASAH2 caused decreases in SPH in HCT116 as expected, but SPH in HT29 cells unexpectedly increased (Supplemental Fig. 1B, A, respectively). No significant changes in S1P were observed in either cell line (Supplemental Fig. 1). Knockdown of ASAH2 also reduced cell viability, as evidenced by the MTT assay (Fig. 1B). In both cell lines, MTT values decreased by approximately 50% (2000 ± 18 vs. 1114 ± 25% at 72 h in HT29 cells and 956 ± 18 vs. 429 ± 20% at 48 h in HCT116 cells).
Figure 1.
Effects of suppression of ASAH2 expression by siRNA on colon cancer cell lines. A) Total ceramide levels after suppression of ASAH2 in HT29 and HCT116 cells. Cells were treated with 20 nM AllStart or siRNA for ASAH2 for 48 h and collected for lipid analysis using high performance liquid chromatography-electrospray ionization-mass spectrometry (HPLC-ESI-MS) at the Lipidomics Core Facility at Stony Brook University. Lipid basal levels for HT29 were 4.8 ± 0.98 pmol/nmol Pi for total ceramide, 0.21 ± 0.055 pmol/nmol Pi for SPH, and 0.016 ± 0.005 pmol/nmol Pi for S1P. Lipid basal levels for HCT116 were 2.32 ± 0.438 pmol/nmol Pi for total ceramide, 0.30 ± 0.078 pmol/nmol Pi for SPH, and 0.007 ± 0.0014 pmol/nmol Pi for S1P. B) Effect of siRNA for ASHA2 in MTT values in HT29 and HCT116 cells. The efficacy of siRNA transfection is shown in insets. C, D) Cells treated with 20 nM AllStart or nCDase siRNA for 48 h were collected and evaluated for caspase-3 (C) and LC3-I and LC3-II (D) quantified by Western blot. Data are shown as means ± sem. A 2-tailed Student’s t test was used to analyze data in A, and a 2-way ANOVA was used to analyze data in B. Results are representative of 3 experiments. ***P < 0.0001.
Increased ceramide and decreased MTT values were accompanied by activation of caspase-3 (reduced uncleaved caspase-3) and accumulation of autophagosomes (increased LC3-II) as shown by Western blots (Fig. 1C, D). Together, these results establish a role of nCDase in lipid regulation in colon cancer cells and that this is accompanied by decreased cell viability, activation of caspase-3, and increased LC3-II.
Inhibition of nCDase increases ceramide in colon cancer cells
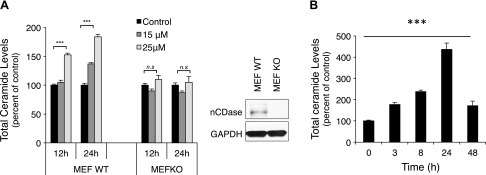
C6 urea-ceramide, a competitive inhibitor of nCDase developed based on structure-activity analysis (18, 31), was used to elucidate the roles of nCDase. Initially, studies were conducted to establish nCDase specificity by evaluating the effects C6 urea-ceramide on lipid generation in WT mouse embryonic fibroblasts (MEFs) vs. MEFs from nCDase-KO (nCDase−/−) mice. The right panel in Fig. 2A confirms loss of nCDase in the nCDase−/− MEFs. As shown in Fig. 2A, in WT MEFs, C6 urea-ceramide induced increases in total ceramide at 12 h, which peaked 24 h after treatment with 25 μM (152 ± 3 and 184 ± 4%, respectively, compared with untreated WT MEFs). It is noteworthy that this increase was not observed in nCDase−/− MEFs. These results provide strong evidence that nCDase is the primary target for C6 urea-ceramide and is responsible for increased ceramide because cells lacking the enzyme did not respond to this inhibition.
Figure 2.
Effects of nCDase inhibition on lipid levels. A) MEF WT and MEF KO for nCDase cells were treated with 15 and 25 μM C6 urea-ceramide, and total ceramide levels were measured at 12 and 24 h (right panel confirms the MEF KO for nCDase by Western blot). Lipid basal levels for MEF WT were 2.06 ± 0.58 pmol/nmol Pi for total ceramide, 0.141 ± 0.0325 pmol/nmol Pi for SPH, and 0.0164 ± 0.004 pmol/nmol Pi for S1P. Lipid basal levels for MEF KO were 2.98 ± 0.43 pmol/nmol Pi for total ceramide, 0.102 ± 0.025 pmol/nmol Pi for SPH, and 0.0176 ± 0.003 pmol/nmol Pi for S1P. B) HT29 cells were treated with C6 urea-ceramide (10 μM), and lipids were evaluated at the indicated times. Lipid analysis was performed using high-performance liquid chromatography-electrospray ionization-mass spectrometry (HPLC-ESI-MS) at the Lipidomics Core Facility at Stony Brook University. Data are shown as means ± sem. A 2-way ANOVA was used to analyze the data in A, and a 1-way ANOVA was used to analyze the data in B. Results are representative of 3 experiments. ***P < 0.0001.
Next, the effects of C6 urea-ceramide on ceramide in colon cancer cells (HT29) were evaluated at different times after treatment. The results in Fig. 2B show that C6 urea-ceramide (10 μM) caused a 4-fold increase in ceramide after 24 h and a 2-fold increase after 48 h. Therefore, as with suppression with siRNA, pharmacological inhibition of nCDase promoted ceramide accumulation. No significant changes in either SPH or S1P were observed after C6 urea-ceramide treatment (Supplemental Fig. 2).
Selective effects of inhibition of nCDase on colon cancer cells versus noncancerous cells
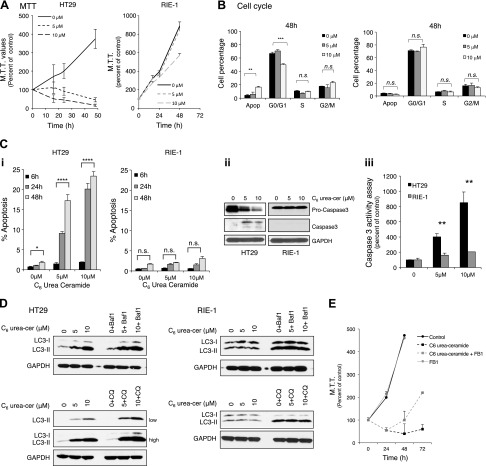
Many studies have shown that ceramide accumulation regulates cell death, apoptosis, autophagy, and/or cell cycle arrest (3, 32, 33). To investigate whether inhibition of nCDase by C6 urea-ceramide induced any of these biological functions, HT29 colon cancer cells and RIE-1 noncancerous untransformed, intestinal epithelial cells derived from rat small intestine (34) were treated with C6 urea-ceramide, and MTT values were evaluated at different time points. Inhibition of nCDase in HT29 cells decreased MTT values in a dose- and time-dependent manner after 24 h, and this was highly significant at 48 h (Fig. 3A, left panel). In contrast to its effects on colon cancer cells, C6 urea-ceramide did not have pronounced effects on RIE-1 cell growth. Indeed, at 5 μM, MTT values were similar to controls at the times studied, and at 10 μM, MTT decreases in RIE-1 cells were not as dramatic as those in HT29 (Fig. 3A, right panel).
