Abstract
Recent studies indicated that cell-based therapy could be a promising approach to treat intervertebral disc degeneration. Though the harsh microenvironment in disc is still challenging to implanted cells, it could be overcome by pre-conditioning graft cells before transplantation, suggested by previous literatures. Therefore, we designed this study to identify the potential effect of chondrogenic pre-differentiation on adipose-derived mesenchymal stem cells in intervertebral disc-like microenvironment, characterized by limited nutrition, acidic, and high osmosis in vitro. Adipose-derived mesenchymal stem cells of rat were divided into five groups, embedded in type II collagen scaffold, and cultured in chondrogenic differentiation medium for 0, 3, 7, 10, and 14 days. Then, the adipose-derived mesenchymal stem cells were implanted and cultured in intervertebral disc-like condition. The proliferation and differentiation of adipose-derived mesenchymal stem cells were evaluated by cell counting kit-8 test, real-time quantitative polymerase chain reaction, and Western blotting and immunofluorescence analysis. Analyzed by the first week in intervertebral disc-like condition, the results showed relatively greater proliferative capability and extracellular matrix synthesis ability of the adipose-derived mesenchymal stem cells pre-differentiated for 7 and 10 days than the control. We concluded that pre-differentiation of rat adipose-derived mesenchymal stem cells in chondrogenic culture medium for 7 to 10 days could promote the regeneration effect of adipose-derived mesenchymal stem cells in intervertebral disc-like condition, and the pre-differentiated cells could be a promising cell source for disc regeneration medicine.
Keywords: Intervertebral disc, adipose-derived mesenchymal stem cells, differentiation, microenvironment, type II collagen, chondrocyte-like cells
Introduction
Low back pain (LBP) is a major health concern worldwide. Between 70% and 85% of people would suffer from LBP at some time of their life, which may induce varying degrees of disability. The etiology of LBP is complex and multifactorial, and the intervertebral disc (IVD) degeneration is one of its major causes.1 Current therapy strategies mainly include physical therapy, pain medication, and invasive surgical methods, which would alleviate symptom in short term. Since these treatments do not address the underlying causes, high rate of recurrent pain in long-term outcome occurs.2,3
The IVD is a fibrocartilaginous articulation. It provides flexibility to daily activities and absorbs load from body weight and muscular activities. The mechanical functions of the disc are achieved by the extracellular matrix (ECM), mainly aggrecan and type II collagen, which maintains tissue hydration and provides tensile strength to the disc respectively.1 The process of degeneration is involved with the loss of nucleus pulposus (NP) cells and the breakdown of the balance between the synthesis and degradation of ECM. The degenerated disc is characterized as reduction in cell viability, loss of NP cells, loss of proteoglycan, development of NP dehydration, and accumulation of waste products.4,5
To repair the disc degeneration, researchers have been utilizing cell-based therapies, which turn out to be a possible approach from animal trials and clinical reports. Researchers found increased magnetic resonance imaging (MRI) T2 signal and preservation of disc height in discs received cell therapy. And in histological assessment, there was increased matrix restoration in the discs received cell treatment, including proteoglycan and collagen, when compared to controls.5–8
Many attempts have been made to achieve disc regeneration by cell-based therapy, especially on candidate cell sources. NP cells of adult humans could produce abundant ECM, rich in aggrecan and type II collagen. However, the population itself is sparse and any procedure to obtain cells from the disc necessitates the damage to the structure of annulus fibrosus, which would lead to degeneration in healthy discs. Due to the relative ease of access, comparative abundance, and the multi-lineage differential potential, mesenchymal stem cells (MSCs) present an attractive cell source for disc cell therapies. MSCs can be isolated from numerous tissues derived from mesodermal tissues and organs including bone marrow (BMSC) and adipose tissue (ADSC).2,6,8 An in vivo study of a rat model reported restoration of disc height and MRI signal intensity after treated with ADSC, indicating the regeneration effect of ADSC.8 Evaluated at two years after surgery, two patients with LBP who received autologous BMSC transplantation reported alleviated symptom and favorable radiological results.9 However, MSCs were observed with poor ability to survive and function in vivo of a porcine model, indicating inferior property of undifferentiated MSCs for tissue engineering in IVD repair.10 And the potential of osteophyte formation caused by leakage into spinal canal or inappropriate differentiation was a realistic concern in tissue engineering application.11
To regenerate degenerated IVDs, it is essential to guarantee the survival of implanted cells and their ability to function normally amidst the disc microenvironment. The condition in degenerated disc is characterized by low oxygen tension, limited nutrition, acidic pH, and high osmolarity, which may negatively influence the function or even survival of any implanted cells.4,6,12–14
To overcome harsh condition in disc, previous literatures proposed pre-conditioning cells before implantation6 and appropriately differentiated cells could survive and synthesize matrix in situ.10 With this in mind, we designed this study to investigate the potential effect of chondrogenic pre-differentiation on the regeneration of adipose-derived mesenchymal stem cells (ADSCs) in a harsh IVD-like condition. And to our knowledge, it is the first in vitro study to investigate the response of pre-conditioned cells to the IVD-like condition with limited nutrition, acidity, and high osmosis. In this study, ADSCs embedded in type II collagen microsphere were cultured in chondrogenic differentiation medium for 0, 3, 7, 10, and 14 days and then maintained in IVD-like condition of low nutrition, acidity, and hyperosmosis. The proliferation and ECM synthesis of all groups including the control were evaluated after one week culture in IVD-like condition.
