Abstract
This study aimed to evaluate differences in plasma and cerebrospinal fluid (CSF) levels of Aβ peptides in older adults with late-life depression compared to non-depressed older controls. We conducted a systematic review and meta-analysis of the literature using PubMed, Web of science and Scopus databases with no search limits for publication dates or languages. Two independent reviewers extracted data and assessed quality. Six hundred references were retrieved, and we included 12 studies in the meta-analysis after eligibility screening. Older adults with late-life depression (LLD) had a higher plasma Aβ40:Aβ42 ratio compared to non-depressed participants (SMD= 1.10, CI95% [0.28; 1.96], p=0.01), and marginally significant reduction of CSF Aβ42 levels (SMD= −1.12, CI95% [−2.47; 0.22], p=0.1). The present results evidence that older adults with depression have significant differences in Aβ metabolism, in the same direction observed in individuals with AD. These differences in the Aβ metabolism may help identify a subgroup of subjects with LLD at higher risk of developing AD.
Keywords: Late-Life Depression, dementia, amyloid-β, plasma, cerebrospinal fluid, meta-analysis
Introduction
Late-life depression (LLD) is a common disorder in the elderly and is associated with cognitive impairment and a higher risk of dementia, especially Alzheimer’s disease (AD) and vascular dementia (Byers and Yaffe, 2011; da Silva et al., 2013; Diniz et al., 2013). The mechanisms linking depression and the risk of dementia are unknown, but may involve abnormalities in multiple biological cascades, including the metabolism of amyloid-β (Aβ) peptide in the brain (Butters et al., 2008a). Neuroimaging studies using in vivo brain amyloid ligands show higher Aβ load in older adults with LLD, in particular, those with late-onset depression, compared with healthy controls (Butters et al., 2008b; Tateno et al., 2014). Nonetheless, other studies reported no significant differences in Aβ burden between LLD and healthy controls (Madsen et al., 2012) what is in line with recent neuropathological studies (Royall and Palmer, 2013; Wilson et al., 2014).
The metabolism of the amyloid precursor protein (APP) yields two common Aβ peptides, the Aβ40 and Aβ42, that can be readily measured in the cerebrospinal (CSF) and plasma (Blennow et al., 2010). Reduced CSF Aβ42 levels is associated with an increased risk of progression of mild cognitive impairment (MCI) to AD (Diniz et al., 2008). Previous clinico and epidemiological studies have reported that increased Aβ42 levels and lower plasma Aβ42:Aβ40 ratio can be an indicator of a higher risk of progression from MCI to AD; though other studies have also found no association between plasma Aβ biomarkers and increased risk of progression (Hansson et al., 2012; Fei et al., 2011; Koyama et al., 2012; Gabelle et al., 2013). Previous studies investigated the levels of Aβ peptides in the CSF and plasma of LLD patients. Pomara and colleagues (2006) showed that the CSF Aβ42 levels are reduced in older adults with depression compared to healthy controls. In contrast, other studies did not find significant differences or even found increased CSF Aβ42 levels in older depressed individuals (Gudmundsson et al., 2007; Kramberger et al., 2012; Reis et al., 2012). Other studies evaluated the plasma levels of Aβ peptides. Most studies found a significant reduction of plasma Aβ42 and increased Aβ40:Aβ42 ratio in LLD; although non-significant results have also been reported (Sun et al., 2008; Baba et al., 2012; Benitez et al., 2009; Kita et al., 2009).
The current knowledge about the dynamics of Aβ peptides in the CSF and plasma of LLD patients are limited by the small sample size of individual studies that are generally underpowered to detect small, but significant, group differences. Due to the importance of understanding the role of abnormalities of Aβ metabolism in LLD, and the lack of power of individual studies, we aim to carry out a meta-analysis on the levels of CSF and plasma Aβ peptides in LLD.
Methods
Search Strategy
This study followed the guidelines for conducting and reporting systematic reviews and meta-analysis methods proposed by the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) working group (Moher et al., 2009). We conducted a comprehensive literature search for potentially relevant studies in the electronic databases PubMed, Scopus and Web of Science. There were no search limits for publication language. We used the following string term for the literature search: “(depression OR depressive disorder OR major depressive disorder) AND (amyloid)”. Additionally, we carried out a manual search for relevant articles in the references of the original articles included in the meta-analysis, as well as in review articles about this subject. We conducted the literature search in January 2015, and all papers published until December 31, 2014 were included.
Study Selection, Data Extraction and Quality Assessment
We selected studies for data extraction and analysis based on the following criteria: (a) identification of depression “caseness”; (b) age over 50 years at baseline assessment for participants with major depression and controls; (c) assessment of human plasma and/or CSF of Aβ peptide levels (Aβ40, Aβ42, and/or Aβ40:Aβ42 ratio) in participants with depression as compared with participants without depression (regarded as controls in the original studies). Two investigators (K.K.F.N and K.S.P) independently reviewed the title and abstract of each article retrieved from the literature search to identify potentially relevant studies. The selected articles were revised to verify whether they fulfilled the inclusion criteria for data extraction. If there was any disagreement in study selection, a third investigator (B.S.D.) made the final decision on the inclusion of the selected article. If different publications reported data from the same population, we included data from the publication with the larger sample size.
Data was extracted by two independent investigators (K.K.F.N and K.S.P) using a standardized data extraction form. The following data were extracted for each study: year of publication, country, study design, depression assessment method, demographic variables, sample size and mean and standard deviation, or median and interquartile range, for each analyte. When the study provided only the median and interquartile range, we transformed these values into mean and standard deviation (Hozo et al., 2005). We used the Newcastle–Ottawa Scale (NOS) to assess the scientific method quality of each study selected for inclusion in the meta-analysis (Wells et al., 2013). This scale assesses methodological aspects of non-randomized observational studies such as selection criteria for inclusion of cases and controls, comparability of population ascertainment of exposure to risk, quality of case ascertainment and outcome assessment.
