Abstract
Patients with chronic kidney disease (CKD) are frequently afflicted with neurological complications. These complications can potentially affect both the central and peripheral nervous systems. Common neurological complications in CKD include stroke, cognitive dysfunction, encephalopathy, peripheral and autonomic neuropathies. These conditions have significant impact not only on patient morbidity but also on mortality risk through a variety of mechanisms. Understanding the pathophysiological mechanisms of these conditions can provide insights into effective management strategies for neurological complications. This review describes clinical management of neurological complications in CKD with reference to the contributing physiological and pathological derangements. Stroke, cognitive dysfunction and dementia share several pathological mechanisms that may contribute to vascular impairment and neurodegeneration. Cognitive dysfunction and dementia may be differentiated from encephalopathy which has similar contributing factors but presents in an acute and rapidly progressive manner and may be accompanied by tremor and asterixis. Recent evidence suggests that dietary potassium restriction may be a useful preventative measure for peripheral neuropathy. Management of painful neuropathic symptoms can be achieved by pharmacological means with careful dosing and side effect considerations for reduced renal function. Patients with autonomic neuropathy may respond to sildenafil for impotence. Neurological complications often become clinically apparent at end-stage disease, however early detection and management of these conditions in mild CKD may reduce their impact at later stages.
Keywords: Chronic kidney disease, neurological complications, uraemic neuropathy, uraemic encephalopathy, cognitive dysfunction, peripheral neuropathy, autonomic neuropathy
Introduction
Chronic kidney disease (CKD) is a significant global health concern with a prevalence of ∼15% in developed countries.1 CKD is defined as decreased kidney function that persists for three or more months and encompasses a continuum of disease from mild kidney damage to end-stage disease. Disease severity is classified using a five-stage system (Table 1) based on the estimated glomerular filtration rate (eGFR), a calculation of waste cleared by the kidneys per minute.2 The aetiology of CKD may be due to a primary renal disorder or as a complication of a multisystem disorder related to comorbidities, such as diabetes which is now the leading cause of CKD worldwide. Irrespective of cause, neurological complications are highly prevalent in CKD. Injury can occur at all levels of the nervous system including central nervous system (CNS) disorders such as stroke, cognitive dysfunction and encephalopathy, through to peripheral nervous system (PNS) conditions such as autonomic and peripheral neuropathies. The presence of these complications has a significant impact on patient morbidity and mortality. As such, clinical management of neurological complications in CKD requires an understanding of the contributing physiological and pathological derangements. While neurological complications often become clinically apparent in end-stage disease, detection and management of these conditions in earlier stages of CKD may reduce their impact at later stages. This review will discuss common neurological complications in CKD with reference to their functional presentation, classified as either ‘altered metal status’ or ‘physical disability’. Potential causes of these functional disorders will then be discussed along with current recommendations for clinical care.
Table 1.
Classification of chronic kidney disease.
| Stage 1 | Evidence of kidney damage with normal eGFR >90 mL/min/1.73 m2 |
| Stage 2 | Evidence of kidney damage with mild reduction of eGFR 60–89 mL/min/1.73 m2 |
| Stage 3 | Moderately reduced eGFR 30–59 mL/min/1.73 m2 |
| Stage 4 | Severely reduced eGFR 15–29 mL/min/1.73 m2 |
| Stage 5 | Renal failure or dialysis eGFR <15 mL/min/1.73 m2 |
Classification as defined by the KDOQI Clinical Practice Guidelines.2
Methods
The information contained in this review was collected from recent peer-reviewed literature related to the neurological complications that occur in the context of CKD. Aspects of interest were epidemiology, pathophysiology, diagnosis and management options. Where appropriate, current recommended clinical guidelines were referenced for the diagnosis and treatment of complications.
Altered mental status
CKD patients are susceptible to a variety of conditions affecting the CNS. From a functional standpoint, CNS disorders typically manifest as altered mental status and can be clinically subdivided into chronic or acute presentation (Table 2).
Table 2.
Summary of neurological complications and potential contributors.
| Condition | Presentation | Contributing factors | Distinguishing tests | Treatments | |
|---|---|---|---|---|---|
| Altered mental status | |||||
| Chronic | Stroke Brain, spinal cord, or retinal cell death attributable to ischemia, based on neuropathological, neuroimaging, and/or clinical evidence of permanent injury. Silent infarction – causes no known symptoms | Neurological deficit will depend on the brain region affected and the type of stroke but features may include: headache, nausea, vertigo, altered mental status, altered vision, aphasia, weakness, facial droop, paralysis. Asymptomatic Mild cognitive impairment | Common between stroke, cognitive impairment and dementia Direct effects of Uraemia Vascular damage, endothelial dysfunction, inflammation, oxidative stress, anaemia, hyperhomocysteinaemia, hypercoagulable states, parathyroid hormone, guanidine compounds, cystatin-C Secondary causes Hypertension, dyslipidaemia, atrial fibrillation, diabetes, increased age, smoking status, secondary hyperparathyroidism Iatrogenic causes EPO-stimulating agents Dialysis via perfusion-related insults | Imaging: CT or MRI Common features include: silent cerebral infarcts, cerebral microbleeds, white matter abnormalities or atrophy | Acute; see Dad and Weiner12 Long-term reduce risk factors: Hypertension Lipid profile Anaemia* Atrial fibrillation |
| Cognitive impairment A new deficit in two or more areas of cognitive function | Altered memory, executive function, attention, concentration, perception and/or language skills | MMSE | As above Renal Tx | ||
| Dementia Persistent cognitive decline and behavioural disturbance | As above with a severity that interferes with independence and daily functioning | Formal Neuro-psychological assessment | Patient/ family education and support plans | ||
| Dialysis dementia Progressive dementia related to chronic Aluminium exposure | Dysarthria, dysphasia, dysgraphia, apathy and depression progressing to convulsions, psychosis and frank dementia | Iatrogenic cause: Chronic exposure to aluminium in dialysis water over years. Now rare due to routine removal of aluminium from dialysis water using reverse osmosis. | Progressive when untreated. | ||
