Abstract
IGF-binding protein-3 (IGFBP-3) is a liver-derived, anti-inflammatory molecule that is decreased in obesity, a key risk factor for nonalcoholic fatty liver disease (NAFLD). It was not known whether IGFBP-3 levels were altered in NAFLD, whether such alterations could be the result of lipotoxicity, and whether altered IGFBP-3 could affect pathways that are involved in hepatic and systemic inflammation. Serum IGFBP-3 was decreased in patients with NAFLD, whereas liver and circulating IL-8 levels were increased. Palmitate inhibited IGFBP-3 secretion by THP-1 macrophages and enhanced IL-8 expression. Exposure of palmitate-treated THP-1 macrophages to IGFBP-3–deficient conditioned medium led to a 20-fold increase in palmitate-induced IL-8 expression by hepatocytes. Conversely, overexpression of IGFBP-3 suppressed JNK and NF-κB activation and blocked palmitate-induced IL-8 expression in hepatocytes. Silencing IGFBP-3 in Huh7 cells enhanced JNK and NF-κB activity and increased palmitate-induced IL-8 secretion. These data indicate that IGFBP-3 serves as an anti-inflammatory brake in hepatocytes against JNK and NF-κB and limits their activation and downstream production of proinflammatory cytokines. Under lipotoxic conditions, palmitate inhibits hepatic macrophage secretion of IGFBP-3, thereby releasing the brake and enhancing palmitate-induced IL-8 synthesis and secretion.—Min, H.-K., Maruyama, H., Jang, B. K., Shimada, M., Mirshahi, F., Ren, S., Oh, Y., Puri, P., Sanyal, A. J. Suppression of IGF binding protein-3 by palmitate promotes hepatic inflammatory responses.
Keywords: NAFLD, nonalcoholic steatohepatitis, lipotoxicity, interleukin-8, IGFBP-3
IGFs are growth-promoting peptides that are members of the insulin superfamily of growth-promoting peptides (1, 2). A major difference between insulin and IGF is the binding of IGFs to specific circulating proteins, called IGF binding proteins (IGFBPs), that reduce the availability of IGF to tissues and, thus, modulate their biologic effects (3, 4). IGFBP-3 is the most abundant IGFBP and is synthesized almost exclusively by Kupffer cells (5). Recently, IGFBPs have been shown to be present inside cells and to have direct biologic effects on cellular pathways that are not related to their binding to IGFs (6, 7). IGFBPs have been shown to modulate tumor signaling pathways (8, 9). IGF/IGF-1 receptor–independent anti-inflammatory and antitumor actions of IGFBP-3 have also been demonstrated in a variety of human diseases, including asthma, other metabolic diseases, and cancer (6, 10, 11).
Nonalcoholic fatty liver disease (NAFLD) is the most common cause of chronic liver disease and affects approximately 30% of the adult population (12, 13). Nonalcoholic steatohepatitis (NASH), the aggressive form of NAFLD, affects 3–4% of the population and progresses to cirrhosis in 15–20% of affected individuals (13, 14). NASH is closely linked to insulin resistance and type 2 diabetes mellitus (15, 16). IGFBP-3 levels have been reported to be decreased in a single study of type 2 diabetes mellitus (17), and IGFBP-3 has also been reported to reduce glucose uptake by interfering with insulin signaling in a tissue-specific manner in adipocytes (11, 18). However, recent studies have shown that IGFBP-3 inhibits TNF-α–induced NF-κB activity, thereby restoring insulin signaling and negating TNF-α–induced inhibition of glucose uptake in human primary adipocytes (19).
The relationship between the histologic pattern of NAFLD and IGFBP-3 is not known. Activation of the innate immune system and downstream inflammatory pathways are considered to play an important role in the progression of NASH to cirrhosis (20, 21). Toxicity from increased circulating saturated fatty acids, for example, palmitate, is also considered to play an important role in promoting inflammation in NASH (22, 23). Some key proinflammatory cytokines synthesized in the liver in response to palmitate include IL-8, IL-6, and TNF-α (24, 25). The roles of altered IGFBP-3 on inflammatory responses in NASH are not known, nor are the potential effects of lipotoxicity by palmitate on IGFBP-3 synthesis and secretion. Furthermore, cross-talk between palmitate, IGFBP-3, and synthesis of cytokines related to the innate immune system is not unknown.
The overall goal of this study was to begin to define if and how IGFBP-3 affected cellular pathways that are considered relevant for NASH-related hepatic inflammatory responses. Aims were to define: 1) whether NAFLD, and especially NASH, was associated with altered IGFBP-3 levels; 2) the effects of palmitate on IGFBP-3 synthesis by hepatic macrophages; 3) the effects of IGFBP-3 on intracellular proinflammatory pathways; 4) the effects of altered IGFBP-3 synthesis on secretion of inflammatory cytokines by hepatocytes and macrophages; and 5) the potential mechanisms by which IGFBP-3 alters cytokine production by hepatocytes.
MATERIALS AND METHODS
Reagents
Rabbit anti–poly (ADP ribose) polymerase, rabbit anti–IκB-α, rabbit anti–p-NF-κB, rabbit anti–p-JNK, rabbit anti–t-JNK, rabbit anti-IKK2, rabbit anti–p-ERK, rabbit anti–t-ERK, recombinant IGFBP-3 protein, signal-silence control small interfering RNA (siRNA), signal-silence IKK2 siRNA I, SP600125, and ERK inhibitor PD98059 were purchased from Cell Signaling Technology (Beverly, MA, USA). Anti–β-actin antibody, RIPA buffer, phorbol 12-myristate 13-acetate (PMA), protease inhibitor mixture, cryovials, and free fatty acid–free bovine serum albumin (BSA) were purchased from Sigma-Aldrich (St. Louis, MO, USA). Primary antibodies, rabbit anti–IGFBP-3 and rabbit anti–NF-κB were purchased from Santa Cruz Biotechnology (Dallas, TX, USA). Horseradish peroxidase–conjugated secondary antibody and SuperSignal chemiluminescence kits were purchased from Pierce Biotechnology (Rockford, IL, USA). Cell culture medium, insulin, Trizol, Opti-MEM medium, Lipofectamine 2000, BSA, and Western blot supplies were obtained from Thermo Fisher Scientific (Waltham, MA, USA).
Human study population
Four groups of subjects were enrolled in this study: 1) lean healthy normal controls, 2) obese controls without NAFLD, 3) patients with nonalcoholic fatty liver (NAFL), and 4) patients with NASH. Lean normal (body mass index < 25) and obese (body mass index >30) control patients were defined by normal liver histology, liver enzymes, and function. Liver histology was evaluated by a single experienced liver pathologist. Fatty liver was defined by the presence of >5% steatosis. Steatohepatitis was defined by the presence of predominantly macrovesicular steatosis, inflammation, and cytologic ballooning with or without Mallory’s hyaline or pericellular fibrosis with a zone III distribution (26). The nonalcoholic nature of disease was established clinically by using a daily intake cutoff of <20 g/d of alcohol for females and <30 g/d for males within the past 5 yr, as used in prior studies (27).
Exclusion criteria for the study included failure to obtain informed consent, concurrent pregnancy, presence of advanced liver fibrosis (stage 3 or 4 disease), liver failure (elevated bilirubin or INR > 1.4), >5% weight gain or loss within prior 3 mo, HIV infection, concomitant presence of other liver diseases (e.g., hepatitis B or C, hemochromatosis, Wilson disease, autoimmune hepatitis, primary biliary cirrhosis, and α1-antitrypsin deficiency), and use of drugs known to affect lipid metabolism (e.g., statins, fibrates, polyunsaturated fatty acids, etc.) or impact NASH (e.g., vitamin E, thiazolidinediones, and pentoxifylline). Concurrent patient with metabolic syndrome and suspected NAFLD were considered for this study before a clinically indicated liver biopsy. NAFLD was suspected from one or more of the following: hepatomegaly, elevated liver enzymes, and abnormal hepatic imaging. In addition, asymptomatic patients with normal liver enzymes and functions and normal hepatic ultrasound who underwent elective abdominal surgery consented to provide liver biopsies for the control group. Liver tissues for control groups were also obtained from an anonymous tissue repository at the investigators’ institution. Liver biopsy was performed via a percutaneous route by using a 16-gauge needle device. For those who underwent a biopsy during surgery, the biopsy was obtained at the beginning of the operation before the gut was manipulated. A 2-cm core was sent for histologic assessment, whereas the rest was snap frozen in liquid nitrogen and stored at −80°C for analysis in 0.5-cm segments in separate cryovials. All participants provided informed consent, and the study was approved by the Virginia Commonwealth Institute Institutional Review Board (#1960).
