Abstract
Iron is an important micronutrient, but it can also act as a dangerous element by interfering with glucose homeostasis and inflammation, two features that are already disturbed in obese subjects. In this work, we study the effects of systemic iron supplementation on metabolic and inflammatory responses in mice with hypoferremia induced by obesity to better characterize whether iron worsens the parameters that are already altered after 24 weeks of a high-fat diet (HFD). Mice were maintained on a control diet or a HFD for 24 weeks and received iron-III polymaltose (50 mg/kg/every 2 days) during the last two weeks. Glucose homeostasis (basal glucose and insulin test tolerance) and systemic and visceral adipose tissue (VAT) inflammation were assessed. Iron levels were measured in serum. The Prussian blue reaction was used in isolated macrophages to detect iron deposition. Iron supplementation resulted in an increased number of VAT macrophages that were positive for Prussian blue staining as well as increased serum iron levels. Systemic hepcidin, leptin, resistin, and monocyte chemoattractant protein-1 (MCP-1) levels were not altered by iron supplementation. Local adipose tissue inflammation was also not made worse by iron supplementation because the levels of hepcidin, MCP-1, leptin, and interleukin (IL)-6 were not altered. In contrast, iron supplementation resulted in an increased production of IL-10 by adipose tissue and VAT macrophages. Leukocytosis and VAT plasminogen activator inhibitor-1 (PAI-1) level were reduced, but insulin resistance was not altered after iron supplementation. In conclusion, systemic iron supplementation in mice with hypoferremia induced by obesity did not worsen inflammatory marker or adipose tissue inflammation or the metabolic status established by obesity. Iron deposition was observed in adipose tissue, mainly in macrophages, suggesting that these cells have mechanisms that promote iron incorporation without increasing the production of inflammatory mediators.
Keywords: Hepcidin, adipokine, adipose tissue, adipose tissue macrophage
Introduction
The first associations between adiposity and low plasma iron were described more than 50 years ago.1,2 The concept of obesity as a chronic low-grade systemic inflammatory condition has recently been linked to the reduction in plasma iron observed previously. Adipose tissue, mainly visceral adipose tissue (VAT), plays a role in the release of pro-inflammatory cytokines, such as interleukin (IL)-6, when it is locally infiltrated by a large number of macrophages.3,4 IL-6 has been recognized as a link between inflammation and iron levels because it induces hepcidin mRNA transcription through signal transducer and activator of transcription (STAT)-3.5 Iron is the main regulator of hepcidin expression in the liver and, when it is present in excess in the blood, hepatic hepcidin production is increased, which prevents additional absorption by the gut.6 Although iron is the main regulator of hepcidin production by the liver, inflammation appears to be important in regulating hepcidin production in macrophages, and it decreases iron release from these cells in an autocrine and/or paracrine manner.7 Adipocytes appear to be able to produce hepcidin, and inflammation is also recognized as the main stimulus linking hepcidin production by adipose tissue and hypoferremia of inflammation in obese patients and experimental models.8,9
Iron is one of the most important micronutrients, and it plays a role in DNA synthesis, respiration, and energy production; however, iron is also able to produce deleterious hydroxyl radicals in the presence of reactive oxygen species (ROS).10 Due to this dual nature, iron absorption, utilization, and storage are tightly regulated in mammals.6 Interestingly, iron metabolism and glucose metabolism are interrelated. Insulin is able to induce iron uptake in hepatocytes through the induction of transferrin receptor-1 (TfR1) synthesis.11 Isolated adipocytes also respond to insulin stimulation by increasing cell-surface transferrin receptors and the uptake of diferric transferrin12 In turn, iron interferes with insulin signaling in hepatocytes and adipocytes.13 In vitro data has shown that iron decreases insulin receptor expression and insulin binding in HepG2 cells.14 In isolated rat adipocytes, iron impairs insulin-mediated glucose uptake and increases lipolysis.13 Excess iron has been associated with reduced insulin secretion to impair the function of pancreatic β cells.15 Therefore, iron could negatively impact glucose homeostasis.
Adipose tissue engulfs resident M2 macrophages with a physiological role, and an infiltration of M1 macrophages has been linked to metabolic consequences of obesity. These distinct phenotypes support different biological functions. M1 macrophages accumulate iron as a well described bacteriostatic mechanism, while M2 macrophages recycle iron, and normally low intracellular iron levels have been found in association with this phenotype.16 Macrophage phenotype polarization is also modified by iron; data suggests that iron accumulation in macrophages, as observed when hepcidin signal is present, could result in a pro-inflammatory M1 phenotype.17 Additionally, a low iron-diet, chelation therapy or phlebotomy improve insulin sensitivity in obese animal models and humans.15,18,19 Obesity induced by a high-fat diet (HFD) in mice results in reduced duodenal iron absorption,20 suggesting that it could play a protective role in controlling iron export to metabolic tissues.