Material and methods
Cell culture
Rat adipose-derived stem cells (ADSC) were purchased from Cyagen Biosciences (Guangzhou, China). And the basic culture medium (Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% fetal bovine serum (FBS), 1% penicillin-streptomycin solution, and 1% glutamine) bought from Cyagen Biosciences was used for MSC cultivation. Type II collagen (Sigma, St Louis, MO, USA) was dissolved in 0.05 M acetic acid at a final concentration of 6 mg/ml and stored at 4℃.
The ADSCs were planted into 25-cm2 cell culture flasks supplemented with basic culture medium and maintained in a humidified incubator at 37℃ and 5% CO2. ADSCs were expanded to passage 4, then detached with 0.25% Trypsin ethylenediaminetetraacetic acid, and resuspended with basic culture medium. The type II collagen scaffold was prepared according to the introduction of type II collagen. With the addition of 1 M sodium hydroxide aqueous solution, type II collagen solution of 6 mg/ml, phosphate-buffered saline 10 × (PBS), and ADSCs suspension at cell density of 2.5 × 106/ml, the mixture solution was adjusted to isotonic and pH 7.4, at the final concentration of type II collagen solution of 3 mg/ml. Each 100 μl of the mixture solution aforesaid was removed into 15-ml polypropylene conical centrifuge tubes. The centrifuge tube was incubated at 37℃ with 5% CO2, and the cap of the tube was loosened to allow air exchanging. After 2 h incubation, the collagen solution was gelated into micro-masses at the button of tubes. The micro-masses were maintained in 1 ml of basic culture medium overnight and then in chondrogenic differentiation medium (high glucose DMEM (Gibco, Carlsbad, CA, USA) supplemented with 5 µg/ml insulin, 1.25 mg/ml bovine serum albumin, 50 ng/ml ascorbic acid, and 10 µg/ml transforming growth factor-β3). We divided the experiment into five groups (D0, D3, D7, D10, and D14), pre-cultured in chondrogenic differentiation culture medium for 0, 3, 7, 10, and 14 days, respectively, and then were maintained in IVD-like culture medium, which was low glucose DMEM (Gibco) supplemented with 5 µg/ml insulin, 1.25 mg/ml bovine serum albumin, and 10 µg/ml transforming growth factor-β3, with an addition of mixed solution of 5 M sodium chloride and 0.4 M potassium chloride to increase the osmolality of the medium to 485 mOsm, and 1 M hydrochloric acid to adjust the pH of the medium to 6.8. The cells of all groups were cultured for one week in IVD-like condition above with medium changed twice a week.
Cell proliferation assay
Cell proliferation was assessed by cell counting kit-8 (CCK-8, Dojindo Molecular Technologies, Kumamoto, Japan) after pre-differentiation (Day 0), and at the third day (Day 3) and seventh day (Day 7) after transplantation as well, at three time points in each group. The supernatant was discarded, 500 μl of 5% CCK-8 solution in high glucose DMEM was added into each tube, and then ADSCs were incubated for 2 h at 37℃ with 5% CO2. The absorbance of each group was quantified at 450 nm by removing supernatant into 96-well plate 100 μl per well. All data were calculated from triplicate samples.
Real-time quantitative polymerase chain reaction
RNA was extracted from micro-masses of five groups using TRIzol reagent (TAKARA, Dalian, China) after complete grinding after one week differentiation in IVD-like condition. Total RNA was reversely transcripted to cDNA utilizing a Double-Strand cDNA Synthesis Kit (TAKARA) according to the manufacturer’s instructions. SYBR Green PCR assays (TAKARA) were used to perform real-time PCR in StepOnePlus (Applied Biosystem, USA), and three independent samples were set to ensure validity. 18S rRNA was used as internal control, and four target genes were detected (Table 1). Primers were synthesized by Sangon Biotech (Shanghai, China), and quantitative real-time PCR data were calculated by the 2−ΔΔCt method.
Table 1.
Primers used in quantitative RT-PCR
| Gene | Forward primer (5′ to 3′) | Reverse primer (5′ to 3′) |
|---|---|---|
| SOX-9 | AGGAAGCTGGCAGACCAGTACC | GGGTCTCTTCTCGCTCTCGTTCA |
| Collagen-II | CTGGTGGAGCAGCAAGAGC | GTGGACAGTAGACGGAGGAAAG |
| Aggrecan | CTAGCTGCTTAGCAGGGATAACG | GATGACCCGCAGAGTCACAAAG |
| Rn18S | GAATTCCCAGTAAGTGCGGGTCATA | CGAGGGCCTCACTAAACCATC |
F: forward; R: reverse.
