Abstract
Background.
Our objective was to study the prognostic value of liver stiffness (LS) in HIV-infected patients with chronic hepatitis C (CHC).
Methods.
We analyzed HIV-infected patients with compensated CHC and at least 1 determination of LS. The primary outcome was the occurrence of liver-related events (LRE), namely, decompensation or hepatocellular carcinoma, whichever occurred first. We selected patients without sustained viral response (SVR) or end-of-treatment response (ETR) during follow-up and allocated them to an estimation cohort (EC) and a validation cohort (VC).
Results.
The study population comprised 1292 patients. After a median follow-up of 5.8 years, 90 patients experienced LRE and 73 died. In the subgroup of 957 patients without SVR or ETR, the area under the receiver operating characteristic curves (AUROCs) (95% confidence interval [CI]) of LS for prediction of LRE in the EC (n = 634) and the VC (n = 323) were 0.87 and 0.88, respectively. The best cutoff value of LS to rule out LRE in the EC was 12 kPa, with a negative predictive value of 98.3% in the EC and 98.2% in the VC. Per each 1 kPa and 5 kPa increase above 12 kPa, the hazard ratio of LRE (taking into account death as a competing risk) was 1.07 (95% CI, 1.05–1.08) and 1.38 (95% CI, 1.31–1.46), respectively.
Conclusions.
Liver stiffness is very accurate for predicting LRE in coinfected patients. Patients with an LS <12 kPa had a 98% probability of not developing LRE after a median follow-up of almost 6 years. Above the 12-kPa cutoff, the hazard of LRE increases proportionally with LS.
Keywords. coinfection, hepatitis C, human immunodeficiency virus infection, liver stiffness, transient elastography.
Staging of liver fibrosis is an essential element of the care of patients with hepatitis C virus (HCV) infection, because it provides useful prognostic data and facilitates treatment decisions [1]. For many years, liver biopsy has been the staging method of choice; however, its invasive nature and the limitations derived from sampling error and observer variation have led to its replacement by noninvasive methods including serum markers and transient elastography (TE) [2].
Transient elastography, a technique based on the observation that liver elasticity is highly correlated with the stage of liver fibrosis in patients with chronic hepatitis C [3], is very accurate for identifying patients with mild or absent fibrosis and those with advanced fibrosis or cirrhosis [4]. Liver stiffness values of between 2.5 and 7 kPa indicate mild or absent fibrosis, and those above 12.5 kPa indicate cirrhosis [4]. However, results should always be interpreted according to the clinical context; for example, higher liver stiffness cutoffs for cirrhosis have been proposed for patients with alcoholic liver disease, in whom coexisting steatohepatitis markedly increases liver stiffness independently of fibrosis stage [5, 6].
Transient elastography is also a method that is potentially useful for estimating portal pressure [7–14], detecting the presence of esophageal varices [11, 12, 15–24], and predicting clinical endpoints. The prognostic capacity of TE has been evaluated in patients with chronic liver diseases of varying etiologies [9, 25–27], in compensated HCV-related liver cirrhosis with or without human immunodeficiency virus (HIV)-coinfection [28–31], and in liver transplantation [32]. All of these studies reported a good ability of TE for the identification of clinical outcomes including liver-related events (LRE) [9, 26, 27, 29–31], death [26, 30, 33], hepatocellular carcinoma [28], and composite endpoints [25]. However, no validated prediction model based on TE has been developed for LRE in patients with chronic hepatitis C with or without cirrhosis, and very little is known about TE cutoffs for stratification of the risk of LRE.
We designed this study to assess the prognostic ability of TE in patients coinfected with HIV and HCV. In particular, we aimed to define clinically useful liver stiffness cutoff points for prediction of LRE in this population.
PATIENTS AND METHODS
Design and Patient Selection
This study was designed during the first half of 2014. We performed a multicenter retrospective study at 3 large teaching hospitals in Spain in which TE was routinely introduced for staging of liver fibrosis in coinfected patients in 2006. Transient elastography was performed by trained operators with a FibroScan device (EchoSens, Paris, France). We reviewed the computerized records of the FibroScan device and identified all patients older than 18 years attended at these institutions and who met the following criteria: (1) HIV infection confirmed by enzyme immunoassay and Western blotting, (2) HCV infection confirmed by detection of HCV-ribonucleic acid (RNA) in plasma, (3) at least 1 valid TE measurement taken when the patient had confirmed HCV infection, and (4) compensated liver disease when the first TE measurement was taken. We excluded patients with acute hepatitis C, decompensated liver disease, or a prior diagnosis of hepatocellular carcinoma at the time of the TE study. The diagnosis of cirrhosis was confirmed by liver biopsy or by liver stiffness >12.5 kPa [4]. The local ethics committees approved the analysis of anonymous routine clinical data without written informed consent with a view to scientific publication.
Investigations
All of the information was recorded at each institution using an online application that satisfied local requirements of data confidentiality. This database included all demographic, clinical, virological, and laboratory data. We extracted the following baseline data from the hospital records: (1) demographics; (2) HIV-related data (HIV transmission category, Centers for Disease Control and Prevention [CDC] clinical category, nadir CD4+ T-cell count, the most recent CD4+ T-cell count, the most recent HIV-RNA load, and whether or not patients were receiving combination antiretroviral therapy [cART]); (3) liver disease-related data (HCV genotype, HCV-RNA load, hepatitis B surface antigen [HBsAg], and anti-HCV therapy); and (4) history of substance abuse including alcohol consumption >50 g/d, smoking, and treatment with methadone. Trained operators performed all FibroScan examinations. We considered 10 acquisitions with a success rate ≥60% and an interquartile range (IQR) <30% of the median value as representative measurements of liver stiffness [4]. Fasting was not routinely required before the examination.
Follow-up and Clinical Endpoints
The length of the study was calculated for each patient from the date of the first TE study to the last follow-up visit or death. The censoring date was December 31, 2014.
In patients who received anti-HCV therapy during follow-up, treatment response was classified into 3 categories: (1) sustained viral response, defined as an undetectable serum HCV-RNA level 24 weeks after discontinuation of therapy; (2) viral relapse, defined as an undetectable serum HCV-RNA level at the end of programmed therapy, with subsequent relapse; and (3) no response, when patients did not fulfill the criteria for sustained viral response or viral relapse.
