Abstract
The fat-soluble diterpenoids tanshinone IIA (TSA) is the major active element of Danshen, which has widespread cardioprotective effect. However, the mechanism of its beneficial effect on cardiomyocytes has not been fully investigated. Here, we aim to demonstrate that TSA ameliorates apoptosis of cardiomyocytes activated by endoplasmic reticulum stress (ERS). Primary cultures of neonatal rat cardiomyocytes are used, in which ERS-mediated apoptosis is induced by tunicamycin (Tm). Apoptosis of cardiomyocytes are detected by Hoechst staining and caspase 3 activity analysis. Protein expression of ERS markers are detected by Western blot, and level of miroRNA-133 (miR-133) is detected by real-time polymerase chain reaction. Tm treatment significantly triggers the apoptosis and ERS of cardiomyocytes. TSA dramatically ameliorates apoptosis and ERS of cardiomyocytes induced by Tm. Interestingly, level of miR-133 is reduced by Tm treatment, which is reversed by TSA. The cardioprotective effect of TSA on apoptosis and ERS of cardiomyocytes is blocked by anti-miR-133. These results suggest that TSA protects cardiomyocytes through ameliorated ERS-mediated apoptosis, which may be resulted from upregulation of miR-133.
Keywords: Tanshinone IIA, cardiomyocytes, apoptosis, endoplasmic reticulum stress
Introduction
Endoplasmic reticulum stress (ERS) is activated because of a disturbance of homeostasis in the endoplasmic reticulum (ER), resulting from accumulation of unfolded/misfolded proteins in the ER lumen. To restore the homeostasis of ER, ERS triggers a series of accommodative mechanisms known as the unfolded protein response (UPR), including attenuation of translation, elevated expression of ER chaperones and associated proteins, and degradation of unfolded/misfolded proteins by quality-control system.1 However, when the ER function is seriously damaged, ERS stimulates apoptotic signals pathway,2,3 which has been implicated in many cardiovascular pathological processes such as ischemic heart disease, atherosclerosis, myocardial ischemia/reperfusion (I/R) injury, cardiomyopathy, and heart failure.4 Previous study has demonstrated that ERS contributed to myocardial injury, and some factors also improved impairment of myocardium by inhibiting ERS.5,6 Therefore, ERS has be considered as one of main target for prevention and therapy of myocardial injury and diseases.
The fat-soluble diterpenoids tanshinone IIA (TSA) is the major active element of Danshen, the dry roots of Salvia miltiorrhiza Bunge, which constitutes 15% of the weight of advantageous ingredients.7,8 TSA has a multi-dimensional beneficial actions on the cardiovascular system through anti-atherosclerosis, anti-arrhythmia, anti-inflammation, anti-smooth muscle hyperplasia, anti-myocardial hypertrophy, and so on.9–12 The protective effect of TSA on impairment of myocardium has been extensively reported,13–16 and the cardioprotection is mainly related to anti-apoptosis of cardiomyocytes.17–19 However, its detailed mechanism for anti-apoptosis has not been fully demonstrated.
Recently, several published articles have demonstrated the prevented effect of TSA on ERS. In the kidneys preserved with TSA, the protein markers of ERS, caspase 12, and CHOP are decreased compared with that in the kidneys maintained with Celsior solution solely.20 In L6 myotubes, TSA prevents the activation of ERS induced by tunicamycin (Tm), a widespread used activator of ERS.21 In cardiac fibroblasts, TSA treatment also weakens the radiation-induced ERS and fibrosis damage.22 All these results demonstrate that TSA reduces the stimulation of ERS, resulting in amelioration of tissue impairment. Given the key role of ERS in cardiomyocytes apoptosis, we hypothesize that TSA improves cardiomyocytes apoptosis through inhibiting ERS. Presently, primary cardiomyocytes of neonatal rats were used to study the ameliorative role of TSA in ERS and apoptosis induced by Tm.
Materials and methods
Animals and agents
One- to three-day-old Sprague-Dawley (SD) rats (180–200 g) were obtained from the Animal Center, Huazhong University of Science and Technology (Wuhan, China). All animal procedures were complied with the Animal Management Rule of the Ministry of Health, People’s Republic of China (documentation No. 55, 2001) and the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH Publication No. 85-23, revised 1996), and approved by the Animal Care Committee of Huazhong University of Science and Technology.