Serum sample collection and ELISA assay
Participants underwent a peripheral venipuncture collection of a 10-ml blood sample in red topped tubes. The blood sample was centrifuged and the serum fraction was isolated. Serum samples were frozen at −80°C for ELISA analysis. Human IL-8, IGF-1, and human IGFBP-3 ELISA kits were purchased from Thermo Fisher Scientific and were measured in serum samples by highly specific ELISA kits according to manufacturer instructions.
Cell cultures and treatments
Frozen vials (2 × 106 cells/ml; HemaCare, Van Nuys, CA, USA) of M1 or M2 macrophages were derived from purified monocytes. In brief, CD14 monocytes were positively selected via immunomagnetic beads. M1 macrophages were cultured in the presence of granulocyte-macrophage colony-stimulating factor (20 ng/ml) for 10 d, and M2 macrophages were cultured in the presence of macrophage colony-stimulating factor (10 ng/ml) for 12 d. M1 and M2 macrophages were confirmed by morphology and surface marker expression of specific biomarkers, which were monitored by flow cytometry. Both macrophages were incubated in X-VIVO 15 serum-free medium overnight and then treated in the presence or absence of 0.25 or 0.5 mM palmitate for 8 h.
Human Huh-7 and THP-1 cell lines were grown in DMEM and RPMI 1680, respectively, that contained 10% fetal bovine serum, 100 IU/ml penicillin, and 100 μg/ml of streptomycin in CO2 incubator at 37°C. THP-1 cells were exposed to PMA, and both CD14 and CD68 marker for Kupffer cells were confirmed (28). THP-1 cells (0.8 × 106 cells) were differentiated into macrophages in 6-well plates that contained 2.5 ml RPMI 1640 medium and 10 or 20 ng/ml PMA, then incubated for 2 d as previously described (28). The 6-well plates were washed 3 times with PBS to remove unattached cells. Before palmitate (C16:0) treatment, 100 mM palmitate solution preheated to 60°C was slowly dissolved in a 50°C preheated BSA solution (essentially fatty acid free; Sigma-Aldrich) (29). Palmitate-BSA complex solutions were freshly diluted in DMEM without serum to a final concentration of 0.5 mM palmitate/1% BSA. Cells were treated with palmitate-BSA complex solution after overnight serum deprivation. Palmitate-BSA complexes were incubated in heat block at 40°C for 30 min before cell exposure. After incubation for 12 h, media were replaced by palmitate-free medium for another 12 h, after which cells and supernatant were analyzed. For the IGFBP-3 protein treatment, Huh-7 cells were incubated in DMEM serum-free medium overnight and treated with 0.5 or 1.0 µg/ml recombinant IGFBP-3 protein for 1 h, then treated in the presence or absence of 0.5 mM palmitate for 12 h.
Adenovirus-mediated gene transduction
Adenoviral vector that expresses IGFBP-3 (Ad-IGFBP-3; provided by Y.O.), silencing IGFBP-3 (Ad-shIGFBP-3), or control GFP (Ad-GFP) were purchased from Vector BioLabs (Malvern, PA, USA). In brief, for infection of Ad-shIGFBP-3 or Ad-GFP in PMA-activated THP-1 macrophages, 8 × 105 cells/well were seeded onto 6-well plates that contained RPMI 1640 medium and were allowed to adhere for 2 d with PMA, then cells were infected with multiplicity of infection (MOI) of Ad-shIGFBP-3 (100 MOI) or Ad-GFP (1 MOI) for 24 h. For Huh-7 cells, 5 × 105 cells were seeded onto 6-well plates that contained DMEM medium. The following day, cells were infected with MOI of Ad-IGFBP-3 (100 MOI) or Ad-GFP (1 MOI) for 24 h.
siRNA transfection
Cells (5 × 105) were plated to 50% confluency and were transfected with 100 nM control siRNA or 100 nM IKK2 siRNA by Lipofectamine 2000 in Opti-MEM medium (Thermo Fisher Scientific) according to manufacturer protocol. Transfection efficiency was monitored with fluorescein-labeled nontargeted siRNA control (Thermo Fisher Scientific). siRNA-treated cells were incubated for 12 h, then treated in the presence or absence of palmitate (0.5 mM) for 8 h. After incubation, cells were harvested and lysed for analysis.
RNA extraction and gene expression analysis by real-time PCR
Primers specific for human IL-8 and glyceraldehyde 3-phosphate dehydrogenase (GAPDH) were as follows: human IL-8: forward, 5′-CTAGGACAAGAGCCAGGAAG-3′; reverse, 5′-GGTGGAAAGGTTTGGA GTATG-3′; and GAPDH: forward, 5′-ACAGTCAGCCGCATCTTC-3′; reverse, 5′-CTCCGACCTTCACCTTCC-3′ [designed from gene sequences (GenBank/European Molecular Biology Laboratory, National Center for Biotechnology Information, Bethesda, MD, USA; http://www.ncbi.nlm.nih.gov/genbank/) as previously described and prepared by the Nucleic Acid Core Facility at Virginia Commonwealth University (30)]. TaqMan probes for GAPDH and IGFBP-3 were purchased from Thermo Fisher Scientific (assay identification Hs04420697_g1 for GAPDH and Hs00365742_g1 for IGFBP-3). Total RNA from human liver samples and cell cultures was extracted by using a commercial RNA isolation kit (Trizol) according to manufacturer instructions. In addition, samples were treated with RNase-free DNase to remove genomic DNA, and cDNA synthesis was performed as previously described (30). RT-PCR was performed by using SYBR Green PCR master mix on a CFX 96 Real-Time System ABI Prism 7300 Sequence Detection System (Bio-Rad, Hercules, CA, USA) using the following protocol: 50°C for 2 min, then 95°C for 10 min, followed by 40 cycles of amplification (95°C for 15 s; 60°C for 30 s; 80°C for 30 s). GAPDH was used as the endogenous normalizer. Ct values were normalized to GAPDH and comparative quantification of target mRNA was done by using the ∆∆Ct method with integrated software with CFX Manager software (version 2.1; Bio-Rad).
Protein extraction and Western blot analysis
Human cell lines were lysed by using RIPA lysis buffer, and cell lysates were microcentrifuged at 12,000 g at 4°C for 10 min. Western blot analysis was performed as described previously (31). In brief, sample proteins were electrophoretically separated by using 4–12% NuPAGE Novex Bis-Tris Mini Gels and were transferred to a nitrocellulose membrane for 1 h at 40 V using a Western blot apparatus. Membrane was blocked for 2 h in 5% nonfat dry milk in Tris-buffered saline–Tween 20 buffer at room temperature. Primary antibodies were incubated overnight at 4°C and then removed. Membrane was washed 3 times (5 min each) with Tris-buffered saline, 0.1% Tween 20. Membranes were then incubated with horseradish peroxidase–conjugated secondary antibody and were detected by using the SuperSignal chemiluminescence kit. All immunoblots were scanned by using a model Fluorchem M imaging system (ProteinSimple, San Jose, CA, USA). Densitometry analysis for the expression of proteins was performed by using ImageJ software (National Institutes of Health, Bethesda, MD, USA). Protein levels were normalized for β-actin or total protein as appropriate.
Statistical analysis and sample size estimation
Sample size estimations were performed by using nQuery Advisor 7.0 (Statistical Solutions, Cork, Ireland). To test the hypothesis that change occurred incrementally from controls to NAFL to NASH with an effect size (mean levels of controls vs. disease) of 1.25 for NAFL and 1.5 for NASH, and assuming an sd of 25% for each of the groups and a power of 80% to detect changes at P = 0.05, a total of 6 patients would be required in each arm. If the effect size was a 2- and 1.5-fold change in NASH and NAFL, respectively, the sample size dropped to 3 patients in each arm. Not knowing the exact sd for each of the biologic parameters to be measured, a minimum sample size of 10–12 patients in each group was planned. Western blot data for phosphorylated proteins were expressed as phosphorylated to total protein, whereas data for unphosphorylated proteins were normalized with β-actin. RNA and protein levels for a given gene were compared across groups by using Kruskal-Wallis ANOVA, a distribution-free test. Dunn’s post hoc test was used for multiple comparisons. Significance was set at P = 0.05.
