Abstract
Macrophage activation is an important feature of primary biliary cholangitis (PBC) pathogenesis and other cholestatic liver diseases. Galectin-3 (Gal3), a pleiotropic lectin, is produced by monocytic cells and macrophages. However, its role in PBC has not been addressed. We hypothesized that Gal3 is a key to induce NOD-like receptor family, pyrin domain containing 3 (NLRP3) inflammasome in macrophages and in turn to propagate proinflammatory IL-17 signaling. In liver tissues from patients with PBC and dnTGF-βRII mice, a model of autoimmune cholangitis, the expression of Gal3, NLRP3, and the adaptor protein adaptor apoptosis-associated speck-like protein was induced, with the downstream activation of caspase-1 and IL-1β. In wild-type hepatic macrophages, deoxycholic acid induced the association of Gal3 and NLRP3 with direct activation of the inflammasome, resulting in an increase in IL-1β. Downstream retinoid-related orphan receptor C mRNA, IL-17A, and IL-17F were induced. In Gal3−/− macrophages, no inflammasome activation was detected. To confirm the key role of Gal3 in the pathogenesis of cholestatic liver injury, we generated dnTGF-βRII/galectin-3−/− (dn/Gal3−/−) mice, which showed impaired inflammasome activation along with significantly improved inflammation and fibrosis. Taken together, our data point to a novel role of Gal3 as an initiator of inflammatory signaling in autoimmune cholangitis, mediating the activation of NLRP3 inflammasome and inducing IL-17 proinflammatory cascades. These studies provide a rationale to target Gal3 in autoimmune cholangitis and potentially other cholestatic diseases.—Tian, J., Yang, G., Chen, H.-Y., Hsu, D. K., Tomilov, A., Olson, K. A., Dehnad, A., Fish, S. R., Cortopassi, G., Zhao, B., Liu, F.-T., Gershwin, M. E., Török, N. J., Jiang, J. X. Galectin-3 regulates inflammasome activation in cholestatic liver injury.
Keywords: primary biliary cholangitis, NLRP3, galectin-3, IL-17
Primary biliary cholangitis (PBC), formerly known as “primary biliary cirrhosis,” is an autoimmune disorder characterized by the destruction of the small intrahepatic bile ducts that leads to cholestasis, progressive inflammation, and fibrosis. Dysregulation of innate immunity plays a major role in its pathogenesis. The induction of Th1/Th17 signaling is linked to disease progression (1), and activation of natural killer T cells has been shown to accelerate PBC in murine models (2). Despite major advances delineating the changes in innate and adaptive immunity in PBC, the early inflammatory events are not well understood. Incubation of biliary epithelial cell apoptotic bodies that carry the E2 component of the pyruvate dehydrogenase complex (PDC-E2) with macrophages from patients with PBC led to an intense production of inflammatory cytokines (3). However, the mechanistic aspects of macrophage activation in PBC have not been well described. Therefore, in this study we focused on elucidating how inflammasome activation leads to exacerbation of proinflammatory cascades. We have previously demonstrated that galectin-3 (Gal3), a pleiotropic lectin, is required for phagocytosis of apoptotic debris by mediating integrin crosslinking, thereby facilitating the tethering and uptake of apoptotic bodies (4). Gal3 is a 30-kDa protein with a unique chimeric structure containing a C-terminal that binds to N-glycan residues of other molecules and an N-terminal domain containing proline/tyrosine/glycine-rich motifs (5). It is mainly produced by activated macrophages and stellate cells in the liver and is thought to play an important profibrogenic role. Therefore, we hypothesized that Gal3 is a key element that is required for the activation of the NLR family, pyrin domain containing 3 (NLRP3) inflammasome, and proinflammatory activation of macrophages.
Accumulating evidence shows that inflammasome signaling in hepatic macrophages contributes to liver injury, inflammation, and fibrosis (6, 7). NLRP3, also known as cryopyrin and NALP3, bound with the adaptor apoptosis-associated speck-like protein (ASC), triggers the cleavage of procaspase-1 to form the mature caspase-1, which catalyzes the activation of IL-1β, contributing to the activation of innate immune responses (8). Transgenic mice expressing constitutively active NLRP3 showed severe hepatocyte pyroptosis, inflammation, and fibrosis (6), and NLRP3 depletion was protective (7, 9). In addition, the NLRP3 inflammasome-derived IL-1β synergized with IL-23/IL-17 signaling in T cells in murine autoimmune encephalomyelitis (10).
In this study, we showed for the first time increased activity of the NLPR3 inflammasome in patients with PBC and in our animal model. Activation of NLRP3 in macrophages was Gal3 dependent, resulting in IL-17 responses via an autocrine mechanism. Furthermore, we found that inflammatory injury and fibrosis were significantly improved in dnTGF-βRII/gal3−/− mice. These findings suggest that the induction of Gal3/inflammasome/IL-17 signaling in macrophages could be an important pathogenic pathway in PBC that contributes to fibrosis.
MATERIALS AND METHODS
PBC liver samples
Liver tissues from patients with PBC were kindly provided by M.E.G. These samples were deidentified and therefore institutional review board exempted. The tissues were processed for Western blot assay and real-time quantitative PCR (qPCR).
Animals and cell cultures
The dnTGF-βRII mice with a C57/B6 background were provided by M.E.G. (11). These mice express dominant negative TGF-β receptor II driven by a CD4 promoter resulting in a knockdown of TGF-β signaling exclusively in T cells. They spontaneously develop autoimmune cholangitis with the presence of serum antimitochondrial antibody (AMA), similarly to human PBC (11–13). Gal3−/− mice (kindly provided by F.-T.L.) in a C57/B6 background were generated by gene targeting technology as previously described (14). To generate dn/Gal3−/− mice, the dnTGF-βRII mice were crossed with the Gal3−/− mice. The offspring with the desired genotype were euthanized at 12 wk of age for tissue collection. The age- and sex-matched littermates were used as controls. NLRP3−/− mice were from The Jackson Laboratory (Bar Harbor, ME, USA).
To isolate hepatic macrophages, a standard in situ perfusion procedure was conducted as described previously (15). In brief, the liver was sequentially perfused with collagenase and protease. The digested liver was suspended into Gey’s balanced salt solution (Sigma-Aldrich, St. Louis, MO, USA). A 25.6%/13% two-step nycodenz (Invitrogen, Carlsbad, CA, USA) gradient was made, and the cell suspension was added on the top. After centrifugation (1500 g, 15 min), macrophages were collected on the top of the 25.6% nycodenz layer and kept in RPMI 1640 medium with 10% fetal bovine serum and antibiotics. The cells were allowed to settle and treated with deoxycholic acid (DCA) (100 μM) with or without recombinant Gal3 (1 μM for 24 h). The cells were then collected for RNA extraction, real-time qPCR, coimmunoprecipitation, and other biochemistry assays.
Immunohistochemistry and confocal microscopy
Cryostat sections from PBC liver (4 µm) were fixed in 4% formaldehyde and permeabilized in 0.2% Triton X-100/PBS. After washing and blocking with 2% bovine serum albumin, the sections were incubated with antibodies for NLRP3 (1:200; Adipogen Corporation, San Diego, CA, USA) and Gal3 (1:2000; provided by F.-T.L.) or ASC (1:200, Adipogen Corp.) for 16 h at 4°C. After washing, the appropriate secondary antibodies were applied (1:2000; Invitrogen). The images were analyzed by confocal microscopy.
H&E staining and Sirius red staining were conducted by the Department of Pathology of UC Davis following standard protocols. The images were analyzed with ImageJ (NIH, Bethesda, MD, USA) to quantify the fibrotic area.
RNA extraction and real-time qRT-PCR
RNA was extracted from the liver tissues or hepatic macrophages using an RNeasy Mini kit (Qiagen, Valencia, CA, USA) following the manufacturer’s instruction. cDNA was synthesized using an iScript cDNA Synthesis kit (Bio-Rad, Hercules, CA, USA). Absolute real-time PCR was conducted with the SYBR Green DNA Master mix (Applied Biosystems, Foster City, CA, USA) using the Applied Biosystems 7900HT real-time PCR system (Invitrogen). The cycler was programmed at 50°C for 2 min and 95°C for 10 min for system activation, followed by 40 cycles at 95°C for 15 s and 60°C for 1 min for melt/annealing/extension. The absolute amount of target genes was calculated based on the standard curves generated from different amount of templates and adjusted to the housekeeping gene. The wild-type (WT) control was set as 1-fold. The primers used are listed in Table 1 and have been described previously (4).
TABLE 1.