Immunocytochemical staining evaluation
ADSCs were divided into five groups, cultured in chondrogenic differentiation medium for 0, 3, 7, 10, and 14 days, respectively, in a 24-well cell culture plate, and then were maintained in IVD-like culture medium. Cells of all groups were fixed with 4% paraformaldehyde for 10 minutes at room temperature after differentiation. The cells were washed three times with PBS and then blocked with TBS containing 5% bovine serum albumin and 0.1% Triton X-100 for 10 minutes at room temperature. After incubating with primary antibody at 4℃ overnight, the samples were washed three times with PBS and incubated with fluorescent-conjugated secondary antibody (1:200; Abcam, UK) for 2 h. Then, the samples were washed three times with PBS and incubated with 4′,6-diamidino-2-phenylindole (DAPI) staining for 10 minutes. Polyclonal antibodies against anti-aggrecan (1:200; Abcam, UK) and collagenase type II (1:200; Abcam, UK) were used as primary antibodies. Stained cells were observed under a fluorescence microscope (Leica, Germany).
Western blot analysis
Cells were washed three times with ice-cold PBS, and total proteins were extracted with RIPA buffer containing 1% PMSF after completely grinded. Protein concentrations were measured using a BCA Protein Quantification Kit (Takara). Proteins were electrophoresed by 10% sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) and then transferred onto poly-vinylidene fluoride (PVDF) membranes (Millipore, Massachusetts, MA, USA). After blocked with 5% skim milk in Tris-buffered saline with 0.1% Tween-20 (TBST) at room temperature for 1 h, membranes were maintained overnight at 4℃ in TBST with the appropriate primary antibody: anti-Sox-9 antibody (1:1000; Santa Cruz) and anti-GAPDH antibody (1:1000; Santa Cruz). Membranes were then incubated with horseradish peroxidase (HRP)-labeled secondary Immunoglobulin G (1:1000, Santa Cruz) for 1 h at room temperature. After the membrane was washed three times with TBST, the immunoreactivity was detected with enhanced chemiluminescence (ECL, Millipore) substrate, and densitometry was performed using Quantity One Software (Bio-Rad Laboratories Inc., Munich, Germany). GAPDH served as a loading control.
Statistical analysis
ADSCs without pre-conditioning served as a control group. A one-way analysis of variance following post-hoc with Bonferroni comparisons was used to analyze. Statistical analyses were performed with SPSS 17.0 for Windows. Results were expressed as mean ± standard deviation (SD). Statistical significance was set at P < 0.05.
Results
The proliferation of ADSCs
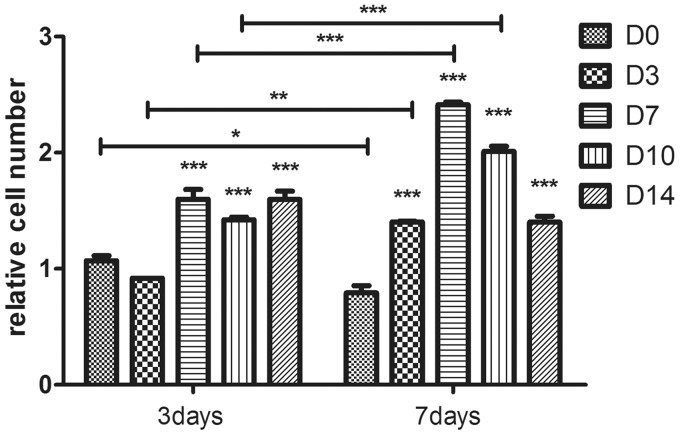
ADSCs were maintained in IVD-like condition for one week culture after chondrogenic differentiation. The relative cell numbers of five groups were close before but varied after the pre-differentiation (Day 0), measured by CCK-8. The cell number in the control was close to D10 group, more than D3 group (P < 0.01) and D7 group (P < 0.001), less than D14 group (P < 0.01) at Day 0. The range among these groups was less than twofolds (D14 group was 1.85 folds to D7, P < 0.001).
The results in IVD-like condition cultivation were presented using the proliferation ratios instead of the CCK-8 values (Figure 1), which were the ratios of the CCK-8 values at Day 3 and Day 7 to Day 0 of each group, respectively. And it showed a slight decrease in the cell number of the control group (D0) at Day 7 (P < 0.05). Compared to the control group, D7, D10, and D14 group showed significant increase until Day 7 (P < 0.001), but D3 group at Day 7 (P < 0.001). The cell number of D7 group and D10 group increased gradually till Day 7 (P < 0.001) but other groups did not. In addition, the relative cell number of D7, D10, and D14 group showed no significant difference on Day 3, but D3 group was less (P < 0.05), while on Day 7, the D7 group showed a significant increase compared to D10 group (P < 0.05) and D14 group (P < 0.001). Therefore, the D7 group showed the best proliferation capability among these groups (2.412 ± 0.0315-fold to D0 group, P < 0.001) during the culture period.