We also assessed the response to cART during follow-up as follows: (1) virological failure, defined as the presence of HIV RNA >200 copies/mL on 2 consecutive occasions in patients taking cART; (2) discontinuation of cART, when cART was suspended for any reason for more than 4 weeks during follow-up; (3) suboptimal immune response, defined as the presence of a CD4+ T-cell count <350 cells/mm3 in 2 consecutive measurements, outside periods of interferon-based anti-HCV therapy; (4) immunosuppression, defined as a CD4+ T-cell count <200 cells/mm3 on 2 consecutive measurements outside periods of interferon-based anti-HCV therapy.
We assessed the following outcome events: (1) LRE, including liver decompensation (ascites, hepatic encephalopathy, variceal bleeding, and nonobstructive jaundice) and hepatocellular carcinoma; (2) progression of HIV infection, defined as the occurrence of any new acquired immune deficiency syndrome (AIDS)-defining conditions during follow-up according to CDC criteria [34]; (3) mortality, classified as liver-related death (when the train of events that ended in death was caused by liver decompensation or hepatocellular carcinoma), AIDS-related death (when death was directly related to an AIDS-defining condition), and nonliver-related non-AIDS-related deaths.
Statistics
The primary endpoint was the occurrence of LRE; for patients who had more than 1 LRE, only the first was included in the analysis. We also assessed death and the composite variable of death or LRE, whichever occurred first. According to our definition, all patients with liver-related deaths were considered to have experienced a LRE before their death.
We first evaluated the independent role of TE in predicting LRE, considering death as a competitive risk in the full dataset of patients using the Fine and Gray method [35]; covariates for adjustment were those with a P value <.1 in the univariate analysis.
Next, we evaluated the ability of TE to predict LRE; for this purpose, we excluded from the full dataset all patients who received anti-HCV therapy during follow-up and achieved a sustained viral response or end-of-treatment response with subsequent relapse, because the natural history of chronic hepatitis C in HIV/HCV-coinfected patients is modified in responders and relapsers [36]. We randomly allocated these patients to an estimation cohort (two thirds of the patients) and a validation cohort (one third of the patients). We used receiver operating characteristic curves to assess the diagnostic capacity of liver stiffness to predict LRE. To identify a cutoff value of TE to separate risk populations, we decided a priori that it would be preferable to identify patients who would not develop LRE. To estimate the hazard of LRE for TE values above the cutoff, we first assessed the assumption of linearity between TE and the proportion of LRE and then assessed the hazard of LRE according to different liver stiffness values above the cutoff using the Fine and Gray proportional hazards model. The covariates for adjustment were those with a P value <.1 in the univariate analysis.
The statistical analyses were performed using IBM SPSS Statistics for Windows, Version 21.0 (IBM Corp., Armonk, NY). The R package was used to plot the cumulative incidence curves and to run the competing risks regression analysis [37].
RESULTS
Patient Characteristics
The baseline characteristics of the 1292 patients included in the study are shown in Table 1. In brief, 78.6% were male, the median age was 44 years, and 80.1% acquired HIV by injection drug use. A current high alcohol intake was reported by 10.9% of patients, and 23.0% were in methadone maintenance programs. Prior AIDS-defining conditions were recorded in 36.5% of individuals, the median nadir CD4+ T-cell count was 176 cells/mm3, and 86.8% were on cART. The most frequently used cART regimens were a protease inhibitor plus 2 nucleoside reverse-transcriptase inhibitors (NRTIs) (38.4%) and a non-NRTI plus 2 NRTIs (32.5%). The median CD4+ T-cell count at baseline was 442 cells/mm3. Human immunodeficiency virus viral suppression (<50 copies/mL) was noted in 75.5% of the whole population and in 86.7% of those receiving cART. Most patients (58.6%) were infected by HCV genotype 1, and the median HCV-RNA was 6.3 log IU/mL. Transient elastography findings showed that 24.6% of patients had cirrhosis.
Table 1.
Characteristics of 1292 HIV/HCV-Coinfected Patients With Compensated Liver Disease Studied Using Transient Elastography
| Variable | No Liver-Related Events | Liver-Related Events | All Patients |
|---|---|---|---|
| 1202 | 90 | N = 1292 | |
| Male sex, n (%) | 943 (78.5) | 72 (80.0) | 1015 (78.6) |
| Age years, median (IQR) | 44 (41–48) | 44 (41–48) | 44 (41–48) |
| HIV transmission category | |||
| Injection drug use | 959 (79.8) | 76 (84.6) | 1035 (80.1) |
| Heterosexual relations | 91 (7.6) | 5 (5.6) | 96 (7.4) |
| Sexual relations between men | 52 (4.3) | 1 (1.1) | 53 (4.1) |
| Mother-to-child transmission | 4 (0.3) | 0 (0.0) | 4 (0.3) |
| Other/unknown | 96 (8.0) | 8 (8.9) | 104 (8.0) |
| Alcohol intake >50 g/d, n (%) | |||
| Yes | 118 (9.8) | 23 (25.6)* | 141 (10.9) |
| No | 835 (69.5) | 55 (61.1) | 890 (68.9) |
| Unknown | 249 (20.7) | 12 (13.3) | 261 (20.2) |
| Current methadone use, n (%) | |||
| Yes | 273 (22.7) | 24 (26.7) | 297 (23.0) |
| CDC clinical categorya, n (%) | |||
| A | 548 (45.6) | 31 (34.4) * | 579 (44.8) |
| B | 173 (14.4) | 14 (15.6) | 187 (14.5) |
| C | 432 (35.9) | 39 (43.3) | 471 (36.5) |
| Unknown | 49 (4.1) | 6 (6.7) | 55 (4.3) |
| cART, n (%) | |||
| Yes | 1044 (86.9) | 77 (85.6) | 1121 (86.8) |
| No | 154 (12.8) | 13 (14.4) | 167 (12.9) |
