Abstract
This review explains the main effects exerted by sex steroids and other hormones on the adolescent brain. During the transition from puberty to adolescence, these hormones participate in the organizational phenomena that structurally shape some brain circuits. In adulthood, this will propitiate some specific behavior as responses to the hormones now activating those neural circuits. Adolescence is, then, a critical “organizational window” for the brain to develop adequately, since steroid hormones perform important functions at this stage. For this reason, the adolescent years are very important for future behaviors in human beings. Changes that occur or fail to occur during adolescence will determine behaviors for the rest of one's lifetime. Consequently, understanding the link between adolescent behavior and brain development as influenced by sex steroids and other hormones and compounds is very important in order to interpret various psycho-affective pathologies.
Lay Summary: The effect of steroid hormones on the development of the adolescent brain, and therefore, on adolescent behavior, is noticeable. This review presents their main activational and organizational effects. During the transition from puberty to adolescence, organizational phenomena triggered by steroids structurally affect the remodeling of brain circuits. Later in adulthood, these changes will be reflected in behavioral responses to such hormones. Adolescence can then be seen as a fundamental “organizational window” during which sex steroids and other hormones and compounds play relevant roles. The understanding of the relationship between adolescent behavior and the way hormones influence brain development help understand some psychological disorders.
Keywords: Activational effects, Adolescence, Adolescent behavior, Brain development, Contraceptive steroids, Hormones, Sex steroids, Organizational effects, Puberty
Introduction
The physical changes experienced during adolescence are remarkable and, most of them evident, especially those leading to the manifestation of secondary sex characters and contributing to the differentiation between male and female phenotype, as well as the acquisition of human reproductive capacity. However, at the psychic level, many of the mental faculties are still under development in the adolescent (either exacerbated or hindered as compared to adults) since his or her brain is undergoing a process of maturation characterized by a number of physiological changes that will modulate its activity, a process associated to individual, social, and psychological development (Lerner and Steinberg 2004). Currently, we know that the levels of endogenous hormones such as gonadal and adrenal steroids (e.g., androgens, estrogens, and progestagens) represent important factors when explaining the differences between males and females. The understanding of the mechanisms underlying such differences has been at the core of neuroendocrinological research and the cause of controversy for decades (Lombardo et al. 2012). The steroids, whose levels start to increase significantly during adolescence, exert a crucial role in the processes of maturation and subsequent modulation of brain activity in the youngsters. A similar effect is observed regarding exogenous sex hormone formulations, as will be explained later.
The aim of this updated review is to provide evidence of the effects exerted by steroids and other hormones and compounds on the development of the adolescent brain, and to explain why this period in the person's life constitutes a window of unique plasticity, during which the hormone-triggered changes in the encephalon determine some behavioral patterns in adulthood.
Search for Bibliographic Information
Articles were searched for in the following bibliographic databases: WoS (Web of Science, ex-ISI), PubMed, SCOPUS Database, Scientific Electronic Library Online (SciELO), ScienceDirect, Google Scholar, and Google Books. Google Chrome was the search engine used. Search languages used were English, Spanish, and French; among the words used when searching were: “activational effects” and “brain,” “adolescent brain” and “hormones,” “adolescent brain” and “contraceptives,” “adolescent brain” and “steroids,” “brain development” and “adolescence,” “brain development” and “hormones,” “brain development” and “puberty,” “organizational effects” and “brain,” and “organizational window” and “brain.” Finally, fifty-nine references on these topics were reviewed depending on whether they were physically or electronically available from the library. In addition, fifty-two references were added since they were seminal papers or other relevant articles needed to provide a clear rationale and concluding remarks.
Puberty and Associated Endocrine Changes
Adolescent growth and development is influenced by highly coordinated endocrine changes beginning in puberty (from the Latin pūbertās, “pubis with hair”), defined as the stage in which an individual reaches sexual maturity (Schulz and Sisk 2006), marking the end of infancy. Puberty is preceded by adrenarche, marking an increase of adrenal androgen production (mainly dehydroepiandrosterone and dehydroepiandrosterone sulfate; Havelock, Auchus, and Rainey 2004) between ages 6 and 10. In girls, puberty begins between ages 8 and 10, ending with menarche between ages 12 and 13 (Miller and Styne 1999). In boys, puberty normally begins a year later and ends between ages 12 and 15 with the full development of the spermatogenic line (Santen 1999; Vigil 2013). Hormonal changes in this period lead to the release of mature oocites from the ovary (first ovulation) and of mature spermatozoa from the testicles (first ejaculation), in women and men, respectively (Vigil 2013).
Even though endocrine mechanisms underlying the onset of puberty have not been totally elucidated, we now know that one of the milestones that signal its beginning is the activation of the hypothalamic–hypophyseal–gonadal axis (Sigel 2005; Vigil et al. 2006), a process in which a number of signaling biomolecules play a crucial role, among them, peptide hormones, such as the neurohormones kisspeptin and neurokinin B, gonadotropin-releasing hormone (GnRH), the adipocytokine leptin, and growth hormone (together with its main effector, insulin-like growth factor 1). Current evidence shows that puberty begins with an increase in the child's adiposity (i.e., an increase in the size and number of adipocytes); such change causes adipocytes to release more leptin, with a subsequent increase of this hormone on the blood (García-Mayor et al. 1997). At the hypothalamus, more specifically at the kisspeptinergic nuclei, when leptin binds leptin receptor-expressing neurons, these cells increase the expression of the gene encoding kisspeptin (Cortés et al. 2015; Latronico 2012). On the other hand, the neurons that secrete GnRH possess kisspeptin receptors (also known as GPR54). Kisspeptin, when binding, causes an increase in the GnRH secretion pulses to the anterior portion of the pituitary gland (adenohypophysis) (Teles et al. 2011). This, in turn, leads to release of follicle stimulating hormone and luteinizing hormone, which, in general, exert their action at the gonadal level increasing biosynthesis and secretion of sex steroid hormones (Sigel 2005). Finally, the steroid surge triggers the expression of the secondary sex characters, e.g., breast development and widening of the hips in women, and appearance of facial hair and broadening of the torso (especially chest and shoulders) in men (Vigil 2013).
Adolescence and Its Related Behaviors
Adolescence (from Latin adolescentem, “the one who is growing”) is not the same as puberty, even though both concepts are frequently confused. Adolescence comprises the adaptive period between childhood and adulthood, and involves changes in psychological, social and physiological development including the capacity to acquire affective and sexual behaviors typically feminine or masculine (Lerner and Steinberg 2004; Vigil et al. 2006). Related psychological changes appear from 12 to 21 years of age. This period is characterized by the modulation of the limbic-cortical circuits associated to the acquisition of adult cognition and the establishment of neural information pathways required to promote emotional and social development (Lerner and Steinberg 2004). Hence, during adolescence individuals evidence a variety of behavioral patterns, which often disappear with adulthood.
Human behaviors can be the consequence of stimuli external (exogenous) or internal to the person (endogenous). Exogenous behavior is triggered by sensory perceptions or emotional responses to situations, which may or may not be real, and involves spontaneous reactions. On the other hand, endogenous behavior results from a process of cognitive control involving more than one brain region to focus behavior towards a previously established objective (Goldman-Rakic, Chafee, and Friedman 1993). Acquired during infancy, this endogenous behavior continues progressing in adolescence and, probably, during the whole life of the person. Some researchers have stated that adolescents can be expected to evidence a predominantly exogenous behavior (Luna 2009); in fact, adolescents are easily overwhelmed by stressful situations, a fact evidenced by their lack of control over stress. This explains the commonplace phrase, “they react first, and they think second” when referring to teenagers. Then, since adolescents are highly vulnerable to stressful factors, this transitional period of their development can be particularly stressing for them (Spear 2008b). In addition, they show great difficulty in performing tasks involving inhibition of impulsive behavior (Yurgelun-Todd 2007) or continuous attention. Since youngsters seem to be unable to perform such selective inhibition, they prefer pleasant activities offering immediate reward, even if risky, because those activities appear to be more attractive and easily gratifying than those offering long-term rewards (Luna 2009).