Figure 3.
Effects of inhibition of nCDase by C6 urea-ceramide on cell viability, apoptosis, cell cycle, and autophagy in colon cancer cells vs. noncancerous cells. A) Effect of nCDase inhibition on cell growth was measured by MTT assay in HT29 cells (left) and RIE-1 cells (right). B) Effects of nCDase inhibition on cell cycle distribution in HT29 cells (left) and RIE-1 (right) evaluated by staining with propidium iodide and analyzed by FACScan flow cytometry at 48 h. C) Effect of nCDase inhibition on apoptosis in HT29 and RIE-1 cells evaluated by the following methods. i) Morphologic changes in nuclear chromatin using bis-benzimide staining in HT29 and RIE-1 cells (cells were scored as apoptotic when they displayed at least 3 apoptotic bodies/nucleus). ii) Caspase-3 cleavage activation after treatment with C6 urea-ceramide evaluated by Western blot. iii) Caspase-3 activity after treatment with C6 urea-ceramide using Caspase-3 Fluormetric Assay Kit from BioVision. D) Effect of nCDase inhibition on LC3-II levels in HT29 (left panel) and RIE-1 cells (right panel) assessed by Western blot for LC3. To evaluate and confirm total autophagic flux, control cells and cells incubated with C6 urea-ceramide were treated with either Baf-1 (100 nM) or CQ (50 μM) for 4 h before harvesting and evaluated by Western blot for LC3-II. Low and high exposure for cells treated with CQ is included for HT29 cells. E) FB1 pretreatment partially reverses MTT values induced by inhibition of nCDase. Colon cancer cells were pretreated with FB1 (50 μM) for 4 h before nCDase inhibition, and MTT values were evaluated as before. Data are shown as means ± sem. A 2-way ANOVA was used to analyze the data in A–C and E. Results are representative of 3 experiments.
Because treatment of HT29 with C6 urea-ceramide inhibited cell viability, the effects of nCDase inhibition on the cell cycle and on apoptosis were assessed. Inhibition of nCDase did not alter the cell cycle after 12 or 24 h at either 5 or 10 μM (data not shown). However, at 48 h, there was a significant decrease in G0/G1 (from 66 ± 2 to 50 ± 1%; P < 0.0001) at 10 μM that was accompanied by increased sub-G0/G1 from 4 ± 1.4 to 16 ± 1% (P < 0.001) (Fig. 3B, left panel), suggesting an increase of apoptosis at 48 h. To confirm apoptosis, morphological changes in nuclear chromatin were measured with bis-benzamide trihydrochloride, and C6 urea-ceramide induced more apoptosis that was time and dose dependent (23 ± 2% at 48 h) (Fig. 3Ci, left panel). The treatment with C6 urea-ceramide increased activated forms of caspase-3, as shown by Western blot (Fig. 3Cii, left panel), and increased caspase activity (at 5 and 10 μM), which was dose dependent and was increased by 8.5-fold with 10 μM compared with untreated cells (Fig. 3Ciii).
Autophagy was evaluated using lysosomal turnover of endogenous LC3-II by Western blot. Treatment with C6 urea-ceramide (5 and 10 μM) increased LC3-II (Fig. 3D, upper panel) in a dose-dependent manner, indicating an accumulation of autophagosomes. To confirm total autophagic flux, control cells and cells treated with C6 urea-ceramide were incubated with either Baf-1 (35) (an inhibitor of autophagosome-lysosome fusion) or CQ [an agent that blocks autophagy by inhibiting lysosomal proteases and autophagosome-lysosomal fusion (36)]. Figure 3D shows that treatment with C6 urea-ceramide and either Baf-1 or CQ increased the accumulation of LC3-II compared with cells treated with the inhibitor alone, confirming autophagic flux.
Increased ceramide is also associated with senescence in several cell types (37–40). Thus, to determine whether nCDase inhibition was associated with senescence, β-galactosidase activity was measured in HT29 cells treated with C6 urea-ceramide. No blue β-galactosidase staining was detected at 48 h at any indicated dose, suggesting that nCDase inhibition does not induce cell senescence in HT29 cells at the indicated time and doses (data not shown).
This approach was also performed in RIE-1 cells, and here, C6 urea-ceramide had no effects on cell cycle at 12, 24, and 48 h at the concentrations studied (0, 5, and 10 μM) (in Fig. 3B, right panel; cell cycle is shown at 48 h). Treatment with C6 urea-ceramide did not significantly increase the apoptotic fraction (Fig. 3Ci, right panel), as confirmed by few cells with morphological changes in nuclear chromatin assessed by bis-benzimide trihydrochloride. In RIE-1 cells, caspase-3 was not cleaved (Fig. 3Cii, right panel), and increased caspase-3 activity was not observed after C6 urea-ceramide treatment (Fig. 3Ciii). Inhibition of nCDase in these cells was also evaluated by lysosomal turnover of endogenous LC3-II, and LC3-II did not increase after treatment (Fig. 3D, lower panel). To evaluate total autophagic flux and to confirm no LC3-II degradation that may mask these results, RIE-1 cells were treated with C6 urea-ceramide and Baf-1 or CQ as described above. RIE-1 cells did not show an increase of LC3-II after treatment with Baf-1 or CQ when treated in combination with the inhibitor, confirming that C6 urea-ceramide does not induce autophagic flux in these cells. RIE-1 cells did not have greater senescence as measured by β-galactosidase activity at pH 6 after nCDase inhibition, even though occasional positive cells were noted (data not shown). These results show that RIE-1 cells are mostly resistant to biological actions of both knockdown [by CRISPR (clustered regularly interspaced short palindromic repeats); data not shown] and inhibition of nCDase.
To determine whether the effects of nCDase inhibition in colon cancer cells are mediated by the accumulation of ceramide, we used fumonisin B1, an inhibitor of ceramide synthases. Inhibition of ceramide synthase after preincubation with 50 μM fumonisin B1 within 4 h partially reversed MTT values induced by nCDase inhibition (Fig. 3E).
Next, the generalizability of the effects of inhibition of nCDase on colon cancer cells was evaluated in a panel of colon cancer cell lines. The results showed that cell viability was decreased significantly in numerous colon cancer cell lines after treatment with C6 urea-ceramide (see Table 1 for data as well as results from RIE-1 cells). Only 1 colon cancer cell line, SW620, was as resistant to C6 urea-ceramide as RIE-1 cells.
TABLE 1.