| Unknown | 4 (0.3) | 0 (0.0) | 4 (0.3) |
| Type of cART regimen, n (%) | |||
| 2 NRTI + 1 PI | 462 (38.4) | 34 (37.8) | 496 (38.4) |
| 2 NRTI + 1 NNRTI | 396 (32.9) | 24 (26.7) | 420 (32.5) |
| 2 NRTI + 1 INSTI | 18 (1.5) | 1 (1.1) | 19 (1.5) |
| PI-based monotherapy | 23 (1.9) | 0 (0.0) | 23 (1.8) |
| PI-based bitherapy | 23 (1.9) | 3 (3.3) | 26 (2.0) |
| Other regimens | 122 (10.1) | 15 (16.7) | 137 (10.6) |
| Unknown | 4 (0.3) | 0 (0.0) | 4 (0.3) |
| HIV-RNA <50 copies/mL, n (%) | |||
| Yes | 909 (75.6) | 66 (73.3) | 975 (75.5) |
| No | 266 (22.1) | 21 (23.3) | 287 (22.2) |
| Unknown | 27 (2.2) | 3 (3.3) | 30 (2.3) |
| HIV-RNA <50 copies/mL in cART, n (%) | 909/1044 (87.1) | 66/77 (85.7) | 975/1121 (86.7) |
| CD4+ T cells/µL, median (IQR) | 452 (276–649) | 373 (181–553)* | 442 (270–646) |
| Nadir CD4+ T cells/µL, median (IQR) | 180 (79–288) | 130 (55–266) | 176 (76–287) |
| HCV genotype, n (%) | |||
| 1 | 707 (58.8) | 50 (55.6) | 757 (58.6) |
| 2 | 20 (1.7) | 1 (1.1) | 21 (1.6) |
| 3 | 222 (18.5) | 18 (20.0) | 240 (18.6) |
| 4 | 214 (17.8) | 10 (11.1) | 224 (17.3) |
| Unknown | 39 (3.2) | 11 (12.2)* | 50 (3.9) |
| HCV-RNA, log (IU/mL), median (IQR) | 6.3 (5.6–6.7) | 6.3 (5.4–6.8) | 6.3 (5.6–6.7) |
| HBsAg, n (%) | |||
| Negative | 1053 (87.6) | 67 (74.4)* | 1120 (86.7) |
| Positive | 44 (3.7) | 8 (8.9)* | 52 (4.0) |
| Unknown | 105 (8.7) | 15 (16.7)* | 120 (9.3) |
| Liver biopsy, n (%) | |||
| No | 1020 (84.9) | 82 (91.1) | 1102 (85.3) |
| Yes | 180 (15.0) | 7 (7.8) | 187 (14.5) |
| METAVIR fibrosis stage (174 patients) | |||
| 0–1 | 76 (45.5) | 3 (42.9) | 79 (45.4) |
| 2 | 57 (34.1) | 0 (0.0) | 57 (32.8) |
| 3 | 24 (14.4) | 2 (28.6) | 26 (14.9) |
| 4 | 10 (6.0) | 2 (28.6) | 12 (6.9) |
| Variable | No Liver-Related Events | Liver-Related Events | All Patients |
| Liver stiffness, kPa, median (IQR) | 8 (6–11) | 26 (15–40)* | 8 (6–12) |
| Liver stiffness, kPa, n (%) | |||
| <7.1 | 588 (48.9) | 5 (5.6)* | 593 (45.9) |
| 7.1–9.5 | 211 (17.6) | 3 (3.3)* | 214 (16.6) |
| 9.6–12.5 | 159 (13.2) | 8 (8.9) | 167 (12.9) |
| >12.5 | 244 (20.3) | 74 (82.2)* | 318 (24.6) |
| FIB-4 value, n (%) (1150) patients | |||
| <1 | 420 (39.1) | 26 (34.7) | 446 (38.8) |
| 1–3.5 | 507 (47.2) | 13 (17.3)* | 520 (45.2) |
| ≥3.5 | 148 (13.8) | 36 (48.0)* | 184 (16.0) |
Abbreviations: AIDS, acquired immune deficiency syndrome; cART, combination antiretroviral therapy; CDC, US Centers for Disease Control and Prevention; FIB, fibrosis; HBsAg, hepatitis B surface antigen; HCV, hepatitis C virus; HIV, human immunodeficiency virus; INSTI, integrase strand transfer inhibitor; IQR, interquartile range; NRTI, nucleoside reverse-transcriptase inhibitor; NNRTI, non-NRTI; PI, protease inhibitor; RNA, ribonucleic acid.aA, asymptomatic HIV or persistent generalized lymphadenopathy; B, symptomatic non-C conditions; C, AIDS-defining conditions.
*P < .05 compared with the no liver-related events group.
Follow-up
The median follow-up time was 5.8 years (IQR, 3.4–7.1); a total of 129 (10%) patients were lost to follow-up after a median of 3.3 years (IQR, 1.6–5.1) after baseline. In comparison with patients not lost to follow-up, those lost to follow-up were younger (median age 43 years vs 44 years; P < .05) and more frequently on methadone maintenance programs (30.2% vs 22.2%; P < .05). No differences were found between them in sex, route of HIV-acquisition, alcohol consumption, prior CDC clinical category C conditions, cART use, HIV viral suppression on cART, baseline and nadir CD4+ T-cell counts, HCV genotype, HCV-RNA, or liver stiffness.
Treatment of Human Immunodeficiency Virus and Hepatitis C Virus
A total of 110 (8.5%) patients had interruptions of cART of more than 4 weeks during follow-up, and 247 (19.1%) experienced at least 1 episode of viral failure. A total of 415 patients (32.1%) experienced a suboptimal immune response, and 203 patients (15.7%) experienced immunosuppression.
During follow-up, 631 (48.8%) patients received 765 different anti-HCV regimens (some patients were retreated): 525 (83.2%) received pegylated interferon plus ribavirin, 67 (10.6%) received pegylated interferon plus ribavirin plus telaprevir or boceprevir, 106 (16.8%) other regimens, and in 22 (3.5%) the regimen was unknown. Overall, 277 (43.9%) of 631 treated patients achieved a sustained viral response, and 58 (9.2%) achieved an end-of-treatment response with a subsequent relapse.
Incident Events and Mortality
A total of 56 (4.3%) patients experienced a new AIDS-defining condition at a median of 20 months (IQR 9–38 months) from baseline. The most frequent AIDS-defining conditions were as follows: recurrent pneumonia, 13; esophageal candidiasis, 10; and non-Hodgkin lymphoma, 5 (Supplementary Material).
Liver-related events were documented in 90 patients (7.0%). A total of 80 patients (6.2%) experienced a first episode of liver decompensation at a median of 34 months (IQR, 15–56 months) from baseline. Overall, there were 87 episodes of liver decompensation, because some patients experienced more than 1. The most frequent types were as follows: ascites, 58; hepatic encephalopathy, 11; variceal bleeding, 7; nonobstructive jaundice, 7; and other, 4. A total of 22 (1.7%) patients were diagnosed with hepatocellular carcinoma at a median of 37 months (IQR, 18–59 months) from baseline. The diagnosis was established by imaging procedures in 21 patients and was histologically confirmed in 1 patient (Supplementary Material).
A total of 73 patients (5.7%) died during follow-up. The causes of death were as follows: 35 nonliver-related non-AIDS-related deaths, 26 liver-related deaths, 6 AIDS-related deaths, and 6 deaths from an unknown cause (Supplementary Material).