| Acute | Encephalopathy Altered brain function induced by an agent or condition Posterior Reversible Encephalopathy Syndrome (PRES) | Altered metal status sometimes accompanied by generalised or focal motor disturbances. Altered mental status: sensorial clouding, delirium, fatigue, apathy, impaired concentration. Motor disturbances: tremor, fasciculations, asterixis. Late signs: hallucinations, seizures, coma. | Direct effects of Uraemia Uraemic metabolites, guanidino compounds, parathyroid hormone, fluid and electrolyte disturbances Secondary Causes Hypertension Thiamine deficiency Iatrogenic Causes EPO induced hypertension, Polypharmacy, Transplant rejection and Acute aluminium induced encephalopathy (may occur in dialysis patients given desferrioxamine as a chelating agent) | Blood tests including complete blood count, electrolyte panel, glucose, urea, vitamin B12, folic acid, thyroid function, liver enzymes and ammonia. EEG Imaging | Rectify underlying cause. Dialysis to normalise uraemia. Normalise overt hypertension. Vitamins for thiamine deficiency. |
| Dialysis disequilibrium syndrome Cerebral oedema during or after dialysis | Mild: Headache, nausea, disorientation, dizziness, restlessness, blurred vision and/or asterixis Severe: Seizures, central pontine demyelination, coma | Iatrogenic cause: Rapid changes in urea and other osmolites induced by dialysis and causing cerebral oedema. Most common in ESKD patients following initiation of dialysis treatment or following a sudden change of dialysis regime. | DOE During or immediately after dialysis. Papilloedema CT or MRI | Preventative: gradual reduction in BUN reduced duration and blood flow rate. | |
| Physical disability | |||||
| Chronic | Peripheral neuropathy Disease or death of the peripheral nerves | Altered sensation such as: numbness, paraesthesia and pain progressing to weakness and wasting maximal distally with greater involvement of lower limbs than upper. | Direct effects of uraemia Uraemic metabolites, Potassium Secondary causes Diabetes, peripheral vascular disease Iatrogenic causes Immunosuppressive medications (CNI) | Nerve conduction studies Stocking and glove distribution. Chronic disease course | Potassium restriction Pharmacological pain management |
| Autonomic neuropathy Disease or death of autonomic nerves | Orthostatic intolerance, syncope, brady-or-tachyarrhythmia, palpitations, nausea, pallor, reduced capacity for exercise, impotence, bladder and/or bowel dysfunction, thermoregulatory and secretomotor abnormalities. | Direct effects of uraemia Cardiovascular autonomic dysfunction is thought to be independent of uraemia-related toxins Secondary causes Hypertension, diabetes | Cardiac and pupillary reflexes, sudomotor function and blood pressure control. Heart rate variability | Renal Tx Midodrine for intradialytic hypotension Sildenafil for impotence | |
| Myopathy Disease of muscle tissue | Proximal muscle weakness in the muscles of the lower limb Reduced endurance and capacity for exercise | Direct effects of uraemia Uraemic metabolite, hyperparathyroidism, impaired potassium regulation, carnitine deficiency, oxidative stress, metabolic bone disease with vitamin D deficiency Secondary causes Diabetes | DOE on clinical neurological examination | Adequate dialysis Exercise program Nutrition | |
CT: computed tomography; MRI: magnetic resonance imaging; EPO: erythropoiesis; MMSE: Mini-Mental State Examination; Tx: transplant; PRES: posterior reversible encephalopathy syndrome; DOE: diagnosis of exclusion; EEG: electroencephalogram; ESKD: end-stage kidney disease; CNI: calcineurin inhibitor. Anaemia*: correction of anaemia within the KDOQI guidelines of haemoglobin levels between 11 and 12 g/dl.
Chronic manifestations of altered mental status
Causes of chronic alterations of mental status in CKD patients typically include stroke, cognitive impairment and dementia. These disorders are manifestations of chronic CNS injury and as such they share several pathophysiological mechanisms.
Pathophysiology
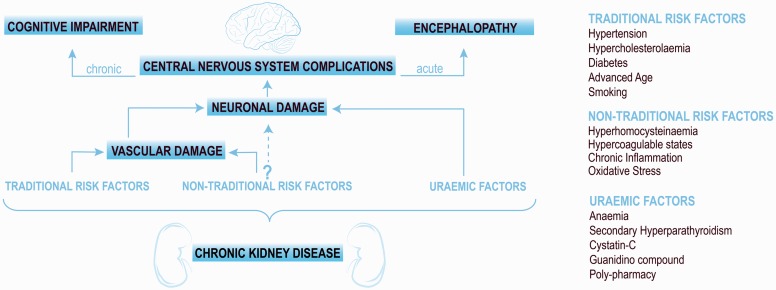
CNS injury in CKD is likely to be multifactorial (Figure 1). However, the proposed mechanisms may be subdivided into two emerging hypotheses, namely vascular and neurodegenerative.
Figure 1.
Flow chart of the exposures in CKD associated with central nervous system damage.
Vascular hypothesis
CKD populations present with a high prevalence of traditional cardiovascular risk factors such as hypertension, hypercholesterolaemia, diabetes, increased age and smoking status.3 These risk factors are often considered to be the primary cause of vascular injury in this cohort. However, vascular damage may also influenced and/or accelerated by non-traditional risk factors common in CKD such as metabolic disorders, particularly related to calcium and phosphate, hyperhomocysteinaemia, hypercoagulable states, inflammation and oxidative stress3 (Figure 1). These influences are thought to exacerbate endothelial dysfunction and accelerate atherosclerosis. Failure of phosphate excretion leading to elevated serum phosphate, calcium-phosphate product and parathyroid hormone (PTH) lead to accelerated vascular calcification. This milieu is particularly damaging to the vasculature of the brain and kidneys as they are both low resistance end organs that are exposed to high volume blood flow.
Further support for a vascular aetiology of CNS injury in CKD is evidenced by the high prevalence of cerebrovascular disease in the form of stroke, intracerebral microbleeds and white matter disease in dialysis patients. Even in non-dialysis CKD patients, imaging studies consistently demonstrate greater evidence of silent infarcts, white matter disease and atrophy than the general population. While vascular injury plays an important role in the pathology of cognitive impairment in CKD, it only partially explains the high prevalence of cognitive disorders in CKD.
Neurodegenerative hypothesis
It has long been recognised that uraemic toxins are likely to contribute to CNS injury either directly or indirectly (Figure 1). Indirect effects of the uraemic milieu include their contribution to systemic inflammation, endothelial dysfunction and atherosclerosis as mentioned above. Many uraemic toxins have been investigated for a possible role in direct neurotoxicity in the context of CKD. Cerebrorenal interactions have been suggested for several compounds including uric acid, indoxyl sulphate, p-cresyl sulphate, interleukin-1β (IL-1β), IL-6 and tumour necrosis factor-α (TNF-α).4 Recent studies have demonstrated that elevated serum levels of cystatin-C are associated with lower cognitive scores independent of age, race, education and medical comorbidities.5 It is hypothesized that cystatin-C may contribute to neurodegeneration through amyloid plaque formation although further longitudinal studies are required.5
Anaemia may have an important role in mediating both cognitive dysfunction and increasing the risk of stroke in CKD patients. It is hypothesized that anaemia leads to reduced delivery of oxygen and subsequent altered brain metabolism. However, correction of anaemia with erythropoietin-stimulating agents has yielded conflicting results on cognition and has in fact worsened the risk of stroke.6 Secondary hyperparathyroidism has also been identified as a potential risk factor for cognitive impairment in CKD.7 It is postulated that elevated PTH levels interfere with neurotransmission in the CNS by increasing brain calcium content.