Egocentrism, defined as loss of differentiation in some area of the subject–object interaction, is a characteristic which is present in the main stages of human cognitive development (Piaget 1995), and quite evident in adolescence. Adolescents are frequently observed supporting their opinions or actions as response to a given situation, paying no attention or remaining indifferent to the logical counter-argumentation of their parents’ advice—a behavior known as “adolescent deafness” (Hall, Holmqvist, and Sherry 2004; Vigil, Molina, and Cortés 2009). Elkind (1967) suggested that this particular adolescent egocentrism is characterized by the youngsters’ belief that they are special and unique, and that the others are excessively worried about their behavior and physical appearance—they are quite influenced by the so-called “cult of the body” (Vigil, Molina, and Cortés 2009). This is typically evidenced by the attention they give to their choice of garments, hair style and accessories; additionally, teenagers need to readjust the image they have of themselves, facing their own body suddenly appearing as an alien as a consequence of its rapid growth. Such egocentrism is paired to the development of formal operational thought (increasingly based on propositional logic) which provides a tool to perform all the possible combinations of thought, linked in turn to the acquisition of new mental abilities in the adolescent. Two fundamental components of this egocentrism are: (i) the construction of an imaginary audience, whose attention adolescents imagine to be on themselves; and (ii) the construction of a personal fable, a personal history which, far from true, is based on the perception of their feelings and experiences as unique, special, different from those of any other; in addition, they believe the unfortunate events affecting their peers will never reach them (cf. Elkind 1967). Thus, typical adolescent behaviors, such as being excessively worried about what others may think of their behavior and appearance, the tendency to develop some degree of paranoia, indifference towards other people's opinions and life experiences, their predilection for risky behavior and finally, a feeling of omnipotence and invulnerability (e.g., against the risks of sexually transmitted infections, Vigil et al. 2009), are for the most part explained by the aforementioned mental constructions. However, adolescent invulnerability is questioned by Reyna and Farley (2007), who state that, on the contrary, teenagers often overestimate the dangers and thus do not feel invulnerable. When assessing danger, adolescent recklessness results from considering that the potential advantages brought about by risky behavior outweigh and override their feeling risk (Reyna and Farley 2007).
Characteristics of the Adolescent Brain
In general terms, the brain of adolescents is characterized by incomplete development of the encephalic functions determining endogenous (voluntary) behavior; this limits the ability to recognize erroneous information, sustain voluntary control, and become interested and motivated on the basis of future rewards. The latter results from immature neuronal connections, not yet able to determine endogenous behavior. However, Epstein (2008) has stated that this so-called immaturity of the adolescent brain is nothing but a myth, and that its behavioral problems rather result from cultural factors. Thus, some researchers affirm that adolescence is a recent construct product of the impact caused by industrialization and technology; in fact, in some societies youngsters went directly from childhood to adulthood (Weiten 2010).
Brain development is a process ongoing through the lifespan (Spear 2008a), and the changes undergone by the brain of adolescents are particularly interesting. From an anatomo-physiological perspective, the adolescent brain is not static, but highly dynamic and plastic, its neurological development being characterized by a gradual acquisition of cognitive control and affective modulation, with a general predominance of the emotional behavioral component. Positron emission tomography studies indicate that the nucleus accumbens and the brain amygdala (linked to emotional behavior, e.g., modulation of affective information and generation of feelings) evidence increased activity during adolescence as opposed to childhood and adulthood (Ernst et al. 2002). The motivational circuitry, where the limbic-cortical neural pathways interact, develops through adolescence, and the connections that regulate it, in late adolescence. The cortical system acts as a “filter” that selects the stimuli from the limbic system, inhibiting those that might lead to inconvenient or risky actions and responding favorably to those that lead to gratification for the individual or their immediate environment (Kail 1993). This adolescent predominance of the emotional component not only results from the absence of inhibition of certain stimuli due to some immature cortical regions; it also could be explained by the activity of the limbic system, which far exceeds that of the cortical system (Casey, Jones, and Hare 2008).
Gamma-aminobutyric acid (GABA), the main inhibitory neurotransmitter in the central nervous system (CNS), plays a substantial role in the adolescent brain. Its inhibitory capacity is explained by GABA which, when faced by an excitatory input, generates an anion chloride influx through the GABAA receptor, triggering neuron hyper polarizations, which prevents action potentials from unleashing and, therefore, the stimuli from propagating as nerve impulses. There is evidence that, during the puberty of female rats, the substitution of GABA receptor α1 subunit by α4 subunit alters the normal inhibition exerted by the GABAergic system. This change increases input resistance and excitation of hippocampal pyramidal neurons, thus promoting anxiety states (McCarthy 2007; Shen et al. 2007). This change at GABAergic modulation level during puberty and adolescence is responsible for impulsiveness in teens. This has been evidenced by the effect of tetrahydropregnenolone (also known as allopregnanalone), a neurosteroid which in this stage binds to GABAA receptors triggering increased anxiousness, as opposed to the inhibition of impulsive behavior (i.e., anxiolytic effect) caused by the same hormone in pre-puberty and adulthood (McCarthy 2007; Shen et al. 2007).
It has been suggested that by the end of adolescence, the neural activity in the limbic and cortical systems reaches similar levels, and thus the balance required to provide integrated responses to the limbic-cortical stimuli (Casey, Tottenham, and Fossella 2002). Because of the maturation of the prefrontal cortex—the executive control center and the last region to reach complete maturation, as late as in the mid-20s (Weiten 2010)—impulsivity shows a decreasing level from childhood to the end of adolescence (Casey, Tottenham, and Fossella 2002; Galvan et al. 2007). Almost an adult, the youngster has acquired a bigger level of abstraction, internal planning, and a sense of guilt (barely developed in children and absent in individuals suffering from certain psychopathologies).
Neural Plasticity and Activational and Organizational Effects
As mentioned above, brain development constitutes an ongoing process (Spear 2008a) and hence the importance of neural plasticity. The concept of neural plasticity refers to the capacity of the nervous system to adapt to constant changes of the internal and external environments, to previous experience and to traumatic experiences (Kanpolat 2012). Therefore, neural plasticity is of utmost importance in the learning process. Estrogens, progestagens, and androgens, end products of the hypothalamic–hypophyseal–gonadal axis, can affect the CNS by modulating neural plasticity and thus exerting a powerful influence on brain structures (Peper and Koolschijn 2012). According to the hypothetical framework of the classical studies of Phoenix et al. (1959), such effects have been classified as activational or organizational. The former modify neural activity favoring a given behavior in a specific context (Sisk and Zehr 2005), are not permanent and appear due to the action of hormones (endogenous or exogenous). Organizational effects, on the other hand, determine the structure of the nervous system during its development, remain in time after hormonal exposure and allow for the generation of activational responses when exposed to such stimuli in adulthood (Sisk and Zehr 2005).
As a consequence of the action of sex steroid hormones, important modifications take place in the CNS during the embryonic stage, also observed in puberty and adolescence (Schulz et al. 2005). During this period, sex steroids and other compounds structurally remodel the circuits that determine behavioral responses to hormones or sensory stimuli in adulthood (Sisk and Zehr 2005). Hence, adolescence represents a second stage in the development of CNS where steroid hormones, increasing during puberty, trigger permanent structural changes.
Organizational effects are explained through changes occurring in the CNS due to five mechanisms: myelination, neural pruning, apoptosis, dendritic spine remodeling, and epigenetic changes (figure 1).
Figure 1.
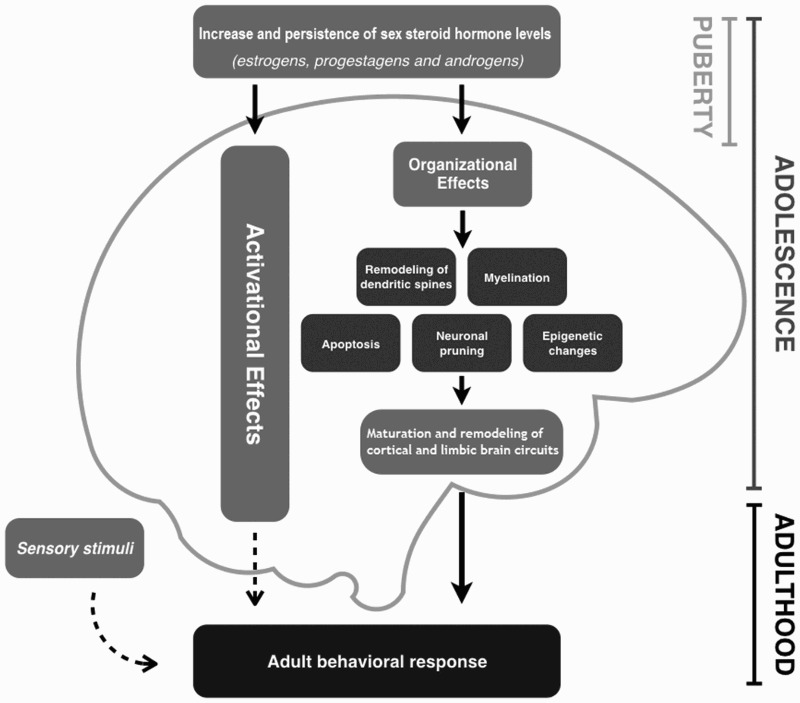
Influence of sex steroids on the organizational and activational effects that shape the adolescent brain. Organizational effects such as epigenetic mechanisms, neuronal (and receptor) pruning, remodeling of dendritic spines, myelination, and apoptosis are common during adolescence. These effects lead to the maturation and remodeling of cortical and limbic circuits, a fact that favors the expression of certain behaviors in adulthood, if activational effects occur.
