Abstract
Inflammatory molecules and matrix metalloproteinase (MMPs) have been found over-expressed in the tear film of patients with keratoconus. However, the mechanistic link between inflammatory molecules and MMPs in the pathogenesis of keratoconus remains still elusive. Therefore, we investigated the effect of tumor necrosis factor-α (TNF-α) and interleukin-6 (IL-6) on MMP-1 expression and used IL-6 antibody (IL-6 Ab) to examine the role of IL-6 on TNF-α mediated regulation of MMP-1 in fibroblasts of normal cornea and keratoconus. Real-time polymerase chain reaction, Enzyme-linked immunosorbent assay, and Western blot data demonstrated that MMP-1 and IL-6 were expressed in fibroblasts of normal cornea and keratoconus. Levels of MMP-1 and IL-6 were significantly higher in keratoconus than normal cornea. TNF-α treatment led to a significant increase in IL-6 levels. IL-6 treatment induced MMP-1 synthesis in normal cornea and keratoconus. TNF-α increased MMP-1 expression in a dose- and time-dependent manner and this response was completely inhibited by the IL-6 Ab. In conclusion, these results indicate that fibroblasts of keratoconus shows increased levels of IL-6 and MMP-1 gene and protein expression and IL-6 mediates the TNF-α-induced MMP-1 expression.
Keywords: Corneal fibroblasts, keratoconus, matrix metalloproteinases (MMPs), inflammatory cytokine
Introduction
Keratoconus is defined as a non-inflammatory disease of the cornea, however, in recent years, several groups have demonstrated that the tear film in keratoconus showed increased levels of inflammatory molecules (IL-4, -5, -6, -8, TNF-α, -β) compared with normal controls. The extent of the increase was found to be associated with the severity of keratoconus. This suggested that the pathogenesis of keratoconus may involve chronic inflammatory events.1–4
Interleukin-6 (IL-6) is a pleiotropic cytokine with a wide range of biological activities in immune regulation, hematopoiesis, inflammation, and oncogenesis.5,6 IL-6 could play an important role in the generation of inflammation within the cornea.7,8 They are secreted mainly by lymphocytes and macrophages, but other cell types including corneal epithelial cells and corneal fibroblasts also secret them.9,10 Tumor necrosis factor-α (TNF-α) is widely considered an important cytokine mediator of inflammation and immune regulation that can cause a range of local and systemic biological effects in various cell types.11,12 TNF-α was also one of the cytokines capable of inducing IL-6 expression in human corneal epithelial cells.13,14 Furthermore, recent studies have demonstrated that TNF-α plays an important role in a variety of corneal diseases.15–17
Matrix metalloproteinases (MMPs) are a family of zinc-dependent enzymes that are responsible for the degradation of extracellular matrix (ECM) and connective tissue protein.18 In the human cornea, MMPs can be secreted by epithelial cells, stromal cells, and neutrophils.19 Among MMPs, MMP-1, -2, -3, -7, -9, and -13 have been found to be elevated in the tear film of patients with keratoconus, indicating that MMPs may be involved in the pathogenesis of keratoconus.20–23
These findings suggest that the development of keratoconus may involve chronic inflammatory reaction and matrix degradation and there may be a potential link between inflammatory molecules and MMPs. Inflammatory cytokines such as IL-6 and TNF-α can modulate the expression of MMPs by human corneal epithelial cells.24,25 MMP-1 is mainly used for the degradation of collagen type I and III which are the major components of corneal ECM.26 Therefore, in our study, we treated cultured fibroblasts of normal cornea and keratoconus with TNF-α, IL-6, and IL-6 antibody (IL-6 Ab) in vitro. We examined the MMP-1 levels in order to investigate the potential link between inflammatory molecules and MMPs during keratoconus.
Materials and methods
Isolation and culture of fibroblasts
Keratoconus corneas (n = 3. age / age range = 18 or 16–20) were collected from patients with keratoconus who were undergoing corneal transplantation within 6–12 h following surgery from Shanxi Eye Hospital (Tai yuan, China). Normal corneas (n = 3; age unknown) were obtained from discarded corneal remnants after corneal transplantation. The study was undertaken with the approval of the local ethics committee and after obtaining written informed consent from each patient. The epithelium and endothelium of corneas were mechanically removed. The corneal stroma was cut into several pieces with diameter of 1.0 mm. Subsequently, the small tissue pieces were digested into single cells with DMEM/F12 medium containing 2 mg/mL of type II collagenase. Cells were cultured in DMEM/F12 containing fetal bovine serum and in an air-5% CO2 incubator at 37℃. Experiments were performed by using cells from passages 3–5.
For each experiment, fibroblasts were seeded at six-well plates with an initial density of 5 × 105/well. Cells were then treated with one of the following protocols: (1) TNF-α (1, 10, or 50 ng/mL; R&D Systems, Minneapolis, MN) for 12 h or with various stimulation time courses (2, 6, or 12 h) to test for MMP-1 and IL-6 expression; (2) IL-6 (6.25, 12.5, 25 or 50 ng/mL; R&D Systems) for 12 h or with various incubation times (2, 6, or 12 h) to test for MMP-1 expression; (3) 1 ng/mL TNF-α, or 25 ng/mL IL-6 or 1 ng/mL TNF-α + 25 ng/mL IL-6 for 12 h to detect for MMP-1 expression; (4) 1 ng/mL TNF-α or 1 ng/mL TNF-α + 40 ng/mL IL-6 Ab (Peprotech, Rocky Hill, NJ) for 12 h to test the production of IL-6 and MMP-1 expression. No significant cytotoxic effect on cell proliferation (data not shown). At the end of each experiment, the cells and supernatants from cell cultures were collected for gene or protein detection, respectively.