Primer sequences used
| Gene | Primers, 5′–3′ |
|
|---|---|---|
| Forward | Reverse | |
| Human Gal3 | GCCTTATAACCTGCCTTTGC | ACCGTGCCCAGAATTGTTAT |
| Human NLRP3 | GGAGAGACCTTTATGAGAAAGCAA | GCTGTCTTCCTGGCATATCACA |
| Human IL-1β | AGCTGAGGAAGATGCTGGTT | GGAAAGAAGGTGCTCAGGTC |
| Human ASC | GTCCTGACGGATGAGCAGTA | CACCAGGTAGGACTGGGACT |
| Human GAPDH | TCAAGAAGGTGGTGAAGCAG | CGCTGTTGAAGTCAGAGGAG |
| Mouse Gal3 | GCCCTTGCCTGGAGGAGTCATG | CATTGAAGCGGGGGTTAAAGTGG |
| Mouse NLRP3 | CCCTTGGAGACACAGGACTC | GAGGCTGCAGTTGTCTAATTCC |
| Mouse ASC | GAGCAGCTGCAAACGACTAA | GTCCACAAAGTGTCCTGTTCTG |
| Mouse IFN-γ | GGAGGAACTGGCAAAAGGATGG | TGTTGCTGATGGCCTGATTGTC |
| Mouse IL-1β | CAACCAACAAGTGATATTCTCCATG | GATCCACACTCTCCAGCTGCA |
| Mouse IL-10 | GGTTGCCAAGCCTTATCGGA | ACCTGCTCCACTGCCTTGCT |
| Mouse IL-18 | CAAACCTTCCAAATCACTTCCT | TCCTTGAAGTTGACGCAAGA |
| Mouse IL-17A | TCCAGAAGGCCCTCAGACTA | TGAGCTTCCCAGATCACAGA |
| Mouse IL-17F | GAGGATAACACTGTGAGAGTTGAC | GAGTTCATGGTGCTGTCTTCC |
| Mouse RORc | TGCAAGACTCATCGACAAGGC | AGCTTTTCCACATGTTGGCTG |
| Mouse IL-23p19 | GCCCAGCCTGAGTTCTAGTC | AGGGCTCAGTCAGAGTTGCT |
| Mouse 18S rRNA | GGAGGAACTGGCAAAAGGATGG | TGTTGCTGATGGCCTGATTGTC |
Coimmunoprecipitation and Western blot analysis
The macrophages were treated as indicated and were collected in the lysis buffer (150 mM NaCl, 5 mM EDTA, 5 mM EGTA, 10 mM HEPES, pH 7.4, and 0.5% Triton X-100) containing protease inhibitors and lysed with repeated freeze-thaw cycles. The protein concentration was determined with the BCA protein assay kit (Thermo Fisher Scientific, Waltham, MA, USA), and 100 μg of protein was incubated with protein A-agarose beads mixed with either anti-NLRP3 or anti-Gal3 rabbit antibodies. After extensive washing with the lysis buffer, the beads were boiled in 40 µl of sample buffer. The supernatant was then separated by SDS-PAGE. The blots were incubated with the appropriate antibodies for 16 h at 4°C. The membranes were washed and incubated with horseradish peroxidase-conjugated secondary antibodies (Santa Cruz Biotechnology, Santa Cruz, USA), and the blots were developed by enhanced chemiluminescence (Thermo Fisher Scientific). As an internal control, GAPDH was blotted using a polyclonal antibody (1:2000; Trevigen, Gaithersburg, MD, USA). The image quantification was performed with ImageJ (NIH). The Western blot data showed are representative, and similar data were obtained at least 3 times.
NLRP3/Gal3 binding assay
The interaction of NLRP3 and Gal3 was analyzed with cell-free biolayer interferometry (BLI) assay using the Octet Red system (Pall ForteBio, Menlo Park, CA, USA). In brief, Octet Red biosensors (antiglutathione S-transferase) were coated with recombinant human NLRP3-GST (Novus Biologicals, Littleton, CO, USA) and incubated with either WT or a truncated Gal3 mutant lacking N-terminal proline-rich domain (Gal3-C) (provided by F.-T.L.) (16), and the binding was measured for 600 s. Biosensors were then moved into the blank buffer without Gal3 or Gal3-C to measure the dissociation for 600 s. The resulting sensogram of NLRP3-Gal3 interaction was presented as response (nanomoles of Gal3) plotted against time.
Caspase-1 activity assay
Macrophages were processed for caspase-1 activity assay using a kit purchased from Abcam (Cambridge, MA, USA) following the manufacturer’s instruction. In brief, the cells were lysed and incubated with fluorescence-conjugated YVAD, the caspase-1 substrate. When cleaved by caspase-1, fluorescence would be emitted at 400 nm. The data were normalized with protein concentration decided by the BCA method.
ELISA
Mouse serum, liver tissues, and macrophage culture medium were collected for ELISA to detect IL-1β, IL-17, and Gal3. The liver tissues were homogenized in the lysis buffer (50 mM Tris-HCl, pH 7.4, 150 mM NaCl, 1 mM EDTA, 1% Triton X-100 containing protease inhibitor cocktail) and quantified using the BCA method. One microgram of protein was used. Classic sandwich ELISA was performed following the standard protocol. All the antibodies and reagents for IL-1β and IL-17 were from Affymetrix eBioscience (San Diego, CA, USA). Mouse serum Gal3 was measured by an ELISA kit from Abcam following the provided instructions.
Hydroxyproline assay
Liver tissue was boiled in 6 N HCl for 16 h. After washing with H2O, the denatured tissue was resuspended in H2O and mixed with 50 mM chloramines-T and incubated at room temperature for 20 min, and then perchloric acid (3.15 M; Sigma-Aldrich) and p-dimethylaminobenzaldehyde (20%; Sigma-Aldrich) were added. After incubation at 60°C for 20 min, 557-nm absorbance was recorded. The result was obtained based on a standard curve generated from a serial dilution of the hydroxyproline standard and expressed as milligrams of hydroxyproline per gram of wet liver.
Statistical analysis
The data shown represent at least 3 experiments and are expressed as the means ± sem. Differences between 2 groups were compared using 1-way ANOVA associated with the Dunnett’s test. The 2-tailed, unpaired Student’s t test was used to analyze the differences between 2 groups. Comparisons between more than 2 groups were done with the Kruskal-Wallis test followed by Dunn’s multiple comparison test. Statistical significance was considered at P < 0.05.