TSA (purity 99%) was from Xian Guanyu Bio-tech Co. Ltd (Xian, CN). Tunicamycin (Tm) was from Cayman Chemical (Ann Arbor, MI). Antibody against GRP78 was from Epitomic Inc (Burlingame, CA). Antibodies against active-caspase 12 and beta-actin were from GeneTex Inc (Irvine, CA). Antibody against CHOP was from Affinity Biosciences Inc (Cincinnati, OH). The second antibody was from Kirkegaard & Perry Laboratories Inc (Gaithersburg, MD). Potent enhanced chemiluminescence (ECL) kit was from MultiSciences Biotech Co (Hangzhou, China). Hoechst staining kit and caspase 3 activity kit was from Applygen (Beijing, China). Dulbecco’s modified eagle medium (DMEM) and fetal bovine serum (FBS) were from Hyclone (Logan, UT). Bromodeoxyuridine was from Sigma (St Louis, MO). Trizol and lipofectamine 2000 were from Invitrogen (Carlsbad, CA). miRNA Detection Kit was from Ambion (Austin, TX). anti-microRNA-133 (miR-133) antisense oligonucleotides (5′-ACAGCUGGUUGAAGGGGACCAA-3′) were synthesized by Guangzhou Ribo Bio Co., Ltd (Guangzhou, China). Others chemicals and reagents were of analytical grade.
Isolation and culture of neonatal rat cardiomyocytes
Primary cultures of neonatal rat cardiomyocytes were separated from one- to three-day-old SD rats by trypsin. The ventricular myocardium was minced in DMEM, which contains 25 × 10−3 mol/L D-glucose and 4 ×10−3 mol/L L-glutamine. After each of six continuing 10 min incubation, the cells were suspended in DMEM involving 10% FBS and centrifuged. Amalgamative cells were plated onto cultured dishes at a density of 105 cells/cm2, cultivated at 37℃ in humidified air with 5% CO2 for 72 h, and bromodeoxyuridine (100 × 10−6 mol/L) was added into the medium to remove non-myocytes. Before the test, the cells starved to serum-free DMEM for another 24 h, and were then administrated with varieties of reagents. Neonatal cardiomyocytes were cultured under a condition of Tm (1 × 10−9 mol/L) for 24 h. TSA (10 ×10−6 mol/L) were given 30 min preceding Tm treatment.
Hoechst staining and caspase 3 activity analysis
Cardiomyocytes apoptosis were detected by fluorescent staining with Hoechst 33342, and colorimetric assay of caspase 3 activity. The operation was accorded to manufacturer’s instruction.
Western blot
To prepare whole cell lysate, cultured primary cardiomyocytes were washed with PBS, collected by scraping and centrifugation, and then resuspended in 100 µL/106 cells RIPA buffer. Equivalent quantity of protein specimens were loaded on 10% SDS gels, and then transferred to the nitrocellulose membrane. Nonspecific proteins were blockaded with 5% nonfat dried milk for 1 h. The membranes were hatched with the special primary antibodies overnight at 4℃, and with the accordingly secondary antibodies (horseradish peroxidase [HRP]-conjugated anti-goat or anti-rabbit IgG) for 1 h at room temperature. The reaction was visualized by ECL, and autoradiograph was scanned. Protein expressions were assessed by NIH image software, and normalized to that of β-actin. All results were repeated at least three times.
Quantitative real-time PCR
Total RNA samples were extracted using Trizol from cultural cardiomyocytes. The mirVana quantitative real-time polymerase chain reaction (Qrt-PCR) miRNA Detection Kit in conjunction with SYBR Green I was used to quantify miR-133 levels. The following primers were utilized for Qrt-PCR detection: 5′-GGGTTTGGTCCCCTTCAA-3′ (forward); 5′-AGTGCGTGTCGTGGAGTC-3′ (reverse). U6 was utilized as the internal control. The levels of miR-133 were normalized by the levels of U6.
Transfection of miRNA
After starved in serum-free medium for 24 h, cardiomyocytes (1 × 105 per well) were transfected with anti-miR-133 by Lipofectamine 2000 according to the manufacturer’s instructions.
Statistical analysis
Statistical analysis was operated by the Graphpad software (v5.00 for Windows; GraphPad Software Inc., San Diego, CA). Unpaired Student’s t-test was used to analyze comparisons between two groups. One-way analysis of variance (ANOVA) followed by Newman–Keuls test was used to analyze comparisons among more than two groups. Data are expressed as mean ± standard deviation (SD). A P < 0.05 was considered statistically significant.