Real-time polymerase chain reaction (real-time PCR)
RNA extraction was performed according to the protocol reported previously. One microgram of RNA was converted to cDNA with the PrimeScript™ RT reagent Kit (TaKaRa Biotechnology CO., Dalian, China) by gradient PCR device (Eppendorf, Germany). Two microliters of 30-fold diluted cDNA products were amplified with SYBR® Premix Ex Taq™ II (TaKaRa Biotechnology CO) by the StepOnePlus™ Real-Time PCR System (Applied Biosystems, USA) using the following gene specific primers designed by Shanghai Sangon Biotechnology Co., Ltd. (Shanghai, China).
For quantification, IL-6 and MMP-1 mRNA expression were normalized to the expressed housekeeping gene glyceraldehyde-3-phosphate dehydrogenase (GAPDH). Real-time PCR conditions were as follows: 95℃ for 30 s and then 40 cycles at 95℃ for 5 s, 60℃ for 30 s, and 72℃ for 1 min, followed by 72℃ for 10 min. The relative quantity of mRNA was calculated using the 2−ΔΔCt method.
| Primer | 5′–3′ sequence (forward; reverse) |
| MMP-1 | For: GGGAGATCATCGGGACAACTC Rev: GGGCCTGGTTGAAAAGCAT |
| IL-6 | For: CCTGAACCTTCCAAAGATGGC Rev: CTTGGGGTTCTTGCTGATGT |
| GAPDH | For: AAGGTCGGAGTGAACGGATTTG Rev: TTCACCAGGCAAGTCTCCTCA |
Enzyme-linked immunosorbent assay (ELISA)
A human IL-6 ELISA kit (BioSource International, Camarillo, CA) was used to measure the IL-6 production in samples (10 µL cell-culture supernatant containing equal amounts of protein [15 µg]) according to the manufacturer's recommendations. The optical density was measured at 450 nm in a microplate reader (Multiskan Go, Thermo Scientific, USA).
Western blot
Equal amounts of protein (25 µg), as determined by the bicinchoninic acid (BCA) protein assay kit (Shanghai Sangon Biotechnology Co.), were loaded into a 10% sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS–PAGE). Protein samples were mixed with loading buffer (20% glycerol, 10% 2-mercaptoethanol, 4% SDS, and 0.2 mg/mL bromophenol blue in 0.1 mol/L Tris-HCl [pH 6.8]), boiled and then separated by SDS–PAGE. Proteins were transferred to polyvinylidene difluoride (PVDF) membrane (Millipore, Billerica, MA). After blocking and washing, membranes were incubated overnight at 4℃ with the primary antibody (Anti-MMP1 antibody, EP1247Y, ab52631, Abcam, Cambridge, MA) as indicated (1:2000 dilution) and then followed by incubation with a 1:7500 dilution of Goat Anti-Mouse IgG (Abcam) at room temperature. The membranes with protein bands were visualized by enhanced chemiluminescence detection reagents (Applygen Technologies Inc., Beijing, China) and exposed to X-ray film. Finally, densitometry of bands is measured by Image-Pro Plus 5.1.
Statistical analysis
All results were reported as mean ± standard deviation of three independent experiments and statistically performed by using SPSS v.19.0 software and one-way analysis of variance (ANOVA) analysis. Specific percentage changes have been provided in the online supplementary Tables 1–8. A P value < 0.05 was considered to indicate a statistically significant difference.
