Abstract
Synaptic dysfunction occurs early in senile dementias, presumably as a result of decreased levels of functional synaptic proteins as found in autopsied brains of patients with Alzheimer’s disease (AD) or frontotemporal dementia (FTD). Plasma neuronal-derived exosomes (NDEs) were recovered by precipitation and immunoabsorption from 12 patients with AD, 16 with FTD, and 28 controls in a cross-sectional study, and from 9 patients with AD, 10 with FTD, and 19 controls in a longitudinal study. Six synaptic proteins in NDE extracts were quantified by ELISAs and normalized for exosome amounts. NDE levels of synaptophysin, synaptopodin, synaptotagmin-2, and neurogranin were significantly lower in patients with FTD and AD than in controls, but those of growth-associated protein 43 and synapsin 1 were reduced only in patients with AD. Functionally relevant phosphorylation of synapsin 1 serine 9 was reduced in patients with FTD and AD, although total synapsin 1 protein was higher in FTD than in controls. NDE levels of synaptotagmin, synaptophysin, and neurogranin were decreased years before dementia in patients with FTD and AD. NDE levels of synaptopodin, synaptotagmin, and synaptophysin, but not of amyloid β-peptide 42 or P-T181-tau, were correlated significantly with cognition assessed by mini-mental state examination or AD assessment scale-cognitive subscale. NDE synaptic proteins may be useful preclinical indices and progression measures in senile dementias.—Goetzl, E. J., Kapogiannis, D., Schwartz, J. B., Lobach, I. V., Goetzl, L., Abner, E. L., Jicha, G. A., Karydas, A. M., Boxer, A., Miller, B. L. Decreased synaptic proteins in neuronal exosomes of frontotemporal dementia and Alzheimer’s disease.
Keywords: cognition, proteinopathy, neurotransmission, biomarkers
Synapses between neurons are vital to their interconnection in all functional circuits. An array of environmental factors may interact detrimentally with proteins that regulate synapse functions to permanently damage neural connectivity and plasticity necessary for higher brain functions (1, 2). In the earliest stages of Alzheimer’s disease (AD), neuropathology may be limited to subtle disorders of synaptic function in the hippocampus and frontal cortex (3, 4). The extent of diminished synaptic activities assessed by decreased levels of functional synaptic proteins in autopsied brain tissues correlates significantly with the severity of memory loss, and the distribution of synaptic dysfunction in the brain is similar to that of neurotoxic proteins characteristic of AD and some other senile neurodegenerative diseases (4–6). Synaptic proteins found to be decreased in brain tissues of patients with AD compared with those of age-matched controls include synaptotagmins, synaptophysin, Rab3A, GAP43 (growth-associated protein 43), synaptobrevin, neurogranin, and synaptopodin (7, 8). In addition, neuropathologic studies of frontotemporal dementia (FTD) have revealed early losses of some of the same functional synaptic proteins in the temporal lobes (4).
The recent development of methods for isolation of neuronal-derived exosomes (NDEs) from plasma has permitted quantification of neuronal proteins relevant to the pathogenesis of human neurodegenerative diseases. Abnormal levels of proteins of several functional clusters have been detected in plasma NDEs of patients with clinically manifest AD and FTD compared with those of matched controls (9–12). The spectrum of NDE proteins detected at altered levels in patients with AD and FTD encompasses those implicated in primary neurotoxicity, insulin resistance, disordered autophagy, and deficient mechanisms of protection of brain neurons. Most NDE protein abnormalities represent early biomarker candidates in AD, as they are significantly different from matched controls in preclinical stages ≤10 yr before recognizable memory loss. Levels of some NDE proteins also strongly distinguish between AD and FTD, including P-S396-tau, one form of tyrosine-phosphorylated type 1 insulin receptor substrate (IRS-1), and the repressor element 1-silencing transcription (REST) factor (9, 11, 12).
We have now identified in human plasma NDEs several neuronal proteins with known synaptic functions on the basis of disordered neural activity in rodents with insertions of natural mutants or engineered genetic deletions (13–16). Brain contents of these synaptic proteins, including synaptophysin, synaptopodin, synaptotagmins, GAP43, and neurogranin, are lower in autopsied tissues from patients with AD or FTD than in those from cognitively normal controls (4, 7, 8). We show here that levels of some of these synaptic proteins in plasma NDEs of patients with FTD or AD are significantly lower than those of age-matched controls in both overt disease and preclinical stages ≤10 yr before neurologic manifestations, that there are differences between NDE levels of some synaptic proteins in patients with FTD and AD, and that levels of some synaptic proteins correlate significantly with mental status.
MATERIALS AND METHODS
Experimental design and patient evaluation
For cross-sectional studies, we retrospectively identified 12 patients with early AD who had been evaluated in the Clinical Research Unit of the U.S. National Institute on Aging (NIA; Baltimore, MD, USA), 16 patients with behavioral variant FTD at the Memory and Aging Center of the University of California, San Francisco (UCSF; San Francisco, CA, USA), and 28 age- and gender-matched cognitively normal controls who had donated blood at the Jewish Home of San Francisco (JHSF) in the same time period as the patients (Table 1). Concurrent mental status assessments and measurements of synaptic proteins in NDEs were carried out in 24 patients with early AD who had been evaluated in the Clinical Research Unit of the NIA, as well as on 4 patients with early AD at JHSF. For longitudinal studies, we identified 9 patients from the University of Kentucky with moderate AD and 10 additional patients with a behavioral variant of FTD from UCSF who had provided blood at 2 times: first when cognitively intact (AD1) or with mild cognitive impairment (MCI; FTD1) and again 1–10 yr later, after diagnosis of dementia (AD2 and FTD2; Table 1). Plasmas from 19 matched controls at JHSF were found that had been obtained in the same time period as those of the first sample from the patients with AD and FTD. One investigator (E.J.G.) supervised harvesting and storage of all plasmas by the same methods and processed all plasmas by the same procedures. Plasmas from patients in the longitudinal studies were analyzed without knowledge of the clinical data.
