Abstract
Snakebites inflicted by the arboreal viperid snake Bothriechis schlegelii in humans are characterized by pain, edema, and ecchymosis at the site of the bite, rarely with blisters, local necrosis, or defibrination. Herein, a comparative study of Bothriechis schlegelii snake venoms from Colombia (BsCo) and Costa Rica (BsCR) was carried out in order to compare their main activities and to verify the efficacy of Bothrops antivenom produced in Brazil to neutralize them. Biochemical (SDS-PAGE and zymography) and biological parameters (edematogenic, lethal, hemorrhagic, nociceptive, and phospholipase A2 activities) induced by BsCo and BsCR snake venoms were evaluated. The presence of antibodies in Bothrops antivenom that recognize BsCo and BsCR snake venoms by enzyme-linked immunosorbent assay and Western blotting, as well as the ability of this antivenom to neutralize the toxic activities were also verified. SDS-PAGE showed differences between venoms. Distinctive caseinolytic and hyaluronidase patterns were detected by zymography. BsCo and BsCR showed similar phospholipase A2 activity. Strong cross-reactivity between BsCo and BsCR was detected using Bothrops antivenom with many components located between 150 and 35 kDa. BsCR was more edematogenic and almost fourfold more hemorrhagic than BsCo, and both venoms induced nociception. BsCR (LD50 5.60 mg/kg) was more lethal to mice than BsCo (LD50 9.24 mg/kg). Bothrops antivenom was effective in the neutralization of lethal and hemorrhagic activities of BsCo and BsCR and was partially effective in the neutralization of edematogenic and nociceptive activities. In conclusion, geographic distribution influences the composition and activities of Bothriechis schlegelii venoms. Bothrops antivenom cross-reacted with these venoms and was partially effective in neutralizing some toxic activities of BsCo and BsCR.
Keywords: Bothriechis schlegelii, eyelash pit viper, snake venom, toxic activities, Bothrops antivenom, antiserum
Introduction
It is estimated that the overall number of venomous snakebites is about two million/year worldwide and approximately 100,000 deaths occur annually.1,2 Recently, the World Health Organization (WHO)3 classified snakebites as a neglected tropical disease, and tropical and subtropical countries in Africa, Asia and Latin America are the most affected by this injury.2–5 In South and Central America, approximately 130,000 to 150,000 snakebites have been reported annually. The majority of snakebite cases in this region are due to species classified in the genus Bothrops.2,6
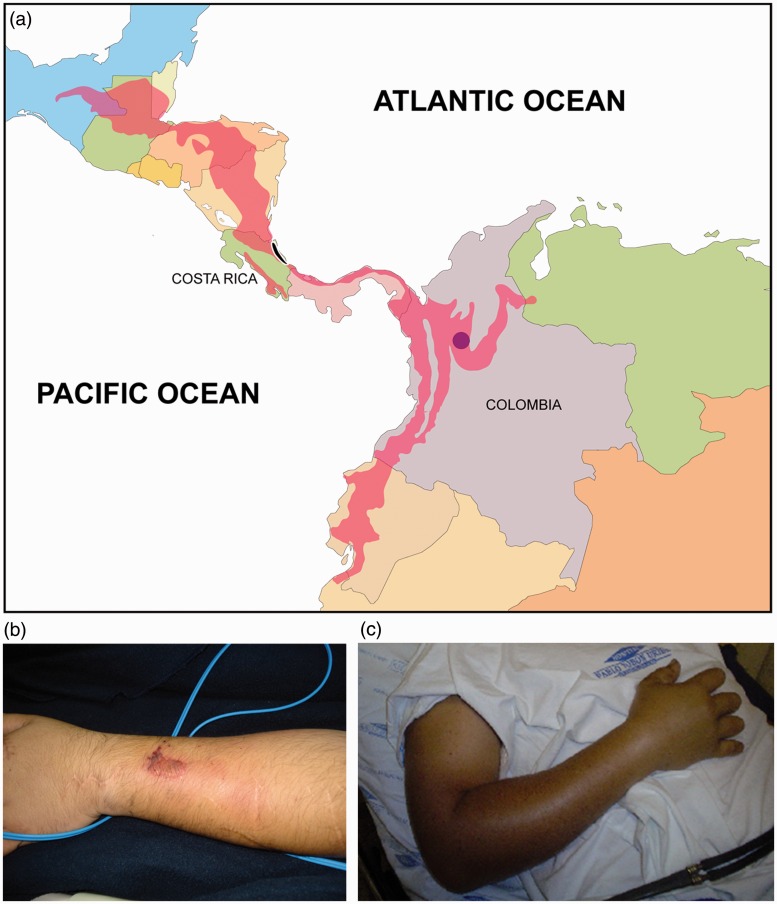
Although less frequently reported, Bothriechis snakes can also cause snakebites.7 Bothriechis comprise a clade of arboreal snakes which includes 10 species. Among them, Bothriechis schlegelii (eyelash pit viper) is distributed in tropical rainforests, from southern Mexico to northwestern South America, and reaches altitudes as high as 2640 m in Colombia8 (Figure 1(a)). Clinical manifestations of envenomation by B. schlegelii (Figure 1(b) and (c)) are characterized mostly by local changes at the site of the bite, such as pain, edema, hemorrhage, and necrosis (in severe cases).7 Systemic manifestations, e.g. hemostatic disturbances, occur less frequently, although they have been also described.9–11 Treatment for viperid envenomation in Latin America is based on intravenous administration of mono- or polyvalent antivenoms.6 So far, antivenom remains the specific treatment used in snakebites, and it is therefore recommended by the WHO.3
Figure 1.
(a) The geographic distribution of B. schlegelii snakes (pink areas) was adapted from Campbell and Lamar.8 The marked areas where snakes were collected from Costa Rica (black ellipsis, Caribbean region) and Colombia (Vegachi, purple circle) are also depicted. (b) and (c) Local manifestations of envenomation in patients bitten by B. schlegelii in Colombia. Note that the edema and inflammatory reaction at the site of the bite. Photos from patients were donated by Dr Rafael Otero. (A color version of this figure is available in the online journal.)
Local pathological manifestations induced by Bothrops spp. and Bothropoides spp. (family Viperidae) envenomation are a consequence of the composition of venoms. Snake venoms contain a wide variety of enzymes and proteins, including toxic and non-toxic proteins. Myotoxins, phospholipase A2 (PLA2), lectins, serine-proteinases, L-amino oxidases, and metalloproteinases, among others, have been reported in these venoms.12–15 However, few studies characterized the toxins present in the venoms of Bothriechis snakes. Angulo et al.16 isolated and characterized a myotoxic protein (PLA2) from B. schlegelii species venom from Costa Rica, named myotoxin I. Lomonte et al.14 analyzed and compared by a proteomic approach the venoms of two snakes from Costa Rica, B. schlegelii and Bothriechis lateralis, and it was observed that both venoms contained bradykinin-potentiating peptides (BPPs), metalloproteinases, PLA2, serine proteases, and L-amino oxidase. Comparative proteomic studies of species of the genus Bothriechis have shown a great variation in the predominant toxin families between the venoms of different species,14,17,18 hence highlighting a wide spectrum of strategies to accomplish the same trophic purpose. Proteomic analyses of the venom of Costa Rican B. schlegelii revealed the presence of predominant components such as BPPs and other vasoactive peptides, Kazal-type inhibitor, metalloproteinases, cysteine-rich secretory proteins, PLA2, serine proteases, and L-amino acid oxidase.14,16,19,20
The composition of snake venoms is under the control of multiple factors including genetic, geographical, seasonal, sexual, and dietary.21–27 Such complexity may influence the potential toxicity of venoms, and consequently the clinical features evoked by snakebites.