Figure 1.
The relative cell number of each group after transplantation was presented with the proliferation ratios on Days 3 and 7 compared to Day 0. Data were presented as mean ± SD (n = 3). Statistically significant differences were indicated with asterisks, *P < 0.05, **P < 0.01, and ***P < 0.001
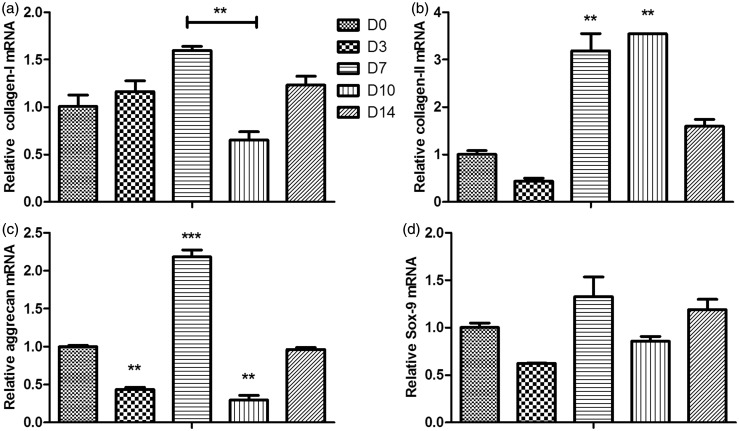
The mRNA expression of ECM
The mRNA expression of type II collagen in D7 group (3.189 ± 0.511-fold, P < 0.001) and D10 group (3.546 ±0.0103-fold, P < 0.001) was significantly higher than the control (Figure 2(b)), and the D3 and D14 group showed no significant difference with the control. D7 group showed higher mRNA expression of aggrecan than the control (2.185 ± 0.128-fold, P < 0.001; Figure 2(c)), but the mRNA expression in D3 and D10 groups was lower than the control (P < 0.05). Compared to the control, the mRNA expression of collagen I in other four groups showed no significant difference, except a bit higher expression in D7 group compared to D10 group (P < 0.01; Figure 2(a)). No significant difference on the mRNA expression of SOX-9 between five groups (Figure 2(d)).
Figure 2.
The mRNA expression of type I collagen (a), type II collagen (b), aggrecan (c), and Sox9 (d). Statistically significant differences were indicated with asterisks, **P < 0.01 and ***P < 0.001
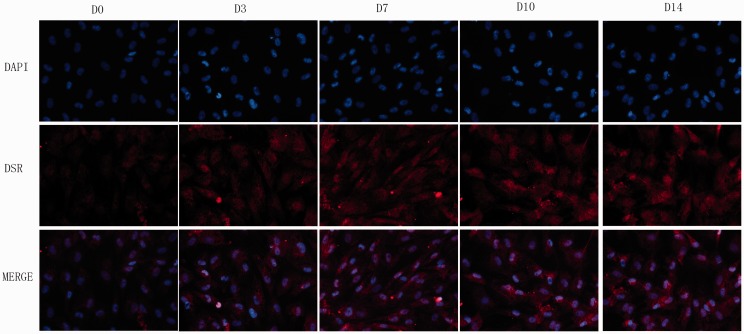
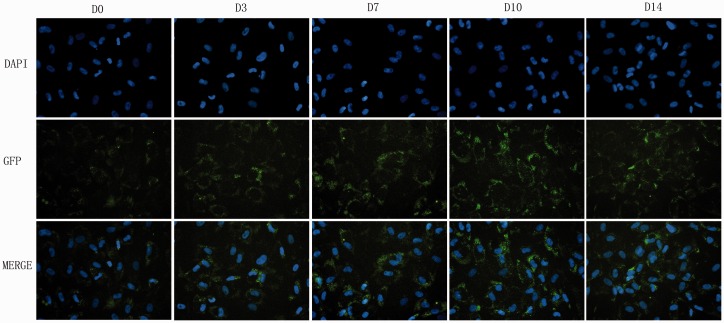
Immunofluorescence of ECM
The ECM protein expression in monolayer culture was detected by immunofluorescence staining (40×). Positive staining of type II collagen and aggrecan in each group was observed under a fluorescence microscope (Figures 3 and 4). Weak staining for aggrecan was observed in D0 group. The D3 group showed stronger positive staining for aggrecan than the control. The D7, D10, and D14 group showed remarkably positive staining for aggrecan compared to the control (Figure 3).
Figure 3.
The matrix staining for aggrecan: Photomicrographs (40×) of DAPI staining for cell nucleus and immunofluorescence for aggrecan of ADSCs pre-differentiated for 0, 3, 7, 10, and 14 days. (A color version of this figure is available in the online journal.)
Figure 4.