Statistical Analysis
We carried out the meta-analysis using the standardized mean difference (SMD) method with a Hedges’ correction for bias in small samples to evaluate differences between LLD and control subjects for plasma Aβ40, Aβ42 levels, and Aβ40:Aβ42 ratio; and for CSF Aβ40, Aβ42 levels (Hedges and Olkin 1985). We assessed heterogeneity in the analysis with the Q-test and I2 index. If the p-value was equal to or below 0.05 in the Q-test and/or the I2 index was higher than 50%, the pooled analysis was considered significantly heterogeneous. Random- or fixed-effect model was used based on the statistical evidence of heterogeneity. We performed sensitivity analyses by excluding one study at a time and recalculating the summary effect (i.e. ‘leave-one-out’ technique) to evaluate whether any individual study biased the result of the meta-analysis. Publication bias was ascertained by visual inspection of a funnel plot. All analyses were carried with the statistical software RevMan 5.1 for Windows 7 (The Nordic Cochrane Centre, Copenhagen, Denmark, http://ims.cochrane.org/revman/download).
Results
Study Selection and Description of Studies
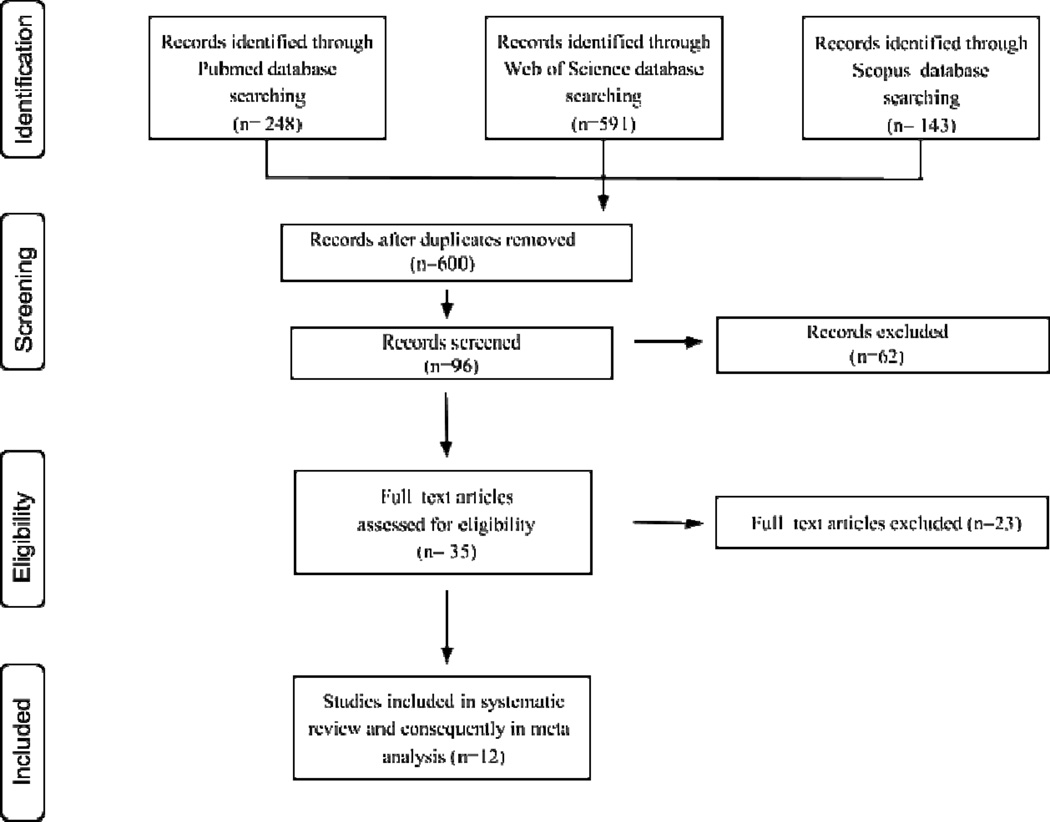
Two hundred forty-eight studies were retrieved from PubMed, 591 from the Web of Science and 143 from Scopus databases. After removing duplicate studies, we included 600 studies for revision. Twelve studies met all inclusion criteria and were included in the meta-analysis. The flowchart shows all steps for the study assessment and selection (figure 1). The main characteristics of studies included are summarized in Tables 1 and 2.
Figure 1.
Flow diagram of study search and selection for inclusion in the meta-analysis.
Table 1.
Characteristics of included studies in systematic review for plasma Aβ peptides levels.
| Study | Control Group (n) |
Mean Age ± SD |
Aβ40 (pg/mL) | Aβ42 (pg/mL) |
Aβ40Aβ 42 ratio |
LLD Group (n) | Mean Age ± SD |
Aβ40 (pg/mL) | Aβ42 (pg/mL) | Aβ40Aβ 42 ratio |
Method | DSM criteria |
Study setting |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Baba et al., 2012 | 160 | 69.7 ± 3.8 | 32.4 ± 4.9 | 3.1 ± 0.8 | 10.3 ± 1.6 | 64 | 72.0 ± 5.5 | 29.0 ± 4.6 | 2.2 ± 0.8 | 15.0 ± 3.9 | Elisa | yes | Clinical |
| Benitez et al., 2009 | 31 | 73.7 ± 6.6 | 90.2 ± 40.4 | - | - | 4 | 73.7 ± 6.6 | 120.6 ± 59.5 | - | - | Elisa | no | Clinical |
| Blasko et al., 2010 | 104 | 75.8 ± 0.5 | - | 62.8 ± 52.8 | - | 38 | 75.8 ± 0.5 | - | 87.8 ± 67.5 | - | Elisa | yes | Clinical |
| Kita et al., 2009 | 30 | 69.7 ± 4.7 | 28.6 ± 15.5 | 3.9 ± 2.8 | 9.4 ± 5.5 | 30 | 68.2 ± 5.6 | 28.0 ± 11.9 | 2.6 ± 2.6 | 16.9 ± 8.9 | Elisa | yes | Clinical |
| Namekawa et al., 2013 | 81 | 66.9 ± 4.9 | 24.3 ± 3.4 | 2.6 ± 0.5 | 9.2 ± 1.4 | 54 | 68.3 ± 5.0 | 25.1 ± 5.8 | 2.3 ± 0.6 | 10.8 ± 1.2 | Elisa | no | Clinical |
| Sun et al., 2008 | 647 | 76.0 ± 8.3 | 133.8 ± 19.0 | 20.3 ± 4.2 | 7.1 ± 1.6 | 348 | 73.8 ± 8.5 | 132.6 ± 20.9 | 18.5 ± 4.1 | 7.6 ± 1.7 | Elisa | yes | Clinical |
| Total number |
1052 | 538 |
Abbreviations: LLD: Late-Life Depression; ELISA: Enzyme-Linked Immunosorbent Assay; DSM: Diagnostic and Statistical Manual of Mental Disorders; CES-D: Centre for Epidemiological Studies - Depression Scale; HDRS: Hamilton Depression Rating Scale; GDS: Geriatric Depression Scale; PRIME-MD: Primary Care Evaluation of Mental Disorders
Table 2.