There are several manufactures of snake antivenoms in Latin America.28 A number of studies have been performed on the ability of some of these antivenoms to immunoreact and neutralize venoms from a variety of snake species in the region.28–33 Since venoms of Bothriechis species are generally not included in the immunization mixtures to produce antivenoms, it is relevant to further investigate whether antivenoms are effective in the neutralization of toxic and enzymatic activities of Bothriechis sp. venoms.
Particularly in Colombia, 5.5% of the bites are caused by B. schlegelii.34 The fact that the intraspecies variability in B. schlegelii venom has not been studied prompted us to establish an international collaboration to evaluate venoms obtained of B. schlegelii from Colombia (BsCo) and Costa Rica (BsCR). Moreover, the ability of Bothrops antivenom (BAV) manufactured at Butantan Institute, in Brazil, was also investigated for its ability to cross-react with these venoms and to neutralize some of their toxic activities.
Materials and methods
Animals
Adult male Swiss mice (18–20 g) were obtained from Butantan Institute Animal House. All procedures involving mice were carried out in conformity with national and international laws and policies, controlled by Butantan Institute Animal Investigation Ethical Committee, protocol number 689/2009.
Antivenom
BAV (batch 1001003/D, expiry date: 12/2012) was produced by Butantan Institute by immunization of horses with a mixture of venoms from Bothrops jararaca (50%), B. jararacussu (12.5%), B. moojeni (12.5%), B. neuwiedi (12.5%), and B. alternatus (12.5%) venoms. It is composed of F(ab′)2 fragments of antibodies.35 The antivenom was used before the expiry date. As a control, IgG isolated from normal horse serum (NHS) was used in experiments for venom neutralization.
Venoms
The venoms of BsCo and BsCR were provided kindly by the Serpentarium of the Antioquia University and Instituto Clodomiro Picado, University of Costa Rica, respectively. BsCo was obtained from 12 specimens collected in the rural zone of Vegachí (Antioquia) on the eastern side of the Cordillera Central of Colombia, between 500 and 1500 m of altitude (Figure 1(a)). BsCR was obtained from more than 20 specimens collected in the Caribbean region of Costa Rica (Figure 1(a)). Several venom samples of each specimen from Colombian B. schlegelii were collected by manual extraction, centrifuged at 5000 g during 30 min and the supernatants were frozen, pooled, lyophilized, and stored at −80℃. A similar protocol was used to study venom from Costa Rica, except that the venom was stored after lyophilization at −20℃. A pool of lyophilized Bothrops jararaca venom (Bj), used as a control, was provided by Butantan Institute. At the moment of use, venom was dissolved in phosphate-buffered saline (PBS). The protein concentration of venoms was determined using bicinchoninic acid36 interpolating data in a standard curve prepared with bovine serum albumin (BSA; Sigma-Aldrich Chemical Co., St. Louis, MO, USA).
Sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE)
Samples of BsCo, BsCR, and Bj (5 µg) venoms were separated in 12% SDS-PAGE gels37 under non-reducing conditions, and then stained by silver.38 Kaleidoscope pre-stained standards (Bio-Rad, Hercules, CA, USA) were used as the molecular mass markers.
Enzyme-linked immunosorbent assay and Western blotting
Titration of BAV was accomplished by enzyme-linked immunosorbent assay (ELISA), as described previously,25 using BsCo, BsCR, and Bj venoms (10 µg/mL) to coat plates (Nunc, USA). The absorbance was read using an ELISA plate reader (Titertek Multiskan plus) and the titer determined as the reciprocal of the highest dilution that shows an absorbance greater than 0.050 at 492 nm, as non-specific reactions were observed below this value. For Western blotting,39 BsCo, BsCR, and Bj venoms (20 µg) were first separated by SDS-PAGE, as described above, and transferred to nitrocellulose membranes at 385 mA for 2 h. After transfer, the membranes were blocked with PBS containing 5% BSA and incubated with BAV or NHS (1:250) for 1 h at room temperature. Immunoreactive proteins were detected using peroxidase-labeled anti-horse IgG and then blots were developed with 0.05% 4-chloro-1-napthol in 15% methanol (v/v), in the presence of 0.03% H2O2 (v/v). NHS was used as a control.
Protease, hyaluronidase, and PLA2 activities
Zymography was used to assay protease and hyaluronidase activities, using casein (2 mg/mL), gelatin (2 mg/mL) or fibrinogen (0.5 mg/mL), and hyaluronic acid (170 µg/mL) from rooster comb (Sigma, USA), respectively, as substrates.40,41 PLA2 activity was carried out as described previously.25 A unit of PLA2 activity was defined as the amount of venom that gives ΔA558nm = 0.3/min. The specific activity was then expressed as U/mg lyophilized venom.
Evaluation of edematogenic activity and neutralization by BAV
Edema-forming activity was evaluated in mice by plethysmometry (7141 Plethysmometer—Ugo Basile, IT) at different times (0.5, 1, 2, 4, 24, 48, and 72 h) as the difference of foot pad volume (µL) between the right foot paw (n = 6) injected intraplantarly (i.pl.) with either PBS (negative control) or different doses (0.5, 1, 2, 4, or 8 µg) of BsCo, BsCR, and Bj diluted in PBS, and the left paw (not injected). To evaluate the edema neutralization, BAV (25, 12.5, or 6.25 µL) was previously incubated at 37℃ for 1 h with 8 µg of Bj, BsCo, or BsCR, centrifuged at 2000 g for 5 min, and the supernatant was immediately injected in mouse footpad. IgG isolated from NHS was used as negative control.
Evaluation of hemorrhagic activity and neutralization by BAV
The hemorrhagic activity was evaluated by the method described by Kondo et al.42 with some modifications43 to determine the minimum hemorrhagic dose (MHD). This corresponds to the dose that causes a hemorrhagic area of 1 cm2. Groups of six mice were shaved on the back and then intradermally (i.d.) injected with different doses of BsCo, BsCR (4, 8, 16, 32, or 64 µg) and Bj (0.1, 0.2, 0.4, 0.8, or 1.6 µg) dissolved in PBS. Mice were sacrificed and skins were excised 2 h later and the areas of hemorrhagic spots were measured on the internal surfaces. PBS was used as negative control. To evaluate the neutralization of the hemorrhagic activity, BAV (80, 40, or 20 µL) was previously incubated at 37℃ for 1 h with two MHD of Bj, BsCo, or BsCR, centrifuged at 2000 g for 5 min, and the supernatant was immediately injected as described previously. Controls included mice injected with venoms incubated with PBS or NHS instead of antivenom.
Evaluation of nociceptive activity and neutralization by BAV
To detect the nociceptive activity, mice (n = 6–8) were injected in the right footpad with 30 µL of PBS containing different doses of Bj, BsCo, or BsCR (0.5, 1, 2, 4, or 8 µg). Animals were placed individually under glass funnels on a mirror. Afterwards, the reactivity of animals to lick the injected foot was measured, in seconds, during 30 min of experimental evaluation.44 Animals injected only with PBS were used as negative controls. To evaluate the nociception neutralization, BAV (25, 12.5, or 6.25 µL) was previously incubated at 37℃ for 1 h with 4 µg of venoms, and samples centrifuged at 2000 g for 5 min, and the supernatant was immediately injected afterwards in the footpads of mice, as described. IgG isolated from NHS was used as a control.
Evaluation of lethality and neutralization by BAV
To characterize and compare the lethal activity induced by Bj, BsCo, and BsCR, five different concentrations (10, 20, 40, 50, or 80 µg—Bj and 60, 90, 120, 180, or 240 µg—BsCo and BsCR/500 µL) or PBS (control) were injected intraperitoneally (i.p.) into mice (n = 6). Mortality was determined 48 h after the injection of venoms and used for calculating the median lethal doses (LD50) and its 95% confidence intervals.45 For neutralization experiments, three LD50 of each venom were incubated for 30 min at 37℃ with BAV (400 µL), centrifuged (5 minutes), and aliquots of the supernatants (500 µL) were injected i.p. into mice (n = 6–8). After injection of the samples, observations were made within 48 h to assess mortality.