IC50 of C6 urea-ceramide in MTT values in different cell lines
| Cell line | MTT IC50, 48 h (μM) |
|---|---|
| HT29 | 3.5 |
| T84 | 3.5 |
| HCT116 | 5 |
| SW837 | 3.5 |
| DLD-1 | 6 |
| HCA-7 | 5 |
| SW480 | 5 |
| SW620 | >10 |
| RIE-1 | >10 |
Effects of nCDase inhibition on HT29 xenografts
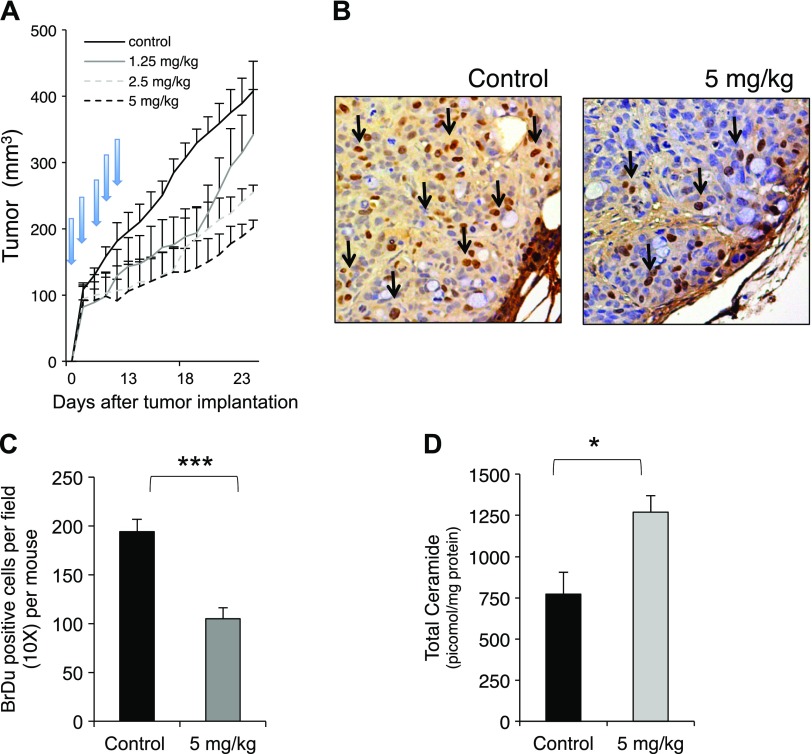
Because cell studies indicated an important and differential role of nCDase in colon cancer cell survival by decreasing MTT values and increasing ceramide after genetic and pharmacologic inhibition, the in vivo effects of nCDase inhibition were evaluated next in a colon cancer xenograft animal model. Specifically, HT29 cells were implanted subcutaneously into Nu/J mice. After solid tumors were established (100–150 mm3), animals were distributed randomly into 4 different groups and received daily C6 urea-ceramide treatments (1.25, 2.5, or 5 mg/kg, i.p. or vehicle control) for 5 consecutive days. Figure 4A shows that nCDase inhibition induced growth delay in a dose-dependent manner, decreasing tumor volume by 16, 37, and 49% at the end of the experiment (24 d), respectively. Arrows indicate treatment days (vehicle control or nCDase inhibitor). Proliferation was assessed with BrdU immunostaining. Figure 4B shows representative fields of HT29 xenografts control vs. treated with C6 urea-ceramide (positive cells are stained brown). Tumors treated with C6 urea-ceramide had significantly less BrdU-positive cells per field (from 194.83 ± 9.1 to 105.33 ± 7.3 positive cells per field per mouse in each group; P < 0.0001) (Fig. 4C). To determine whether treatment response was accompanied by induction of apoptosis, TUNEL assay was performed, and the results (Supplemental Fig. 3A) revealed a rare positive staining (brown staining indicated by arrows) in both control and treated tumors, suggesting that apoptosis is not relevant for tumor growth delay observed after nCDase inhibition or that it is too fleeting to capture. Senescence was not detected in untreated tumors or in tumors after treatment (Supplemental Fig. 3B), and positive cells for β-galactosidase activity at pH 6 (in blue) were found only occasionally (indicated by arrows). No changes in animal weight were observed between groups during these studies (data not shown).
Figure 4.
Effects of nCDase inhibition on HT29 xenografts. A) Growth pattern of HT29 xenografts after treatment with C6 urea-ceramide. Mice were administered subcutaneous injections of HT29 cells (5 × 106 cells resuspended in PBS) into the right hind limb and treated with C6 urea-ceramide daily (vehicle, 1.25, 2.5, and 5 mg/kg, i.p.) for 5 consecutive days (as indicated by arrows) when tumors reached 100 mm3. Tumor volume, measured with calipers, was calculated daily. At least 5 mice were used in each group. B) Proliferation of HT29 xenografts treated with either control vehicle or with C6 urea-ceramide assessed by BrDu staining. Representative 5-μm histologic xenograft sections were stained with BrdU. Arrows indicate positive staining. C) Quantification of positive BrDu cells: brown positive cells were quantified manually, and data represent positive cells per field (original magnification, ×10) per mouse (5 control mice; 6 treated mice). At least 20 random fields were evaluated for each mouse. D) Ceramide levels in control xenografts vs. treated with C6 urea-ceramide (5 mg/kg). Xenografts from control (n = 5) and treated (n = 5) mice were evaluated for ceramide content using high-performance liquid chromatography-electrospray ionization-mass spectrometry (HPLC-ESI-MS) at the Lipidomics Core Facility at Stony Brook University. Data are shown as means ± sem. A 2-way ANOVA was used to analyze the data in A, and a 2-tailed Student’s t test was used to analyze the data in C and D. *P < 0.01; ***P < 0.0001.
Tumor growth delay and decreased proliferation after nCDase inhibition were accompanied by a significant increase in ceramide in tumor samples harvested 1 h after the last dose of C6 urea-ceramide (Fig. 4D, upper panel), revealing an increase from 772.0 ± 108 pmol/mg in controls to 1271 ± 85 pmol/mg protein in the treated group (P < 0.01). In particular, C6 urea-ceramide treatment generated a significant increase of C16:0, C18:0, C20:0, and C24:0 ceramide species. These results indicated that nCDase is important for the growth of a colon xenograft and regulates lipids and tumor cell proliferation. Inhibition of nCDase with C6 urea-ceramide did not affect complete blood counts in mice (Supplemental Fig. 3C). Although neutrophils and platelets decreased in treated mice, values were in the normal range for these animals. In addition, the inhibitor did not affect body weight and movement of treated mice compared with control.
Deletion of nCDase inhibits colon carcinogenesis induced by AOM
Because inhibition of nCDase by C6 urea-ceramide suppressed growth in human xenografts, we evaluated the effects of genetic deletion of the Asah2 gene (23) in a model of inducible colon carcinogenesis. Thus, AOM, an alkylating agent that induces development of tumors mostly in the distal colon and that recapitulates the pathogenesis of human sporadic colon cancer (41), was administered to nCDase−/− mice (n = 30) and WT mice (n = 30), and animals were euthanized at the times indicated in Fig. 5A. The results (summarized in Table 2) show that 57% of WT mice treated with AOM developed tumors (17/30), with 14 of 30 animals developing adenocarcinomas and 3 developing adenomas. In contrast, only 3 of the 28 nCDase−/− mice treated with AOM developed colon tumors, including 2 mice that developed adenomas and 1 mouse that developed an adenocarcinoma. Loss of nCDase significantly reduced AOM-induced colon carcinogenesis (93% in adenocarcinoma and 82% in total colon tumors). This resistance to AOM-induced colonic carcinogenesis provides evidence that nCDase plays an important role regulating the pathogenesis of colon cancer development.