Characteristics of included studies in systematic review for CSF Aβ peptides levels.
| Study | Control Group (n) |
Mean Age ± SD |
Aβ40 (pg/mL) | Aβ42 (pg/mL) | LLD Group (n) |
Mean Age ± SD |
Aβ40 (pg/mL) | Aβ42 (pg/mL) | Method | DSM criteria |
Study setting |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Diniz et al., 2014 | 25 | 71.0 ± 3.7 | - | 464.7 ± 166.5 | 25 | 69.2 ± 5.5 | - | 462.1 ± 208.0 | Luminex | yes | Clinical |
| Gudmundsson et al., 2007 | 70 | 72.6 ± 3.1 | - | 794.0 ± 234.4 |
11 | 72.6 ± 3.1 | - | 973.3 ± 184.1 | Elisa | yes | Population-based |
| Hertze et al., 2010 | 38 | 77.0 ± 8.2 | 11036.0 ± 2613.0 |
1019.0 ± 435.0 |
28 | 58.0 ± 8.4 | 8235.0 ± 2535.0 |
862.0 ± 386.0 | Luminex | yes | Population-based |
| Kramberger et al., 2012 | 51 | 70.7 ± 6.3 | - | 883.0 ± 93.0 | 41 | 71.3 ± 6.1 | - | 504.0 ± 40.0 | Elisa | no | Clinical |
| Pomara et al., 2012 | 19 | 68.1 ± 7.3 | 6518.0 ± 2687.0 |
335.4 ± 182.7 | 28 | 66.5 ± 5.4 | 5146.0 ± 2369.0 |
224.7 ± 125.1 | Luminex | yes | Population- based |
| Reis et al., 2012) | 8 | 65.0 ± 5.6 | - | 818.8 ± 141.0 | 20 | 62.7 ± 3.0 | - | 639.6 ± 105.3 | Elisa | yes | Clinical |
| Total number | 211 | 153 |
Abbreviations: LLD: Late-Life Depression; ELISA: Enzyme-Linked Immunosorbent Assay; DSM: Diagnostic and Statistical Manual of Mental Disorders; CES-D: Centre for Epidemiological Studies - Depression Scale; HDRS: Hamilton Depression Rating Scale; MADRS: Montgomery–Asberg Depression Rating Scale; CPRS: Comprehensive Psychopathological Rating Scale.
Plasma Aβ peptides (Aβ40, Aβ42, and Aβ40:Aβ42 ratio)
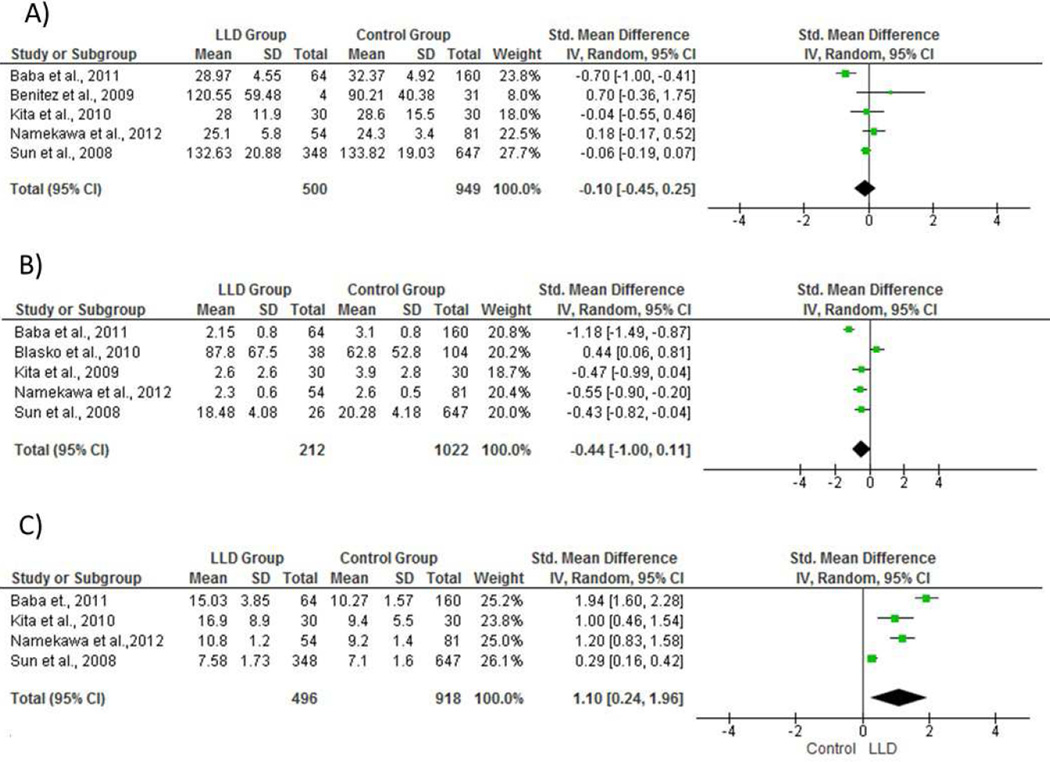
The LLD group had a higher Aβ40:Aβ42 ratio compared to non-depressed participants (SMD= 1.10, CI95% [0.28, 1.96], z=2.52, p=0.01; Q=92.50, p<0.00001; I2 =97%). We found no significant differences in plasma Aβ42 (SMD= −0.44, CI95% [−1.00, 0.11], z=1.57, p=0.1; Q=42.8, p<0.001; I2=91%) or plasma Aβ40 levels (SMD= −0.10, CI95% [−0.45, 0.25], z=0.54, p=0.59; Q=20.82, p=0.00003; I2 =81%) between groups.