RESULTS
Gal3 and NLRP3 inflammasome signaling are induced in patients with PBC and in a mouse model of autoimmune cholangitis
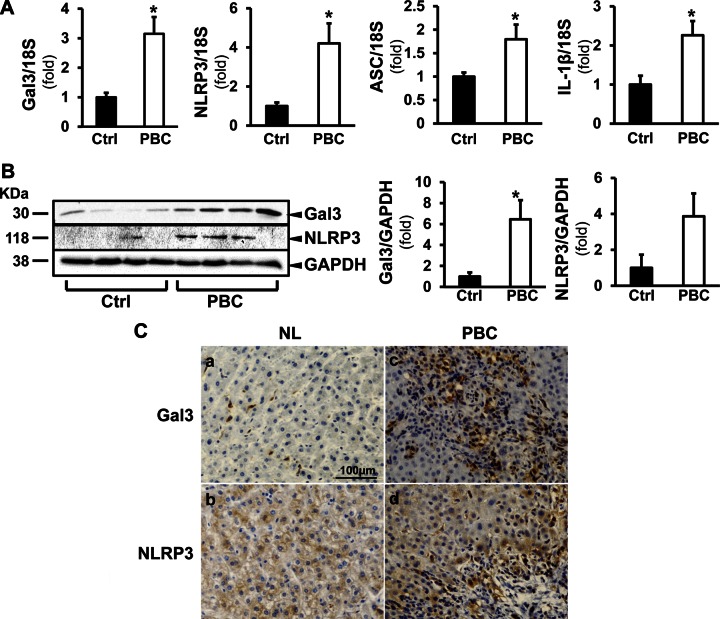
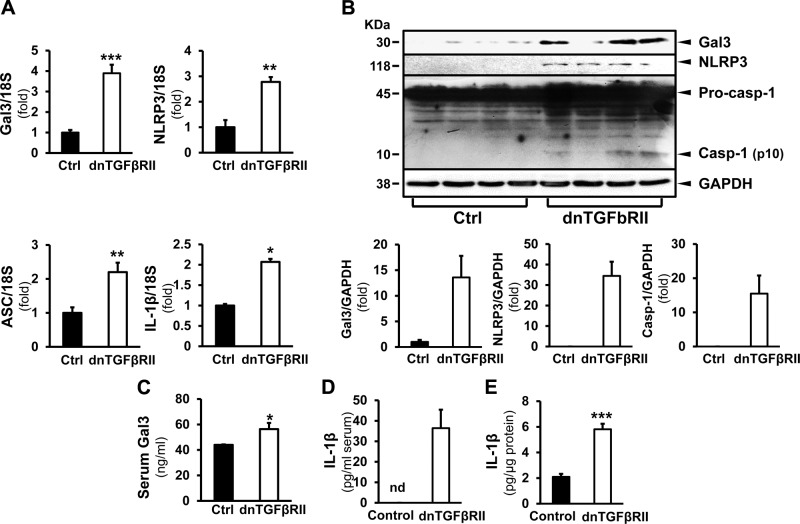
In the normal liver, Gal3 is expressed at a low level, mainly in macrophages. Here, we examined Gal3 expression in the liver of patients with PBC and in dnTGF-βRII mice. In patients with PBC, Gal3 was significantly induced at the mRNA (3.15 ± 0.57-fold; P < 0.05) (Fig. 1A) and protein levels (6.49 ± 1.81-fold; P < 0.05) (Fig. 1B). NLRP3 expression was also significantly increased in PBC livers (4.21 ± 1.02-fold in transcript, P < 0.05; 3.90 ± 1.30-fold in protein) (Fig. 1A, B, respectively). ASC transcript was induced by 1.80 ± 0.31-fold and IL-1β by 2.26 ± 0.36-fold (P < 0.05) (Fig. 1A). Immunohistochemical studies showed enhanced signals of Gal3 and NLRP3 in livers of patients with PBC (Fig. 1C). These results were corroborated in the dnTGF-βRII mice, which showed increased Gal3 and NLRP3 at the transcript (3.49 ± 0.42-fold, P < 0.001; 2.78 ± 0.19-fold, P < 0.01) and protein levels (13.83 ± 0.43-fold; 34.00 ± 7.00-fold, P < 0.001) compared with the WT littermates (Fig. 2A, B). The transcripts of ASC and IL-1β were induced as well (2.20 ± 0.28-fold, P < 0.01; 2.07 ± 0.07-fold, P < 0.05). The cleavage of caspase-1 was more pronounced in the livers of the transgenic mice (15.50 ± 5.30-fold; P < 0.001), indicating inflammasome activation (Fig. 2B). Serum ELISA showed that the transgenic mice had significantly higher Gal3 levels (56.35 ± 4.86 ng/ml; P < 0.05) (Fig. 2C). The activation of inflammasome in the livers of dnTGF-βRII mice was confirmed by IL-1β ELISA. IL-1β was significantly higher in the serum (36.44 ± 8.99 pg/ml) and in the liver homogenates of these mice (5.81 ± 0.43 pg/µg protein) compared with WT mice (nondetectable in serum, P < 0.001; 2.10 ± 0.23 pg/µg protein, P < 0.001) (Fig. 2D, E).
Figure 1.
The expression of Gal3 and NLRP3 inflammasome components are induced in patients with PBC. A) Liver tissues from healthy subjects (Ctrl) and patients with PBC were processed for RT-qPCR to evaluate the expression of Gal3, NLRP3, ASC, and IL-1β. A) The mRNA levels of Gal3, NLRP3, the adaptor ASC and the downstream effector IL-1β were all significantly elevated in livers from patients with PBC. *P < 0.05; means ± sem; n = 4. B) Western blot and densitometry analyses showed that the protein levels of Gal3 and NLRP3 were increased in PBC livers. *P < 0.05; means ± sem; n = 4. C) Immunohistochemistry revealed enhanced signals for Gal3 (a, c) and NLRP3 (b, d) in the livers from patients with PBC compared with normal livers (NL) from healthy control subjects.
Figure 2.
The expression of Gal3 and NLRP3 inflammasome components are induced in dnTGF-βRII mice. A) In transgenic dnTGF-βRII mice that spontaneously develop PBC-like cholangitis, the transcripts of Gal3, NLRP3, ASC, and IL-1β were significantly induced in the livers compared with WT (Ctrl) livers. *P < 0.05, **P < 0.01, ***P < 0.001; means ± sem; n = 4. B) Western blots on liver homogenates depicted increased Gal3 and NLRP3 in the dnTGF-βRII mice and enhanced caspase-1 cleavage. ***P < 0.001; data are representative. C) Serum levels of Gal3 were examined by ELISA. The dnTGF-βRII mice had significantly higher levels of Gal3. *P < 0.05; means ± sem; n = 5, 13. D, E) Sera (D) and the liver homogenates (E) from WT and dnTGF-βRII mice were processed for IL-1β ELISA. Significantly higher levels of IL-1β were seen in both serum and livers from the transgenic mice. ***P < 0.001; means ± sem; n = 6.
Deoxycholic acid induces the expression of Gal3 and NLRP3 and their association in hepatic macrophages
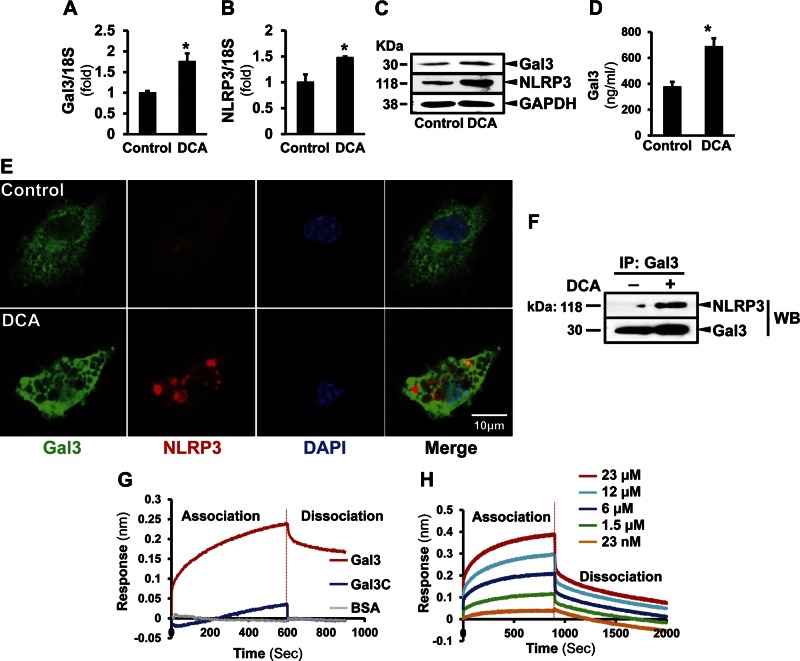
To study whether Gal3 modulates the inflammasome activation, we treated hepatic macrophages with DCA, a hydrophobic dihydroxy bile acid known to mediate hepatotoxicity (17, 18), and macrophage activation (19). DCA increased the transcripts of Gal3 by 1.75 ± 0.20-fold (P < 0.05) and NLRP3 by 1.48 ± 0.02-fold (P < 0.05) (Fig. 3A, B). The induction of Gal3 and NLRP3 by DCA was confirmed by Western blot (Fig. 3C). The cell culture medium from the DCA-treated cells also had higher level of Gal3 (687.00 ± 64.50 ng/ml) compared with the control (375.75 ± 39.00 ng/ml, P < 0.05) (Fig. 3D). Immunofluorescence studies showed intense Gal3 and NLRP3 signals in DCA-treated macrophages compared with that in the nontreated cells (Fig. 3E). Immunoprecipitation revealed the association of Gal3 and NLRP3 in macrophages, and this was enhanced by DCA (Fig. 3F), suggesting that Gal3 modulates inflammasome activation. To further confirm the direct association of Gal3 to the NLRP3 inflammasome, we next performed a cell-free BLI study (Fig. 3G, H), which showed direct binding of Gal3 to NLRP3 in a dose-dependent pattern. Interestingly, Gal3-C, the mutant without N-terminal domain, did not associate to NLRP3, suggesting that the binding site is at the N-terminal motif.
Figure 3.