Results
Tm induced ERS and apoptosis in cardiomyocytes
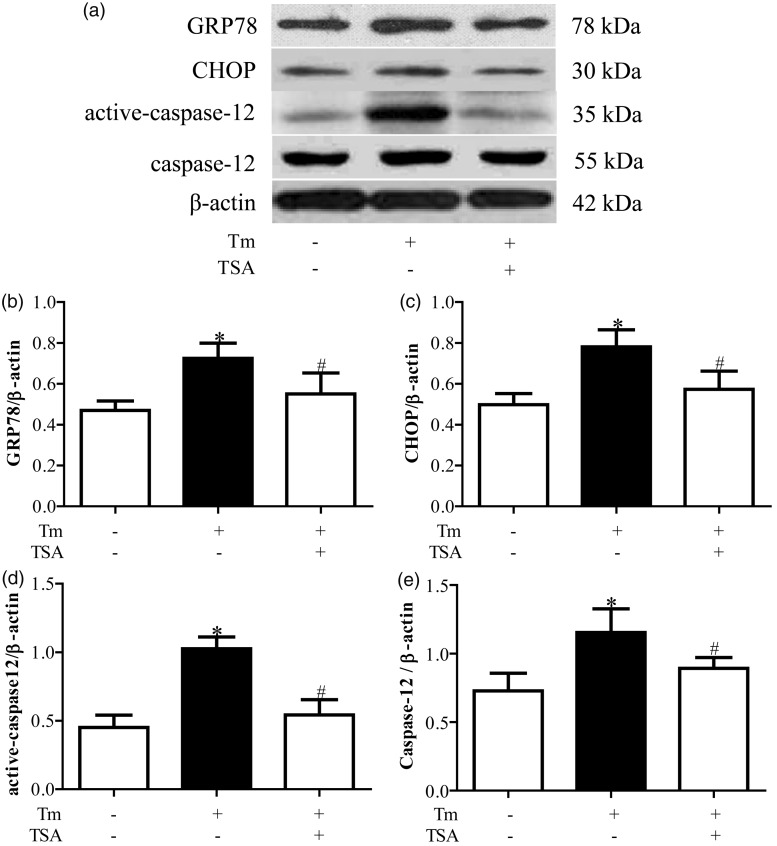
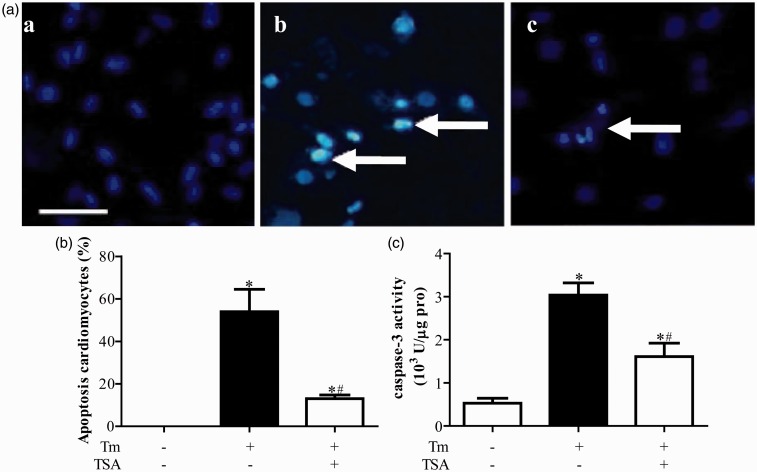
Compared with control group, protein expressions of GRP78, CHOP, and active-caspase 12 were upregulated in Tm treatment group (P < 0.05, Figure 1). Hoechst staining showed that Tm treatment induced apoptosis in cardiomyocytes (Figure 2a and b). Accordingly, caspase 3 activity was also significantly more increased in Tm treatment group than that in control group (P < 0.05, Figure 2c).
Figure 1.
Tanshinone IIA (TSA) prevented tunicamycin (Tm)-induced endoplasmic reticulum stress (ERS). (a) is the representative imaging of Western blot. (b), (c), and (d) are the statistical analysis of protein expressions of GRP78, CHOP, and active-caspase 12, respectively. *P < 0.05 vs. Con group; #P < 0.05 vs. Tm alone treatment group (n = 6)
Figure 2.
Tanshinone IIA (TSA) ameliorated apoptosis in primary neonatal rat cardiomyocytes induced by tunicamycin (Tm). (a) is the representative imaging of Hoechst staining, and (b) is the statistical analysis. (c) is the caspase-3 activity. *P < 0.05 vs. Con group; #P < 0.05 vs. Tm alone treatment group (n = 6). (A color version of this figure is available in the online journal.)
TSA ameliorated the ERS and apoptosis in cardiomyocytes caused by Tm
Compared with Tm treatment group, protein expressions of GRP78, CHOP, and active-caspase 12 were downregulated in Tm + TSA group (P < 0.05, Figure 1). Tm treatment induced apoptosis of cardiomyocytes were also prevented by TSA treatment determined by Hoechst staining (Figure 2a and b and caspase 3 activity analysis (Figure 2c).