Sensitivity analysis showed no significant effect of individual studies on results for plasma Aβ40:Aβ42 ratio or Aβ40 levels. On the other hand, sensitivity analysis showed that after the exclusion of Blasko et al. (2010) study, plasma Aβ42 levels were significantly reduced in the LLD group (SMD= −0.68 CI95% [−1.07, −0.29], z=3.42, p<0.001). This result suggests that the data from Blasko et al. (2010) is biasing the meta-analysis results for plasma Aβ42 levels.
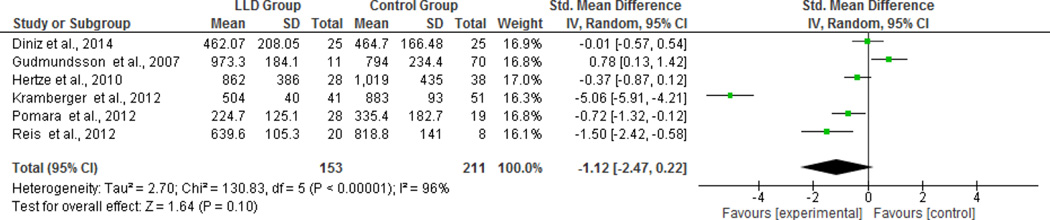
CSF peptide (Aβ40 and Aβ42)
As there were only two studies that evaluated CSF Aβ40 levels, we did not carry out a meta-analysis for this peptide. There was no significant difference in the CSF Aβ42 levels between groups (SMD= −1.12, CI95% [−2.47; 0.22], z=1.64 p=0.10; Q=130.83, p<0.00001; I2 =96%, figure 3). Nonetheless, sensitivity analysis revealed that after excluding one study (Gudmundsson et al., 2007) depressed participants had a marginally significant lower level of CSF Aβ42 compared to non-depressed participants (SMD= −1.51, CI95% [−3.00; −0.01], z=1.97, p=0.05, Q=107.32, p=0.00001; I2=96%). The visual inspection of funnel plots showed no evidence of publication bias for the CSF Aβ40 and Aβ42.
Figure 3.
Forest plot of CSF for individual studies and its respective weight for Aβ42 levels for all individual studies and its respective weight.
Additional Analysis
Age is one of the most important risk factor for AD, and there is evidence of a positive correlation between age and increased deposition of Aβ peptide in the brain of non-demented older adults. We carried out additional analysis to assess whether there were significant differences in the age of depressed and non-depressed subjects that could bias the results of the analyses. We found no significant differences in the age of depressed and non-depressed participants in the studies included in the meta-analysis of plasma Aβ peptides (SMD= 0.44, CI95% [−0.35; 1.22], z=1.09, p=0.28) or CSF Aβ (SMD= −0.67, CI95% [−1.40; 0.06], z=1.79, p=0.07).
Cognitive impairment is also an important risk factor for the development of AD in older adults with depression (Modrego and Ferrández, 2004; Aizenstein et al., 2011). However, only one study included in this meta-analysis specifically evaluated whether cognitive impairment in subjects with LLD was associated with differences in the AD-related biomarker in plasma or CSF (Diniz et al., 2014). Thus, it was not possible to carry out a meta-analysis on the influence of this risk factor.
Discussion
This present study showed that older adults with LLD have significantly different Aβ peptide metabolism, identified by a significantly lower level of plasma Aβ42 level and higher Aβ40:Aβ42 ratio. This pattern of differences in plasma Aβ peptides is similar to those observed in subjects with AD and those with MCI, who progress to AD upon follow-up (Forlenza et al., 2010). However, the magnitude of change in individuals with LLD is less than that observed in individuals with AD. Therefore, we can hypothesize that abnormalities in the Aβ metabolism may be one of the possible mechanisms by which a depressive episode in the elderly increases the risk of AD.
The measurement of Aβ in the plasma and CSF is a potential biomarker for the diagnosis of AD (Blennow et al., 2010). Low CSF levels of Aβ42, along with high total and phosphorylated tau protein may be helpful in differentiating AD from other dementia syndromes and can predict the conversion to from MCI to AD (Diniz et al., 2008). As depression in older adults may increase the risk of AD on follow-up, the systematic evaluation of AD-related biomarkers can help identify a subgroup of depressed older adults, perhaps over and above the presence of cognitive impairment, that are at increased risk of developing AD. It should be noted that depression is also a common feature of other dementia syndromes, as vascular dementia or Lewy body dementia (Ballard et al., 2000; Klatka et al., 1996). These syndromes have also been associated with altered levels of Aβ peptides in the CSF, though at a lesser degree compared to AD (Schoonenboom et al., 2012; Kaerst et al., 2014). Therefore, the current results can in part explain the increased association between depression and other dementia syndromes like vascular dementia or Lewy body dementia.
The Aβ peptides are the result of sequential proteolytic cleavage of the type I transmembrane amyloid precursor protein (APP) by β and γ secretase, producing various peptides with different lengths. The concentration of Aβ species in human plasma and CSF is Aβ40 (~90%) followed by Aβ42 (~10%). Despite the higher concentration of Aβ40, Aβ42 is a major constituent of amyloid plaques, what suggest that Aβ42 aggregation plays a critical role in plaque formation in AD (Iwatsubo et al., 1994; Tamaoka et al., 1994; Gravina et al., 1995; Mcgowan et al., 2005). The dynamics of Aβ peptides in the CSF is better understood than in plasma. CSF Aβ peptides have large diurnal variability, and its metabolism is significantly influenced by sleep-wake cycle disruptions (Bateman et al., 2006; Kang et al., 2009). Nonetheless, the dynamics and the pathological relevance of circulating Aβ species are less understood. Plasma Aβ peptides can be derived from CSF clearance or from APP metabolism in the platelets as they have the full biological machinery to cleave APP into Aβ peptides (Smith et al., 2009; Zainaghi et al., 2012). Plasma and CSF Aβ40 and Aβ42 have a weak to moderate correlation, though it is not clear whether plasma Aβ peptides can cross the blood-brain barrier (reverse transport), influencing CSF levels and deposit into neuritic plaques (Barten et al., 2005; Toledo et al., 2011; Huang et al., 2012). Thus, additional studies are necessary to improve knowledge about the dynamics and biological significance of plasma Aβ peptides in physiological and pathological conditions, to improve its use as diagnostic and prognostic markers in AD or LLD.