Stroke
Stroke is defined as ‘brain, spinal cord, or retinal cell death attributable to ischemia, based on neuropathological, neuroimaging, and/or clinical evidence of permanent injury’.8 In long-term dialysis patients stroke has a prevalence of 17% compared to 10% for non-dialysis CKD patients and 4% for the general population.9 In addition, post-stroke outcomes in dialysis patients are poor with mortality rates 3–5 times higher than non-CKD patients.10
Recent meta-analysis data have confirmed that there is a strong inverse relationship between renal function and stroke with a 7% increase in risk per 10 mL/min decline in eGFR.11 As such, non-dialysis CKD patients are also at a heightened risk of stroke. This risk is related partially to the over-representation of risk factors such as hypertension, hypercholesterolaemia, atrial fibrillation, bleeding diatheses and blood vessel wall fragility as well as CKD specific processes such as anaemia, bone mineral disorder and dialysis itself.10
Brain lesions and subclinical cerebrovascular disease are also highly prevalent in this cohort with ∼50% of advanced CKD patients estimated to have evidence of silent brain infarcts on magnetic resonance imaging (MRI).9
Diagnosis and management
Clinical features of stroke will depend on the brain region affected and the type of stroke but may include: headache, nausea, vertigo, altered mental status, altered vision, aphasia, weakness, facial droop and paralysis. Prompt recognition, early assessment and admission to dedicated stroke units and rapid initiation of treatments are crucial elements to achieve optimal outcomes.
Few studies have examined the acute treatment of stroke specifically in patients with CKD (see Arnold et al.10 and Dad and Weiner12 for a review) but based on interventions for other cardiovascular diseases, acute treatment is similar between non-dialysis CKD patients and the general population.12 However, for CKD patients on dialysis, there is a higher risk of intracerebral haemorrhage compared to the general population when administering tissue plasminogen activator, possibly due to endothelial and platelet dysfunction in these patients.12 The efficacy of mechanical thrombectomy as an alternative to thrombolysis in CKD patients is yet to be adequately established. Reduction of modifiable risk factors and, optimally, stroke prevention are attractive management strategies in CKD patients. Risks that are prominent in CKD population include hypertension, hypercholesterolaemia, atrial fibrillation, hyperhomocysteinaemia, anaemia and dialysis.
The most prominent modifiable risk factor for stroke, in both CKD and the general population, is hypertension.12 Evidence suggests that the risk of major cardiovascular events, including stroke, is reduced by decreasing blood pressure in patients with an eGFR below 60 mL/min.12 This relationship is however complicated in advanced CKD/dialysis patients where blood pressure may change drastically pre-, intra- and post-dialysis and thus in this cohort optimal blood pressure has yet to be determined.10
Lowering levels of lipids in patients with CKD has shown to significantly reduce the risk of ischaemic stroke.10 As with hypertension, this relationship is not as clear in severe CKD. However, lipid lowering therapies are typically safe and given their preventative role in general cardiovascular events should be undertaken as a routine measure.
Atrial fibrillation, which may occur in approximately 20% of non-dialysis CKD patients, is an important cause of thromboembolic stroke.12 The benefit of thromboprophylaxis for CKD patients with atrial fibrillation is yet to be fully established. Evidence suggests warfarin is appropriate for stage 3 CKD but not dialysis patients and that anti-coagulation reduces the risk of stroke.12
Hyperhomocysteinaemia is highly prevalent in CKD and has been associated with stroke. However, clinical trials have failed to provide compelling evidence that vitamin therapy with folic acid provides benefit in reducing stroke risk.12
With reference to iatrogenic risk factors for stroke, evidence suggests that treatment of anaemia with erythropoietin-stimulating agents and haemodialysis itself increases the risk of stroke. Correction of anaemia should remain within the KDOQI guidelines of haemoglobin levels between 11 and 12 g/dl. Large randomised clinical trials have consistently demonstrated that using higher haemoglobin targets has resulted in worse outcomes including death.10,13 Finally, the process of dialysis causes significant haemodynamic shifts, these changes in blood flow are hypothesized to adversely affect end-organs and result in perfusion related brain injury.12 As such, strategies that improve haemodynamic stability such as cooling the dialysate, haemodiafiltration or lower ultrafiltration rate may be beneficial.12
Cognitive impairment and dementia
Cognitive impairment is defined as a new deficit in two or more areas of cognitive function. In milder forms, cognitive impairment may not interfere with daily functioning. In contrast, dementia is characterised by persistent cognitive decline and behavioural disturbance that is severe enough to interfere with independence and daily functioning. CKD is an independent risk factor for progressive cognitive impairment and dementia.14 Dementia presents an important clinical complication as it can result in poor health literacy, medical adherence and is a powerful predictor of mortality in dialysis patients.3,15 While cognitive impairment is recognised as a common complication of CKD, it remains poorly identified. The reported prevalence of cognitive impairment in dialysis is estimated at between 30% and 60% while less than 5% of patients have clinically documented histories of cognitive impairment.3
Evidence suggests that both the prevalence and progression of cognitive impairment are inversely associated with the level of kidney function. Several large population-based studies have demonstrated an increased risk of cognitive decline in the presence of moderate CKD including an 11% increased prevalence of cognitive impairment per 10 mL/min/1.73 m2 decrease in eGFR.16
Furthermore, longitudinal studies have demonstrated a more rapid decline in cognitive function over time in the presence of CKD. The greatest dysfunction has been reported in the cognitive domains of orientation, attention and executive function.15 ‘Dialysis dementia’, a term used to describe a chronic progressive dementia secondary to aluminium exposure in dialysis patients, is rarely encountered in the modern era due to the effectiveness of water treatments aimed at removing aluminium from the dialysis water and the limitation of oral aluminium use as a phosphate binder.17
Diagnosis and management
Recognition and documentation are the crucial first steps in managing cognitive dysfunction or dementia in patients with CKD. The Mini-Mental State Examination (MMSE) is a widely used method of assessment for cognitive impairment in the general population.3,15 This instrument is effective as a screening tool that provides a brief, standardised method that can be useful to assess the severity of cognitive impairment and cognitive changes over time. However, the MMSE has low sensitivity for mild cognitive dysfunction. Additionally, it has a focus on the assessment of memory and attention at the expense of other cognitive domains, such as executive function. There are several other screening tools that have been utilised in CKD patients including the Modified Mini-Mental State Examination, the Kidney Disease Quality of Life Cognitive Function subscale, the Montreal Cognitive Assessment, to name a few.3,15 If suspicion for cognitive impairment is high, referral to a neuropsychology service is recommended for comprehensive cognitive assessment. MRI is also essential and may identify silent brain infarcts, microbleeds and white matter disease which are highly prevalent in this cohort and are associated with cognitive impairment. Importantly, cerebral imaging will exclude space-occupying lesions which may represent a treatable cause of cognitive impairment. Other causes of cognitive impairment, including B12 deficiency and hypothyroidism, should be investigated through blood test screening.