TABLE 1.
Characteristics of patients and control subjects
| Age (yr) | MMSE score | |||||
|---|---|---|---|---|---|---|
| Diagnosis | Total (N) | Male/female (n) | Mean ± sem | sd | Mean ± sem | sd |
| AD | 12 | 6/6 | 74.4 ± 1.98 | 6.84 | 26.3 ± 0.99* | 3.45 |
| AC | 12 | 6/6 | 74.4 ± 1.98 | 6.84 | 29.8 ± 0.11 | 0.39 |
| AD1 | 9 | 2/7 | 81.7 ± 2.06 | 6.18 | 28.7 ± 0.47 | 1.41 |
| AC1 | 9 | 2/7 | 82.2 ± 2.28 | 6.50 | 28.3 ± 0.96 | 2.75 |
| AD2 | 9 | 2/7 | 87.8 ± 2.50 | 7.51 | 21.4 ± 1.60** | 4.80 |
| FTD | 16 | 12/4 | 63.6 ± 1.82 | 7.27 | 19.7 ± 2.57*** | 10.3 |
| FTC | 16 | 12/4 | 63.6 ± 1.82 | 7.27 | 29.9 ± 0.09 | 0.34 |
| FTD1 | 10 | 5/5 | 62.9 ± 1.93 | 6.10 | 23.4 ± 2.74 | 8.67 |
| FTC1 | 10 | 5/5 | 63.6 ± 2.04 | 5.98 | 29.1 ± 2.20 | 4.98 |
| FTD2 | 10 | 5/5 | 66.5 ± 1.82 | 5.74 | 17.7 ± 3.46* | 11.0 |
The significance of differences between cognitive state [mini-mental state examination (MMSE)] values of the groups was calculated by an unpaired Student’s t test for AD vs. AD control (AC) and FTD vs. FTD control (FTC), and by a paired Student’s t test for AD when cognitively intact (AD1) vs. AD with diagnosis of dementia (AD2) and FTD with mild cognitive impairment (FTD1) vs. FTD with diagnosis of dementia (FTD2). *P < 0.05; **P < 0.01; ***P < 0.001.
Patients with AD had mental status testing at the time of each blood sampling. Mini-mental state examination (MMSE) and the AD assessment scale-cognitive subscale (ADAS-cog) were conducted as described (17, 18). Patients from the NIA had amnestic MCI from AD or mild dementia with high probability of AD and a Clinical Dementia Rating global score of 0.5 or 1.0 according to the Petersen and Dubois criteria (19, 20). Patients from the University of Kentucky had probable AD and mild-to-moderate dementia by NINCDS-ADRDA criteria and had a Clinical Dementia Rating global score of ≥1.0 at the time of the second blood collection (21, 22). All patients with AD had abnormal CSF levels of amyloid β-peptides (Aβ) 1–42 and P-T181-tau that supported their diagnosis (23).
Patients with FTD were evaluated at the Memory and Aging Center of UCSF at the time of each blood sampling. Their diagnosis and assessment of dementia (Table 1) were based on standard clinical and mental status criteria, including discriminant analyses of neuropsychiatric and other elements that distinguish FTD from AD (24, 25). All patients who were studied and some patient designates signed a consent form approved with the protocol at each institution.
Blood and CSF sampling of patients and control participants
Venous blood (10 ml) was drawn into 0.5 ml saline with EDTA or 100 U/ml of heparin, incubated for 10 min at room temperature, and centrifuged for 15 min at 2500 g. Plasmas were stored in 0.5-ml aliquots at −80°C. CSF levels of P-T181-tau and Aβ1–42 were quantified by xMAP Technology (Luminex Corp., Austin, TX, USA) using Inno-Bia AlzBio3 kits (Innogenetics, Ghent, Belgium).
Isolation of plasma exosomes for extraction and ELISA quantification of NDE proteins
Aliquots of 0.25 ml plasma were incubated with 0.1 ml thromboplastin D (Thermo Fisher Scientific, Waltham, MA, USA), followed by addition of calcium- and magnesium-free Dulbecco’s balanced salt solution with protease inhibitor cocktail (Roche, Indianapolis, IN, USA) and phosphatase inhibitor cocktail (Thermo Fisher Scientific) (10). After centrifugation at 3000 g for 30 min at 4°C, supernatants were incubated with ExoQuick exosome precipitation solution (System Biosciences, Mountain View, CA, USA), and resultant suspensions were centrifuged at 1500 g for 30 min at 4°C. Each pellet was resuspended in 350 μl distilled water with inhibitor cocktails for immunochemical enrichment of exosomes from neuronal sources (10).
Exosome suspensions were incubated with 1.5 µg mouse anti-human CD171 (L1CAM neural adhesion protein) biotinylated antibody (clone 5G3; eBiosciences, San Diego, CA, USA) in 50 µl of 3% bovine serum album (BSA), followed by incubation with 10 μl Streptavidin-Plus UltraLink resin (Thermo Fisher Scientific) in 40 µl of 3% BSA (10). After centrifugation at 400 g and removal of supernatant, each pellet was resuspended in 100 µl of 0.05 M glycine-HCl (pH 3.0), incubated at 4°C for 10 min, and centrifuged at 4°C for 10 min at 4000 g. Supernatants were transferred to prechilled Eppendorf tubes that contained 10 µl of 1 M Tris-HCl (pH 8.0) and 25 µl of 10% BSA, mixed before addition of 365 µl M-PER mammalian protein extraction reagent (Thermo Fisher Scientific) that contained protease and phosphatase inhibitors, and stored at −80°C.