Results
TNF-α induces MMP-1 mRNA expression and protein synthesis
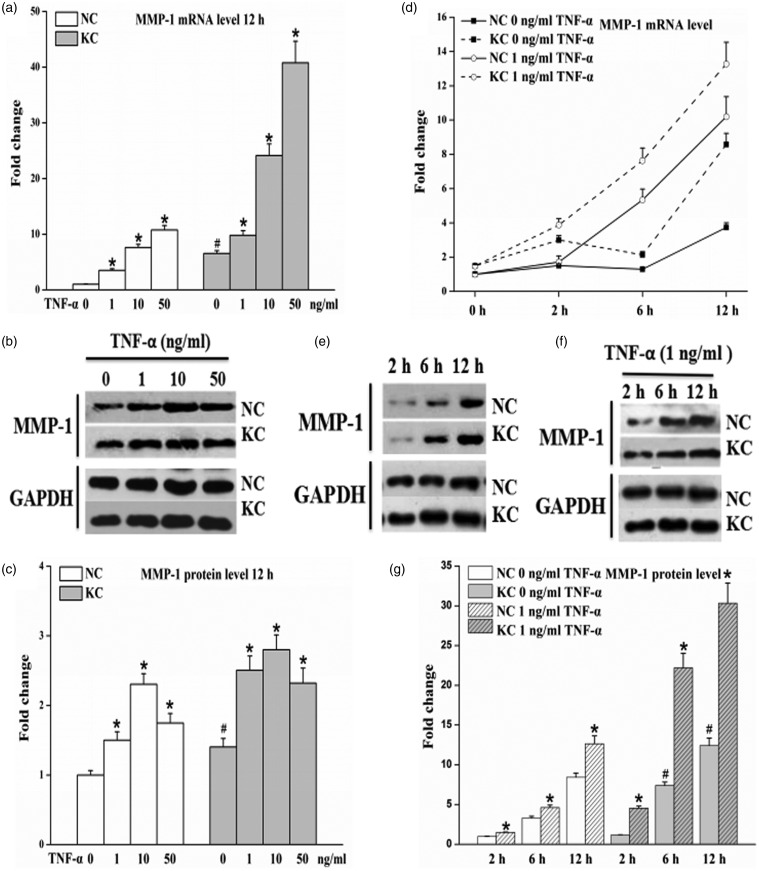
Levels of MMP-1 mRNA in fibroblasts of keratoconus cultured without TNF-α were significantly higher than that in normal cornea and TNF-α up-regulated MMP-1 mRNA in a dose-dependent manner (P < 0.05) (Figure 1a). MMP-1 protein levels in keratoconus without TNF-α were significantly higher than that in normal cornea and the levels in cells with TNF-α for 12 h began to increase at 1 ng/mL TNF-α, remained increased with concentration of 10 ng/mL TNF-α, and then decreased at 50 ng/mL TNF-α (P < 0.05) (Figure 1b and c). Levels of MMP-1 mRNA in fibroblasts of keratoconus without TNF-α were higher than that in normal cornea at 2, 6 or 12 h; Higher levels of MMP-1 mRNA in cultured cells under 1 ng/mL TNF-α for various stimulation times than that without TNF-α incubation (Figure 1d); TNF-α up-regulated MMP-1 protein synthesis levels in cells incubated with 1 ng/mL TNF-α than that without TNF-α incubation and the levels increased in a time-dependent manner (P < 0.05) (Figure 1e–g).
Figure 1.
Effect of TNF-α on MMP-1 mRNA expression and protein synthesis in fibroblasts of normal cornea (n = 3) and keratoconus (n = 3). Cells were incubated for 12 h in the presence of various concentrations of TNF-α and stimulated with the absence or presence of 1 ng/mL TNF-α for the indicated times intervals. Total RNA and cell-culture supernatants were then collected. (a and d) Levels of MMP-1 mRNA measured by real-time PCR from total RNA. (b, e, and f) Representative Western blot for MMP-1 protein in cell-culture supernatants. (c and g) Quantitative analysis of MMP-1 protein levels by Image-Pro Plus. The results are presented as mean ± standard deviation of three independent experiments. KC: keratoconus, NC: normal cornea. Cells without TNF-α treatment were used as control. * P < 0.05, compared with their own control group; #P < 0.05, KC control group vs. NC control group
TNF-α up-regulates the levels of IL-6
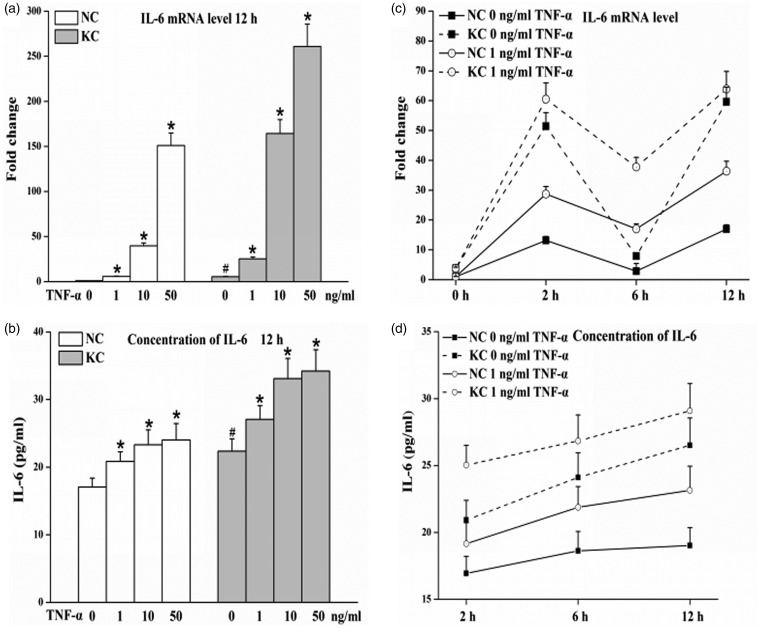
Either IL-6 mRNA or protein levels in fibroblasts of keratoconus cultured without TNF-α for 12 h were significantly higher than that in normal cornea; TNF-α induced IL-6 mRNA and protein levels in a dose-dependent manner in cultured fibroblasts (P < 0.05) (Figure 2a and b). Levels of IL-6 mRNA and protein in fibroblasts of keratoconus without TNF-α were higher than that in normal cornea at 2, 6 or 12 h; IL-6 mRNA expression in the presence of TNF-α (1 ng/mL) was increased than that in the absence of TNF-α at any indicated times intervals; moreover, incubation with 1 ng/mL TNF-α for 2 h increased the IL-6 protein levels than that without TNF-α, and greater up-regulation in IL-6 protein levels were found on stimulation with 1 ng/mL TNF-α for 6 h and 12 h (Figure 2c and d).
Figure 2.