Myelination
This is the process of forming a myelin sheath around the neuron axon. Myelin, formed mainly by lipids and, to some extent, by proteins, is a dielectric insulator that allows nerve impulses to travel in a “saltatory conduction,” thus increasing the speed of propagation along the axon. Myelin confers on axons their characteristic white appearance, and thus myelination is responsible for the increase in white matter in the brain during adolescence (Morriss et al. 1999). In the CNS, myelin is synthesized by oligodendrocytes, while in the peripheral nervous system synthesis is performed by Schwann cells (Curry and Heim 1966). In males, testosterone is fundamental in myelination since its administration seems to partly induce maturation of oligodendrocytes and formation of myelin (Kafitz et al. 1992). In females, on the other hand, estradiol and progesterone facilitate myelination, inducing the synthesis of myelin basic protein (Jung-Testas et al. 1992). The relevance of an adequate myelination process, therefore, lies in the fact that the development of the myelin sheath enhances the flow of information along the axons, and thus, leads to a faster and more efficient processing of information (Spear 2008a). Dayan and Guillery-Girard (2013) state that the development of white fibers resulting from myelination increases exponentially during adolescence, a process comparable to the resumption of the development tendencies observed during the first 5 years of life.
Neuronal Pruning
Neuronal pruning (or synaptic pruning) refers to the decrease in synaptic density (decrease in the number of synapses per unit of brain tissue) (Luna 2009) which had increased during the process of synaptogenesis during the individual's early development. In the prepuberal stage, the volume of gray matter increases significantly (i.e., a period of growth or “arborization”) linked to the last waves of synaptogenesis, followed by a considerable reduction in gray matter volume (Dayan and Guillery-Girard 2013). This phenomenon is associated with neuronal pruning, which “is believed to be essential for the fine-tuning of functional networks of brain tissue” (Blakemore and Choudhury 2006). During neural pruning, frequently used synaptic connections are reinforced and conserved, while seldom activated synapses degenerate. Hence, nerve circuits acquire a much more efficient synaptic configuration. In other words, neural pruning eliminates unwanted connections and solidifies brain circuitry (Ruben and Caskey 2007). Synaptic pruning is then a neural mechanism reflecting the elimination of the least active synapses, explaining the decrease of gray matter in adolescence (Weiten 2010). Neuronal pruning takes place, for example, in students with good bodily-kinesthetic skills who are not encouraged to move or exercise (Teele 2004). Most probably, the processes of human neuronal pruning (and “receptor pruning,” discussed later) involves sex steroids, among other hormones and compounds.
Apoptosis
Apoptosis (programed cell death) and other types of cell death allow for variation in the volume of different brain regions; they constitute mechanisms of neural plasticity and operate mainly in the embryonic stage. In fact, the developing nervous system produces more new neurons than are actually used, and selective pruning occurs by apoptosis (Gore et al. 2014), evidencing that both mechanisms are related. Estradiol shows a dual effect in this process: it acts as an anti-apoptotic agent, promoting division and proliferation of cortical neurons in rats (Brinton et al. 1997); and as a pro-apoptotic agent, triggering a reduction in the volume of the preoptic area (a region in the hypothalamus) in some mammals (Arai, Sekine, and Murakami 1996). In effect, one of the first nuclei showing sexual dimorphism (a concept to be discussed later) was identified in the preoptic area, and it is several times bigger in male than in female rats; such dimorphism is partially caused by steroid hormones on the apoptopic processes at play during brain development (Gore et al. 2014; Simerly 2002).
Dendritic Spine Remodeling
Dendritic spines, the small cytoplasmic extensions where synapses occur (Diamond, Gray, and Yasargil 1969), cover neuronal dendrites. These structures constitute a quite dynamic type of dendrite (they grow, persist, or degenerate according to the stimuli they receive), so each dendrite has a different spine remodeling, which can be altered by endocrine-metabolic pathologies, steroid hormone fluctuations, and ageing, among others (Coke and Wolley 2005), as well as by other exogenous elements such as drugs, synthetic hormones, and their derivatives. Among adult female rats, dendritic spine density changes along the normal ovarian cycle, being higher in the proestrus than in the estrus and diestrus (Chen et al. 2009). This indicates that estradiol could also promote dendritic spine formation and remodeling in other species such as humans.
Epigenetic Control of Gene Expression
Some of the changes observed in the adolescent brain are believed to be controlled by epigenetic phenomena, regulated in different ways by sex steroid hormones. Epigenetic changes allow for modifications in the expression of certain genes (and, thus, changes in brain structures or even in behavior) and are manifested through the following mechanisms: (i) DNA methylation: a way of repressing gene transcription, whose products may be peptidic intermediates participating in cell signaling, or promoters or repressors of the expression of other genes (Morrison et al. 2014). DNA methylation has been linked to a decrease in the expression of estrogen and progesterone receptors in some brain regions known to undergo changes during puberty (Prewitt and Wilson 2007; Westberry, Trout, and Wilson 2011). In castrated murine models, alterations are observed in the methylation patterns in the anteroventral and periventricular nucleus, which can be reverted by injecting testosterone. The expression of kiss1, the kisspeptin encoding-gene, has proved to be epigenetically regulated by DNA methylation. An alteration in the gene methylation can lead to an early onset of the rat fertile cycle (Lomniczi et al. 2013; Morrison et al. 2014). (ii) Histone acetylation: histones are proteins whose acetylation produces laxity in certain DNA regions, allowing for these regions to be transcribed and their products expressed. Brain histone acetylation changes when exposed to sex steroids, while inhibition of acetylation leads to a series of changes in reproductive behavior. In addition, a dimorphism (concept discussed later) may be observed in acetylation patterns, which could explain certain differences between the sexes (Morrison et al. 2014). (iii) Changes in microRNA levels: microRNA participates in post-transcriptional mechanisms of regulation of gene expression. Brain microRNA micro milieu changes in response to sex steroids and has a completely different pattern in males and females once puberty begins. This mechanism could exert a relevant role in the sexual dimorphisms developed during adolescence and puberty (Morrison et al. 2014).
Sexual Dimorphism in the Adolescent Brain
Sexual dimorphism is defined as the set of differences in the anatomic and physiological attributes of males and females of the same species, e.g., the dissimilar hormone concentrations of women and men. The female and male brains are known to be physically and biologically distinct. Current evidence suggests that most of these differences originate during adolescence due to changes triggered by the amount of female and male sex steroids released, which vary from puberty onwards. Ingalhalikar et al. (2014), in a study involving 949 individuals, showed that one of the most relevant changes that occurs during adolescence is linked to the connectivity between and within brain hemispheres. There is higher intrahemispheric connection in men, which could be associated to a higher coordination between perception and execution. On the other hand, women evidence increased interhemispheric connectivity, which could relate to a more efficient communication between analytic and intuitive processing. Thus, as established by Baron-Cohen (2004, 1), “the female brain is predominantly hard-wired for empathy. The male brain is predominantly hard-wired for understanding and building systems.” Koolschijn, Peper, and Crone (2014) investigated, in 215 individuals ages 8 to 25, the effect on the brain of the sex steroids testosterone and estradiol along the progression from childhood to adulthood. Results suggest that this stage of development presents morphological changes in the brain associated to hormone levels. Men show correlation between high testosterone levels and lower volume of gray matter in the anterior singular cortex; women, however, show no change from childhood to adolescence in this region, which is linked to the assessment of options before taking action. On the other hand, women who were found to possess high testosterone levels had a higher volume of gray matter in the frontal orbital cortex, a fact exerting a major influence on decision making both when risk is involved and when decisions are based on assessment of rewards. In order to determine to what extent changes in the adolescent brain are dependent on hormones, Herting et al. (2014) researched 126 adolescents aged 10 to 14 who underwent magnetic resonance imaging (MRI) to measure their hormone level in two stages, with a two-year lapse between measurements. Results show that, regardless of age, variations in cortical volume and in some subcortical regions (such as the amygdala and caudate) relate to the levels of testosterone and estradiol (Herting et al. 2014).
The sexual dimorphisms found are not only structural, but also cognitive and functional. One of the circuits experiencing significant changes is that known as the “empathy circuitry.” Both men and women show a recruitment of mirror neurons in the inferior gyrus; women, however, evidence higher recruitment from the right inferior gyrus and the superior temporal suculus, while the opposite is true for men. Such distinction corresponds to differences in estrogen levels. In addition, it has been suggested that when we respond emotionally in reaction to somebody else's emotion, women's empathic response is determined for the most part by the human mirror neuron system; in men, on the other hand, the response is linked to the theory of mind, and thus related to a cognitive strategy (Schulte-Rüther et al. 2008). This would explain the distinctive emotional circuits of men and women. Some neuroimaging studies suggest the existence of a series of activational effects in the amygdala and prefrontal cortex; in fact, both show higher sexual dimorphism during puberty and adolescence because of their dissimilar levels of gonadal hormones. Such effects would partially explain the differences in emotion and affection between both sexes (Van Wingen et al. 2011).