Predictors of Liver-Related Events and Mortality
In the univariate analysis (Table 2), several variables were associated with the development of LRE including alcohol intake, baseline and nadir CD4+ T-cell counts, suboptimal immune function, immunosuppression, HBsAg positivity, liver stiffness, and sustained viral response after anti-HCV therapy. When all variables with a P value <.1 in the univariate analysis were entered into a multivariate model, the only variables that remained independent predictors of LRE were liver stiffness and achievement of sustained viral response after anti-HCV therapy. As for mortality, the variables that were independently associated with mortality included liver stiffness, cART, and achievement of a sustained viral response (Table 2).
Table 2.
Factors Associated With Liver-Related Events and With Mortality in 1292 HIV/HCV-Coinfected Patients
| Liver-Related Events | ||||||
|---|---|---|---|---|---|---|
| Univariate Analysis | Multivariate Analysis | |||||
| Variables | sHR | 95% CI | P | sHR | 95% CI | P |
| Alcohol intake >50 g/d | 2.64 | 1.62–4.30 | <.001 | 1.26 | 0.65–2.44 | .488 |
| Baseline CD4+ (100 cells) | 0.87 | 0.79–0.96 | .003 | 0.91 | 0.80–1.04 | .172 |
| Nadir CD4+ (100 cells) | 0.88 | 0.76–1.01 | .071 | 0.97 | 0.81–1.15 | .687 |
| Suboptimal immune function | 1.72 | 1.13–2.61 | .011 | 1.25 | 0.71–2.22 | .442 |
| Immunosuppression | 1.81 | 1.11–2.95 | .018 | 0.98 | 0.51–1.88 | .961 |
| HBsAg positivity | 3.10 | 1.43–6.74 | .004 | 2.12 | 0.73–6.14 | .168 |
| Liver stiffness per 1 kPa increase | 1.06 | 1.05–1.07 | <.001 | 1.06 | 1.05–1.08 | <.001 |
| Response to anti-HCV therapy | ||||||
| No SVR | Ref. | Ref. | Ref. | Ref. | ||
| Untreated | 0.90 | 0.58–1.41 | .657 | 0.80 | 0.46–1.40 | .428 |
| Viral relapse | 0.86 | 0.34–2.22 | .760 | 1.20 | 0.42–3.10 | .726 |
| Sustained viral response | 0.13 | 0.04–0.36 | <.001 | 0.12 | 0.04–0.37 | <.001 |
| Overall mortality | ||||||
| Univariate Analysis | Multivariate Analysis | |||||
| Variables | HR | 95% CI | P | HR | 95% CI | P |
| Methadone | 1.60 | 0.97–2.64 | .066 | 1.36 | 0.78–2.38 | .276 |
| Nadir CD4+ (100 cells) | 0.81 | 0.68–0.97 | .019 | 0.84 | 0.71–0.99 | .035 |
| Injection drug use | 1.91 | 0.92–3.98 | .084 | 1.91 | 0.79–4.61 | .151 |
| Immunosuppression | 1.79 | 1.04–3.08 | .035 | 0.89 | 0.45–1.76 | .736 |
| HBsAg positivity | 3.57 | 1.68–7.60 | .001 | 1.86 | 0.83–4.16 | .151 |
| Prior AIDS-defining conditions | 1.59 | 1.01–2.53 | .048 | 1.11 | 0.65–1.89 | .701 |
| New AIDS-defining conditions | 2.62 | 1.19–5.76 | .016 | 1.42 | 0.59–3.43 | .440 |
| Liver stiffness per 1 kPa increase | 1.04 | 1.03–1.06 | <.001 | 1.06 | 1.04–1.07 | <.001 |
| cART | 0.50 | 0.29–0.88 | .016 | 0.32 | 0.18–0.56 | <.001 |
| Response to anti-HCV therapy | ||||||
| No SVR | Ref. | Ref. | ||||
| Untreated | 1.58 | 0.91–2.73 | .102 | 1.40 | 0.71–2.77 | .330 |
| Viral relapse | 1.52 | 0.56–4.07 | .409 | 1.79 | 0.63–5.05 | .274 |
| Sustained viral response | 0.11 | 0.02–0.46 | .003 | 0.11 | 0.02–0.49 | .004 |
Abbreviations: AIDS, acquired immune defficiancy syndrome; cART, combination antiretroviral therapy; CI, confidence interval; IQR, interquartile range; HBsAg, hepatitis B surface antigen; HCV, hepatitis C virus; HIV, human immunodeficiency virus; HR, hazard ratio; Ref., reference; sHR, sub-HR; SVR, sustained viral response.
Ability of Transient Elastography to Predict Outcomes
From the full dataset, the 957 patients without a sustained viral response or end-of-treatment response and subsequent relapse during follow-up were included in the analysis. Of these, 634 were randomly allocated to the estimation cohort and 323 to the validation cohort. No significant differences in baseline characteristics or outcomes were detected between the groups (Table 3).
Table 3.
Baseline Characteristics and Outcomes of 957 HIV/HCV-Coinfected Patients Analyzed to Assess the Ability of Transient Elastography to Predict Outcomes, 634 of Whom Were Randomly Assigned to an Estimation Cohort and 323 to a Validation Cohort
| Variable | Estimation Cohort n = 634 | Validation Cohort n = 323 | Total N = 957 |
|---|---|---|---|
| Baseline variables | |||
| Male sex, n (%) | 483 (76.2) | 263 (81.4) | 746 (78.0) |
| Age, years, median (IQR) | 44 (41–48) | 44 (40–48) | 44 (41–48) |
| HIV acquired by injection drug use, n (%) | 506 (79.8) | 261 (80.8) | 767 (80.1) |
| CDC clinical category C, n (%) | 240 (37.9) | 133 (41.2) | 373 (39.0%) |
| cART, n (%) | 547 (86.3) | 284 (87.9) | 831 (86.8) |
| HIV-RNA <50 copies/mL, n (%) | 457 (72.1) | 243 (75.2) | 700 (73.1) |
| CD4+ T cells/µL, median (IQR) | 432 (254–625) | 429 (273–621) | 430 (261–624) |
| HCV genotype 1, n (%) | 385 (60.7) | 195 (60.4) | 580 (60.6) |
| Liver stiffness, kPa, median (IQR) | 8 (6–11) | 8 (6–14) | 8 (6–12) |
| Follow-up variables | |||
| Follow-up, years, median (IQR) | 5.3 (2.7–6.9) | 5.3 (2.5–7.0) | 5.3 (2.7–6.9) |
| New AIDS-defining condition, n (%) | 30 (4.7) | 19 (5.9) | 49 (5.1) |
| Liver decompensation, n (%) | 44 (6.9) | 22 (6.8) | 66 (6.9) |
| Ascites | 29 | 18 | 47 |
| Other | 18 | 6 | 24 |
| Hepatocellular carcinoma, n (%) | 12 (1.9) | 7 (2.2) | 19 (2.0) |
| Death, n (%) | 45 (7.1) | 21 (6.5) | 66 (6.9) |
| Liver-related | 14 | 9 | 23 |
| AIDS-related | 4 | 1 | 5 |
| Nonliver-related, non-AIDS-related | 25 | 8 | 33 |
| Unknown | 2 | 3 | 5 |
Abbreviations: AIDS, acquired immune defficiancy syndrome; cART, combination antiretroviral therapy; CDC, Centers for Disease Control and Prevention; HCV, hepatitis C virus; HIV, human immunodeficiency virus; IQR, interquartile range; RNA, ribonucleic acid.