Statistical analyses
Two-way analysis of variance (ANOVA) was used to analyze data from nociception (factors: snake species and venom doses), nociception neutralization (factors: treatments and doses for each snake species), hemorrhagic area (factors: Bothriechis origin and venom doses), and neutralization of edematogenic activity evoked by the dose of 8 µg (factors: time intervals and Bothriechis origin). In order to evaluate the data from mice that received only one treatment and were observed at more than one time interval, two-way repeated measure ANOVA was employed to compare the following variables: edema extension (factors: time intervals and venom doses) and edema neutralization (factors: time intervals and antivenom doses), using one snake species for each analysis. Whenever the ANOVA table showed statistically significant F tests, analyses were followed by Holm-Sidak test. Statistical analyses were performed using the software Stata™, version 8.0, and SigmaPlot, version 12, and differences with p < 0.05 were considered statistically significant. The results for continuous data were presented as mean ± SEM.
LD50’s and statistical comparisons among their values were computed as described elsewhere,45 using a program created in Stata™. The comparison of ELISA titers to evidence cross-neutralization among venoms by BAV was carried out as described elsewhere.46 Titer values above twofold or more were considered significant, based on previous experience from our group.
Results
Comparison of venom profiles by SDS-PAGE
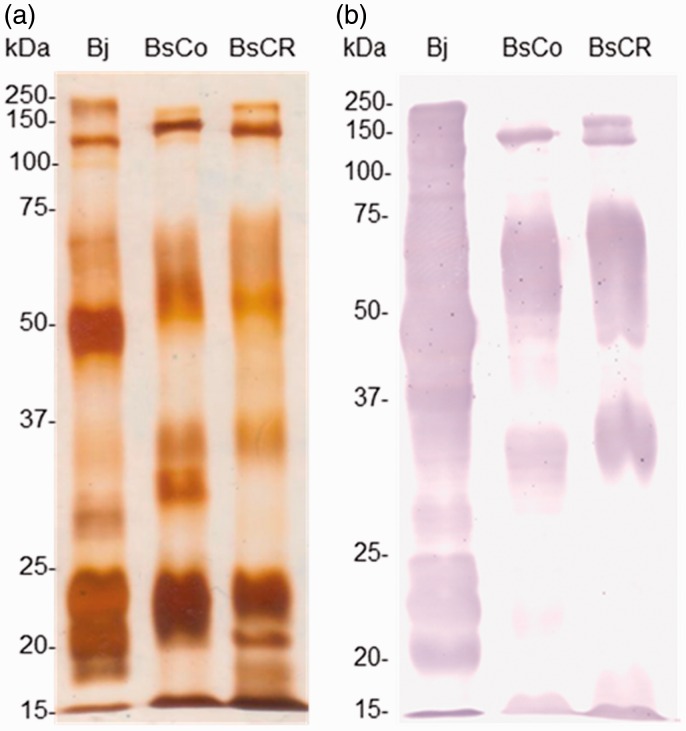
After SDS-PAGE, under non-reducing conditions, many components with similar molecular masses around 150 kDa and between 75 and 50 kDa were detected in BsCo and BsCR (Figure 2(a)). Moreover, it was observed that below 37 kDa venoms showed noticeable differences in protein profile, and components in the region of 32 kDa in BsCo were not observed in BsCR. On the other hand, bands between 20 and 15 kDa were visible mainly in BsCR. The electrophoretic profile of Bj was different, with intensely stained bands in the region of 50 kDa and between 25 and 20 kDa.
Figure 2.
(a) Electrophoretic profiles of the venoms of B. jararaca (Bj) and B. schlegelii from Colombia (BsCo) and Costa Rica (BsCR). Venoms (5 µg) were separated in SDS-PAGE (12%). Proteins were silver stained. (b) Recognition of components of venoms from B. jararaca (Bj) and BsCo and BsCR after incubation with BAV (1:250 dilution) by Western blotting. The numbers on the left correspond to molecular mass markers. (A color version of this figure is available in the online journal.)
Cross-reactivity of antivenom determined by ELISA and Western blotting
Intense cross-reactivity between BAV and venoms was observed by ELISA, with antibody titers of 256,000 against BsCo and BsCR, and 1,024,000 against Bj venom. Hence, there was no significant difference between the titers of BAV among the venoms, since variation in ELISA titers below twofold serial dilutions are not considered significant. Venom components observed around 150 kDa, between 75 and 50 kDa and around 35 kDa in BsCo and BsCR were recognized using BAV by Western blotting (Figure 2(b)). However, BAV weakly recognized components of lower molecular mass (below 25 kDa) in both venoms. In addition, BAV recognized bands between the regions from 250 to 15 kDa of Bj (positive control). There was no recognition of components of venoms by IgGs from NHS (results not shown).
Enzymatic activities of venoms
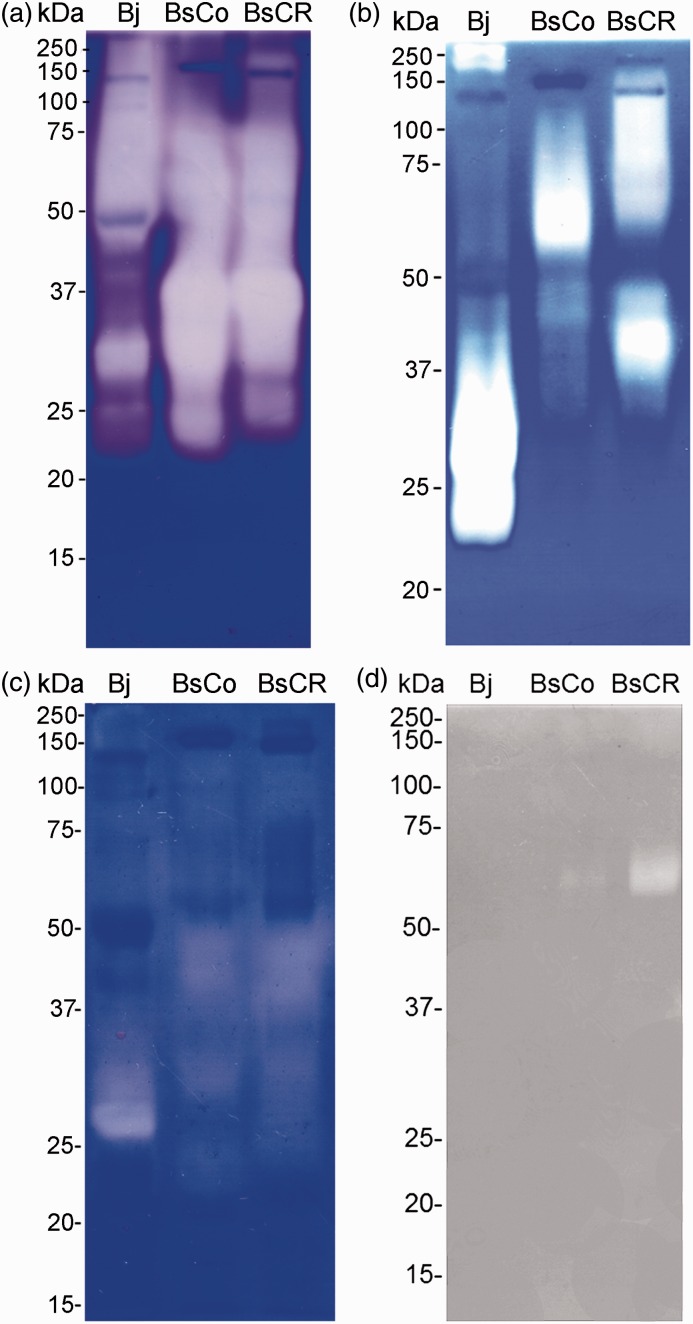
Zymography was used to detect proteolytic and hyaluronidase activities in BsCo, BsCR, and Bj, using casein, gelatin, fibrinogen, and hyaluronic acid as substrates (Figure 3). BsCo and BsCR showed similar profiles for gelatinolytic activity, with bands mainly between 75 and 23 kDa, and intense areas of hydrolysis around 40 to 27 kDa. A different profile was observed in Bj, which showed high gelatinolytic activity above 46 kDa, and less intense hydrolysis areas around 30 kDa (Figure 3(a)). However, remarkable differences between these three venoms were noticed when casein was used as a substrate. BsCo showed enzymes, mainly around 60 kDa, that hydrolyzed casein, whereas BsCR showed activity above 63 kDa and another intense area at 40 kDa. Bj exhibited caseinases of high molecular mass, around 250 kDa, and others with an intense activity between 35 and 22 kDa (Figure 3(b)). Fibrinogenolytic activity was observed in BsCR and BsCo, with components around 50 to 37 kDa and between 33 and 21 kDa; Bj showed fibrinogenolytic areas around 30 to 27 kDa (Figure 3(c)). Hyaluronidase activity around 62 kDa was observed in BsCR and BsCo, but BsCR exhibited a stronger activity when compared to BsCo. In the experimental protocol used, Bj did not hydrolyze hyaluronic acid (Figure 3(d)).