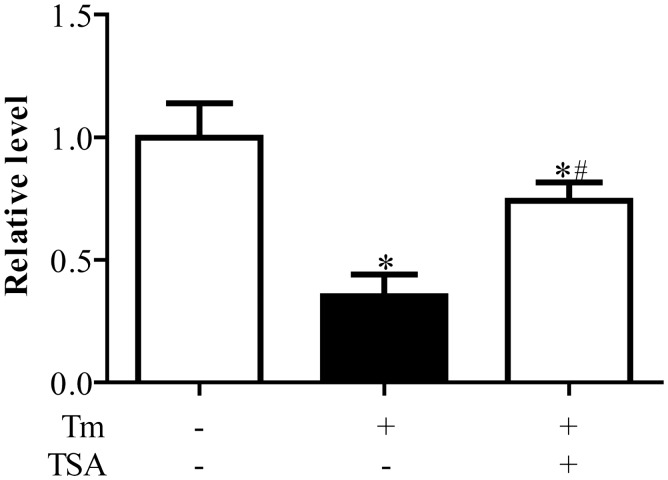
Expression of miR-133 in cardiomyocytes
Compared with control group, expressions of miR-133 were significantly downregulated in cardiomyocytes treated with Tm (P < 0.05). TSA could reverse the decreased level of miR-133 caused by Tm treatment (P < 0.05, Figure 3).
Figure 3.
Expressions of miR-133 in primary neonatal rat cardiomyocytes. *P < 0.05 vs. Con group; #P < 0.05 vs. Tunicamycin (Tm) alone treatment group (n = 6)
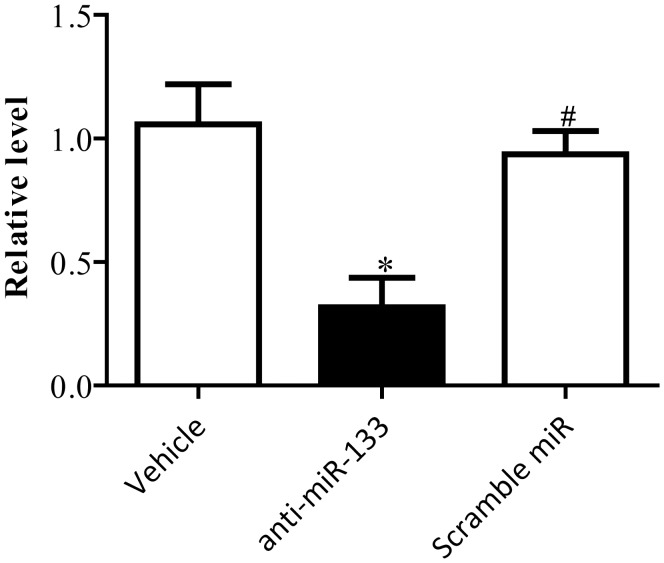
Anti-miR-133 antagonized the ameliorated effect of TSA on ERS and apoptosis in cardiomyocytes
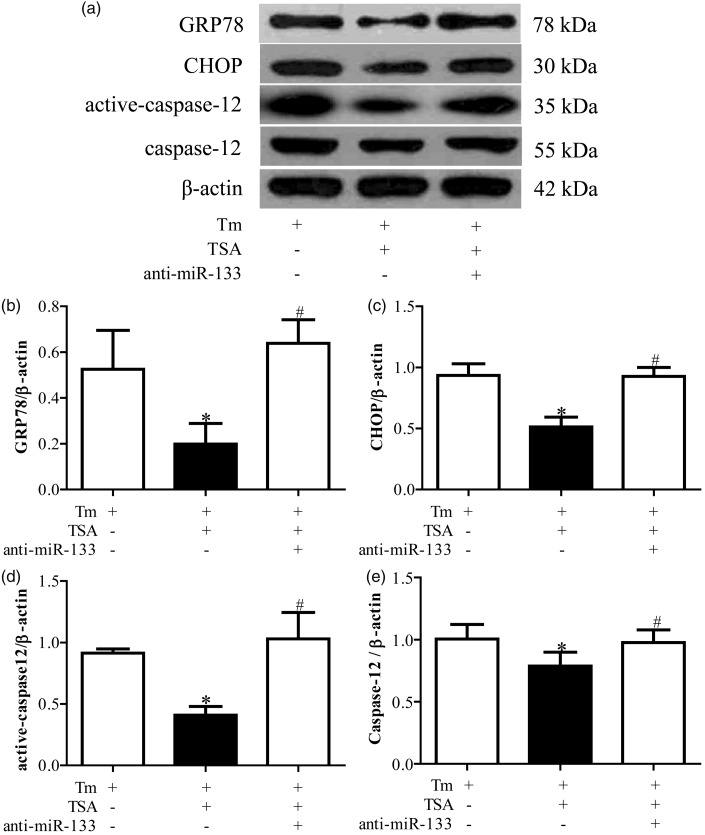
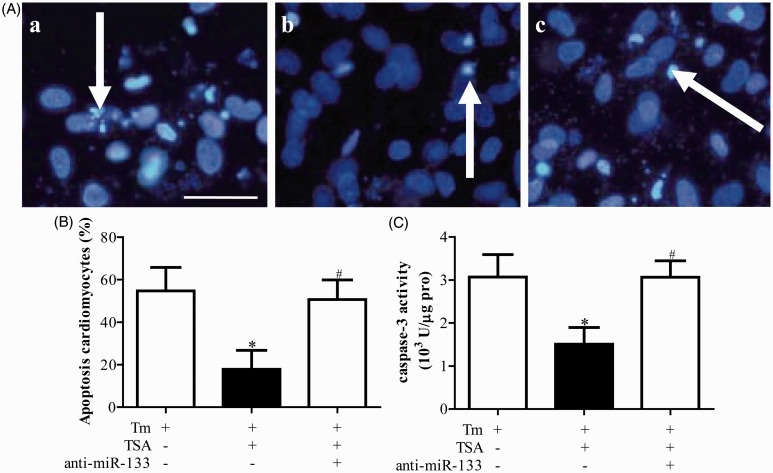
Compared with vehicle treatment group, the expressions of miR-133 were significantly downregulated in anti-miR-133 treatment group (P < 0.05, Figure 4). There was no difference between vehicle and scramble miR group (P < 0.05, Figure 4). The preventive effect of TSA on activation of ERS was blocked by the anti-miR-133 (P < 0.05, Figure 5). Accordingly, anti-miR-133 dramatically reduced the ameliorative impact of TSA on apoptosis in cardiomyocytes (P < 0.05, Figure 6).
Figure 4.
Anti-miR-133 inhibited the expressions of miR-133 in primary neonatal rat cardiomyocytes. *P < 0.05 vs. scramble miR treatment group; #P < 0.05 vs. anti-miR-133 treatment group (n = 6)
Figure 5.
Anti-miR-133 blocked the inhibited effect of tanshinone IIA (TSA) on tunicamycin (Tm)-induced endoplasmic reticulum stress (ERS). (a) is the representative imaging of Western blot. (b), (c), and (d) are the statistical analysis of protein expressions of GRP78, CHOP, and active-caspase 12, respectively. *P < 0.05 vs. Tm alone treatment group; #P < 0.05 vs. Tm plus TSA treatment group (n = 6)
Figure 6.
Anti-miR-133 prevented the ameliorated effect of tanshinone IIA (TSA) on apoptosis in primary neonatal rat cardiomyocytes induced by tunicamycin (Tm). (a) is the representative imaging of Hoechst staining, and (b) is the statistical analysis. (c) is the caspase-3 activity. *P < 0.05 vs. Tm alone treatment group; #P < 0.05 vs. Tm plus TSA treatment group (n = 6). (A color version of this figure is available in the online journal.)