DCA induces Gal3 and NLRP3 expression and their association in hepatic macrophages. Primary macrophages were isolated from mouse livers and treated with DCA (100 µM for 24 h). A, B) RT-qPCR showed significantly induced Gal3 (A) and NLRP3 (B) expression after DCA treatment (means ± sem; n = 4). *P < 0.05. C) The induction at protein levels was also detected by Western blot analysis. D) Culture medium was collected for ELISA to examine Gal3. The DCA-challenged cells released significantly higher levels of Gal3 (means ± sem; n = 6). *P < 0.05. E) To visualize Gal3 and NLRP3 in macrophages, immunofluorescence studies were done in the DCA-treated macrophages. The signals for Gal3 (green) and NLRP3 (red) were dispersed and weak in nontreated cells. In the DCA-challenged cells, enhanced Gal3 and NLRP3 were seen, and more NLRP3 clusters were observed. F) Immunoprecipitation of Gal3 and NLRP3 Western blot showed their association in DCA-treated macrophages. BLI was conducted to confirm the association. Recombinant NLRP3 loaded sensors were incubated with either recombinant WT Gal3 or N-terminal truncated Gal3 (Gal3-C) (18 μM). The association was recorded for 600 s. G) The sensors were moved to the blank buffer to measure the dissociation. Gal3 showed an obvious association/dissociation pattern. This was not present in Gal3-C. Thus, Gal3 directly interacts with NLRP3, and its N-terminal motif is crucial for this interaction. H) When the NLRP3-loaded sensors were incubated with Gal3 at a series of concentrations, a dose-dependent association was observed.
DCA induces the activation of NLRP3 signaling in WT but not in Gal3−/− macrophages
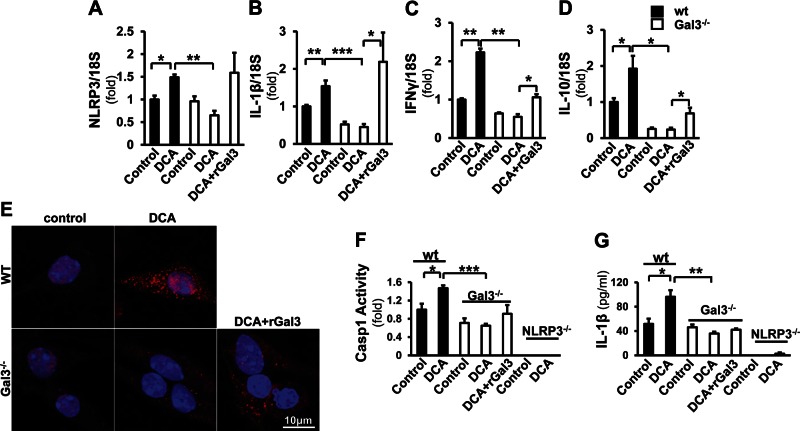
To study whether Gal3 is required for the activation of NLRP3 inflammasome, hepatic macrophages were isolated from WT and Gal3−/− mice and treated with DCA. Because recombinant Gal3 (rGal3) could be taken up by macrophages (Supplemental Fig. 1), a separate group of Gal3−/− cells was incubated with DCA in combination with rGal3 (Fig. 4). Real-time qPCR showed that DCA significantly increased the mRNA levels of NLRP3 (1.49 ± 0.06-fold, P < 0.05), IL-1β (1.54 ± 0.15-fold, P < 0.01), IFN-γ (2.23 ± 0.10-fold, P < 0.01), and IL-10 (1.93 ± 0.36-fold, P < 0.05). The expression of these genes was significantly reduced in the Gal3−/− cells (NLRP3, 0.65 ± 0.10-fold, P < 0.01; IL-1β, 0.45 ± 0.08-fold, P < 0.001; IFN-γ, 0.55 ± 0.07-fold, P < 0.01; IL-10, 0.24 ± 0.05-fold, P < 0.05). Administration of rGal3 reversed the expression of NLRP3 to 1.59 ± 0.44-fold, IL-1β to 2.19 ± 0.78-fold (P < 0.05), and partially for IFN-γ (1.06 ± 0.08-fold, P < 0.05) and IL-10 (0.69 ± 0.16-fold, P < 0.05) (Fig. 4A–D). To visualize the activation of inflammasome in macrophages, WT and Gal3−/− cells treated with DCA were stained for ASC (Fig. 4E). DCA increased the formation of ASC foci in WT cells. Fewer foci were detected in the knockout cells, and this was increased after the exposure to rGal3. To confirm that the DCA-induced inflammasome activation is Gal3 dependent, caspase-1 activity and IL-1β were measured in culture medium (Fig. 4F, G). As a control, macrophages from NLRP3−/− mice were included. Caspase-1 activity significantly increased in DCA-treated WT cells (1.47 ± 0.66-fold, P < 0.05). No induction was seen in Gal3−/− cells. Caspase-1 activity was not detected in NLRP3−/− cells. IL-1β ELISA showed a similar trend: it was induced by DCA in WT cells (96.30 ± 10.70 pg/ml) compared with the untreated cells (51.80 ± 8.50 pg/ml, P < 0.05); this effect was abolished in Gal3−/− cells. A minimal level of IL-1β was detected in NLRP3−/− cells.
Figure 4.
Gal3 is required for the induction of NLRP3 inflammasome signaling by DCA in macrophages. Macrophages from WT and Gal3−/− mice were incubated with DCA (100 μM) and with recombinant Gal3 (rGal3; 1 µM for 24 h) in the knockout cells. A–D) Real-time qPCR revealed that the expression of NLRP3 (A), IL-1β (B), IFN-γ (C), and IL-10 (D) was significantly induced in WT cells (means ± sem; n = 4) but not in the Gal3−/− cells. When the knockout cells were exposed to rGal3, the expression of these transcripts was partially reversed (means ± sem; n = 4). *P < 0.05, **P < 0.01. E) The immunofluorescent staining probing ASC (red) revealed that there were no ASC foci in nontreated cells, whereas DCA induced more ASC focus formation in WT cells than in the knockout cells. Application of rGal3 in the knockout cells enhanced the ASC signal and focus formation. F, G) To confirm that the activation of inflammasome by DCA is Gal3 dependent, caspase-1 activity (F) and IL-1β in medium (G) were assessed in WT, Gal3−/−, and NLRP3−/− macrophages. DCA significantly induced caspase-1 activity in WT cells but not in Gal3−/− cells (F). In NLRP3−/− cells, caspase-1 activity could not be detected in either DCA-treated or nontreated groups (means ± sem; n = 4). IL-1β ELISA was conducted with cell culture medium from the above cells. WT cells released more IL-1β upon the challenge of DCA; this was not seen in the Gal3−/− cells (G). A minimal amount of IL-1β was detected in NLRP3−/− cells (means ± sem; n = 4).
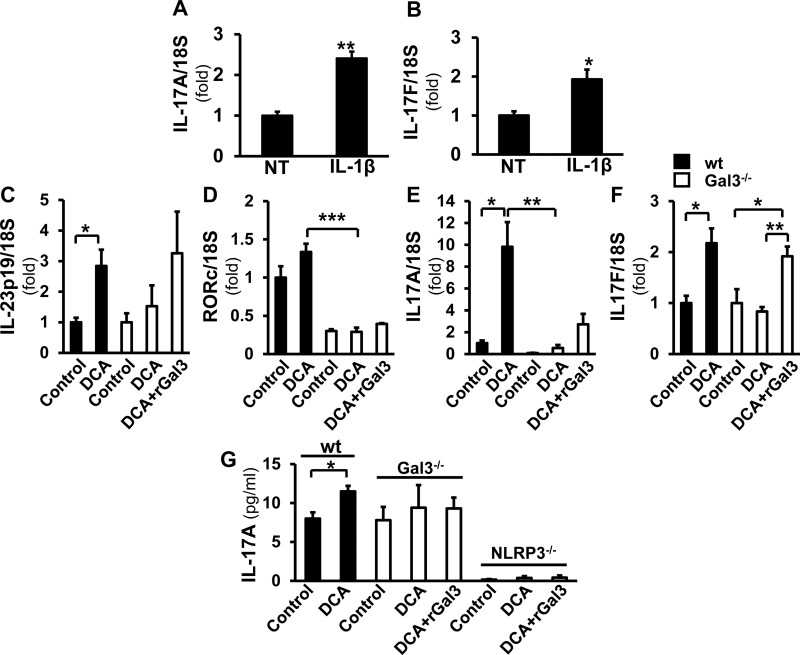
Gal3 mediates IL-23/IL-17 signaling in hepatic macrophages
IL-17 is considered a proinflammatory and profibrogenic cytokine (20). Because IL-1β is known to induce IL-23/IL-17 signaling and Th17 polarization (21, 22) and because macrophages produce significant IL-17 (23), we hypothesized that IL-1β resulting from NLRP3 inflammasome activation mediates IL-17 production by macrophages. Next, we examined whether DCA-induced IL-17 signaling is Gal3 mediated (Fig. 5). First, WT hepatic macrophages treated with IL-1β showed significantly higher levels of IL-17A (2.41 ± 0.17-fold; P < 0.01) and IL-17F (1.93 ± 0.25-fold; P < 0.05) mRNA, suggesting that IL-1β could induce IL-17 production in hepatic macrophages (Fig. 5A, B). When treated with DCA, WT macrophages displayed increased expression of IL-23p19, the IL-17 inducer (2.84 ± 0.53-fold; P < 0.05), retinoid-related orphan receptor C (RORc), the transcription factor mediating IL-17 (1.34 ± 0.11-fold), IL-17A (9.82 ± 2.27-fold; P < 0.05), and IL-17F (2.17 ± 0.29-fold; P < 0.05). This was not found in Gal3−/− cells. When Gal3−/− cells were treated with rGal3 in addition to DCA, the mRNA levels of IL-23p (3.26 ± 1.36-fold), IL-17A (2.73 ± 0.95-fold), and IL-17F (1.92 ± 0.19-fold; P < 0.05) increased (Fig. 5C–F). The IL-17A in the medium from WT cells was also increased by DCA (11.50 ± 0.30 pg/ml; P < 0.05), but no induction was found in Gal3−/− culture medium. A minimal level of IL-17A was detected in the medium from NLRP3−/− cells, and it was not altered by DCA or DCA plus rGal3 (Fig. 5G). These data suggest that extra- or intracellular Gal3 mediates the assembly and activation of NLRP3 inflammasome, resulting in IL-1β activation, which in turn activates IL-17 production by macrophages.