We previously demonstrated that mice fed a HFD for 24 weeks, but not less than this length of time, is a suitable model to study alterations in iron homeostasis associated with obesity. This is because mice presenting with high hepcidin serum and adipose tissue levels associated with high IL-6 levels in the adipose tissue, as well as alterations in hematological parameters, suggests the establishment of anemia associated with chronic disease.9 In this study, we investigated the effects of iron supplementation, administered through the parenteral route to avoid interference from intestinal absorption, in metabolic and inflammatory responses associated with obesity in mice fed a HFD for 24 weeks. The aim of this study was to characterize whether iron worsens the parameters that are already altered by obesity and to contribute with data to assist therapeutic decisions in the management of anemia during obesity.
Materials and methods
Animals
Free of specific pathogens male Swiss mice (8-week-old; 26.1 ± 0.8 g) were obtained from Multidisciplinary Center for Biological Research from Campinas University (Unicamp), Campinas, SP, Brazil. All experiments received approval from the Ethics Committee of São Francisco University, Bragança Paulista, SP, Brazil (Protocol CEA/USF 001.02.10). The animals were maintained on a 12:12 h artificial light–dark cycle in individual cages.
Diet-induced obesity, iron supplementation, and metabolic status
Mice were introduced to either a control (AIN-93; 15% energy from fat source) or HFD (60% energy from fat source; see Supplementary Material). Body weights were assessed weekly. The mice were evaluated after 24 weeks on a HFD. Animals receiving the HFD were initially divided into two experimental groups, those who received only the HFD diet or those that received the HFD diet supplemented with iron-III polymaltose (Noripurum®, Nycomed Pharma, SP, Brazil) 50 mg/kg/every two days intramuscularly (i.m.) during the last two weeks (HFD + iron). Two days before the feeding protocols ended, glucose and insulin tolerance tests were performed as described by Pinto et al.21 The basal glucose level was also determined at the end of the feeding protocol before the animals were euthanized.
Blood and tissue collection
After 6 h of fasting, the mice were anesthetized with xylazine/ketamine (1:1 v/v of xylazine 2%–ketamine 10%), and blood samples were collected by cardiac puncture. Hematological parameters were analyzed using blood collected with ethylenediaminetetraacetic acid (EDTA) using an autoanalyser (ABX Pentra 120, Horiba Instruments Brazil, Jundiai, SP, Brazil) and serum samples were employed for enzyme immunoassay (EIA) quantification of hepcidin and serum iron dosages. Epididymal adipose tissue, liver, and gastrocnemius muscle were carefully dissected and weighed. Fragments of adipose tissue were homogenized and homogenate samples were stored for later quantification of cytokines.21
Macrophage isolation from adipose tissue, M1/M2 markers, and Prussian blue reaction
Stromal vascular fraction (SVF) was obtained from epididymal adipose tissue after collagenase digestion as described before.9 Macrophages (CD11b + cells) were obtained from the SVF by sorting using magnetic-selected cell (Miltenyi Biotec, Germany).
Macrophages RNA were isolated, cDNA was synthesize, and the expression of the Tnf, IL10, Nos2, and Mrc1 (formerly CD206) was analyzed using real-time PCR as described9 (see Supplementary Material for primers information). All reactions were performed in duplicate, and the average Ct value was used to assess gene expression. Relative expression was calculated according to previously described methods.22
Isolated macrophages underwent the Prussian blue reaction as described.9 Cells were classified as Prussian blue positive or negative and counted in five aleatory high power fields using an optical microscope.
Measurement of cytokines, hepcidin, and serum iron
Hepcidin, interleukin (IL)-10, IL-6, insulin, leptin, monocyte chemoattractant protein-1 (MCP-1), plasminogen activator inhibitor-1 (PAI-1), resistin, and tumor necrosis factor-α (TNF-α) were quantified in serum and adipose tissue samples using commercial EIA kits (Hepcidin from USCN Life Science, Wuhan, China and IL-10 from R&D Systems, MN) and Multiplex/Luminex (Adipokine [serum] and Adipocyte Panel [adipose tissue homogenate], Millipore, MA). Measurements of iron in serum and iron-binding capacity were performed by colorimetric tests using commercial kits (K017-Iron serum and K009-Iron binding capacity Bioclin, Belo Horizonte, MG, Brazil).
Statistical analysis
All of the data are expressed as the means ± standard error mean (SEM). Group comparisons were performed using Dunnett test. An associated probability of less than 5% was considered to be significant.