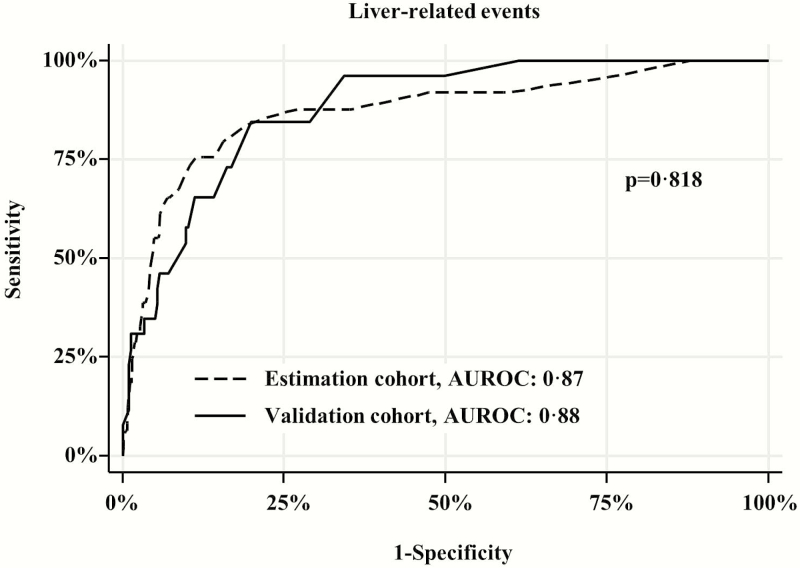
The AUROC of TE to predict LRE was 0.87 in the estimation cohort and 0.88 in the validation cohort (Figure 1). Of all the possible values of liver stiffness up to 75 kPa, the best cutoff value to rule out LRE was 12 kPa, which was also the optimum cutoff value according to Youden’s J (Supplementary Material). The values of the 12 kPa cutoff for the prediction of LRE in the estimation and validation cohorts are shown in Table 4. The negative predictive value for this cutoff was 98.3% (95% confidence interval [CI], 97.3–99.3) in the estimation cohort and 98.2% (95% CI, 96.8–99.7) in the validation cohort.
Figure 1.
Ability of transient elastography to predict liver-related events. The area under the receiver operating characteristic curve (AUROC) to predict liver-related events was 0.87 (95% confidence interval [CI], 0.84–0.90) in the estimation cohort and 0.88 (95% CI, 0.84–0.91) in the validation cohort.
Table 4.
Predictive Values of the Liver Stiffness Cutoff of 12 kPa for the Prediction of Liver-Related Events in the Estimation Cohort and in the Validation Cohort
| Estimation Cohort | |||
|---|---|---|---|
| Liver-Related Event | |||
| Liver Stiffness (kPa) | No | Yes | Total |
| <12 | 472 | 8 | 480 |
| ≥12 | 113 | 41 | 154 |
| Total | 585 | 49 | 634 |
| Value | 95% CI | ||
| Sensitivity (%) | 83.7 | 80.8–86.5 | |
| Specificity (%) | 80.7 | 77.6–83.8 | |
| Positive predictive value (%) | 26.6 | 23.2–30.1 | |
| Negative predictive value (%) | 98.3 | 97.3–99.3 | |
| Positive likelihood ratio | 4.33 | 3.52–5.33 | |
| Negative likelihood ratio | 0.20 | 0.11–0.38 | |
| Validation cohort | |||
| Liver-related event | |||
| Liver stiffness (kPa) | No | Yes | Total |
| <12 | 220 | 4 | 224 |
| ≥12 | 77 | 22 | 99 |
| Total | 297 | 26 | 323 |
| Value | 95% CI | ||
| Sensitivity (%) | 84.6 | 80.7–88.6 | |
| Specificity (%) | 74.1 | 69.3–78.8 | |
| Positive predictive value (%) | 22.2 | 17.7–26.8 | |
| Negative predictive value (%) | 98.2 | 96.8–99.7 | |
| Positive likelihood ratio | 3.26 | 2.54–4.20 | |
| Negative likelihood ratio | 0.21 | 0.08–0.51 | |
Abbreviations: CI, confidence interval.