RESULTS
IGFBP-3 and IGF-1 are decreased in NAFLD
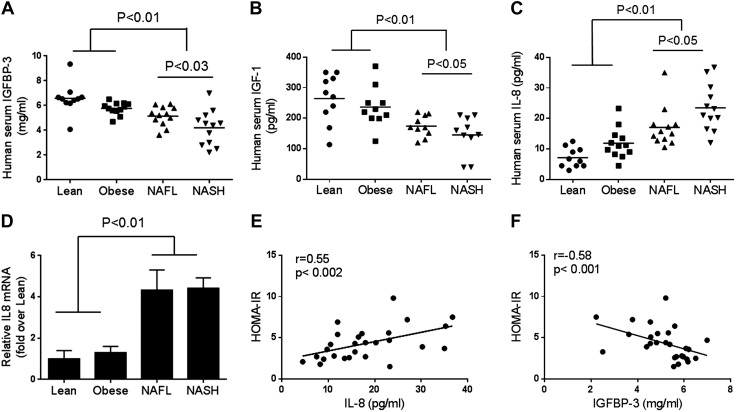
A total of 12 patients each with NASH or NAFL were compared with 10 lean and 12 obese controls with normal liver histology. The summary of demographic, clinical, and laboratory data is shown in Table 1. Groups were well matched with respect to age, gender, and racial distribution. As expected, patients with NAFL or NASH had higher alanine aminotransferase levels than in obese and lean controls (P < 0.008). Hepatic synthetic functions were normal across all groups. There was a progressive decrease in mean free IGFBP-3 levels in circulation, from lean and obese controls to patients with NAFL, then to patients with NASH (Fig. 1A; P < 0.01). Mean levels were comparable in both control groups and were higher than those in patients with either NAFL or NASH; levels in patients with NASH were lower than in those with NAFL (Fig. 1A; P < 0.03). This was accompanied by a similar decrease in levels of free IGF-1 (Fig. 1B; P < 0.01), the principal IGF that binds IGFBP-3 (17, 32).
TABLE 1.
Baseline demographic, clinical, and laboratory data
| Parameter | Lean normal, n = 10 | Obese normal, n = 12 | NAFL, n = 12 | NASH, n = 12 | P |
|---|---|---|---|---|---|
| Age (yr) | 52.1 ± 4.0 | 49.8 ± 11.0 | 49.9 ± 14.1 | 57.4 ± 10.3 | n.s. |
| Male-to-female ratio (n) | 5:5 | 2:10 | 4:8 | 5:7 | n.s. |
| Caucasian (%) | 65 | 60 | 75 | 100 | n.s. |
| BMI (kg/m2) | 24.8 ± 2.3 | 41.6 ± 6.3 | 37.1 ± 7.0 | 41.8 ± 5.4 | <0.0001a |
| Type 2 diabetes mellitus (n) | 0 | 3 | 4 | 4 | n.s.b |
| Hypertension (n) | 0 | 5 | 6 | 6 | 0.02a,b |
| AST (IU/L) | 24.5 ± 6.2 | 28.2 ± 9.5 | 30.8 ± 14.7 | 41.7 ± 17.8 | <0.005c |
| ALT (IU/L) | 26.3 ± 15.3 | 49.4 ± 31.5 | 70.1 ± 44.4 | 80.1 ± 16.8 | <0.008c |
| Alkaline phosphate (IU/L) | 87 ± 24 | 90 ± 9 | 97 ± 18 | 101 ± 21 | n.s. |
| Bilirubin (mg/dl) | 0.2 ± 0.09 | 0.3 ± 0.1 | 0.2 ± 0.1 | 0.3 ± 0.1 | n.s. |
| Albumin (g/dl) | 4.1 ± 0.4 | 4 ± 0.4 | 3.9 ± 0.3 | 4 ± 0.3 | n.s. |
| Fasting blood sugar (mg/dl) | 90 ± 9 | 95 ± 8 | 98 ± 11 | 99 ± 12 | n.s. |
| Fasting insulin (uIU/dl) | 6.5 ± 3 | 16.2 ± 9 | 20 ± 11 | 22 ± 7 | <0.005a |
| Hemoglobin A1C (%) | 5.3 ± 0.6 | 6.1 ± 1.3 | 6.2 ± 2.1 | 6.5 ± 1.4 | n.s. |
| Total cholesterol (mg/dl) | 195.6 ± 31.2 | 199.0 ± 51.1 | 206.6 ± 70.7 | 216.0 ± 28.9 | n.s. |
| LDL cholesterol (mg/dl) | 107.4 ± 19.4 | 116.6 ± 38.5 | 109.8 ± 57.3 | 139.8 ± 17.8 | <0.01a |
| HDL cholesterol (mg/dl) | 56.3 ± 14.8 | 50.0 ± 7.6 | 43.6 ± 13.7 | 43.6 ± 8.8 | <0.05c |
| Triglycerides (mg/dl) | 97.7 ± 38.7 | 102.8 ± 48.2 | 171.8 ± 81.6 | 211.3 ± 44.7 | <0.01a |
Data are given as mean ± sd unless otherwise noted. ALT, alanine aminotransferase; AST, aspartate aminotransferase; BMI, body mass index; n.s., not significant. aLean vs. other groups. bχ2 for trend. cNAFL or NASH vs. either control group.
Figure 1.
Human serum IGFBP-3, IGF-1, plasma IL-8, and hepatic IL-8 mRNA levels. IGFBP-3 and IGF-1 levels were examined in patients with NAFL and NASH (n = 12 each) and were compared with lean (n = 10) and weight-matched controls (n = 12) with normal liver histology. A, B) Serum IGFBP-3 (A) and IGF-1 (B) decreased progressively from lean normal to obese controls, to patients with NAFL, then to those with NASH (controls vs. NAFL or NASH; P < 0.01). C, D) Circulating IL-8 protein (C) and hepatic IL-8 mRNA (D) were increased in both patients with NAFL and NASH compared with lean and obese controls (P < 0.01). D, E) Circulating IL-8 was directly related to homeostatic model assessment–insulin resistance (HOMA-IR; E), whereas IGFBP-3 was inversely related to HOMA-IR (F). Individual data points or means ± sd are shown.
Circulating IL-8 levels are inversely related to IGFBP-3
In contrast to IGFBP-3, levels of circulating IL-8, a largely liver-derived proinflammatory cytokine (33), increased progressively from lean normal to obese controls, to patients with NAFL, then to those with NASH (Fig. 1C; P < 0.01). Levels in both those with NAFL and with NASH were significantly higher than in either control group. In addition, the levels in those with NASH were higher than in those with NAFL (P < 0.05). Liver tissue expression of IL-8 mRNA was also significantly higher in those with NAFL or NASH compared with either control group (Fig. 1D; P < 0.01). IL-8 and IGFBP-3 levels were directly and indirectly, respectively, related to homeostatic model assessment–insulin resistance (Fig. 1E, F).
Palmitate suppresses IGFBP-3 protein secretion in PMA-activated THP-1 macrophages
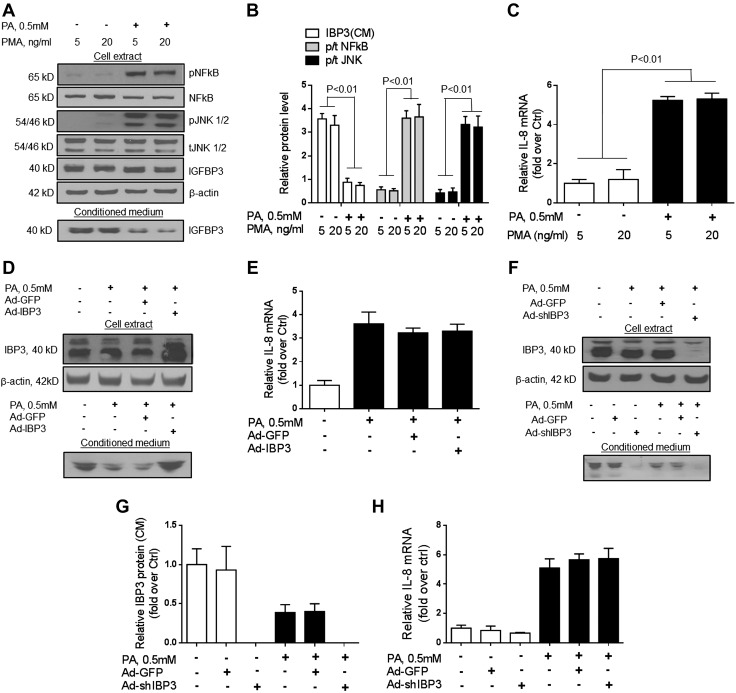
Exposure to palmitate (0.5 mM) led to a significant decrease in IGFBP-3 protein secretion into the culture medium by PMA-activated THP-1 macrophages (Fig. 2A, B; P < 0.01), but intracellular IGFBP-3 protein levels were unchanged (Fig. 2A). This was not affected by the concentration of PMA (5 or 20 ng/ml) in the medium. The suppression of IGFBP-3 by palmitate in PMA-activated THP-1 macrophages was accompanied by a significant increase in both NF-κB and JNK activation (Fig. 2A, B; P < 0.01). IGFBP-3 mRNA was minimally expressed in both peripheral monocyte-derived type I (M1) and type II (M2) macrophages regardless of palmitate treatment (data not shown).