Management of CKD patients with dementia should include prompt patient and family education, along with non-pharmacological support plans. Pharmacological interventions are of limited utility in cognitive dysfunction due to CKD and are not widely recommended.
Acute manifestations of altered mental status
Acute alterations of metal status in CKD are often indicative of encephalopathy which may be rapidly progressive requiring urgent treatment/rectification of causative agent/s. Patients with CKD are exposed to several factors that may contribute to the development of encephalopathy. Aside from ‘uraemia’ per se other mechanisms include thiamine deficiency, hypertension, transplant rejection, fluid and electrolyte disturbances and polypharmacy.18 Prompt recognition and identification of the causative factor/s are crucial as encephalopathies may be reversible with treatment.
Encephalopathy and delirium
Encephalopathy and delirium are terms that are frequently used interchangeably when describing the toxic metabolic encephalopathy that occurs in CKD. Both refer to an acute diffuse alteration of brain function or structure induced by a toxic or metabolic disturbance, though encephalopathy can be supported by changes in electroencephalography (EEG).3
Features of uraemic encephalopathy are wide ranging, from mild sensorial clouding to delirium and coma.18 Uraemic encephalopathy may have insidious onset and early features can be non-specific such as fatigue, apathy, irritability and impaired concentration which makes early identification difficult. Alterations in mental status can be accompanied by generalised or focal motor disturbances including tremor, fasciculations, asterixis and seizures. Later features are more severe including confusion, disorientation, delirium, hallucinations, coma and seizures.18
Pathophysiology
Uraemic encephalopathy has a complex pathophysiology presumably related to the retention of uraemic metabolites. Given the large number of compounds known to accumulate with kidney failure, the relative importance of individual uraemic toxins has been difficult to elucidate. Moreover, the pathophysiological investigations of compounds that are elevated in serum are further complicated by the intricate dynamics and transport systems of the blood–brain barrier.
Guanidino compounds have long been implicated in uraemic encephalopathy.19 Specific guanidino compounds such as guanidinosuccinic acid, methylguanidine, guanidine and creatinine have been shown to be elevated in serum, brain and cerebrospinal fluid of uraemic patients and have been the focus of many experimental investigations in vivo and in vitro. These compounds are known to induce convulsions in the experimental setting and several mechanisms have been proposed based on animal studies.19 PTH has also been implicated in uraemic encephalopathy via similar mechanisms discussed in reference to cognitive dysfunction, i.e. excess PTH may disrupt cerebral function via increased brain calcium content.7
Several of the toxic metabolic encephalopathies that can occur in CKD patients have very specific pathological mechanisms and will be described briefly as follows. Wernicke’s encephalopathy is a result of thiamine (vitamin B1) deficiency and is classically reported to present with a triad of ophthalmoplegia, ataxia and disturbances to cognition or consciousness.20 It has been suggested that poor nutrition and loss of water-soluble vitamins through dialysis place CKD patients at high risk of this type of encephalopathy.
Hypertensive encephalopathy in CKD patients presents as severe hypertension in combination with encephalopathic symptoms and may be reversible with immediate anti-hypertensive treatment.18 Specific to CKD patients, treatment of anaemia with erythropoietin is known to cause hypertension in ∼35% of patients and presents an important consideration in CKD patients with hypertensive encephalopathy.21 Acute hypertension in end-stage kidney disease patients may also result in posterior reversible encephalopathy syndrome (PRES), which manifests as decreased consciousness, headache and seizures associated with abnormalities of the posterior white matter on neuroimaging.22 Calcineurin inhibitors such as cyclosporine and tacromilus, which are used for immunosuppression in transplant recipients, are also known to cause PRES.22 Rejection encephalopathy may occur in patients with acute graft rejection and is thought to be induced by cytokine production subsequent to graft rejection.18 As such, recovery is typically rapid and complete following treatment of the rejection episode. Sepsis may be another cause of impaired consciousness in patients receiving dialysis treatment. Sepsis is a challenge in end-stage renal failure as uraemia is associated with a degree of immunosuppression and fever may not be prominent. Besides obvious sites of infection, the dialysis access site should be excluded as a cause.
Fluid and electrolyte disturbances are frequent in patients with CKD and have an adverse effect on CNS function. Dialysis disequilibrium syndrome results from rapid changes in urea and other osmolites during acute dialysis especially when commencing dialysis for severe uraemia. The osmotic gradient between blood and brain causes cerebral oedema and the presentation includes headache, tremor, disturbed conscientiousness and convulsions.23 Electrolytes of pathological importance in the CNS include calcium, magnesium and sodium along with the associated changes in osmolality.18
Drug toxicity and polypharmacy are also common problems in patients with CKD. A large proportion of drugs are metabolised or excreted by the kidney and thus CKD patients have an increased risk of drug-induced encephalopathy. In addition to filtration and half-life considerations,24 protein binding and drug interactions25 may also be important factors in managing drug toxicity in CKD.