Results
Obesity and alterations in metabolic parameters
Swiss mice that were fed a HFD for 24 weeks became obese and contained large amounts of VAT. Insulin resistance was well established (Table 1). Systemic iron supplementation during the last two weeks did not alter the body weight at the end of experimental protocol or adiposity (Table 1). The final liver and gastrocnemius muscle weights, as well as insulin resistance, were not altered in obese mice after iron supplementation (Table 1).
Table 1.
Clinical parameters of the control, obese (HFD), and iron supplemented (HFD + iron) mice
| Control | HFD | HFD + iron | |
|---|---|---|---|
| Final body weight (g) | 44.8 ± 1.7 | 72.4 ± 4.01a | 70.8 ± 4.3a |
| Epididymal fat (g) | 1.2 ± 0.1 | 2.7 ± 0.1a | 2.2 ± 0.3a |
| Epididymal fatb | 2.9 ± 0.1 | 3.8 ± 0.2a | 3.0 ± 0.4 |
| Liver (g) | 3.7 ± 0.2 | 5.8 ± 0.5a | 5.2 ± 0.2a |
| Gastrocnemius muscleb | 0.3 ± 0.0 | 0.2 ± 0.0a | 0.3 ± 0.0 |
| Basal glucose (mg/dL) | 134 ± 12 | 207 ± 30a | 174 ± 22 |
| Insulin (pg/mL) | 193 ± 111 | 3748 ± 1086a | 4553 ± 1675a |
| ITT (k) | 3.7 ± 0.4 | 1.2 ± 0.4a | 1.1 ± 0.5a |
HFD, high-fat diet.
P < 0.05 when compared with control group (n = 5). ITT (k) rate constant for glucose disappearance during an insulin tolerance test.
Expressed as % of body weight.
Alterations in hematological parameters and serum iron levels
Hematologic parameters of obese mice were altered resulting in a reduced hemoglobin concentration and an increased number of circulating leukocytes when compared with lean mice (Table 2). Iron supplementation normalized the number of circulating blood leukocytes (Table 2). Hypoferremia was observed in obese mice concomitant with increased latent iron binding capacity of transferrin (Table 3). Our protocol of iron supplementation resulted in increased iron levels and decreased latent iron binding capacity, as well as increased transferrin saturation (Table 3).
Table 2.
Hematological parameters of control, obese (HFD), and iron supplemented (HFD + iron) mice
| Control | HFD | HFD + iron | |
|---|---|---|---|
| Red blood cells (10/µL) | 7.7 ± 0.3 | 6.8 ± 0.6 | 7.1 ± 0.2 |
| Hemoglobin (g/dL) | 16.02 ± 0.3 | 13.1 ± 1.2a | 14.9 ± 0.15 |
| Hematocrit (%) | 32.9 ± 1.3 | 26.4 ± 2.5 | 31.8 ± 1.08 |
| MCV (fL) | 42.8 ± 0.4 | 42.7 ± 6 ± 0.7 | 44.7 ± 0.6a |
| MCH (pg/cell) | 20.7 ± 0.7 | 21.2 ± 1.4 | 21 ± 0.6 |
| MCHC (g/dL) | 48.9 ± 1.6 | 49.7 ± 2.9 | 46.9 ± 1.18 |
| White blood cells (cells/µL) | 7184 ± 1650 | 16,100 ± 2910a | 8125 ± 1518b |
HFD, high-fat diet; MCV, mean corpuscular volume; MCH, mean corpuscular hemoglobin; MCHC, mean cell hemoglobin concentration.
P < 0.05 when compared with the control group.
P < 0.05 when compared with HFD group (n = 5).
Table 3.
Iron parameters of control, obese (HFD), and iron supplemented (HFD + iron) mice
| Control | HFD | HFD + iron | |
|---|---|---|---|
| Serum iron (µg/dL) | 217.16 ± 16.9 | 172.5 ± 13.3a | 376.5 ± 34.6a,b |
| Latent iron-binding capacity (mcg/dL) | 68.4 ± 32.1 | 239.7 ± 40.6a | <0.1a,b |
| Total iron-binging capacity (mcg/dL) | 362.5 ± 33.8 | 401.4 ± 48.7 | 371 ± 38.5 |
| Transferrin saturation index (%) | 79.8 ± 10.09 | 69.29 ± 15.6 | 104.4 ± 12.05b |
P < 0.05 when compared with control group.
P < 0.05 when compared with HFD group (n = 5).
Hepcidin and systemic inflammatory markers
Serum leptin, MCP-1, and resistin were increased in obese mice when compared with control mice (Table 5). Serum TNF-α and IL-6 levels did not differ between the groups. Iron supplementation did not result in serum alterations in inflammatory markers (Table 4). Hepcidin was also increased in the serum of obese mice when compared with lean mice, but again, hepcidin levels were not modified by iron supplementation (Table 4).