TABLE 2.
nCDase deficiency inhibits AOM-induced colon carcinogenesis
| Tissue | nCDase−/−, 28 mice [n (%)] | Wild-type, 30 mice [n (%)] |
|---|---|---|
| Adenoma | 2 (7) | 3 (10) |
| Adenocarcinoma | 1 (4) | 14 (47) |
| Total | 3 (11) | 17 (57) |
Total indicates incidence of both adenomas and carcinomas. Values in parentheses represent percentage of mice that developed tumors. P < 0.0005 (Fisher’s exact test).
Deletion of nCDase inhibits ACF formation induced by AOM
Colon carcinogens, such as AOM, induce the development of ACFs in rodents, early pathologic formations that are considered to be indicators of increased risk for progression to CRC. Even though it remains controversial whether all ACFs are precursors for colon cancer, it has been reported that at least some of these lesions have this capacity (42). To investigate whether nCDase plays a role in the formation of these putative cancer “precursors,” the effects of nCDase deficiency in early stages of AOM-induced colon carcinogenesis were evaluated. WT and nCDase−/− mice received an AOM injection (10 mg/kg, i.p.) once weekly for 3 consecutive weeks, and animals were euthanized 2 wk after the last treatment (indicated in the cartoon in Fig. 5A). Mice deficient in nCDase had significantly fewer ACFs per colon (49%), with mice having an average of 9.11 ± 0.73 ACFs per colon in WT vs. 4.67 ± 0.41 in nCDase−/− mice (P < 0.0001) (Fig. 5B). Thus, these results show that nCDase plays a role in the early phases in the response to AOM; however, most ACFs generated in nCDase−/− mice did not progress to colon cancer development as demonstrated above. No changes in animal weight were observed during these studies (data not shown).
nCDase inhibition regulates key pathways of colon carcinogenesis
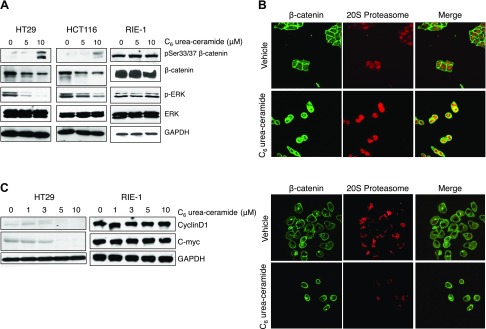
To determine the effects of nCDase in pathways relevant to colon cancer, we evaluated the effects of nCDase inhibition on the canonical Wnt/β-catenin pathway. Treatment of HT29 and HCT116 cells with C6 urea-ceramide reduced total β-catenin. This finding correlated with increased phosphorylation of Ser33 and Ser37 (Fig. 6A), which promotes ubiquitination of β-catenin and targets it for proteasomal destruction. Thus, inhibition of nCDase appears to regulate the Wnt/β-catenin pathway upstream of β-catenin.
Figure 6.
Effects of nCDase inhibition on pathways relevant for initiation and formation of colon carcinoma in colon cancer cells and noncancerous cells. A) HT29, HCT116, and RIE cells were treated with 5 and 10 μM C6 urea-ceramide, and lysates were evaluated by Western blot to measure proteins relevant to colon cancer as indicated. B) Immunofluorescent staining of β-catenin and 20S proteasome subunit in HT116 (upper panel) and RIE-1 (lower panel) treated with C6 urea ceramide. Cells were treated with the inhibitor or vehicle, and the presence and location of β-catenin (green) and 20S proteasome (red) were evaluated by immunofluorescent staining and visualized with a confocal microscope. Results are representative of 3 experiments. C) HT29 and RIE-1 cells were treated with different doses of C6 urea-ceramide and were evaluated for Cyclin D1 and c-myc by Western blot.
The K-RAS/ERK pathway is also important in colon cancer and particularly in the AOM-induced model of carcinogenesis (43). Phosphorylation of ERK is up-regulated in CRC, and this correlates closely with CRC progression (44). C6 urea-ceramide decreased ERK phosphorylation in HT29 and HCT116 cells (Fig. 6A). In contrast, in RIE-1 cells, neither activation of the WNT/β-catenin pathway nor phosphorylation of ERK was decreased by C6 urea-ceramide (Fig. 6A, right panel), confirming that these cells do not respond to inhibition of nCDase (Fig. 3).
To further evaluate the effects of nCDase inhibition on the WNT/β-catenin pathway in colon cancer cells, immunostaining of β-catenin and 20S proteasome was performed. In HCT116 cells treated with vehicle, β-catenin was mostly found in the plasma membrane (green) and did not colocalize with the 20S proteasome (red), whereas treatment with C6 urea-ceramide induced mobilization of β-catenin and colocalization with proteasome (in yellow) (Fig. 6B, upper panel). On the other hand, C6 urea-ceramide did not affect the location of β-catenin (green; mostly in the membrane) and did not induce colocalization of β-catenin with 20S proteasome (red) in the RIE-1 cells, confirming the resistance of noncancer cells to nCDase inhibition (Fig. 6B, lower panel).
Accumulation of nuclear β-catenin, together with the DNA-binding protein transcription factor 4 (TCF-4), functions as a transcriptional activator of target genes, notably c-myc and cyclin D1 (45). To confirm the effects of nCDase inhibition downstream of β-catenin, the levels of cyclin D1 and c-myc were evaluated in colon cancer cells. Results (Fig. 6C) show a significant decrease of cyclin D1 and c-myc in a dose-dependent manner, whereas no change was detected in RIE-1 cells.
Taken together, these results strongly suggest a critical role for nCDase in regulating the responses of the Wnt/β-catenin and the K-ras/ERK pathways.
DISCUSSION
The results from this study reveal a key role for nCDase in CRC. Molecular and pharmacologic inhibition of nCDase induced an increase in ceramide, accompanied by decreased survival, increased apoptosis, and autophagy of colon cancer cells. In contrast, nCDase inhibition caused minimal effects in noncancerous intestinal cells. These results were extended to a cancer xenograft model in which treatment of animals with an inhibitor of nCDase delayed tumor growth, increased ceramide, and decreased tumor cell proliferation. These results prompted an investigation of the role of nCDase in vivo using a murine model of colon carcinogenesis. Whereas AOM treatment induced tumors in the distal colon in 57% WT mice, only 11% of mice deficient in nCDase had tumor formation. Moreover, nCDase−/− mice were protected from AOM-induced ACF development. Mechanistically, data revealed important effects of nCDase inhibition with respect to down-regulation of key signaling pathways relevant to the development of colon cancer (WNT/β-catenin and ERK). Taken together, the results strongly suggest a key role for nCDase in regulating the initiation and development of colon cancer, specifically pointing to nCDase as a potential unique chemotherapeutic target.
