Introduction
It is recognized that chronic right ventricular pacing (RVP) has deleterious effects on cardiac function, including decreased global left ventricular (LV) function and worsening functional status.1,2 This is a result of abnormal myocardial activation due to slow muscle-to-muscle conduction, compared to the rapid conduction of the His-Purkinje system (HPS).3 Similarly, left bundle branch block (LBBB) results in dyssynchronous contraction, with delayed electrical activation of the lateral wall of the left ventricle relative to the interventricular septum.
Cardiac resynchronization therapy (CRT) addresses the dyssynchronous activation created by RVP as well as LBBB, with earlier activation of the LV by a lead placed via the coronary venous system. CRT has been shown to improve mortality and non-fatal heart failure events.4–6 His bundle pacing (HBP) has emerged as a means of restoring normal myocardial activation through the native conduction system in those with proximal HPS disease. Early studies have supported HBP as non-inferior to traditional CRT, and better than RVP when it comes to clinical outcomes.7,8
Although the idea of utilizing the native conduction system and its clinical benefits are intuitive, the mechanisms behind HBP are still not well understood. Traditionally, the success of HBP has been posited on the theory of longitudinal dissociation, that fibers within the His Bundle (HB) are pre-destined for their respective bundles. Therefore a block that occurred within the HB could be bypassed by pacing distal to the block but proximal to the bifurcation of the bundles.9 However, more recent studies have shown success in patients that may not be expected to have a block within the proximal HB, suggesting there may be alternative, and likely complimentary mechanisms underlying HBP.10
Further details of the history, technique, criteria, and benefits of HBP are described elsewhere in this symposium on HBP. In this section, we will focus on the potential mechanisms underlying QRS narrowing with HBP, including the classic understanding of longitudinal dissociation and possible alternative mechanisms, including penetration of the proximal HPS, output dependence, and virtual electrode polarization effect. Furthermore, we describe how these mechanisms may translate to non-selective versus selective HBP and result in narrowing versus normalization of the QRS interval.
Longitudinal dissociation
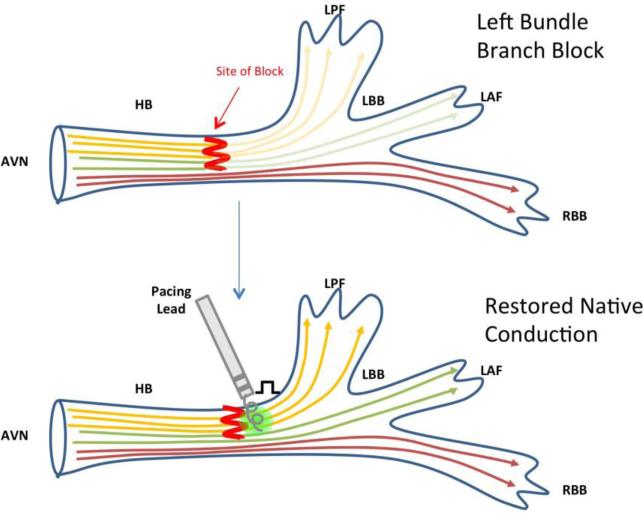
Longitudinal dissociation is the theory that bundle branch block may originate within the proximal HB when block occurs in the fibers predestined for the left or right bundle branches (Figure 1). This concept dates back the early 1900s when Kaufman and Rothberger, and later Condorelli (1930s), and Sciacca & Sangiorgi (1950s) demonstrated that traumatic HB lesions could result in bundle branch block patterns.11,12 Further evaluation of the anatomy and electrophysiological properties of the HPS as evaluated in human and canine hearts in the 1970s by James and Sherf identified fibers separated by collagen sheaths longitudinally directly into the bundle branches.13 Based on this, El-Sherif et al. suggested that block could occur more proximally, within the HB, but still manifest as a bundle branch block pattern. This theory was demonstrated in canine experiments, where conduction delay within the HB identified by split HB potential was associated with the appearance of a bundle branch block pattern following anterior septal artery ligation.14
Figure 1. Restoration of normal conduction in left bundle branch block due to longitudinal dissociation.