Discussion
We presently objected to study whether the cardioprotective effect of TSA is mediated by ameliorating ERS. TSA directly inhibits ERS and apoptosis triggered by Tm in primary cardiomyocytes. Additionally, expression of miRNA133 in cardiomyocytes is decreased by Tm treatment, and TSA reverses the downregulation of miRNA133 induced by Tm. Anti-miR-133 blocks the above beneficial effect of TSA on cardiomyocytes.
The ERS signaling triggers a set of pro-apoptosis programs while the cells fail to successfully adapt to restore homeostasis of ER.23 CHOP and caspase 12 function as the main mediators mediating ERS-induced apoptosis, and many factors ameliorate apoptosis in cardiomyocytes through inhibiting the upregulation of CHOP24–26 or the activation of caspase 12.27–29 Here, our results also demonstrate that Tm induces apoptosis in cardiomyocytes resulted from triggering ERS, including upregulation of CHOP and activated caspase-12. It is suggested that Tm can induce cardiomyocytic apoptosis via activating ERS.
TSA is a plentiful lipophilic abietane diterpene element which was separated from Danshen (Salvia miltiorrhiza), a Traditional Chinese Medicine usually utilized for therapy of cardiovascular diseases.30–32 Its protective mechanisms have been broadly investigated. Several reports reveal the inhibited effect of TSA on ERS. TSA attenuates radiation-induced upregulation of ERS-related molecules, GRP78, and CHOP in cardiac fibroblasts.22 In Tm-treated L6 myotubes, TSA suppresses the activation of ERS.21 It is also demonstrated that ameliorative effect of TSA on kidneys of rat during hypothermic preservation is mediated by inhibiting ERS.20 In this experiment, TSA improved Tm-induced apoptosis and activation of ERS in cardiomyocytes. By our knowledge, these results first suggest that TSA protects cardiomyocytes against ERS mediated apoptosis.