Increasing evidence suggests roles for bioactive sphingolipids in CRC. Specifically, studies suggest that colon cancer pathology may be associated with decreased ceramide and increased S1P. Thus, initial studies showed that human colon cancer samples had 50% less ceramide compared with adjacent normal colon mucosa (8). Furthermore, application of short-chain ceramides, ceramide analogs, or modulators of ceramide metabolism to different colon cancer cell lines induced rapid cell death (46). Moreover, both C16-pyridinium ceramide and B13 analog decreased tumor growth in a CT26 subcutaneous tumor mouse model (47) and completely prevented liver metastasis induced by colon cancer cells in nude mice, respectively (46). In contrast, mice null for alkaline sphingomyelinase (alk-SMase) (48) showed decreased ceramide and increased S1P in the small intestine and colon and had enhanced colonic tumorigenesis when treated with AOM/dextran sulfate sodium (DSS) (48). In contrast, SK1 expression was elevated in human colon adenocarcinomas compared with normal adjacent tissue (8), and this increase correlated with advanced tumor stage and increased mortality (10). The relevance of the SK1/S1P pathway in colon cancer was confirmed with mice null for SK1 for which treatment with AOM/DSS induced fewer ACFs but also reduced colon cancer development (9). The importance of S1P in colon cancer models has also been described in ApcMin/+ mice; polyps generated in this model have greater S1P compared with surrounding tissues. In addition, loss of SK1 in this model resulted in smaller tumors (49). Subsequent studies indicate that animals with epithelium-specific KO for SPL, which clears S1P, promoted susceptibility to AOM/DSS-induced tumor formation and crypt cell proliferation (50). Moreover, SK2-KO mice developed more and larger tumors when treated with AOM/DSS, and this was attributed to a reciprocal increase in SK1 (51).
Mammalian nCDase was first purified, characterized, and cloned by our group and identified as a novel nonlysosomal CDase (52). Functional studies have implicated the enzyme in response to stress stimuli and in regulating growth in various cellular models. Wu et al. (19) reported that the chemotherapeutic gemcitabine caused both a selective reduction in nCDase protein and a concomitant reduction in its activity in a polyoma middle T-transformed murine endothelial cell line. This was accompanied by an increase in specific very-long-chain ceramides, resulting in cell growth inhibition and cell cycle arrest at the G0/G1 phase. In rat mesangial cells, IL-1β treatment led to a biphasic increase of neutral SMase activity accompanied by delayed de novo synthesis of nCDase counteracting SMase-induced apoptosis (53). Long-term exposure of renal mesangial cells to nitric oxide resulted in less nCDase activity and increased SMase activity, and as a consequence, ceramide was increased significantly (54, 55). In addition, overexpression of nCDase in hepatocytes prevented TNF-α-induced apoptosis by reducing C16-ceramide and by activation of AKT through S1P generation. Moreover, overexpression of nCDase in vivo inhibited liver injury and hepatocyte apoptosis in mice treated with d-galactosamine and TNF-α by way of C16-ceramide reduction and S1P formation (22). Likewise, conversion of ceramide into SPH by acidic and/or neutral CDases protected keratinocytes from ultraviolet radiation B-induced apoptosis (21). Furthermore, in neuroblastoma cells, all-trans-retinoic acid induced ceramide accumulation, cell growth arrest, and differentiation accompanied by down-regulation of mRNA, protein, and activity of nCDase (20).
It is noteworthy that nCDase is highly expressed in the intestine (23), and recent studies have shown an increase in the protein levels of nCDase measured by immunohistochemistry in 8 of 11 human colon cancer samples compared with normal tissues (www.proteinatlas.org, Asah2 gen in cancer, www.proteinatlas.org/ENSG00000188611-ASAH2/cancer); however, its relevance in colon cancer has not been investigated. The current results suggest a critical role for the enzyme in colon cancer pathogenesis as supported from studies in cancer cell lines, xenografts, and in vivo models. Cell-based studies revealed a clear effect of down-regulation of nCDase in colon cancer cell growth inhibition. Indeed, most colon cancer cell lines evaluated, with the exception of SW620, were sensitive to inhibition of nCDase. Of note, Voelkel-Johnson and colleagues (56, 57) reported that SW620 cells are also resistant to the action of TNF-related apoptosis-inducing ligand in a ceramide-dependent manner, ascribing this to less endogenous ceramide synthesis because of reduced ceramide synthase 6. Thus, this cell type may represent a cell line with intrinsic resistance to ceramide-generating pathways of growth suppression.
In vivo, nCDase−/− mice have increased intestinal C16 ceramide and show defective intestinal digestion of dietary sphingolipids, underscoring the importance of this enzyme in the metabolism of dietary sphingolipids (23). However, on normal chow these mice were resistant to AOM-induced colon cancer, indicating that, in this model, colon cancer formation is nCDase dependent and probably the result of an increase of intestinal C16 ceramide. This result is profound in that nCDase activity is required for the full development of colon cancer, and this has several implications. First, it suggests that nCDase is important to early phases of colon cancer development in the AOM model. Data with respect to ACF formation partly support this conjecture; however, the effects of nCDase were intermediate: nCDase−/− mice had 50% fewer ACFs, suggesting a role for nCDase early in tumor initiation. However, the proposed role of ACFs as an early mediator of adenocarcinoma development in the AOM model is disputed (43, 58). Second, the significance of deletion of nCDase in colon cancer development, but not for normal intestine and colon development, suggests that oncogenically transformed cells become “addicted” to the action of nCDase. Accordingly, inhibition of nCDase causes substantial inhibition of growth only in transformed cells. These results are mechanistically supported by a significant decrease of c-myc and cyclin D1 (both target genes of the β-catenin/TCF-4 complex) in colon cancer cells, with no decrease observed in normal intestinal cells. Inactivation of the WNT/β-catenin pathway by ceramide has been shown by our group and others to occur in breast and colon cancer cells, respectively (59, 60). This effect of sphingolipids on β-catenin is relevant to colon cancer cell growth because β-catenin is critical for growth of colon cancer cells because suppression by siRNA decreases cell proliferation and increases apoptosis (61–63).
The results from this work also have implications for the advancement of nCDase as a potentially novel target for therapeutic developments to treat colon cancer. nCDase−/− mice do not have basal whole-animal or colon defects, suggesting that inhibition of the enzyme is not deleterious to growth and development of normal tissue. This is further supported by the minimal effects of inhibition of nCDase in noncancer intestinal cell lines and in vivo. Mechanistically, whereas nCDase inhibition induced a decrease in the activation of relevant pathways for colon cancer, such as WNT/β-catenin and ERK in colon cancer cells, these pathways were not affected in noncancerous cells.
The effect of nCDase inhibition in a xenograft model also supports the development of nCDase as a novel therapeutic target. Our data confirm increased tumor ceramide in animals treated with C6 urea-ceramide and that inhibition of nCDase delayed tumor growth and decreased tumor cell proliferation. Although inhibition of nCDase in colon cancer cells induced caspase3-mediated apoptosis, this effect was not observed in xenografts, suggesting that perhaps this death mechanism is not relevant for tumor growth delay or that the generated apoptotic cells are transitory and difficult to detect in vivo under the studied conditions. Thus, our results extend earlier studies implicating ceramide in tumor suppression in the colon and define nCDase as a specific vulnerability in the pathogenesis of CRC (46, 47, 64).