Brain Developmental Window
Some decades ago, it was generally agreed that brain organizational changes took place only during embryonic development, while activational changes (hormone-regulated) occurred during puberty. However, both types of changes have been subsequently associated with puberty and adolescence (Schulz et al. 2004, 2005; Schulz and Sisk 2006). Research assessing copulatory behavior in the male hamster in the presence of a female hamster has provided relevant information in this respect (Schulz et al. 2004). In these experiments, the prepuberal rodents who were administered testosterone did not copulate. The same happened with adult males under the same conditions, but castrated in the prepuberal stage. However, adult hamster males who had been castrated during their postpuberal stage immediately generated copulatory responses following testosterone administration. Thus, copulatory response in adult rodents requires the presence of testosterone both in puberty and in adulthood (Schulz et al. 2004). Therefore, this behavior results from a hormonal effect at an activational level. The behavior, however, only occurs provided there has been a previous organizational change triggered by exposure to testosterone in puberty; the latter coordinates the neural circuitry required to exhibit the characteristic adult sexual behavior in the presence of sex steroid hormones and a female individual. The proof that such organizational events did not take place during the embryonic stage lies in that hamsters did not show sexual behavior under the same circumstances in the prepuberal period (Schulz et al. 2004). Copulatory behavior was only evidenced in adults given that, in their puberal stage, they possessed gonads able to secrete physiological concentrations of sex steroid hormones. This suggests that certain organizational processes occur during adolescence.
The effect of androgens, such as testosterone and dihydrotestosterone, is strong from the embryonic and fetal periods. Lombardo et al. (2012) studied twenty-eight human fetuses and embryos, determining the presence of fetal testosterone in the amniotic fluid (amniocentesis) during the critical period of brain masculinization (between 13 and 20 weeks of gestation). At 8 and 11 years of age, these children were given structural MRIs; voxel-based morphometry showed that the volume of matter in the right temporoparietal junction/posterior superior temporal sulcus and bilateral somatopsensory, motor and pre-motor cortex positively correlated with fetal testosterone levels for all twenty-eight children. In addition, androgens can affect cognitive abilities. A classic study on verbal and spatial ability tests conducted on men by Hier and Crowley (1982) showed no differences in verbal skills, but lower scores in subjects with prepuberal idiopathic hypogonadism and postpuberal hypogonadism, as compared to control. Androgen replacement therapy through administration of testosterone after puberty did not affect the scores of either hypogonadic group (Hier and Crowley 1982). The latter suggests that organizational changes determining the development of spatial skills are also activated during adolescence, which requires physiological levels of androgens (testosterone or its derived metabolites) produced by functional gonads.
Based on the aforementioned, during adolescence there is a “plasticity window” allowing for the occurrence of the structural (organizational) changes required to trigger certain activational effects. This organizational window is limited, so it is possible to suggest that puberty constitutes the critical period when the multiple changes affecting the encephalon could affect adult behavior (figure 1). The effect of steroid hormones in the process results, in the first place, from their interaction with their receptors in the neurons, and, next, from the coordination and execution of specific cell responses. This phenomenon can be better understood by studying the intracellular signaling pathways used by sex steroids, both genomic effects (also known as canonical), in which hormonal action involves genetic transcription (Jensen and DeSombre 1973; Sherman, Corvol, and O'Malley 1970), and non-genomic (non-canonical) effects, where membrane receptors act through secondary messengers such as calcium cation, inositol triphosphate, cyclic adenosine monophosphate and diacylglycerol, among others (Falkenstein and Wehling 2000).
Extense research is currently underway to determine which steroidal effects are genomic, non-genomic, or result from a combination of both. Interestingly, spermatozoa constitute an excellent model to study such effects (Luconi et al. 2004; Meizel 2004; Vigil et al. 2009). During its development, this cell loses its gene transcription ability; therefore, non-genomic signaling pathways are the only ones available. The fact that steroid hormone receptors in both spermatozoa and neurons are uncommonly alike in mammals represents another advantage, suggesting that both cell types possess a similar capacity to react to the same environmental hormone changes (Meizel 2004), for example, showing comparable responses to the interactions between steroid hormones and the GABAergic system (Vigil et al. 2009; Vigil, Orellana, and Cortés 2011). GABA together with progesterone (neuroactive steroid) would influence mood swings in women undergoing hormone replacement therapy by inducing a negative mood (Andreen et al. 2009); in addition, it triggers the acrosomic reaction (AR) in human spermatozoa (Vigil et al. 2009; Vigil, Orellana, and Cortés 2011). Estradiol, on the other hand, would present the opposite effect together with GABA, both at the CNS and in spermatozoa, leading to a state of euthymia and inhibiting AR, respectively (Andreen et al. 2009; Vigil, Orellana, and Cortés 2011).
It seems reasonable to assume that in puberty changes in sex steroid levels possess a relevant role, since they not only exert their influence on the reproductive system, but would also impact brain development and organization during this stage. In the adolescence, steroid hormones also play a part in brain organizational development through the mechanisms of plasticity aforementioned. Thus, puberty and adolescence appear to be closely linked, considering that the brain constitutes a target organ that responds to the presence of a number of hormones whose concentration increases during puberty. To date, solid evidence suggests that gonadal hormones have a different influence on the socio-sexual behavior of men and women during adolescence (Sisk 2016). Thus, during male adolescent development, testosterone programs the ability to make behavioral adaptations to social experience that improve reproductive efficience and avoid aggression, leading to better reproductive fitness. High levels of ovarian hormones during puberty, on the other hand, seem to organize fertility-related behaviors, which is contingent on metabolic signals predicting sufficient energy availability to sustain pregnancy, lactation, and maternal care (Sisk 2016).
Psychological Alterations Associated with Endocrine Disorders
The understanding of the effects of sex steroids on behavior is still limited; however, there seems to be a correlation between the presence of endocrine disorders and certain behavioral alterations. During adolescence, sex steroids modify the structure of neuronal connections and the number of neurotransmitter receptors present in neurons since the prenatal period (Weiner, Primeau, and Ehrmann 2004). Such changes would affect the CNS ability to respond to exogenous and endogenous variables, leading to affective and mood disorders (Goel and Bale 2009; Weiner, Primeau, and Ehrmann 2004). Studies have validated this hypothesis, showing the link between free testosterone (and other steroids) and both premenstrual syndrome and depression in women (Baischer et al. 1995; Dalton 1981; Eriksson et al. 1992; Godoy and Cortés 2014a; Vogel, Klaiber, and Broverman 1978).
One pathology that can be associated to psychological alterations is polycystic ovary syndrome (PCOS), an endocrine disorder defined as an ovulatory dysfunction resulting from hyperandrogenism and/or hyperandrogenemia, frequently linked to endocrine-metabolic disorders such as hyperinsulinemia, insulin resistance, and its co morbidities (Godoy and Cortés 2014b; Vigil et al. 2007). PCOS women show high correlation between their abnormal androgen levels and mood disorders, which range from emotional problems to sexual identity problems and depression (Ghaziuddin 1989; Orenstein et al. 1986; Rasgon et al. 2002). Studies carried out by researchers in the field of nursing and midwifery have found that PCOS significantly affects women's quality of life, mainly in the areas of menstrual problems, emotions, body hair, body weight, and infertility (Aguirre, Benvenuto, and Urrutia 2005; Muñoz et al. 2010). Fontecilla and Worthington (2005) used the Rorschach test with PCOS women, evidencing distortions in the configuration of the psychological self: they tended to think they stood at a disadvantage as compared to others, and to have an extremely self-critical and intolerant approach to non-standard factors. An interesting review on this topic by Krępuła et al. (2012) states that among PCOS women depression is often found, sometimes accompanied by suicidal ideation, bipolar disorder (hypothymia and major depression disorders), and eating disorders such as anorexia nervosa, bulimia nervosa, and periodic overeating (binge-eating disorder), with pervasive effects on the patients’ quality of life (Krępuła et al. 2012). Considering that among PCOS women metabolic disorders such as insulin resistance and hyperinsulinemia, hyperglycemia, and/or obesity usually coexist, and that many of them show exacerbated stress levels—manifested in the ovary as an increase in sympathetic secreting activity of noradrenalin (norepinephrine), a hormone affecting synthesis of sex steroids and, therefore, follicular development (Araya, Jara, and Lara 2004)—it is necessary to elucidate the existence of a common pathogenic disorder underlying these altered metabolic conditions and the psychological problems frequently observed in PCOS women, as well as to specifically determine the effects of hyperandrogenemia on these women's brain circuits.
Prolactin, the peptid hormone released by the adenohypophysis (pituitary anterior lobe), would also modulate human mood. An interesting study by Reavley et al. (1997) compared sixty-five women suffering from hyperprolactinemia (pituitary adenoma or idiopathic or “functional” hyperprolactinemia) to a twenty-six woman control group with pituitary normoprolactinemia (acromegalia or non-functional hypophyseal adenoma). The Hospital Anxiety and Depression questionnaire was used with both groups. As a result, 54 percent hyperprolactinemia patients showed defined or borderline anxiety as compared to 27 percent of the normoprolactinemia control group. These results evidence significant presence of anxiety among a proportion of women suffering from hyperprolactinemia. On the other hand, an interesting study carried out by Lee et al. (1986) concluded that steroidogenesis by the granulosa lutein cells of preovulatory human follicles is probably not influenced by the quantities of prolactin that are normally present in the blood or follicular fluid. Higher doses, however, may suppress production of the sex steroids estradiol and progesterone.