Figure 2.
Effects of palmitate (PA) and PMA on production of IGFBP-3 (IBP3) and IL-8 in THP-1 cells. A, B) Palmitate activated JNK and NF-κB as decreased secretion of IGFBP-3 into the medium without affecting its intrahepatic levels. C) This was accompanied by increased IL-8 mRNA expression. PMA had no effect on IL-8 expression. D, E) Overexpression of IGFBP-3 using an adenovirus vector (D) did not impact palmitate-induced IL-8 mRNA expression in PMA-activated THP-1 macrophages (E). F–H) Conversely, after silencing of IGFB-P3, there was no change in palmitate-induced IL-8 mRNA expression in PMA-activated THP-1 macrophages. This indicates that palmitate reduces IGFBP-3 secretion by PMA-activated THP-1 macrophages. However, this is not linked to palmitate-induced IL-8 mRNA expression in these cells. Ctrl, control. Representative blots and means of 3 experiments are shown for graphical data.
Palmitate enhances IL-8 mRNA expression by PMA-activated THP-1 macrophages in an IGFBP-3–independent manner
After incubation with palmitate (0.5 mM), there was a significant increase in IL-8 mRNA expression by PMA-activated THP-1 macrophages, whereas IGFBP-3 protein secretion was decreased (Fig. 2A–C). To determine whether the increase in IL-8 mRNA by macrophages was linked to decreased IGFBP-3 secretion, the ability to abrogate palmitate-induced increases in IL-8 mRNA by overexpression of IGFBP-3 (Ad-IGFBP-3) was assessed (Fig. 2D, E). Ad-IGFBP-3 did not block palmitate-induced increase in IL-8 mRNA (Fig. 2E). Furthermore, silencing IGFBP-3 in PMA-activated THP-1 macrophages led to a nonsignificant decrease in IL-8 mRNA under baseline conditions and did not augment palmitate-induced IL-8 mRNA increase (Fig. 2F–H). These data indicate that the palmitate-induced increase in IL-8 synthesis by PMA-activated THP-1 macrophages is independent of IGFBP-3 suppression by palmitate.
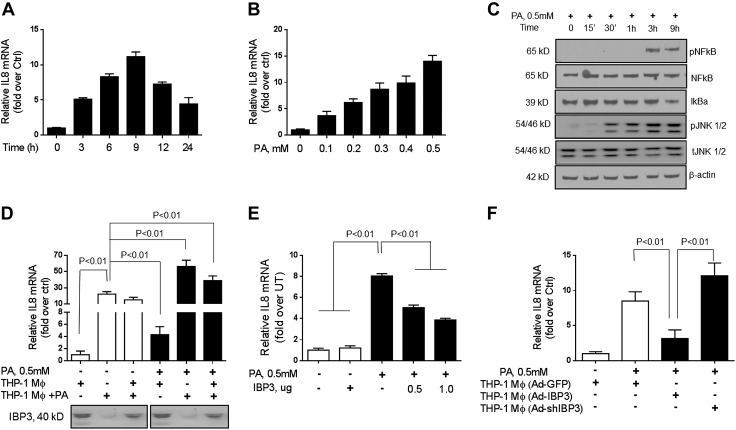
Conditioned media from palmitate-treated, but not untreated, PMA-activated THP-1 macrophages increase hepatocyte IL-8 mRNA expression
Palmitate (0.5 mM) increased IL-8 mRNA expression in Huh-7 cells with a maximal increase at 9 h (Fig. 3A). This effect was dose dependent from 0.1 to 0.5 mM palmitate (Fig. 3B). This was accompanied by increased phosphorylation of NF-κB and JNK; however, IκB-α levels were decreased (Fig. 3C). Next, to evaluate the effects of macrophage-derived IGFBP-3 on hepatocyte IL-8 secretion, both in the presence and absence of palmitate, Huh-7 cells were exposed to conditioned media from PMA-activated THP-1 macrophages in the presence or absence of palmitate in the hepatocyte medium. Conditioned media were obtained from macrophages with or without prior palmitate pretreatment. IGFBP-3 protein was abundant in conditioned media from macrophages under baseline conditions, whereas media from macrophages that were pretreated with palmitate had little detectable IGFBP-3 (Fig. 3D, lanes 2 and 5). After 12 h of incubation, IL-8 mRNA expression was measured in hepatocytes. Low levels of IL-8 mRNA expression were seen upon exposure of Huh-7 cells to PMA-activated THP-1 macrophage conditioned medium under basal conditions (Fig. 3D, lane 4). In contrast, addition of IGFBP-3–deficient conditioned medium from palmitate-treated THP-1 cells led to a 20-fold increase in IL-8 mRNA expression (P < 0.01; Fig. 3D, lane 1 vs. 2). Admixture of conditioned media from both untreated and palmitate-treated, PMA-activated THP-1 macrophages also had lower IGFBP-3 levels compared with untreated medium alone and was associated with an increase in hepatocyte IL-8 mRNA expression compared with lane 1 (Fig. 3D, lane 3 vs. 1). Direct addition to palmitate to the Huh-7 cell culture medium led to higher IL-8 mRNA levels compared with basal THP-1 conditioned medium alone; however, it remained significantly lower than that seen with exposure to conditioned medium from palmitate-treated THP-1 cells (Fig. 3D, lane 4 vs. 2). Addition of palmitate to conditioned medium from palmitate-treated THP-1 cells led to further augmentation of IL-8 mRNA expression by Huh-7 cells (Fig. 3D, lane 5 vs. 2). Direct addition of palmitate to Huh-7 medium derived from THP-1 conditioned media from both untreated and palmitate-pretreated cells also led to increased IL-8 mRNA expression (Fig. 3D, lane 6). This was higher than that seen with palmitate-pretreated THP-1 conditioned medium (Fig. 3D, lane 6 vs. 2).
Figure 3.
Effects of palmitate (PA) and IGFBP-3 (IBP3) on IL-8 expression in Huh-7 hepatocytes. A, B) Palmitate produced a dose-dependent increase in IL-8 mRNA (B), which peaked at 9 h after exposure (A). C) This was associated with activation of JNK and NF-κB. Conditioned medium from PMA-activated THP-1 macrophages that were preconditioned with palmitate that was deficient in IGFBP-3 led to a 20-fold increase in IL-8 mRNA, whereas conditioned medium from PMA-activated THP-1 macrophages that were without prior preconditioning had no significant effects. D) Addition of palmitate to PMA-activated THP-1 macrophage conditioned medium led to an increase in IL-8 mRNA (bar 4 vs. 1). Similarly, addition of palmitate to IGFBP-3–deficient medium from PMA-activated THP-1 macrophages that were preconditioned with palmitate led to a further increase in IL-8 mRNA. E) Exposure to recombinant IGFBP-3 led to a marked suppression of palmitate-induced IL-8 mRNA expression. F) Effects of silencing or overexpression of IGFBP-3 in THP-1 cells on Huh-7 cells IL-8 mRNA expression was also evaluated. Control vectors had no effect on palmitate-induced IL-8 expression, whereas silencing and overexpression had an inverse relationship to hepatocyte IL-8 mRNA expression. Ctrl, control; UT, untreated cells. Means ± sd from 3 independent experiments are shown for all graphical data.