In summary, we have defined a previously unappreciated role for nCDase in colon cancer pathogenesis, and the results raise the distinct possibility that this sphingolipid enzyme may emerge as a novel target for colon cancer treatment.
ACKNOWLEDGMENTS
This work was supported by U.S. National Institutes of Health, National Cancer Institute Grants R01-CA172517 (to Y.A.H.), P01-CA97132 (to L.M.O.), and R01-CA172113 (to V.W.Y.). The authors thank the Lipidomics Core facility at the State University of New York at Stony Brook for lipid analysis, the Lipidomic Facility at the Medical University of South Carolina for providing the nCDase inhibitor, the Flow Cytometry Core facility for assistance with cell cycle analysis, the Research Core Histology Laboratory at Stony Brook University for technical assistance, and Dr. Takuji Tanaka (certified pathologist and toxicologist; Gifu Municipal Hospital, Gifu, Japan) for assistance with AOM-induced tumors diagnosis. The laboratory of Dr. Y.A.H. has research support from ONO Co., for a distinct project.
Glossary
- ACF
aberrant crypt focus
- AOM
azoxymethane
- Baf-1
bafilomycin A1
- BrdU
5-bromo-2-deoxyuridine
- CQ
chloroquine
- CRC
colorectal cancer
- DSS
dextran sodium sulfate
- KO
knockout
- MEF
mouse embryonic fibroblast
- MTT
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
- nCDase
neutral ceramidase
- S1P
sphingosine-1-phosphate
- siRNA
small interfering RNA
- SK
sphingosine kinase
- SM
sphingomyelin
- SMase
sphingomyelinase
- SPH
sphingosine
- SPL
sphingosine-1-phosphate lyase
- TCF-4
transcription factor 4
- WT
wild-type
Footnotes
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
AUTHOR CONTRIBUTIONS
M. García-Barros, N. Coant, V. W. Yang, L. M. Obeid, and Y. A. Hannun designed the research studies; M. García-Barros acquired and analyzed the data; A. J. Snider provided materials; M. García-Barros, N. Coant, T. Kawamori, M. Wada, A. J. Snider, J.-P. Truman, B. X. Wu, H. Furuya, C. J. Clarke, A. B. Bialkowska, and A. Ghaleb conducted the experiments; and M. García-Barros and Y. A. Hannun wrote the manuscript.
REFERENCES
- 1.Vogelstein B., Fearon E. R., Hamilton S. R., Kern S. E., Preisinger A. C., Leppert M., Nakamura Y., White R., Smits A. M., Bos J. L. (1988) Genetic alterations during colorectal-tumor development. N. Engl. J. Med. 319, 525–532 [DOI] [PubMed] [Google Scholar]
- 2.Arnold C. N., Goel A., Blum H. E., Boland C. R. (2005) Molecular pathogenesis of colorectal cancer: implications for molecular diagnosis. Cancer 104, 2035–2047 [DOI] [PubMed] [Google Scholar]
- 3.Hannun Y. A., Obeid L. M. (2011) Many ceramides. J. Biol. Chem. 286, 27855–27862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hannun Y. A. (1996) Functions of ceramide in coordinating cellular responses to stress. Science 274, 1855–1859 [DOI] [PubMed] [Google Scholar]
- 5.Nikolova-Karakashian M. N., Rozenova K. A. (2010) Ceramide in stress response. Adv. Exp. Med. Biol. 688, 86–108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stancevic B., Kolesnick R. (2010) Ceramide-rich platforms in transmembrane signaling. FEBS Lett. 584, 1728–1740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.El Alwani M., Wu B. X., Obeid L. M., Hannun Y. A. (2006) Bioactive sphingolipids in the modulation of the inflammatory response. Pharmacol. Ther. 112, 171–183 [DOI] [PubMed] [Google Scholar]
- 8.Kawamori T., Osta W., Johnson K. R., Pettus B. J., Bielawski J., Tanaka T., Wargovich M. J., Reddy B. S., Hannun Y. A., Obeid L. M., Zhou D. (2006) Sphingosine kinase 1 is up-regulated in colon carcinogenesis. FASEB J. 20, 386–388 [DOI] [PubMed] [Google Scholar]
- 9.Kawamori T., Kaneshiro T., Okumura M., Maalouf S., Uflacker A., Bielawski J., Hannun Y. A., Obeid L. M. (2009) Role for sphingosine kinase 1 in colon carcinogenesis. FASEB J. 23, 405–414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tan S. S., Khin L. W., Wong L., Yan B., Ong C. W., Datta A., Salto-Tellez M., Lam Y., Yap C. T. (2014) Sphingosine kinase 1 promotes malignant progression in colon cancer and independently predicts survival of patients with colon cancer by competing risk approach in South Asian population. Clin. Transl. Gastroenterol. 5, e51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Oskouian B., Saba J. (2007) Sphingosine-1-phosphate metabolism and intestinal tumorigenesis: lipid signaling strikes again. Cell Cycle 6, 522–527 [DOI] [PubMed] [Google Scholar]
- 12.Hertervig E., Nilsson A., Nyberg L., Duan R. D. (1997) Alkaline sphingomyelinase activity is decreased in human colorectal carcinoma. Cancer 79, 448–453 [PubMed] [Google Scholar]
- 13.Dillehay D. L., Webb S. K., Schmelz E. M., Merrill A. H. Jr (1994) Dietary sphingomyelin inhibits 1,2-dimethylhydrazine-induced colon cancer in CF1 mice. J. Nutr. 124, 615–620 [DOI] [PubMed] [Google Scholar]
- 14.Schmelz E. M., Dillehay D. L., Webb S. K., Reiter A., Adams J., Merrill A. H. Jr (1996) Sphingomyelin consumption suppresses aberrant colonic crypt foci and increases the proportion of adenomas versus adenocarcinomas in CF1 mice treated with 1,2-dimethylhydrazine: implications for dietary sphingolipids and colon carcinogenesis. Cancer Res. 56, 4936–4941 [PubMed] [Google Scholar]
- 15.Symolon H., Bushnev A., Peng Q., Ramaraju H., Mays S. G., Allegood J. C., Pruett S. T., Sullards M. C., Dillehay D. L., Liotta D. C., Merrill A. H. Jr (2011) Enigmol: a novel sphingolipid analogue with anticancer activity against cancer cell lines and in vivo models for intestinal and prostate cancer. Mol. Cancer Ther. 10, 648–657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.García-Barros M., Coant N., Truman J. P., Snider A. J., Hannun Y. A. (2014) Sphingolipids in colon cancer. Biochim. Biophys. Acta 1841, 773–782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ito M., Okino N., Tani M. (2014) New insight into the structure, reaction mechanism, and biological functions of neutral ceramidase. Biochim. Biophys. Acta 1841, 682–691 [DOI] [PubMed] [Google Scholar]