Cortisol (hydrocortisone) is a glucocorticoid steroid known as “the major stress hormone”; cortisol can also affect the developing brain of children and adolescents. According to the classical proposal by Selye (1950), “anything that causes stress endangers life, unless it is met by adequate adaptive responses; conversely, anything that endangers life causes stress and adaptive responses.” Nowadays, toxic stress is defined as the strong, frequent, or prolonged activation of the body's stress management system. Most investigations evidence that toxic stress can have an adverse effect on brain architecture. During the sensitive stages of brain development, toxic stress can produce an excessive amount of neuronal connections in the brain regions involved in fear, anxiety, and impulse responses; at the same time, it can reduce the number of neuronal connections in the areas devoted to reasoning, planning, and behavioral control (NSCDC 2014). Extreme exposure to toxic stress can even alter the stress system to respond at lower thresholds to events other people would not see as stressful, and thus, more frequently and for a longer period than necessary. Such wearing of the system increases the risk of stress-related physical and mental illnesses in adulthood (NSCDC 2014).
Adolescence is a stage when response to sex hormones and stress is enhanced; the maturation of dopaminergic neuronal circuits is deeply influenced by these factors (Sinclair et al. 2014). In youngsters exposed to stress, increased production and secretion of glucocorticoids like cortisol partly contribute to the modern diathesis-stress hypothesis which suggests that cortisol secretion would exert a significant pathophysiological role in the etiology of depression (Gillespie and Nemeroff 2005). Testosterone, estrogens, and glucocorticoids interact, having different region-specific impacts on the dopaminergic neurotransmission in the adolescent brain, shaping brain maturation, and cognitive function in adolescence and adulthood. Some effects of sex/stress hormones on the levels of cortical and subcortical dopamine show similarities to dopaminergic abnormalities described in schizophrenia, suggesting a possible participation of sex/stress hormones during adolescence triggering risk of psychiatric disorder through the modulation of dopamine neurotransmission. A marked decrease in the density of dopamine receptors (by a process of receptor pruning) in male rats during the periadolescent period has been reported (Andersen et al. 2000), which leads to the interesting question of the influence of stress on this process. For this and other reasons, sex and stress hormones constitute easy targets for future strategies aiming at prevention and treatment of psychiatric disorders (Sinclair et al. 2014).
Effects of the Use of Contraceptives on the Adolescent Brain
Exogenous sex hormones can also have effects at metabolic and psychic levels in adolescents and adults (Cortés and Alfaro 2014; Klaus and Cortés 2015). In the past few years, research has contributed significant evidence on the effect exerted by the active compounds of hormone-based contraceptives (ethinyl estradiol and progestins, as well as chemically related compounds) on brain excitability, and thus on the mood of the users (Gingnell et al. 2013; Pluschino, Caruso, and Daino 2012). Even though hormonal contraceptives are among the most widely studied drugs in the history of medicine, Cobey and Buunk (2012) argue that little research has focused on determining the consequences of these drugs on female psychological welfare; this has led to users and health professionals being misinformed regarding the consequences of their use. For decades, hormonal contraceptives such as oral formulations have been thought to produce psychological disorders in female users, e.g., depression, mood swings, changes in the libido, and unstable affective relationships (Klaus and Cortés 2015). This appears to be especially relevant considering that many adolescents are currently being prescribed such hormone formulations as contraceptive methods (Feldman 2006). The effects of hormonal contraceptives have not been taken seriously; and, moreover, the possible influences on young girls’ brains and behavior, and the social effects of the intake of hormonal contraceptives are generally being overlooked.
Pletzer et al. (2010) have studied young women's brains (both using and not using contraceptives) through high-resolution structural images, and have found that contraceptive users had larger prefrontal cortices, pre- and post-central gyri, parahippocampal and fusiform gyri, and temporal regions. An interesting study of female university students (18–35 years old) carried out by Nielsen et al. (2011) found alterations in the emotional memory of hormonal contraceptive users as opposed to naturally cycling women (non-contraceptive users). Considering that the effects of stress hormones (e.g., cathecolamines such as norepinephrine) on memory depend on the levels of sex hormones, these researchers attribute such finding to the alteration produced by hormone contraceptives on sex/stress hormone interactions on memory formation (Nielsen et al. 2011). In addition, other investigations show that the use of contraceptives by adult users affects performance in numerical cognitive tests, presenting masculinized answers (Pletzer et al. 2014). The same researcher tested androgenic and anti-androgenic contraceptives, obtaining different results for both types of drugs. The strongest changes both in structure and performance in the face recognition test were evidenced in users of androgenic contraceptives such as levonorgestrel (Pletzer, Kronbichler, and Kerschbaum 2015). Another interesting observation was made by Abler et al. (2013), who described that women using oral contraceptives present a weaker response to erotic stimuli than women with menstrual cycles in their follicular phase.
The question arises whether the changes resulting from steroidal contraceptive use add their effects and risks to other noxious exogenous factors like sleep deprivation and consumption of drugs such as nicotine and alcohol, common behaviors among some adolescent girls. Together, the latter would affect the organizational processes in the brain during the adolescence, resulting in behavioral and psycho-affective disorders in the adulthood (Baischer et al. 1995; Vogel, Klaiber, and Broverman 1978).
Concluding Remarks
Adolescent behavior is characterized by irrational decisions, impulsivity and lack of emotional control, evidencing a particular neurological phase. Two relevant events take place at this stage: (i) increase in the level of sex steroid hormones, triggering secondary sex characters as a systemic effect, and (ii) a “plasticity window” in which permanent organizational brain processes take place. Both events are closely linked due to the physiological level of steroid hormones in adolescence, which allow for a timely and adequate remodeling of the brain circuits, determining the existence of activational effects in adulthood. Several adult behavioral patterns, such as copulatory sexual conduct and spatial ability, are dependent upon this endocrine modulation.
A number of neural plasticity mechanisms have been described through which sex steroids exert organizational changes in the brain: neuronal (and receptor) pruning, myelination, apoptosis, dendritic spine remodeling, and epigenetic changes. These mechanisms could exert permanent changes at different levels in the CNS (figure 1). It is of utmost importance to consider the effects of internal and external factors capable of altering the hormonal balance during adolescence, and therefore, interfering with such plasticity mechanisms. These could negatively influence CNS organizational modifications, triggering permanent effects which would become evidence later in adulthood, such as some endocrine-metabolic disturbances, for example, PCOS.
In the face of studies evidencing that hormonal contraceptives can cause psychological disorders in women, it is important to consider these facts in under-age users of hormonal contraceptives, specifically in teenage girls whose brains are undergoing a process of remodeling by the action of sex steroids. We believe that the scientific community has not devoted enough effort to studying the effects of exogenous steroids on their users’ brain development. These hormones are probably triggering permanent organizational changes in users’ brains, with consequences on their adult behavioral patterns; and such effects probably remain through life. As a way of example, the human prefrontal cortex is an area whose final volume is not reached until the early twenties (Yurgelun-Todd 2007), hence, the use of contraceptives before this age could lead to changes in this region's permanent circuitry (Pletzer and Kerschbaum 2014). The latter has several implications, both from an ethical point of view and from the perspective of pediatric medicine. Cortés and Alfaro (2014) state that it is the duty and ethical responsibility of physicians and other health specialists, in the light of the risks linked to the use of hormonal contraceptives, to offer advice and guidance to users, and to warn them of the secondary effects these drugs involve. Worth considering, one of the fundamental principles of health professionals and health educators should always be the promotion of health maintenance and the prevention of risky behaviors (Cortés and Alfaro 2014). Parra (2013) argues that every professional is morally obliged to properly inform the patient; in fact, patients are legally entitled to be informed. Moreover, Peck and Norris (2012), in an interesting article published in The Linacre Quarterly, state that prescribing hormonal contraceptives without proper warning of its risks to the user violates the Hippocratic Oath to “do no harm.” In addition, these researchers argue that while physicians feel they cannot ethically “impose” their own Catholic morality they should rightly insist that their patients be given information about all the risks of hormonal contraceptives so that they may give opportune, adequate, and complete informed consent (Peck and Norris 2012). As mentioned above, many adolescents are currently using various types of steroid contraceptives, either as a contraceptive per se, or prescribed as treatment for menstrual pathologies (Béliard 2016; Feldman 2006); and for this reason, research on the effects these hormonal formulations have on human physiology, especifically on young girls’ brain development should be of special clinical interest to pediatricians, gynecologists, midwives, and other health specialists—the same as a timely, adequate, and straightforward sharing of the information obtained. In addition, biomedical researchers, health educators (e.g., high school teachers), as well as the patients themselves (and their parents and guardians if they are minors) are actively encouraged to take interest in this issue.
In view of the aforementioned facts, during adolescence there is a unique plasticity window, strongly influenced by endogenous and exogenous steroid hormones, among other factors. The effect produced by these hormones over this plasticity window is directly associated with patterns of emotional behavior in adult life.