IGFBP-3 decreases palmitate-induced IL-8 mRNA expression by hepatocytes
In a separate set of studies, the effect of an addition of recombinant IGFBP-3 on the palmitate-induced increase in IL-8 mRNA expression in Huh-7 cells was studied (Fig. 3E). There was a dose-dependent impairment of palmitate-induced IL-8 mRNA levels when recombinant IGFBP-3 was added to the medium (P < 0.01). Of importance, IGFBP-3 alone in the absence of palmitate had no effect on IL-8 mRNA expression (Fig. 3E, lane 2). To further evaluate whether THP-1 secretion of IGFBP-3 could modulate hepatocyte IL-8 expression, IGFBP-3 was either overexpressed or silenced in THP-1 cells. The effect of conditioned media from these cells on palmitate-induced hepatocyte IL-8 expression was studied. On the one hand, control vectors had no effect (Fig. 3F, lane 1), whereas medium from IGFBP-3-overexpressing THP-1 cells suppressed palmitate-induced IL-8 mRNA expression (Fig. 3F, lane 3). On the other hand, medium from THP-1 cells with silenced IGFBP-3 led to a further increase in palmitate-induced IL-8 expression in Huh-7 cells (Fig. 3F, lane 4).
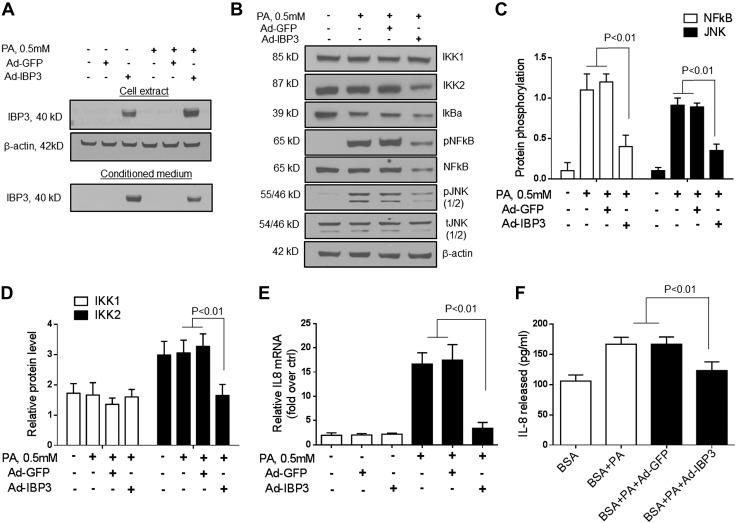
Mechanism of IGFBP-3–mediated suppression of palmitate-induced IL-8 expression
To evaluate the potential mechanism of IGFBP-3–mediated suppression of palmitate-induced IL-8 expression, IGFBP-3 was overexpressed in the presence of palmitate (0.5 mM) in Huh-7 cells (Fig. 4A). Overexpression of IGFBP-3 inhibited IKK2 levels, as well as both JNK and NF-κB activation in palmitate-treated Huh-7 cells (Fig. 4B, C; P < 0.01 for JNK and NF-κB; and Fig. 4D; P < 0.01 for IKK2). IKK1 protein levels remained unchanged. This was accompanied by a decrease in IL-8 mRNA and secreted protein (Fig. 4E, F).
Figure 4.
A) To evaluate the direct effect of IGFBP-3 (IBP3) on palmitate (PA)-induced IL-8 expression, IGFBP-3 was overexpressed in the presence of palmitate (0.5 mM) in Huh-7 cells. B–D) Overexpression of IGFBP-3 suppressed IKK2 protein and both JNK and NF-κB activation in palmitate-treated Huh-7 cells. E, F) This was accompanied by decreased IL-8 mRNA expression (E) and IL-8 protein secretion (F) into the medium. Ctrl, control. Means ± sd from 3 independent experiments are shown for all graphical data.
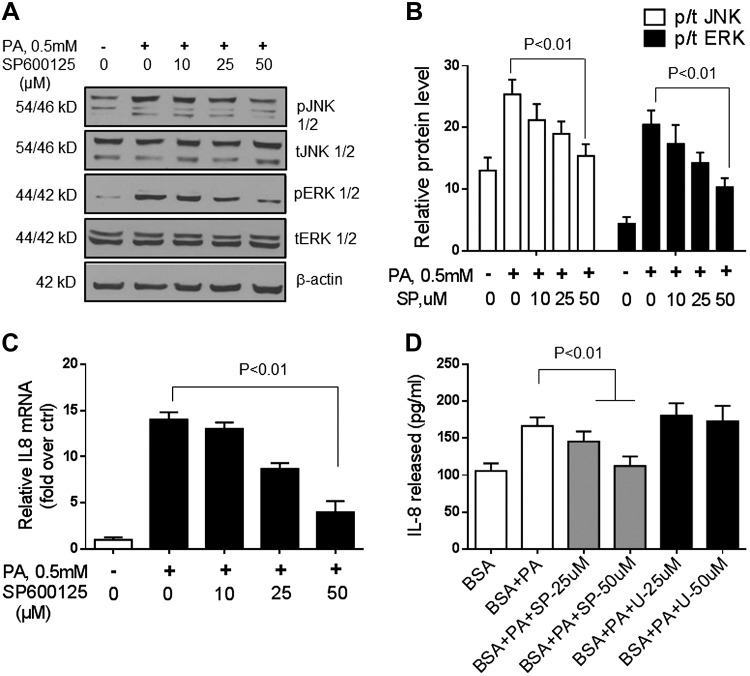
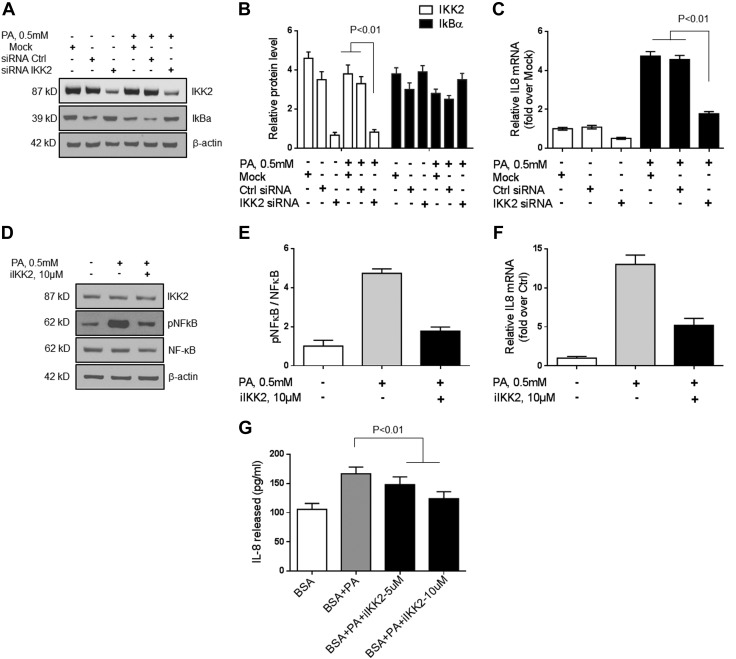
To further verify the role of JNK, ERK, and IKK2 in palmitate-induced IL-8 mRNA and protein expression in hepatocytes, these pathways were blocked by using specific compounds or siRNA (Fig. 5). On the one hand, after JNK inhibition, palmitate exposure no longer produced an increase in IL-8 mRNA and protein (Fig. 5; P < 0.01 for IL-8 mRNA; and Fig. 5D; P < 0.01 for IL-8 protein). On the other hand, ERK inhibition did not affect palmitate-induced IL-8 expression (Fig. 5D). Silencing of IKK2 by siRNA (Fig. 6A–C) or by an IKK2 inhibitor (Fig. 6D–G) decreased IKK2 protein expression levels and led to a decrease in palmitate-induced IL-8 mRNA and protein levels in hepatocytes. Together, these data indicate that IGFBP-3 acts via both JNK and NF-κB to modulate hepatocyte IL-8 gene and protein expression.
Figure 5.
A, B) JNK and ERK were inhibited by SP600125 (SP). C) Subsequent exposure to palmitate (PA) led to a dose-dependent decrease in IL-8 mRNA and protein release into the medium. D) Exposure to the specific ERK inhibitor U0126 (U) did not affect palmitate-induced IL-8 production by Huh-7 cells. These data indicate that IL-8 expression in hepatocytes is enhanced by JNK activation. Ctrl, control. Means ± sd from 3 independent experiments is shown for all graphical data.
Figure 6.