NDE proteins were quantified by ELISA kits for human synaptophysin, GAP43, Aβ42, and the tetraspanning exosome marker CD81 (American Research Products–Cusabio, Waltham, MA, USA), with verification of the CD81 antigen standard curve using human purified recombinant CD81 antigen (Origene Technologies, Rockville, MD, USA), P-T181-tau (Thermo Fisher Scientific Life Sciences), neurogranin (American Research Products–Cloud Clone Corp.), synapsin 1 (LifeSpan Biosciences, Seattle, WA, USA) without and with biotin-anti–(P-S9) synapsin 1 antibody (USBiologicals, Salem, MA, USA), and human synaptopodin and synaptotagmin-2 (Biomatik, Wilmington, DE, USA) according to supplier directions. Mean value for all determinations of CD81 in each assay group was set at 1.00 and relative values of CD81 for each sample were used to normalize their recovery.
Statistical analyses
A Shapiro-Wilks test showed that data in all sets were distributed normally, although neurogranin levels in the AD control (AC) group had a borderline value. Statistically significant differences between means for cross-sectional patient groups and between each patient group and their respective control group were determined with an unpaired Student’s t test, including a Bonferroni correction (Prism 6; GraphPad Software, La Jolla, CA, USA). The discriminatory ability of each exosomal protein is presented by using receiver operating characteristic (ROC) analyses with confidence intervals estimated on the basis of the binomial exact distribution (STATA 13.1; StataCorp, College Station, TX, USA). Discriminant classifier analyses were performed to evaluate the conjoint ability of exosomal proteins to differentiate the diagnostic groups (STATA 13.1). For longitudinal analyses, significant differences between serial values for AD1−AD2 and FTD1−FTD2 patients taken before and after onset of MCI or dementia were calculated with a paired Student’s t test (GraphPad Software). Correlations between cognitive scores and NDE protein values were determined in 28 patients with AD with MCI or mild-to-moderate dementia using Pearson correlation coefficients (r) with 95% confidence intervals (CIs) and stratification on median NDE protein values to exclude any impact of ceiling effects.
RESULTS
Patients with FTD were a younger group than the patients with AD, as expected, and had more severe cognitive losses than those with early AD in cross-sectional studies (Table 1). For the longitudinal studies, patients in the FTD1 and AD1 groups were at their respective initial stages of MCI and normal cognition. By the second blood sampling, both patients at the FTD2 and AD2 stages had progressed to moderate dementia.
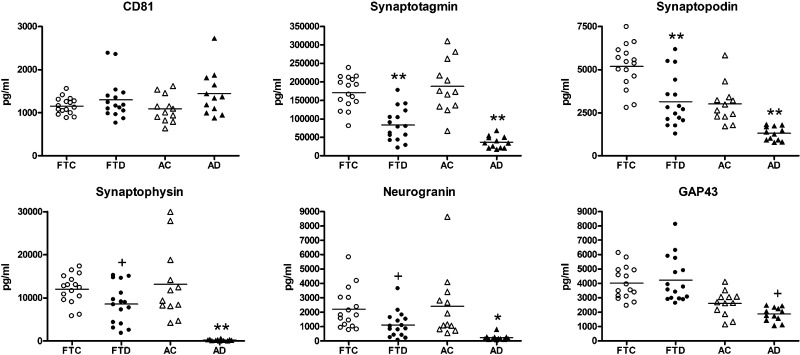
Compared with values for their respective control groups, plasma NDE levels of synaptotagmin and synaptopodin were significantly lower for the FTD and AD groups (Fig. 1). NDE levels of synaptophysin and neurogranin showed greater reductions for patients with AD than for those with FTD compared with those of their respective control groups. For GAP43 levels, there was no difference between FTD and FTC groups, and those of the AD group were only modestly significantly lower than those of the AC group. The AD group had significantly lower values than did the FTD group for synaptotagmin, synaptopodin, synaptophysin, neurogranin, GAP43, and synapsin 1, with respective P values of 0.0019, 0.0003, <0.0001, 0.0052, 0.0014, and <0.0001 (Fig. 1 and Table 2). There were no differences among patient or control groups in total levels of NDEs recovered from plasma on the basis of the content of CD81 exosomal marker protein that was used to normalize concentrations of protein analytes (Fig. 1).
Figure 1.
Levels of plasma NDE synaptic proteins in cross-sectional studies of FTD and AD. Each point in a frame depicts the value for a single subject, and the horizontal line among points represents the mean value for that group. Subject group abbreviations and the meaning of statistical symbols are the same as in Table 1. Statistical symbols above FTD and AD points show the significance level of differences from their respective control groups. Means ± sem for synaptotagmin, synaptopodin, synaptophysin, neurogranin, and GAP43, respectively, were as follows: FTC: 171,116 ± 10,876; 5178 ± 321; 12,009 ± 841; 2208 ± 354; and 4019 ± 282; FTD: 83,307 ± 11,154; 3145 ± 372; 8541 ± 1157; 1117 ± 227; and 4515 ± 630; AC: 187,650 ± 20,480; 3808 ± 333; 13,148 ± 2417; 2413 ± 658; and 2607 ± 250; AD: 36,555 ± 4682; 1299 ± 116; 240 ± 47.1; 232 ± 56.5; and 1863 ± 140. All differences between AD and FTD groups in values of synaptotagmin, synaptopodin, synaptophysin, neurogranin, and GAP43 were significant at respective P values of 0.0019, 0.0003, <0.0001, 0.0052, and 0.0014. +P < 0.05; *P < 0.01; **P < 0.001.