Table 5.
Visceral adipose tissue inflammation markers in control, obese (HFD), and iron supplemented (HFD + iron) mice
| Control | HFD | HFD + iron | |
|---|---|---|---|
| IL-6 (pg/mg protein) | 1.8 ± 0.1 | 2.5 ± 0.2a | 2.8 ± 0.4a |
| TNF-α (pg/mg protein) | 0.5 ± 0.1 | 0.5 ± 0.1 | 0.8 ± 0.2 |
| MCP-1 (pg/mg protein) | 1.8 ± 0.5 | 12.9 ± 2.0a | 11.0 ± 1.7a |
| Leptin (pg/mg protein) | 399 ± 53 | 1891 ± 240a | 1298 ± 250a |
| Adiponectin (pg/mg protein) | 5560 ± 118 | 5648 ± 101 | 5245 ± 237 |
| IL-10 (pg/mg protein) | 2.4 ± 0.5 | 2.3 ± 0.3 | 3.5 ± 0.3b |
| PAI-1 (pg/mg protein) | 53 ± 11 | 220 ± 76a | 104 ± 21a,c |
| Hepcidin (pg/mg protein) | 168 ± 37 | 355 ± 22a | 351 ± 19a |
P < 0.05 when compared with control group.
P < 0.05 when compared with HFD group.
P = 0.06 when compared with HFD group (n = 5).
Table 4.
Inflammation serum markers in control, obese (HFD), and iron supplemented (HFD + iron) mice
| Control | HFD | HFD + iron | |
|---|---|---|---|
| IL-6 (pg/mL) | 4.1 ± 0.5 | 9.3 ± 2.8 | 13.1 ± 3.3a |
| TNF-α (pg/mL) | 6.5 ± 0.9 | 9.1 ± 1.5 | 11.2 ± 2.1 |
| MCP-1 (pg/mL) | 24.3 ± 7.2 | 53.45 ± 6.5a | 58.9 ± 20.7a |
| Leptin (pg/mL) | 5251 ± 1864 | 15,060 ± 1979a | 13,183 ± 596a |
| Resistin (pg/mL) | 376.4 ± 110.2 | 714.7 ± 137.07a | 966.3 ± 192.8a |
| Hepcidin (pg/mL) | 1782.6 ± 239.9 | 3432.8 ± 754.7a | 3819.02 ± 1083.8a |
P < 0.05 when compared with the control group (n = 5).
Inflammatory markers in adipose tissue
VAT from obese mice exhibited a higher level of leptin, IL-6, MCP-1, and PAI-1 when compared with lean mice (Table 5). Iron supplementation did not modify the levels of adipokines altered by obesity, but a tendency towards decreased PAI-1 levels was observed in VAT after iron supplementation (P = 0.06; Table 5). IL-10 level was not altered by obesity but it was increased after iron supplementation. Hepcidin was measured in VAT, and it was higher and remained at the same level in obese mice after iron supplementation (Table 5).
Macrophages phenotype markers and iron deposition
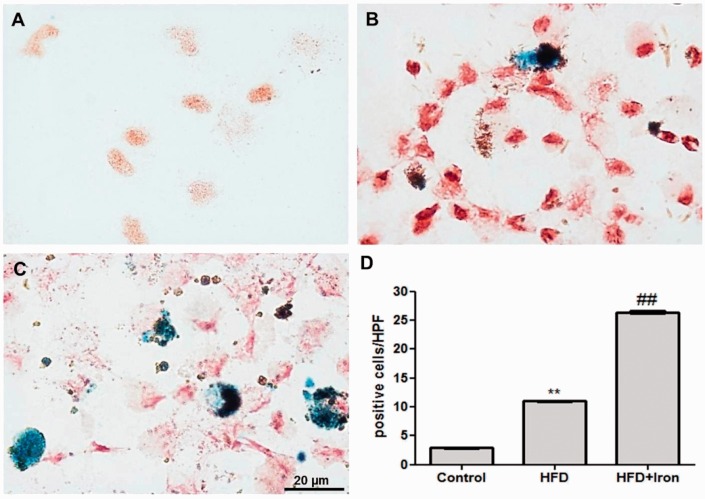
Macrophages isolated from the white adipose tissue (ATMs) of obese mice had more Prussian blue-positive cells when compared with cells from the adipose tissue of lean mice (Figure 1). Iron administration resulted in a significant increase in Prussian blue-positive macrophages (Figure 1). The analysis of gene expression revealed that obese ATM presented higher level of Tnf and Mrc1 expression and lower level of IL10 when compared with ATM of lean mice. Iron supplementation increased the IL10 expression (Table 6).
Figure 1.