The matrix staining of type II collagen: Photomicrographs (40×) of DAPI staining for cell nucleus and immunofluorescence for type II collagen of ADSCs pre-differentiated for 0, 3, 7, 10, and 14 days. (A color version of this figure is available in the online journal.)
As for the Type II collagen staining (Figure 4), weak green-stained type II collagen was observed in the control group. Pre-differentiation slightly increased the protein expression in type II collagen in ADSCs of four experimental groups. Notably, D10 group showed relatively stronger positive than the control. Identical settings were used for the images to ensure that the subtle differences noted were not because of different exposure times.
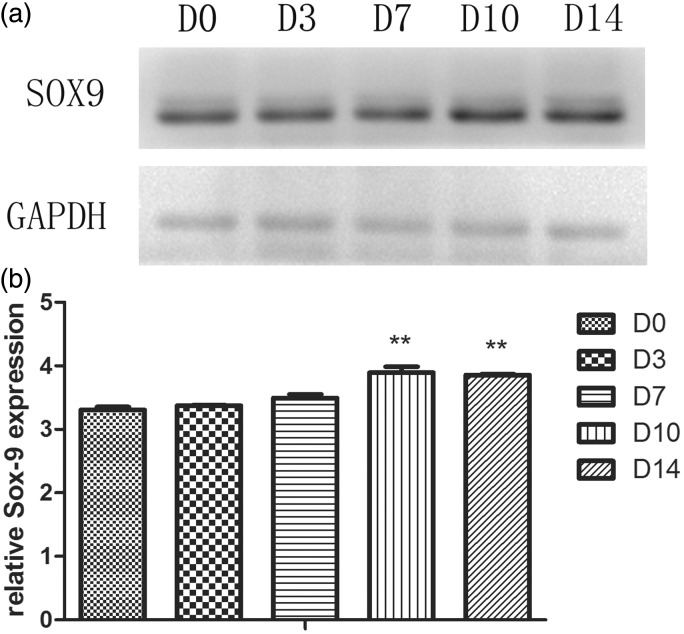
Western blotting analysis of Sox-9 expression
Western blotting was performed to analyze the expression of Sox-9, a transcription factor for chondrogenesis (Figure 5). There was no significant difference on the protein expression of Sox-9 in D3 and D7 group compared to the control. A slight increase in Sox-9 expression in D10 and D14 group compared with the control (P < 0.01).
Figure 5.
Sox-9 expression of ADSCs of five groups by western blotting (a). GAPDH served as the loading control. Statistically significant differences were indicated with asterisks (b), **P < 0.01
Discussion
Recent studies have been emphasized on the cell-based therapy to regenerate disc by replenishing cells in site and correcting the deficiencies of ECM and disc structure.2,5 In vitro and in vivo studies have showed that stem cells have the capacity to regenerate degenerative discs by differentiating toward chondrocyte-like cells, capable of producing proteoglycans and type II collagen.8,15,16 However, the microenvironment condition in degenerated disc is characterized of hypoxia, low nutrition, acidic pH, high mechanical loading, high osmolarity, and a complicated protease and cytokine network.1,4,6,12 It is quite different from normal disc and may have negative impact to graft cells. It is reported that 60% of bovine BM-MSCs survived after initial injection in to a cryopreserved IVD tissue in vitro, but only 20% of cells remained alive after seven days.12,17 Besides, the state of disc cells was found affected by the degeneration process that NP cells from degenerative disc displayed decreased synthesis of matrix components and increased expression of matrix catabolic and degrading enzymes.18 Consistently, researchers have reported inhibited cell viability and proliferation and decreased expression of aggrecan and collagen-I in IVD-like pH and high osmosis condition. Despite the IVD-like glucose condition was found a positive factor for ADSCs-based IVD regeneration, the cell viability and proliferation and the matrix biosynthesis capability were inhibited in combined IVD condition.4,19,20 From this respect, the IVD condition is still an obstacle for the application of cell-based therapy to treat degenerative diseases.
Type II collagen has been found to improve the cell attachment and distribution in the constructs via integrins and affect the chondrogenic differentiation by interaction with TGF-β1 as well. Appropriate content of type II collagen together with aggrecan might facilitate the chondrogenic differentiation, while the ECM scaffold rich in type II collagen and aggrecan inhibited the chondrogenesis of MSCs. Our previous study found great biocompatibility of type II collagen scaffold by inducing ADSC proliferation and differentiation toward a NP-like phenotype, consistent with previous literatures. Therefore, we established a type II collagen constructs for ADSCs culture.21–23
In this study, we found a reduction in cell density after one week culture in IVD-like condition in control group, which consistent with previous studies of the effect of chemical environment to ADSCs.4,13,19 In contrast, a significant increase of cell density after implantation was observed in pre-differentiated ADSCs, especially in D7 and D10 groups, which supported the opinion that pre-differentiated cells might function better than undifferentiated cells. This result suggested that chondrogenic pre-differentiation could enhance the viability of ADSCs in IVD-like condition, and the optimum time of pre-differentiation was seven days.