TABLE 2.
Plasma NDE levels of total synapsin 1 protein and P-S9-synapsin 1
| Protein | FTC | FTD | AC | AD |
|---|---|---|---|---|
| Synapsin 1 protein (pg/ml) | 2295 ± 138 | 7781 ± 1379** | 2048 ± 219 | 1058 ± 99** |
| P-S9-synapsin 1 (OD450 nm/ng synapsin 1) | 0.511 ± 0.050 | 0.118 ± 0.021** | 0.545 ± 0.081 | 0.270 ± 0.049* |
Each value is the mean ± sem for levels of the 16 patients with FTD and controls (FTC) or 12 patients with AD and controls (AC) in Fig. 1. Group differences were determined by an unpaired Student’s t test. *P < 0.01; **P < 0.001.
ROC plots were constructed to assess the extent of distinction between patient groups for synaptic protein levels individually and collectively (Supplemental Fig. 1). Discriminant analyses of matched control vs. patient groups correctly classified 91.7% of AC subjects and 100% of patients with AD (Supplemental Fig. 1A), as well as 93.8% of FTC subjects and 81.3% of patients with FTD (Supplemental Fig. 1B). Values of synaptophysin and GAP43 show no overlap between AD and FTD groups (Fig. 1 and Supplemental Fig. 1C). The range of synaptophysin values in patients with AD was 35.2–617 pg/ml, whereas that of patients with FTD was 1767–15,154 pg/ml. The range of GAP43 values in patients with AD was 1079–2479 pg/ml, whereas that of patients with FTD was 2616–6286 pg/ml. ROC analyses identified a cutoff value for synaptophysin of 1767 pg/ml and for GAP43 of 2616 pg/ml that resulted in a sensitivity of 1 (95% CI, 0.79–1), a specificity of 1 (95% CI, 0.74–1), and a correct classification of 1 (95% CI, 0.88–1) for both exosomal proteins. When considered collectively, ROC analyses of the remaining proteins synaptotagmin, synaptopodin, and neurogranin all show overlapping values, but correctly classified 100% of patients with AD and 75% of those with FTD (Supplemental Fig. 1C).
NDE levels of synapsin 1 were significantly lower than those of controls in AD, but were unexpectedly higher than those of corresponding controls in FTD (Table 2). Phosphorylation of serine 9 in synapsin 1, which is required for release of synaptic vesicles from the cytoskeleton and normal impulse transmission, was also reduced significantly in AD compared with controls but was even more reduced in FTD (26, 27).
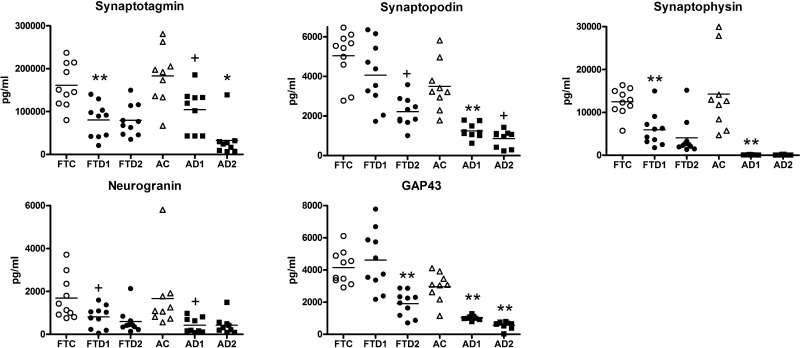
In the complex patterns of NDE synaptic protein levels of the longitudinal studies, values for synaptotagmin, synaptophysin, and neurogranin for patients in the FTD1 and AD1 groups were lower than that of their respective control groups (Fig. 2). However, progression of these decreases with declining cognition was observed only for the AD2 group. For synaptopodin and GAP43 levels, decreases relative to those of the control group were only significant at the lower level of cognition in FTD2, whereas decreases were significant at the AD1 stage and declined significantly further with decreasing cognition to the AD2 stage. Despite their similar levels of diminished cognition, the AD2 group had significantly lower values than did the FTD2 group for NDE synaptotagmin, synaptopodin, synaptophysin, and GAP43, with respective P values of 0.0175, 0.0002, 0.0004, and <0.0001; however, this difference was not observed for neurogranin.
Figure 2.
Levels of plasma NDE synaptic proteins in longitudinal studies of FTD and AD. Points, subject group abbreviations, and the meaning of statistical symbols are the same as in Table 1 and Fig. 1. Statistical symbols above FTD1 and AD1 values show the significance level of differences from their respective control groups, FTC and AC, whereas symbols above FTD2 and AD2 values show significant differences from the corresponding FTD1 and AD1 groups. Mean ± sem for synaptotagmin, synaptopodin, synaptophysin, neurogranin, and GAP43, respectively, were as follows: FTC: 161,039 ± 16,113; 5043 ± 402; 12,469 ± 989; 1690 ± 322; and 4149 ± 328; FTD1: 80,256 ± 12,829; 4067 ± 511; 5902 ± 1238; 810 ± 164; and 4608 ± 593; FTD2: 79,507 ± 11,569; 2216 ± 235; 3157 ± 661; 596 ± 182; and 3319 ± 237; AC: 183,214 ± 21,994; 3491 ± 417; 14,427 ± 2906; 1691 ± 537; and 2960 ± 301; AD1: 104,296 ± 16,898; 1250 ± 126; 95.5 ± 11.6; 459 ± 129; and 1034 ± 47.4; AD2: 32,622 ± 13,711; 857 ± 142; 95.3 ± 25.1; 430 ± 144; and 570 ± 78.2. Differences between AD2 and FTD2 groups in values of synaptotagmin, synaptopodin, synaptophysin, neurogranin, and GAP43 showed significance with respective P values of 0.0175, 0.0002, 0.0004, not significant, and <0.0001. +P < 0.05; *P < 0.01; **P < 0.001.