Isolated adipose tissue macrophages from control (a), obese (b: high-fat diet [HFD]), and iron supplemented (c; HFD + Iron). Prussian blue reaction assessing the presence of ferric iron and ferritin. Magnification: 1000x. d. Positive Prussian blue cells count in isolated adipose tissue macrophages (CD11+ cells). Cells were counted in five random high-power fields (HPF; n = 5 per group). **P < 0.01 when compared with control group and ##P < 0.01 when compared with HFD group (n = 5). (A color version of this figure is available in the online journal.)
Table 6.
Gene expression analysis in isolated adipose tissue macrophages from control, obese (HFD), and iron supplemented (HFD + iron) mice
| Control | HFD | HFD + iron | |
|---|---|---|---|
| Tnf | 0.25 ± 0.04 | 0.45 ± 0.04a | 0.68 ± 0.23 |
| Nos2 | 0.01 ± 0.01 | 0.01 ± 0.01 | 0.01 ± 0.01 |
| IL10 | 0.16 ± 0.05 | 0.03 ± 0.00a | 0.26 ± 0.14b |
| Mrc1 | 0.16 ± 0.08 | 0.64 ± 0.16a | 0.36 ± 0.09 |
P < 0.05 when compared with control group.
P < 0.05 when compared with HFD group.
Discussion
An optimal iron status is essential for human health because energetic efficiency is directly affected by iron deficiency. The prevalence of iron deficiency increases concomitant with increases in body mass index (BMI).23 There are no differences between the intake of dietary factors that can affect iron levels in obese subjects when compared with lean subjects.23 The most probable link between obesity and hypoferremia was originally described by Bekri et al.8 who found hepcidin expression in human adipose tissue. Hepcidin inhibits iron release from macrophages and iron absorption by enterocytes through ferroportin degradation.24 The hepatic production of hepcidin is substantially higher than that produced by adipose tissue, but in obese subjects, adipose tissue mass exceeds the liver mass by about 20 times.25 Mice fed a HFD provide a suitable model to study obesity, and when Swiss mice were fed a HFD for 24 weeks, they presented with high hepcidin levels in the blood and adipose tissue in addition to hypoferremia.9 The HFD was also shown to reduce intestinal iron absorption by a hepcidin-independent mechanism involving a reduction in the expression of oxidoreductases and metal uptake in the gut.20 Thus, in this study, we employed an experimental model of hypoferremia induced by obesity in mice and described the effects of iron supplementation via the parenteral route on adipose tissue inflammation, glucose homeostasis, and iron status previously altered by obesity. In spite of treatment with deferoxamine, an iron chelating agent, for two weeks in spontaneous obese KKAy mice, which results in improvements in metabolic status and a reduction in adiposity,18 two weeks of iron supplementation in obese Swiss mice fed a HFD did not modify adiposity or insulin resistance. Hypoferremia was corrected, but hemoglobin levels were not. Our hypothesis is that iron sequestration by adipose tissue could exacerbate local inflammation and subsequently worsen systemic inflammation and metabolic status of obese individuals; however, local and systemic inflammation were not additionally made worse by iron supplementation in this experimental study, even though we observed iron deposition in adipose tissue (data not shown) and in ATMs. ATMs play a role in iron metabolism in adipose tissue. Approximately 25% of ATMs from lean mice had elevated expression of iron metabolism-related genes relative to recycling, and not storage ability, in parallel with M2 phenotype markers.26 In the context of obesity, ATM recruiting has a more pro-inflammatory phenotype (M1) but does not play a role in iron handling as observed in lean ATMs, resulting in adipocyte iron overload and reduced adiponectin expression as described by Orr et al.26 M1-polarized macrophages were classically described as activated by hepcidin and able to sequester iron during inflammation,27 but as cited before, M2-polarized macrophages are also able to play a role in iron storage. Evaluation of phenotype markers in isolated ATM in this work revealed an increased expression of gene that encoding cytokine TNF-α and lower expression of gene for IL-10 characterizing a M1 macrophage. Mannose receptor expression (Mrc1) was higher and these results are in agreement with published results that ATM consist a specific type presenting surface markers as phenotype M2 and capability to produce pro-inflammatory cytokines.28 More important than