Levels of IL-6 in fibroblasts of normal cornea (n = 3) and keratoconus (n = 3) cultured with TNF-α. Cells were treated with various TNF-α concentrations for 12 h or incubated with the absence or presence of 1 ng/mL TNF-α for various incubation times. Total RNA and cell-culture supernatants were then collected. (a and c) IL-6 mRNA levels determined by real-time PCR from total RNA. (b and d) IL-6 protein in supernatants measured using a human IL-6 ELISA kit. Values are presented as mean ± standard deviation of three independent experiments. KC: keratoconus, NC: normal cornea. Cells without TNF-α treatment were used as control. *P < 0.05, compared with their own control group; #P < 0.05, KC control vs. NC control
IL-6 induces MMP-1 expression
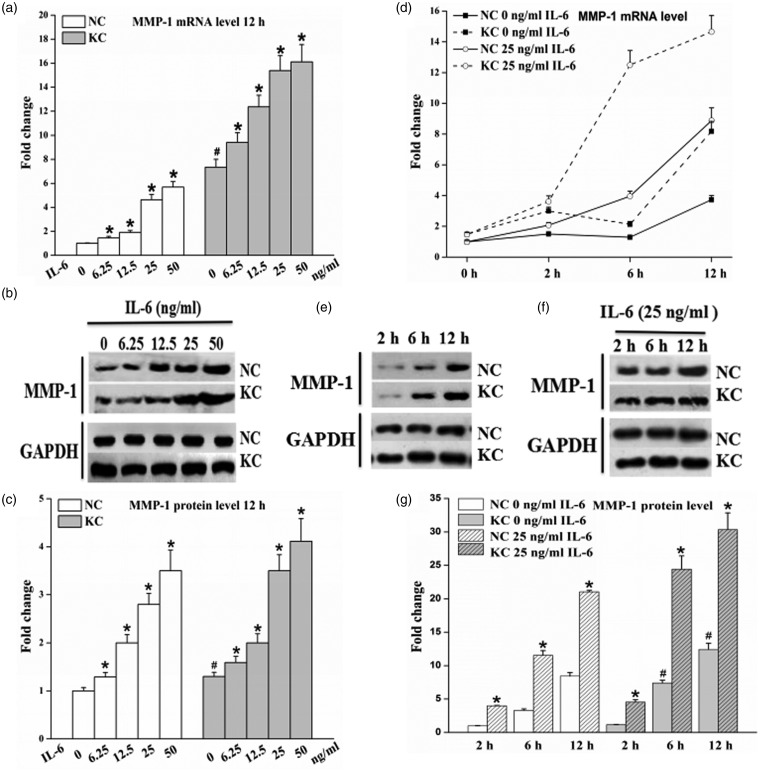
IL-6 stimulation of fibroblasts induced a significant increase in MMP-1 mRNA and protein expression, at concentrations as low as 6.25 ng/mL for 12 h and with further increases upon stimulation with 12.5, 25, or 50 ng/mL for 12 h (P < 0.05, Figure 3a–c). Levels of MMP-1 mRNA were increased in cultured fibroblasts without IL-6 at 2 h and then reduced at 6 h, but later on, the levels were up-regulated at 12 h; IL-6 (25 ng/mL) up-regulated the levels of MMP-1 mRNA in cultured cells compared to their own control and the levels increased in a time-dependent manner (Figure 3d). Moreover, incubation with 25 ng/mL for 2 h elevated the MMP-1 protein level, and greater increases in MMP-1 protein levels were observed upon treatment with 25 ng/mL IL-6 for 6 h and 12 h (P < 0.05, Figure 3e–g). These results demonstrate that IL-6 up-regulates the mRNA and protein expression of MMP-1 in a dose- and time-dependent manner in cultured fibroblasts of normal cornea and keratoconus.
Figure 3.
Effect of IL-6 on MMP-l levels in fibroblasts of normal cornea (n = 3) and keratoconus (n = 3). Cells were treated with IL-6 for 12 h at the indicated concentration and incubated in the absence or presence of 25 ng/mL IL-6 for various times. Total RNA and cell-culture supernatants were then collected. (a and d) MMP-1 mRNA expression detected by real-time PCR from total RNA. (b, e, and