IKK2 was silenced in Huh-7 cells. A–C) Silencing of IKK2 by siRNA decreased IKK2 protein expression level (B) and led to a decrease in palmitate-induced IL-8 mRNA level in Huh-7 cells (C). D–G) Furthermore, pharmacologic inhibition of IKK2 blocked NF-κB activation (E), as well as palmitate-induced IL-8 mRNA expression (F) and protein release into the medium (G). These data demonstrate that IL-8 expression in hepatocytes is modulated by NF-κB. Ctrl, control. Means ± sd from 3 independent experiments is shown for all graphical data.
DISCUSSION
The functions of IGFBP-3 have largely been explored in the context of cancer as a pivot that can determine cell fate, that is, the likelihood of survival and proliferation vs. death (2, 34). In the current study, we demonstrate that IGFBP-3 may have a general anti-inflammatory effect under conditions of lipotoxic stress and that deficiency of IGFBP-3 increases IL-8, a well-known proinflammatory cytokine, and would thus be expected to contribute to the hepatic and systemic inflammatory state in patients with NAFL or NASH.
Patients with NAFL and NASH had decreased IGFBP-3 and increased IL-8 levels in circulation, whereas obese controls did not demonstrate these changes, which indicates that the changes were specific for NASH. Palmitate suppressed secretion of IGFBP-3 by THP-1 (CD68+) macrophages, and palmitate-conditioned medium from these macrophages increased hepatocyte IL-8 expression. Conversely, recombinant IGFBP-3 as well as overexpression of IGFBP-3 inhibited palmitate-induced IL-8 expression by hepatocytes. NAFL and NASH are well known to be associated with lipotoxic stress from palmitate and stearate (22, 24, 31). On the basis of these results, one may model a role for palmitate-induced suppression of Kupffer cell IGFBP-3 secretion as a driver of increased IL-8 and IL-8–mediated inflammatory responses in NAFLD. However, it is recognized that there are many other fatty acids in circulation and that their individual effects on IGFBP-3 remain to be clarified. Given the overall decrease in IGFBP-3 in humans with NASH and the well-known increase in palmitate in such cases, as well as our demonstration of the effects of palmitate on IGFBP-3, it is reasonable to postulate a role for palmitate as a driver of the low levels of IGFBP-3 observed in our patients.
The liver is the principal site of synthesis of both IGFBP-3 and IL-8 (5, 33). A key finding is that macrophage-derived IGFBP-3 may modulate hepatocyte IL-8 expression and may thus provide a novel proinflammatory mechanism in NASH. This conclusion is based on: 1) the demonstration of IGFBP-3 secretion by THP-1 cells; 2) suppression of hepatocyte IL-8 expression by conditioned medium from such cells; 3) suppression of IGFBP-3 secretion by THP-1 cells upon palmitate exposure; 4) enhanced hepatocyte IL-8 expression by IGFBP-3–deficient, palmitate-pretreated THP-1 medium; 5) restoration of the suppressive effect of THP-1 medium with readdition of IGFBP-3 to the conditioned medium from palmitate-exposed THP-1 medium; and 6) the reciprocal relationship between THP-1 medium IGFBP-3 level and hepatocyte IL-8 expression in experiments in which IGFBP-3 was overexpressed or silenced in THP-1 cells. IGFBP-3 suppressed palmitate-induced IL-8 synthesis via decreased transcription of the IL-8 gene by inhibiting both the JNK and NF-κB pathways in hepatocytes. Of note, under baseline conditions, exposure to IGFBP-3 did not affect hepatocyte IL-8 expression or secretion. This suggests that IGFBP-3 serves as an anti-inflammatory brake, and a decrease in IGFBP-3 releases the JNK and NF-κB pathways in hepatocytes from their normal inhibitory tone, thus activating these proinflammatory pathways. This drives the downstream transcriptional activation of IL-8 in hepatocytes. IL-8 is a key chemoattractant for neutrophils, and NASH is classically associated with either a neutrophilic or mixed lobular inflammation (33, 35). The severity of lobular inflammation has been associated with risk of disease progression (36). The demonstration that diminished IGFBP-3 may turn on JNK- and NF-κB–related proinflammatory pathways suggests that future strategies to increase IGFBP-3 may represent a novel approach to limit inflammatory responses in NASH.
NAFLD, and especially NASH, is well known to be associated with a systemic inflammatory state in addition to hepatic inflammation (37), and NAFLD is also a key component of metabolic syndrome (38). Decreased levels of IGFBP-3 have been reported in type 2 diabetes (17). In our study, IGFBP-3 levels in patients with NAFL/NASH with or without type 2 diabetes were similar. This suggests that NAFLD is independently associated with altered IGFBP-3 levels. In addition, IGFBP-3 was not expressed by circulating M1 and M2 macrophages. It is estimated that 70–80% of individuals with type 2 diabetes have NAFLD (39). It is thus feasible that the previously observed decrease in IGFBP-3 in type 2 diabetes may be a result of underlying NAFLD, and the findings of this study indicate that this may be a driver of the systemic inflammatory state associated with type 2 diabetes. This further raises the novel possibility that, in the future, IGFBP-3–enhancing strategies may be used to further reduce systemic inflammatory consequences of metabolic syndrome.
Another potential implication of our study relates to a major complication of NASH, that is, the development of hepatocellular cancer. Progression to cirrhosis is a classic risk factor for development of hepatocellular cancer for all chronic liver diseases, including NASH. Over the past few years, the number of reports of hepatocellular cancer in the absence of cirrhosis in patients with NAFLD has increased (40, 41). IGFBP-3 promotes ceramide-induced autophagic cell death (42). It also has complex interactions with the retinoid X receptor, vitamin D receptor, TNF-related apoptosis-inducing ligand, and TGF-β signaling pathways (43–45). All of these contribute to a general antioncogenic effect. Furthermore, a novel IGFBP-3–specific receptor has been identified, which is expressed in a variety of human tissues and mediates the anti-inflammatory and antitumor functions of IGFBP-3 (6, 7, 10). It is therefore tempting to postulate that a decrease in IGFBP-3 may be a potential mechanism driving this predisposition to hepatocellular cancer. It may also explain, at least in part, the general predisposition to malignancies, especially hepatocellular cancer, in those with type 2 diabetes (45). If so, in future studies, strategies designed to increase IGFBP-3 may be used for primary prophylaxis of hepatocellular cancer; however, IGFBP-3 has also pro-oncogenic effects (46, 47) and much additional work is required to clarify the implications of decreased IGFBP-3 in NASH for hepatocellular cancer.
Another previously unknown finding of the current study is that palmitate decreases IGFBP-3 protein secretion in PMA-activated THP-1 macrophages. NAFLD is well known to be associated with adipose tissue insulin resistance (48). Adipose tissue insulin resistance increases circulating free fatty acids, including palmitate (49). We hypothesize that the observed decrease in circulating IGFBP-3 in NAFLD is the result of increased palmitate-associated lipotoxic stress. The current study was not designed or powered to specifically relate IGFBP-3 levels to the severity of adipose tissue insulin resistance, a subject for future study.
Mechanisms of a palmitate-induced decrease in IGFBP-3 seem to involve NF-κB and JNK. In a model of bronchial asthma, it has been shown that IGFBP-3 inhibits NF-κB via IGFBP-3 receptor–mediated activation of caspases (6, 21). A detailed analysis of cross-talk between, on one hand, palmitate-induced activation of these pathways and, on the other hand, IGFBP-3–mediated suppression in NAFLD also remains a topic of future research.
A potential limitation of our study is the in vitro system used and the possibility that IGFBP-3 concentrations may not represent intrahepatic levels; however, we were limited by a lack of data on intrahepatic levels in the vicinity of hepatocytes in the space of Disse, and we assumed it to be lower than that in circulation. In addition, we used PMA-activated THP-1 macrophages as a surrogate for Kupffer cells. Human Kupffer cells are difficult to obtain, and we were unable to find enough cells to perform our studies; however, given that CD14 and CD68 are both biomarkers of human Kupffer cells and that our cells were both CD14 and CD68 positive, we used these cells instead; others have also used these cells to study Kupffer cell biology (50). Thus, it is likely that the findings from our studies are relevant and the mechanistic studies that relate palmitate to IGFBP-3 suppression will provide potential future opportunities to translate these into novel approaches to limit systemic and hepatic inflammation in NAFLD and metabolic syndrome.
In summary, the current study demonstrates that IGFBP-3 levels are decreased in NASH, and IGFBP-3 is associated with increased IL-8. It further demonstrates that palmitate suppresses IGFBP-3, which inhibits basal and palmitate-induced IL-8 synthesis by hepatocytes. In the future, these findings may provide novel IGFBP-3–based approaches for the treatment of NASH, to decrease the systemic inflammatory consequences of metabolic syndrome, and potentially to prevent hepatocellular cancer in those with NAFLD and metabolic syndrome. A large amount of additional work will be needed to realize these concepts, and the current study represents a critical first step in this process.