Acknowledgments
We would like to thank the librarians of SIBUC (Pontifical Catholic University of Chile Library Service) for their support, as well as CFR-Abbott-Chile librarians, who kindly granted access to some of the references cited in this manuscript.
Biographies
Dr. Pilar Vigil is associate professor at the Pontificia Universidad Católica de Chile, Santiago, and medical director of the Reproductive Health Research Institute (RHRI), Santiago, Chile. In addition, Dr. Vigil is a member of the Pontifical Academy for Life, Vatican City, and president of Teen STAR International.
Juan Pablo del Río is currently pursuing a degree in medicine and surgery and studying philosophy at the Universidad de los Andes, Santiago, Chile.
Dr. Manuel E. Cortés is professor of human physiology, researcher and head of the Departamento de Ciencias Químicas y Biológicas, Universidad Bernardo O Higgins, and a postdoctoral researcher at the RHRI, Santiago, Chile.
Mrs. Bárbara Carrera is a biologist and RHRI research coordinator.
Dr. Hernán Rioseco is a physician and researcher at RHRI.
Mrs. Florencia C. Aránguiz is a biologist and researcher at RHRI.
References
- Abler B., Kumpfmüller D., Grön G., Walter M., Stingl J., and Seeringer A.. 2013. Neural correlates of erotic stimulation under different levels of female sexual hormones. PLoS One 8: e54447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aguirre A., Benvenuto G., and Urrutia M. T.. 2005. Calidad de vida en mujeres con síndrome de ovario poliquístico. Revista Chilena de Obstetricia y Ginecología 70: 103–7. [Google Scholar]
- Andersen S. L., Thompson A. T., Rutstein M., Hostetter J. C., and Teicher M. H.. 2000. Dopamine receptor pruning in prefrontal cortex during the periadolescent period in rats. Synapse 37: 167–69. [DOI] [PubMed] [Google Scholar]
- Andreen L., Nyberg S., Turkmen S., van Wingen G., Fernández G., and Bäckström T.. 2009. Sex steroid induced negative mood may be explained by the paradoxical effect mediated by GABAA modulators. Psychoneuroendocrinology 34: 1121–32. [DOI] [PubMed] [Google Scholar]
- Arai Y., Sekine Y., and Murakami S.. 1996. Estrogen and apoptosis in the developing sexually dimorphic preoptic area in female rats. Neurochemical Research 25: 403–7. [DOI] [PubMed] [Google Scholar]
- Araya V., Jara P., and Lara H. E.. 2004. Cerebro, estrés y ovario poliquístico. Participación de la inervación simpática en el desarrollo del síndrome de ovario poliquístico. Endocrinología y Nutrición 51: 473–7. [Google Scholar]
- Baischer W., Koinig G., Hartmann B., Huber J., and Langer G.. 1995. Hypothalamic-pituitary-gonadal axis in depressed premenopausal women: Elevated blood testosterone concentrations compared to normal controls. Psychoneuroendocrinology 20: 553–59. [DOI] [PubMed] [Google Scholar]
- Baron-Cohen S. 2004. The essential difference: Male and female brains and the truth about autism. New York: Basic Books. [Google Scholar]
- Béliard A. 2016. Première consultation de contraception chez les adolescentes. Revue Médicale de Liège 71: 28–33. [PubMed] [Google Scholar]
- Blakemore S. J., and Choudhury S.. 2006. Development of the adolescent brain: Implications for executive function and social cognition. Journal of Child Psychology and Psychiatry 47: 296–312. [DOI] [PubMed] [Google Scholar]
- Brinton R. D., Tran J., Proffitt P., and Montoya M.. 1997. 17 β-estradiol enhances the outgrowth and survival of neocortical neurons in culture. Neurochemical Research 22: 1339–51. [DOI] [PubMed] [Google Scholar]
- Casey B., Tottenham N., and Fossella J.. 2002. Clinical, imaging, lesion, and genetic approaches toward a model of cognitive control. Developmental Psychobiology 40: 237–54. [DOI] [PubMed] [Google Scholar]
- Casey B. J., Jones R. M., and Hare T. A.. 2008. The adolescent brain. Annals of the New York Academy of Sciences 1124: 111–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J. R., Yan Y. T., Wang T. J., Chen L. J., Wang Y. J., and Tseng G. F.. 2009. Gonadal hormones modulate the dendritic spine densities of primary cortical pyramidal neurons in adult female rat. Cerebral Cortex 19: 2719–27. [DOI] [PubMed] [Google Scholar]
- Cobey K. D., and Buunk A. P.. 2012. Conducting high-quality research on the psychological impact of oral contraceptive use. Contraception 86: 330–331. [DOI] [PubMed] [Google Scholar]
- Coke B. M., and Wolley C. S.. 2005. Gonadal hormone modulation of dendrites in the mammalian CNS. Journal of Neurobiology 64: 34–46. [DOI] [PubMed] [Google Scholar]
- Cortés M. E., and Alfaro A. A.. 2014. The effects of hormonal contraceptives on glycemic regulation. Linacre Quarterly 81: 209–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cortés M. E., Carrera B., Rioseco H., del Río J. P., and Vigil P.. 2015. The role of kisspeptin in the onset of puberty and in the ovulatory mechanism: A Mini-review. Journal of Pediatric & Adolescent Gynecology 28: 286–91. [DOI] [PubMed] [Google Scholar]
- Curry J. J., and Heim L. M.. 1966. Brain myelination after neonatal administration of oestradiol. Nature 209: 915–6. [DOI] [PubMed] [Google Scholar]
- Dalton M. E. 1981. Sex hormone-binding globulin concentrations in women with severe premenstrual syndrome. Postgraduate Medical Journal 57: 560–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dayan J., and Guillery-Girard B.. 2013. Psychopathologie de l'adolescent et neurosciences: Congruences et incongruences. Archives de Pédiatrie 20: 206–7. [Google Scholar]
- Diamond J., Gray E. G., and Yasargil G. M.. 1969. The function of dendritic spines: An analysis. Journal of Physiology 202: 116P. [PubMed] [Google Scholar]
- Elkind D. 1967. Egocentrism in Adolescence. Child Development 38: 1025–34. [PubMed] [Google Scholar]
- Epstein R. 2008. El mito del cerebro adolescente. Mente y Cerebro 32: 22–9. [Google Scholar]
- Eriksson E., Sundblad C., Lisjö P., Modigh K., and Andersch B.. 1992. Serum levels of androgens are higher in women with premenstrual irritability and dysphoria than in controls. Psychoneuroendocrinology 17: 195–204. [DOI] [PubMed] [Google Scholar]
- Ernst M., Bolla K., Mouratidis M., Contoreggi C., Matochik J. A., Kurian V., Cadet J. L., Kimes A. S., and London E. D.. 2002. Decision-making in a risk-taking task: A PET study. Neuropsychopharmacology 26: 682–91. [DOI] [PubMed] [Google Scholar]
- Falkenstein E., and Wehling M.. 2000. Nongenomically initiated steroid actions. European Journal of Clinical Investigation 30: 51–54. [DOI] [PubMed] [Google Scholar]
- Feldman E. 2006. Contraceptive care for the adolescent. Primary Care: Clinics in Office Practice 33: 405–31. [DOI] [PubMed] [Google Scholar]
- Fontecilla A., and Worthington S.. 2005. Análisis de la función de identidad en mujeres diagnosticadas con síndrome de ovario poliquístico a través del test de Rorschach. Tesis Título Profesional de Psicología, Universidad de Chile.