Sox-9 is a chondrocyte marker and a key transcription factor for chondrogenesis that positively controls type II collagen synthesis.24 The result of RT-PCR showed no significant trend of Sox-9 expression among five groups, indicating the similar chondrocytic phenotype of ADSCs after differentiation. The result of Western blotting was partially inconsistent with RT-PCR with a slight increase in Sox-9 expression in D10 and D14 group compared with the control. So far, there were rare researches that had investigated the effect of IVD-like condition to the expression of Sox-9. Previous studies found increased expression of Sox-9 of MSCs when cultured in hypoxic condition.14,25,26 Therefore, we speculate hypoxia may be one of the major factors in IVD condition affecting the expression of Sox-9. And it may promote the expression of Sox-9 of ADSCs when chondrogenic pre-differentiated for 10 to 14 days.
Aggrecan is the major type of proteoglycan, which is responsible for the maintaining of tissue hydration.1 In contrast to the downregulation of aggrecan in previous study,19 we found a significant increase in mRNA expression of aggrecan by ADSCs in IVD-like condition in D7 group when compared with the control. The immunofluorescence showed relatively stronger positive in aggrecan staining in pre-differentiated ADSCs, remarkably in D7, D10, and D14 group when compared to the control. The results of this study suggested that it could enhance the aggrecan expression of ADSCs in IVD-like condition when chondrogenic pre-differentiated before implantation for seven days, or 10 to 14 days. Since the most significant biochemical change to occur in degenerated disc is the loss of proteoglycan, and it results in a fall of osmotic pressure and the dehydration of NP tissue. Therefore, it is important for disc regeneration with increased synthesis of aggrecan after implantation by graft cells.
Type II collagen is an important member of collagen fibrils in discs. Increased amount of type II collagen was denatured and ruptured with degeneration, which resulted in an alteration in the type and distribution of collagen.1 The gene analysis showed significant up-regulation in the mRNA expression of type II collagen in D7 and D10 group. The immunofluorescence staining for collagen was weak in five groups and showed no significant promotion in type II collagen expression. Importantly, to eliminate the interference signal from the type II collagen scaffold, the immunofluorescence analysis was performed on monolayer cultured ADSCs in this study. And previous studies have confirmed that type II collagen hydrogel could direct on the cartilage/NP lineage differentiation of ADSCs efficiently.27,28 Besides, it is reported that the expression of chondrogenic markers increased when grown in three-dimensional (3D) structure compared to monolayer.29 Type I collagen is another component of collagen fibril in discs. The result of RT-PCR showed similar expression between the control and pre-differentiated ADSCs, partially consistent with previous studies.16 The similar expression to the control reflected positively on disc health, because the increased expression of type I collagen had been linked to a decrease in aggrecan and a resulting assessment of disc degeneration.30 The results indicated that chondrogenic pre-differentiation for 7 to 10 days could up-regulate type II collagen expression, meanwhile did not regulate type I collagen expression of ADSCs. Though the inner mechanism was unclear, we speculated that the up-regulation of disialoganglioside 2 (GD2) downstream the differentiation of stem cells could primarily lead to the promotion of matrix expression in this study. GD2 was the marker relevant to the capability to form spherical cell colonies, which were strongly positive for type II collagen and aggrecan.31
There are several limitations for this study. The microenvironment condition in disc is highly complicated. The IVD-like low nutrition, acidity, and hyperosmosis were achieved in this study, but the mechanical loading, hypoxia, and protease and cytokine network could have played essential roles in the proliferation and differentiation of ADSCs. Immunofluorescence staining was performed on monolayer cultured cells, which could result in relatively lower expression of type II collagen and aggrecan. The 3D nucleus-like type II collagen hydrogel microspheres were relatively simplified scaffold for graft cells with poor mechanical properties and stability, which could be promoted in further study. Though a significant promotion was observed in the viability and matrix synthesis ability of pre-differentiated ADSCs in IVD-like condition in this study, long-term outcome as well as the inner mechanism of pre-differentiation remained our further investigation.
So far, there has been a dramatic improvement in the understanding of the cell-based therapy to treat degenerated disc diseases, while it is still at an early stage to the translation into clinical application. In this study, the process of pre-differentiation presented positive effects on the regeneration of ADSCs in IVD harsh environment, which could provide clues to the development of stem cell therapy for disc degenerative diseases. However, the present study is still preliminary. To develop a new kind of cell candidates for cell-based regenerative medicine and tissue engineering, further investigations are absolutely necessary both in vitro and in vivo in this field.
Conclusion
We concluded that chondrogenic pre-differentiation for 7 to 10 days before implantation could enhance the regeneration effect of ADSCs in IVD harsh environment, by promoting the proliferation ability and chondrogenic differentiation. Besides, the pre-differentiated ADSCs could be a potential candidate for disc regeneration medicine.