f) Representative Western blot for evaluation of MMP-1 protein in cell-culture supernatants. (c and g) Quantitative analysis of MMP-1 protein levels using Image-Pro Plus. Data are expressed as mean ± standard deviation of three independent experiments. KC: keratoconus, NC: normal cornea. Cells without IL-6 incubation were used as control. *P < 0.05, compared with their own control group; #P < 0.05, KC control vs. NC control
TNF-α and IL-6 have a synergistic effect on the MMP-1 mRNA expression and protein synthesis
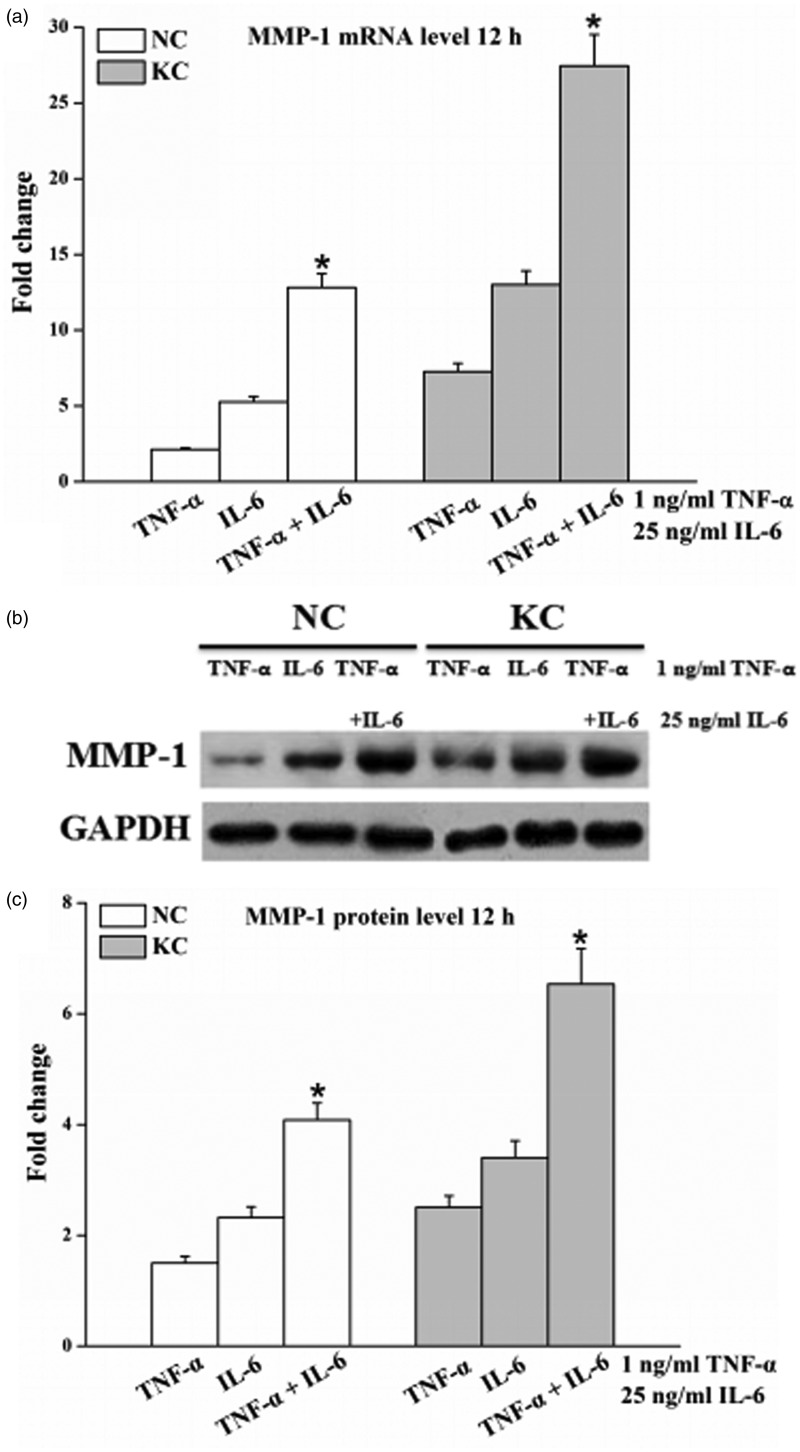
MMP-1 mRNA (Figure 4a) and protein (Figure 4b and c) levels in the cells treated with 1 ng/mL TNF-α together with 25 ng/mL IL-6 for 12 h were significantly higher (P < 0.05) than that in the cells cultured with 1 ng/mL TNF-α or 25 ng/mL IL-6 alone. The synergistic effect in fibroblasts of keratoconus was more obvious than normal cornea.
Figure 4.
Effect of TNF-α and IL-6 on the levels of MMP-1. Cells were cultured with 1 ng/mL TNF-α or 25 ng/mL IL-6 individually and jointly for 12 h. Total RNA and cell-culture supernatants were then collected. (a) The mRNA levels of MMP-1 determined by real-time PCR from total RNA. (b) Representative Western blot for MMP-1 protein in cell-culture supernatants. (c) Quantitative analysis of MMP-1 protein levels by Image-Pro Plus. Values are expressed as mean ± standard deviation of three independent experiments. KC: keratoconus (n = 3), NC: normal cornea (n = 3). *P < 0.05, compared with cells stimulated by TNF-α or IL-6 individually
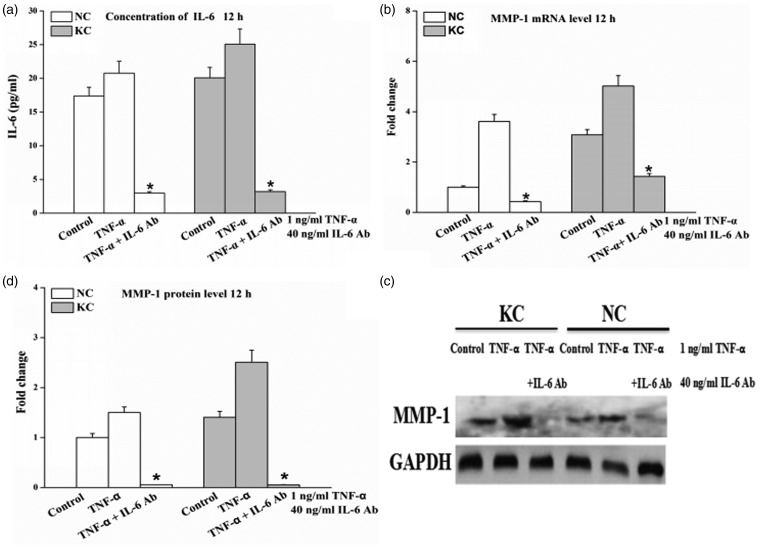
IL-6 Ab treatment significantly inhibits TNF-α-induced the expression of MMP-1. The production of IL-6 protein in cell-culture supernatants from cells incubated with the addition of IL-6 Ab (40 ng/mL) was significantly combined (P < 0.05) (Figure 5a). IL-6 Ab almost completely inhibited (P < 0.05) the TNF-α-mediated increases of MMP-1 mRNA expression (Figure 5b) and protein synthesis (Figure 5c and d) in cultured cells. The inhibitory effect on MMP-1 protein by IL-6 Ab in fibroblasts of keratoconus was more significant compared with normal cornea (Figure 5c and d).