Interestingly, the converse effect of TSA on ERS has been reported in carcinoma cells. TSA prevents proliferation of carcinoma cell and stimulates its apoptosis through exaggerated ERS in gastric carcinoma AGS cells,33 advanced cervix carcinoma CaSki cells,34 human prostate cancer cells,35 and so on. All the results indicate the biphasic effect of TSA on ERS, which may be depended on different kinds of cells. It is necessary to broadly investigate the effect of TSA on ERS in different cells, and thoroughly study its mechanism.
MicroRNAs are small non-coding RNAs that take part in various physiological and pathophysiological processes, and provide delicate comprehensive regulation of cellular pathways, usually by controlling elements of signaling pathways. miR-133 is one of the canonical myomiRs that are primary to the health and development of mammalian cardiac and skeletal muscles.36 Downregulation of miR-133 involves in cardiomyocyte apoptosis,37 whereas upregulation of miR-133 protects cardiomyocytes against apoptosis.38 Our results also confirm the association of miR-133 and cardiomyocytic apoptosis. Expression of miR-133 significantly reduces in Tm-treated cardiomyocytic apoptosis. It has been reported that TSA can reverse the downregulation of miR-133, and ameliorate apoptosis of cardiomyocytes.39 Furthermore, the cardioprotective effect of TSA on apoptosis is blocked by anti-miR-133 that effectively reduced expression of miR-133.39 Therefore, impacts of miR-133 on the cardioprotection of TSA against ERS-induced apoptosis are investigated presently. Our results indicate the possible ameliorated effect of miR-133 on ERS. However, there is less knowledge about the association of miR-133 and ERS, which need further investigate in future.
In conclusion, we originally demonstrate that TSA ameliorates ERS and apoptosis in cardiomyocytes, which may be resulted from upregulation of miRNA133. Our results might provide new strategy for prevention and therapy of myocardial injury and ERS.
Acknowledgments
This experiment was funded by the National Natural Science Foundation of China (No. 81202825).
Author contributions
All authors participated in the design, interpretation of the studies and analysis of the data and review of the manuscript; JF and SL conducted the experiments, JF and HC wrote the manuscript.
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- 1.Gupta A, Read DE, Gupta S. Assays for induction of the unfolded protein response and selective activation of the three major pathways. Methods Mol Biol 2015; 1292: 19–38. [DOI] [PubMed] [Google Scholar]
- 2.Hiramatsu N, Chiang WC1, Kurt TD1, Sigurdson CJ, Lin JH. Multiple mechanisms of unfolded protein response-induced cell death. Am J Pathol 2015; 185: 1800–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li Y, Guo Y, Tang J, Jiang J, Chen Z. New insights into the roles of CHOP-induced apoptosis in ER stress. Acta Biochim Biophys Sin 2015; 47: 146–7. [DOI] [PubMed] [Google Scholar]
- 4.Cominacini L, Mozzini C, Garbin U, Pasini A, Stranieri C, Solani E, Vallerio P, Tinelli IA, Fratta Pasini A. Endoplasmic reticulum stress and Nrf2 signaling in cardiovascular diseases. Free Radic Biol Med 2015; 88: 233–42. [DOI] [PubMed] [Google Scholar]
- 5.Zhang GG, Teng X, Liu Y, Cai Y, Zhou YB, Duan XH, Song JQ, Shi Y, Tang CS, Yin XH, Qi YF. Inhibition of endoplasm reticulum stress by ghrelin protects against ischemia/reperfusion injury in rat heart. Peptides 2009; 30: 1109–16. [DOI] [PubMed] [Google Scholar]
- 6.Teng X, Song J, Zhang G, Cai Y, Yuan F, Du J, Tang C, Qi Y. Inhibition of endoplasmic reticulum stress by intermedin(1-53) protects against myocardial injury through a PI3 kinase-Akt signaling pathway. J Mol Med 2011; 89: 1195–205. [DOI] [PubMed] [Google Scholar]
- 7.Xu S, Liu P. Tanshinone II-A: new perspectives for old remedies. Expert Opin Ther Pat 2013; 23: 149–53. [DOI] [PubMed] [Google Scholar]