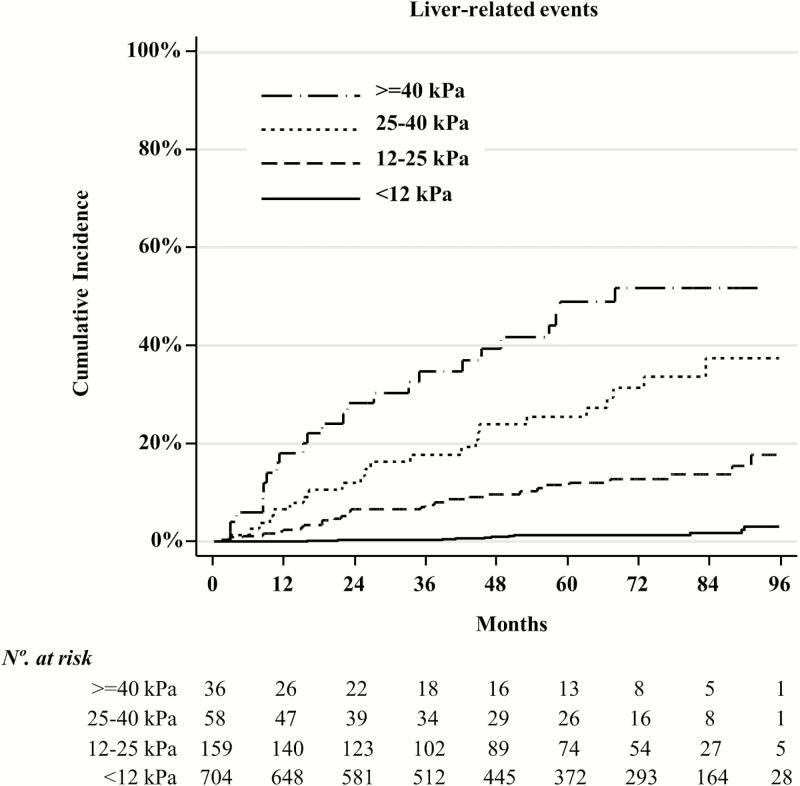
When we analyzed the 957 patients included in the estimation and validation cohorts, we found that above the 12 kPa cutoff, the relationship between liver stiffness and the proportion of LRE was linear. For each 1 kPa and 5 kPa increase above the 12 kPa cutoff, the sHR of LRE was 1.07 (95% CI, 1.05–1.08) and 1.38 (95% CI, 1.31–1.46), respectively. Furthermore, when we took the 12 kPa cutoff as the reference, the sHR of LRE in patients with liver stiffness values of 12–24 kPa, 25–39 kPa, and ≥40 kPa were 9.42, 28.0, and 37.8, respectively (Table 5). The cumulative incidence of LRE for these liver-stiffness strata is shown in Figure 2.
Table 5.
Hazard of Liver-Related Events by Fine and Gray Proportional Hazard Regression Analysis, Considering Death as a Competitive Risk in the Full Data Set of Patients (Estimation and Validation Cohorts, N = 957)
| Liver Stiffness kPa | sHR | 95% CI | P |
|---|---|---|---|
| <12 | Ref. | — | — |
| 12–24 | 9.42 | 4.72–17.15 | <.001 |
| 25–39 | 28.00 | 13.99–56.01 | <.001 |
| ≥40 | 37.76 | 17.87–79.80 | <.001 |
Abbreviations: CI, confidence interval; Ref., reference; sHR, subhazard ratio.
Figure 2.
Cumulative incidence plots of liver-related events for different liver-stiffness strata.
DISCUSSION
Our study of 1292 HIV/HCV-coinfected patients with compensated liver disease revealed that liver stiffness and sustained viral response were predictors of LRE considering death a competing risk. In a subgroup of 957 patients without sustained viral response or viral relapse after anti-HCV therapy who were randomly allocated to an estimation cohort and a validation cohort, we found that TE was highly accurate for prediction of LRE. We sought to identify a threshold value of liver stiffness to separate various risk populations, as did Ripoll et al [38] when studying the predictive ability of hepatic venous pressure gradient in patients with compensated cirrhosis. We found that the cutoff value of 12 kPa identified 2 populations at risk of developing LRE, with a negative predictive value of 98% for patients with baseline values below this value. Other authors have sought a liver stiffness cutoff value to rule out clinical outcomes in patients with chronic liver disease. In one study (667 patients with chronic liver disease of varying etiologies), a cutoff of 10.5 kPa was valid for identifying individuals with a low risk of experiencing a composite endpoint of death, LRE, increase in Child-Pugh score, or listing for liver transplant over 2 years [25]. In a study of 984 Japanese patients with chronic hepatitis C treated or not with interferon-based anti-HCV therapy, hepatocellular carcinoma was rarely detected in patients with liver stiffness <10 kPa after a mean follow-up of 3 years [28].
Having identified the 12 kPa cutoff as the threshold for identification of patients with a very low risk of progression, we found that the hazard of LRE increased proportionally with liver stiffness values above this cutoff. Thus, per each 1 kPa and 5 kPa increase in liver stiffness above the 12 kPa cutoff, the hazard of LRE considering death as a competing risk increased by 7% and 38%, respectively. When we took the 12 kPa cutoff as the reference, the adjusted hazard of LRE considering death as a competing risk for liver stiffness values in the 12–24 kPa, 25–39 kPa, and ≥40 kPa strata were 9.42, 28.0, and 37.8, respectively. In a meta-analysis based on studies of patients with chronic liver diseases, each kPa increase in liver stiffness was associated with an incremental risk of decompensation and hepatocellular carcinoma of 7% and 11%, respectively [39]. A study of 2052 patients with diverse hepatic conditions not included in this meta-analysis showed that for every 1 kPa increase in liver stiffness, the adjusted risk of complications increased by 5% [26].
Our study is limited by its retrospective design. However, we believe that it is unlikely that the results differ considerably from those that would have been obtained in a prospective study, because patients were followed-up in the 3 participating centers by the same physicians throughout the course of the disease, with standard clinical and laboratory parameters assessed every 3 to 6 months, as is common practice with HIV-infected patients in Spain. In addition, the complications of cirrhosis were managed and prevented in the 3 clinical centers after protocols based on contemporary clinical practice guidelines. Another limitation of the study is that TE was not always performed in the fasting state, as is currently recommended, because food intake increases liver stiffness in patients with HCV infection and healthy controls [40].
Our study has several strengths, namely, its large sample size, the inclusion of patients with cirrhosis and patients with different stages of fibrosis, and the exclusion of patients who achieved sustained viral response or viral relapse after anti-HCV therapy, because these factors clearly modify the natural history of hepatitis C [36]. Our choice of LRE as the primary outcome variable is also relevant, because it is considered to be the most appropriate outcome in patients with compensated liver disease, whereas death is the most relevant outcome in those with decompensated liver disease [41]. In addition, the TE cutoff value for identification of patients who would not develop LRE was obtained in a training cohort and confirmed in a validation cohort; this approach has never been used when assessing the prognostic utility of TE in patients with chronic hepatitis C. Finally, we wish to emphasize our use of a competing risk analysis considering death as a competing risk to reduce error when assessing the ability of TE to predict liver-related complications. This is of particular relevance in HIV/HCV-coinfected patients, a group characterized by an increased risk not only of liver-related deaths, but also AIDS-related deaths and nonliver-related non-AIDS-related deaths [42].
We believe that our findings have substantial implications for practice, because they show that TE is an excellent tool for stratifying the risk of liver-related outcomes in HIV/HCV-coinfected patients and suggest that those with a liver stiffness below the 12 kPa cutoff could be followed-up less closely than patients with higher liver stiffness values. Nevertheless, we acknowledge that the limitations inherent to the retrospective design of our study imply that this proposed cutoff should be evaluated prospectively with large datasets and cost-effectiveness analyses before it can be endorsed for specific recommendations, including hepatocellular carcinoma screening. The high accuracy of TE for predicting the clinical outcomes found in our study also suggests that this technique could be used to estimate the residual risk of LRE, in patients with HCV-related cirrhosis who achieve sustained viral response with the new interferon-free regimens for treatment of HCV infection.