Figure 5.
DCA-induced IL-23/IL-17 signaling is mediated by Gal3. RT-qPCR was performed on macrophages treated with IL-1β (5 ng/ml). A, B) The transcripts of IL-17A (A) and IL-17F (B) were significantly increased (means ± sem; n = 4). *P < 0.05, **P < 0.01. C–F) DCA induced IL-23p19 (C), RORc (D), IL-17A (E), and IL-17F (F) in WT cells but not in the knockout cells. This effect was partially reversed by rGal3 (means ± sem; n = 4). *P < 0.05, **P < 0.01, ***P < 0.001. G) IL-17A in medium from DCA-treated WT, Gal3−/−, and NLRP3−/− cells was evaluated by ELISA. The DCA-treated WT cells, but not the Gal3−/− cells, released significantly more IL-17A. Only a trace amount of IL-17A was detected in NLRP3−/− cells, and rGal3 did not change this (means ± sem; n = 4). *P < 0.05.
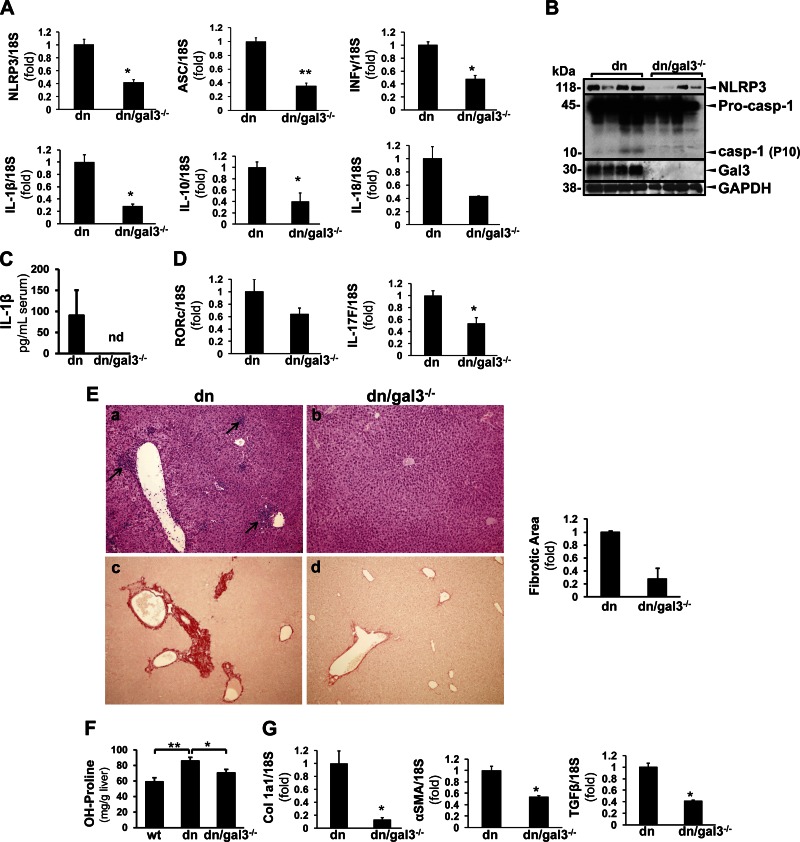
Gal3-deficient dnTGF-βRII mice display reduced inflammasome activation and collagen deposition
To assess the role of Gal3 in vivo, we crossed dnTGF-βRII with Gal3−/− mice to generate Gal3-deficient transgenic mice (dn/Gal3−/−). These mice had similar levels of the characteristic serologic hallmark AMA, anti-PDC-E2 as the Gal3-sufficient dnTGF-βRII mice (Supplemental Fig. 2A). The dnTGF-βRII mice are known to develop inflammatory bowel disease (24), and the severity of colitis was scored based on histology (Supplemental Fig. 2B, C). The dn and dn/Gal3−/− mice showed similar severity of colon inflammation.
However, in the liver, we found that knocking down Gal3 significantly reduced the transcripts of NLRP3 (0.42 ± 0.04-fold; P < 0.05), ASC (0.36 ± 0.04-fold; P < 0.05), IFN-γ (0.48 ± 0.05-fold; P < 0.05), IL-1β (0.28 ± 0.04-fold; P < 0.05), IL-10 (0.40 ± 0.15-fold; P < 0.05), and IL-18 (0.43 ± 0.01-fold; P < 0.05) (Fig. 6A). Less NLRP3 protein and caspase-1 cleavage was observed in the livers of these animals as well (Fig. 6B). The serum level of IL-1β was reduced in these mice (Fig. 6C). We next examined IL-17 signaling and found reduced RORc and IL-17F in the dn/Gal3−/− mice (0.64 ± 0.10-fold and 0.53 ± 0.10-fold, P < 0.05) (Fig. 6D). Liver histology showed improved inflammation with less inflammatory focus formation and less collagen deposition (Fig. 6E). In parallel, Gal3 deficiency significantly reduced hydroxyproline from 85.80 ± 4.58 to 70.55 ± 4.36 mg/g liver (P < 0.05) (Fig. 6F). We and others have previously reported that Gal3 is profibrogenic in BDL and CCl4 models (4, 25). The dn/Gal3−/− mice also had suppressed profibrogenic genes, including procollagen Iα1, α-SMA, and TGF-β (0.13 ± 0.03-fold, P < 0.05; 0.54 ± 0.02-fold, P < 0.05; and 0.41 ± 0.02-fold, P < 0.05, respectively) (Fig. 6G). These data support our hypothesis that Gal3 modulates the activation of NLRP3 inflammasome/IL-1β production and IL-17 signaling, contributing to the inflammatory injury and fibrogenesis in cholestatic liver injury.
Figure 6.
Gal3 deficiency prevents inflammasome activation and reduces collagen deposition in dnTGF-βRII mice. dn/Gal3−/− mice were generated by crossing Gal3−/− and dnTGF-βRII mice. A) RT-qPCR showed that dn/Gal3−/− mice expressed significantly lower levels of NLRP3, ASC, INF-γ, IL-1β, IL-10, and IL-18 compared with dnTGF-βRII (dn) mice (means ± sem; n = 6). *P < 0.05, **P < 0.01. B) Western blots showed less hepatic NLRP3 and caspase-1 cleavage (representative data). C) The dn/Gal3−/− mice also had undetectable serum IL-1β (representative data), suggesting suppressed NLRP3 inflammasome activation. D) The transcripts of RORc and IL-17F were lower in these mice as well (n = 6). *P < 0.05. E) H&E showed inflammatory granulomas in the dnTGF-βRII mice (a, arrows), whereas this was not seen in the dn/Gal3−/− group (b). Picrosirius red staining and image morphometric analysis revealed less fibrosis in the dn/Gal3−/− mice (c, d). F) Liver tissue was subjected to hydroxyproline assay. The dnTGF-βRII mice showed significantly higher amount of hydroxyproline compared with their WT littermates, and this was significantly reduced in dn/Gal3−/− mice (means ± sem; n = 9). *P < 0.05, **P < 0.01. G) Fibrogenic transcripts procollagen Ia1, α-SMA, and TGF-β also significantly decreased in the dn/Gal3−/− mice (means ± sem, n = 6). *P < 0.05.