Diagnosis and management
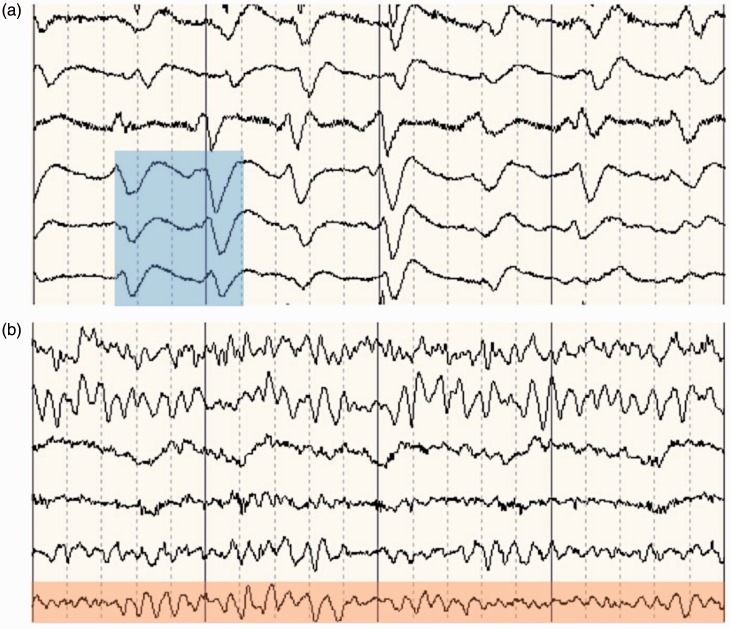
The first step in the treatment of uraemic encephalopathy is to identify the underlying metabolic disturbance. Laboratory blood tests should be undertaken and include a complete blood count, electrolyte panel, glucose, urea, creatinine, vitamin B12, folic acid, thyroid function, liver enzymes and ammonia. Though symptoms of uraemic encephalopathy rarely correlate with laboratory values, these test results may provide insight into causes related to anaemia, elevated PTH concentrations, electrolyte abnormalities or glucose levels. EEG findings can be of diagnostic value as the degree of EEG changes correlate with severity of encephalopathy. The typical features of an EEG in uraemic neuropathy are often non-specific such as a slowing of the alpha rhythm with excess delta and theta waves.26 The presence of triphasic sharp waves on EEG is considered a specific feature of metabolic encephalopathies (Figure 2).
Figure 2.
(a) Electroencephalogram of a chronic kidney disease patient who presented with drowsiness and confusion. Triphasic waves as typically seen in uraemic encephalopathy are highlighted in blue. (b) Electroencephalogram of a healthy patient. Normal background alpha rhythm is highlighted in red.
Cerebral imaging with CT or MRI is required to exclude a space-occupying lesion, haemorrhage or ischaemic stroke.27 It may be necessary to conduct a lumbar puncture if the patient becomes febrile to investigate the possibility of meningitis or encephalitis.26
Symptoms associated with uraemic encephalopathy are usually alleviated by adequate dialysis or successful transplantation.26 Care should be taken to avoid rapid shifts in electrolyte concentrations, particularly sodium, as this may exacerbate symptoms. Similarly, dialysis implementation should aim for gradual clearance of urea to avoid dialysis disequilibrium syndrome.23
Practical approach to the confused patient
In summary, it is evident that patients with advanced CKD are prone to disorders of consciousness and that specialised long-term management is required to accurately identify acute vs. chronic manifestations. Chronic conditions require referral to specialised care such as neuropsychology or stroke clinics. The most common causes of acute presentations are often related to hypertension, electrolyte disturbances, dialysis and polypharmacy (Table 2). Distinguishing the various causes of altered mental status typically requires a diagnosis of exclusion. The most paramount tests include blood tests, blood pressure monitoring and imaging. Careful recognition of the timing of symptoms will help exclude dialysis itself as a cause and thorough management of medications from renal physicians is crucial to minimising the complications of polypharmacy.
Physical disability
Physical disability is a common problem in CKD patients with a significant impact on activities of daily living and independence. Physical impairment may be a manifestation of various PNS disorders (Table 2). The most common PNS disorders in CKD patients are peripheral neuropathy, autonomic neuropathy and myopathy.
Peripheral neuropathy
Peripheral neuropathy in CKD, also known as uraemic neuropathy, is the most common neurological complication of CKD and affects ∼90% of dialysis patients.27 Patients typically experience functionally disabling symptoms such as pain, loss of sensation and weakness.28 Neuropathy is also a significant contributor to ulceration and amputation.
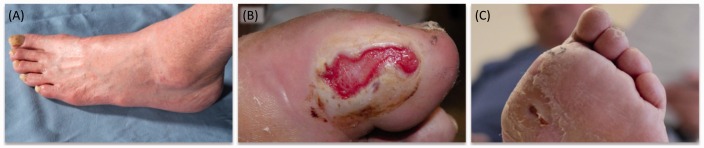
Signs and symptoms in the initial stages of uraemic neuropathy include distal sensory loss to pinprick and vibration as well as reduced or absent tendon reflexes in the lower limbs.28 With disease progression, sensory loss extends proximally in the lower limb and similar signs and symptoms may manifest in the distal upper limb. In advanced cases, motor nerves can be affected resulting in weakness and atrophy in distal muscles of the lower limb28 (Figure 3). This should be differentiated from the pattern of weakness in myopathy in uraemic myopathy, in which weakness is maximal proximally with muscles of the hip girdle particularly affected.26 In addition to the damage of large motor and sensory fibres in uraemic neuropathy, small fibre neuropathy may also occur. In diabetic CKD patients, small fibre neuropathy may result in burning and sharp pain as well as altered perception of temperature.29
Figure 3.
Clinical features of advanced uraemic neuropathy: (a) atrophy of musculature in the distal lower limb, (b) ulceration and (c) amputation.
Traditionally, uraemic neuropathy was thought to only become clinically apparent when GFRs fell below 12 mL/min. However, studies in contemporary cohorts have demonstrated that neuropathy is apparent in up to 70% of pre-dialysis patients.30
Pathophysiology
The systemic effects of uraemia on peripheral nerves is emphasised by the generalised nature of nerve conduction slowing which has been demonstrated in upper and lower extremities, and in both sensory and motor nerves. As such, it is possible that while structural changes occur in a length-dependent manner, uraemia may have a universal neurotoxic effect caused by an unknown uraemic toxin.
While many substrates have been investigated as a potential uraemic neurotoxin including; urea, creatinine, guanidine, methylguanidine, guanidinosuccinic acid, uric acid, oxalic acid, phenols, aromatic hydroxyacids, indican, amines, myo-inositol, ‘middle molecules’, β-2 microglobulin, PTH, amino acids and neurotransmitters, none of these have yielded evidence of causality.