- 18.Novgorodov S. A., Wu B. X., Gudz T. I., Bielawski J., Ovchinnikova T. V., Hannun Y. A., Obeid L. M. (2011) Novel pathway of ceramide production in mitochondria: thioesterase and neutral ceramidase produce ceramide from sphingosine and acyl-CoA. J. Biol. Chem. 286, 25352–25362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wu B. X., Zeidan Y. H., Hannun Y. A. (2009) Downregulation of neutral ceramidase by gemcitabine: Implications for cell cycle regulation. Biochim. Biophys. Acta 1791, 730–739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tanaka K., Tamiya-Koizumi K., Hagiwara K., Ito H., Takagi A., Kojima T., Suzuki M., Iwaki S., Fujii S., Nakamura M., Banno Y., Kannagi R., Tsurumi T., Kyogashima M., Murate T. (2012) Role of down-regulated neutral ceramidase during all-trans retinoic acid-induced neuronal differentiation in SH-SY5Y neuroblastoma cells. J. Biochem. 151, 611–620 [DOI] [PubMed] [Google Scholar]
- 21.Uchida Y., Houben E., Park K., Douangpanya S., Lee Y. M., Wu B. X., Hannun Y. A., Radin N. S., Elias P. M., Holleran W. M. (2010) Hydrolytic pathway protects against ceramide-induced apoptosis in keratinocytes exposed to UVB. J. Invest. Dermatol. 130, 2472–2480 [DOI] [PubMed] [Google Scholar]
- 22.Osawa Y., Uchinami H., Bielawski J., Schwabe R. F., Hannun Y. A., Brenner D. A. (2005) Roles for C16-ceramide and sphingosine 1-phosphate in regulating hepatocyte apoptosis in response to tumor necrosis factor-alpha. J. Biol. Chem. 280, 27879–27887 [DOI] [PubMed] [Google Scholar]
- 23.Kono M., Dreier J. L., Ellis J. M., Allende M. L., Kalkofen D. N., Sanders K. M., Bielawski J., Bielawska A., Hannun Y. A., Proia R. L. (2006) Neutral ceramidase encoded by the Asah2 gene is essential for the intestinal degradation of sphingolipids. J. Biol. Chem. 281, 7324–7331 [DOI] [PubMed] [Google Scholar]
- 24.Bielawski J., Pierce J. S., Snider J., Rembiesa B., Szulc Z. M., Bielawska A. (2010) Sphingolipid analysis by high performance liquid chromatography-tandem mass spectrometry (HPLC-MS/MS). Adv. Exp. Med. Biol. 688, 46–59 [DOI] [PubMed] [Google Scholar]
- 25.Hernández-Corbacho M. J., Canals D., Adada M. M., Liu M., Senkal C. E., Yi J. K., Mao C., Luberto C., Hannun Y. A., Obeid L. M. (2015) Tumor necrosis factor-α (TNFα)-induced ceramide generation via ceramide synthases regulates loss of focal adhesion kinase (FAK) and programmed cell death. J. Biol. Chem. 290, 25356–25373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Garzotto M., White-Jones M., Jiang Y., Ehleiter D., Liao W. C., Haimovitz-Friedman A., Fuks Z., Kolesnick R. (1998) 12-O-tetradecanoylphorbol-13-acetate-induced apoptosis in LNCaP cells is mediated through ceramide synthase. Cancer Res. 58, 2260–2264 [PubMed] [Google Scholar]
- 27.Gandy K. A., Canals D., Adada M., Wada M., Roddy P., Snider A. J., Hannun Y. A., Obeid L. M. (2013) Sphingosine 1-phosphate induces filopodia formation through S1PR2 activation of ERM proteins. Biochem. J. 449, 661–672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kim J. H., Alfieri A. A., Kim S. H., Young C. W. (1986) Potentiation of radiation effects on two murine tumors by lonidamine. Cancer Res. 46, 1120–1123 [PubMed] [Google Scholar]
- 29.Bird R. P. (1987) Observation and quantification of aberrant crypts in the murine colon treated with a colon carcinogen: preliminary findings. Cancer Lett. 37, 147–151 [DOI] [PubMed] [Google Scholar]
- 30.Krutovskikh V. A., Turusov V. S. (1994) Tumours of the intestines. IARC Sci. Publ. 111, 195–221 [PubMed] [Google Scholar]
- 31.Usta J., El Bawab S., Roddy P., Szulc Z. M., Yusuf, Hannun A., Bielawska A. (2001) Structural requirements of ceramide and sphingosine based inhibitors of mitochondrial ceramidase. Biochemistry 40, 9657–9668 [DOI] [PubMed] [Google Scholar]
- 32.Hannun Y. A., Obeid L. M. (2008) Principles of bioactive lipid signalling: lessons from sphingolipids. Nat. Rev. Mol. Cell Biol. 9, 139–150 [DOI] [PubMed] [Google Scholar]
- 33.Pattingre S., Bauvy C., Levade T., Levine B., Codogno P. (2009) Ceramide-induced autophagy: to junk or to protect cells? Autophagy 5, 558–560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Blay J., Brown K. D. (1985) Functional receptors for epidermal growth factor in an epithelial-cell line derived from the rat small intestine. Biochem. J. 225, 85–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mizushima N., Yoshimori T. (2007) How to interpret LC3 immunoblotting. Autophagy 3, 542–545 [DOI] [PubMed] [Google Scholar]
- 36.Kung C. P., Budina A., Balaburski G., Bergenstock M. K., Murphy M. (2011) Autophagy in tumor suppression and cancer therapy. Crit. Rev. Eukaryot. Gene Expr. 21, 71–100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Venable M. E., Lee J. Y., Smyth M. J., Bielawska A., Obeid L. M. (1995) Role of ceramide in cellular senescence. J. Biol. Chem. 270, 30701–30708 [DOI] [PubMed] [Google Scholar]
- 38.Venable M. E., Yin X. (2009) Ceramide induces endothelial cell senescence. Cell Biochem. Funct. 27, 547–551 [DOI] [PubMed] [Google Scholar]
- 39.Castro M. E., Ferrer I., Cascón A., Guijarro M. V., Lleonart M., Ramón y Cajal S., Leal J. F., Robledo M., Carnero A. (2008) PPP1CA contributes to the senescence program induced by oncogenic Ras. Carcinogenesis 29, 491–499 [DOI] [PubMed] [Google Scholar]
- 40.Kraveka J. M., Li L., Bielawski J., Obeid L. M., Ogretmen B. (2003) Involvement of endogenous ceramide in the inhibition of telomerase activity and induction of morphologic differentiation in response to all-trans-retinoic acid in human neuroblastoma cells. Arch. Biochem. Biophys. 419, 110–119 [DOI] [PubMed] [Google Scholar]
- 41.Johnson R. L., Fleet J. C. (2013) Animal models of colorectal cancer. Cancer Metastasis Rev. 32, 39–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pretlow T. P., Pretlow T. G. (2005) Mutant KRAS in aberrant crypt foci (ACF): initiation of colorectal cancer? Biochim. Biophys. Acta 1756, 83–96 [DOI] [PubMed] [Google Scholar]