Figure 5.
Expression of MMP-1 in fibroblasts of normal cornea (n = 3) and keratoconus (n = 3) under both TNF-α and IL-6 Ab. Cells were treated with 1 ng/mL TNF-α and 40 ng/mL IL-6 Ab during 12 h. Total RNA and cell-culture supernatants were then collected. (a) IL-6 protein detected in cell-culture supernatants by ELISA. (b) IL-6 mRNA expression from total RNA determined by real-time PCR. (c) Representative Western blot for MMP-1 protein in cell-culture supernatants. (d) Quantitative analysis of MMP-1 protein levels by Image-Pro Plus. Results are presented as mean ± standard deviation of three independent experiments. KC: keratoconus, NC: normal cornea. Cells without IL-6 Ab or TNF-α treatment were used as control. *P < 0.05, TNF-α + IL-6 Ab vs. control
Discussion
In the present study, we have found that human corneal fibroblasts cultured in vitro could express IL-6 and MMP-1. Moreover, the expression of IL-6 and MMP-1 was significantly higher in cultured fibroblasts of keratoconus compared to normal cornea. This is consistent with the results of previous studies in the tear film of patients with keratoconus.2,3,22 The MMP-1 overexpression might weaken the stability of structure of the cornea, by reason of its ability to degrade collagen type I and III, which are the main components of ECM in the cornea.20,26 Additionally, the synthesis of larger amounts of IL-6 by epithelial cells could influence a number of immunity and inflammation activities which could lead to corneal damage within the eye.7 Since keratoconus is characterized by the thinning of the corneal stroma, taken together, these results of higher expressions on IL-6 and MMP-1 provide further support for that the pathogenesis of keratoconus may involve chronic inflammatory events and matrix degradation.
Previous reports have shown that MMPs levels are associated with increased concentration of inflammatory cytokines in a wide variety of cells.24,25 However, to date, there have been no reports regarding the correlation between inflammatory molecules and MMPs in fibroblasts of keratoconus. We further examined the mechanism of MMP-1 induction by inflammatory stimuli in a human corneal fibroblasts model in vitro. Our data show that either TNF-α or IL-6 can up-regulate MMP-1 mRNA expression and protein synthesis in a dose-dependent manner and both TNF-α and IL-6 have a synergistic effect on the expression of MMP-1. A recent study has indicated that TNF-α and IL-6 may rank as important factors in the pathogenesis of keratoconus.22 In addition, previous studies have showed that MMP-1, -2, -9 might be involved in the pathology of keratoconus; The thinning and ectasia of a keratoconus cornea have been mainly attributed to the increased degradation of ECM.20,22,27,28 Our study indicates that the overexpression of TNF-α and IL-6 in fibroblasts of keratoconus may be reflective of a chronic inflammatory situation by inducing MMP-1 expression, leading to degradation of corneal ECM.
It has been proposed that TNF-α is capable of inducing the expression of IL-6 in human corneal epithelial cells.13,14 Our results show that TNF-α can enhance the IL-6 mRNA expression and protein synthesis in cultured fibroblasts of normal cornea and keratoconus in vitro. In addition, our experiments dada demonstrate that MMP-1 mRNA and protein expression induced by TNF-α significantly are inhibited after treatment with IL6 Ab and the inhibitory effect on MMP-1 protein in keratoconus was more significant than normal cornea.
The coculture of nerve cells with corneal fibroblasts increased the MMP-1 mRNA expression and protein synthesis with no effect on MMP-2 and MMP-9 expression in human corneal fibroblasts and furthermore, only the cytokine IL-6 in cocultures of the two cell types was significantly higher than that in cultures of corneal fibroblasts alone. These suggest that inflammatory cytokines and MMPs may play an important role in corneal inflammation, wound healing, and ECM degradation.29 A report that showed lipopolysaccharide (LPS) stimulated MMP-1 and IL-6 secretion from macrophages and also found that LPS and IL-6 had a synergistic effect on MMP-1 expression, suggesting that IL-6 enhanced production of LPS-induced MMP-1.30 Previous work suggested that heat shock can increase MMP-1, -3, and IL-6 at both the mRNA and protein levels and the expression of MMP-1,-3 increased by heat shock significantly was blocked after treatment with a monoclonal antibody against IL-6, indicating that IL-6 mediates the heat shock-induced MMP-1, -3 expression.31 Similarly, in our experiment, IL-6 Ab was added to examine the effect of IL-6 Ab treatment on TNF-α-induced MMP-1 expression in fibroblasts of keratoconus and found that MMP-1 expression could be significantly inhibited in fibroblasts of keratoconus.