In contrast, a substantial body of research has recently demonstrated that hyperkalaemia has a pivotal role in uraemic neuropathy. These studies provided evidence that hyperkalaemia impairs nerve function in a dose-dependent manner and this dysfunction can be normalised with the removal of excess serum potassium.31 These studies also suggest that maintaining normal levels of potassium in CKD patients may prevent peripheral nerve injury.31
Diagnosis and management
The gold standard for diagnosis of neuropathy is clinical neurological examination including nerve conduction studies, which examine conduction velocity and amplitude. Nerve conduction studies in patients with uraemic neuropathy typically demonstrate changes of an axonal neuropathy, with reduced sensory amplitudes initially and reduced motor amplitudes in later disease stages while conduction velocities are only minimally affected.28
Clinical diagnosis of neuropathy in CKD requires eliminating other causes of neuropathy such as glucose dysmetabolism and connective tissue disease. The presence of glucose dysmetabolism is significant in diabetic CKD patients who may have pre-existing neuropathy. Connective tissue disorders such as peripheral nerve vasculitis may be associated with a rapid progression of neuropathy. Other causes of rapidly progressive neuropathy in CKD patients include inflammatory demyelinating neuropathies, such as chronic inflammatory demyelinating polyneuropathy, which may occur in the context of glomerulonephritis.27
For dialysis patients there is some evidence for enhanced dialysis strategies such as haemodiafiltration may improve nerve function.32 Renal transplantation has previously been recognised as the most effective treatment to improve clinical outcomes. However, some immunosuppressive medications such as calcineurin inhibitors may hinder neurological improvements.33
Recent evidence suggests that maintaining normal levels of potassium in CKD patients may prevent peripheral nerve injury.31 Given the results of pathophysiological studies suggesting that hyperkalaemia may cause nerve dysfunction in CKD,31 lowering serum potassium levels may be a potential strategy to prevent or treat neuropathy in CKD. A Phase 2 clinical trial has recently been completed which will provide information on the potential benefits of dietary potassium restriction on neuropathy progression in stage 3-4 CKD (Australian and New Zealand Clinical Trials Registry: ACTRN12610000538044).
Neuropathic pain can be managed pharmacologically with membrane-stabilising treatments such as tricyclic antidepressants and anticonvulsants.29 However, these drugs have many potential side effects and dosing restrictions.26 First-line treatment for painful neuropathy usually consists of tricyclic antidepressants, such as amitriptyline. However, these medications are poorly tolerated by older patients and should be avoided in patients with cardiac arrhythmias, congestive heart failure, orthostatic hypotension and urinary retention.29 Anticonvulsants such as pregabalin or gabapentin provide an alternative although both have dosing restrictions in patients according to creatinine clearance.29
Autonomic neuropathy
Autonomic dysfunction refers to a disorder in which autonomic outflow is altered, resulting in sympathetic overdrive and a decrease of parasympathetic function. Autonomic dysfunction is highly prevalent in CKD and occurs in more than 50% of patients on haemodialysis.34,35 Diabetes, vascular disease and hypertension are also associated with autonomic dysfunction and as these are commonly also present in patients with CKD it is difficult to attribute a causal relationship with the CKD. However, recent studies have shown that autonomic dysfunction may be detectable in the early stages of CKD and that the magnitude of sympathetic overdrive corresponds to the severity of renal failure.36 Cardiovascular manifestations of autonomic neuropathy such as higher resting heart rate and lower heart rate variability have been associated with both incident end-stage kidney disease and CKD hospital admissions.37
A common symptom of autonomic neuropathy in CKD is impotence, which develops in the majority of male patients.38 Other clinical manifestations may include gastrointestinal complications, such as impaired gastric motility and dyspepsia, impaired sudomotor function and intradialytic hypotension.27,34,39 Patients with cardiovascular autonomic dysfunction may exhibit orthostatic intolerance, cardiac arrhythmia and reduced capacity for exercise. Cardiac autonomic dysfunction is potentially life-threatening with manifestations such as cardiac arrhythmia, silent myocardial ischemia and sudden cardiac death.26,27
Pathophysiology
Sympathetic overactivity may contribute to development and progression of renal disease.36 In cardiovascular autonomic dysfunction, this overdrive is potentially mediated by renal sensory afferents in damaged kidneys and is potentiated by impaired chemosensor function, elevated circulating humoral and metabolic factors such as angiotensin II and cardiovascular remodelling.36,40 The mechanisms contributing to altered autonomic outflow in CKD are incompletely understood but the several that have been proposed are reviewed in Salman.40 Of particular interest however, clinical studies have indicate that elevated sympathetic outflow in cardiovascular autonomic dysfunction appears to be independent of uraemia-related toxins.40
Diagnosis and management
Clinical assessment of autonomic function can be undertaken by examining cardiac and pupillary reflexes, sudomotor function and blood pressure control. Heart rate variability has been shown to provide an indication of patients prone to intradialytic hypotension.34 Cardiac autonomic neuropathy can be assessed using tests such as heart rate variability, change in heart rate upon postural change and the Valsalva manoeuvre.26
Renal transplantation has shown to improve parasympathetic function and in some cases sympathetic function.40 Impotence can be effectively treated with phosphodiesterase type 5 inhibitors such as sildenafil or vardenafil and is well tolerated.38 Gastrointestinal complications such as impaired motility may improve with haemodialysis.39 Intradialytic hypotension may be improved with midodrine; an alpha 1-receptor agonist producing an increase in vascular tone, administered prior to dialysis. Modifiable risk factors that may reduce intradialytic hypertension episodes include a low dry weight and hypertension.41
For cardiovascular autonomic dysfunction observational studies have shown that angiotensin receptor blockers combined with another antihypertensive medication, with the exception of angiotensin-converting enzyme inhibitors, may improve cardiovascular mortality in haemodialysis patients.42 Retrospective studies have shown beta-blockers, which prevent arrhythmia and improve heart rate variability, lower the risks sudden cardiac death in patients receiving haemodialysis.43 However, the use of beta-blockers requires caution because of potential side effects such as hyperkalaemia and bradycardia.43 The combined alpha/beta-blocker carvedilol may provide cardiovascular benefits with fewer side-effects.26 In patients with diabetic CKD, optimising glycaemic control remains an important preventative approach against the progression of autonomic and peripheral neuropathy.26
Myopathy
Approximately 50% of CKD patients on dialysis are affected by myopathy. Myopathy is characterised by proximal muscle weakness in the muscles of the lower limbs. Patients with myopathy are substantially limited in function due to reduced endurance and capacity for exercise, leading to an increase in mortality and morbidity.44
Pathophysiology
The pathophysiology of uraemic myopathy is unclear as there is an interplay of various mechanisms such as oxidative stress.45 Symptoms typically appear when glomerular filtrations rates drop below 25 mL/min and progression corresponds with a decline of kidney function.26,28 Possible aetiologies of myopathy include hyperparathyroidism, metabolic bone disease with vitamin D deficiency, impaired potassium regulation, accumulation of uraemic toxins and carnitine deficiency.28 Atrophy of skeletal muscle via metabolic acidosis in uraemia is likely due to protein metabolism abnormalities and evidence suggests increase of adequate amino acid intake does not seem to improve these abnormalities.44 The prevalence of uraemic myopathy is higher in patients with diabetic CKD, suggesting that insulin resistance plays a role in the development of myopathy.44 This is probably due to the decrease of glucose as an energy source and insulin resistance also associated with protein catabolism.