CONCLUSIONS
In conclusion, we found that TE was very accurate for predicting clinical outcomes in HIV/HCV-coinfected patients. Patients with a liver stiffness value <12 kPa had a 98% probability of not developing LRE after a median follow-up of almost 6 years; above this threshold value, the hazard of LRE increased proportionally with liver stiffness.
Supplementary Data
Supplementary material is available online at Open Forum Infectious Diseases (http://OpenForumInfectiousDiseases.oxfordjournals.org/).
Acknowledgments
We thank Thomas O’Boyle for writing assistance during the preparation of the manuscript.
FibroScan devices were available at the 3 institutions by a donation from Abbvie Spain to “Grupo de Estudio del SIDA” (AIDS Study Group [GESIDA]) of the “Sociedad Española de Enfermedades Infecciosas y Microbiología Clínica” (Spanish Society of Infectious Diseases and Clinical Microbiology [SEIMC]).
Disclaimer. The study sponsors had no role in the design of the study, the collection, analysis, and interpretation of data, the writing of the report, or the decision to submit the paper for publication.
Financial support. This work was funded by a grant from GILEAD Spain (Ref. no. 14_0099) and by grants from Instituto de Salud Carlos III (ISC-III) (Ref. nos. PI11/01556, PI14/01094, and PI14/01581). Research in the field of human immunodeficiency virus in all the institutions is supported by the RD12/0017 project as part of the Plan Nacional R + D + I and cofunded by ISCIII-Subdirección General de Evaluación and Fondo Europeo de Desarrollo Regional. J. B. is supported by Programa de Intensificación de la Actividad Investigadora en el Sistema Nacional de Salud (I3SNS) (Ref. no. INT15/00079). A. R.-J. has been the recipient of a Post-Doctoral Perfection grant from the Fundación Progreso y Salud, Consejería de Salud y Bienestar Social de la Junta de Andalucía (Ref. no. RH-2013-0024).
Potenial conflicts of interest. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest.
References
- 1. European Association for the Study of the Liver. EASL recommendations on treatment of hepatitis C 2015. J Hepatol 2015; 63:199–236. [DOI] [PubMed] [Google Scholar]
- 2. Patel K, Bedossa P, Castera L. Diagnosis of liver fibrosis: present and future. Semin Liver Dis 2015; 35:166–83. [DOI] [PubMed] [Google Scholar]
- 3. Sandrin L, Fourquet B, Hasquenoph JM, et al. Transient elastography: a new noninvasive method for assessment of hepatic fibrosis. Ultrasound Med Biol 2003; 29:1705–13. [DOI] [PubMed] [Google Scholar]
- 4. Castera L, Forns X, Alberti A. Non-invasive evaluation of liver fibrosis using transient elastography. J Hepatol 2008; 48:835–47. [DOI] [PubMed] [Google Scholar]
- 5. Mueller S, Millonig G, Sarovska L, et al. Increased liver stiffness in alcoholic liver disease: differentiating fibrosis from steatohepatitis. World J Gastroenterol 2010; 16:966–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Janssens F, de Suray N, Piessevaux H, et al. Can transient elastography replace liver histology for determination of advanced fibrosis in alcoholic patients: a real-life study. J Clin Gastroenterol 2010; 44:575–82. [DOI] [PubMed] [Google Scholar]
- 7. Hong WK, Kim MY, Baik SK, et al. The usefulness of non-invasive liver stiffness measurements in predicting clinically significant portal hypertension in cirrhotic patients: Korean data. Clin Mol Hepatol 2013; 19:370–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Berzigotti A, Seijo S, Arena U, et al. Elastography, spleen size, and platelet count identify portal hypertension in patients with compensated cirrhosis. Gastroenterology 2013; 144:102–11. [DOI] [PubMed] [Google Scholar]
- 9. Robic MA, Procopet B, Métivier S, et al. Liver stiffness accurately predicts portal hypertension related complications in patients with chronic liver disease: a prospective study. J Hepatol 2011; 55:1017–24. [DOI] [PubMed] [Google Scholar]
- 10. Lemoine M, Katsahian S, Ziol M, et al. Liver stiffness measurement as a predictive tool of clinically significant portal hypertension in patients with compensated hepatitis C virus or alcohol-related cirrhosis. Aliment Pharmacol Ther 2008; 28:1102–10. [DOI] [PubMed] [Google Scholar]
- 11. Bureau C, Metivier S, Peron JM, et al. Transient elastography accurately predicts presence of significant portal hypertension in patients with chronic liver disease. Aliment Pharmacol Ther 2008; 27:1261–8. [DOI] [PubMed] [Google Scholar]
- 12. Vizzutti F, Arena U, Romanelli RG, et al. Liver stiffness measurement predicts severe portal hypertension in patients with HCV-related cirrhosis. Hepatology 2007; 45:1290–7. [DOI] [PubMed] [Google Scholar]
- 13. Sánchez-Conde M, Montes Ramírez ML, Bellón Cano JM, et al. Impact of liver steatosis on the correlation between liver stiffness and fibrosis measured by transient elastography in patients coinfected with human immunodeficiency virus and hepatitis C virus. J Viral Hepat 2011; 18:e278–83. [DOI] [PubMed] [Google Scholar]
- 14. Carrión JA, Navasa M, Bosch J, et al. Transient elastography for diagnosis of advanced fibrosis and portal hypertension in patients with hepatitis C recurrence after liver transplantation. Liver Transpl 2006; 12:1791–8. [DOI] [PubMed] [Google Scholar]
- 15. Kazemi F, Kettaneh A, N’kontchou G, et al. Liver stiffness measurement selects patients with cirrhosis at risk of bearing large oesophageal varices. J Hepatol 2006; 45:230–5. [DOI] [PubMed] [Google Scholar]
- 16. Castéra L, Le Bail B, Roudot-Thoraval F, et al. Early detection in routine clinical practice of cirrhosis and oesophageal varices in chronic hepatitis C: comparison of transient elastography (FibroScan) with standard laboratory tests and non-invasive scores. J Hepatol 2009; 50:59–68. [DOI] [PubMed] [Google Scholar]
- 17. Pineda JA, Recio E, Camacho A, et al. Liver stiffness as a predictor of esophageal varices requiring therapy in HIV/hepatitis C virus-coinfected patients with cirrhosis. J Acquir Immune Defic Syndr 2009; 51:445–9. [DOI] [PubMed] [Google Scholar]