macrophage phenotype is the role of secondary or local factors that could influence iron status. For example, nitric oxide is able to activate IRP1 and IRP2 resulting in macrophage iron retention29 as well as local hepcidin production.9 In fact, we observed that the number of ATMs with incorporated iron increases after systemic iron supplementation, but no additional effects were observed in the pro-inflammatory mediators produced by the adipose tissue of obese mice. In contrast, iron supplementation resulted in an increased production of IL-10 by adipose tissue and ATMs, independently of obesity. The ingestion of hemoglobin:haptoglobin complex results in an additional macrophage phenotype, M(Hb) or Mhem, very competent in IL-10 production.30,31 Superparamagnetic iron oxide nanoparticles were also able to increase IL-10 production in M2 macrophages.32 Therefore, we hypothesized that ATMs could also be able to respond to the increase of intracellular iron producing IL-10. Iron supplementation did not interfere with local or systemic hepcidin production in our experimental model. In addition, we assessed liver hepcidin production and found that it was not altered by obesity or iron supplementation (data not shown). However, leukocytosis was reduced by iron supplementation in obese mice. Obesity is recognized as a cause of leukocytosis, and it contributes to the low-grade inflammatory systemic status of obese subjects.33 Leptin released by bone marrow adipocytes was identified as being responsible for increasing the differentiation of granulocytes from hematopoietic progenitors.34,35 Additionally, it has recently been demonstrated that adipose tissue containing hematopoietic progenitors is able to reconstitute normal blood cell levels in irradiated mice.35,36 In the present study, we did not determine whether leukocytosis was reduced by the effects of iron supplementation on bone marrow or adipose tissue, but iron excess induced in different ways has been shown to damage bone marrow stromal cells as well as other vital organs that support hematopoiesis through the generation of ROS.37 Conversely, an increase of ROS in adipose tissue is not consistent with the local reduction in PAI-1 we observed.38 PAI-1 has a broad range of biological activities, and cellular iron status is known to regulate PAI-1 expression by inducing mRNA stability,39 suggesting that local PAI-1 reduction could be one of the effects resulting from iron deposition in adipose tissue.
In conclusion, systemic iron supplementation in mice with hypoferremia induced by obesity did not result in improvements in hematological parameters, but it also did not negatively affect inflammatory marker or adipose tissue inflammation or the metabolic status established by obesity. Iron deposition was observed in adipose tissue, mainly in macrophages, suggesting that these cells have mechanisms to promote iron sequestration, but this did not modify their ability to produce proinflammatory mediators.
Supplementary Material
Acknowledgment
Financial Support: (470404/2012-4) National Counsel of Technological and Scientific Development—CNPq/Brazil.
Author contributions
All authors participated in the design, interpretation of the studies, and analysis of the data and review of the manuscript; EMFG, CRPC, and CCO conducted the experiments, TR provided support for histological analysis, MLR and AG wrote the manuscript.
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- 1.Seltzer CC, Mayer J. Serum iron and iron-binding capacity in adolescents. II. Comparison of obese and nonobese subjects. Am J Clin Nutr 1963; 13: 354–61. [DOI] [PubMed] [Google Scholar]
- 2.Wenzel BJ, Stults HB, Mayer J. Hypoferraemia in obese adolescents. Lancet 1962; 2: 327–8. [DOI] [PubMed] [Google Scholar]
- 3.Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW., Jr Obesity is associated with macrophage accumulation in adipose tissue. J Clin Investig 2003; 112: 1796–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sarvari AK, Doan-Xuan QM, Bacso Z, Csomos I, Balajthy Z, Fesus L. Interaction of differentiated human adipocytes with macrophages leads to trogocytosis and selective IL-6 secretion. Cell Death Dis 2015; 6: e1613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wrighting DM, Andrews NC. Interleukin-6 induces hepcidin expression through STAT3. Blood 2006; 108: 3204–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang X, Rovin BH. Beyond anemia: hepcidin, monocytes and inflammation. Biol Chem 2013; 394: 231–8. [DOI] [PubMed] [Google Scholar]