DISCUSSION
Ursodeoxycholic acid, a hydrophilic bile acid, is the only known treatment for PBC. However, 20 to 30% of patients with PBC do not respond to ursodeoxycholic acid; thus, a large patient population still does not have adequate therapy (26). Understanding the mechanisms of key proinflammatory pathways may offer a potential new treatment approach. Herein we focused on describing a novel pathway with Gal3 as an important modulator of inflammasome signaling in hepatic macrophages, triggering IL-17 production.
In physiological conditions, Gal3 is predominantly expressed by macrophages (27, 28) in the liver. Gal3 expression is enhanced in macrophages and active stellate cells in different liver diseases (4, 25, 27, 29, 30), and its profibrogenic role has been emphasized in several studies. Gal3 deficiency or inhibition was shown to exert antifibrogenic effects in BDL, CCl4, thioacetamide, concanavalin A, and NASH diet-induced liver injuries in rodent models (4, 25, 29–32). Gal3 also plays a pivotal role in regulating innate and adaptive immune functions, modulating T-cell functions by inhibiting T-cell apoptosis, and regulating T-cell receptor signaling (33, 34). We found that Gal3 expression was increased in livers of patients with PBC and in a murine model of autoimmune cholangitis. For in vitro studies, we chose DCA to challenge the cells because it is one of the major hydrophobic cytotoxic bile acids and is highly related to the hepatotoxicity that follows the immunologic injury in autoimmune liver diseases (35, 36). Exposure of hepatic macrophages to DCA increased their Gal3 expression. DCA is known to activate NF-κB and Ap-1 (37, 38), which are known transcriptional activators of Gal3 (39–41), hence, this could represent the potential mechanism.
The key role of macrophages in PBC has been noted (42, 43). A common compound used in cosmetics and food, 2-octynoic acid could induce a PBC-like histology in mice lacking CD4+ or CD8+ T cells, with a significant increase of hepatic macrophages (44). Monocyte-derived macrophages from patients with PBC were shown to display a more inflammatory profile (43), and the exposure to apotopes led to proinflammatory cytokine production in macrophages from patients with PBC (3). To gain more mechanistic insights into the role of Gal3 in cholestatic liver injury, in this study we focused on a novel role for Gal3 as an important regulator of inflammasome activation. NLRP3 is at the crossroads of many different inflammatory pathways, however, activation of inflammasomes in autoimmune-driven cholestatic liver injury has not been evaluated. NLRP3 was shown to be activated by danger-associated molecular pattern (DAMP) molecules (8), and Gal3 is considered to be a DAMP (45). In this study, we showed that activation of NLRP3 was Gal3 dependent. We also discovered a direct binding of Gal3 N-terminal domain to NLRP3, as shown in immunoprecipitation and BLI assay, and, as a result, the activation of the inflammasome. In Gal3−/− cells, inflammasome signaling was significantly reduced, and this could be reversed by recombinant Gal3, suggesting that extracellular Gal3 also plays a role. A putative mechanism for this could be Gal3-mediated integrin crosslinking and formation of a “lattice,” as we have previously demonstrated (4), which culminates in more potent inflammasome activation.
It was previously reported that Gal3 induced the alternative activation of macrophages in the presence of T-helper (Th)2 cytokines and contributed to fibrogenesis (46). Our findings are more consistent with an early inflammatory classic activation pathway and point to the fact that Gal3 could mediate distinct effects depending on the etiology, the phase of liver injury, and cellular cross talks. A recent study in the thioacetamide model also showed that the regulatory role of Gal3 on macrophage activation could indeed be either M1 or M2 oriented (47).
Because IL-17 is a significant proinflammatory and profibrogenic factor in the liver (20) and plays a key role in PBC (48), we postulated that inflammasome-mediated IL-1β signaling can trigger Th17 responses. Indeed, a recent study showed increased hepatic Th17 in patients with advanced PBC (49), and the induction of Th17 in PBC was preferential to the liver (48). Here we showed that Gal3-induced inflammasome activation resulted in the production of IL-1β, and this in turn enhanced IL-17 production by macrophages in an autocrine manner. Whether Gal3 mediates Th17 differentiation is unknown and requires further study.
Nitrosative stress is another pathogenic factor in cholestatic liver injury, and the accumulation of nitrotyrosine correlates with bile duct damage in PBC, reflecting disease severity (50). Because IL-1β can induce nitrosative stress (51), this could be an alternative pathway by which the Gal3/inflammasome/IL-1β axis contributes to the disease pathogenesis.
The dnTGF-βRII mice spontaneously develop intrahepatic bile duct destruction and are positive for AMA. This model has been used to study the pathogenesis of PBC, although it is not a perfect model because it does not mimic all aspects of the human disease (11–13). However, to study PBC there is a paucity of available models that would satisfy all criteria. These mice are also known to develop colitis, but global knockdown of Gal3 did not protect against bowel inflammation (Supplemental Fig. 2B, C). It was reported recently that Gal3 may exert a protective role in colon inflammatory diseases (52). M.E.G.’s group also showed that autoimmune cholangitis and colitis may have distinct pathogenic pathways in dnTGF-βRII mice (53).
In summary, NLRP3 inflammasome activation is a key to early proinflammatory injury in autoimmune-driven biliary injury, and Gal3 plays an important role in inflammasome activation. Interrupting the signaling cascade with Gal3 or NLRP3 inhibitors could be potential therapeutic strategies for PBC and other chronic cholestatic liver diseases.
Supplementary Material
ACKNOWLEDGMENTS
This study was supported by the U.S. National Institutes of Health (NIH) National Institute of Diabetes and Digestive and Kidney Diseases Grants DK090121 and DK106467 (to J.X.J.), DK083283 (to N.J.T.), DK39588 and DK090019 (to M.E.G.); U.S. Veterans Administration Grant BX002418 (to N.J.T.); National Natural Science Foundation of China Grant NNSFC21525730 (to B.Z.); and NIH National Institute on Aging Grant AG025532 (to G.C.); and Taiwan Ministry of Science and Technology Grant MOST104-2321-B-001-036 (to H.-Y.C.). The authors declare no conflicts of interest.
Glossary
- AMA
antimitochondria antibody
- ASC
adaptor apoptosis-associated speck-like protein
- BLI
biolayer interferometry
- DCA
deoxycholic acid
- Gal3
galectin-3
- NLRP3
NOD-like receptor family, pyrin domain containing 3
- PBC
primary biliary cholangitis
- qPCR
quantitative PCR
- rGal3
recombinant galectin-3
- RORc
retinoid-related orphan receptor C
- Th
T helper
- WT
wild-type
Footnotes
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
AUTHOR CONTRIBUTIONS
J. Tian and G. Yang performed the experiments; J. Tian and J. X. Jiang wrote the manuscript; G. Yang, H.-Y. Chen, D. K. Hsu, G. Cortopassi, B. Zhao, N, J. Török, and J. X. Jiang analyzed the data; H.-Y. Chen, D. K. Hsu, and G. Cortopassi participated in the discussion; A. Tomilov performed the BLI assay; K. A. Olson analyzed the histology data; A. Dehnad conducted colon sample processing and analysis; S. R. Fish conducted genotyping and participated in colony management and language correction; B. Zhao interpreted the data; M. E. Gershwin and F.-T. Liu collaborated on this study; M. E. Gershwin provided dnTGF-βRII mice and samples from PBC patients; F.-T. Liu provided Gal3−/− mice, Gal3 antibody, and recombinant proteins; N. J. Török and J. X. Jiang designed the experiment; N. J. Török edited the manuscript; and J. X. Jiang acquired funding for this experiment.