Figure 3.
Gelatinolytic, caseinolytic, fibrinogenolytic and hyaluronidase activities of the venoms of B. jararaca (Bj) and B. schlegelii from Colombia (BsCo) and Costa Rica (BsCR). Venoms (20 µg for (a) and (b); 40 µg for (c) and (d)) were separated by SDS-PAGE (12%) containing gelatin (a), casein (b), fibrinogen (c) or hyaluronic acid (d). After electrophoresis, gels were stained with Coomassie Blue ((a)–(c)) or Alcian Blue (d). Clear areas in the gels indicated enzymatic activity. The numbers on the left correspond to molecular mass markers (kDa). (A color version of this figure is available in the online journal.)
PLA2 activity
BsCo and BsCR had similar PLA2 activity, which was much higher than Bj. The specific activities for BsCo, BsCR, and Bj were 1273, 1255, and 33 U/mg, respectively (Figure 4).
Figure 4.
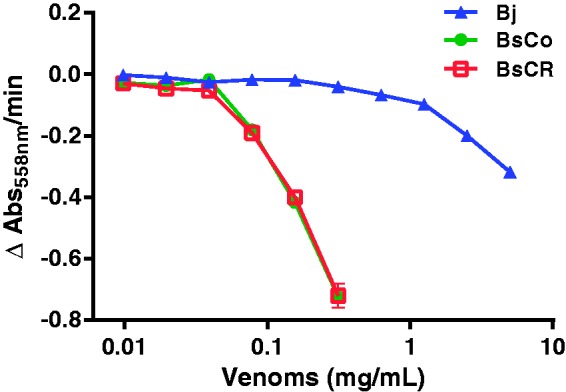
Curves of PLA2 activity of venoms from B. jararaca (Bj), B. schlegelii from Colombia (BsCo) and Costa Rica (BsCR). Note the strong activity of BsCo and BsCR compared to Bj. Each point represents the mean ± SEM of triplicate determinations of two different experiments. (A color version of this figure is available in the online journal.)
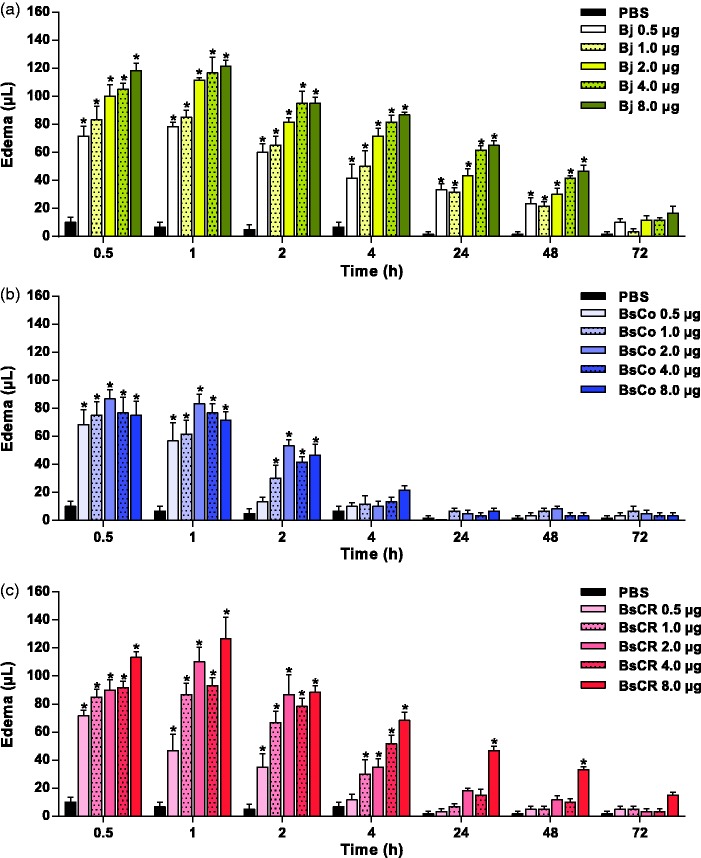
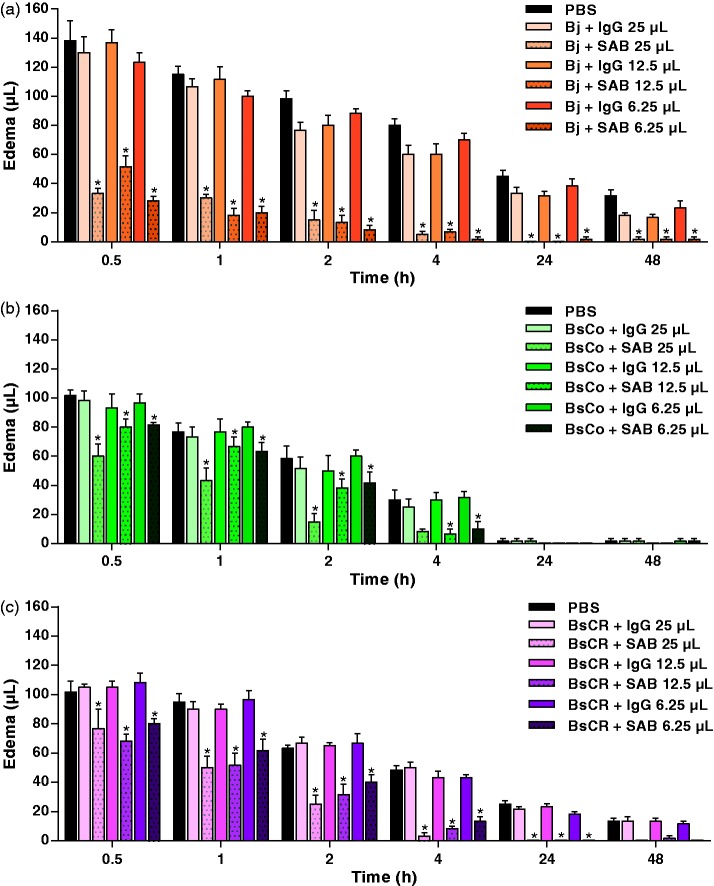
Edematogenic activity of venoms and neutralization by BAV
As shown in Figure 5(a), Bj used as a positive control evoked severe and persistent paw swelling in all concentrations of venoms used up to 48 h. BsCo and BsCR (Figure 5(b) and (c)) induced edematogenic activity in all concentrations tested during the initial time periods, reaching a maximum at 0.5 and 1 h. At 4 h, there was a marked decrease in edema induced by BsCo (Figure 5(b)), and values were similar to those injected with PBS (negative control). Reduction in BsCR-induced edema was slower than that induced by BsCo, so that edema was still evidenced at 4 h (for 1–8 µg); later on, edema was observed only with the dose of 8 µg. The ability of BAV to neutralize the edematogenic activity induced by Bj, BsCo, and BsCR (8 µg) was also evaluated. In Figure 6(a), edema induced by Bj (control) was partially inhibited by BAV, with values higher than 60% at 0.5 and 1 h and above 80% after 2 h. Figure 6(b) shows that edema caused by BsCo was better neutralized (around 70%) only in 2 h with 25 µL of BAV. When BsCR was incubated with BAV (Figure 6(c)), edema was inhibited partially by all doses of BAV at 2 h (range 26%–63%) and 4 h (above 70%). At 24 h, edema in the paw of the animals injected with BAV + BsCR was not detected.