Put together, our study demonstrates that TNF-α up-regulated MMP-1 expression via IL-6 in fibroblasts of keratoconus. A recent research has found that subsequent treatment of keratoconus patients with cyclosporine A (CyA) reduced tear MMP-9 levels significantly and led to local reduction in corneal curvatures, indicating that CyA might be a novel treatment strategy in keratoconus.32 It is worth mentioning that several IL-6 blocking agents such as a humanized anti-IL-6 receptor antibody has been developed and successfully applied in clinical trials for the treatment of several diseases.33–35 MMP-1 reduction by IL-6 Ab might provide a new modality to reduce elevated MMP-1 levels in keratoconus.
Further studies are required to determine which signaling pathway being involved in the process: TNF-α → IL-6 → MMP-1 in keratoconus. It is possible that inhibiting IL-6 may help to dampen inflammatory responses mediated by TNF-α thereby reducing damage to the cornea. This may be realized by inhibiting IL-6 receptors or inhibition of targets downstream of the IL-6 signaling pathway or other pathways targeting IL-6 for treating keratoconus.
In conclusion, our findings indicate that fibroblasts of keratoconus show increased levels of IL-6 and MMP-1 gene and protein expression; IL-6 mediates the TNF-α-induced MMP-1 expression in keratoconus.
Supplementary Material
Acknowledgments
This study was financially supported by the National Natural Science Foundation of China (grant No. 11402162, 11402161, 31300770, 31271005).
Author contributions
W-YC, X-NL, and RH contributed to the design and review of the research. G-LD and W-WL conducted the experiments. C-XL, X-JW, and P-FF analyzed the data. G-LD and C-XL wrote the manuscript. All authors approved the final version of the manuscript for publication.
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- 1.Rabinowitz YS. Keratoconus. Surv Ophthalmol 1998; 42: 297–319. [DOI] [PubMed] [Google Scholar]
- 2.Lema I, Durán JA. Inflammatory molecules in the tears of patients with keratoconus. Ophthalmology 2005; 112: 654–9. [DOI] [PubMed] [Google Scholar]
- 3.Lema I, Sobrino T, Duran JA, Brea D, Diez-Feijoo E. Subclinical keratoconus and inflammatory molecules from tears. Br J Ophthalmol 2009; 93: 820–4. [DOI] [PubMed] [Google Scholar]
- 4.McMonnies CW. Inflammation and keratoconus. Optom Vis Sci 2015; 92: e35–41. [DOI] [PubMed] [Google Scholar]
- 5.Naka T, Nishimoto N, Kishimoto T. The paradigm of IL-6: from basic science to medicine. Arthritis Res 2002; 4(Suppl 3): S233–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Song M, Kellum JA. Interleukin-6. Crit Care Med 2005; 33: S463–5. [DOI] [PubMed] [Google Scholar]
- 7.Moqattash S, Lutton JD. Leukemia cells and the cytokine network: therapeutic prospects. Exp Biol Med 2004; 229: 121–37. [DOI] [PubMed] [Google Scholar]
- 8.Imanishi J, Kamiyama K, Iguchi I, Kita M, Sotozono C, Kinoshita S. Growth factors: importance in wound healing and maintenance of transparency of the cornea. Prog Retin Eye Res 2000; 19: 113–29. [DOI] [PubMed] [Google Scholar]
- 9.Akira S. IL-6 and NF-IL6 in acute-phase response and viral infection. Immunol Rev 1992; 127: 25–50. [DOI] [PubMed] [Google Scholar]
- 10.Kawano H, Ito T, Maruyama I, Hashiguchi T, Miyata K, Sakamoto T. The use of hyaluronan in phacoemulsification protects human corneal endothelial cells from the noxious effect of extracellular histones. Invest Ophthalmol Vis Sci 2014; 55: 2049–2049.24609623 [Google Scholar]
- 11.Tracey KJ, Cerami A. Tumor necrosis factor and regulation of metabolism in infection: role of systemic versus tissue levels. Exp Biol Med 1992; 200: 233–9. [DOI] [PubMed] [Google Scholar]
- 12.Cubitt CL, Lausch RN, Oakes JE. Differences in interleukin-6 gene expression between cultured human corneal epithelial cells and keratocytes. Invest Ophthalmol Vis Sci 1995; 36: 330–330. [PubMed] [Google Scholar]
- 13.Li D-Q, Lokeshwar BL, Solomon A, Monroy D, Ji Z, Pflugfelder SC. Regulation of MMP-9 production by human corneal epithelial cells. Exp Eye Res 2001; 73: 449–59. [DOI] [PubMed] [Google Scholar]
- 14.Ueta M, Kinoshita S. Ocular surface inflammation mediated by innate immunity. Eye Contact Lens 2010; 36: 269–81. [DOI] [PubMed] [Google Scholar]
- 15.Yang Y-N, Wang F, Zhou W, Wu Z-Q, Xing Y-Q. TNF-α stimulates MMP-2 and MMP-9 activities in human corneal epithelial cells via the activation of FAK/ERK signaling. Ophthal Res 2012; 48: 165–70. [DOI] [PubMed] [Google Scholar]