Depression and AD often co-occur and share some clinical symptoms such as memory impairment, executive dysfunction, and behavioral symptoms as apathy, suggesting that these disorders may share common pathophysiologic changes. A recent animal study showed that the injection of amyloid-β oligomers leads to depressive-like phenomena in mice (Ledo et al., 2013). Also, the deposition of Aβ in the brain leads to microglial activation, increased pro-inflammatory markers, and reduced neurotrophic support (Azevedo et al., 2013). These changes are observed in both AD and LLD subjects (Thorsell et al., 2010; Naismith et al., 2012). On the other hand, long-term antidepressant treatment can modulate Aβ metabolism (Sheline et al., 2014). Thus, the changes in Aβ metabolism may not only reflect the emergence of neurodegenerative changes in older adults with depression, but also, may reflect a primary pathophysiologic event in a subgroup of LLD subjects, i.e. the amyloid-related depression (Sun et al., 2008).
Other neurobiological abnormalities, in addition to abnormalities in the Aβ metabolism, can contribute to the higher risk of AD in older adults with major depression. Pomara and colleagues (Pomara et al., 2012) found higher F2-isoprostane CSF levels indicating pro-oxidative stress status in LLD. Kern and colleagues (Kern et al., 2014) found higher CSF levels of IL-6 and IL-8 in older adults with LLD suggesting older adults with LLD present with pro-inflammatory status. In a recent study, we found that despite no significant differences in AD-related biomarkers, older adults with LLD had significantly lower CSF BDNF levels compared to non depressed control subjects (Diniz et al., 2014). The reduction in CSF BDNF levels was greater in those with LLD and MCI. Finally, a recent data-driven proteomic-based study showed that cognitive impairment in LLD was associated with abnormalities in multiple biological pathways, e.g., regulation of the immune-inflammatory processes, lipid and protein metabolism, metabolic control, cell survival and neurotrophic support (Diniz et al., 2015). These changes have been reported in AD and may help to explain why LLD increases the risk of AD and other dementias in the elderly.
The present results should be viewed in light of some limitations. The individual study samples were very heterogeneous with relatively small samples of depressed and non-depressed subjects, with methodological difference in the diagnosis of depression, recruitment settings, and the laboratorial methods used to measure plasma and CSF Aβ peptides. Moreover, depression in older adults has a very heterogeneous clinical presentation that may reflect abnormalities in multiple biological pathways. These factors may have introduced significant heterogeneity in the meta-analysis, influencing its results. Nonetheless, we addressed study heterogeneity by using random effects models in all analyses. Also, due to the cross-sectional design of the included studies we could not evaluate whether plasma or CSF Aβ peptides can predict the risk of AD among older adults with depression. Some studies did not find a significant correlation between plasma and CSF Aβ peptides concentration or brain amyloid deposition (Mehta et al., 2001; Fagan et al., 2006), which limits the interpretation of the role of plasma in AD or LLD. Finally, the studies included in the meta-analysis, except one (Diniz et al., 2014), did not specifically address the impact of cognitive impairment co-occurring with depression on CSF or plasma levels of Aβ peptides. This is important since the comorbidity of depression and MCI significantly increases the risk of developing AD in older adults (Modrego and Ferrández, 2004; Aizenstein et al., 2011).
In conclusion, the present meta-analysis provides additional evidence that LLD is associated with significant changes in the metabolism of plasma and CSF Aβ peptide. The findings also provide insights into the potential role for Aβ in the pathophysiology of LLD. Population-based studies with systematic assessment of cognitive performance, are necessary to determine whether the systematic measurement of plasma and CSF Aβ peptides can identify a subgroup of older adults with major depression that are at increased risk for developing AD and the possibility to adjust for future dementias.
Figure 2.
Forest plot of plasma amyloid-β levels for individual studies and its respective weight for (A) Aβ40 levels (B) Aβ42 levels and (C) Aβ40:Aβ42 ratio levels
Acknowledgments
None
ROLE OF FUNDING SOURCES:
This work was partially funded by CNPq grant n° 472138/2013-8 and 466623/2014-3 (Dr. Diniz) and UFMG Intramural Research Grant (Dr. Diniz). Dr. Butters acknowledges support from the National Institutes of Health through Grant Numbers R01 MH080240, P30 MH090333 (ACISR), and UL1 TR000005 (CTSI). The funding sources did not have any influence in the data acquisition and analysis, interpretation of the findings, manuscript writing and in the decision to publish this study.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
CONFLICT OF INTEREST
The authors do not have any conflict of interest to report regarding this manuscript.
AUTHOR AND CONTRIBUTORS
All authors contributed with the study design, data analysis, interpretation of results, drafting and revising the manuscript.