- 8.Adams JD, Wang R, Yang J. Preclinical and clinical examinations of Salvia miltiorrhiza and its tanshinones in ischemic conditions. Chin Med 2006; 1: 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen M, Liu Y, Yi D. Tanshinone IIA promotes pulmonary artery smooth muscle cell apoptosis in vitro by inhibiting the JAK2/STAT3 signaling pathway. Cell Physiol Biochem 2014; 33: 1130–8. [DOI] [PubMed] [Google Scholar]
- 10.Pang H, Han B, Yu T. The complex regulation of tanshinone IIA in rats with hypertension-induced left ventricular hypertrophy. PLoS One 2014; 9: e92216–e92216. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 11.Wu WY, Yan H, Wang XB. Sodium Tanshinone IIA silate inhibits high glucose-induced vascular smooth muscle cell proliferation and migration through activation of AMP-activated protein kinase. PLoS One 2014; 9: e94957–e94957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xu S, Liu Z, Huang Y. Effectiveness of combination therapy of atorvastatin and non lipid-modifying tanshinone IIA from Danshen in a mouse model of atherosclerosis. Int J Cardiol 2014; 174: 878–80. [DOI] [PubMed] [Google Scholar]
- 13.Zhang MQ, Zheng YL, Chen H. Sodium tanshinone IIA sulfonate protects rat myocardium against ischemia–reperfusion injury via activation of PI3K/Akt/FOXO3A/Bim pathway. Acta Pharmacol Sin 2013; 34: 1386–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hu H, Zhai C, Qian G, Gu A, Liu J, Ying F, Xu W, Jin D, Wang H, Hu H, Zhang Y, Tang G. Protective effects of tanshinone IIA on myocardial ischemia reperfusion injury by reducing oxidative stress, HMGB1 expression, and inflammatory reaction. Pharm Biol 2015; 53: 1752–8. [DOI] [PubMed] [Google Scholar]
- 15.Wei B, Li WW, Ji J, Hu QH, Ji H. The cardioprotective effect of sodium tanshinone IIA sulfonate and the optimizing of therapeutic time window in myocardial ischemia/reperfusion injury in rats. Atherosclerosis 2014; 235: 318–27. [DOI] [PubMed] [Google Scholar]
- 16.Zhang Y, Wei L, Sun D, Cao F, Gao H, Zhao L, Du J, Li Y, Wang H. Tanshinone IIA pretreatment protects myocardium against ischaemia/reperfusion injury through the phosphatidylinositol 3-kinase/Akt-dependent pathway in diabetic rats. Diabetes Obes Metab 2010; 12: 316–22. [DOI] [PubMed] [Google Scholar]
- 17.Jin HJ, Xie XL, Ye JM, Li CG. Tanshinone IIA and cryptotanshinone protect against hypoxia-induced mitochondrial apoptosis in H9c2 cells. PLoS One 2013; 8: e51720–e51720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wu WY, Wang WY, Ma YL, Yan H, Wang XB, Qin YL, Su M, Chen T, Wang YP. Sodium tanshinone IIA silate inhibits oxygen-glucose deprivation/recovery-induced cardiomyocyte apoptosis via suppression of the NF-κB/TNF-α pathway. Br J Pharmacol 2013; 169: 1058–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fu J, Huang H, Liu J, Pi R, Chen J, Liu P. Tanshinone IIA protects cardiac myocytes against oxidative stress-triggered damage and apoptosis. Eur J Pharmacol 2007; 568: 213–21. [DOI] [PubMed] [Google Scholar]
- 20.Zhang X, He D, Xu L, Ling S. Protective effect of tanshinone IIA on rat kidneys during hypothermic preservation. Mol Med Rep 2012; 5: 405–9. [DOI] [PubMed] [Google Scholar]
- 21.Hwang SL, Yang JH, Jeong YT, Kim YD, Li X, Lu Y, Chang YC, Son KH, Chang HW. Tanshinone IIA improves endoplasmic reticulum stress-induced insulin resistance through AMP-activated protein kinase. Biochem Biophys Res Commun 2013; 430: 1246–52. [DOI] [PubMed] [Google Scholar]
- 22.Gu J, Li HL, Wu HY, Gu M, Li YD, Wang XG, Ming HX, Dong XL, Liu K. Sodium tanshinone IIA sulfonate attenuates radiation-induced fibrosis damage in cardiac fibroblasts. J Asian Nat Prod Res 2014; 16: 941–52. [DOI] [PubMed] [Google Scholar]