Simplified construct of fibers coursing through the AVN surrounded by parallel sheaths that extend into the bundle branches, making each fiber “pre-destined.” Pacing beyond the level of the block restores conduction through the His-Purkinje system.25
This theory was supported by Narula, who demonstrated that advancing a catheter more distally within the HB could restore the native QRS, suggesting a block within the HB could be bypassed.9 Barba-Pichardo et al. explored the feasibility of permanent HBP and concluded that the success of HBP in normalizing the QRS was determined by how proximal the block was, making the block bypassable.15 As depicted in Figure 1, pacing from a catheter distal to this site of block restores normal conduction via the HPS.
Although it is likely that location of the block and the ability to bypass it plays a role in the mechanism behind HBP, Teng et al. recently studied the relationship between QRS axis and QRS narrowing with HBP. It was hypothesized that a left axis deviation in the setting of strictly defined complete LBBB signifies escape of left posterior fascicular fibers prior to the level of the block, therefore representing a more distal block that would not be bypassed. However, there was no correlation between absolute QRS narrowing and QRS axis using this paradigm. Further, 83% of the 29 patients studied exhibited narrowing.10 Similar success rates have been found in other recent studies.7,8 While it is possible that a high percentage of LBBBs may be within the proximal HB, or that HB lead placement is actually extending beyond the HB and has the ability to capture and bypass more distal blocks than traditionally considered, it is more likely that there are complementary mechanisms at play. Lazzara et al. studied the effects of incisions within the HB followed by intracellular stimulation. Restoration of native conduction via intracellular stimulation suggested transverse connections exist and allow for signal to travel across fibers, challenging the theory of longitudinal dissociation. However, the authors concluded that perhaps these concepts could be reconciled by recognizing that under certain circumstances transverse interconnections do not function.16 Still the question remains that if transverse connections do coexist with longitudinal tracts, then it should follow that native conduction would be able to circumnavigate around a block, negating the need for an external pacing stimulus. These questions highlight the limitations of these theories and suggest that additional mechanisms must exist.
Output dependence
It has been observed that slight increases in stimulus strength during distal HBP can result in normalization of bundle branch block. While the focus remained on the theory of longitudinal dissociation and the need for high pacing outputs to penetrate the HB sheath or to overcome a physical structural block (for example scar). It is possible that applying increasing stimulus strength could recruit fibers closely bordering the abnormal myocardium causing functional block, and mimic native conduction through the HPS.9
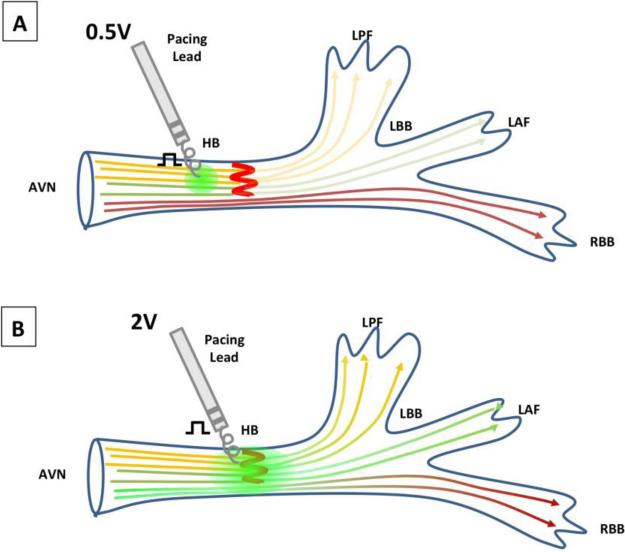
Thus perhaps, HBP pacing relies not only on bypassing a structural block, but may also depend on overcoming a block in local propagation by applying a high effective output to a region of the block. In general, HBP requires higher pacing thresholds than traditional RVP.17 In early feasibility studies on permanent HBP implantation, Desmukh et al. reported mean pacing thresholds of 2.4 ±0.9V. At the time, using pacing leads designed for traditional RVP, they noted challenges in precisely directing the stylet to the small area of the HB and the short length of the helix preventing deep enough penetration.17 Similarly, Barba-Pichardo et al. reported pacing thresholds required to be 2.2 ± 1.1V, while Kronberg et al. noted average thresholds of 2.3V ± 1 in selective HBP and 1.7 ± 1.5V in non-selective HBP at the time of implantation.15,18 However, as technology has improved with pacing leads and sheaths specifically designed for HBP, and operator experience has improved, voltage requirements have decreased, with equal or higher success rates.7,19 Vijayaraman et al. achieved a successful HBP rate of 84% in a group of 100 patients with AV nodal or infranodal disease, only requiring pacing thresholds of 1.3 ±0.7V.7 By current standards, pacing thresholds less than 2V are considered acceptable.19 As demonstrated in figure 2, higher levels of output correspond with a larger area of capture, thereby reaching beyond the block. As mentioned earlier, an important and alternative explanation for higher thresholds certainly includes the need to overcome the resistivity of the fibrous sheath encasing the HB.