REFERENCES
- Aizenstein HJ, Andreescu C, Edelman KL, Cochran JL, Price J, Butters MA, et al. fMRI correlates of white matter hyperintensities in late-life depression. Am J Psychiatry. 2011;168:1075–1082. doi: 10.1176/appi.ajp.2011.10060853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azevedo EP, Ledo JH, Barbosa G, Sobrinho M, Diniz L, Fonseca AC, et al. Activated microglia mediate synapse loss and short-term memory deficits in a mouse model of transthyretin-related oculoleptomeningeal amyloidosis. Cell Death Dis. 2013;4:e789. doi: 10.1038/cddis.2013.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baba H, Nakano Y, Maeshima H, Satomura E, Kita Y, Suzuki T, Arai H. Metabolism of amyloid-â protein may be affected in depression. J Clin Psychiatry. 2012;73:115–120. doi: 10.4088/JCP.10m06766. [DOI] [PubMed] [Google Scholar]
- Ballard C, Neill D, O'Brien J, McKeith IG, Ince P, Perry R. Anxiety, depression and psychosis in vascular dementia: prevalence and associations. J Affect Disord. 2000;59(2):97–106. doi: 10.1016/s0165-0327(99)00057-9. [DOI] [PubMed] [Google Scholar]
- Barten DM, Guss VL, Corsa JA, Loo A, Hansel SB, Zheng M, et al. Dynamics of {beta}-amyloid reductions in brain, cerebrospinal fluid, and plasma of {beta}-amyloid precursor protein transgenic mice treated with a {gamma}-secretase inhibitor. J Pharmacol Exp Ther. 2005;312:635–643. doi: 10.1124/jpet.104.075408. [DOI] [PubMed] [Google Scholar]
- Bateman RJ, Munsell LY, Morris JC, Swarm R, Yarasheski KE, Holtzman DM. Human amyloid-beta synthesis and clearance rates as measured in cerebrospinal fluid in vivo. Nat Med. 2006;12:856–861. doi: 10.1038/nm1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benitez A, Gunstad J, Hughes J, Glickman E, Alexander T, Spitznagel MB, et al. Troponin and S100 are associated with depression in healthy older adults. Aging Ment Health. 2009;13:894–898. doi: 10.1080/13607860903046438. [DOI] [PubMed] [Google Scholar]
- Blasko I, Kemmler G, Jungwirth S, Wichart I, Krampla W, Weissgram S, et al. Plasma amyloid beta-42 independently predicts both late-onset depression and Alzheimer disease. Am J Geriatr Psychiatry. 2010;18:973–982. doi: 10.1097/JGP.0b013e3181df48be. [DOI] [PubMed] [Google Scholar]
- Blennow K, Hampel H, Weiner M, Zetterberg H. Cerebrospinal fluid and plasma biomarkers in Alzheimer disease. Nat Rev Neurol. 2010;6(3):131–144. doi: 10.1038/nrneurol.2010.4. [DOI] [PubMed] [Google Scholar]
- Butters MA, Young JB, Lopez O, Aizenstein HJ, Mulsant BH, Reynolds CF, 3rd, et al. Pathways linking late-life depression to persistent cognitive impairment and dementia. Dialogues Clin Neurosci. 2008a;10(3):345–357. doi: 10.31887/DCNS.2008.10.3/mabutters. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butters MA, Klunk WE, Mathis CA, Price JC, Ziolko SK, Hoge JA, et al. Imaging Alzheimer pathology in late-life depression with PET and Pittsburgh Compound-B. Alzheimer Dis Assoc Disord. 2008b;22:261–268. doi: 10.1097/WAD.0b013e31816c92bf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byers AL, Yaffe K. Depression and risk of developing dementia. Nat Rev Neurol. 2011;7(6):323–331. doi: 10.1038/nrneurol.2011.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- da Silva J, Gonçalves-Pereira M, Xavier M, Mukaetova-Ladinska EB. Affective disorders and risk of developing dementia: systematic review. Br J Psychiatry. 2013;202:177–186. doi: 10.1192/bjp.bp.111.101931. [DOI] [PubMed] [Google Scholar]
- Diniz BS, Butters MA, Albert SM, Dew MA, Reynolds CF. Late-life depression and risk of vascular dementia and Alzheimer's disease: systematic review and meta-analysis of community-based cohort studies. Br J Psychiatry. 2013;202:329–335. doi: 10.1192/bjp.bp.112.118307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diniz BS, Pinto Júnior JA, Forlenza OV. Do CSF total tau, phosphorylated tau, beta-amyloid 42 help to predict progression of mild cognitive impairment to Alzheimer's disease? A systematic review and meta-analysis of the literature. World J Biol Psychiatry. 2008;9:172–182. doi: 10.1080/15622970701535502. [DOI] [PubMed] [Google Scholar]
- Diniz BS, Sibille E, Ding Y, Tseng G, Aizenstein HJ, Lotrich F, et al. Plasma biosignature and brain pathology related to persistent cognitive impairment in late-life depression. Mol Psychiatry. 2015;20:594–601. doi: 10.1038/mp.2014.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diniz BS, Teixeira AL, Machado-Vieira R, Talib LL, Radanovic M, Gattaz WF, Forlenza OV. Reduced Cerebrospinal Fluid Levels of Brain-Derived Neurotrophic Factor Is Associated With Cognitive Impairment in Late-Life Major Depression. J Gerontol B Psychol Sci Soc Sci. 2014;69:845–851. doi: 10.1093/geronb/gbu096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagan AM, Mintun MA, Mach RH, Lee SY, Dence CS, Shah AR, et al. Inverse relation between in vivo amyloid imaging load and cerebrospinal fluid Abeta42 in humans. Ann Neurol. 2006;59:512–519. doi: 10.1002/ana.20730. [DOI] [PubMed] [Google Scholar]
- Fei M, Jianghua W, Rujuan M, Wei Z, Qian W. The relationship of plasma Aβ levels to dementia in aging individuals with mild cognitive impairment. J Neurol Sci. 2011;305(1–2):92–96. doi: 10.1016/j.jns.2011.03.005. [DOI] [PubMed] [Google Scholar]