Acknowledgments
This study was partly supported by grants from the National Nature Science Foundation of China (81401822, 81472114, 81501908 and 81171756), and the key Science and Technology Planning Project (2012C13G2010083 and 2012C33121) and Nature Science Foundation (Q14H060007 and Y13H060001) of Zhejiang Province.
Author contributions
All authors participated in the design, interpretation of the studies, and analysis of the data and review of the manuscript. JW, YT, XZ, HL, and CL conducted the experiments; JW, YT, XZ, and FL performed the statistical analysis and drafted the manuscript; and Q-xC supervised the study. All authors read and approved the final manuscript.
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- 1.Urban JP, Roberts S. Degeneration of the intervertebral disc. Arthrit Res Ther 2003; 5: 120–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gou S, Oxentenko SC, Eldrige JS, Xiao L, Pingree MJ, Wang Z, Perez-Terzic C, Qu W. Stem cell therapy for intervertebral disk regeneration. Am J Phys Med Rehabili 2014; 93: S122–31. [DOI] [PubMed] [Google Scholar]
- 3.Karppinen J, Shen FH, Luk KD, Andersson GB, Cheung KM, Samartzis D. Management of degenerative disk disease and chronic low back pain. Orthop Clin N Am 2011; 42: 513–28. [DOI] [PubMed] [Google Scholar]
- 4.Liang C, Li H, Tao Y, Zhou X, Li F, Chen G, Chen Q. Responses of human adipose-derived mesenchymal stem cells to chemical microenvironment of the intervertebral disc. J Transl Med 2012; 10: 49–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Oehme D, Goldschlager T, Ghosh P, Rosenfeld JV, Jenkin G. Cell-based therapies used to treat lumbar degenerative disc disease: a systematic review of animal studies and human clinical trials. Stem Cell Int 2015; 2015: 946031–946031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kregar Velikonja N, Urban J, Frohlich M, Neidlinger-Wilke C, Kletsas D, Potocar U, Turner S, Roberts S. Cell sources for nucleus pulposus regeneration. Eur Spine J 2014; 23: S364–74. [DOI] [PubMed] [Google Scholar]
- 7.Hudson KD, Alimi M, Grunert P, Hartl R, Bonassar LJ. Recent advances in biological therapies for disc degeneration: tissue engineering of the annulus fibrosus, nucleus pulposus and whole intervertebral discs. Curr Opin Biotechnol 2013; 24: 872–9. [DOI] [PubMed] [Google Scholar]
- 8.Jeong JH, Lee JH, Jin ES, Min JK, Jeon SR, Choi KH. Regeneration of intervertebral discs in a rat disc degeneration model by implanted adipose-tissue-derived stromal cells. Acta Neurochir 2010; 152: 1771–7. [DOI] [PubMed] [Google Scholar]
- 9.Yoshikawa T, Ueda Y, Miyazaki K, Koizumi M, Takakura Y. Disc regeneration therapy using marrow mesenchymal cell transplantation: a report of two case studies. Spine 2010; 35: E475–80. [DOI] [PubMed] [Google Scholar]
- 10.Acosta FL, Jr, Metz L, Adkisson HD, Liu J, Carruthers-Liebenberg E, Milliman C, Maloney M, Lotz JC. Porcine intervertebral disc repair using allogeneic juvenile articular chondrocytes or mesenchymal stem cells. Tissue Eng A 2011; 17: 3045–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Crevensten G, Walsh AJ, Ananthakrishnan D, Page P, Wahba GM, Lotz JC, Berven S. Intervertebral disc cell therapy for regeneration: mesenchymal stem cell implantation in rat intervertebral discs. Ann Biomed Eng 2004; 32: 430–4. [DOI] [PubMed] [Google Scholar]
- 12.Huang YC, Leung VY, Lu WW, Luk KD. The effects of microenvironment in mesenchymal stem cell-based regeneration of intervertebral disc. Spine J 2013; 13: 352–62. [DOI] [PubMed] [Google Scholar]
- 13.Li H, Liang C, Tao Y, Zhou X, Li F, Chen G, Chen QX. Acidic pH conditions mimicking degenerative intervertebral discs impair the survival and biological behavior of human adipose-derived mesenchymal stem cells. Exp Biol Med 2012; 237: 845–52. [DOI] [PubMed] [Google Scholar]
- 14.Li H, Tao Y, Liang C, Han B, Li F, Chen G, Chen Q. Influence of hypoxia in the intervertebral disc on the biological behaviors of rat adipose- and nucleus pulposus-derived mesenchymal stem cells. Cells Tissues Organs 2013; 198: 266–77. [DOI] [PubMed] [Google Scholar]
- 15.Hiyama A, Mochida J, Iwashina T, Omi H, Watanabe T, Serigano K, Tamura F, Sakai D. Transplantation of mesenchymal stem cells in a canine disc degeneration model. J Orthop Res 2008; 26: 589–600. [DOI] [PubMed] [Google Scholar]