- 7.Rochette L, Gudjoncik A, Guenancia C, Zeller M, Cottin Y, Vergely C. The iron-regulatory hormone hepcidin: a possible therapeutic target? Pharmacol Ther 2015; 146C: 35–52. [DOI] [PubMed] [Google Scholar]
- 8.Bekri S, Gual P, Anty R, Luciani N, Dahman M, Ramesh B, Iannelli A, Staccini-Myx A, Casanova D, Ben Amor I, Saint-Paul MC, Huet PM, Sadoul JL, Gugenheim J, Srai SK, Tran A, Le Marchand-Brustel Y. Increased adipose tissue expression of hepcidin in severe obesity is independent from diabetes and NASH. Gastroenterology 2006; 131: 788–96. [DOI] [PubMed] [Google Scholar]
- 9.Gotardo EM, Santos AN, Miyashiro RA, Gambero S, Rocha T, Ribeiro ML, Gambero A. Mice that are fed a high-fat diet display increased hepcidin expression in adipose tissue. J Nutr Sci Vitaminol 2013; 59: 454–61. [DOI] [PubMed] [Google Scholar]
- 10.Zafon C, Lecube A, Simo R. Iron in obesity. An ancient micronutrient for a modern disease. Obes Rev 2010; 11: 322–8. [DOI] [PubMed] [Google Scholar]
- 11.Biswas S, Tapryal N, Mukherjee R, Kumar R, Mukhopadhyay CK. Insulin promotes iron uptake in human hepatic cell by regulating transferrin receptor-1 transcription mediated by hypoxia inducible factor-1. Biochim Biophys Acta 2013; 1832: 293–301. [DOI] [PubMed] [Google Scholar]
- 12.Davis RJ, Corvera S, Czech MP. Insulin stimulates cellular iron uptake and causes the redistribution of intracellular transferrin receptors to the plasma membrane. J Biol Chem 1986; 261: 8708–11. [PubMed] [Google Scholar]
- 13.Rumberger JM, Peters T, Jr., Burrington C, Green A. Transferrin and iron contribute to the lipolytic effect of serum in isolated adipocytes. Diabetes 2004; 53: 2535–41. [DOI] [PubMed] [Google Scholar]
- 14.Fargion S, Dongiovanni P, Guzzo A, Colombo S, Valenti L, Fracanzani AL. Iron and insulin resistance. Aliment Pharmacol Ther 2005; 22: 61–3. [DOI] [PubMed] [Google Scholar]
- 15.Cooksey RC, Jones D, Gabrielsen S, Huang J, Simcox JA, Luo B, Soesanto Y, Rienhoff H, Abel ED, McClain DA. Dietary iron restriction or iron chelation protects from diabetes and loss of beta-cell function in the obese (ob/ob lep-/-) mouse. Am J Physiol Endocrinol Metab 2010; 298: E1236–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Recalcati S, Locati M, Gammella E, Invernizzi P, Cairo G. Iron levels in polarized macrophages: regulation of immunity and autoimmunity. Autoimmun Rev 2012; 11: 883–9. [DOI] [PubMed] [Google Scholar]
- 17.Zhang Z, Zhang F, An P, Guo X, Shen Y, Tao Y, Wu Q, Zhang Y, Yu Y, Ning B, Nie G, Knutson MD, Anderson GJ, Wang F. Ferroportin1 deficiency in mouse macrophages impairs iron homeostasis and inflammatory responses. Blood 2011; 118: 1912–22. [DOI] [PubMed] [Google Scholar]
- 18.Tajima S, Ikeda Y, Sawada K, Yamano N, Horinouchi Y, Kihira Y, Ishizawa K, Izawa-Ishizawa Y, Kawazoe K, Tomita S, Minakuchi K, Tsuchiya K, Tamaki T. Iron reduction by deferoxamine leads to amelioration of adiposity via the regulation of oxidative stress and inflammation in obese and type 2 diabetes KKAy mice. Am J Physiol Endocrinol Metab 2012; 302: E77–86. [DOI] [PubMed] [Google Scholar]
- 19.Valenti L, Fracanzani AL, Dongiovanni P, Rovida S, Rametta R, Fatta E, Pulixi EA, Maggioni M, Fargion S. A randomized trial of iron depletion in patients with nonalcoholic fatty liver disease and hyperferritinemia. World J Gastroenterol 2014; 20: 3002–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sonnweber T, Ress C, Nairz M, Theurl I, Schroll A, Murphy AT, Wroblewski V, Witcher DR, Moser P, Ebenbichler CF, Kaser S, Weiss G. High-fat diet causes iron deficiency via hepcidin-independent reduction of duodenal iron absorption. J Nutr Biochem 2012; 23: 1600–8. [DOI] [PubMed] [Google Scholar]
- 21.Pinto LdF, Compri CM, Fornari JV, Bartchewsky W, Cintra DE, Trevisan M, Carvalho Pde O, Ribeiro ML, Velloso LA, Saad MJ, Pedrazzoli J, Jr., Gambero A. The immunosuppressant drug, thalidomide, improves hepatic alterations induced by a high-fat diet in mice. Liver Int 2010; 30: 603–10. [DOI] [PubMed] [Google Scholar]
- 22.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001; 25: 402–8. [DOI] [PubMed] [Google Scholar]