- Forlenza OV, Diniz BS, Gattaz WF. Diagnosis and biomarkers of predementia in Alzheimer's disease. BMC Med. 2010:89. doi: 10.1186/1741-7015-8-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabelle A, Richard F, Gutierrez LA, Schraen S, Delva F, Rouaud O, et al. Plasma amyloid-β levels and prognosis in incident dementia cases of the 3-City Study. J Alzheimers Dis. 2013;33(2):381–391. doi: 10.3233/JAD-2012-121147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gravina SA, Ho L, Eckman CB, Long KE, Otvos L, Younkin LH, Suzuki N, Younkin SG. Amyloid beta protein (A beta) in Alzheimer's disease brain. Biochemical and immunocytochemical analysis with antibodies specific for forms ending at A beta 40 or A beta 42(43) J Biol Chem. 1995;270:7013–7016. doi: 10.1074/jbc.270.13.7013. [DOI] [PubMed] [Google Scholar]
- Gudmundsson P, Skoog I, Waern M, Blennow K, Palsson S, Rosengren L, Gustafson D. The relationship between cerebrospinal fluid biomarkers and depression in elderly women. Am J Geriatr Psychiatry. 2007;15:832–838. doi: 10.1097/JGP.0b013e3180547091. 2007. [DOI] [PubMed] [Google Scholar]
- Hansson O, Stomrud E, Vanmechelen E, Östling S, Gustafson DR, Zetterberg H, Blennow K, Skoog I. Evaluation of plasma Aβ as predictor of Alzheimer's disease in older individuals without dementia: a population-based study. J Alzheimers Dis. 2012;28(1):231–238. doi: 10.3233/JAD-2011-111418. [DOI] [PubMed] [Google Scholar]
- Hedges L, Olkin I. Statistical methods for meta-analysis. San Diego, CA: Academic Press; 1985. [Google Scholar]
- Hertze J, Minthon L, Zetterberg H, Vanmechelen E, Blennow K, Hansson O. Evaluation of CSF biomarkers as predictors of Alzheimer's disease: A clinical follow-up study of 4.7 years. J Alzheimer Dis. 2010;21:1119–1128. doi: 10.3233/jad-2010-100207. [DOI] [PubMed] [Google Scholar]
- Hozo SP, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol. 2005;5:13. doi: 10.1186/1471-2288-5-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y, Potter R, Sigurdson W, Kasten T, Connors R, Morris JC, et al. β-amyloid dynamics in human plasma. Arch Neurol. 2012;69:1591–1597. doi: 10.1001/archneurol.2012.18107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwatsubo T, Odaka A, Suzuki N, Mizusawa H, Nukina N, Ihara Y. Visualization of A beta 42(43) and A beta 40 in senile plaques with end-specific A beta monoclonals: evidence that an initially deposited species is A beta 42(43) Neuron. 1994;13:45–53. doi: 10.1016/0896-6273(94)90458-8. [DOI] [PubMed] [Google Scholar]
- Kaerst L, Kuhlmann A, Wedekind D, Stoeck K, Lange P, Zerr I. Using cerebrospinal fluid marker profiles in clinical diagnosis of dementia with Lewy bodies, Parkinson's disease, and Alzheimer's disease. J Alzheimers Dis. 2014;38(1):63–73. doi: 10.3233/JAD-130995. [DOI] [PubMed] [Google Scholar]
- Kang JE, Lim MM, Bateman RJ, Lee JJ, Smyth LP, Cirrito JR, et al. Amyloid-beta dynamics are regulated by orexin and the sleep-wake cycle. Science. 2009;326:1005–1007. doi: 10.1126/science.1180962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kern S, Skoog I, Börjesson-Hanson A, Blennow K, Zetterberg H, Ostling S, et al. Higher CSF interleukin-6 and CSF interleukin-8 in current depression in older women. Results from a population-based sample. Brain Behav Immun. 2014;41:55–58. doi: 10.1016/j.bbi.2014.05.006. [DOI] [PubMed] [Google Scholar]
- Kita Y, Baba H, Maeshima H, Nakano Y, Suzuki T, Arai H. Serum amyloid beta protein in young and elderly depression: a pilot study. Psychogeriatrics. 2009;9:180–185. doi: 10.1111/j.1479-8301.2009.00293.x. [DOI] [PubMed] [Google Scholar]
- Klatka LA, Louis ED, Schiffer RB. Psychiatric features in diffuse Lewy body disease: a clinicopathologic study using Alzheimer's disease and Parkinson's disease comparison groups. Neurology. 1996;47(5):1148–1152. doi: 10.1212/wnl.47.5.1148. [DOI] [PubMed] [Google Scholar]
- Koyama A, Okereke OI, Yang T, Blacker D, Selkoe DJ, Grodstein F. Plasma amyloid-β as a predictor of dementia and cognitive decline: a systematic review and meta-analysis. Arch Neurol. 2012;69:824–831. doi: 10.1001/archneurol.2011.1841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramberger MG, Jelic V, Kareholt I, Enache D, Eriksdotter Jonhagen M, et al. Cerebrospinal Fluid Alzheimer Markers in Depressed Elderly Subjects with and without Alzheimer's Disease. Dement Geriatr Cogn Dis. 2012;2:48–56. doi: 10.1159/000334644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ledo JH, Azevedo EP, Clarke JR, Ribeiro FC, Figueiredo CP, Foguel D, et al. Amyloid-β oligomers link depressive-like behavior and cognitive deficits in mice. Mol Psychiatry. 2013;18:1053–1054. doi: 10.1038/mp.2012.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madsen K, Hasselbalch BJ, Frederiksen KS, Haahr ME, Gade A, Law I, et al. Lack of association between prior depressive episodes and cerebral [11C]PiB binding. Neurobiol Aging. 2012;33(10):2334–2342. doi: 10.1016/j.neurobiolaging.2011.11.021. [DOI] [PubMed] [Google Scholar]
- McGowan E, Pickford F, Kim J, Onstead L, Eriksen J, Yu C, et al. Abeta42 is essential for parenchymal and vascular amyloid deposition in mice. Neuron. 2005;47:191–199. doi: 10.1016/j.neuron.2005.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta PD, Pirttila T, Patrick BA, Barshatzky M, Mehta SP. Amyloid beta protein 1–40 and 1–42 levels in matched cerebrospinal fluid and plasma from patients with Alzheimer disease. Neurosci Lett. 2001;18(304):102–106. doi: 10.1016/s0304-3940(01)01754-2. 2001. [DOI] [PubMed] [Google Scholar]