ACKNOWLEDGMENTS
This work was supported by U.S. National Institutes of Health Grants DK081450 and T32 DK07150 (National Institute of Diabetes and Digestive and Kidney Diseases) and AA020758 (National Institute on Alcohol Abuse and Alcoholism) (to A.J.S.). This is original work and is not under consideration for publication elsewhere. It has been previously presented in part at the 2012 annual meeting of the American Association for Study of Liver Diseases (Digestive Diseases Week; San Diego, CA, USA; May 2012).
Glossary
- BSA
bovine serum albumin
- GAPDH
glyceraldehyde 3-phosphate dehydrogenase
- IGFBP-3
IGF binding protein-3
- MOI
multiplicity of infection
- NAFL
nonalcoholic fatty liver
- NAFLD
nonalcoholic fatty liver disease
- NASH
nonalcoholic steatohepatitis
- PMA
phorbol 12-myristate 13-acetate
- siRNA
small interfering RNA
AUTHOR CONTRIBUTIONS
H.-K. Min, Y. Oh, and A. J. Sanyal designed the study; H.-K. Min, S. Ren, and A. J. Sanyal analyzed the data; H.-K. Min, H. Maruyama, B. K. Jang, M. Shimada, F. Mirshahi, and P. Puri performed research; H.-K. Min, Y. Oh, and A. J. Sanyal performed statistical analysis using appropriate software; H.-K. Min and A. J. Sanyal wrote the paper; and all authors have read and approved the final manuscript.
REFERENCES
- 1.Roelfsema V., Clark R. G. (2001) The growth hormone and insulin-like growth factor axis: its manipulation for the benefit of growth disorders in renal failure. J. Am. Soc. Nephrol. 12, 1297–1306 [DOI] [PubMed] [Google Scholar]
- 2.Baxter R. C. (2014) IGF binding proteins in cancer: mechanistic and clinical insights. Nat. Rev. Cancer 14, 329–341 [DOI] [PubMed] [Google Scholar]
- 3.Oy G. F., Slipicevic A., Davidson B., Solberg Faye R., Maelandsmo G. M., Florenes V. A. (2010) Biological effects induced by insulin-like growth factor binding protein 3 (IGFBP-3) in malignant melanoma. Int. J. Cancer 126, 350–361 [DOI] [PubMed] [Google Scholar]
- 4.Butt A. J., Firth S. M., Baxter R. C. (1999) The IGF axis and programmed cell death. Immunol. Cell Biol. 77, 256–262 [DOI] [PubMed] [Google Scholar]
- 5.Arany E., Afford S., Strain A. J., Winwood P. J., Arthur M. J. P., Hill D. J. (1994) Differential cellular synthesis of insulin-like growth factor binding protein-1 (IGFBP-1) and IGFBP-3 within human liver. J. Clin. Endocrinol. Metab. 79, 1871–1876 [DOI] [PubMed] [Google Scholar]
- 6.Lee Y. C., Jogie-Brahim S., Lee D. Y., Han J., Harada A., Murphy L. J., Oh Y. (2011) Insulin-like growth factor-binding protein-3 (IGFBP-3) blocks the effects of asthma by negatively regulating NF-κB signaling through IGFBP-3R-mediated activation of caspases. J. Biol. Chem. 286, 17898–17909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jogie-Brahim S., Feldman D., Oh Y. (2009) Unraveling insulin-like growth factor binding protein-3 actions in human disease. Endocr. Rev. 30, 417–437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Martin J. L., de Silva H. C., Lin M. Z., Scott C. D., Baxter R. C. (2014) Inhibition of insulin-like growth factor-binding protein-3 signaling through sphingosine kinase-1 sensitizes triple-negative breast cancer cells to EGF receptor blockade. Mol. Cancer Ther. 13, 316–328 [DOI] [PubMed] [Google Scholar]
- 9.Ibanez de Caceres I., Cortes-Sempere M., Moratilla C., Machado-Pinilla R., Rodriguez-Fanjul V., Manguán-García C., Cejas P., López-Ríos F., Paz-Ares L., de CastroCarpeño J., Nistal M., Belda-Iniesta C., Perona R. (2010) IGFBP-3 hypermethylation-derived deficiency mediates cisplatin resistance in non-small-cell lung cancer. Oncogene 29, 1681–1690 [DOI] [PubMed] [Google Scholar]
- 10.Ingermann A. R., Yang Y. F., Han J., Mikami A., Garza A. E., Mohanraj L., Fan L., Idowu M., Ware J. L., Kim H. S., Lee D. Y., Oh Y. (2010) Identification of a novel cell death receptor mediating IGFBP-3-induced anti-tumor effects in breast and prostate cancer. J. Biol. Chem. 285, 30233–30246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim H. S. (2013) Role of insulin-like growth factor binding protein-3 in glucose and lipid metabolism. Ann. Pediatr. Endocrinol. Metab. 18, 9–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Browning J. D., Szczepaniak L. S., Dobbins R., Nuremberg P., Horton J. D., Cohen J. C., Grundy S. M., Hobbs H. H. (2004) Prevalence of hepatic steatosis in an urban population in the United States: impact of ethnicity. Hepatology 40, 1387–1395 [DOI] [PubMed] [Google Scholar]
- 13.Younossi Z. M., Koenig A. B., Abdelatif D., Fazel Y., Henry L., Wymer M. (2016) Global epidemiology of non-alcoholic fatty liver disease-meta-analytic assessment of prevalence, incidence and outcomes. Hepatology 64, 73–84 [DOI] [PubMed] [Google Scholar]
- 14.Ekstedt M., Franzen L. E., Mathiesen U. L., Thorelius L., Holmqvist M., Bodemar G., Kechagias S. (2006) Long-term follow-up of patients with NAFLD and elevated liver enzymes. Hepatology 44, 865–873 [DOI] [PubMed] [Google Scholar]
- 15.Chitturi S., Abeygunasekera S., Farrell G. C., Holmes-Walker J., Hui J. M., Fung C., Karim R., Lin R., Samarasinghe D., Liddle C., Weltman M., George J. (2002) NASH and insulin resistance: Insulin hypersecretion and specific association with the insulin resistance syndrome. Hepatology 35, 373–379 [DOI] [PubMed] [Google Scholar]
- 16.Sanyal A. J., Campbell-Sargent C., Mirshahi F., Rizzo W. B., Contos M. J., Sterling R. K., Luketic V. A., Shiffman M. L., Clore J. N. (2001) Nonalcoholic steatohepatitis: association of insulin resistance and mitochondrial abnormalities. Gastroenterology 120, 1183–1192 [DOI] [PubMed] [Google Scholar]
- 17.Frystyk J., Skjaerbaek C., Vestbo E., Fisker S., Orskov H. (1999) Circulating levels of free insulin-like growth factors in obese subjects: the impact of type 2 diabetes. Diabetes Metab. Res. Rev. 15, 314–322 [DOI] [PubMed] [Google Scholar]
- 18.Kim H. S., Ali O., Shim M., Lee K. W., Vuguin P., Muzumdar R., Barzilai N., Cohen P. (2007) Insulin-like growth factor binding protein-3 induces insulin resistance in adipocytes in vitro and in rats in vivo. Pediatr. Res. 61, 159–164 [DOI] [PubMed] [Google Scholar]
- 19.Mohanraj L., Kim H. S., Li W., Cai Q., Kim K. E., Shin H. J., Lee Y. J., Lee W. J., Kim J. H., Oh Y. (2013) IGFBP-3 inhibits cytokine-induced insulin resistance and early manifestations of atherosclerosis. PLoS One 8, e55084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hoque R., Vodovotz Y., Mehal W. (2013) Therapeutic strategies in inflammasome mediated diseases of the liver. J. Hepatol. 58, 1047–1052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ganz M., Szabo G. (2013) Immune and inflammatory pathways in NASH. Hepatol. Int. 7, 771–781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Alkhouri N., Dixon L. J., Feldstein A. E. (2009) Lipotoxicity in nonalcoholic fatty liver disease: not all lipids are created equal. Expert Rev. Gastroenterol. Hepatol. 3, 445–451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fuchs M., Sanyal A. J. (2012) Lipotoxicity in NASH. J. Hepatol. 56, 291–293 [DOI] [PubMed] [Google Scholar]