REFERENCES
- 1.He X. S., Ansari A. A., Ridgway W. M., Coppel R. L., Gershwin M. E. (2006) New insights to the immunopathology and autoimmune responses in primary biliary cirrhosis. Cell. Immunol. 239, 1–13 [DOI] [PubMed] [Google Scholar]
- 2.Chuang Y. H., Lian Z. X., Yang G. X., Shu S. A., Moritoki Y., Ridgway W. M., Ansari A. A., Kronenberg M., Flavell R. A., Gao B., Gershwin M. E. (2008) Natural killer T cells exacerbate liver injury in a transforming growth factor beta receptor II dominant-negative mouse model of primary biliary cirrhosis. Hepatology 47, 571–580 [DOI] [PubMed] [Google Scholar]
- 3.Lleo A., Bowlus C. L., Yang G. X., Invernizzi P., Podda M., Van de Water J., Ansari A. A., Coppel R. L., Worman H. J., Gores G. J., Gershwin M. E. (2010) Biliary apotopes and anti-mitochondrial antibodies activate innate immune responses in primary biliary cirrhosis. Hepatology 52, 987–998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jiang J. X., Chen X., Hsu D. K., Baghy K., Serizawa N., Scott F., Takada Y., Takada Y., Fukada H., Chen J., Devaraj S., Adamson R., Liu F.-T., Török N. J. (2012) Galectin-3 modulates phagocytosis-induced stellate cell activation and liver fibrosis in vivo. Am. J. Physiol. Gastrointest. Liver Physiol. 302, G439–G446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liu F.-T. (1990) Molecular biology of IgE-binding protein, IgE-binding factors, and IgE receptors. Crit. Rev. Immunol. 10, 289–306 [PubMed] [Google Scholar]
- 6.Wree A., Eguchi A., McGeough M. D., Pena C. A., Johnson C. D., Canbay A., Hoffman H. M., Feldstein A. E. (2014) NLRP3 inflammasome activation results in hepatocyte pyroptosis, liver inflammation, and fibrosis in mice. Hepatology 59, 898–910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wree A., McGeough M. D., Peña C. A., Schlattjan M., Li H., Inzaugarat M. E., Messer K., Canbay A., Hoffman H. M., Feldstein A. E. (2014) NLRP3 inflammasome activation is required for fibrosis development in NAFLD. J. Mol. Med. 92, 1069–1082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Davis B. K., Wen H., Ting J. P. (2011) The inflammasome NLRs in immunity, inflammation, and associated diseases. Annu. Rev. Immunol. 29, 707–735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhu P., Duan L., Chen J., Xiong A., Xu Q., Zhang H., Zheng F., Tan Z., Gong F., Fang M. (2011) Gene silencing of NALP3 protects against liver ischemia-reperfusion injury in mice. Hum. Gene Ther. 22, 853–864 [DOI] [PubMed] [Google Scholar]
- 10.Lalor S. J., Dungan L. S., Sutton C. E., Basdeo S. A., Fletcher J. M., Mills K. H. (2011) Caspase-1-processed cytokines IL-1beta and IL-18 promote IL-17 production by gammadelta and CD4 T cells that mediate autoimmunity. J. Immunol. 186, 5738–5748 [DOI] [PubMed] [Google Scholar]
- 11.Oertelt S., Lian Z. X., Cheng C. M., Chuang Y. H., Padgett K. A., He X. S., Ridgway W. M., Ansari A. A., Coppel R. L., Li M. O., Flavell R. A., Kronenberg M., Mackay I. R., Gershwin M. E. (2006) Anti-mitochondrial antibodies and primary biliary cirrhosis in TGF-beta receptor II dominant-negative mice. J. Immunol. 177, 1655–1660 [DOI] [PubMed] [Google Scholar]
- 12.Kawata K., Yang G. X., Ando Y., Tanaka H., Zhang W., Kobayashi Y., Tsuneyama K., Leung P. S., Lian Z. X., Ridgway W. M., Ansari A. A., He X. S., Gershwin M. E. (2013) Clonality, activated antigen-specific CD8(+) T cells, and development of autoimmune cholangitis in dnTGFβRII mice. Hepatology 58, 1094–1104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang J., Yang G. X., Tsuneyama K., Gershwin M. E., Ridgway W. M., Leung P. S. (2014) Animal models of primary biliary cirrhosis. Semin. Liver Dis. 34, 285–296 [DOI] [PubMed] [Google Scholar]
- 14.Hsu D. K., Yang R. Y., Pan Z., Yu L., Salomon D. R., Fung-Leung W. P., Liu F.-T. (2000) Targeted disruption of the galectin-3 gene results in attenuated peritoneal inflammatory responses. Am. J. Pathol. 156, 1073–1083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Geerts A., Niki T., Hellemans K., De Craemer D., Van Den Berg K., Lazou J. M., Stange G., Van De Winkel M., De Bleser P. (1998) Purification of rat hepatic stellate cells by side scatter-activated cell sorting. Hepatology 27, 590–598 [DOI] [PubMed] [Google Scholar]
- 16.Hsu D. K., Zuberi R. I., Liu F.-T. (1992) Biochemical and biophysical characterization of human recombinant IgE-binding protein, an S-type animal lectin. J. Biol. Chem. 267, 14167–14174 [PubMed] [Google Scholar]
- 17.Qiao L., Studer E., Leach K., McKinstry R., Gupta S., Decker R., Kukreja R., Valerie K., Nagarkatti P., El Deiry W., Molkentin J., Schmidt-Ullrich R., Fisher P. B., Grant S., Hylemon P. B., Dent P. (2001) Deoxycholic acid (DCA) causes ligand-independent activation of epidermal growth factor receptor (EGFR) and FAS receptor in primary hepatocytes: inhibition of EGFR/mitogen-activated protein kinase-signaling module enhances DCA-induced apoptosis. Mol. Biol. Cell 12, 2629–2645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ferreira D. M., Afonso M. B., Rodrigues P. M., Simão A. L., Pereira D. M., Borralho P. M., Rodrigues C. M., Castro R. E. (2014) c-Jun N-terminal kinase 1/c-Jun activation of the p53/microRNA 34a/sirtuin 1 pathway contributes to apoptosis induced by deoxycholic acid in rat liver. Mol. Cell. Biol. 34, 1100–1120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ljubuncic P., Fuhrman B., Oiknine J., Aviram M., Bomzon A. (1996) Effect of deoxycholic acid and ursodeoxycholic acid on lipid peroxidation in cultured macrophages. Gut 39, 475–478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Meng F., Wang K., Aoyama T., Grivennikov S. I., Paik Y., Scholten D., Cong M., Iwaisako K., Liu X., Zhang M., Osterreicher C. H., Stickel F., Ley K., Brenner D. A., Kisseleva T. (2012) Interleukin-17 signaling in inflammatory, Kupffer cells, and hepatic stellate cells exacerbates liver fibrosis in mice. Gastroenterology 143, 765–776 e1-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Besnard A. G., Togbe D., Couillin I., Tan Z., Zheng S. G., Erard F., Le Bert M., Quesniaux V., Ryffel B. (2012) Inflammasome-IL-1-Th17 response in allergic lung inflammation. J. Mol. Cell Biol. 4, 3–10 [DOI] [PubMed] [Google Scholar]
- 22.Acosta-Rodriguez E. V., Napolitani G., Lanzavecchia A., Sallusto F. (2007) Interleukins 1beta and 6 but not transforming growth factor-beta are essential for the differentiation of interleukin 17-producing human T helper cells. Nat. Immunol. 8, 942–949 [DOI] [PubMed] [Google Scholar]
- 23.Bosmann M., Sarma J. V., Atefi G., Zetoune F. S., Ward P. A. (2012) Evidence for anti-inflammatory effects of C5a on the innate IL-17A/IL-23 axis. FASEB J. 26, 1640–1651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gorelik L., Flavell R. A. (2000) Abrogation of TGFbeta signaling in T cells leads to spontaneous T cell differentiation and autoimmune disease. Immunity 12, 171–181 [DOI] [PubMed] [Google Scholar]
- 25.Henderson N. C., Mackinnon A. C., Farnworth S. L., Poirier F., Russo F. P., Iredale J. P., Haslett C., Simpson K. J., Sethi T. (2006) Galectin-3 regulates myofibroblast activation and hepatic fibrosis. Proc. Natl. Acad. Sci. USA 103, 5060–5065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Eisa N. H., Ebrahim M. A., Ragab M., Eissa L. A., El-Gayar A. M. (2014) Galectin-3 and matrix metalloproteinase-9: Perspective in management of hepatocellular carcinoma. J. Oncol. Pharm. Pract.°21, 323–330 [DOI] [PubMed] [Google Scholar]