As patients from group AD1 had significantly decreased levels of all NDE synaptic proteins when they were cognitively normal, these proteins may be early biomarkers of proteinopathy in AD. In contrast, levels of only 3 of the NDE synaptic proteins, synaptotagmin, synaptophysin, and neurogranin, but not synaptopodin or GAP43, were significantly decreased at this early proteinopathic stage for patients of group FTD1, despite mild cognitive losses. There were no significant differences in mean levels of any synaptic protein biomarkers between subsets of patients in the AD2 group with 2- to 5- and 6- to 10-yr clinical courses or patients in the FTD2 group with 1- to 2- and 6- to 10-yr clinical courses to moderate dementia.
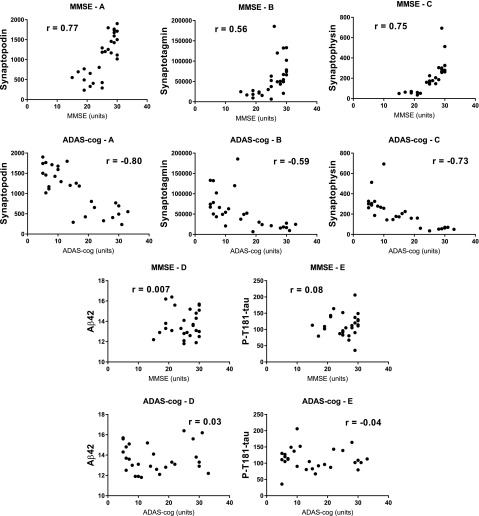
To further examine relationships between diminished cognition and decreased NDE synaptic protein values in AD (Fig. 2), correlations were sought for NDE levels of synaptopodin, synaptotagmin, and synaptophysin with values of MMSE or ADAS-cog in a total of 28 patients with AD with MCI to moderate dementia. Correlations of synaptopodin and synaptophysin levels, but less so for synaptotagmin levels, with both MMSE and ADAS-cog values were significant (Fig. 3). In contrast, neither correlations of MMSE or ADAS-cog values with levels of AD primary neurotoxic proteins Aβ42 or P-T181-tau were significant.
Figure 3.
Correlations of MMSE and ADAS-cog cognitive function values with NDE synaptic protein levels, but not of primary neurotoxic proteins in AD. MMSE frames show significant correlations between MMSE and synaptopodin (MMSE-A; P < 0.001), synaptotagmin (MMSE-B; P = 0.002), and synaptophysin (MMSE-C; P < 0.001), but not Aβ42 (MMSE-D; P = 0.97) or P-T181-tau (MMSE-E; P = 0.70). ADAS-cog frames show significant correlations between ADAS-cog and synaptopodin (ADAS-cog-A; P < 0.001), synaptotagmin (ADAS-cog-B; P = 0.009), and synaptophysin (ADAS-cog-C; P < 0.001), but not Aβ42 (ADAS-cog-D; P = 0.86) or P-T181-tau (ADAS-cog-E; P = 0.82). All protein analyte concentrations are given as picograms per milliliter after normalization with the corresponding level of CD81.
DISCUSSION
The synaptic proteins found at decreased levels in NDEs of patients with FTD and AD, as contrasted with levels of matched cognitively normal controls, differ in synaptic localization and functions (Fig. 1). The presynaptic vesicle protein synaptophysin binds synaptobrevin and regulates vesicle fusion and recycling (28, 29). Synaptotagmins also are presynaptic vesicle proteins that bind calcium and thereby initiate vesicle fusion (30). Synaptic membrane protein GAP43 seems to share functions with synaptophysin and synaptotagmins (31). The 2 postsynaptic proteins, synaptopodin and neurogranin, regulate intracellular calcium concentration as well (8). Presynaptic protein synapsin 1 is decreased in AD and paradoxically increased in FTD, but significantly less phosphorylated than in both AC and FTC groups (Table 2). Several of these proteins are required for distinct synaptic functions; for example, synaptotagmin is required for synaptic facilitation and maintenance of the readily releasable pool of synaptic vesicles (32, 33). Mutations in human synaptophysin are recognized in X-linked intellectual disability and mutations in synaptotagmin-1 are found in altered states of cognition and movement (15, 16). The extent of loss of these synaptic proteins in hippocampal and cortical tissues at autopsy of patients with AD parallels decreases in the number of synapses and correlates with decreases in premortem cognitive functions (4, 8).
Differences between FTD and AD NDE levels of functional synaptic proteins are as complex as those previously described for NDE levels of other functional protein clusters (9–12). Despite a 10-yr-younger mean age for FTC group vs. AC group, only the mean level of synaptopodin was significantly lower for AC than for FTC (Fig. 1). Against this control background, the AD group had significantly lower values than did AC subjects for all synaptic proteins, whereas the FTD group showed no difference from FTC controls for GAP43 and higher levels for synapsin 1 (Fig. 2 and Table 2). Furthermore, the AD group had significantly lower values compared with the FTD group for all synaptic proteins (Fig. 1). Similarly, greater alterations in NDE levels of other functional protein clusters were found for patients with AD compared with patients with FTD. Mean NDE level of P-serine 312-type 1 IRS-1 was higher, that of P-tyrosine-IRS-1 was lower, and the ratio of P-S312-IRS-1 to P-Y-IRS-1, which represents a calculated level of insulin resistance, was much higher for the AD group than for the FTD group up to 10 yr before onset of memory loss (9). Indices of neuronal lysosomal dysfunction, reflected in elevated NDE levels of cathepsin D, lysosome-associated membrane protein 1, and total ubiquitinylated proteins, were also significantly higher for the AD group than for the FTD group up to 10 yr before onset of memory loss (10). NDE levels of LDL receptor-related protein 6, heat shock factor-1, and REST factor, which mediate neuronal defenses against diverse forms of stress, were all significantly lower in the AD group compared with the AC and FTD groups, but REST was higher in patients with FTD than in controls (11).