Figure 5.
Edematogenic activity induced by the venoms of (a) B. jararaca (Bj), (b) B. schlegelii from Colombia (BsCo) and (c) Costa Rica (BsCR). Edema was calculated by pletysmometry at different times by the difference of volume displaced (µL) by the paws of animals (n = 6) before and after sample injection. The results were expressed as mean ± SEM. PBS was used as negative control. *Differences were statistically significant (p < 0.05) when the edema induced by venom concentrations and controls (PBS or venom + IgG) was compared. (A color version of this figure is available in the online journal.)
Figure 6.
Neutralization by anti-Bothrops antivenom (BAV) of the edematogenic activity induced by 8 µg of (a) B. jararaca (Bj), (b) B. schlegelii from Colombia (BsCo) and (c) Costa Rica (BsCR) venoms. Edema was calculated by pletysmometry at different times by the difference of volume displaced (µL) by the paws of animals (n = 6) before and after sample injection. The results were expressed as mean ± SEM. PBS or IgG isolated from normal horse serum were used as negative controls. *Differences were statistically significant (p < 0.05) when the edema induced by venom concentrations and controls (PBS or venom + IgG) was compared. (A color version of this figure is available in the online journal.)
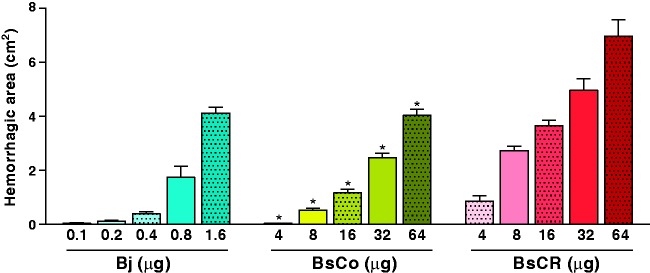
Hemorrhagic activity of venoms and neutralization by BAV
BsCR (MHD 3.9 µg) was more hemorrhagic than BsCo (MHD 14.2 µg), and both of them were less hemorrhagic than Bj (MHD 0.6 µg) (Figure 7). Regarding neutralization of this activity by BAV, all three doses of BAV tested (80, 40, and 20 µL) neutralized completely two MHD of all three venoms tested. As positive control, two MHD of each venom were incubated with NHS or PBS and showed hemorrhagic activity as expected (data not shown).
Figure 7.
Hemorrhagic activity induced by the venoms of B. jararaca (Bj) and B. schlegelii from Colombia (BsCo) and Costa Rica (BsCR). Animals (n = 6) were injected (i.d.) on the back with different concentrations of venoms. After 2 h of injection, the animals were euthanized and the skin of the back folded to measure the hemorrhagic area (cm2). The results were expressed as mean ± SEM. *Statistically significant difference (p < 0.05) between the hemorrhagic activity induced by each dose of BsCo and BsCR. (A color version of this figure is available in the online journal.)
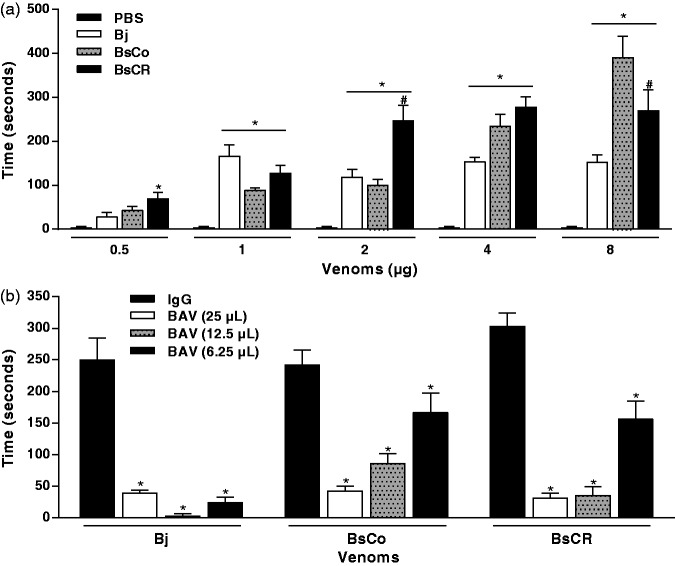
Nociceptive activity of venoms and neutralization by BAV
Nociception was induced by both BsCo (1–8 µg) and BsCR (0.5–8 µg); at 2 and 8 µg, the difference was statistically significant. At higher venom concentrations, BsCo and BsCR were more nociceptive than Bj (Figure 8(a)). The peak of nociceptive activity caused by Bj was observed when 1 µg of venom was injected. The nociception induced by Bj (4 µg) was reduced (>85%) by BAV in all concentrations used (Figure 8(b)). The nociceptive activity induced by BsCo and BsCR (4 µg) was partially neutralized by BAV, with reduction of 83% (25 µL), 64% (12.5 µL) in BsCo, and around 90% in BsCR at both doses of BAV. At the lowest dose of BAV (6.25 µL), the reduction of nociceptive activity was 31% and 48% for BsCo and BSCR, respectively.
Figure 8.
(a) Nociceptive activity induced by the venoms of B. jararaca (Bj) and B. schlegelii from Colombia (BsCo) and Costa Rica (BsCR); (b) neutralization of nociceptive activity induced by Bj, BsCo and BsCR by anti-Bothrops antivenom (BAV); IgG isolated from NHS was used as a control. The reactivity of animals to lick the injected foot was measured, in seconds, during 30 min of experimental evaluation (see Materials and methods for details). The results were expressed as mean ± SEM. *Statistically significant difference (p < 0.05) between the nociception induced by venoms when compared to control (PBS or IgG). #Statistically significant difference (p < 0.05) between BsCo and BsCR
Lethal activity of venoms and neutralization by BAV
As can be seen in Table 1, BsCR was more lethal when compared to the BsCo (p < 0.05), with LD50 estimated as 5.60 and 9.24 mg/kg, respectively. Bj (control) was more lethal than BsCo and BsCR (p < 0.05), with a LD50 of 1.74 mg/kg. In regard to neutralization of three LD50 by BAV (400 µL), the antivenom protected 75% and 86% of animals injected with BsCR and BsCo, respectively. All animals injected with Bj (positive control) were protected by BAV.
Table 1.
Evaluation of lethality induced by B. jararaca (Bj) and B. schlegelii from Colombia (BsCo) and Costa Rica (BsCR) snake venoms
| Venom dose | Bj | Venom dose | BsCo | BsCR |
|---|---|---|---|---|
| (µg) | (mortality) | (µg) | (mortality) | (mortality) |
| 10 | 0/6a | 60 | 0/6 | 0/6 |
| 20 | 1/6 | 90 | 0/6 | 1/6 |
| 40 | 4/6 | 120 | 0/6 | 5/6 |
| 50 | 4/6 | 180 | 2/6 | 5/6 |
| 80 | 5/6 | 240 | 6/6 | 6/6 |
| LD50 (µg/animal)b | 34.8 | LD50 (µg/animal) | 184.8 | 112 |
| LD50 (mg/kg)b | 1.74 | LD50 (mg/kg) | 9.24 | 5.60 |
| 95% confidence intervals (mg/kg) | 1.15–2.63 | 95% confidence intervals (mg/kg) | 8.67–9.83 | 4.50–6.98 |
Number of animals dead/injected.