- 16.Chastre A, Bélanger M, Beauchesne E, Nguyen BN, Desjardins P, Butterworth RF. Inflammatory cascades driven by tumor necrosis factor-alpha play a major role in the progression of acute liver failure and its neurological complications. 2012; 7: e49670–e49670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kimura K, Orita T, Morishige N, Nishida T, Sonoda K-H. Role of the JNK signaling pathway in downregulation of Connexin43 by TNF-α in human corneal fibroblasts. Curr Eye Res 2013; 38: 926–32. [DOI] [PubMed] [Google Scholar]
- 18.Kleiner DE, Stetler-Stevenson WG. Matrix metalloproteinases and metastasis. Cancer Chemother Pharmacol 1999; 43: S42–51. [DOI] [PubMed] [Google Scholar]
- 19.Bauvois B. New facets of matrix metalloproteinases MMP-2 and MMP-9 as cell surface transducers: outside-in signaling and relationship to tumor progression. Biochim Biophys Acta 2012; 1825: 29–36. [DOI] [PubMed] [Google Scholar]
- 20.Collier SA. Is the corneal degradation in keratoconus caused by matrix-metalloproteinases? Clin Exp Ophthalmol 2001; 29: 340–4. [DOI] [PubMed] [Google Scholar]
- 21.Balasubramanian SA, Pye DC, Willcox MD. Are proteinases the reason for keratoconus? Curr Eye Res 2010; 35: 185–91. [DOI] [PubMed] [Google Scholar]
- 22.Balasubramanian SA, Mohan S, Pye DC, Willcox MDP. Proteases, proteolysis and inflammatory molecules in the tears of people with keratoconus. Acta Ophthalmol 2012; 90: e303–9. [DOI] [PubMed] [Google Scholar]
- 23.Balasubramanian SA, Pye DC, Willcox MDP. Levels of lactoferrin, secretory IgA and serum albumin in the tear film of people with keratoconus. Exp Eye Res 2012; 96: 132–7. [DOI] [PubMed] [Google Scholar]
- 24.Lokeshwar BL, Pflugfelder SC. Regulated expression of collagenases MMP-1,-8, and-13 and stromelysins MMP-3,-10, and-11 by human corneal epithelial cells. Invest Ophthalmol Vis Sci 2003; 44: 2928–36. [DOI] [PubMed] [Google Scholar]
- 25.Wisithphrom K, Windsor LJ. The effects of tumor necrosis factor-α, interleukin-1β, interleukin-6, and transforming growth factor-β1 on pulp fibroblast mediated collagen degradation. J Endod 2006; 32: 853–61. [DOI] [PubMed] [Google Scholar]
- 26.Pilcher BK, Dumin JA, Sudbeck BD, Krane SM, Welgus HG, Parks WC. The activity of collagenase-1 is required for keratinocyte migration on a type I collagen matrix. J Cell Biol 1997; 137: 1445–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brookes N, Loh I-P, Clover G, Poole C, Sherwin T. Involvement of corneal nerves in the progression of keratoconus. Exp Eye Res 2003; 77: 515–24. [DOI] [PubMed] [Google Scholar]
- 28.Matthews FJ, Cook SD, Majid MA, Dick AD, Smith VA. Changes in the balance of the tissue inhibitor of matrix metalloproteinases (TIMPs)-1 and-3 may promote keratocyte apoptosis in keratoconus. Exp Eye Res 2007; 84: 1125–34. [DOI] [PubMed] [Google Scholar]
- 29.Ko J-A, Chikama T-i, Sonoda K-H, Kiuchi Y. Up-regulation of matrix metalloproteinase-1 and interleukin-6 expression in cocultures of corneal fibroblasts and neural cells. Biochem Biophys Res Commun 2012; 419: 537–42. [DOI] [PubMed] [Google Scholar]
- 30.Sundararaj KP, Samuvel DJ, Li Y, Sanders JJ, Lopes-Virella MF, Huang Y. Interleukin-6 released from fibroblasts is essential for up-regulation of matrix metalloproteinase-1 expression by U937 macrophages in coculture cross-talking between fibroblasts and U937 macrophages exposed to high glucose. J Biol Chem 2009; 284: 13714–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Park C-H, Lee MJ, Ahn J, Kim S, Kim HH, Kim KH, Eun HC, Chung JH. Heat shock-induced matrix metalloproteinase (MMP)-1 and MMP-3 are mediated through ERK and JNK activation and via an autocrine interleukin-6 loop. J Invest Dermatol 2004; 123: 1012–9. [DOI] [PubMed] [Google Scholar]
- 32.Shetty R, Ghosh A, Lim RR, Subramani M, Mihir K, AR R, Ranganath A, Nagarai S, Beuerman R, Das D, Chaurasia SS, Roy AS, Ghosh A. Elevated expression of matrix metalloproteinase-9 and inflammatory cytokines in keratoconus patients is inhibited by cyclosporine A. Invest Ophthalmol Vis Sci 2015; 56: 738–50. [DOI] [PubMed] [Google Scholar]
- 33.Nishimoto N, Kishimoto T. Interleukin 6: from bench to bedside. Nat Clin Pract Rheumatol 2006; 2: 619–26. [DOI] [PubMed] [Google Scholar]
- 34.Jones SA. Directing transition from innate to acquired immunity: defining a role for IL-6. J Immunol 2005; 175: 3463–8. [DOI] [PubMed] [Google Scholar]
- 35.Lu L, Reinach PS, Kao WW-Y. Corneal epithelial wound healing. Exp Biol Med 2001; 226: 653–64. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.