Furthermore, some of the plasma NDE functional proteins that were found to be at abnormal levels in AD are at oppositely abnormal levels in the CSF of patients with AD (11, 12). Here, the low plasma NDE levels of neurogranin in AD (Figs. 1 and 2) are the opposite of elevations reported in the CSF of patients with AD (34, 35). In AD, this seems to be attributable to decreases in brain tissue levels reflected in NDE levels compared with the concurrent greater transport into CSF.
NDE levels of the primary neurotoxic proteins, Aβ42 and P-T181-tau, were elevated in AD compared with those of matched cognitively normal controls in early preclinical phases of disease (12). However, little or no progression of these increases was observed with development of clinical AD, and values of these putatively pathogenic proteins did not correlate with those of cognitive function measures. This lack of correlation between cognition and NDE levels of Aβ42 and P-T181-tau is seen here again in another group of 28 patients with AD, whereas there are highly significant correlations between MMSE and ADAS-cog indices of cognition and NDE levels of synaptic proteins (Fig. 3).
The etiological relationships between neuronal accumulation of primary neurotoxic proteins and decreases in synaptic proteins that lead to early synaptic dysfunction are now being unraveled definitively. Recent evidence from several lines of research has shown intensive localization of tau with a distinctive pattern of phosphorylation at neuronal synapses in AD and several other pathophysiologic tauopathies (36). The capacity of synaptic P-tau to alter synaptic physiology is particularly evident in long-term depression of synaptic signaling in hippocampal neurons. Investigations of synaptic deposition of other neuropathologic proteins and weakening of synaptic signaling are now warranted. An emerging capacity to quantify via plasma NDEs the levels of multiple functionally relevant synaptic proteins will permit more precise investigations of the roles of such abnormalities in the development of dementia.
ACKNOWLEDGMENTS
The authors thank Sonya Anderson (University of Kentucky Alzheimer’s Disease Center) for organizing and distributing biospecimens and Judith H. Goetzl (Jewish Home of San Francisco) for expert production of graphic illustrations. This work was supported by a grant from the BAND2 Program of the Michael J. Fox Foundation for Parkinson’s Research, the Alzheimer’s Association, Alzheimer’s Research UK, and the Weston Brain Institute (Toronto, ON, Canada), by a grant for methodological development from Nanosomix (to E.J.G.), the Intramural Program of the U.S. National Institutes of Health, National Institute on Aging (NIA; to D.K.), and NIA Grant P30 AG028383 (to E.L.A.). E.J.G. has filed an application with the U.S. Patent Office for the platform and methodologies described in this report; he is a founder of Nanosomix, but now receives no salary or consulting fees, but only support for laboratory supplies. E.L.A. received travel support from Nanosomix for the 2015 annual meeting of the American Association for the Advancement of Science. A.B. declares that outside the submitted studies, he has grants from the NIA, Tau Research Consortium, Allon Therapeutics, Genentech, Bristol-Myers Squibb, TauRx, Alzheimer’s Association, Bluefield Project to Cure FTD, the Association for Frontotemporal Degeneration, Alzheimer’s Drug Discovery Foundation, EnVivo, C2N Diagnostics, Pfizer, Eli Lilly, and Corticobasal Degeneration Solutions; grants, personal fees, and nonfinancial support from Archer Biosciences; personal fees from Acetylon; and personal fees from iPierian. The remaining authors declare no conflicts of interest.
Glossary
- Aβ
amyloid β-peptide
- AC
Alzheimer’s disease control
- AD
Alzheimer’s disease
- AD1
Alzheimer's disease when cognitively intact
- AD2
Alzheimer's disease with diagnosis of dementia
- ADAS-cog
Alzheimer’s disease assessment scale-cognitive subscale
- BSA
bovine serum albumin
- CI
confidence interval
- FTC
frontotemporal dementia control
- FTD
frontotemporal dementia
- FTD1
frontotemporal dementia with mild cognitive impairment
- FTD2
frontotemporal dementia with diagnosis of dementia
- GAP43
growth-associated protein 43
- IRS-1
type 1 insulin receptor substrate
- JHSF
Jewish Home of San Francisco
- MCI
mild cognitive impairment
- MMSE
mini-mental state examination
- NDE
neuron-derived exosome
- NIA
U.S. National Institute on Aging
- REST
repressor element 1-silencing transcription
- ROC
receiver operating characteristic
- UCSF
University of California, San Francisco
Footnotes
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
AUTHOR CONTRIBUTIONS
E. J. Goetzl, J. B. Schwartz, L. Goetzl, A. Boxer, and B. L. Miller constructed and critiqued the study design; D. Kapogiannis, E. L. Abner, G. A. Jicha, A. M. Karydas, and B. L. Miller provided clinical material; E. J. Goetzl developed analytical methods; E. J. Goetzl, D. Kapogiannis, E. L. Abner, and A. M. Karydas acquired data; I. V. Lobach conducted statistical analyses; and E. J. Goetzl, J. B. Schwartz, L. Goetzl, and A. Boxer drafted and edited the manuscript.