Figure 2. Output dependence as a mechanism of native QRS restoration in bundle branch block.
Simplified schematic demonstrating that with increasing output voltages, the size of local depolarization increases. In figure 2A, 0.5V is not enough to overcome the block. However, with increasing output (to 2V) as shown in 2B, the region of local capture extends beyond the level of HB block, and restores native His-Purkinje conduction.
Virtual Electrode Polarization
An alternative theory underlying HBP-mediated QRS narrowing rests on the mechanism of virtual electrode polarization initially studied in the setting of defibrillation.20 The delivery of charge to an area of tissue creates virtual electrodes, with an anode and cathode locally, which can redistribute charges within the myocardium and alterations in transmembrane polarization.20 Sepulveda et al. used a bidomain model, where cardiac tissue is composed of fibers with different conductivities across their lengths, to study the effect of applying a current to cardiac tissue. They described that point stimulation from a catheter creates a pattern of polarization in a “dog-bone” shape characterized by two hyperpolarized virtual anode regions running parallel to the fibers being stimulated.21,22 As applied to pacing, local virtual electrodes are created with depolarized and hyperpolarized regions surrounding the pacing electrode tip, which can result in recovery from inexcitability of diseased local tissue impeding propagation.7
Implications of different pacing mechanisms: selective vs. non-selective and QRS narrowing vs. normalization
The effects of HBP may also lend hints to the mechanisms of HBP. First, selective and non-selective HBP are two subgroups of HBP. Selective HBP is indicated by stimulus to ventricular activation is equal to the intrinsic His-ventricular interval, and paced QRS morphology is identical to the intrinsic QRS complex. Conversely, non-selective pacing represents fusion of captured neighboring ventricular myocardium and the HB. It is characterized by a shorter stimulus to ventricular activation compared to the intrinsic His-ventricular (HV) interval, and QRS morphology can vary depending on output.19.
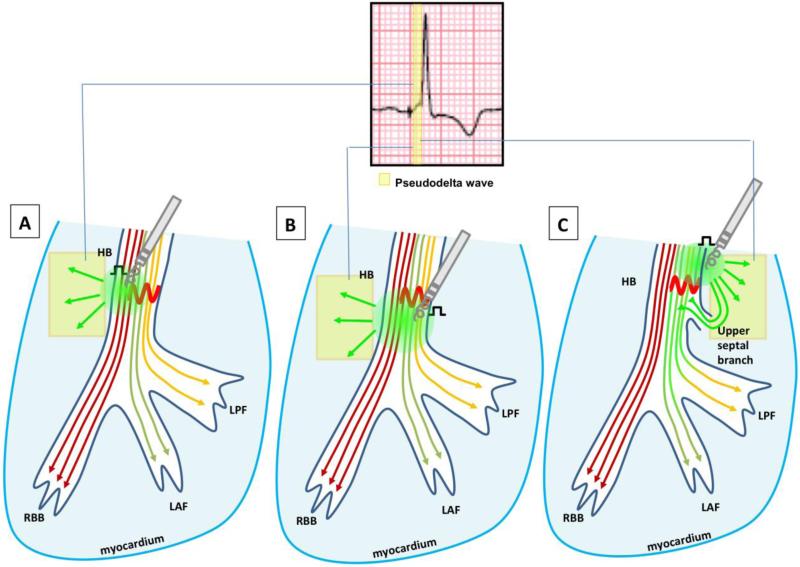
Non-selective HBP is related to proximity to conducting HB tissue, i.e. the pacing lead captures the HB and local myocardium, but is close enough that the paced wavefront engages the HPS relatively quickly, creating a pseudo-delta wave (Figure 3). It relies more heavily on output, as pacing amplitude in the setting of non-selective pacing can dramatically affect the QRS. When high output is applied to non-selective pacing it can preferentially recruit the HB with progressive widening as output is reduced. Alternatively, the high output can capture more of the local ventricular tissue, and the QRS narrows as output is lessened due to less fusion.19 As such, non-selective HBP is influenced by output due to the interaction of nearby ventricular myocardium with His capture. (Figure 3)
Figure 3. Potential mechanisms of non-selective His bundle capture and QRS narrowing or normalization.