- 16.Le Maitre CL, Baird P, Freemont AJ, Hoyland JA. An in vitro study investigating the survival and phenotype of mesenchymal stem cells following injection into nucleus pulposus tissue. Arthrit Res Ther 2009; 11: R20–R20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chan SC, Gantenbein-Ritter B, Leung VY, Chan D, Cheung KM, Ito K. Cryopreserved intervertebral disc with injected bone marrow-derived stromal cells: a feasibility study using organ culture. Spine J 2010; 10: 486–96. [DOI] [PubMed] [Google Scholar]
- 18.Rodrigues-Pinto R, Richardson SM, Hoyland JA. An understanding of intervertebral disc development, maturation and cell phenotype provides clues to direct cell-based tissue regeneration therapies for disc degeneration. Eur Spine J 2014; 23: 1803–14. [DOI] [PubMed] [Google Scholar]
- 19.Wuertz K, Godburn K, Neidlinger-Wilke C, Urban J, Iatridis JC. Behavior of mesenchymal stem cells in the chemical microenvironment of the intervertebral disc. Spine 2008; 33: 1843–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Naqvi SM, Buckley CT. Extracellular matrix production by nucleus pulposus and bone marrow stem cells in response to altered oxygen and glucose microenvironments. J Anat 2015; 227: 757–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ragetly G, Griffon DJ, Chung YS. The effect of type II collagen coating of chitosan fibrous scaffolds on mesenchymal stem cell adhesion and chondrogenesis. Acta Biomater 2010; 6: 3988–97. [DOI] [PubMed] [Google Scholar]
- 22.Cai R, Nakamoto T, Kawazoe N, Chen G. Influence of stepwise chondrogenesis-mimicking 3D extracellular matrix on chondrogenic differentiation of mesenchymal stem cells. Biomaterials 2015; 52: 199–207. [DOI] [PubMed] [Google Scholar]
- 23.Li YY, Choy TH, Ho FC, Chan PB. Scaffold composition affects cytoskeleton organization, cell-matrix interaction and the cellular fate of human mesenchymal stem cells upon chondrogenic differentiation. Biomaterials 2015; 52: 208–20. [DOI] [PubMed] [Google Scholar]
- 24.Yang Z, Huang CY, Candiotti KA, Zeng X, Yuan T, Li J, Yu H, Abdi S. Sox-9 facilitates differentiation of adipose tissue-derived stem cells into a chondrocyte-like phenotype in vitro. J Orthop Res 2011; 29: 1291–7. [DOI] [PubMed] [Google Scholar]
- 25.Ni L, Liu X, Sochacki KR, Ebraheim M, Fahrenkopf M, Shi Q, Liu J, Yang H. Effects of hypoxia on differentiation from human placenta-derived mesenchymal stem cells to nucleus pulposus-like cells. Spine J 2014; 14: 2451–8. [DOI] [PubMed] [Google Scholar]
- 26.Feng G, Jin X, Hu J, Ma H, Gupte MJ, Liu H, Ma PX. Effects of hypoxias and scaffold architecture on rabbit mesenchymal stem cell differentiation towards a nucleus pulposus-like phenotype. Biomaterials 2011; 32: 8182–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lu ZF, Doulabi BZ, Wuisman PI, Bank RA, Helder MN. Influence of collagen type II and nucleus pulposus cells on aggregation and differentiation of adipose tissue-derived stem cells. J Cell Mol Med 2008; 12: 2812–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Calderon L, Collin E, Velasco-Bayon D, Murphy M, O’Halloran D, Pandit A. Type II collagen-hyaluronan hydrogel – a step towards a scaffold for intervertebral disc tissue engineering. Eur Cell Mater 2010; 20: 134–48. [DOI] [PubMed] [Google Scholar]
- 29.Merceron C, Portron S, Masson M, Lesoeur J, Fellah BH, Gauthier O, Geffroy O, Weiss P, Guicheux J, Vinatier C. The effect of two- and three-dimensional cell culture on the chondrogenic potential of human adipose-derived mesenchymal stem cells after subcutaneous transplantation with an injectable hydrogel. Cell Transplant 2011; 20: 1575–88. [DOI] [PubMed] [Google Scholar]
- 30.Ganey T, Hutton WC, Moseley T, Hedrick M, Meisel HJ. Intervertebral disc repair using adipose tissue-derived stem and regenerative cells: experiments in a canine model. Spine 2009; 34: 2297–304. [DOI] [PubMed] [Google Scholar]
- 31.Sakai D, Nakamura Y, Nakai T, Mishima T, Kato S, Grad S, Alini M, Risbud MV, Chan D, Cheah KS, Yamamura K, Masuda K, Okano H, Ando K, Mochida J. Exhaustion of nucleus pulposus progenitor cells with ageing and degeneration of the intervertebral disc. Nat Commun 2012; 3: 1264–1264. [DOI] [PMC free article] [PubMed] [Google Scholar]