Lethal dose 50%.
Bj: B. jararaca venom; BsCo: B. schlegelii from Colombia; BsCR: B. schlegelii from Costa Rica.
Discussion
B. schlegelii is a species with a wide distribution in Latin America,8 however, there are few reports of injuries in humans by bites of this snake, whose clinical picture is mainly characterized by edema and other inflammatory events at the site of the bite. Thus, this study aimed to characterize and compare the composition and various toxic activities of B. schlegelii snake venoms from Colombia (BsCo) and Costa Rica (BsCR), as well as to evaluate the neutralization of some biological activities by BAV.
Differences were noticed in the venom profiles by SDS-PAGE and variability was observed in biological and enzymatic activities between BsCR and BsCo. Similar gelatinolytic and fibrinogenolytic profiles were noticed between BsCo and BsCR, whereas caseinolytic activity was more intense in BsCR, which showed two lytic areas in different regions. In regard to hyaluronidase activity, both B. schlegelii venoms showed lytic areas in the 62 kDa region, and BsCR was more lytic than BsCo. Hyaluronidases in the B. schlegelii venom have already been described in BsCR.47 On the other hand, the absence of hyaluronidase activity by zymography had been already reported in Bj, corroborating the data presented herein.32 In regard to PLA2, BsCR, and BsCo had higher activities than Bj. In fact, high PLA2 activity has been described and associated with the myonecrosis and clinical signs observed in the envenomation by B. schlegelii.14,18
As our results pointed out, geographical differences in protein and enzymatic activities did influence the toxic activities of BsCR and BsCo. Our results showed that BsCR was more toxic than BsCo in almost all biological tests. The edema caused by BsCR was more intense and lasted longer. In addition, BsCR induced higher hemorrhage, around fourfold as compared to that induced by BsCo. Results obtained by Kuch et al.48 demonstrated that B. schlegelii snake venom from Ecuador did not induce hemorrhage, even when used at high concentrations (10 µg), indicating great variability in toxicity in the venom of this species. It was also verified that edema and hemorrhage in Bj, used as a control, proved to be more toxic, with more persistent edema and higher hemorrhagic activity.
In regard to the nociceptive activity, we observed that BsCR induced a higher response in animals when 2 µg of venom was injected. On the other hand, an inverse result was observed at the highest venom concentration (8 µg), where the response induced by BsCo was the most evident. Results also showed that Bj exhibited no dose–response curve, and maximum responses were recorded from 1 µg; in higher doses (4 and 8 µg), nociceptive activity induced by Bj was lower than that of BsCo and BsCR, respectively. For lethality, the results showed that higher doses of BsCR and BsCo were required to induce death when compared to Bj. Furthermore, differences were also noticed between both B. schlegelii venoms, since BsCR was 1.6-fold more lethal than BsCo. LD50’s of B. schlegelii venoms described in literature have been reported to be between around 4 and 10.3 mg/kg,10,18,48,49 which are close to our results, taking into consideration that geographical variability and other parameters (protein quantification of venoms, mouse strains, and weight of animals) may influence the results. Taken together, our observations indicated differences in toxic activities between B. schlegelii venoms from different locations. B. schlegelii thus represents an interesting species to investigate patterns of geographically based variations in venom composition, owing to its wide range of distribution.14,18 In addition to geographical distribution, other factors may contribute in the diversity of venoms, such as ontogeny, diet, sex, and seasonal variation.21,50
In this study, we also evaluated the ability of BAV produced by Butantan Institute to recognize venom components, as well as to neutralize the toxic activities of BsCo and BsCR, since administration of antivenom is the treatment recommended for snakebites by WHO.3,5 Since the immunizing mixture to produce this antivenom does not include venoms of Bothriechis species, it was of interest to assess whether BAV is able to neutralize these venoms. Initially, we verified by ELISA the antigenic cross-reactivity of BAV to BsCo and BsCR, and minor differences between the antibody titer obtained against Bj. By Western blotting, components of BsCo and BsCR were recognized by BAV, especially above 35 kDa. However, proteins in the range of 20–25 kDa were weakly recognized by BAV, although they were strongly recognized in Bj. In fact, BAV has been reported to cross react with other venoms that do not participate in the immunization pool, such as Bothrops atrox, Bothrops insularis, and Bothriopsis taeniata venoms.30,32,51–54 Given that BAV cross reacts with components of BsCo and BsCR, in vivo assays were carried out to evaluate the neutralization of their toxic activities. BAV partially neutralized edema, nociception, and lethality induced by BsCo and BsCR, whereas it completely neutralized local hemorrhage, in agreement with previous observations.30 In summary, BAV was effective to neutralize some toxic activities from BsCo and BsCR venoms. Our results suggest that this antivenom may be effective for the treatment of envenomations by B. schlegelii, but this assertion needs to be further explored at the preclinical and clinical levels. It has been previously shown that a heterologous polyvalent antivenom manufactured by Instituto Clodomiro Picado (Costa Rica) also neutralized several toxic and enzymatic activities induced by BsCR.19,43 Since neither of these antivenoms include venoms of Bothriechis sp in the immunizing mixtures, it is likely that other venoms included in the mixtures, particularly those of Bothrops sp provide cross-protection against the venom of B. schlegelii.
In conclusion, our results show intraspecific variation in the composition and enzymatic and toxicological profiles of venoms of BsCo and BsCR, underscoring the effect of geographical isolation and adaptation to local conditions in the composition of snake venoms. These variations in the toxicological profile may have implications in the clinical manifestations in envenomations by these two populations of this species. The ability of BAV to neutralize some toxic effects of these venoms suggests that the venoms included in the immunization mixture to generate this antivenom have immunological relatedness with components in B. schlegelii venoms. This opens the possibility of using BAV in the treatment of envenomations by B. schlegelli, a hypothesis that awaits further experimental and clinical analyses.
Acknowledgments
The authors thank CNPq for the grant to KCB. (305359/2010-0, 305719/2013-0) and CAPES for the grant to JPPN. This research was, in part, supported by a São Paulo Research Foundation (FAPESP, grant #2010/08162-1), and INCTTox. Training of Ana María Suárez in Butantan Institute was partially supported by the Faculty of Medicine, Antioquia University.
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Author contributions
All authors participated in the design, interpretation of the studies, analysis of the data, and review of the manuscript. JPPN, LFK, AFA, JMG, RO, MLS, and KCB conceived and designed the experiments. JPPN, LFK, AFA, MLS, and KCB performed the experiments. JPPN, LFK, AFA, JMG, RO, AMS, MLS, and KCB analyzed the data. KCB supplied reagents, analysis tools, and materials. JMG, RO, AMS supplied B. schlegelii snake venoms. JPPN, LFK, AFA, JMG, RO, AMS, MLS, and KCB wrote the manuscript.