- 27.Sano H., Hsu D. K., Apgar J. R., Yu L., Sharma B. B., Kuwabara I., Izui S., Liu F.-T. (2003) Critical role of galectin-3 in phagocytosis by macrophages. J. Clin. Invest. 112, 389–397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hsu D. K., Dowling C. A., Jeng K. C., Chen J. T., Yang R. Y., Liu F.-T. (1999) Galectin-3 expression is induced in cirrhotic liver and hepatocellular carcinoma. Int. J. Cancer 81, 519–526 [DOI] [PubMed] [Google Scholar]
- 29.Nomoto K., Nishida T., Nakanishi Y., Fujimoto M., Takasaki I., Tabuchi Y., Tsuneyama K. (2012) Deficiency in galectin-3 promotes hepatic injury in CDAA diet-induced nonalcoholic fatty liver disease. ScientificWorldJournal 2012, 959824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Iacobini C., Menini S., Ricci C., Blasetti Fantauzzi C., Scipioni A., Salvi L., Cordone S., Delucchi F., Serino M., Federici M., Pricci F., Pugliese G. (2011) Galectin-3 ablation protects mice from diet-induced NASH: a major scavenging role for galectin-3 in liver. J. Hepatol. 54, 975–983 [DOI] [PubMed] [Google Scholar]
- 31.Wijesundera K. K., Izawa T., Tennakoon A. H., Murakami H., Golbar H. M., Kato-Ichikawa C., Tanaka M., Kuwamura M., Yamate J. (2014) M1- and M2-macrophage polarization in rat liver cirrhosis induced by thioacetamide (TAA), focusing on Iba1 and galectin-3. Exp. Mol. Pathol. 96, 382–392 [DOI] [PubMed] [Google Scholar]
- 32. doi: 10.1371/journal.pone.0083481. T raber, P. G., and Zomer, E. (2013) Therapy of experimental NASH and fibrosis with galectin inhibitors. PloS One 8, e83481. [DOI] [PMC free article] [PubMed]
- 33.Hsu D. K., Chen H. Y., Liu F.-T. (2009) Galectin-3 regulates T-cell functions. Immunol. Rev. 230, 114–127 [DOI] [PubMed] [Google Scholar]
- 34.Chen H. Y., Fermin A., Vardhana S., Weng I. C., Lo K. F., Chang E. Y., Maverakis E., Yang R. Y., Hsu D. K., Dustin M. L., Liu F.-T. (2009) Galectin-3 negatively regulates TCR-mediated CD4+ T-cell activation at the immunological synapse. Proc. Natl. Acad. Sci. USA 106, 14496–14501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Beuers U., Boyer J. L., Paumgartner G. (1998) Ursodeoxycholic acid in cholestasis: potential mechanisms of action and therapeutic applications. Hepatology 28, 1449–1453 [DOI] [PubMed] [Google Scholar]
- 36.Rodrigues C. M., Fan G., Wong P. Y., Kren B. T., Steer C. J. (1998) Ursodeoxycholic acid may inhibit deoxycholic acid-induced apoptosis by modulating mitochondrial transmembrane potential and reactive oxygen species production. Mol. Med. 4, 165–178 [PMC free article] [PubMed] [Google Scholar]
- 37.Jenkins G. J., Harries K., Doak S. H., Wilmes A., Griffiths A. P., Baxter J. N., Parry J. M. (2004) The bile acid deoxycholic acid (DCA) at neutral pH activates NF-kappaB and induces IL-8 expression in oesophageal cells in vitro. Carcinogenesis 25, 317–323 [DOI] [PubMed] [Google Scholar]
- 38.Looby E., Abdel-Latif M. M., Athié-Morales V., Duggan S., Long A., Kelleher D. (2009) Deoxycholate induces COX-2 expression via Erk1/2-, p38-MAPK and AP-1-dependent mechanisms in esophageal cancer cells. BMC Cancer 9, 190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hsu D. K., Hammes S. R., Kuwabara I., Greene W. C., Liu F.-T. (1996) Human T lymphotropic virus-I infection of human T lymphocytes induces expression of the beta-galactoside-binding lectin, galectin-3. Am. J. Pathol. 148, 1661–1670 [PMC free article] [PubMed] [Google Scholar]
- 40.Liu L., Sakai T., Sano N., Fukui K. (2004) Nucling mediates apoptosis by inhibiting expression of galectin-3 through interference with nuclear factor kappaB signalling. Biochem. J. 380, 31–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kim K., Mayer E. P., Nachtigal M. (2003) Galectin-3 expression in macrophages is signaled by Ras/MAP kinase pathway and up-regulated by modified lipoproteins. Biochim. Biophys. Acta 1641, 13–23 [DOI] [PubMed] [Google Scholar]
- 42.Leicester K. L., Olynyk J. K., Brunt E. M., Britton R. S., Bacon B. R. (2006) Differential findings for CD14-positive hepatic monocytes/macrophages in primary biliary cirrhosis, chronic hepatitis C and nonalcoholic steatohepatitis. Liver Int. 26, 559–565 [DOI] [PubMed] [Google Scholar]
- 43.Mao T. K., Lian Z. X., Selmi C., Ichiki Y., Ashwood P., Ansari A. A., Coppel R. L., Shimoda S., Ishibashi H., Gershwin M. E. (2005) Altered monocyte responses to defined TLR ligands in patients with primary biliary cirrhosis. Hepatology 42, 802–808 [DOI] [PubMed] [Google Scholar]
- 44.Chang C. H., Chen Y. C., Yu Y. H., Tao M. H., Leung P. S., Ansari A. A., Gershwin M. E., Chuang Y. H. (2014) Innate immunity drives xenobiotic-induced murine autoimmune cholangitis. Clin. Exp. Immunol. 177, 373–380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sato S., St-Pierre C., Bhaumik P., Nieminen J. (2009) Galectins in innate immunity: dual functions of host soluble beta-galactoside-binding lectins as damage-associated molecular patterns (DAMPs) and as receptors for pathogen-associated molecular patterns (PAMPs). Immunol. Rev. 230, 172–187 [DOI] [PubMed] [Google Scholar]
- 46.MacKinnon A. C., Farnworth S. L., Hodkinson P. S., Henderson N. C., Atkinson K. M., Leffler H., Nilsson U. J., Haslett C., Forbes S. J., Sethi T. (2008) Regulation of alternative macrophage activation by galectin-3. J. Immunol. 180, 2650–2658 [DOI] [PubMed] [Google Scholar]
- 47.Wijesundera K. K., Izawa T., Tennakoon A. H., Murakami H., Golbar H. M., Katou-Ichikawa C., Tanaka M., Kuwamura M., Yamate J. (2014) M1- and M2-macrophage polarization in rat liver cirrhosis induced by thioacetamide (TAA), focusing on Iba1 and galectin-3. Exp. Mol. Pathol. 96, 382–392 [DOI] [PubMed] [Google Scholar]
- 48.Lan R. Y., Salunga T. L., Tsuneyama K., Lian Z. X., Yang G. X., Hsu W., Moritoki Y., Ansari A. A., Kemper C., Price J., Atkinson J. P., Coppel R. L., Gershwin M. E. (2009) Hepatic IL-17 responses in human and murine primary biliary cirrhosis. J. Autoimmun. 32, 43–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yang C. Y., Ma X., Tsuneyama K., Huang S., Takahashi T., Chalasani N. P., Bowlus C. L., Yang G. X., Leung P. S., Ansari A. A., Wu L., Coppel R. L., Gershwin M. E. (2014) IL-12/Th1 and IL-23/Th17 biliary microenvironment in primary biliary cirrhosis: implications for therapy. Hepatology 59, 1944–1953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sanz-Cameno P., Medina J., García-Buey L., García-Sánchez A., Borque M. J., Martín-Vílchez S., Gamallo C., Jones E. A., Moreno-Otero R. (2002) Enhanced intrahepatic inducible nitric oxide synthase expression and nitrotyrosine accumulation in primary biliary cirrhosis and autoimmune hepatitis. J. Hepatol. 37, 723–729 [DOI] [PubMed] [Google Scholar]
- 51.Geller D. A., de Vera M. E., Russell D. A., Shapiro R. A., Nussler A. K., Simmons R. L., Billiar T. R. (1995) A central role for IL-1 beta in the in vitro and in vivo regulation of hepatic inducible nitric oxide synthase. IL-1 beta induces hepatic nitric oxide synthesis. J. Immunol. 155, 4890–4898 [PubMed] [Google Scholar]
- 52.Tsai H. F., Wu C. S., Chen Y. L., Liao H. J., Chyuan I. T., Hsu P. N. (2016) Galectin-3 suppresses mucosal inflammation and reduces disease severity in experimental colitis. J. Mol. Med. 94, 545–556 [DOI] [PubMed] [Google Scholar]
- 53.Zhang W., Tsuda M., Yang G. X., Tsuneyama K., Rong G., Ridgway W. M., Ansari A. A., Flavell R. A., Coppel R. L., Lian Z. X., Gershwin M. E. (2010) Deletion of interleukin-6 in mice with the dominant negative form of transforming growth factor beta receptor II improves colitis but exacerbates autoimmune cholangitis. Hepatology 52, 215–222 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.