- 23.Menzie CM, Yanoff LB, Denkinger BI, McHugh T, Sebring NG, Calis KA, Yanovski JA. Obesity-related hypoferremia is not explained by differences in reported intake of heme and nonheme iron or intake of dietary factors that can affect iron absorption. J Am Diet Assoc 2008; 108: 145–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nemeth E, Tuttle MS, Powelson J, Vaughn MB, Donovan A, Ward DM, Ganz T, Kaplan J. Hepcidin regulates cellular iron efflux by binding to ferroportin and inducing its internalization. Science 2004; 306: 2090–3. [DOI] [PubMed] [Google Scholar]
- 25.Becker C, Orozco M, Solomons NW, Schumann K. Iron metabolism in obesity: how interaction between homoeostatic mechanisms can interfere with their original purpose. Part I: underlying homoeostatic mechanisms of energy storage and iron metabolisms and their interaction. J Trace Elem Med Biol 2015; 30: 195–201. [DOI] [PubMed] [Google Scholar]
- 26.Orr JS, Kennedy A, Anderson-Baucum EK, Webb CD, Fordahl SC, Erikson KM, Zhang Y, Etzerodt A, Moestrup SK, Hasty AH. Obesity alters adipose tissue macrophage iron content and tissue iron distribution. Diabetes 2014; 63: 421–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hubler MJ, Peterson KR, Hasty AH. Iron homeostasis: a new job for macrophages in adipose tissue? Trends Endocrinol Metab 2015; 26: 101–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zeyda M, Farmer D, Todoric J, Aszmann O, Speiser M, Gyori G, Zlabinger GJ, Stulnig TM. Human adipose tissue macrophages are of an anti-inflammatory phenotype but capable of excessive pro-inflammatory mediator production. Int J Obes 2007; 31: 1420–8. [DOI] [PubMed] [Google Scholar]
- 29.Drapier JC, Hirling H, Wietzerbin J, Kaldy P, Kuhn LC. Biosynthesis of nitric oxide activates iron regulatory factor in macrophages. EMBO J 1993; 12: 3643–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Boyle JJ, Harrington HA, Piper E, Elderfield K, Stark J, Landis RC, Haskard DO. Coronary intraplaque hemorrhage evokes a novel atheroprotective macrophage phenotype. Am J Pathol 2009; 174: 1097–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Finn AV, Nakano M, Polavarapu R, Karmali V, Saeed O, Zhao X, Yazdani S, Otsuka F, Davis T, Habib A, Narula J, Kolodgie FD, Virmani R. Hemoglobin directs macrophage differentiation and prevents foam cell formation in human atherosclerotic plaques. J Am Coll Cardiol 2012; 59: 166–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rojas JM, Sanz-Ortega L, Mulens-Arias V, Gutierrez L, Perez-Yague S, Barber DF. Superparamagnetic iron oxide nanoparticle uptake alters M2 macrophage phenotype, iron metabolism, migration and invasion. Nanomedicine 2016;12:1127–38. [DOI] [PubMed]
- 33.Herishanu Y, Rogowski O, Polliack A, Marilus R. Leukocytosis in obese individuals: possible link in patients with unexplained persistent neutrophilia. Eur J Haematol 2006; 76: 516–20. [DOI] [PubMed] [Google Scholar]
- 34.Laharrague P, Oppert JM, Brousset P, Charlet JP, Campfield A, Fontanilles AM, Guy-Grand B, Corberand JX, Penicaud L, Casteilla L. High concentration of leptin stimulates myeloid differentiation from human bone marrow CD34+ progenitors: potential involvement in leukocytosis of obese subjects. Int J Obes Relat Metab Dis 2000; 24: 1212–6. [DOI] [PubMed] [Google Scholar]
- 35.Cousin B, Casteilla L, Laharrague P, Luche E, Lorsignol A, Cuminetti V, Paupert J. Immuno-metabolism and adipose tissue: the key role of hematopoietic stem cells. Biochimie 2016;124:21–6. [DOI] [PubMed]
- 36.Cousin B, Andre M, Arnaud E, Penicaud L, Casteilla L. Reconstitution of lethally irradiated mice by cells isolated from adipose tissue. Biochem Biophys Res Commun 2003; 301: 1016–22. [DOI] [PubMed] [Google Scholar]
- 37.Chai X, Li D, Cao X, Zhang Y, Mu J, Lu W, Xiao X, Li C, Meng J, Chen J, Li Q, Wang J, Meng A, Zhao M. ROS-mediated iron overload injures the hematopoiesis of bone marrow by damaging hematopoietic stem/progenitor cells in mice. Sci Rep 2015; 5: 10181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lubrano C, Valacchi G, Specchia P, Gnessi L, Rubanenko EP, Shuginina EA, Trukhanov AI, Korkina LG, De Luca C. Integrated haematological profiles of redox status, lipid, and inflammatory protein biomarkers in benign obesity and unhealthy obesity with metabolic syndrome. Oxid Med Cell Longevity 2015; 2015: 490613, . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Radha KS, Sugiki M, Harish Kumar M, Omura S, Maruyama M. Post-transcriptional regulation of plasminogen activator inhibitor-1 by intracellular iron in cultured human lung fibroblasts–interaction of an 81-kDa nuclear protein with the 3′-UTR. J Thromb Haemost 2005; 3: 1001–8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.