References
- 1.Chippaux JP. Snake-bites: appraisal of the global situation. Bull World Health Organ 1998; 76: 515–24. [PMC free article] [PubMed] [Google Scholar]
- 2.Kasturiratne A, Wickremasinghe AR, De Silva N, Gunawardena NK, Pathmeswaran A, Premaratna R, Savioli L, Lalloo DG, De Silva HJ. The global burden of snakebite: a literature analysis and modelling based on regional estimates of envenoming and deaths. PloS Med 2008; 5: 1591–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.World Health Organization (WHO). Guidelines for the production, control and regulation of antivenom immunoglobulins, Geneva: WHO, 2010. [Google Scholar]
- 4.Gutiérrez JM, Theakston RDG, Warrell DA. Confronting the neglected problem of snake bite envenoming: the need for a global partnership. PloS Med 2006; 3: 727–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Warrell DA. Snake bite. Lancet 2010; 375: 77–88. [DOI] [PubMed] [Google Scholar]
- 6.Gutiérrez JM, León G, Burnouf T. Antivenoms for the treatment of snakebite envenomings: the road ahead. Biologicals 2011; 39: 129–42. [DOI] [PubMed] [Google Scholar]
- 7.Otero R, Gutiérrez J, Mesa MB, Duque E, Rodríguez O, Arango JL, Gómez F, Toro A, Cano F, Rodríguez LM, Caro E, Martínez J, Cornejo W, Gómez LM, Uribe FL, Cárdenas S, Núñez V, Díaz A. Complications of Bothrops, Porthidium, and Bothriechis snakebites in Colombia. A clinical and epidemiological study of 39 cases attended in a university hospital. Toxicon 2002; 40: 1107–14. [DOI] [PubMed] [Google Scholar]
- 8.Campbell JA, Lamar WW. The venomous reptiles of the western hemisphere, Ithaca, NY: Comstock Publishing Associates, 2004, pp. p. 870–p. 870. [Google Scholar]
- 9.Warrell DA. Snakebites in Central and South America: epidemiology, clinical features, and clinical management. In: Campbell JA, Lamar WW. (eds). The venomous reptiles of the western hemisphere, Ithaca, NY: Comstock Publishing Associates, 2004, pp. 709–61. [Google Scholar]
- 10.Otero R, Osorio RG, Valderrama R, Giraldo CA. Efectos farmacológicos y enzimáticos de los venenos de serpientes de Antioquia y Chocó (Colombia). Toxicon 1992; 30: 611–20. [DOI] [PubMed] [Google Scholar]
- 11.Otero R. Envenenamiento Ofídico. In: Correa JA, Gómez JF, Posada R. (eds). Fundamentos de Pediatría, Tomo V, Urgencias, Neurología, Otorrinolaringología, Ortopedia, Medellín: Corporación para Investigaciones Biológicas (CIB), 2014, pp. 73–119. [Google Scholar]
- 12.Pérez AV, Saravia P, Rucavado A, Sant’Ana CD, Soares AM, Gutiérrez JM. Local and systemic pathophysiological alterations induced by a serine proteinase from the venom of the snake Bothrops jararacussu. Toxicon 2007; 49: 1063–9. [DOI] [PubMed] [Google Scholar]
- 13.Stábeli RG, Sant’Ana CD, Ribeiro PH, Costa TR, Ticli FK, Pires MG, Nomizo A, Albuquerque S, Malta-Neto NR, Marins M, Sampaio SV, Soares AM. Cytotoxic L-amino acid oxidase from Bothrops moojeni: biochemical and functional characterization. Int J Biol Macromol 2007; 41: 132–40. [DOI] [PubMed] [Google Scholar]
- 14.Lomonte B, Escolano J, Fernández J, Sanz L, Angulo Y, Gutiérrez JM, Calvete JJ. Snake venomics and antivenomics of the arboreal neotropical pitvipers Bothriechis lateralis and Bothriechis schlegelii. J Prot Res 2008; 7: 2445–57. [DOI] [PubMed] [Google Scholar]
- 15.Zychar BC, Dale CS, Demarchi DS, Gonçalves LR. Contribution of metalloproteases, serine proteases and phospholipases A2 to the inflammatory reaction induced by Bothrops jararaca crude venom in mice. Toxicon 2010; 55: 227–34. [DOI] [PubMed] [Google Scholar]
- 16.Angulo Y, Chaves E, Alape A, Rucavado A, Gutiérrez JM, Lomonte B. Isolation and characterization of a myotoxic phospholipase A2 from the venom of the arboreal snake Bothriechis schlegelii from Costa Rica. Arch Biochem Biophys 1997; 339: 260–6. [DOI] [PubMed] [Google Scholar]
- 17.Fernández J, Lomonte B, Sanz L, Angulo Y, Gutiérrez JM, Calvete JJ. Snake venomics of Bothriechis nigroviridis reveals extreme variability among palm pitviper venoms: different evolutionary solutions for the same trophic purpose. J Proteome Res 2010; 9: 4234–41. [DOI] [PubMed] [Google Scholar]
- 18.Lomonte B, Tsai WC, Bonilla F, Solorzano A, Solano G, Ângulo Y, Gutiérrez JM, Calvete JJ. Snake venomics and toxicological profiling of the arboreal pitviper Bothriechis supraciliaris from Costa Rica. Toxicon 2012; 59: 592–9. [DOI] [PubMed] [Google Scholar]
- 19.Gutiérrez JM, Lomonte B, Sanz L, Calvete JJ, Pla D. Immunological profile of antivenoms: preclinical analysis of the efficacy of a polyspecific antivenom through antivenomics and neutralization assays. J Proteomics 2014; 105: 340–50. [DOI] [PubMed] [Google Scholar]
- 20.Muñoz LJV, Estrada-Gomez S, Núñez V, Sanz L, Calvete JJ. Characterization and cDNA sequence of Bothriechis schlegelii-amino acid oxidase with antibacterial activity. Int J Biol Macromol 2014; 69: 200–7. [DOI] [PubMed] [Google Scholar]
- 21.Chippaux JP, Williams V, White J. Snake venom variability: methods of study, results and interpretation. Toxicon 1991; 29: 1279–303. [DOI] [PubMed] [Google Scholar]
- 22.Moura-da-Silva AM, Della-Casa MS, David AS, Assakura MT, Butera D, Lebrun I, Shannon JD, Serrano SMT, Fox JW. Evidence for heterogeneous forms of the snake venom metalloproteinase Jararhagin: a factor contributing to snake venom variability. Arch Biochem Biophys 2003; 409: 395–401. [DOI] [PubMed] [Google Scholar]
- 23.Furtado MFD, Travaglia-Cardoso SR, Rocha MMT. Sexual dimorphism in venom of Bothrops jararaca (Serpentes: Viperidae). Toxicon 2006; 48: 401–10. [DOI] [PubMed] [Google Scholar]
- 24.Menezes MC, Furtado MF, Travaglia-Cardoso SR, Camargo ACM, Serrano SMT. Sex-based individual variation of snake venom proteome among eighteen Bothrops jararaca siblings. Toxicon 2006; 47: 304–12. [DOI] [PubMed] [Google Scholar]
- 25.Antunes TC, Yamashita KM, Barbaro KC, Saiki M, Santoro ML. Comparative analysis of newborn and adult Bothrops jararaca snake venoms. Toxicon 2010; 56: 1443–58. [DOI] [PubMed] [Google Scholar]
- 26.Zelanis A, Tashima AK, Rocha MMT, Furtado MFD, Camargo ACM, Ho PL, Serrano SMT. Analysis of the ontogenetic variation in the venom proteome/peptidome of Bothrops jararaca reveals different strategies to deal with prey. J Prot Res 2010; 9: 2278–91. [DOI] [PubMed] [Google Scholar]
- 27.Santoro ML, do Carmo T, Cunha BH, Alves AF, Zelanis A, Serrano SM, Grego KF, Sant’Anna SS, Barbaro KC, Fernandes W. Ontogenetic Variation in Biological Activities of Venoms from Hybrids between Bothrops erythromelas and Bothrops neuwiedi Snakes. PLoS One 2015; 10: e0145516–e0145516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gutiérrez JM, Higashi HG, Wen FH, Burnouf T. Strengthening antivenom production in Central and South American public laboratories: report of a workshop. Toxicon 2007; 49: 30–5. [DOI] [PubMed] [Google Scholar]