- Galvan A., Hare T., Voss H., Glover G., and Casey B. J.. 2007. Risk-taking and the adolescent brain: Who is at risk? Developmental Science 10: F8–14. [DOI] [PubMed] [Google Scholar]
- García-Mayor R. V., Andrade M. A., Ríos M., Lage M., Diéguez C., and Casanueva F. F.. 1997. Serum leptin levels in normal children: Relationship to age, gender, body mass index, pituitary-gonadal hormones, and pubertal stage. Journal of Clinical Endocrinology and Metabolism 82: 2849–55. [DOI] [PubMed] [Google Scholar]
- Ghaziuddin M. 1989. Polycystic ovary disease, manic-depressive illness and mental retardation. Journal of Mental Deficiency Research 33: 335–8. [DOI] [PubMed] [Google Scholar]
- Gillespie C. F., and Nemeroff C. B.. 2005. Hypercortisolemia and depression. Psychosomatic Medicine 67: S26–8. [DOI] [PubMed] [Google Scholar]
- Gingnell M., Engman J., Frick A., Moby L., Wikström J., Fredrikson M., and Sundström-Poromaa I.. 2013. Oral contraceptive use changes brain activity and mood in women with previous negative affect on the pill—a double-blinded, placebo-controlled randomized trial of a levonorgestrel-containing combined oral contraceptive. Psychoneuroendocrinology 38: 1133–44. [DOI] [PubMed] [Google Scholar]
- Godoy S., and Cortés M. E.. 2014a. Cuidados de la mujer con síndrome de ovario poliquístico. In Enfermería Ginecológica, ed. Urrutia María Teresa, Riquelme Giselle, and Araya Alejandra, 139–49. Santiago: Editorial Mediterráneo. [Google Scholar]
- Godoy S., and Cortés M. E.. 2014b. Cuidados de la mujer con síndrome premenstrual. In Enfermería Ginecológica, ed. Urrutia María Teresa, Riquelme Giselle, and Araya Alejandra, 151–60. Santiago: Editorial Mediterráneo. [Google Scholar]
- Goel N., and Bale T. L.. 2009. Examining the intersection of sex and stress in modelling neuropsychiatric disorders. Journal of Neuroendocrinology 21: 415–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman-Rakic P. S., Chafee M., and Friedman H.. 1993. Allocation of function in distributed circuits. In Brain mechanisms of perception and memory: From neuron to behaviour, ed. Ono Taketoshi, Fukuda Masaji, Squire Larry R., Raichle Marcus E., and Perrett David I., 445–56. New York: Oxford University Press. [Google Scholar]
- Gore A. C., Martien K. M., Gagnidze K., and Pfaff D.. 2014. Implications of prenatal steroid perturbations for neurodevelopment, behavior, and autism. Endocrine Review 35: 961–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall P. A., Holmqvist M., and Sherry S. B.. 2004. Risky adolescent sexual behavior: A psychological perspective for primary care clinicians. Topics in Advanced Practice Nursing Journal 4. http://www.medscape.com/viewarticle/467059.
- Havelock J. C., Auchus R. J., and Rainey W. E.. 2004. The rise in adrenal androgen biosynthesis: Adrenarche. Seminars in Reproductive Medicine 22: 337–47. [DOI] [PubMed] [Google Scholar]
- Herting M. M., Gautam P., Spielberg J. M., Kan E., Dahl R. E., and Sowell E. R.. 2014. The role of testosterone and estradiol in brain volume changes across adolescence: A longitudinal structural MRI. Human Brain Mapping 35: 5633–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hier D. B., and Crowley W. F.. 1982. Spatial ability in androgen-deficient men. New England Journal of Medicine 306: 1202–5. [DOI] [PubMed] [Google Scholar]
- Ingalhalikar M., Smith A., Parker D., Satterhwaite T. D., Elliot M. A., Ruparel K., Hakonarson H., Gur R. E., and Verma R.. 2014. Sex differences in the structural connectome of the human brain. Proceedings of the National Academy of Sciences 111: 823–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen E. V., and DeSombre E. R.. 1973. Estrogen-receptor interaction: Estrogenic hormones effect transformation of specific receptor proteins to a biochemically functional form. Science 182: 126–34. [DOI] [PubMed] [Google Scholar]
- Jung-Testas I., Renoir M., Bugnard H., Greene G. L., and Baulieu E. E.. 1992. Demonstration of steroid hormone receptors and steroid action in primary cultures of rat glial cells. Journal of Steroid Biochemistry and Molecular Biology 41: 621–31. [DOI] [PubMed] [Google Scholar]
- Kafitz K. W., Herth G., Bartsch U., Güttinger H. R., and Schachner M.. 1992. Application of testosterone accelerates oligodendrocyte maturation in brains of zebra finches. Neuroreport 3: 315–18. [DOI] [PubMed] [Google Scholar]
- Kail R. 1993. Processing time decreases globally at an exponential rate during childhood and adolescence. Journal of Experimental Child Psychology 56: 254–65. [DOI] [PubMed] [Google Scholar]
- Kanpolat Yücel. 2012. Research and publishing in neurosurgery. Vienna: Springer Science & Business Media. [Google Scholar]
- Klaus H., and Cortés M. E.. 2015. Psychological, social, and spiritual effects of contraceptive steroid hormones. Linacre Quarterly 82: 283–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koolschijn P. C. M. P., Peper J. S., and Crone E. A.. 2014. The influence of sex steroids on structural brain maturation in adolescence. PLoS One 9: e83929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krępuła K., Bidzińska-Speichert B., Lenarcik A., and Tworowska-Bardzińska U.. 2012. Psychiatric disorders related to polycystic ovary syndrome. Endokrynologia Polska 62: 488–91. [PubMed] [Google Scholar]
- Latronico A. C. 2012. Kisspeptin: Switching on puberty. Endocrine News, August, 14–18. [Google Scholar]
- Lee M. S., Ben-Rafael Z., Meloni F., Mastroianni L., and Flickinger G. L.. 1986. Effects of prolactin on steroidogenesis by human luteinized granulosa cells. Fertility and Sterility 46: 32–6. [DOI] [PubMed] [Google Scholar]
- Lerner Richard M., and Steinberg Laurence. 2004. Handbook of adolescent psychology. New York: Wiley. [Google Scholar]
- Lombardo M. V., Ashwin E., Auyeung B., Chakrabarti B., Taylor K., Hackett G., Bullmore E. T., and Baron-Cohen S.. 2012. Fetal testosterone influences sexually dimorphic gray matter in the human brain. Journal of Neuroscience 32: 674–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lomniczi A., Loche A., Castellano J. M., Ronnekleiv O. K., Bosch M., Kaidar G., Knoll J. G., Wright H., Pfeifer G. P., and Ojeda S. R.. 2013. Epigenetic control of female puberty. Nature Neuroscience 16: 281–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luconi M., Francavilla F., Porazzi I., Macerola B., Fortu G., and Baldi E.. 2004. Human spermatozoa as a model for studying membrane receptors mediating rapid nongenomic effects of progesterone and estrogens. Steroids 69: 553–59. [DOI] [PubMed] [Google Scholar]
- Luna Beatriz. 2009. The maturation of cognitive control and the adolescent brain. In From attention to goal-directed behavior: Neurodynamical, methodological and clinical trends, ed. Aboitiz Francisco, and Cosmelli Diego, 249–67. Berlin: Springer-Verlag. [Google Scholar]
- McCarthy M. 2007. GABA receptors make teens resistant to input. Nature Neuroscience 10: 397–9. [DOI] [PubMed] [Google Scholar]
- Meizel S. 2004. The sperm, a neuron with a tail: “neuronal” receptors in mammalian sperm. Biological Reviews of the Cambridge Philosophical Society 79: 713–32. [DOI] [PubMed] [Google Scholar]
- Miller Walter L., and Styne Dennis M.. 1999. Female puberty and its disorders. In Reproductive endocrinology: Physiology, pathophysiology, and clinical management, ed. Yen Samuel C. C., and Barbieri Robert, 388–412. Philadelphia: W.B. Saunders Company. [Google Scholar]
- Morrison K. E., Rodgers A. B., Morgan C. P., and Bale T. L.. 2014. Epigenetic mechanisms in puberal brain maturation. Neuroscience 264: 17–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morriss M. C., Zimmerman R. A., Bilaniuk L. T., Hunter J. V., and Haselgrove J. C.. 1999. Changes in brain water diffusion during childhood. Neuroradiology 41: 929–34. [DOI] [PubMed] [Google Scholar]
- Muñoz L., Villa L., Araya A., and Urrutia M. T.. 2010. Calidad de vida en mujeres con síndrome de ovario poliquístico. Horizonte de Enfermería 21: 11–8. [Google Scholar]
- Nielsen S. E., Ertman N., Lakhani Y. S., and Cahill L.. 2011. Hormonal contraception usage is associated with altered memory for an emotional story. Neurobiology of Learning and Memory 96: 378–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NSCDC 2014. National scientific council on the developing child. (2005/2014). Excessive stress disrupts the architecture of the developing brain. Working Paper 3. Updated edition. http://www.developingchild.harvard.edu.