Diagnosis and management
No diagnostic tool or biochemical parameter can be used to diagnose uraemic myopathy and electromyography as well as levels of muscle enzymes are typically normal in these patients.44 However, demonstration of weakness in proximal pelvic muscles is a strong indicator of myopathy. Muscle biopsy studies have shown atrophy of type II fibres and fibre splitting,44,45 but biopsy is rarely undertaken due to the invasive nature of the procedure. No specific treatment exists for uraemic myopathy however management of the potential contributing factors such as hyperparathyroidism, vitamin D and anaemia may be beneficial. Exercise programs and renal transplantation has been shown to improve exercise tolerance and neuromuscular function.28
Practical approach to a patient with physically disabling symptoms
In brief summary, many PNS disorders may present with physically disabling features such as weakness, pain and dizziness. The location of symptoms, such as distal vs. proximal leg weakness, can be distinguishing for peripheral neuropathy vs. myopathy, respectively. Autonomic neuropathy often accompanies peripheral neuropathy but presents with distinct cardiovascular features that are clearly identifiable. These conditions have a chronic disease course that typically parallels the decline in kidney function. As such, similar symptoms with an acute onset or rapid progression may indicate a different causality such as Guillian Barre Syndrome or vasculitic neuropathy or mimic disorders such as myelinolysis and polyradiculopathies requiring referral for specialist neurological diagnosis. Multi-disciplinary management combining nephrology and neurology care is a useful way of monitoring the severity and progression of neurological complications with reference to kidney function in CKD and thus minimising risk of misdiagnosis.
Conclusion
Neurological complications are highly prevalent in CKD and are a major cause of morbidity and mortality. Presentations of altered mental status may be differentiated according to acute vs. chronic CNS derangements. Chronic conditions such as stroke, cognitive impairment and dementia require long-term risk management strategies to optimise outcomes in CKD patients. Acute encephalopathies may be caused by a wide variety of metabolic and pharmacologic exposures common in CKD and require rapid treatment to avoid escalation to seizures or coma. Physical disability in CKD is highly prevalent most commonly caused by peripheral neuropathy, autonomic neuropathy and myopathy. Both CNS and PNS complications are most apparent at end-stage disease, though they are likely to be present at much earlier stages of CKD. As such, early detection and management of these conditions in mild CKD may represent a window of opportunity to reduce their impact at later stages.
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: AK was supported by a Career Development Award of the National Health and Medical Research Council of Australia (grant number 1065663). RA was supported by an Early Career Post-Doctoral Fellowship of the National Health and Medical Research Council of Australia (#1091006).
Ethical approval
Not applicable.
Guarantor
RA is the guarantor for all the content presented in this paper.
Contributorship
RA, AVK and BP were involved in planning and design of the manuscript. RA and TI participated in the literature review. AVK, RA, TI and BP critically revised the manuscript.
References
- 1.Couser WG, Remuzzi G, Mendis S, et al. The contribution of chronic kidney disease to the global burden of major noncommunicable diseases. Kidney Int 2011; 80: 1258–1270. [DOI] [PubMed] [Google Scholar]
- 2.National Kidney F. K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis 2002; 39: S1–266. [PubMed] [Google Scholar]
- 3.McQuillan R, Jassal SV. Neuropsychiatric complications of chronic kidney disease. Nat Rev Nephrol 2010; 6: 471–479. [DOI] [PubMed] [Google Scholar]
- 4.Watanabe K, Watanabe T, Nakayama M. Cerebro-renal interactions: impact of uremic toxins on cognitive function. Neurotoxicology 2014; 44: 184–193. [DOI] [PubMed] [Google Scholar]
- 5.Yaffe K, Kurella-Tamura M, Ackerson L, et al. Higher levels of cystatin C are associated with worse cognitive function in older adults with chronic kidney disease: the chronic renal insufficiency cohort cognitive study. J Am Geriatr Soc 2014; 62: 1623–1629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chillon JM, Massy ZA, Stengel B. Neurological complications in chronic kidney disease patients. Nephrol Dial Transplant 2016; 31: 1606–1614. [DOI] [PubMed] [Google Scholar]
- 7.Pereira AA, Weiner DE, Scott T, et al. Cognitive function in dialysis patients. Am J Kidney Dis 2005; 45: 448–462. [DOI] [PubMed] [Google Scholar]
- 8.Sacco RL, Kasner SE, Broderick JP, et al. An updated definition of stroke for the 21st century: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2013; 44: 2064–2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bugnicourt JM, Godefroy O, Chillon JM, et al. Cognitive disorders and dementia in CKD: the neglected kidney-brain axis. J Am Soc Nephrol 2013; 24: 353–363. [DOI] [PubMed] [Google Scholar]
- 10.Arnold J, Sims D, Ferro CJ. Modulation of stroke risk in chronic kidney disease. Clin Kidney J 2016; 9: 29–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Masson P, Webster AC, Hong M, et al. Chronic kidney disease and the risk of stroke: a systematic review and meta-analysis. Nephrol Dial Transplant 2015; 30: 1162–1169. [DOI] [PubMed] [Google Scholar]
- 12.Dad T, Weiner DE. Stroke and chronic kidney disease: epidemiology, pathogenesis, and management across kidney disease stages. Semin Nephrol 2015; 35: 311–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Madero M, Gul A, Sarnak MJ. Cognitive function in chronic kidney disease. Semin Dial 2008; 21: 29–37. [DOI] [PubMed] [Google Scholar]
- 14.Seliger SL, Siscovick DS, Stehman-Breen CO, et al. Moderate renal impairment and risk of dementia among older adults: the Cardiovascular Health Cognition Study. J Am Soc Nephrol 2004; 15: 1904–1911. [DOI] [PubMed] [Google Scholar]