- 24.Cusi K. (2012) Role of obesity and lipotoxicity in the development of nonalcoholic steatohepatitis: pathophysiology and clinical implications. Gastroenterology 142, 711–725.e6 [DOI] [PubMed] [Google Scholar]
- 25.Feldstein A. E., Werneburg N. W., Canbay A., Guicciardi M. E., Bronk S. F., Rydzewski R., Burgart L. J., Gores G. J. (2004) Free fatty acids promote hepatic lipotoxicity by stimulating TNF-alpha expression via a lysosomal pathway. Hepatology 40, 185–194 [DOI] [PubMed] [Google Scholar]
- 26.Brunt E. M. (1999) Nonalcoholic steatohepatitis (NASH): further expansion of this clinical entity? Liver 19, 263–264 [DOI] [PubMed] [Google Scholar]
- 27.Sanyal A. J., Chalasani N., Kowdley K. V., McCullough A., Diehl A. M., Bass N. M., Neuschwander-Tetri B. A., Lavine J. E., Tonascia J., Unalp A., Van Natta M., Clark J., Brunt E. M., Kleiner D. E., Hoofnagle J. H., Robuck P. R.; NASH CRN (2010) Pioglitazone, vitamin E, or placebo for nonalcoholic steatohepatitis. N. Engl. J. Med. 362, 1675–1685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Park E. K., Jung H. S., Yang H. I., Yoo M. C., Kim C., Kim K. S. (2007) Optimized THP-1 differentiation is required for the detection of responses to weak stimuli. Inflamm. Res. 56, 45–50 [DOI] [PubMed] [Google Scholar]
- 29.Schulz N., Kluth O., Jastroch M., Schürmann A. (2013) Minor role of mitochondrial respiration for fatty-acid induced insulin secretion. Int. J. Mol. Sci. 14, 18989–18998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Min H. K., Kapoor A., Fuchs M., Mirshahi F., Zhou H., Maher J., Kellum J., Warnick R., Contos M. J., Sanyal A. J. (2012) Increased hepatic synthesis and dysregulation of cholesterol metabolism is associated with the severity of nonalcoholic fatty liver disease. Cell Metab. 15, 665–674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Min H. K., Mirshahi F., Verdianelli A., Pacana T., Patel V., Park C. G., Choi A., Lee J. H., Park C. B., Ren S., Sanyal A. J. (2015) Activation of the GP130-STAT3 axis and its potential implications in nonalcoholic fatty liver disease. Am. J. Physio. Gastrointest. Liver Physiol. 308, G794–G803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lam C. S., Chen M. H., Lacey S. M., Yang Q., Sullivan L. M., Xanthakis V., Safa R., Smith H. M., Peng X., Sawyer D. B., Vasan R. S. (2010) Circulating insulin-like growth factor-1 and its binding protein-3: metabolic and genetic correlates in the community. Arterioscler. Thromb. Vasc. Biol. 30, 1479–1484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Joshi-Barve S., Barve S. S., Amancherla K., Gobejishvili L., Hill D., Cave M., Hote P., McClain C. J. (2007) Palmitic acid induces production of proinflammatory cytokine interleukin-8 from hepatocytes. Hepatology 46, 823–830 [DOI] [PubMed] [Google Scholar]
- 34.Mehta H. H., Gao Q., Galet C., Paharkova V., Wan J., Said J., Sohn J. J., Lawson G., Cohen P., Cobb L. J., Lee K. W. (2011) IGFBP-3 is a metastasis suppression gene in prostate cancer. Cancer Res. 71, 5154–5163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Brunt E. M. (2007) Pathology of fatty liver disease. Mod. Pathol. 20, S40–S48 [DOI] [PubMed] [Google Scholar]
- 36.Argo C. K., Northup P. G., Al-Osaimi A. M., Caldwell S. H. (2009) Systematic review of risk factors for fibrosis progression in non-alcoholic steatohepatitis. J. Hepatol. 51, 371–379 [DOI] [PubMed] [Google Scholar]
- 37.Haukeland J. W., Damås J. K., Konopski Z., Løberg E. M., Haaland T., Goverud I., Torjesen P. A., Birkeland K., Bjøro K., Aukrust P. (2006) Systemic inflammation in nonalcoholic fatty liver disease is characterized by elevated levels of CCL2. J. Hepatol. 44, 1167–1174 [DOI] [PubMed] [Google Scholar]
- 38.Marchesini G., Brizi M., Bianchi G., Tomassetti S., Bugianesi E., Lenzi M., McCullough A. J., Natale S., Forlani G., Melchionda N. (2001) Nonalcoholic fatty liver disease: a feature of the metabolic syndrome. Diabetes 50, 1844–1850 [DOI] [PubMed] [Google Scholar]
- 39.Anstee Q. M., Targher G., Day C. P. (2013) Progression of NAFLD to diabetes mellitus, cardiovascular disease or cirrhosis. Nat. Rev. Gastroenterol. Hepatol. 10, 330–344 [DOI] [PubMed] [Google Scholar]
- 40.Leung C., Yeoh S. W., Patrick D., Ket S., Marion K., Gow P., Angus P. W. (2015) Characteristics of hepatocellular carcinoma in cirrhotic and non-cirrhotic non-alcoholic fatty liver disease. World J. Gastroenterol. 21, 1189–1196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Baffy G., Brunt E. M., Caldwell S. H. (2012) Hepatocellular carcinoma in non-alcoholic fatty liver disease: an emerging menace. J. Hepatol. 56, 1384–1391 [DOI] [PubMed] [Google Scholar]
- 42.Grkovic S., O’Reilly V. C., Han S., Hong M., Baxter R. C., Firth S. M. (2013) IGFBP-3 binds GRP78, stimulates autophagy and promotes the survival of breast cancer cells exposed to adverse microenvironments. Oncogene 32, 2412–2420 [DOI] [PubMed] [Google Scholar]
- 43.Li J., Jin D., Fu S., Mei G., Zhou J., Lei L., Yu B., Wang G. (2013) Insulin-like growth factor binding protein-3 modulates osteoblast differentiation via interaction with vitamin D receptor. Biochem. Biophys. Res. Commun. 436, 632–637 [DOI] [PubMed] [Google Scholar]
- 44.Schedlich L. J., Graham L. D., O’Han M. K., Muthukaruppan A., Yan X., Firth S. M., Baxter R. C. (2007) Molecular basis of the interaction between IGFBP-3 and retinoid X receptor: role in modulation of RAR-signaling. Arch. Biochem. Biophys. 465, 359–369 [DOI] [PubMed] [Google Scholar]
- 45.Williams A. C., Smartt H., H-Zadeh A. M., Macfarlane M., Paraskeva C., Collard T. J. (2007) Insulin-like growth factor binding protein 3 (IGFBP-3) potentiates TRAIL-induced apoptosis of human colorectal carcinoma cells through inhibition of NF-kappaB. Cell Death Differ. 14, 137–145 [DOI] [PubMed] [Google Scholar]
- 46.Martin J. L., Baxter R. C. (1999) Oncogenic ras causes resistance to the growth inhibitor insulin-like growth factor binding protein-3 (IGFBP-3) in breast cancer cells. J. Biol. Chem. 274, 16407–16411 [DOI] [PubMed] [Google Scholar]
- 47.Baxter R. C. (2001) Signalling pathways involved in antiproliferative effects of IGFBP-3: a review. Mol. Pathol. 54, 145–148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lomonaco R., Ortiz-Lopez C., Orsak B., Webb A., Hardies J., Darland C., Finch J., Gastaldelli A., Harrison S., Tio F., Cusi K. (2012) Effect of adipose tissue insulin resistance on metabolic parameters and liver histology in obese patients with nonalcoholic fatty liver disease. Hepatology 55, 1389–1397 [DOI] [PubMed] [Google Scholar]
- 49.Lapointe A., Piché M. E., Weisnagel S. J., Bergeron J., Lemieux S. (2009) Associations between circulating free fatty acids, visceral adipose tissue accumulation, and insulin sensitivity in postmenopausal women. Metabolism 58, 180–185 [DOI] [PubMed] [Google Scholar]
- 50.Cha S. J., Park K., Srinivasan P., Schindler C. W., van Rooijen N., Stins M., Jacobs-Lorena M. (2015) CD68 acts as a major gateway for malaria sporozoite liver infection. J. Exp. Med. 212, 1391–1403 [DOI] [PMC free article] [PubMed] [Google Scholar]