- 29.Otero R, Gutiérrez JM, Rojas G, Núñez V, Díaz A, Miranda E, Uribe AF, Silva JF, Ospina JG, Medina Y, Toro MF, García ME, León G, García M, Lizano S, De La Torre J, Márquez J, Mena Y, González N, Arenas LC, Puzón A, Blanco N, Sierra A, Espinal ME, Arboleda M, Jiménez JC, Ramírez P, Díaz M, Guzmán MC, Barros J, Henao S, Ramírez A, Macea U, Lozano R. A randomized blinded clinical trial of two antivenoms, prepared by caprylic acid or ammonium sulphate fractionation of IgG, in Bothrops and Porthidium snake bites in Colombia: correlation between safety and biochemical characteristics of antivenoms. Toxicon 1999; 37: 895–908. [DOI] [PubMed] [Google Scholar]
- 30.Bogarín G, Morais JF, Yamaguchi IK, Stephano MA, Marcelino JR, Nishikawa AK, Guidolin R, Rojas G, Higashi HG, Gutiérrez JM. Neutralization of crotaline snake venoms from Central and South America by antivenoms produced in Brazil and Costa Rica. Toxicon 2000; 38: 1429–41. [DOI] [PubMed] [Google Scholar]
- 31.Schier JG, Wiener SW, Touger M, Nelson LS, Hoffman RS. Efficacy of crotalidae polyvalent antivenin for treatment of hognosed viper (Porthidium nasutum) envenomation. Ann Emerg Med 2003; 41: 391–5. [DOI] [PubMed] [Google Scholar]
- 32.Lira MS, Furtado MF, Martins LMP, Lopes-Ferreira M, Santoro ML, Barbaro KC. Enzymatic and immunochemical characterization of Bothrops insularis venom and its neutralization by polyspecific Bothrops antivenom. Toxicon 2007; 49: 982–94. [DOI] [PubMed] [Google Scholar]
- 33.Segura A, Castillo MC, Núñez V, Yarlequé A, Gonçalves LR, Villalta M, Bonilla C, Herrera M, Vargas M, Fernández M, Yano MY, Araújo HP, Boller MA, León P, Tintaya B, Sano-Martins IS, Gómez A, Fernández GP, Geoghegan P, Higashi HG, León G, Gutiérrez JM. Preclinical assessment of the neutralizing capacity of antivenoms produced in six Latin American countries against medically-relevant Bothrops snake venoms. Toxicon 2010; 56: 980–9. [DOI] [PubMed] [Google Scholar]
- 34.Otero R, Tobón GS, Gómez LF, Osorio RG, Valderrama R, Hoyos D, Urreta JE, Molina S, Arboleda JJ. Accidente ofídico en Antioquia y Chocó. Aspectos clínicos y epidemiológicos (marzo de 1989-febrero de 1990). Acta Med Colomb 1992; 17: 229–49. [Google Scholar]
- 35.Raw I, Guidolin R, Higashi HG, Kelen EMA. Antivenins in Brazil: preparation. In: Tu AT. (eds). Handbook of natural toxins, vol 5, reptile venoms and toxins, New York: Marcel Dekker, 1991, pp. 557–81. [Google Scholar]
- 36.Smith PK, Krohn RI, Hermanson GT, Mallia AK, Gartner FH, Provenzano MD, Fujimoto EK, Goeke NM, Olson BJ, Klenk DC. Measurement of protein using bicinchoninic acid. Anal Biochem 1985; 150: 76–85. [DOI] [PubMed] [Google Scholar]
- 37.Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970; 227: 680–5. [DOI] [PubMed] [Google Scholar]
- 38.Blum H, Beier H, Gross HJ. Improved silver staining of plant proteins, RNA and DNA in polyacrylamide gels. Eletrophoresis 1987; 8: 93–9. [Google Scholar]
- 39.Towbin H, Staehelin T, Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A 1979; 76: 4350–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Miura RO, Yamagata S, Miura Y, Harada T, Yamagata T. Analysis of glycosaminoglycan-degrading enzymes by substrate gel electrophoresis (Zymography). Anal Biochem 1995; 225: 333–40. [DOI] [PubMed] [Google Scholar]
- 41.Barbaro KC, Knysak I, Martins R, Hogan C, Winkel K. Enzymatic characterization, antigenic cross-reactivity and neutralization of dermonecrotic activity of five Loxosceles spider venoms of medical importance in the Americas. Toxicon 2005; 45: 489–99. [DOI] [PubMed] [Google Scholar]
- 42.Kondo H, Kondo S, Ikezawa I, Murata R, Ohsaka A. Studies of the quantitative method for determination of hemorrhagic activity of habu snake venom. Jpn J Med Sci Bio 1960; 13: 43–51. [DOI] [PubMed] [Google Scholar]
- 43.Gutiérrez JM, Gené JA, Rojas G, Cerdas L. Neutralization of proteolytic and hemorrhagic activities of Costa Rican snake venoms by a polyvalent antivenom. Toxicon 1985; 23: 887–93. [DOI] [PubMed] [Google Scholar]
- 44.Hunskaar S, Fasmer OB, Hole K. Formalin test in mice, a useful technique for evaluating mild analgesics. J Neurosci Meth 1985; 14: 69–76. [DOI] [PubMed] [Google Scholar]
- 45.Litchfield JT, Wilcoxon F. A simplified method of evaluating dose-effect experiments. J Pharmacol Exp Ther 1949; 96: 99–113. [PubMed] [Google Scholar]
- 46.Malta MB, Lira MS, Soares SL, Rocha GC, Knysak I, Martins R, Guizze SP, Santoro ML, Barbaro KC. Toxic activities of Brazilian centipede venoms. Toxicon 2008; 52: 255–63. [DOI] [PubMed] [Google Scholar]
- 47.Gené JA, Gómez M, Gutiérrez JM, Cerdas L. Neutralization of hyaluronidase and indirect hemolytic activities of Costa Rica snake venoms by a polyvalent antivenom. Toxicon 1985; 23: 1015–8. [DOI] [PubMed] [Google Scholar]
- 48.Kuch U, Mebs D, Gutiérrez JM, Freire A. Biochemical and biological characterization of Ecuadorian pitviper venoms (genera Bothriechis, Bothriopsis, Bothrops and Lachesis). Toxicon 1996; 34: 714–7. [DOI] [PubMed] [Google Scholar]
- 49.Bolaños R. Toxicity of Costa Rican snake venoms for the white mouse. Am J Trop Med Hyg 1972; 21: 360–3. [DOI] [PubMed] [Google Scholar]
- 50.Daltry JC, Wüster W, Thorpe RS. Diet and snake venom evolution. Nature 1996; 379: 537–40. [DOI] [PubMed] [Google Scholar]
- 51.Ferreira ML, Moura-da-Silva AM, Mota I. Neutralization of different activities of venoms from nine species of Bothrops snakes by Bothrops jararaca antivenom. Toxicon 1992; 30: 1591–602. [DOI] [PubMed] [Google Scholar]
- 52.Queiróz GP, Pessoa LA, Portaro FCV, Furtado MFD, Tambourgi DV. Interspecific variation in venom composition and toxicity of Brazilian snakes from Bothrops genus. Toxicon 2008; 52: 842–51. [DOI] [PubMed] [Google Scholar]
- 53.Furtado MFD, Cardoso ST, Soares OE, Pereira AP, Fernandes DS, Tambourgi DV, Sant’anna OA. Antigenic cross-reactivity and immunogenicity of Bothrops venoms from snakes of the Amazon region. Toxicon 2010; 55: 881–7. [DOI] [PubMed] [Google Scholar]
- 54.Segura A, Castillo MC, Núñez V, Yarlequé A, Gonçalves LRC, Villalta M, Bonilla C, Herrera M, Vargas M, Fernández M, Yano MY, Araújo HP, Boller MAA, León P, Tintaya B, Sano-Martins IS, Gómez A, Fernández GP, Geoghegan P, Higashi HG, León G, Gutiérrez JM. Preclinical assessment of the neutralizing capacity of antivenoms produced in six Latin American countries against medically-relevant Bothrops snake venoms. Toxicon 2010; 56: 980–9. [DOI] [PubMed] [Google Scholar]