- 43.Rosenberg D. W., Giardina C., Tanaka T. (2009) Mouse models for the study of colon carcinogenesis. Carcinogenesis 30, 183–196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tai C. J., Chang C. C., Jiang M. C., Yeh C. M., Su T. C., Wu P. R., Chen C. J., Yeh K. T., Lin S. H., Chen H. C. (2012) Clinical-pathological correlation of K-Ras mutation and ERK phosphorylation in colorectal cancer. Pol. J. Pathol. 63, 93–100 [PubMed] [Google Scholar]
- 45.Brabletz T., Herrmann K., Jung A., Faller G., Kirchner T. (2000) Expression of nuclear beta-catenin and c-myc is correlated with tumor size but not with proliferative activity of colorectal adenomas. Am. J. Pathol. 156, 865–870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Selzner M., Bielawska A., Morse M. A., Rüdiger H. A., Sindram D., Hannun Y. A., Clavien P. A. (2001) Induction of apoptotic cell death and prevention of tumor growth by ceramide analogues in metastatic human colon cancer. Cancer Res. 61, 1233–1240 [PubMed] [Google Scholar]
- 47.Dahm F., Bielawska A., Nocito A., Georgiev P., Szulc Z. M., Bielawski J., Jochum W., Dindo D., Hannun Y. A., Clavien P. A. (2008) Mitochondrially targeted ceramide LCL-30 inhibits colorectal cancer in mice. Br. J. Cancer 98, 98–105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chen Y., Zhang P., Xu S. C., Yang L., Voss U., Ekblad E., Wu Y., Min Y., Hertervig E., Nilsson Å., Duan R. D. (2015) Enhanced colonic tumorigenesis in alkaline sphingomyelinase (NPP7) knockout mice. Mol. Cancer Ther. 14, 259–267 [DOI] [PubMed] [Google Scholar]
- 49.Kohno M., Momoi M., Oo M. L., Paik J. H., Lee Y. M., Venkataraman K., Ai Y., Ristimaki A. P., Fyrst H., Sano H., Rosenberg D., Saba J. D., Proia R. L., Hla T. (2006) Intracellular role for sphingosine kinase 1 in intestinal adenoma cell proliferation. Mol. Cell. Biol. 26, 7211–7223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Degagné E., Pandurangan A., Bandhuvula P., Kumar A., Eltanawy A., Zhang M., Yoshinaga Y., Nefedov M., de Jong P. J., Fong L. G., Young S. G., Bittman R., Ahmedi Y., Saba J. D. (2014) Sphingosine-1-phosphate lyase downregulation promotes colon carcinogenesis through STAT3-activated microRNAs. J. Clin. Invest. 124, 5368–5384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Liang J., Nagahashi M., Kim E. Y., Harikumar K. B., Yamada A., Huang W. C., Hait N. C., Allegood J. C., Price M. M., Avni D., Takabe K., Kordula T., Milstien S., Spiegel S. (2013) Sphingosine-1-phosphate links persistent STAT3 activation, chronic intestinal inflammation, and development of colitis-associated cancer. Cancer Cell 23, 107–120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.El Bawab S., Bielawska A., Hannun Y. A. (1999) Purification and characterization of a membrane-bound nonlysosomal ceramidase from rat brain. J. Biol. Chem. 274, 27948–27955 [DOI] [PubMed] [Google Scholar]
- 53.Franzen R., Pautz A., Bräutigam L., Geisslinger G., Pfeilschifter J., Huwiler A. (2001) Interleukin-1beta induces chronic activation and de novo synthesis of neutral ceramidase in renal mesangial cells. J. Biol. Chem. 276, 35382–35389 [DOI] [PubMed] [Google Scholar]
- 54.Huwiler A., Pfeilschifter J., van den Bosch H. (1999) Nitric oxide donors induce stress signaling via ceramide formation in rat renal mesangial cells. J. Biol. Chem. 274, 7190–7195 [DOI] [PubMed] [Google Scholar]
- 55.Franzen R., Fabbro D., Aschrafi A., Pfeilschifter J., Huwiler A. (2002) Nitric oxide induces degradation of the neutral ceramidase in rat renal mesangial cells and is counterregulated by protein kinase C. J. Biol. Chem. 277, 46184–46190 [DOI] [PubMed] [Google Scholar]
- 56.Voelkel-Johnson C., Hannun Y. A., El-Zawahry A. (2005) Resistance to TRAIL is associated with defects in ceramide signaling that can be overcome by exogenous C6-ceramide without requiring down-regulation of cellular FLICE inhibitory protein. Mol. Cancer Ther. 4, 1320–1327 [DOI] [PubMed] [Google Scholar]
- 57.White-Gilbertson S., Mullen T., Senkal C., Lu P., Ogretmen B., Obeid L., Voelkel-Johnson C. (2009) Ceramide synthase 6 modulates TRAIL sensitivity and nuclear translocation of active caspase-3 in colon cancer cells. Oncogene 28, 1132–1141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wargovich M. J., Brown V. R., Morris J. (2010) Aberrant crypt foci: the case for inclusion as a biomarker for colon cancer. Cancers (Basel) 2, 1705–1716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Marchesini N., Jones J. A., Hannun Y. A. (2007) Confluence induced threonine41/serine45 phospho-beta-catenin dephosphorylation via ceramide-mediated activation of PP1cgamma. Biochim. Biophys. Acta 1771, 1418–1428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Schmelz E. M., Roberts P. C., Kustin E. M., Lemonnier L. A., Sullards M. C., Dillehay D. L., Merrill A. H. Jr (2001) Modulation of intracellular beta-catenin localization and intestinal tumorigenesis in vivo and in vitro by sphingolipids. Cancer Res. 61, 6723–6729 [PubMed] [Google Scholar]
- 61.Verma U. N., Surabhi R. M., Schmaltieg A., Becerra C., Gaynor R. B. (2003) Small interfering RNAs directed against beta-catenin inhibit the in vitro and in vivo growth of colon cancer cells. Clin. Cancer Res. 9, 1291–1300 [PubMed] [Google Scholar]
- 62.Roh H., Green D. W., Boswell C. B., Pippin J. A., Drebin J. A. (2001) Suppression of beta-catenin inhibits the neoplastic growth of APC-mutant colon cancer cells. Cancer Res. 61, 6563–6568 [PubMed] [Google Scholar]
- 63.Green D. W., Roh H., Pippin J. A., Drebin J. A. (2001) Beta-catenin antisense treatment decreases beta-catenin expression and tumor growth rate in colon carcinoma xenografts. J. Surg. Res. 101, 16–20 [DOI] [PubMed] [Google Scholar]
- 64.Morad S. A., Cabot M. C. (2013) Ceramide-orchestrated signalling in cancer cells. Nat. Rev. Cancer 13, 51–65 [DOI] [PubMed] [Google Scholar]