REFERENCES
- 1.Kalia M. (2008) Brain development: anatomy, connectivity, adaptive plasticity, and toxicity. Metabolism 57, S2–S5 [DOI] [PubMed] [Google Scholar]
- 2.Tau G. Z., Peterson B. S. (2010) Normal development of brain circuits. Neuropsychopharmacology 35, 147–168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Selkoe D. J. (2002) Alzheimer’s disease is a synaptic failure. Science 298, 789–791 [DOI] [PubMed] [Google Scholar]
- 4.Clare R., King V. G., Wirenfeldt M., Vinters H. V. (2010) Synapse loss in dementias. J. Neurosci. Res. 88, 2083–2090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.DeKosky S. T., Scheff S. W. (1990) Synapse loss in frontal cortex biopsies in Alzheimer’s disease: correlation with cognitive severity. Ann. Neurol. 27, 457–464 [DOI] [PubMed] [Google Scholar]
- 6.Masliah E., Terry R. D., Alford M., DeTeresa R., Hansen L. A. (1991) Cortical and subcortical patterns of synaptophysinlike immunoreactivity in Alzheimer’s disease. Am. J. Pathol. 138, 235–246 [PMC free article] [PubMed] [Google Scholar]
- 7.Davidsson P., Blennow K. (1998) Neurochemical dissection of synaptic pathology in Alzheimer’s disease. Int. Psychogeriatr. 10, 11–23 [DOI] [PubMed] [Google Scholar]
- 8.Reddy P. H., Mani G., Park B. S., Jacques J., Murdoch G., Whetsell W. Jr., Kaye J., Manczak M. (2005) Differential loss of synaptic proteins in Alzheimer’s disease: implications for synaptic dysfunction. J. Alzheimers Dis. 7, 103–117, discussion 173–180 [DOI] [PubMed] [Google Scholar]
- 9.Kapogiannis D., Boxer A., Schwartz J. B., Abner E. L., Biragyn A., Masharani U., Frassetto L., Petersen R. C., Miller B. L., Goetzl E. J. (2015) Dysfunctionally phosphorylated type 1 insulin receptor substrate in neural-derived blood exosomes of preclinical Alzheimer’s disease. FASEB J. 29, 589–596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Goetzl E. J., Boxer A., Schwartz J. B., Abner E. L., Petersen R. C., Miller B. L., Kapogiannis D. (2015) Altered lysosomal proteins in neural-derived plasma exosomes in preclinical Alzheimer disease. Neurology 85, 40–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Goetzl E. J., Boxer A., Schwartz J. B., Abner E. L., Petersen R. C., Miller B. L., Carlson O. D., Mustapic M., Kapogiannis D. (2015) Low neural exosomal levels of cellular survival factors in Alzheimer’s disease. Ann. Clin. Transl. Neurol. 2, 769–773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fiandaca M. S., Kapogiannis D., Mapstone M., Boxer A., Eitan E., Schwartz J. B., Abner E. L., Petersen R. C., Federoff H. J., Miller B. L., Goetzl E. J. (2015) Identification of preclinical Alzheimer's disease by a profile of pathogenic proteins in neurally derived blood exosomes: A case-control study. Alzheimers Dement. 11, 600–607.e1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Miyakawa T., Yared E., Pak J. H., Huang F. L., Huang K. P., Crawley J. N. (2001) Neurogranin null mutant mice display performance deficits on spatial learning tasks with anxiety related components. Hippocampus 11, 763–775 [DOI] [PubMed] [Google Scholar]
- 14.Glavan G., Schliebs R., Zivin M. (2009) Synaptotagmins in neurodegeneration. Anat. Rec. (Hoboken) 292, 1849–1862 [DOI] [PubMed] [Google Scholar]
- 15.Gordon S. L., Cousin M. A. (2013) X-linked intellectual disability-associated mutations in synaptophysin disrupt synaptobrevin II retrieval. J. Neurosci. 33, 13695–13700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Baker K., Gordon S. L., Grozeva D., van Kogelenberg M., Roberts N. Y., Pike M., Blair E., Hurles M. E., Chong W. K., Baldeweg T., Kurian M. A., Boyd S. G., Cousin M. A., Raymond F. L. (2015) Identification of a human synaptotagmin-1 mutation that perturbs synaptic vesicle cycling. J. Clin. Invest. 125, 1670–1678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Irizarry M. C., Webb D. J., Bains C., Barrett S. J., Lai R. Y., Laroche J. P., Hosford D., Maher-Edwards G., Weil J. G. (2008) Predictors of placebo group decline in the Alzheimer’s disease Assessment Scale-cognitive subscale (ADAS-Cog) in 24 week clinical trials of Alzheimer’s disease. J. Alzheimers Dis. 14, 301–311 [DOI] [PubMed] [Google Scholar]
- 18.Cano S. J., Posner H. B., Moline M. L., Hurt S. W., Swartz J., Hsu T., Hobart J. C. (2010) The ADAS-cog in Alzheimer’s disease clinical trials: psychometric evaluation of the sum and its parts. J. Neurol. Neurosurg. Psychiatry 81, 1363–1368 [DOI] [PubMed] [Google Scholar]
- 19.Petersen R. C. (2004) Mild cognitive impairment as a diagnostic entity. J. Intern. Med. 256, 183–194 [DOI] [PubMed] [Google Scholar]