Simplified depiction ofpossible mechanisms of non-selective His bundle pacing (HBP). The area of myocardial capture is represented by a slurring in the QRS prior to capture of the His-Purkinje system (HPS). (A) High enough output overcomes His bundle block but also captures neighboring myocardium. (B) Placement of the pacing lead is distal to the site of block and bypasses it, but also captures neighboring myocardium. (C) Placement of the pacing lead in the myocardium within the vicinity of the HB and in close proximity to an upper septal branch results in capture of adjacent local myocardium and HB. 24 The pacing wavefront can then travel the short distance to the upper septal branch, penetrate and activate the normal distal HPS.
Given that selective HBP, on the other hand, mimics the HV interval and QRS morphology of the intrinsic pattern, it can be assumed that this represents placement of the catheter directly within the HB (or if any capture occurs of the local myocardium it is concealed). Therefore, it is likely that selective HBP is more consistent with longitudinal dissociation, bypassing the block within the HB. Both non-selective and selective HBP likely have overlapping mechanisms, and likewise the concept of virtual electrode polarization effect plays a role in both in creating fields of depolarization in or around the areas of block.
Another outcome of HBP is whether the QRS interval narrows or completely normalizes (QRS≤100-120ms) in bundle branch block ECG patterns. While complete QRS normalization likely represents completely bypassing or overcoming a block, partial narrowing could result from lead placement, variations in anatomy, distal or complex block within the HPS as the cause of the QRS delay, or a combination of these factors. Studies of the HPS anatomy have shown that the HB measures only 20mm in length and 4mm in diameter. The HB lies towards the left of the septum in most, but in approximately thirty percent of people it runs for several millimeters down the muscular septum or to the right of the interventricular septal crest.23 Given the inherently imprecise nature of placing the HBP lead, the small target area that is the HB, and the anatomical variation between patients, there are many combinations in which the HB is partially captured and may result in a lesser degree of narrowing.
Conclusions
The precise mechanisms underlying HBP-mediated QRS narrowing remain unclear and are likely multifactorial. Whether its success lies in bypassing a block, overcoming a functional block with effective voltage delivery, hyperpolarizing dormant HB tissue, or an alternative mechanism altogether, it is clear that the success rates of executing HBP, narrowing the QRS interval, and minimizing pacing thresholds and procedure times are rapidly improving over time. The combination of improvement in operator experience and insights into possible mechanisms has led to the development of a wider variety of tools. It is likely that these mechanisms work in tandem, where the ability to place a pacing lead directly into the HB allows for lower required voltage outputs.
With significant QRS narrowing seen in as high as 83% of cases, it raises questions regarding the unsuccessful cases. Do these cases represent conduction disease so distal they cannot be overcome, or block due to severe calcification such that no level of output could penetrate through to the functional fibers? Perhaps the difference is anatomic variation between patients, where the anatomy of the HPS makes accepted lead placement techniques irrelevant. Use of technologies to assess cardiac electrical propagation may improve our understanding of the causes of bundle branch block and may help determine which patients might benefit from HBP. Technologies such as ECG imaging (ECGI) may allow visualization of upper septal activation and suggest which patients have proximal block.24 Despite the limitations in our understanding, HBP remains an important emerging tool in cardiac pacing. Further studies are warranted to improve our understanding, and for patient selection.