- Modrego PJ, Ferrández J. Depression in patients with mild cognitive impairment increases the risk of developing dementia of Alzheimer type: a prospective cohort study. Arch Neurol. 2004;61:1290–1293. doi: 10.1001/archneur.61.8.1290. [DOI] [PubMed] [Google Scholar]
- Moher D, Liberati A, Tetzlaff J, Altman DG, Group P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. J Clin Epidemiol. 2009;62:1006–1012. doi: 10.1016/j.jclinepi.2009.06.005. [DOI] [PubMed] [Google Scholar]
- Naismith SL, Norrie LM, Mowszowski L, Hickie IB. The neurobiology of depression in later-life: clinical, neuropsychological, neuroimaging and pathophysiological features. Prog Neurobiol. 2012;98:99–143. doi: 10.1016/j.pneurobio.2012.05.009. [DOI] [PubMed] [Google Scholar]
- Namekawa Y, Baba H, Maeshima H, Nakano Y, Satomura E, Takebayashi N, et al. Heterogeneity of elderly depression: Increased risk of Alzheimer's disease and A beta protein metabolism. Prog Neuropsychopharmacol Biol Psychiatry. 2013;43:203–208. doi: 10.1016/j.pnpbp.2012.12.016. [DOI] [PubMed] [Google Scholar]
- Pomara N, Bruno D, Sarreal AS, Hernando RT, Nierenberg J, Petkova E, et al. Lower CSF amyloid beta peptides and higher F2-isoprostanes in cognitively intact elderly individuals with major depressive disorder. Am J Psychiatry. 2012;169:523–530. doi: 10.1176/appi.ajp.2011.11081153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pomara N, Doraiswamy PM, Willoughby LM, Roth AE, Mulsant BH, Sidtis JJ, et al. Elevation in plasma Abeta42 in geriatric depression: A pilot study. Neurochem Res. 2006;31:341–349. doi: 10.1007/s11064-005-9029-z. [DOI] [PubMed] [Google Scholar]
- Reis T, Brandao CO, Coutinho ESF, Engelhardt E, Laks J. Cerebrospinal Fluid Biomarkers in Alzheimer's Disease and Geriatric Depression: Preliminary Findings from Brazil. CNS Neurosci Ther. 2012;18:524–529. doi: 10.1111/j.1755-5949.2012.00311.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Royall DR, Palmer RF. Alzheimer's disease pathology does not mediate the association between depressive symptoms and subsequent cognitive decline. Alzheimers Dement. 2013;9:318–325. doi: 10.1016/j.jalz.2011.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoonenboom NS, Reesink FE, Verwey NA, Kester MI, Teunissen CE, van de Ven PM, et al. Cerebrospinal fluid markers for differential dementia diagnosis in a large memory clinic cohort. Neurology. 2012;78(1):47–54. doi: 10.1212/WNL.0b013e31823ed0f0. doi: [DOI] [PubMed] [Google Scholar]
- Sheline YI, West T, Yarasheski K, Swarm R, Jasielec MS, Fisher JR, Ficker WD, Yan P, Xiong C, Frederiksen C, et al. An antidepressant decreases CSF Aβ production in healthy individuals and in transgenic AD mice. Sci Transl Med. 2014;6:236re4. doi: 10.1126/scitranslmed.3008169. 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith CC, Prichard BN, Cooper MB. Platelet alpha- and beta-secretase activities: A preliminary study in normal human subjects. Platelets. 2009;20:29–34. doi: 10.1080/09537100802334434. [DOI] [PubMed] [Google Scholar]
- Strozyk D, Blennow K, White LR, Launer LJ. CSF Abeta 42 levels correlate with amyloid-neuropathology in a population-based autopsy study. Neurology. 2003;60:652–656. doi: 10.1212/01.wnl.0000046581.81650.d0. [DOI] [PubMed] [Google Scholar]
- Sun X, Steffens DC, Au R, Folstein M, Summergrad P, Yee J, et al. Amyloid-associated depression: a prodromal depression of Alzheimer disease? Arch Gen Psychiatry. 2008;65:542–550. doi: 10.1001/archpsyc.65.5.542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamaoka A, Kondo T, Odaka A, Sahara N, Sawamura N, Ozawa K, et al. Biochemical evidence for the long-tail form (A beta 1–42/43) of amyloid beta protein as a seed molecule in cerebral deposits of Alzheimer's disease. Biochem Biophys Res Commun. 1994;205:834–842. doi: 10.1006/bbrc.1994.2740. [DOI] [PubMed] [Google Scholar]
- Tateno A, Sakayori T, Higuchi M, Suhara T, Ishihara K, Kumita S, et al. Amyloid imaging with [(18) F]florbetapir in geriatric depression: early-onset versus late-onset. Int J Geriatr Psychiatry. 2014 Oct 21; doi: 10.1002/gps.4215. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Thorsell A, Bjerke M, Gobom J, Brunhage E, Vanmechelen E, Andreasen N, et al. Neurogranin in cerebrospinal fluid as a marker of synaptic degeneration in Alzheimer's disease. Brain Res. 2010;1362:13–22. doi: 10.1016/j.brainres.2010.09.073. [DOI] [PubMed] [Google Scholar]
- Toledo JB, Vanderstichele H, Figurski M, Aisen PS, Petersen RC, Weiner MW, et al. Factors affecting Aβ plasma levels and their utility as biomarkers in ADNI. Acta Neuropathol. 2011;122:401–413. doi: 10.1007/s00401-011-0861-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells GASB, O'Connell D, Peterson J, Welch V, Losos M, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality if nonrandomized studies in meta-analyses. 2013 http://www.ohri.ca/programs/clinical_epidemiology/oxford.htm. [Google Scholar]
- Wilson RS, Capuano AW, Boyle PA, Hoganson GM, Hizel LP, Shah RC, Nag S, Schneider JA, Arnold SE, Bennett DA. Clinical-pathologic study of depressive symptoms and cognitive decline in old age. Neurology. 2014;83:702–709. doi: 10.1212/WNL.0000000000000715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zainaghi IA, Talib LL, Diniz BS, Gattaz WF, Forlenza OV. Reduced platelet amyloid precursor protein ratio (APP ratio) predicts conversion from mild cognitive impairment to Alzheimer's disease. J Neural Transm. 2012;119:815–819. doi: 10.1007/s00702-012-0807-x. [DOI] [PubMed] [Google Scholar]