- 20.Sperling R. A., Aisen P. S., Beckett L. A., Bennett D. A., Craft S., Fagan A. M., Iwatsubo T., Jack C. R. Jr., Kaye J., Montine T. J., Park D. C., Reiman E. M., Rowe C. C., Siemers E., Stern Y., Yaffe K., Carrillo M. C., Thies B., Morrison-Bogorad M., Wagster M. V., Phelps C. H. (2011) Toward defining the preclinical stages of Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 7, 280–292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dubois B., Feldman H. H., Jacova C., Dekosky S. T., Barberger-Gateau P., Cummings J., Delacourte A., Galasko D., Gauthier S., Jicha G., Meguro K., O’brien J., Pasquier F., Robert P., Rossor M., Salloway S., Stern Y., Visser P. J., Scheltens P. (2007) Research criteria for the diagnosis of Alzheimer’s disease: revising the NINCDS-ADRDA criteria. Lancet Neurol. 6, 734–746 [DOI] [PubMed] [Google Scholar]
- 22.McKhann G., Drachman D., Folstein M., Katzman R., Price D., Stadlan E. M. (1984) Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology 34, 939–944 [DOI] [PubMed] [Google Scholar]
- 23.Shaw L. M., Vanderstichele H., Knapik-Czajka M., Clark C. M., Aisen P. S., Petersen R. C., Blennow K., Soares H., Simon A., Lewczuk P., Dean R., Siemers E., Potter W., Lee V. M., Trojanowski J. Q.; Alzheimer’s Disease Neuroimaging Initiative (2009) Cerebrospinal fluid biomarker signature in Alzheimer’s disease neuroimaging initiative subjects. Ann. Neurol. 65, 403–413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rascovsky K., Hodges J. R., Knopman D., Mendez M. F., Kramer J. H., Neuhaus J., van Swieten J. C., Seelaar H., Dopper E. G., Onyike C. U., Hillis A. E., Josephs K. A., Boeve B. F., Kertesz A., Seeley W. W., Rankin K. P., Johnson J. K., Gorno-Tempini M. L., Rosen H., Prioleau-Latham C. E., Lee A., Kipps C. M., Lillo P., Piguet O., Rohrer J. D., Rossor M. N., Warren J. D., Fox N. C., Galasko D., Salmon D. P., Black S. E., Mesulam M., Weintraub S., Dickerson B. C., Diehl-Schmid J., Pasquier F., Deramecourt V., Lebert F., Pijnenburg Y., Chow T. W., Manes F., Grafman J., Cappa S. F., Freedman M., Grossman M., Miller B. L. (2011) Sensitivity of revised diagnostic criteria for the behavioural variant of frontotemporal dementia. Brain 134, 2456–2477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gorno-Tempini M. L., Hillis A. E., Weintraub S., Kertesz A., Mendez M., Cappa S. F., Ogar J. M., Rohrer J. D., Black S., Boeve B. F., Manes F., Dronkers N. F., Vandenberghe R., Rascovsky K., Patterson K., Miller B. L., Knopman D. S., Hodges J. R., Mesulam M. M., Grossman M. (2011) Classification of primary progressive aphasia and its variants. Neurology 76, 1006–1014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Parks K. M., Sugar J. E., Haroutunian V., Bierer L., Perl D., Wallace W. C. (1991) Reduced in vitro phosphorylation of synapsin I (site 1) in Alzheimer’s disease postmortem tissues. Brain Res. Mol. Brain Res. 9, 125–134 [DOI] [PubMed] [Google Scholar]
- 27.Rosahl T. W., Geppert M., Spillane D., Herz J., Hammer R. E., Malenka R. C., Südhof T. C. (1993) Short-term synaptic plasticity is altered in mice lacking synapsin I. Cell 75, 661–670 [DOI] [PubMed] [Google Scholar]
- 28.Daly C., Ziff E. B. (2002) Ca2+-dependent formation of a dynamin-synaptophysin complex: potential role in synaptic vesicle endocytosis. J. Biol. Chem. 277, 9010–9015 [DOI] [PubMed] [Google Scholar]
- 29.Valtorta F., Pennuto M., Bonanomi D., Benfenati F. (2004) Synaptophysin: leading actor or walk-on role in synaptic vesicle exocytosis? BioEssays 26, 445–453 [DOI] [PubMed] [Google Scholar]
- 30.Sudhof T. C. (2004) The synaptic vesicle cycle. Annu. Rev. Neurosci. 27, 509–547 [DOI] [PubMed] [Google Scholar]
- 31.Haruta T., Takami N., Ohmura M., Misumi Y., Ikehara Y. (1997) Ca2+-dependent interaction of the growth-associated protein GAP-43 with the synaptic core complex. Biochem. J. 325, 455–463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bacaj T., Wu D., Burré J., Malenka R. C., Liu X., Südhof T. C. (2015) Synaptotagmin-1 and -7 are redundantly essential for maintaining the capacity of the readily-releasable pool of synaptic vesicles. PLoS Biol. 13, e1002267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jackman S. L., Turecek J., Belinsky J. E., Regehr W. G. (2016) The calcium sensor synaptotagmin 7 is required for synaptic facilitation. Nature 529, 88–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kvartsberg H., Duits F. H., Ingelsson M., Andreasen N., Öhrfelt A., Andersson K., Brinkmalm G., Lannfelt L., Minthon L., Hansson O., Andreasson U., Teunissen C. E., Scheltens P., Van der Flier W. M., Zetterberg H., Portelius E., Blennow K. (2015) Cerebrospinal fluid levels of the synaptic protein neurogranin correlates with cognitive decline in prodromal Alzheimer’s disease. Alzheimers Dement. 11, 1180–1190 [DOI] [PubMed] [Google Scholar]
- 35.Kester M. I., Teunissen C. E., Crimmins D. L., Herries E. M., Ladenson J. H., Scheltens P., van der Flier W. M., Morris J. C., Holtzman D. M., Fagan A. M. (2015) Neurogranin as a cerebrospinal fluid biomarker for synaptic loss in symptomatic Alzheimer disease. JAMA Neurol. 72, 1275–1280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Regan P., Whitcomb D. J., Cho K. (2016) Physiological and pathophysiological implications of synaptic tau. [E-pub ahead of print] Neuroscientist doi: 10.1177/1073858416633439 [DOI] [PubMed] [Google Scholar]