Highlights.
In this review article, we
discuss known and putative mechanisms of conduction block within the His bundle, resulting in bundle branch block.
discuss physiological mechanisms by which His bundle pacing narrows or normalizes the QRS interval.
discuss the differences and physiological bases for direct vs. indirect His bundle capture.
discuss the physiological mechanisms for QRS narrowing vs. normalization following His bundle pacing.
Acknowledgements
This work was supported by NIH/NHLBI HL125730 to OAA.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: None
REFERENCES
- 1.Wilkoff BL, Kudenchuk PJ, Buxton AE, et al. The DAVID (Dual Chamber and VVI Implantable Defibrillator) II Trial. J Am Coll Cardiol. 2009;53(10):872–880. doi: 10.1016/j.jacc.2008.10.057. [DOI] [PubMed] [Google Scholar]
- 2.Sharma AD, Rizo-Patron C, Hallstrom AP, et al. Percent right ventricular pacing predicts outcomes in the DAVID trial. Hear Rhythm. 2005;2(8):830–834. doi: 10.1016/j.hrthm.2005.05.015. [DOI] [PubMed] [Google Scholar]
- 3.Lee MY, Yeshwant SC, Lustgarten DL. Honing in on Optimal Ventricular Pacing Sites: an Argument for His Bundle Pacing. Curr Treat Options Cardiovasc Med. 2015;17(4):13. doi: 10.1007/s11936-015-0372-3. [DOI] [PubMed] [Google Scholar]
- 4.Moss AJ, Hall J, Cannom DS, et al. Cardiac-resynchronization therapy for the prevention of heart-failure events. N Eng J Med. 2009;361(14):1329–1338. doi: 10.1056/NEJMoa0906431. [DOI] [PubMed] [Google Scholar]
- 5.Bristow MR, Saxon LA, Boehmer J, Krueger S, Kass D a, Marco T De, et al. Cardiac-Resynchronization Therapy with or without an Implantable Defibrillator in Advanced Chronic Heart Failure. N Eng J Med. 2009;350:2140–2150. doi: 10.1056/NEJMoa032423. [DOI] [PubMed] [Google Scholar]
- 6.Cleland JGF, Daubert J-C, Erdmann E, et al. The effect of cardiac resynchronization on morbidity and mortality in heart failure. N Engl J Med. 2005;352(15):1539–1549. doi: 10.1056/NEJMoa050496. [DOI] [PubMed] [Google Scholar]
- 7.Vijayaraman P, Naperkowski A, Ellenbogen KA, Dandamudi G. Electrophysiologic Insights into Site of Atrioventricular Block Lessons from Permanent His Bundle Pacing. JACC Clin Electrophysiol. 2015;1(6):571–581. doi: 10.1016/j.jacep.2015.09.012. [DOI] [PubMed] [Google Scholar]
- 8.Lustgarten DL, Crespo EM, Arkhipova-Jenkins I, et al. His-bundle pacing versus biventricular pacing in cardiac resynchronization therapy patients: A crossover design comparison. Hear Rhythm. 2015;12(7):1548–1557. doi: 10.1016/j.hrthm.2015.03.048. [DOI] [PubMed] [Google Scholar]
- 9.Narula OS. Longitudinal dissociation in the His bundle. Bundle branch block due to asynchronous conduction within the His bundle in man. Circulation. 1977;56(6):996–1006. doi: 10.1161/01.cir.56.6.996. [DOI] [PubMed] [Google Scholar]
- 10.Teng AE, Lustgarten DL, Tung R, Shivkumar K, Wagner GS, Ajijola OA. His Bundle Pacing to Achieve Electrical Resynchronization in Patients with Complete Left Bundle Branch Block: The Relationship Between Native QRS Axis, Duration, and Normalization. Am J Cardiol. 2016 doi: 10.1016/j.amjcard.2016.05.049. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Condorelli L. Schenkelblock durch Laesion des Tawaraknotens. Sonderabdruck aus den Verhandlungen der Dtsch Gesellschaft fur Kreislauorschung. 1932 [Google Scholar]