- 15.O'Lone E, Connors M, Masson P, et al. Cognition in people with end-stage kidney disease treated with hemodialysis: a systematic review and meta-analysis. Am J Kidney Dis 2016; 67: 925–935. [DOI] [PubMed] [Google Scholar]
- 16.Kurella Tamura M, Wadley V, Yaffe K, et al. Kidney function and cognitive impairment in US adults: the Reasons for Geographic and Racial Differences in Stroke (REGARDS) Study. Am J Kidney Dis 2008; 52: 227–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dunea G. Dialysis dementia: an epidemic that came and went. ASAIO J 2001; 47: 192–194. [DOI] [PubMed] [Google Scholar]
- 18.Brouns R, De Deyn PP. Neurological complications in renal failure: a review. Clin Neurol Neurosurg 2004; 107: 1–16. [DOI] [PubMed] [Google Scholar]
- 19.De Deyn PP, D'Hooge R, Van Bogaert PP, et al. Endogenous guanidino compounds as uremic neurotoxins. Kidney Int Suppl 2001; 78: S77–S83. [DOI] [PubMed] [Google Scholar]
- 20.Hung SC, Hung SH, Tarng DC, et al. Thiamine deficiency and unexplained encephalopathy in hemodialysis and peritoneal dialysis patients. Am J Kidney Dis 2001; 38: 941–947. [DOI] [PubMed] [Google Scholar]
- 21.Delanty N, Vaughan C, Frucht S, et al. Erythropoietin-associated hypertensive posterior leukoencephalopathy. Neurology 1997; 49: 686–689. [DOI] [PubMed] [Google Scholar]
- 22.Roth C, Ferbert A. The posterior reversible encephalopathy syndrome: what's certain, what's new? Pract Neurol 2011; 11: 136–144. [DOI] [PubMed] [Google Scholar]
- 23.Patel N, Dalal P, Panesar M. Dialysis disequilibrium syndrome: a narrative review. Semin Dial 2008; 21: 493–498. [DOI] [PubMed] [Google Scholar]
- 24.Doogue MP, Polasek TM. Drug dosing in renal disease. Clin Biochem Rev / Aust Assoc Clin Biochem 2011; 32: 69–73. [PMC free article] [PubMed] [Google Scholar]
- 25.Mallet L, Spinewine A, Huang A. The challenge of managing drug interactions in elderly people. Lancet 2007; 370: 185–191. [DOI] [PubMed] [Google Scholar]
- 26.Arnold R, Krishnan A. Neuropathy and other neurological problems in chronic kidney disease. In: Arici M. (ed). Management of chronic kidney disease, Berlin: Springer, 2014, pp. 343–352. [Google Scholar]
- 27.Krishnan AV, Kiernan MC. Neurological complications of chronic kidney disease. Nat Rev Neurol 2009; 5: 542–551. [DOI] [PubMed] [Google Scholar]
- 28.Krishnan AV, Pussell BA, Kiernan MC. Neuromuscular disease in the dialysis patient: an update for the nephrologist. Semin Dial 2009; 22: 267–278. [DOI] [PubMed] [Google Scholar]
- 29.Pop-Busui R, Roberts L, Pennathur S, et al. The management of diabetic neuropathy in CKD. Am J Kidney Dis 2010; 55: 365–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Arnold R, Kwai N, Pussell BA, et al. Effects of neuropathy on physical function and quality of life in moderate severity chronic kidney disease. Clin Neurophysiol 2014; 125: e4–e4. [Google Scholar]
- 31.Arnold R, Pussell BA, Howells J, et al. Evidence for a causal relationship between hyperkalaemia and axonal dysfunction in end-stage kidney disease. Clin Neurophysiol 2014; 125: 179–185. [DOI] [PubMed] [Google Scholar]
- 32.Arnold R, Kwai N, Lin CS, et al. Axonal dysfunction prior to neuropathy onset in type 1 diabetes. Diabetes Metab Res Rev 2013; 29: 53–59. [DOI] [PubMed] [Google Scholar]
- 33.Arnold R, Pussell BA, Pianta TJ, et al. Association between calcineurin inhibitor treatment and peripheral nerve dysfunction in renal transplant recipients. Am J Transplant 2013; 13: 2426–2432. [DOI] [PubMed] [Google Scholar]
- 34.Chang YM, Shiao CC, Chang KC, et al. Heart rate variability is an indicator for intradialytic hypotension among chronic hemodialysis patients. Clin Exp Nephrol 2015: 1–10. [DOI] [PubMed]
- 35.Kurella M, Chertow GM, Fried LF, et al. Chronic kidney disease and cognitive impairment in the elderly: the health, aging, and body composition study. J Am Soc Nephrol 2005; 16: 2127–2133. [DOI] [PubMed] [Google Scholar]
- 36.Grassi G, Quarti-Trevano F, Seravalle G, et al. Early sympathetic activation in the initial clinical stages of chronic renal failure. Hypertension 2011; 57: 846–851. [DOI] [PubMed] [Google Scholar]
- 37.Brotman DJ, Bash LD, Qayyum R, et al. Heart rate variability predicts ESRD and CKD-related hospitalization. J Am Soc Nephrol 2010; 21: 1560–1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lasaponara F, Sedigh O, Pasquale G, et al. Phosphodiesterase type 5 inhibitor treatment for erectile dysfunction in patients with end-stage renal disease receiving dialysis or after renal transplantation. J Sex Med 2013; 10: 2798–2814. [DOI] [PubMed] [Google Scholar]
- 39.Adachi H, Kamiya T, Hirako M, et al. Improvement of gastric motility by hemodialysis in patients with chronic renal failure. J Smooth Muscle Res 2007; 43: 179–189. [DOI] [PubMed] [Google Scholar]
- 40.Salman IM. Cardiovascular autonomic dysfunction in chronic kidney disease: a comprehensive review. Curr Hypertens Rep 2015; 17: 59–59. [DOI] [PubMed] [Google Scholar]
- 41.Rocha A, Sousa C, Teles P, et al. Frequency of intradialytic hypotensive episodes: old problem, new insights. J Am Soc Hypertens JASH 2015; 9: 763–768. [DOI] [PubMed] [Google Scholar]
- 42.Chan KE, Ikizler TA, Gamboa JL, et al. Combined angiotensin-converting enzyme inhibition and receptor blockade associate with increased risk of cardiovascular death in hemodialysis patients. Kidney Int 2011; 80: 978–985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Matsue Y, Suzuki M, Nagahori W, et al. beta-blocker prevents sudden cardiac death in patients with hemodialysis. Int J Cardiol 2013; 165: 519–522. [DOI] [PubMed] [Google Scholar]
- 44.Fahal IH, Bell GM. Uraemic myopathy: fact or fiction. Int J Artif Organs 1998; 21: 185–187. [PubMed] [Google Scholar]
- 45.Kaltsatou A, Sakkas GK, Poulianiti KP, et al. Uremic myopathy: is oxidative stress implicated in muscle dysfunction in uremia? Front Physiol 2015; 6: 102–102. [DOI] [PMC free article] [PubMed] [Google Scholar]