- Orenstein H., Raskind M. A., Wyllie D., Raskind W. H., and Soules M. R.. 1986. Polysymptomatic complaints and Briquet's syndrome in polycystic ovary disease. American Journal of Psychiatry 143: 768–71. [DOI] [PubMed] [Google Scholar]
- Parra D. 2013. La obligación de informar al paciente. Cuestiones sobre el derecho a ser informado. Revista Médica de Chile 141: 1578–83. [DOI] [PubMed] [Google Scholar]
- Peck R., and Norris C. W.. 2012. Significant risks of oral contraceptives (OCPs): Why this drug class should not be included in a preventive care mandate. Linacre Quarterly 79: 41–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peper J. S., and Koolschijn P. C.. 2012. Sex steroids and the organization of the human brain. Journal of Neuroscience 32: 6745–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phoenix C., Goy R. W., Gerall A. A., and Young W. C.. 1959. Organizing action of prenatally administered testosterone propionate on the tissues mediating mating behavior in the female guinea pig. Endocrinology 65: 369–82. [DOI] [PubMed] [Google Scholar]
- Piaget J. 1995. Commentary on Vygotsky's criticisms of language and thought of the child and judgment and reasoning in the child. New Ideas in Psychology 13: 325–40. [Google Scholar]
- Pletzer B., and Kerschbaum H. H.. 2014. 50 years of hormonal contraception – time to find out, what it does to our brain. Frontiers in Neuroscience 8: 256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pletzer B., Kronbichler M., and Kerschbaum H. H.. 2015. Differential effects of androgenic and anti-androgenic progestins on fusiform and frontal gray matter volumen and face recognition performance. Brain Research 1596: 108–15. [DOI] [PubMed] [Google Scholar]
- Pletzer B., Kronbichler M., Nuerk H. C., and Kerschbaum H. H.. 2014. Hormonal contraceptives masculinize brain activation patterns in the absence of behavioral changes in two numerical tasks. Brain Research 1543: 128–42. [DOI] [PubMed] [Google Scholar]
- Pletzer B., Krombinchler M., Aichhorn M., Bergmann J., Ladurner G., and Kerschbaum H. H.. 2010. Menstrual cycle and hormonal contraceptive use modulate human brain structure. Brain Research 1348: 55–62. [DOI] [PubMed] [Google Scholar]
- Pluschino N., Caruso A., and Daino D.. 2012. How does oral contraception affect mood? Archives of Perinatal Medicine 18: 137–41. [Google Scholar]
- Prewitt A. K., and Wilson M. E.. 2007. Changes in estrogen receptor-alpha mRNA in the mouse cortex during development. Brain Research 1134: 62–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasgon N. L., Carter M., Elman S., Bauer M., Love M., and Korenman S. G.. 2002. Common treatment of polycystic ovarian syndrome and major depressive disorder: Case report and review. Current Drug Targets-Immune, Endocrine & Metabolic Disorders 2: 97–102. [PubMed] [Google Scholar]
- Reavley A., Fisher A. D., Owen D., Creed F. H., and Davis J. R.. 1997. Psychological distress in patients with hyperprolactinaemia. Clinical Endocrinology (Oxford) 47: 343–8. [DOI] [PubMed] [Google Scholar]
- Reyna V. F., and Farley F.. 2007. El cerebro adolescente. Mente y Cerebro 26: 56–63. [Google Scholar]
- Ruben Barbara, and Caskey Micki M.. 2007. Under construction: The young adolescent brain. In The young adolescent and the middle school, ed. Mertens Steven B., Anfara Vincent A., and Caskey Micki M., 7–72. Charlotte: Information Age Publishing. [Google Scholar]
- Santen Richard J. 1999. The testis: Function and dysfunction. In Reproductive endocrinology: Physiology, pathophysiology, and clinical management, ed. Yen Samuel C. C., and Barbieri Robert, 632–68. Philadelphia: W.B. Saunders Company. [Google Scholar]
- Schulte-Rüther M., Markowitsch H. J., Shah N. J., Fink G. R., and Piefke M.. 2008. Gender differences in brain networks supporting empathy. NeuroImage 42: 393–403. [DOI] [PubMed] [Google Scholar]
- Schulz K. M., and Sisk C. L.. 2006. Pubertal hormones, the adolescent brain, and the maturation of social behaviors: Lessons from the Syrian hamster. Molecular and Cellular Endocrinology 254–255: 120–126. [DOI] [PubMed] [Google Scholar]
- Schulz K. M., Zehr J. L., Osetek A. J., and Sisk C. L.. 2005. Exposure to gonadal hormones during puberty influences the cross-sectional area of the adult male ventromedial hypothalamus in response to estradiol and progesterone. In Society for Neuroscience (Program No. 762.5, abstract viewer/Itinerary planner). Washington, DC. [Google Scholar]
- Schulz K. M., Richardson H. N., Zehr J. L., Osetek A. J., Menard T. A., and Sisk C. L.. 2004. Gonadal hormones masculinize and defeminize reproductive behaviors during puberty in the male Syrian hamster. Hormones and Behavior 45: 242–9. [DOI] [PubMed] [Google Scholar]
- Selye H. 1950. Stress and the general adaptation syndrome. British Medical Journal 1: 1383–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen H., Gong Q. H., Aoki C., Yuan M., Ruderman Y., Dattilo M., Williams K., and Smith S. S.. 2007. Reversal of neurosteroid effects at α4β2δ GABAA receptors triggers anxiety at puberty. Nature Neuroscience 10: 469–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman M. R., Corvol P. L., and O'Malley B. W.. 1970. Progesterone-binding components of chick oviduct. I. Preliminary characterization of cytoplasmic components. Journal of Biological Chemistry 245: 6085–96. [PubMed] [Google Scholar]
- Sigel Eric J. 2005. Adolescent growth and development. In Essential adolescent medicine, ed. Greydanus Donald E., Patel Dilip R., and Pratt Helen D., 3–15. New York: McGraw Hill Professional. [Google Scholar]
- Simerly R. B. 2002. Wired for reproduction: Organization and development of sexually dimorphic circuits in the mammalian forebrain. Annual Review of Neuroscience 25: 507–36. [DOI] [PubMed] [Google Scholar]
- Sinclair D., Purves-Tyson T. D., Allen K. M., and Weickert C. S.. 2014. Impacts of stress and sex hormones on dopamine neurotransmission in the adolescent brain. Psychopharmacology (Berlin) 231: 1581–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sisk C. L. 2016. Hormone-dependent adolescent organization of socio-sexual behaviors in mammals. Current Opinion in Neurobiology 38: 63–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sisk C. L., and Zehr J. L.. 2005. Pubertal hormones organize the adolescent brain and behavior. Frontiers in Neuroendocrinology 26: 163–74. [DOI] [PubMed] [Google Scholar]
- Spear L. 2008a. Le développement du cerveau et les patterns de conduits typiques pendant l'adolescence (1re partie). Psychiatrie, Sciences Humaines, Neurosciences 6: 149–54. [Google Scholar]
- Spear L. 2008b. Le développement du cerveau et les patterns de conduits typiques pendant l'adolescence (2e partie). Psychiatrie, Sciences Humaines, Neurosciences 6: 197–204. [Google Scholar]
- Teele S. 2004. Overcoming barricades to reading: A multiple intelligences approach. Thousand Oaks: Corwin Press. [Google Scholar]
- Teles M. G., Silveira L. F., Tusset C., and Latronico A. C.. 2011. New genetic factors implicated in human GnRH-dependent precocious puberty: The role of kisspeptin system. Molecular and Cellular Endocrinology 346: 84–90. [DOI] [PubMed] [Google Scholar]
- Van Wingen G. A., Ossewaarde L., Bäckström T., Hermans E. J., and Fernández G.. 2011. Gonadal hormone regulation of the emotion circuitry in humans. Neuroscience 191: 38–45. [DOI] [PubMed] [Google Scholar]
- Vigil Pilar. 2013. La Fertilidad de la Pareja Humana. Santiago: Ediciones Universidad Católica de Chile. [Google Scholar]
- Vigil P., Molina C. T., and Cortés M. E.. 2009. La sexualidad de las jóvenes chilenas. Ars Medica 18: 195–208. [Google Scholar]
- Vigil P., Orellana R. F., and Cortés M. E.. 2011. Modulation of spermatozoon acrosome reaction. Biological Research 44: 151–9. [PubMed] [Google Scholar]
- Vigil P., Ceric F., Cortés M. E., and Klaus H.. 2006. Usefulness of monitoring fertility from menarche. Journal of Pediatric and Adolescent Gynecology 19: 173–79. [DOI] [PubMed] [Google Scholar]
- Vigil P., Orellana R., Godoy A., Barrientos V., and del Río M. J.. 2009. Effects of gamma-amino butyric acid, progesterone and oestradiol on human spermatozoa acrosome reaction. In Papers contributed to the 9th International Congress of Andrology, ed. Josep Lluís Ballescà Lagarda, and Rafael Oliva Virgili, 107–11. Bologna: Medimond International Proceedings.
- Vigil P., Contreras P., Alvarado J. L., Godoy A., Salgado A. M., and Cortés M. E.. 2007. Evidence of subpopulations with different levels of insulin resistance in women with polycystic ovary syndrome. Human Reproduction 22: 2974–80. [DOI] [PubMed] [Google Scholar]
- Vogel W., Klaiber E. L., and Broverman D. M.. 1978. Roles of the gonadal steroid hormones in psychiatric depression in men and women. Progress in Neuro-Psychopharmacology 2: 487–503. [Google Scholar]
- Weiner C., Primeau M., and Ehrmann D.. 2004. Androgens and mood dysfunction in women: Comparison of women with polycystic ovarian syndrome to healthy controls. Psychosomatic Medicine 66: 356–62. [DOI] [PubMed] [Google Scholar]
- Weiten W. 2010. Psychology: Themes and variations. Wadsworth, CA: Cengage Learning. [Google Scholar]
- Westberry J. M., Trout A. L., and Wilson M. E.. 2011. Epigenetic regulation of estrogen receptor beta expression in the rat cortex during aging. NeuroReport 22: 428–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yurgelun-Todd D. 2007. Emotional and cognitive changes during adolescence. Current Opinion in Neurobiology 17: 251–57. [DOI] [PubMed] [Google Scholar]