- 12.Sciacca A, Sangiorgi M. Trouble de la conduction intraventriculaire droite du a la lesion du tronc common du faiseau de His. Acta Cardiol. 1957;2:486. [PubMed] [Google Scholar]
- 13.James TN, Sherf L. Fine Structure of the His Bundle. Circulation. 1971;44(1):9–28. doi: 10.1161/01.cir.44.1.9. [DOI] [PubMed] [Google Scholar]
- 14.El-Sherif N, Amay-Y-Leon F, Schonfield C, et al. Normalization of bundle branch block patterns by distal His bundle pacing. Clinical and experimental evidence of longitudinal dissociation in the pathologic his bundle. Circulation. 1978;57(3):473–483. doi: 10.1161/01.cir.57.3.473. [DOI] [PubMed] [Google Scholar]
- 15.Barba-Pichardo R, Moriña-Vázquez P, Fernández-Gómez JM, Venegas-Gamero J, Herrera-Carranza M. Permanent His-bundle pacing: Seeking physiological ventricular pacing. Europace. 2010;12(4):527–533. doi: 10.1093/europace/euq038. doi:10.1093/europace/euq038. [DOI] [PubMed] [Google Scholar]
- 16.Lazzara BR, Yeh BK, Samet P. Functional Transverse Interconnections within the His Bundle and the Bundle Branches. Circ Res. Apr. 1973;32(4):509–15. doi: 10.1161/01.res.32.4.509. [DOI] [PubMed] [Google Scholar]
- 17.Deshmukh P, Casavant DA, Romanyshyn M, Anderson K. Permanent, direct His-bundle pacing: a novel approach to cardiac pacing in patients with normal His-Purkinje activation. Circulation. 2000;101(8):869–877. doi: 10.1161/01.cir.101.8.869. [DOI] [PubMed] [Google Scholar]
- 18.Kronborg MB, Mortensen PT, Gerdes JC, Jensen HK, Nielsen JC. His and para-His pacing in AV block: Feasibility and electrocardiographic findings. J Interv Card Electrophysiol. 2011;31(3):255–262. doi: 10.1007/s10840-011-9565-1. [DOI] [PubMed] [Google Scholar]
- 19.Dandamudi G, Vijayaraman P. How To Perform Permanent His Bundle Pacing In Routine Clinical Practice. Heart Rhythm. 2016;13(6):1362–1366. doi: 10.1016/j.hrthm.2016.03.040. [DOI] [PubMed] [Google Scholar]
- 20.Cheng Y, Mowrey K a, Van Wagoner DR, Tchou PJ, Efimov IR. Virtual electrode-induced reexcitation: A mechanism of defibrillation. Circ Res. 1999;85(11):1056–1066. doi: 10.1161/01.res.85.11.1056. [DOI] [PubMed] [Google Scholar]
- 21.Sepulveda NG, Roth BJ, Wikswo JP. Current injection into a two-dimensional anisotropic bidomain. Biophys J. 1989;55(5):987–999. doi: 10.1016/S0006-3495(89)82897-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sambelashvili AT, Nikolski VP, Efimov IR. Virtual electrode theory explains pacing threshold increase caused by cardiac tissue damage. Am J Physiol Heart Circ Physiol. 2004;286(6):H2183–H2194. doi: 10.1152/ajpheart.00637.2003. [DOI] [PubMed] [Google Scholar]
- 23.Syed FF, Hai JJ, Lachman N, DeSimone C V, Asirvatham SJ. The infrahisian conduction system and endocavitary cardiac structurs: relevance for the invasive electrophysiologist. J Interv Card Electrophysiol. 2014;39(1):45–56. doi: 10.1007/s10840-013-9858-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Durrer D, van Dam RT, Freud GE, Janse MJ, Meijler FL, Arzbaecher RC. Total excitation of the isolated human heart. Circulation. 1970;41(6):899–912. doi: 10.1161/01.cir.41.6.899. [DOI] [PubMed] [Google Scholar]
- 25.Demoulin JC, Kulbertus HE. Histopathological examination of concept of left hemiblock. Br Heart J. 1972;34(8):807–814. doi: 10.1136/hrt.34.8.807. [DOI] [PMC free article] [PubMed] [Google Scholar]