- 18. Malik R, Lai M, Sadiq A, et al. Comparison of transient elastography, serum markers and clinical signs for the diagnosis of compensated cirrhosis. J Gastroenterol Hepatol 2010; 25:1562–8. [DOI] [PubMed] [Google Scholar]
- 19. Nguyen-Khac E, Saint-Leger P, Tramier B, et al. Noninvasive diagnosis of large esophageal varices by Fibroscan: strong influence of the cirrhosis etiology. Alcohol Clin Exp Res 2010; 34:1146–53. [DOI] [PubMed] [Google Scholar]
- 20. Sánchez-Conde M, Miralles P, Bellón JM, et al. Use of transient elastography (FibroScan®) for the noninvasive assessment of portal hypertension in HIV/HCV-coinfected patients. J Viral Hepat 2011; 18:685–91. [DOI] [PubMed] [Google Scholar]
- 21. Pritchett S, Cardenas A, Manning D, et al. The optimal cut-off for predicting large oesophageal varices using transient elastography is disease specific. J Viral Hepat 2011; 18:e75–80. [DOI] [PubMed] [Google Scholar]
- 22. Stefanescu H, Grigorescu M, Lupsor M, et al. A new and simple algorithm for the noninvasive assessment of esophageal varices in cirrhotic patients using serum fibrosis markers and transient elastography. J Gastrointestin Liver Dis 2011; 20:57–64. [PubMed] [Google Scholar]
- 23. Montes Ramirez ML, Pascual-Pareja JF, Sánchez-Conde M, et al. Transient elastography to rule out esophageal varices and portal hypertensive gastropathy in HIV-infected individuals with liver cirrhosis. AIDS 2012; 26:1807–12. [DOI] [PubMed] [Google Scholar]
- 24. Hassan EM, Omran DA, El Beshlawey ML, et al. Can transient elastography, Fib-4, Forns Index, and Lok Score predict esophageal varices in HCV-related cirrhotic patients? Gastroenterol Hepatol 2014; 37:58–65. [DOI] [PubMed] [Google Scholar]
- 25. Klibansky DA, Mehta SH, Curry M, et al. Transient elastography for predicting clinical outcomes in patients with chronic liver disease. J Viral Hepat 2012; 19:e184–93. [DOI] [PubMed] [Google Scholar]
- 26. Pang JX, Zimmer S, Niu S, et al. Liver stiffness by transient elastography predicts liver-related complications and mortality in patients with chronic liver disease. PLoS One 2014; 9:e95776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kitson MT, Roberts SK, Colman JC, et al. Liver stiffness and the prediction of clinically significant portal hypertension and portal hypertensive complications. Scand J Gastroenterol 2015; 50:462–9. [DOI] [PubMed] [Google Scholar]
- 28. Masuzaki R, Tateishi R, Yoshida H, et al. Prospective risk assessment for hepatocellular carcinoma development in patients with chronic hepatitis C by transient elastography. Hepatology 2009; 49:1954–61. [DOI] [PubMed] [Google Scholar]
- 29. Merchante N, Rivero-Juárez A, Téllez F, et al. Liver stiffness predicts clinical outcome in human immunodeficiency virus/hepatitis C virus-coinfected patients with compensated liver cirrhosis. Hepatology 2012; 56:228–38. [DOI] [PubMed] [Google Scholar]
- 30. Fernández-Montero JV, Barreiro P, Vispo E, et al. Liver stiffness predicts liver-related complications and mortality in HIV patients with chronic hepatitis C on antiretroviral therapy. AIDS 2013; 27:1129–34. [DOI] [PubMed] [Google Scholar]
- 31. Pérez-Latorre L, Sánchez-Conde M, Rincón D, et al. Prediction of liver complications in patients with hepatitis C virus-related cirrhosis with and without HIV coinfection: comparison of hepatic venous pressure gradient and transient elastography. Clin Infect Dis 2014; 58:713–8. [DOI] [PubMed] [Google Scholar]
- 32. Crespo G, Lens S, Gambato M, et al. Liver stiffness 1 year after transplantation predicts clinical outcomes in patients with recurrent hepatitis C. Am J Transplant 2014; 14:375–83. [DOI] [PubMed] [Google Scholar]
- 33. Vergniol J, Foucher J, Terrebonne E, et al. Noninvasive tests for fibrosis and liver stiffness predict 5-year outcomes of patients with chronic hepatitis C. Gastroenterology 2011; 140:1970–9, 1979.e1–3. [DOI] [PubMed] [Google Scholar]
- 34. 1993 revised classification system for HIV infection and expanded surveillance case definition for AIDS among adolescents and adults. MMWR Recomm Rep 1992; 41:1–19. [PubMed] [Google Scholar]
- 35. Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc 1999; 94:496–509. [Google Scholar]
- 36. Berenguer J, Alvarez-Pellicer J, Carrero A, et al. Clinical effects of viral relapse after interferon plus ribavirin in patients co-infected with human immunodeficiency virus and hepatitis C virus. J Hepatol 2013; 58:1104–12. [DOI] [PubMed] [Google Scholar]
- 37. R Development Core Team. The R Project for Statistical Computing Available at: https://www.r-project.org/ Vienna, Austria: R Foundation for Statistical Computing; 2015. [Google Scholar]
- 38. Ripoll C, Groszmann R, Garcia-Tsao G, et al. Hepatic venous pressure gradient predicts clinical decompensation in patients with compensated cirrhosis. Gastroenterology 2007; 133:481–8. [DOI] [PubMed] [Google Scholar]
- 39. Singh S, Fujii LL, Murad MH, et al. Liver stiffness is associated with risk of decompensation, liver cancer, and death in patients with chronic liver diseases: a systematic review and meta-analysis. Clin Gastroenterol Hepatol 2013; 11:1573–84. e1–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Mederacke I, Wursthorn K, Kirschner J, et al. Food intake increases liver stiffness in patients with chronic or resolved hepatitis C virus infection. Liver Int 2009; 29:1500–6. [DOI] [PubMed] [Google Scholar]
- 41. Zipprich A, Garcia-Tsao G, Rogowski S, et al. Prognostic indicators of survival in patients with compensated and decompensated cirrhosis. Liver Int 2012; 32:1407–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Berenguer J, Alejos B, Hernando V, et al. Trends in mortality according to hepatitis C virus serostatus in the era of combination antiretroviral therapy. AIDS 2012; 26:2241–6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.