- 23.Hiramatsu N, Chiang WC, Kurt TD, Sigurdson CJ, Lin JH. Multiple mechanisms of unfolded protein response-induced cell death. Am J Pathol 2015; 185: 1800–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shen M, Wang L, Yang G, Gao L, Wang B, Guo X, Zeng C, Xu Y, Shen L, Cheng K, Xia Y, Li X, Wang H, Fan L, Wang X. Baicalin protects the cardiomyocytes from ER stress-induced apoptosis: inhibition of CHOP through induction of endothelial nitric oxide synthase. PLoS One 2014; 9: e88389–e88389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nam DH, Han JH, Lee TJ, Shishido T, Lim JH, Kim GY, Woo CH. CHOP deficiency prevents methylglyoxal-induced myocyte apoptosis and cardiac dysfunction. J Mol Cell Cardiol 2015; 85: 168–77. [DOI] [PubMed] [Google Scholar]
- 26.Ji Y, Zhao Z, Cai T, Yang P, Cheng M. Liraglutide alleviates diabetic cardiomyopathy by blocking CHOP-triggered apoptosis via the inhibition of the IRE-α pathway. Mol Med Rep 2014; 9: 1254–8. [DOI] [PubMed] [Google Scholar]
- 27.Chen Y, Tang Y, Xiang Y, Xie YQ, Huang XH, Zhang YC. Shengmai injection improved doxorubicin-induced cardiomyopathy by alleviating myocardial endoplasmic reticulum stress and caspase-12 dependent apoptosis. Biomed Res Int 2015; 2015: 952671–952671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bajaj G, Sharma RK. TNF-alpha-mediated cardiomyocyte apoptosis involves caspase-12 and calpain. Biochem Biophys Res Commun 2006; 345: 1558–64. [DOI] [PubMed] [Google Scholar]
- 29.Jang YM, Kendaiah S, Drew B, Phillips T, Selman C, Julian D, Leeuwenburgh C. Doxorubicin treatment in vivo activates caspase-12 mediated cardiac apoptosis in both male and female rats. FEBS Lett 2004; 577: 483–90. [DOI] [PubMed] [Google Scholar]
- 30.Zhou L, Zuo Z, Chow MS. Danshen: an overview of its chemistry, pharmacology, pharmacokinetics, and clinical use. J Clin Pharm 2005; 45: 1345–59. [DOI] [PubMed] [Google Scholar]
- 31.Shang QH, Xu H, Huang L. Tanshinone IIA: a promising natural cardioprotective agent. Evid Based Complement Alternat Med 2012; 7: 1120–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang MQ, Tu JF, Chen H, Shen Y, Pang LX, Yang XH, Sun RH, Zheng YL. Janus kinase/signal transducer and activator of transcription inhibitors enhance the protective effect mediated by tanshinone IIA from hypoxic/ischemic injury in cardiac myocytes. Mol Med Rep 2015; 11: 3115–21. [DOI] [PubMed] [Google Scholar]
- 33.Su CC. Tanshinone IIA inhibits human gastric carcinoma AGS cell growth by decreasing BiP, TCTP, Mcl-1 and Bcl-xL and increasing Bax and CHOP protein expression. Int J Mol Med 2014; 34: 1661–8. [DOI] [PubMed] [Google Scholar]
- 34.Pan TL, Wang PW, Hung YC, Huang CH, Rau KM. Proteomic analysis reveals tanshinone IIA enhances apoptosis of advanced cervix carcinoma CaSki cells through mitochondria intrinsic and endoplasmic reticulum stress pathways. Proteomics 2013; 13: 3411–23. [DOI] [PubMed] [Google Scholar]
- 35.Chiu SC, Huang SY, Chen SP, Su CC, Chiu TL, Pang CY. Tanshinone IIA inhibits human prostate cancer cells growth by induction of endoplasmic reticulum stress in vitro and in vivo. Prostate Cancer Prostatic Dis 2013; 16: 315–22. [DOI] [PubMed] [Google Scholar]
- 36.Mitchelson KR, Qin WY. Roles of the canonical myomiRs miR-1, -133 and -206 in cell development and disease. World J Biol Chem 2015; 6: 162–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang L, Li X, Zhou Y, Shi H, Xu C, He H, Wang S, Xiong X, Zhang Y, Du Z, Zhang R, Lu Y, Yang B, Shan H. Downregulation of miR-133 via MAPK/ERK signaling pathway involved in nicotine-induced cardiomyocyte apoptosis. Naunyn Schmiedebergs Arch Pharmacol 2014; 387: 197–206. [DOI] [PubMed] [Google Scholar]
- 38.Xu C, Hu Y, Hou L, Ju J, Li X, Du N, Guan X, Liu Z, Zhang T, Qin W, Shen N, Bilal MU, Lu Y, Zhang Y, Shan H. β-Blocker carvedilol protects cardiomyocytes against oxidative stress-induced apoptosis by up-regulating miR-133 expression. J Mol Cell Cardiol 2014; 75: 111–21. [DOI] [PubMed] [Google Scholar]
- 39.Zhang L, Wu Y, Li Y, Xu C, Li X, Zhu D, Zhang Y, Xing S, Wang H, Zhang Z, Shan H. Tanshinone IIA improves miR-133 expression through MAPK ERK1/2 pathway in hypoxic cardiac myocytes. Cell Physiol Biochem 2012; 30: 843–52. [DOI] [PubMed] [Google Scholar]